Abstract
Extreme droughts are expected to increase in frequency and severity in many regions of the world, threatening multiple ecosystem services provided by forests. Effective strategies to adapt forests to such droughts require comprehensive information on the effects and importance of the factors influencing forest resistance and resilience. We used a unique combination of inventory and dendrochronological data from a long‐term (>30 years) silvicultural experiment in mixed silver fir and Norway spruce mountain forests along a temperature and precipitation gradient in southwestern Germany. We aimed at examining the mechanisms and forest stand characteristics underpinning the resistance and resilience to past mild and severe droughts. We found that (i) fir benefited from mild droughts and showed higher resistance (i.e., lower growth loss during drought) and resilience (i.e., faster return to pre‐drought growth levels) than spruce to all droughts; (ii) species identity determined mild drought responses while species interactions and management‐related factors strongly influenced the responses to severe droughts; (iii) intraspecific and interspecific interactions had contrasting effects on the two species, with spruce being less resistant to severe droughts when exposed to interaction with fir and beech; (iv) higher values of residual stand basal area following thinning were associated with lower resistance and resilience to severe droughts; and (v) larger trees were resilient to mild drought events but highly vulnerable to severe droughts. Our study provides an analytical approach for examining the effects of different factors on individual tree‐ and stand‐level drought response. The forests investigated here were to a certain extent resilient to mild droughts, and even benefited from such conditions, but were strongly affected by severe droughts. Lastly, negative effects of severe droughts can be reduced through modifying species composition, tree size distribution and stand density in mixed silver fir‐Norway spruce forests.
Keywords: Abies alba, adaptation strategies, climate change, forest management, inventory data, Picea abies, species interaction, tree rings
We examined mechanisms and stand characteristics underpinning the growth resistance (Rt), recovery (Rc) and resilience (Rs) to past mild and severe droughts in mixed silver fir and Norway spruce mountain forests in southwestern Germany. We found that the forests investigated were to a certain extent resilient to mild droughts, and even benefited from such conditions, but were strongly affected by severe droughts (especially those stands with higher residual basal area and larger trees). Lastly, negative effects of severe droughts can be reduced through modifying species composition, tree size distribution and stand density in mixed silver fir‐Norway spruce forests.

1. INTRODUCTION
Gradual changes in climate and the increasing occurrence of extreme climatic events and disturbances are threatening the ecological stability of forests as well as multiple ecosystem services they provide (McDowell et al., 2018; Seidl et al., 2017). The stability properties of a system can be viewed as being composed of resistance, that is, the degree to which the system state changes following a perturbation, and resilience, that is, the processes that allow the system to return to its pre‐perturbation state (Larsen, 1995; Pimm, 1984). These stability concepts can be applied at different levels, for example, to tree populations or to individual trees (Lloret et al., 2011; Schwarz et al., 2020). Maintaining or improving resistance and resilience is an important goal of sustainable forest management to stabilize forest functions and assure the continuous provisioning of ecosystem services in the face of climate change (Scheffer et al., 2001; Seidl et al., 2016).
Among extreme climatic events, droughts are projected to increase in frequency and severity in many regions of the world (IPCC, 2014), with detrimental impacts on the resistance and resilience of individual trees and forest ecosystems in all climatic regions in which forests occur (Bauhus, Forrester, Gardiner, et al., 2017; McDowell et al., 2018). Drought can affect the carbon and water balance, impair plant functioning, reduce primary and secondary growth, impede tree recruitment and increase tree mortality (Anderegg et al., 2013; Schuldt et al., 2020; Senf et al., 2020). The effects of drought on plant functioning may thus trigger large changes in productivity, structure, composition and distribution of entire forest ecosystems (Ciais et al., 2005; Rigling et al., 2013).
Biodiversity is often considered a key feature supporting the resistance and resilience of ecosystem functions to extreme droughts (Isbell et al., 2015; Oliver et al., 2015). Owing to the complementarity among tree species, forest biodiversity may contribute to climate change mitigation by enhancing and stabilizing the productivity of mixed‐species forests at stand to biome scales (del Río et al., 2017; Jucker et al., 2014). Biodiversity–ecosystem functioning relationships in forests are, however, not always positive and vary considerably across regions (Grossiord, Granier, Ratcliffe, et al., 2014), showing a strong dependence on the environmental context (Ratcliffe et al., 2017) and the actual species composition (Ammer, 2019). This applies also to the resistance and resilience to stress, including drought (Bauhus, Forrester, Gardiner, et al., 2017; Forrester et al., 2016; Grossiord, 2020). A positive effect of species diversity on growth resistance and/or resilience to drought of individual tree species has been observed for European beech (Fagus sylvatica L.; Pretzsch et al., 2013) and silver fir (Abies alba Mill.; Gazol & Camarero, 2016; Vitali, Forrester, et al., 2018), whereas the opposite effect has been documented for Norway spruce (Picea abies (L.) Karst.; Vitali, Forrester, et al., 2018). Only weak interspecific effects were found for silver fir and Norway spruce (Dănescu et al., 2018).
In addition to regulating species composition, forest management can have a profound effect on competition for water and light via changing stand density and tree size distribution. Water is a prime limiting factor for plant functioning in drought‐prone environments particularly during hot or dry periods, whereas competition for light is more important on sites generally not limited by water (Forrester, 2014). However, few studies have quantified both water and light competition along environmental gradients for a given species (Forrester, 2014). Maintaining stands at reduced levels of basal area to avoid strong competition is advocated as a management strategy to impart resistance and/or resilience to drought (e.g., Bottero et al., 2017; Sohn et al., 2016), with potential positive repercussions on the adaptation and/or mitigation potential of forest ecosystems (Brang et al., 2014). Especially under generally dry conditions, stand basal area reductions were found to increase water availability and reduce drought stress of the remaining trees (Giuggiola et al., 2016; Manrique‐Alba et al., 2020). This effect, however, may vary among sites (cf. Simon et al., 2017) and depend on drought severity (Gleason et al., 2017).
In the face of climate change, concerns have been raised about the future performance of economically important tree species and the sustainability of the valuable ecosystem services provided by European forests (Hanewinkel et al., 2013). Silver fir and Norway spruce are two of the most abundant and economically important tree species in Europe (San‐Miguel‐Ayanz et al., 2016), whose distribution has been profoundly affected by millennia of human interventions (Caudullo et al., 2016; Tinner et al., 2013). Potential range shift and dynamic vegetation models indicate that species like Norway spruce are likely to decline in the absence of adaptation measures, with severe economic (Hanewinkel et al., 2013) and ecological repercussions (Mina et al., 2017; Temperli et al., 2012). Silver fir may also be negatively affected by increasing aridity mostly at its south‐western distribution limit but may, in contrast, respond positively to warming outside Mediterranean areas, at high elevation or otherwise cool sites, where its growth is currently temperature‐limited (Gazol et al., 2015; Vitasse, Bottero, Rebetez, et al., 2019). Some of these changes in tree species composition have recently accelerated through the widespread tree mortality triggered by the extreme dry and hot years in 2018 and 2019, and associated with extensive bark beetle outbreaks (cf. Schuldt et al., 2020). There is, hence, a strong interest in developing forest management adaptation strategies based on robust information about the effects and importance of different factors influencing tree resistance and resilience, such as climate, management, species interactions and mechanisms that regulate responses to stress, including drought (Yousefpour et al., 2017).
Numerous dendroecological studies on silver fir and Norway spruce support the higher growth susceptibility of Norway spruce to drought (e.g., Lévesque et al., 2013; Vitasse, Bottero, Cailleret, et al., 2019). Most of these studies, however, focus on dominant and/or codominant trees, or ignore the effects of tree size on the drought response, potentially leading to a large bias of growth rates and trends (Forrester, 2019; Nehrbass‐Ahles et al., 2014) and an overestimation of climate change impacts on forest growth (Klesse et al., 2018). For example, drought may affect different tree size classes to different degrees. However, the evidence for such size effects in uneven‐aged forests is ambiguous (Dănescu et al., 2018; Pretzsch et al., 2018). In addition, many studies have focused on the drought response at the tree level, with no or limited consideration of forest stands, management and other factors (e.g., species interactions and traits related to resource‐use efficiency regarding photosynthetically active radiation or soil resources) that may profoundly affect such responses. Ignoring these effects could also lead to overestimates of the importance of some variables at the expense of others.
Here, we provide an analytical approach to evaluate the drought response at the tree and stand scale that takes advantage of a unique combination of forest inventory and dendrochronological data from a long‐term silvicultural experiment to examine the mechanisms and stand characteristics underpinning the growth resistance and resilience to drought of silver fir and Norway spruce growing in mixed stands along a temperature and precipitation gradient in southwestern Germany. The analyzed stands are part of a research project initiated between 1979 and 1981 (shelterwood experiment; Weise, 1995) and were subjected to different experimental thinning intensities leading to different levels of stand densities.
The three specific objectives of this study were as follows.
To evaluate the tree‐ and stand‐level reactions of silver fir and Norway spruce growing in mixtures to past drought events of different severity; we hypothesized that each species would show different responses to mild and severe drought events, with silver fir being more resistant and resilient to drought than Norway spruce. Additionally, we hypothesized that stand‐level responses would be less pronounced than those at the individual tree level.
To quantify the relative importance of different tree‐, site‐ and drought‐related factors that influence tree‐ and stand‐level growth responses to drought, we hypothesized that different factor combinations would influence tree‐ and stand‐level responses to mild vs. severe droughts, and that the effect of management would be weaker than what was observed in drier environments.
Lastly, to provide quantitative information on stand characteristics and tree growth‐related mechanisms that support the drought resistance and resilience of the two species to advise forest management in the face of climate change.
2. MATERIALS AND METHODS
2.1. Study sites
The six study sites are located in southwestern Germany and distributed over the major natural range of silver fir and Norway spruce in the Black Forest (Schwarzwald), the Swabian‐Franconian Forest (Schwäbisch‐Fränkischer Wald) and the southwestern Swabian Jura (Schwäbische Alb; Weise, 1995; Figure 1). They support mixed‐species mountain forests consisting mainly of silver fir (Abies alba Mill.; hereafter called fir), Norway spruce (Picea abies (L.) Karst.; hereafter called spruce) and European beech (Fagus sylvatica L.; hereafter called beech), with interspersed individuals of Scots pine (Pinus sylvestris L.). The climate is temperate to cool‐temperate and mean total annual precipitation ranges from c. 910 to 1920 mm for the period 1881–2016 (Table 1). The warmest month is July (15.1 ± 1.9℃) and the coldest month is January (−1.8 ± 2.6℃) at all sites. The study sites are part of a long‐term silvicultural experiment initiated between 1979 and 1981 to investigate tree and regeneration responses to the irregular group shelterwood regeneration method (Femelschlag; Puettmann et al., 2009; Weise, 1995). Before the initiation of the experiment, the stands were mature even‐aged, naturally regenerated and no harvesting interventions had taken place in the decade preceding the installation of the plots. At the time of the research installation, the treatments were cut to 75% of the volume of a fully stocked stand. The experimental stands analyzed in this study comprised two Femelschlag regeneration cutting regimes (treatments) differing in terms of the length of the regeneration period (and, thus, the speed of removal of canopy trees, namely slow and medium) and increment controls (stands maintained fully stocked via the periodic removal mostly of dead and damaged trees). The interventions were planned at 5‐year intervals in each stand (for more details on the experiment, see Appendix S1). This resulted in a variety of tree growth conditions reflecting a range of stand basal area (Figure 2a–f), tree stem density (Figure S1), vertical structure (Dănescu et al., 2016) and different levels of intraspecific and interspecific competition (Forrester et al., 2013).
FIGURE 1.
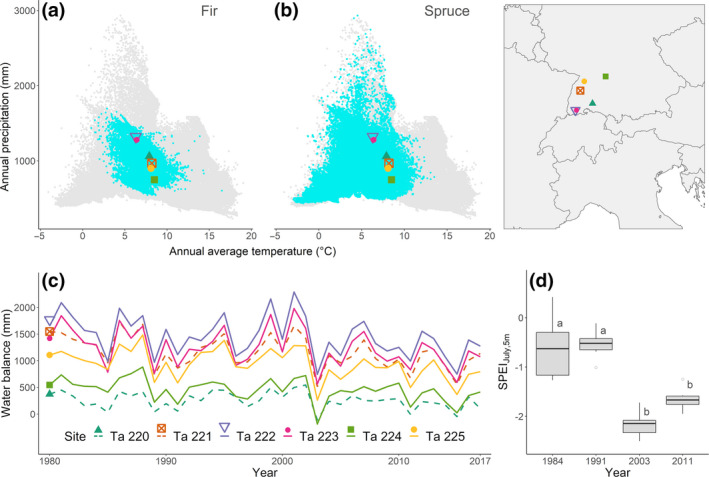
Location of study sites in central Europe, with climate‐space diagrams for (a) fir and (b) spruce; gray dots are all forest field observations in Europe, cyan dots are observed presence of the species in Europe and different symbols denote the six study sites. Presence data and temperature and precipitation data used in the climate‐space diagrams were extracted from the European Atlas of Forest Tree Species (San‐Miguel‐Ayanz et al., 2016), and from WorldClim 2 (Fick & Hijmans, 2017) for the study sites. (c) Annual climatic water balance (annual sum of precipitation – annual potential evapotranspiration) for the period 1980–2016 across study sites. (d) Box plots of the Standardized Precipitation and Evapotranspiration Index of July at the time scale of 5 months (SPEIJuly,5m) for the four drought events analyzed. Different letters indicate significant differences among years (ANOVA test, α < 0.05)
TABLE 1.
Site and stand information of the six study sites in southwestern Germany
| Ta 220 | Ta 221 | Ta 222 | Ta 223 | Ta 224 | Ta 225 | |
|---|---|---|---|---|---|---|
| Region | Swabian Jura | Black Forest | Black Forest | Black Forest | Swabian‐Franconian Forest | Black Forest |
| Latitude | 47°58,4′ | 48°25,8′ | 47°43,9′ | 47°44,0′ | 48°56,2′ | 48°45,7′ |
| Longitude | 8°52,5′ | 8°13,9′ | 7°58,4′ | 8°1,5′ | 9°34,0′ | 8°26,4′ |
| Elevation (m a.s.l.) | 830 | 720 | 1020 | 1020 | 520 | 700 |
| Slope (°) | 3 | 26 | 14 | 12 | 7 | 14 |
| Aspect (°) | 53 | 127 | 170 | 75 | 184 | 63 |
| Parent materiala | Limestone | Sandstone | Gneiss | Granite | Sandstone | Sandstone |
| Soil conditionsa | Well drained, loamy | Well drained, sandy | Well drained, loamy | Well drained, loamy | Sandy top layer over loamy layer | Well drained, sandy, acid |
| MAT (°C) 1881–1979/1980–2016 | 6.8/7.4 | 6.4/7.2 | 4.8/5.5 | 5.3/6.0 | 7.6/8.3 | 6.8/7.5 |
| MAP (mm) 1881–1979/1980–2016 | 900/947 | 1679/1758 | 1887/2019 | 1703/1849 | 1044/1149 | 1441/1550 |
| Mean basal area (m2/ha) 1979 | 47.9 | 42.1 | 41.0 | 44.2 | 39.5 | 39.6 |
| Mean tree agea fir/spruce (years) 1979 | 103/91 | 120/126 | 95/92 | 116/107 | 108/103 | 98/91 |
| Mean tree heighta fir/spruce (m) 1979 | 28.9/29.9 | 25.8/27.1 | 29.8/30.9 | 29.8/30.9 | 29.7/30.4 | 27.4/29.0 |
| Proportiona fir/spruce (%) 1979 | 76/20 | 43/57 | 60/24 | 23/63 | 37/63 | 53/47 |
| Number of stands analyzed | 5 | 2 | 2 | 2 | 3 | 2 |
| Number of trees inventoried | 380 | 214 | 199 | 329 | 345 | 207 |
| Number of discs collected | 81 | 51 | 20 | 31 | 75 | 31 |
Abbreviations: MAP, mean total annual precipitation; MAT, mean annual temperature.
Weise (1995).
FIGURE 2.
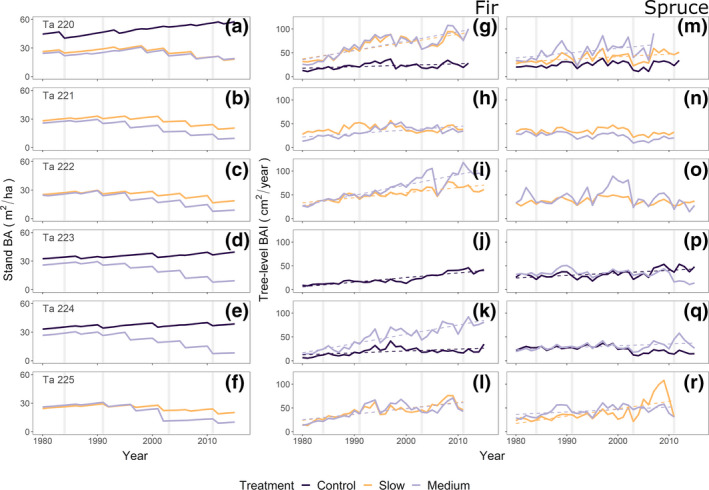
Stand basal area (BA, m2/ha, panels a–f) and mean annual tree‐level basal area increment (BAI, cm2/year) of fir (panels g–l) and spruce (panels m–r) across the analyzed treatments and sites (Ta 220–225) since 1980 (see Figure S9 for tree‐ring width indices). Linear regression lines (dotted) in panels (g–r) show significant growth trends. Vertical gray lines denote the years 1984, 1991, 2003 and 2011
This experiment was chosen for our study because (i) the plots are part of a well‐documented experiment lasting >30 years, which is based on a strict experimental design and measurement protocol; (ii) the analyzed growth responses to drought are based on trees living at the time of sampling and on those progressively harvested over time, in contrast to most dendroecological studies using only living trees at the time of sampling, thereby missing the responses of trees that had previously been harvested or died; (iii) mortality events were precisely recorded at the tree level, thus eliminating uncertainties related to unknown past mortality (Teets et al., 2018); (iv) detailed records of individual‐tree positions and dimensions, including height, crown length and crown radii, were measured repeatedly, therefore allowing also for an estimation of tree–tree competition at the annual level over time; and (v) it was possible to collect additional tree‐ring samples from some of the fully stocked control stands within the experiment, which is often not allowed in managed stands or long‐term experiments.
2.2. Inventory and field data collection, and laboratory analysis
At the onset of the long‐term experiment in 1979, on the respective treatment or control plots (plot area c. 0.25 ha), all trees ≥4.0 cm in diameter at breast height (DBH, 1.3 m height) were mapped, measured (DBH, height, crown length and radii) and tagged, recording c. 2000 trees in total. All tagged living trees were periodically remeasured at c. 5‐year intervals, at the end of the growing season. Tree removal occurred during the winter, before the beginning of the following growing season. Trees of poor quality or health, or that had died or disappeared during the interval between inventories were also recorded. The cause and time of death were assessed and the DBH and height at the time of death reconstructed. Annual tree radial growth was measured on discs collected at breast height from 289 trees harvested progressively between 1980 and 2017 from the treatment and control plots (Table 1). Cross‐sections were air‐dried, sanded with progressively finer sandpaper and visually cross‐dated. Radial increments were measured using a Digitalpositiometer Typ 2 (developed by K. Johann; Biritz GmbH, precision 0.01 mm), and TSAPWinTM (Rinntech, 2003) using the Lintab measuring device (precision 0.01 mm). In total, 2012 individual measurement series were obtained (eight radii per disc for trees measured at the Forest Research Institute of Baden‐Württemberg FVA, three radii per disc for trees measured at the Swiss Federal Institute for Forest, Snow and Landscape Research WSL) across the six sites. Cross‐dating and ring‐width measurements of each series were checked for errors using the COFECHA software (Holmes, 1983). Ring series were converted to annual tree basal area increment (BAI) based on backwards‐reconstructed DBH values derived from DBH inside bark at time of sampling and radial increments over time (Bunn, 2008). The harvested trees were used to develop species‐specific bark factor equations for bark thickness as a function of DBH, then validated against regional bark thickness models (Stängle & Dormann, 2018; Stängle et al., 2016). Bark thickness was subtracted from DBH to obtain the corresponding DBH inside bark. BAI was used instead of ring width because of its lower dependence on stem size and cambial age (Biondi, 1999), and because BAI is more representative of biomass increment (Bouriaud et al., 2005). Stand‐level BAI was quantified as the sum of tree‐level BAI for each stand and year. To obtain annual data for all trees without tree‐ring width series, DBH, height, crown length, crown projections and leaf area were reconstructed using linear and nonlinear least square regression models (for details on the methods see Appendix S1).
2.3. Climate data and drought variables
Downscaled monthly air temperature (minimum, mean and maximum) and precipitation sum for each study site were obtained from the DWD Climate Data Center dataset (1 × 1 km grids over Germany, version v1.0; Kaspar et al., 2013). Based on these variables, the multiscalar Standardized Precipitation and Evapotranspiration Index (SPEI, unitless; Vicente‐Serrano et al., 2010) was used as an indicator for climatic drought, and calculated with the R package SPEI (Beguería & Vicente‐Serrano, 2013). The potential evapotranspiration (PET) used in the function to obtain the series of monthly climatic water balance (water balance = precipitation − PET) was calculated according to the Hargreaves equation (Hargreaves, 1994), and the SPEI was then computed at different time scales. Correlation analyses between SPEI and tree‐ring chronologies showed that the most suitable SPEI time window across all sites was the 5‐month period from March to July (SPEIJuly,5m, Figure S2), which corresponds to the period just before and during which most of the cambial activity occurs for the study species in the region (Dietrich et al., 2018).
Four single‐year climatic drought events, corresponding to radial growth depressions, were selected for analysis. The first two (years 1984 and 1991, hereafter called mild drought events) had the lowest average values of SPEIJuly,5m across sites throughout the first half of the experiment (SPEIJuly,5m = −0.61 ± 0.66 in 1984 and −0.54 ± 0.30 in 1991); the other two events (years 2003 and 2011, hereafter called severe drought events) had the lowest average values of SPEIJuly,5m across sites after 2000 (SPEIJuly,5m = −2.16 ± 0.27 in 2003 and −1.65 ± 0.24 in 2011, Figure 1d; Figure S3). The year 2014, although severe, was not included in the analysis due to a low number of observations in the inventory data.
2.4. Stand structure and composition, tree resource use, and management‐related variables
A wide variety of stand structure‐ and composition‐related variables (e.g., species interaction, species composition), traits related to resource‐use efficiency (absorption of photosynthetically active radiation) and management‐related variables (reflecting the intensity and frequency of thinning and, thus, the experimental treatments and controls) was used as tree‐ and stand‐level predictors of growth responses to drought (Table S1).
At the tree level, a distance‐dependent competition index (NI) was computed:
where NIt is the competition intensity experienced by treet, from n neighboring treesi within a radius of 10 m of treet; BA is the treei basal area (cm2); and distanceti is the distance (m) between the stem center of the central treet and its ith neighbor. Along a continuous range of radii, 10 m was selected because it maximized the R 2 of the relationship between the periodic annual basal area increment and NIt (Forrester et al., 2013). To account for differences in species interactions among neighboring trees, the proportion of competition intensity (NIt) exerted by fir, spruce and other species was computed as a ratio of NIspX to NIt, where NIspX is the NIt calculated only considering fir, spruce or other species as neighboring trees, respectively.
Individual‐tree absorption of photosynthetically active radiation (APAR, GJ/tree/year) was predicted using the 3D tree‐level model Maestra (Medlyn, 2004; Wang & Jarvis, 1990). Maestra APAR predictions have been tested and validated in several monospecific and mixed forests (Forrester et al., 2018; Wang & Jarvis, 1990). Crown architecture (live crown length and radius, leaf area) and species‐specific differences in leaf optical properties, as well as leaf area density and angle distributions were used to predict individual‐tree APAR (Table S2). The model defines tree crown positions by x and y coordinates, considering slope and aspect in both x and y directions, to account for the shading of neighboring trees in the canopy. Individual tree APAR was computed as total annual APAR for evergreen species, or total APAR of the period between leaf unfolding and leaf discoloration in autumn for deciduous species (Table S2). To avoid edge effects, APAR predictions were not used for trees within 10 m of plot boundaries. An additional 20 m wide buffer was simulated around each plot, and contained stands with the average tree spacing, species composition and tree sizes of the given plot (Forrester & Albrecht, 2014).
At the stand level, the Shannon diversity index (Shannon, 1948) was used as a measure of compositional diversity and was calculated with the R package vegan (Oksanen et al., 2013). The relative contribution of fir, spruce and other species to total stand basal area was also considered. The effect of management on drought responses was tested based on variables related to the intensity and frequency of thinning, such as total residual stand basal area at the time of drought (as an expression of the treatment intensity), basal area removed, total number of interventions and the number of years since the last thinning.
2.5. Growth responses to drought
Tree‐ and stand‐level growth responses to drought were expressed as resistance, recovery and resilience (Lloret et al., 2011; Figures S4 and S5). These three indices allow for the examination of growth performance before, during and after periods of stress, and therefore characterize tree‐ and stand‐level growth responses to drought. The indices were calculated as follows:
where BAIDr is the BAI during drought, BAIpreDr is the average BAI for the 2 years preceding drought and BAIpostDr is the average BAI for the 2 years following drought. The same indices, but calculated using detrended tree‐ring series (smoothing spline with 50% frequency response at 2/3 of series’ length), led to similar results (correlation coefficient r > 0.9). A period of 2 years was selected because radial growth autocorrelation >0.5 was found for 1‐ and 2‐year lags (Figure S6), supporting the significant legacies in radial growth observed in non‐arid sites for 1–2 years after drought (Anderegg et al., 2015). Additionally, the short time window pre‐ and post‐drought limits the confounding effect of other biotic and abiotic events that can determine growth anomalies (e.g., the mast year in 2006; Ascoli et al., 2017; Schwarz et al., 2020).
2.6. Statistical analyses
Stand structure and composition, traits related to resource‐use efficiency, and management effects on resistance, recovery and resilience to drought were predicted using linear mixed‐effects models (LMM, at the tree level) and linear models (at the stand level). General individual‐tree APAR conditions—average values of APAR of the 2 years preceding drought—were included in the models. First, all weakly correlated predictors (r < 0.5, cf. Figure S7 for tree‐ and Figure S8 for stand‐level variables) with their interactions were included as fixed effects in the full model. Additionally, multicollinearity among explanatory variables was assessed using the variance inflation factor (VIF), and variables with VIF > 2 were removed from the full model. A total of six models were fitted at both the tree and the stand level. At each level, three models were developed for each drought group (mild and severe drought events). In the tree‐level models, an interaction with species was added to all predictors. Predictors were scaled and centered to improve interpretability and to allow for the direct comparison of the regression coefficients (Schielzeth, 2010). Different random structures were tested to select the optimal random structure and, thus, type of model for analysis (for details, see Appendix S1; Table S3). Including a random structure in the models with “site” as grouping factor did not improve their performance, highlighting similar drought effects among sites irrespective of their structure, composition and management (Table S3). A set of models with all possible combinations of fixed effect terms in the full model was ranked by the second‐order Akaike information criterion (AICc), and used to select the model with lowest AICc (best model). Marginal and conditional R‐squared and coefficient of determination (Nakagawa & Schielzeth, 2013) were calculated to examine the variance explained by the fixed effects and by the entire model, respectively. The assumptions of normality and variance homogeneity of residuals were visually verified. Estimates of the variance components (effect size) for each predictor variable were obtained as the ratio of the variance of the standardized predictor to the total variance of the best model for each index and drought event. Nonparametric Kruskal–Wallis tests were conducted to detect differences in drought response (resistance, recovery and resilience) among drought events (mild and severe), forest components (individual fir, individual spruce and whole stand) and treatments (control, slow and medium). All analyses were performed in the statistical computing software R (version 4.0.1, R Core Team, 2020) using the packages lme4 (Bates et al., 2007), car (Fox et al., 2012), MuMIn (Bartoń, 2015) and rstatix (Kassambara, 2020).
3. RESULTS
3.1. Tree growth
Differences in growth trends were observed for individuals of fir and spruce since 1980. Fir showed significantly increasing growth rates across all treatments and sites (except for the slow treatment at site Ta 221), with differences among treatments (Figure 2g–l). In contrast, spruce showed less pronounced differences among treatments, and several non‐significant growth trends (Figure 2m–r). In accordance with the experimental treatment plans, stand basal area decreased in all harvested stands while it increased in the increment controls (Figure 2a–f).
3.2. Influence of drought event, forest component and treatment on drought responses
Drought responses varied considerably between drought events, with significantly lower values of resistance, recovery and resilience observed during severe droughts (Figure S10; Table S4). Compared with individuals of fir and whole stands, individuals of spruce recorded lower resistance, recovery and resilience to mild droughts, but higher recovery to severe droughts; whole stands were significantly more resistant and resilient to severe droughts than the average individual trees of those stands (Figure S10; Tables S4 and S5).
3.3. Predictors of tree‐level resistance, recovery and resilience
For mild drought events at the tree level, species identity and species interaction were the main predictors of resistance, recovery and resilience (Table 2; Figure 3a). Fir showed significantly higher resistance, recovery and resilience than spruce, and its recovery benefited from intraspecific interactions (Table 2; Figure 3a). Species identity contributed with 85%–100% to the explained variance across all drought responses (Figure 3a).
TABLE 2.
Summary of the linear mixed‐effect models
| Tree‐level | Best model | Best model | Best model | |
|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | ||
| Mild | Resistance | Recovery | Resilience | |
| Intercept | −0.47 (0.15)* | −0.41 (0.11)** | −0.55 (0.13)** | |
| Spfir | 0.74 (0.10)*** | 0.61 (0.10)*** | 0.86 (0.10)*** | |
| NIratio_fir | −0.07 (0.09) | |||
| NIratio fir × Spfir | 0.21 (0.10)* | |||
|
|
0.127 | 0.102 | 0.170 | |
|
|
0.216 | 0.134 | 0.240 | |
| Severe | Resistance | Recovery | Resilience | |
| Intercept | −0.72 (0.18)** | 0.11 (0.17) | −0.56 (0.11)*** | |
| Spfir | 1.20 (0.12)*** | −0.35 (0.14)* | 0.88 (0.13)*** | |
| NIratio_fir | −0.20 (0.10)* | −0.27 (0.10)** | ||
| NIratio other | −0.31 (0.11)** | 0.45 (0.12)*** | ||
| BAstand | 0.03 (0.13) | −0.16 (0.07)° | ||
| SPEI | 0.15 (0.07)* | 0.26 (0.08)*** | 0.27 (0.06)*** | |
| NIratio fir × Spfir | 0.30 (0.12)* | 0.21 (0.13) | ||
| NIratio other × Spfir | 0.32 (0.12)** | −0.46 (0.14)*** | ||
| BAstand × Spfir | −0.29 (0.12)* | |||
|
|
0.349 | 0.172 | 0.250 | |
|
|
0.509 | 0.265 | 0.266 |
Fit of tree‐level resistance, recovery, and resilience (mild and severe events) as a function of different variables (best models). Sp = species (2 levels: fir, and the reference spruce); NIratio fir = ratio of competition intensity of fir to total intensity of competition; NIratio other = ratio of competition intensity of other species (mainly beech) to total intensity of competition; BAstand = residual stand basal area; SPEI = SPEI of July at the time scale of 5 months; x = interaction; = marginal R‐squared (variance explained by the fixed factors); and = conditional R‐squared (variance explained by the fixed and random factors). Significance levels: “***” 0.001, “**” 0.01, “*” 0.05, “°” 0.1. See Table S6 for the full models.
FIGURE 3.
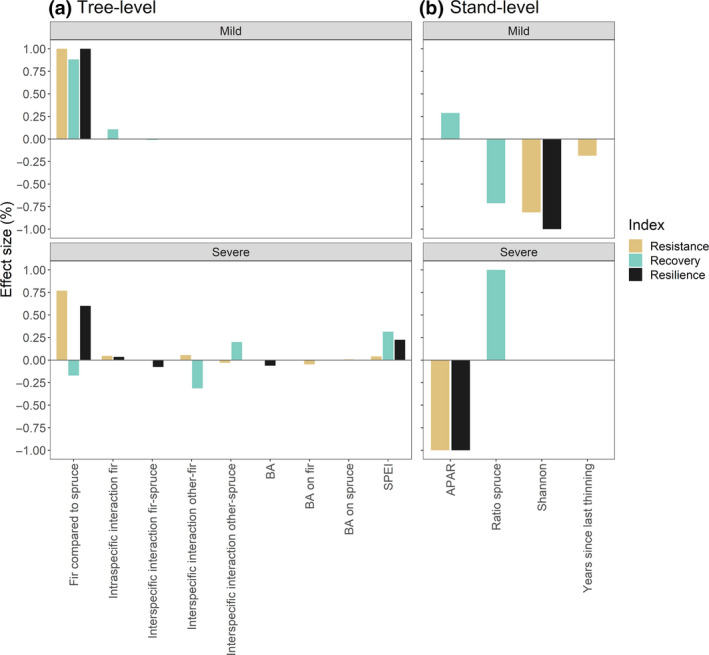
Variance components showing the proportion of total variation in (a) tree‐level and (b) stand‐level resistance, recovery and resilience to mild and severe drought events explained by each predictor variable (effect size). Fir compared to spruce = species comparison: response (resistance, recovery and resilience) of fir compared to spruce; BA = effect of residual stand basal area on both fir and spruce; BA on fir/spruce = effect of residual stand basal area on fir/spruce; Intraspecific interaction fir = effect of intraspecific interaction on fir; Interspecific interaction fir‐spruce = effect of interspecific interaction of fir with spruce; Interspecific interaction other‐fir/spruce = effect of interspecific interaction of other species (mainly beech) with fir/spruce; SPEI = effect of SPEI of July at the time scale of 5 months; APAR = effect of absorption of photosynthetically active radiation; Ratio spruce = effect of ratio of basal area of spruce to total stand basal area; Shannon = effect of Shannon diversity index; Years since last thinning = effect of the number of years since the last thinning
For severe drought events, species identity, species interaction, drought severity and residual stand basal area at the time of drought were the main predictors of the analyzed growth responses (Table 2; Figure 3a). Fir showed significantly higher resistance and resilience than spruce, but had lower recovery under severe drought (Table 2). Species identity contributed most to the total proportion of explained variance in resistance and resilience (77% and 60%, respectively) while it had a smaller effect on recovery (17%, Figure 3a). Intraspecific interaction increased the resistance and resilience of both fir and spruce to severe droughts, whereas the resistance and recovery of the two species were differently affected by interspecific interactions (Table 2). Higher proportions of fir in the neighborhood negatively affected the resistance and resilience of spruce to drought while the presence of other species (excluding fir, i.e., mainly beech) negatively affected the resistance of spruce but favored its recovery. The opposite was observed for fir (Table 2), and interspecific interaction (with other species) explained up to 32% of the variance in recovery of fir (Figure 3a). Species interactions had a lower effect in all other cases (<10%, Figure 3a). SPEI exerted a positive effect on all drought responses, particularly recovery and resilience, which were higher under less severe drought conditions (Table 2). Drought severity explained 30% of the variance in recovery and 20% of the variance in resilience (Figure 3a). Higher values of residual stand basal area were associated with lower resistance and resilience (Table 2) and explained <7% of the total variation in the drought response of both species (Figure 3a).
3.4. Predictors of stand‐level resistance, recovery and resilience
For mild drought events at the stand level, APAR, proportion of spruce, Shannon diversity index and number of years since the last thinning were the most important predictors of the analyzed growth responses (Table 3). The Shannon diversity index and the number of years since the last thinning both had a negative effect on stand‐level resistance, explaining 81% and 19% of its variance, respectively (Table 3; Figure 3b). Stand‐level recovery was positively influenced by APAR and negatively influenced by the proportion of spruce; APAR and the proportion of spruce explained 29% and 71% of the variance in recovery, respectively (Table 3; Figure 3b). The Shannon diversity index negatively affected the resilience to mild drought events, explaining its entire variance (Table 3; Figure 3b).
TABLE 3.
Summary of the linear regression models
| Stand‐level | Best model | Best model | Best model | |
|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | ||
| Mild | Resistance | Recovery | Resilience | |
| Intercept | 0.00 (0.14) | 0.00 (0.17) | 0.00 (0.17) | |
| APAR | 0.31 (0.18)° | |||
| Shannon | −0.64 (0.15)*** | −0.46 (0.18)* | ||
| Ratiospruce | −0.45 (0.18)* | |||
| Yrssince last | −0.30 (0.15)° | |||
|
|
0.436 | 0.192 | 0.178 | |
| Severe | Resistance | Recovery | Resilience | |
| Intercept | 0.00 (0.20) | 0.00 (0.20) | 0.00 (0.20) | |
| APAR | −0.39 (0.20)° | −0.34 (0.21) | ||
| Ratiospruce | 0.38 (0.20)° | |||
|
|
0.114 | 0.107 | 0.074 |
Fit of stand‐level resistance, recovery and resilience (mild and severe events) as a function of different variables (best models). APAR = absorption of photosynthetically active radiation; Shannon = Shannon diversity index; Ratiospruce = ratio of basal area of spruce to total stand basal area; Yrssince last = number of years since the last thinning; and = adjusted R‐squared. Significance levels: “***” 0.001, “**” 0.01, “*” 0.05, “°” 0.1. See Table S7 for the full models.
For severe drought events, APAR and the proportion of spruce were the most important predictors of the analyzed growth responses (Table 3). Stand‐level resistance and resilience were negatively and solely influenced by APAR while recovery was positively affected by higher proportions of spruce (Table 3; Figure 3b).
4. DISCUSSION
Based on a unique long‐term silvicultural experiment in mixed silver fir and Norway spruce mountain forests in central Europe, we examined growth responses to mild and severe drought events at both the tree and the stand level. Furthermore, we evaluated the drivers of such responses, using models containing the most influential tree and stand characteristics as well as management‐related variables, to provide insights for the management of such forests under climate change.
4.1. Growth response of fir and spruce to drought
Our results highlight the higher drought resistance and resilience of fir compared to spruce, although with variability among sites. This is consistent with other studies showing higher drought tolerance of fir at least in the recent past (e.g., Vitali et al., 2017; Vitasse, Bottero, Cailleret, et al., 2019). It should be noted, however, that in the past centuries this was not necessarily the case: recurring decline events were observed for fir in central Europe in the 19th and 20th centuries, with drought often being considered among the possible major drivers (e.g., Vincent & Kantor, 1971; Wiedemann, 1927). However, the ongoing changes in climate and the recovery of forest soils from a century of depletion (e.g., following litter raking; Ganter, 1927) may have changed relevant ecological conditions that, in turn, positively affected the vigor and resistance of fir. In contrast to fir, spruce exhibited higher recovery to severe drought events. This pattern, however, does not imply a better recovery of spruce compared to fir, but reflects (i) that fir, characterized by higher drought resistance, has less growth to recover after drought compared to spruce (see the synthesis scheme presented in Figure 4) and (ii) the negative nature of the relationship between resistance and recovery that is inherent in the method by Lloret et al. (2011), where the growth during drought is the numerator of the fraction used to calculate resistance, and the denominator for recovery. It has been shown for numerous studies which applied the indicators by Lloret et al. (2011) that forest stands with stronger growth reductions during drought (lower resistance) were indeed capable to recover faster than stands with higher resistance (Schwarz et al., 2020).
FIGURE 4.
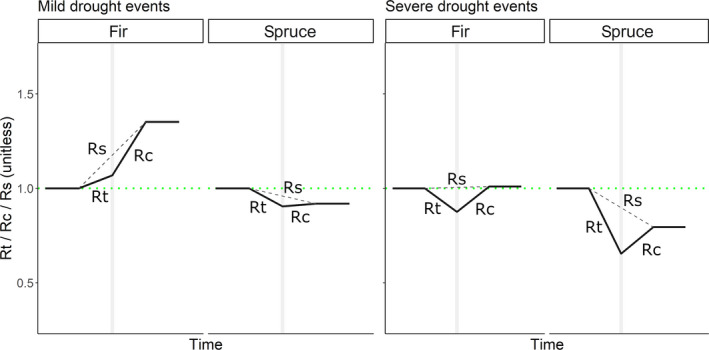
Synthesis scheme of tree‐level growth resistance (Rt), recovery (Rc) and resilience (Rs) of fir and spruce. The graphs are based on the data and overall findings of this study for the two mild (years 1984 and 1991) and severe (years 2003 and 2011) drought events examined. Vertical gray lines denote drought events
In our study, the growth of fir increased during mild drought events compared to previous years without drought stress (resistance >1), and this effect continued after drought (recovery >1). Increased temperatures are the probable cause for the growth increase of fir in relation to these mild drought events. The growth of fir, in fact, likely benefits from warmer conditions, even under low precipitation (Vitasse, Bottero, Rebetez, et al., 2019). Spruce, instead, experienced growth reductions even during mild drought events (resistance <1). Severe drought events indicated clear growth reductions for both species, particularly pronounced for spruce. Spruce is characterized by a smaller hydraulic safety margin and therefore a more risky strategy than fir (Cochard, 1992; Mayr et al., 2003, 2006). If damage occurs in the hydraulic system, water transport is reduced over a longer period even though sufficient water is available (Brodribb et al., 2010). The protracted lower post‐drought growth of spruce may indicate that damage occurred during drought, causing longer‐term legacy effects (cf. Anderegg et al., 2015; Schuldt et al., 2020).
Long‐term growth trends documented for the region of the experiment show that the growth of fir and spruce has been consistently increasing after the growth depression observed in the 1970s. The growth of spruce, however, has restarted to decline from the mid‐1990s (Kohnle et al., 2014; Yue et al., 2011). Our results are in line with the documented trends and the expected future performance of the species: the growth of fir will potentially increase in a warmer climate, in particular if it leads to milder winter and spring conditions (Vitali, Büntgen, et al., 2018), whereas spruce will probably suffer from warmer and drier conditions, with potentially negative repercussions on timber production, especially at lower elevations (Elkin et al., 2013; Pretzsch et al., 2020). Some of the expected difficulties for spruce have become visible with its epochal large‐scale mortality following the extreme summer drought of 2018, which may have profound impacts for the future of European forests and the forestry sector (Hanewinkel et al., 2013; Schuldt et al., 2020).
Finally, we found that stands were overall more resilient than the average individual trees. Stand‐level growth responses to drought are probably buffered by individual growth responses among trees of different species and dimensions, and therefore show averaged responses of lower magnitude than those at the species level. Besides an averaging effect, there is evidence that mixed stands such as those assessed here show complementarity (Ammer, 2019), and the beneficial interactions in admixtures may also convey resilience and adaptability to climate change (Bauhus, Forrester, & Pretzsch, 2017), as observed in mixed stands in temperate forests under drought conditions (Grossiord, Granier, Ratcliffe, et al., 2014).
4.2. Predictors of growth responses to mild and severe drought events
4.2.1. Tree level
Species identity was the sole predictor of the tree‐level drought response to mild drought events. In contrast, in the case of severe drought events, additional factors such as drought severity, intraspecific and interspecific interactions and residual stand basal area at the time of drought co‐determined the growth responses to drought.
Less pronounced water deficits (i.e., a higher SPEI value) were associated with higher values of the growth response (particularly recovery and resilience) of fir and spruce to severe drought events (years 2003 and 2011). Drought severity and seasonality are generally important determinants of forest sensitivity (Huang et al., 2018; Li et al., 2020), and can exceed the magnitude of the influence of stand and soil characteristics (D’Orangeville et al., 2018). At our sites, smaller water deficits occurred during the year 2011, which was characterized by dry conditions in late winter and spring. In this case, thus, not only the severity but also the timing of drought may have played a decisive role for the higher recovery and resilience. A global study on the influence of drought timing on post‐drought stem radial growth, in fact, found that extreme droughts during the dry season had larger negative effects on post‐drought tree growth compared to extreme droughts during the wet season (Huang et al., 2018). Besides these effects, we did not find differences in the resilience components among sites that could be caused by differences in mean climatic conditions. For instance, forest resilience has been reported to decrease with increasing mean annual precipitation (Stuart‐Haëntjens et al., 2018), but the climatic gradient covered by our sites is most likely too narrow to confirm this trend.
Interspecific interactions (mainly with fir and beech) negatively affected the drought resistance of spruce during severe droughts. Broadleaf species such as beech are characterized during the growing season by a high interception of precipitation, stemflow (channel water more to their own rooting system), and may therefore play a negative role especially during periods of water shortage (Staelens et al., 2008). Beyond these aboveground interactions, it is unclear to what extent the drought response of spruce may be driven by changes in rooting depth and intensity in mixtures when compared to pure stands. Observations from beech and spruce mixtures in Austria and Germany showed that the root system of spruce tends to be confined to the upper soil layers, suffering from strong competition by beech (Bolte & Villanueva, 2006; Schume et al., 2004). Another study conducted in southern Germany supported these findings, but found no increase in drought stress or growth reduction of spruce mixtures with beech (Goisser et al., 2016). The pattern observed in our study suggests that spruce subjected to strong interspecific interaction may suffer from higher competition for resources, which would further lower soil moisture availability during droughts, as observed in boreal forests (Grossiord, Granier, Gessler, et al., 2014). In contrast, our results suggest that facilitation and/or resource partitioning may underlie the improved growth resistance of fir subjected to interspecific interaction (cf. Vitali, Forrester, et al., 2018).
The recovery of fir following severe drought events was negatively affected by the presence of other species, including a higher presence of spruce. After a drought, it takes time until deeper soil layers are rewetted. Species with a shallow root system, such as spruce, are thus likely to profit first, at the expense of coexisting species with deeper root systems (Schume et al., 2004). Complementarity in the temporal origin of water used by spruce and beech, which has been observed in temperate forests (Brinkmann et al., 2018), could explain the positive effect of other species (mainly beech) on the recovery to drought of spruce found in our study.
The contrasting effects of species diversity on tree growth resilience to severe droughts observed here and elsewhere show that these effects in mixed stands are often complex and depend strongly on the local environment and the complementarity of species‐specific functional traits (Ammer, 2019; Ratcliffe et al., 2017).
Stand‐level competition directly affects resource availability (Moreno‐Gutiérrez et al., 2012). High stand density (i.e., basal area) is often associated with higher rainfall interception and transpiration (van Dijk & Bruijnzeel, 2001), thus lowering soil water availability and storage (Bréda et al., 1995), as observed in several Norway spruce stands in Europe (e.g., Misson et al., 2003; Sohn et al., 2013) and various species worldwide (e.g., Andrews et al., 2020; Navarro‐Cerrillo et al., 2019; Sohn, Hartig, et al., 2016). However, other studies reported a minor or variable influence of residual stand basal area on the growth response to drought (e.g., Serra‐Maluquer et al., 2018; Sohn et al., 2016). We found a negative influence of residual stand basal area on the tree‐level response to severe droughts, which was significant for the resistance of fir and the resilience of both species, but rather marginal (effect size <7%). The magnitude of the effect of residual basal area on the drought response is not usually examined in studies of this kind. However, the relatively limited effect of residual stand basal area that we observed may be related to local conditions and stand characteristics. At the experimental stands, the relatively narrow range of stand basal area that was removed with each intervention may mask the potential beneficial effect of stand density reduction, as it was clearly observed under more pronounced density reductions (e.g., Sohn et al., 2013).
4.2.2. Stand level
Stand structural characteristics and composition, and years since the last thinning determined growth responses to drought at the stand level.
Stands with larger and taller trees with larger leaf areas (i.e., higher APAR, Figure S11) had higher recovery following mild drought events. Trees growing in mesic and fertile locations, like our study sites, can develop large crowns. Often it is assumed that tall stature and large crowns make trees more susceptible to drought (Bennett et al., 2015; Grote et al., 2016). During the mild droughts assessed here, the positive effects of a large crown (i.e., the provisioning of photosynthates) outweighed potential negative effects (e.g., higher water loss). Furthermore, abundant carbohydrate reserves of these trees may support post‐drought growth (Zweifel et al., 2020), therefore improving the overall resilience of stands on productive sites. For example, individual Scots pine trees growing at low‐productivity sites across Europe showed lower drought recovery and resilience (Bose et al., 2020). In contrast to the situation under mild droughts, in our study the same stands were less resistant and resilient to severe droughts. The higher drought vulnerability of larger and taller trees is caused by higher radiation and evaporative demand of the exposed crowns, and a greater inherent vulnerability to xylem embolism (Bennett et al., 2015; Olson et al., 2018). Thus, the lower resilience may be related to the post‐drought necessity of large trees to restore their hydraulic system and crown (Choat et al., 2018), thus slowing recovery (Brodribb et al., 2010).
We found that resistance to mild droughts was higher in the immediate post‐intervention period. A possible explanation is that the first interventions at the beginning of the experiment led to a more intense competition release than later interventions, when the stands had already been thinned multiple times (Giuggiola et al., 2016; Simon et al., 2017). Additionally, younger trees can respond more strongly with crown expansion to release than older trees (Nyland, 2016).
4.3. Implications for forest management in the face of climate change
The cumulative effects of severe droughts and species composition are likely to have major consequences for mixed fir and spruce forests in central Europe. This study highlights the importance of stand composition for tree‐ and stand‐level drought responses. With adequate water supply and in the absence of disturbances, spruce is more productive than fir and most other species under similar site conditions (Pretzsch, 2005). However, as the growth of spruce under current environmental and climatic conditions is generally less resilient to drought than fir, and biotic and abiotic disturbances are likely to increase with climate change, the productivity of this species may be strongly reduced in the future (Seidl et al., 2017; Temperli et al., 2013). The unprecedented tree mortality triggered by the 2018/2019 drought and the associated bark beetle outbreaks in central European temperate forests (Schuldt et al., 2020) emphasize the importance of developing appropriate adaptation strategies. Reducing the proportion of spruce, for instance by fostering mixtures with more drought‐tolerant broadleaf species, and/or by reducing stand density, would improve the resilience of these forests to future (extreme) droughts and other disturbances, with positive repercussions on the provision of multiple ecosystem services (Bauhus, Forrester, & Pretzsch, 2017). In addition, management strategies aiming for smaller size trees would be beneficial for the resilience of trees and stands to drought, as taller trees are more vulnerable to hydraulic stress (Grote et al., 2016) and larger spruce trees are also more susceptible to bark beetle infestations (Netherer & Nopp‐Mayr, 2005). Our study demonstrates that interventions at short intervals can improve the drought resilience of mature stands, especially in dense stands and during mild droughts.
Although the growth response to drought has been investigated for many tree species at the individual tree level, much remains unknown about the drought response of forest stands. This lack of knowledge results because many studies do not sample the full range of tree sizes, and are not based on random samples of trees. This inhibits the scaling of growth from individual trees across species and dimensions to whole stands. The scaling tasks is further complicated by the fact that typically there are no data for trees that have died or were removed over time, which is particularly relevant in managed forests or when the analysis extends over long periods.
Overall, the mixed silver fir and Norway spruce stands in central Europe that we studied are resilient to mild drought events, and even profit from such conditions, but they suffer from severe droughts. Forest management can support these forests by promoting their growth resistance, recovery and resilience, specifically by controlling or modifying species composition, tree size distribution and stand density. The approach described here provides relevant information for the management of the widespread European mixed mountain forests dominated by fir and spruce in the face of climate change.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank Marek Sławski for field assistance, Andreas Ehring for organizing tree harvesting, Thilo Wolf for providing climate data, Matthias Haeni for statistical assistance, and Daniele Castagneri and Georg von Arx for inputs on a previous version of the manuscript. Funding for this research was provided by the ForRISK project (ERA‐NET Sumforest). Sumforest was funded by the European Union under Grant Agreement No. 606803. The ForRISK project was funded in Switzerland through the Federal Office for the Environment FOEN (Grant No. 05.0602.PZ/P382‐0487), in France through the French National Research Agency ANR (Grant No. ANR‐16‐SUMF‐0001‐01) and in Germany through the Federal Ministry for Food and Agriculture BMEL (FKZ: 2816ERA04S). A.G. acknowledges support from the Swiss National Science Foundation SNF (310030_189109). M.C. acknowledges support from a grant overseen by the ANR as part of the "Investissements d'Avenir" program (ANR‐11‐LABX‐0002‐01). Phenology data were provided by the members of the PEP725 project.
Bottero, A., Forrester, D. I., Cailleret, M., Kohnle, U., Gessler, A., Michel, D., Bose, A. K., Bauhus, J., Bugmann, H., Cuntz, M., Gillerot, L., Hanewinkel, M., Lévesque, M., Ryder, J., Sainte‐Marie, J., Schwarz, J., Yousefpour, R., Zamora‐Pereira, J. C., & Rigling, A. (2021). Growth resistance and resilience of mixed silver fir and Norway spruce forests in central Europe: Contrasting responses to mild and severe droughts. Global Change Biology, 27, 4403–4419. 10.1111/gcb.15737
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ammer, C. (2019). Diversity and forest productivity in a changing climate. New Phytologist, 221(1), 50–66. 10.1111/nph.15263 [DOI] [PubMed] [Google Scholar]
- Anderegg, W. R., Kane, J. M., & Anderegg, L. D. (2013). Consequences of widespread tree mortality triggered by drought and temperature stress. Nature Climate Change, 3(1), 30–36. 10.1038/nclimate1635 [DOI] [Google Scholar]
- Anderegg, W. R., Schwalm, C., Biondi, F., Camarero, J. J., Koch, G., Litvak, M., Ogle, K., Shaw, J. D., Shevliakova, E., Williams, A., Wolf, A., Ziaco, E., & Pacala, S. (2015). Pervasive drought legacies in forest ecosystems and their implications for carbon cycle models. Science, 349(6247), 528–532. [DOI] [PubMed] [Google Scholar]
- Andrews, C. M., D’Amato, A. W., Fraver, S., Palik, B., Battaglia, M. A., & Bradford, J. B. (2020). Low stand density moderates growth declines during hot droughts in semi‐arid forests. Journal of Applied Ecology, 57(6), 1089–1102. 10.1111/1365-2664.13615 [DOI] [Google Scholar]
- Ascoli, D., Maringer, J., Hacket‐Pain, A., Conedera, M., Drobyshev, I., Motta, R., Cirolli, M., Kantorowicz, W., Zang, C., Schueler, S., Croisé, L., Piussi, P., Berretti, R., Palaghianu, C., Westergren, M., Lageard, J. G. A., Burkart, A., Gehrig Bichsel, R., Thomas, P. A., … Vacchiano, G. (2017). Two centuries of masting data for European beech and Norway spruce across the European continent. Ecology, 98(5), 1473. 10.1002/ecy.1785 [DOI] [PubMed] [Google Scholar]
- Bartoń, K. (2015). Package ‘MuMIn’. Version, 1, 18.
- Bates, D., Sarkar, D., Bates, M. D., & Matrix, L. (2007). The lme4 package. R Package Version, 2(1), 74. [Google Scholar]
- Bauhus, J., Forrester, D. I., Gardiner, B., Jactel, H., Vallejo, R., & Pretzsch, H. (2017). Ecological stability of mixed‐species forests. In Mixed‐species forests (pp. 337–382). Springer. [Google Scholar]
- Bauhus, J., Forrester, D. I., & Pretzsch, H. (2017). Mixed‐species forests: the development of a forest management paradigm. In Pretzsch H., Forrester D. I., & Bauhus J. (Eds.), Mixed‐species forests (pp. 1–25). Springer. [Google Scholar]
- Beguería, S., & Vicente‐Serrano, S. M. (2013). SPEI: Calculation of the standardised precipitation‐evapotranspiration index (1.6). [R]. CRAN. https://cran.r‐project.org/package=SPEI
- Bennett, A. C., McDowell, N. G., Allen, C. D., & Anderson‐Teixeira, K. J. (2015). Larger trees suffer most during drought in forests worldwide. Nature Plants, 1(10), 15139. 10.1038/nplants.2015.139 [DOI] [PubMed] [Google Scholar]
- Biondi, F. (1999). Comparing tree‐ring chronologies and repeated timber inventories as forest monitoring tools. Ecological Applications, 9(1), 216–227. [Google Scholar]
- Bolte, A., & Villanueva, I. (2006). Interspecific competition impacts on the morphology and distribution of fine roots in European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). European Journal of Forest Research, 125(1), 15–26. [Google Scholar]
- Bose, A. K., Gessler, A., Bolte, A., Bottero, A., Buras, A., Cailleret, M., Camarero, J. J., Haeni, M., Hereş, A.‐M., Hevia, A., Lévesque, M., Linares, J. C., Martinez‐Vilalta, J., Matías, L., Menzel, A., Sánchez‐Salguero, R., Saurer, M., Vennetier, M., Ziche, D., & Rigling, A. (2020). Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Global Change Biology, 26(8), 4521–4537. 10.1111/gcb.15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottero, A., D’Amato, A. W., Palik, B. J., Bradford, J. B., Fraver, S., Battaglia, M. A., & Asherin, L. A. (2017). Density‐dependent vulnerability of forest ecosystems to drought. Journal of Applied Ecology, 54(6), 1605–1614. 10.1111/1365-2664.12847 [DOI] [Google Scholar]
- Bouriaud, O., Bréda, N., Dupouey, J.‐L., & Granier, A. (2005). Is ring width a reliable proxy for stem‐biomass increment? A case study in European beech. Canadian Journal of Forest Research, 35(12), 2920–2933. 10.1139/x05-202 [DOI] [Google Scholar]
- Brang, P., Spathelf, P., Larsen, J. B., Bauhus, J., Bonc ina, A., Chauvin, C., Drossler, L., Garcia‐Guemes, C., Heiri, C., Kerr, G., Lexer, M. J., Mason, B., Mohren, F., Muhlethaler, U., Nocentini, S., & Svoboda, M. (2014). Suitability of close‐to‐nature silviculture for adapting temperate European forests to climate change. Forestry, 87(4), 492–503. 10.1093/forestry/cpu018 [DOI] [Google Scholar]
- Bréda, N., Granier, A., & Aussenac, G. (1995). Effects of thinning on soil and tree water relations, transpiration and growth in an oak forest (Quercus petraea (Matt.) Liebl.). Tree Physiology, 15(5), 295–306. 10.1093/treephys/15.5.295 [DOI] [PubMed] [Google Scholar]
- Brinkmann, N., Seeger, S., Weiler, M., Buchmann, N., Eugster, W., & Kahmen, A. (2018). Employing stable isotopes to determine the residence times of soil water and the temporal origin of water taken up by Fagus sylvatica and Picea abies in a temperate forest. New Phytologist, 219(4), 1300–1313. 10.1111/nph.15255 [DOI] [PubMed] [Google Scholar]
- Brodribb, T. J., Bowman, D. J. M. S., Nichols, S., Delzon, S., & Burlett, R. (2010). Xylem function and growth rate interact to determine recovery rates after exposure to extreme water deficit. New Phytologist, 188(2), 533–542. 10.1111/j.1469-8137.2010.03393.x [DOI] [PubMed] [Google Scholar]
- Bunn, A. G. (2008). A dendrochronology program library in R (dplR). Dendrochronologia, 26(2), 115–124. 10.1016/j.dendro.2008.01.002 [DOI] [Google Scholar]
- Caudullo, G., Tinner, W., & de Rigo, D. (2016). Picea abies in Europe: Distribution, habitat, usage and threats. In San‐Miguel‐Ayanz J., de Rigo D., Caudullo G., Houston Durrant T., & Mauri A. (Eds.), European atlas of forest tree species (pp. 114–116). Publication Office of the European Union. [Google Scholar]
- Choat, B., Brodribb, T. J., Brodersen, C. R., Duursma, R. A., López, R., & Medlyn, B. E. (2018). Triggers of tree mortality under drought. Nature, 558(7711), 531–539. 10.1038/s41586-018-0240-x [DOI] [PubMed] [Google Scholar]
- Ciais, P., Reichstein, M., Viovy, N., Granier, A., Ogée, J., Allard, V., Aubinet, M., Buchmann, N., Bernhofer, C., & Carrara, A. (2005). Europe‐wide reduction in primary productivity caused by the heat and drought in 2003. Nature, 437(7058), 529–533. [DOI] [PubMed] [Google Scholar]
- Cochard, H. (1992). Vulnerability of several conifers to air embolism. Tree Physiology, 11(1), 73–83. 10.1093/treephys/11.1.73 [DOI] [PubMed] [Google Scholar]
- Dănescu, A., Albrecht, A. T., & Bauhus, J. (2016). Structural diversity promotes productivity of mixed, uneven‐aged forests in southwestern Germany. Oecologia, 182(2), 319–333. 10.1007/s00442-016-3623-4 [DOI] [PubMed] [Google Scholar]
- Dănescu, A., Kohnle, U., Bauhus, J., Sohn, J. A., & Albrecht, A. T. (2018). Stability of tree increment in relation to episodic drought in uneven‐structured, mixed stands in southwestern Germany. Forest Ecology and Management, 415, 148–159. 10.1016/j.foreco.2018.02.030 [DOI] [Google Scholar]
- del Río, M. , Pretzsch, H., Ruíz‐Peinado, R., Ampoorter, E., Annighöfer, P., Barbeito, I., Bielak, K., Brazaitis, G., Coll, L., & Drössler, L. (2017). Species interactions increase the temporal stability of community productivity in Pinus sylvestris–Fagus sylvatica mixtures across Europe. Journal of Ecology, 105(4), 1032–1043. [Google Scholar]
- Dietrich, L., Zweifel, R., & Kahmen, A. (2018). Daily stem diameter variations can predict the canopy water status of mature temperate trees. Tree Physiology, 38(7), 941–952. 10.1093/treephys/tpy023 [DOI] [PubMed] [Google Scholar]
- D'Orangeville, L., Maxwell, J., Kneeshaw, D., Pederson, N., Duchesne, L., Logan, T., Houle, D., Arseneault, D., Beier, C. M., Bishop, D. A., Druckenbrod, D., Fraver, S., Girard, F., Halman, J., Hansen, C., Hart, J. L., Hartmann, H., Kaye, M., Leblanc, D., … Phillips, R. P. (2018). Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Global Change Biology, 24(6), 2339–2351. 10.1111/gcb.14096 [DOI] [PubMed] [Google Scholar]
- Elkin, C., Gutiérrez, A. G., Leuzinger, S., Manusch, C., Temperli, C., Rasche, L., & Bugmann, H. (2013). A 2 °C warmer world is not safe for ecosystem services in the European Alps. Global Change Biology, 19(6), 1827–1840. [DOI] [PubMed] [Google Scholar]
- Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37(12), 4302–4315. 10.1002/joc.5086 [DOI] [Google Scholar]
- Forrester, D. I. (2014). The spatial and temporal dynamics of species interactions in mixed‐species forests: From pattern to process. Forest Ecology and Management, 312, 282–292. 10.1016/j.foreco.2013.10.003 [DOI] [Google Scholar]
- Forrester, D. I. (2019). Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. Forest Ecology and Management, 447, 139–157. 10.1016/j.foreco.2019.05.053 [DOI] [Google Scholar]
- Forrester, D. I., & Albrecht, A. T. (2014). Light absorption and light‐use efficiency in mixtures of Abies alba and Picea abies along a productivity gradient. Forest Ecology and Management, 328(Suppl. C), 94–102. 10.1016/j.foreco.2014.05.026 [DOI] [Google Scholar]
- Forrester, D. I., Ammer, C., Annighöfer, P. J., Barbeito, I., Bielak, K., Bravo‐Oviedo, A., Coll, L., del Río, M. , Drössler, L., & Heym, M. (2018). Effects of crown architecture and stand structure on light absorption in mixed and monospecific Fagus sylvatica and Pinus sylvestris forests along a productivity and climate gradient through Europe. Journal of Ecology, 106(2), 746–760. [Google Scholar]
- Forrester, D. I., Bonal, D., Dawud, S., Gessler, A., Granier, A., Pollastrini, M., & Grossiord, C. (2016). Drought responses by individual tree species are not often correlated with tree species diversity in European forests. Journal of Applied Ecology, 53(6), 1725–1734. 10.1111/1365-2664.12745 [DOI] [Google Scholar]
- Forrester, D. I., Kohnle, U., Albrecht, A. T., & Bauhus, J. (2013). Complementarity in mixed‐species stands of Abies alba and Picea abies varies with climate, site quality and stand density. Forest Ecology and Management, 304(Suppl. C), 233–242. 10.1016/j.foreco.2013.04.038 [DOI] [Google Scholar]
- Fox, J., Weisberg, S., Adler, D., Bates, D., Baud‐Bovy, G., Ellison, S., Firth, D., Friendly, M., Gorjanc, G., & Graves, S. (2012). Package ‘car’. R Foundation for Statistical Computing. [Google Scholar]
- Ganter, K. (1927). Streuversuchsflächen der badischen forstlichen Versuchsanstalt an der Universität Freiburg i. Br. Allgemeine Forst Und Jagdzeitung, 103, 353–358. [Google Scholar]
- Gazol, A., & Camarero, J. J. (2016). Functional diversity enhances silver fir growth resilience to an extreme drought. Journal of Ecology, 104(4), 1063–1075. 10.1111/1365-2745.12575 [DOI] [Google Scholar]
- Gazol, A., Camarero, J. J., Gutiérrez, E., Popa, I., Andreu‐Hayles, L., Motta, R., Nola, P., Ribas, M., Sangüesa‐Barreda, G., & Urbinati, C. (2015). Distinct effects of climate warming on populations of silver fir (Abies alba) across Europe. Journal of Biogeography, 42(6), 1150–1162. [Google Scholar]
- Giuggiola, A., Ogée, J., Rigling, A., Gessler, A., Bugmann, H., & Treydte, K. (2016). Improvement of water and light availability after thinning at a xeric site: Which matters more? A dual isotope approach. New Phytologist, 210, 108–121. 10.1111/nph.13748 [DOI] [PubMed] [Google Scholar]
- Gleason, K. E., Bradford, J. B., Bottero, A., D’Amato, A. W., Fraver, S., Palik, B. J., Battaglia, M. A., Iverson, L., Kenefic, L., & Kern, C. C. (2017). Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere, 8(7), 1–16. 10.1002/ecs2.1849 29552374 [DOI] [Google Scholar]
- Goisser, M., Geppert, U., Rötzer, T., Paya, A., Huber, A., Kerner, R., Bauerle, T., Pretzsch, H., Pritsch, K., Häberle, K. H., Matyssek, R., & Grams, T. (2016). Does belowground interaction with Fagus sylvatica increase drought susceptibility of photosynthesis and stem growth in Picea abies? Forest Ecology and Management, 375, 268–278. 10.1016/j.foreco.2016.05.032 [DOI] [Google Scholar]
- Grossiord, C. (2020). Having the right neighbors: How tree species diversity modulates drought impacts on forests. New Phytologist, 228, 42–49. 10.1111/nph.15667 [DOI] [PubMed] [Google Scholar]
- Grossiord, C., Granier, A., Gessler, A., Jucker, T., & Bonal, D. (2014). Does drought influence the relationship between biodiversity and ecosystem functioning in boreal forests? Ecosystems, 17(3), 394–404. 10.1007/s10021-013-9729-1 [DOI] [Google Scholar]
- Grossiord, C., Granier, A., Ratcliffe, S., Bouriaud, O., Bruelheide, H., Chećko, E., Forrester, D. I., Dawud, S. M., Finér, L., Pollastrini, M., Scherer‐Lorenzen, M., Valladares, F., Bonal, D., & Gessler, A. (2014). Tree diversity does not always improve resistance of forest ecosystems to drought. Proceedings of the National Academy of Sciences of the United States of America, 111(41), 14812–14815. 10.1073/pnas.1411970111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote, R., Gessler, A., Hommel, R., Poschenrieder, W., & Priesack, E. (2016). Importance of tree height and social position for drought‐related stress on tree growth and mortality. Trees, 30(5), 1467–1482. 10.1007/s00468-016-1446-x [DOI] [Google Scholar]
- Hanewinkel, M., Cullmann, D. A., Schelhaas, M.‐J., Nabuurs, G.‐J., & Zimmermann, N. E. (2013). Climate change may cause severe loss in the economic value of European forest land. Nature Climate Change, 3(3), 203–207. 10.1038/nclimate1687 [DOI] [Google Scholar]
- Hargreaves, G. H. (1994). Defining and using reference evapotranspiration. Journal of Irrigation and Drainage Engineering, 120(6), 1132–1139. 10.1061/(ASCE)0733-9437(1994)120:6(1132) [DOI] [Google Scholar]
- Holmes, R. L. (1983). Computer‐assisted quality control in tree‐ring dating and measurement. Tree‐Ring Bulletin, 43(1), 69–78. [Google Scholar]
- Huang, M., Wang, X., Keenan, T. F., & Piao, S. (2018). Drought timing influences the legacy of tree growth recovery. Global Change Biology, 24(8), 3546–3559. 10.1111/gcb.14294 [DOI] [PubMed] [Google Scholar]
- IPCC . (2014). Climate change 2014: Synthesis report. In Pachauri R. K. & Meyer L. A. (Eds.), Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Author. [Google Scholar]
- Isbell, F., Craven, D., Connolly, J., Loreau, M., Schmid, B., Beierkuhnlein, C., Bezemer, T. M., Bonin, C., Bruelheide, H., de Luca, E. , Ebeling, A., Griffin, J. N., Guo, Q., Hautier, Y., Hector, A., Jentsch, A., Kreyling, J., Lanta, V., Manning, P., … Eisenhauer, N. (2015). Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature, 526(7574), 574–577. [DOI] [PubMed] [Google Scholar]
- Jucker, T., Bouriaud, O., Avacaritei, D., & Coomes, D. A. (2014). Stabilizing effects of diversity on aboveground wood production in forest ecosystems: Linking patterns and processes. Ecology Letters, 17(12), 1560–1569. 10.1111/ele.12382 [DOI] [PubMed] [Google Scholar]
- Kaspar, F., Müller‐Westermeier, G., Penda, E., Mächel, H., Zimmermann, K., Kaiser‐Weiss, A., & Deutschländer, T. (2013). Monitoring of climate change in Germany – Data, products and services of Germany’s National Climate Data Centre. Advances in Science and Research, 10(1), 99–106. 10.5194/asr-10-99-2013 [DOI] [Google Scholar]
- Kassambara, A. (2020). rstatix: Pipe‐friendly framework for basic statistical tests. R package version 0.6.0.
- Klesse, S., DeRose, R. J., Guiterman, C. H., Lynch, A. M., O’Connor, C. D., Shaw, J. D., & Evans, M. E. (2018). Sampling bias overestimates climate change impacts on forest growth in the southwestern United States. Nature Communications, 9(1), 5336. 10.1038/s41467-018-07800-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnle, U., Albrecht, A., Lenk, E., Ohnemus, K., & Yue, C. (2014). Zuwachstrends im Spiegel langfristiger Versuchsflächen in Südwestdeutschland. Allg Forst‐ Und Jagdzeitung, 185, 97–117. [Google Scholar]
- Larsen, J. B. (1995). Ecological stability of forests and sustainable silviculture. Forest Ecology and Management, 73(1), 85–96. 10.1016/0378-1127(94)03501-M [DOI] [Google Scholar]
- Lévesque, M., Saurer, M., Siegwolf, R., Eilmann, B., Brang, P., Bugmann, H., & Rigling, A. (2013). Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Global Change Biology, 19(10), 3184–3199. 10.1111/gcb.12268 [DOI] [PubMed] [Google Scholar]
- Li, X., Piao, S., Wang, K., Wang, X., Wang, T., Ciais, P., Chen, A., Lian, X., Peng, S., & Peñuelas, J. (2020). Temporal trade‐off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nature Ecology & Evolution, 4(8), 1075–1083. 10.1038/s41559-020-1217-3 [DOI] [PubMed] [Google Scholar]
- Lloret, F., Keeling, E. G., & Sala, A. (2011). Components of tree resilience: Effects of successive low‐growth episodes in old ponderosa pine forests. Oikos, 120(12), 1909–1920. 10.1111/j.1600-0706.2011.19372.x [DOI] [Google Scholar]
- Manrique‐Alba, À., Beguería, S., Molina, A. J., González‐Sanchis, M., Tomàs‐Burguera, M., Del Campo, A. D., Colangelo, M., & Camarero, J. J. (2020). Long‐term thinning effects on tree growth, drought response and water use efficiency at two Aleppo pine plantations in Spain. Science of the Total Environment, 728, 138536. 10.1016/j.scitotenv.2020.138536 [DOI] [PubMed] [Google Scholar]
- Mayr, S., Hacke, U., Schmid, P., Schwienbacher, F., & Gruber, A. (2006). Frost drought in conifers at the alpine timberline: Xylem dysfunction and adaptations. Ecology, 87(12), 3175–3185. [DOI] [PubMed] [Google Scholar]
- Mayr, S., Schwienbacher, F., & Bauer, H. (2003). Winter at the alpine timberline. Why does embolism occur in Norway spruce but not in stone pine? Plant Physiology, 131(2), 780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, N. G., Michaletz, S. T., Bennett, K. E., Solander, K. C., Xu, C., Maxwell, R. M., & Middleton, R. S. (2018). Predicting chronic climate‐driven disturbances and their mitigation. Trends in Ecology & Evolution, 33(1), 15–27. 10.1016/j.tree.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Medlyn, B. (2004). A maestro retrospective. In Mencuccini M., Grace J., Moncrieff J., & McNaughton K. G. (Eds.), Forests at the land‐atmosphere interface (pp. 105–122). CABI Publishing. [Google Scholar]
- Mina, M., Bugmann, H., Cordonnier, T., Irauschek, F., Klopcic, M., Pardos, M., & Cailleret, M. (2017). Future ecosystem services from European mountain forests under climate change. Journal of Applied Ecology, 54(2), 389–401. 10.1111/1365-2664.12772 [DOI] [Google Scholar]
- Misson, L., Nicault, A., & Guiot, J. (2003). Effects of different thinning intensities on drought response in Norway spruce (Picea abies (L.) Karst.). Forest Ecology and Management, 183(1–3), 47–60. [Google Scholar]
- Moreno‐Gutiérrez, C., Battipaglia, G., Cherubini, P., Saurer, M., Nicolas, E., Contreras, S., & Querejeta, J. I. (2012). Stand structure modulates the long‐term vulnerability of Pinus halepensis to climatic drought in a semiarid Mediterranean ecosystem. Plant, Cell & Environment, 35(6), 1026–1039. 10.1111/j.1365-3040.2011.02469.x [DOI] [PubMed] [Google Scholar]
- Nakagawa, S., & Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed‐effects models. Methods in Ecology and Evolution, 4(2), 133–142. [Google Scholar]
- Navarro‐Cerrillo, R. M., Sánchez‐Salguero, R., Rodriguez, C., Lazo, J. D., Moreno‐Rojas, J. M., Palacios‐Rodriguez, G., & Camarero, J. J. (2019). Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. Sylvestris stands in southern Spain. Forest Ecology and Management, 433, 313–324. 10.1016/j.foreco.2018.11.006 [DOI] [Google Scholar]
- Nehrbass‐Ahles, C., Babst, F., Klesse, S., Nötzli, M., Bouriaud, O., Neukom, R., Dobbertin, M., & Frank, D. (2014). The influence of sampling design on tree‐ring‐based quantification of forest growth. Global Change Biology, 20(9), 2867–2885. 10.1111/gcb.12599 [DOI] [PubMed] [Google Scholar]
- Netherer, S., & Nopp‐Mayr, U. (2005). Predisposition assessment systems (PAS) as supportive tools in forest management—Rating of site and stand‐related hazards of bark beetle infestation in the High Tatra Mountains as an example for system application and verification. Decision Support in Multi Purpose Forestry, 207(1), 99–107. 10.1016/j.foreco.2004.10.020 [DOI] [Google Scholar]
- Nyland, R. D. (2016). Silviculture: Concepts and applications (3rd ed.). Waveland Press. [Google Scholar]
- Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R., Simpson, G. L., Solymos, P., Stevens, M. H. H., & Wagner, H. (2013). Package ‘vegan.’ Community ecology package, version, 2(9).
- Oliver, T. H., Heard, M. S., Isaac, N. J. B., Roy, D. B., Procter, D., Eigenbrod, F., Freckleton, R., Hector, A., Orme, C. D. L., Petchey, O. L., Proença, V., Raffaelli, D., Suttle, K. B., Mace, G. M., Martín‐López, B., Woodcock, B. A., & Bullock, J. M. (2015). Biodiversity and resilience of ecosystem functions. Trends in Ecology & Evolution, 30(11), 673–684. 10.1016/j.tree.2015.08.009 [DOI] [PubMed] [Google Scholar]
- Olson, M. E., Soriano, D., Rosell, J. A., Anfodillo, T., Donoghue, M. J., Edwards, E. J., León‐Gómez, C., Dawson, T., Camarero Martínez, J. J., Castorena, M., Echeverría, A., Espinosa, C. I., Fajardo, A., Gazol, A., Isnard, S., Lima, R. S., Marcati, C. R., & Méndez‐Alonzo, R. (2018). Plant height and hydraulic vulnerability to drought and cold. Proceedings of the National Academy of Sciences of the United States of America, 115(29), 7551. 10.1073/pnas.1721728115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm, S. L. (1984). The complexity and stability of ecosystems. Nature, 307(5949), 321. 10.1038/307321a0 [DOI] [Google Scholar]
- Pretzsch, H. (2005). Diversity and productivity in forests: Evidence from long‐term experimental plots. In Scherer‐Lorenzen M., Körner Ch., & Schulze E.‐D. (Eds.), Forest diversity and function (pp. 41–64). Springer. [Google Scholar]
- Pretzsch, H., Hilmers, T., Biber, P., Avdagić, A., Binder, F., Bončina, A., Bosela, M., Dobor, L., Forrester, D. I., Lévesque, M., Ibrahimspahić, A., Nagel, T. A., del Río, M. , Sitkova, Z., Schütze, G., Stajić, B., Stojanović, D., Uhl, E., Zlatanov, T., & Tognetti, R. (2020). Evidence of elevation‐specific growth changes of spruce, fir, and beech in European mixed mountain forests during the last three centuries. Canadian Journal of Forest Research, 50(7), 689–703. 10.1139/cjfr-2019-0368 [DOI] [Google Scholar]
- Pretzsch, H., Schütze, G., & Biber, P. (2018). Drought can favour the growth of small in relation to tall trees in mature stands of Norway spruce and European beech. Forest Ecosystems, 5(1), 20. 10.1186/s40663-018-0139-x [DOI] [Google Scholar]
- Pretzsch, H., Schütze, G., & Uhl, E. (2013). Resistance of European tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter‐specific facilitation. Plant Biology, 15(3), 483–495. 10.1111/j.1438-8677.2012.00670.x [DOI] [PubMed] [Google Scholar]
- Puettmann, K. J., D’Amato, A. W., Kohnle, U., & Bauhus, J. (2009). Individual‐tree growth dynamics of mature Abies alba during repeated irregular group shelterwood (Femelschlag) cuttings. Canadian Journal of Forest Research, 39(12), 2437–2449. 10.1139/X09-158 [DOI] [Google Scholar]
- R Core Team . (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R‐project.org/ [Google Scholar]
- Ratcliffe, S., Wirth, C., Jucker, T., van der Plas, F. , Scherer‐Lorenzen, M., Verheyen, K., Allan, E., Benavides, R., Bruelheide, H., Ohse, B., Paquette, A., Ampoorter, E., Bastias, C. C., Bauhus, J., Bonal, D., Bouriaud, O., Bussotti, F., Carnol, M., Castagneyrol, B., … Baeten, L. (2017). Biodiversity and ecosystem functioning relations in European forests depend on environmental context. Ecology Letters, 20(11), 1414–1426. 10.1111/ele.12849 [DOI] [PubMed] [Google Scholar]
- Rigling, A., Bigler, C., Eilmann, B., Feldmeyer‐Christe, E., Gimmi, U., Ginzler, C., Graf, U., Mayer, P., Vacchiano, G., Weber, P., Wohlgemuth, T., Zweifel, R., & Dobbertin, M. (2013). Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Global Change Biology, 19(1), 229–240. 10.1111/gcb.12038 [DOI] [PubMed] [Google Scholar]
- San‐Miguel‐Ayanz, J., de Rigo, D. , Caudullo, G., Durrant, T. H., Mauri, A., Tinner, W., Ballian, D., Beck, P., Birks, H., & Eaton, E. (2016). European atlas of forest tree species. Publication Office of the European Union. [Google Scholar]
- Scheffer, M., Carpenter, S., Foley, J. A., Folke, C., & Walker, B. (2001). Catastrophic shifts in ecosystems. Nature, 413(6856), 591–596. [DOI] [PubMed] [Google Scholar]
- Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution, 1(2), 103–113. 10.1111/j.2041-210X.2010.00012.x [DOI] [Google Scholar]
- Schuldt, B., Buras, A., Arend, M., Vitasse, Y., Beierkuhnlein, C., Damm, A., Gharun, M., Grams, T. E. E., Hauck, M., Hajek, P., Hartmann, H., Hiltbrunner, E., Hoch, G., Holloway‐Phillips, M., Körner, C., Larysch, E., Lübbe, T., Nelson, D. B., Rammig, A., … Kahmen, A. (2020). A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic and Applied Ecology, 45, 86–103. 10.1016/j.baae.2020.04.003 [DOI] [Google Scholar]
- Schume, H., Jost, G., & Hager, H. (2004). Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. Journal of Hydrology, 289(1), 258–274. 10.1016/j.jhydrol.2003.11.036 [DOI] [Google Scholar]
- Schwarz, J., Skiadaresis, G., Kohler, M., Kunz, J., Schnabel, F., Vitali, V., & Bauhus, J. (2020). Quantifying growth responses of trees to drought—A critique of commonly used resilience indices and recommendations for future studies. Current Forestry Reports, 6, 185–200. 10.1007/s40725-020-00119-2 [DOI] [Google Scholar]
- Seidl, R., Spies, T. A., Peterson, D. L., Stephens, S. L., & Hicke, J. A. (2016). Searching for resilience: Addressing the impacts of changing disturbance regimes on forest ecosystem services. Journal of Applied Ecology, 53(1), 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl, R., Thom, D., Kautz, M., Martin‐Benito, D., Peltoniemi, M., Vacchiano, G., Wild, J., Ascoli, D., Petr, M., Honkaniemi, J., Lexer, M. J., Trotsiuk, V., Mairota, P., Svoboda, M., Fabrika, M., Nagel, T. A., & Reyer, C. P. O. (2017). Forest disturbances under climate change. Nature Climate Change, 7, 395–402. 10.1038/nclimate3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senf, C., Buras, A., Zang, C. S., Rammig, A., & Seidl, R. (2020). Excess forest mortality is consistently linked to drought across Europe. Nature Communications, 11(1), 6200. 10.1038/s41467-020-19924-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra‐Maluquer, X., Mencuccini, M., & Martínez‐Vilalta, J. (2018). Changes in tree resistance, recovery and resilience across three successive extreme droughts in the northeast Iberian Peninsula. Oecologia, 187(1), 343–354. 10.1007/s00442-018-4118-2 [DOI] [PubMed] [Google Scholar]
- Shannon, C. E. (1948). A mathematical theory of communication. Bell System Technical Journal, 27(3), 379–423. 10.1002/j.1538-7305.1948.tb01338.x [DOI] [Google Scholar]
- Simon, J., Dannenmann, M., Pena, R., Gessler, A., & Rennenberg, H. (2017). Nitrogen nutrition of beech forests in a changing climate: Importance of plant‐soil‐microbe water, carbon, and nitrogen interactions. Plant and Soil, 418(1), 89–114. 10.1007/s11104-017-3293-y [DOI] [Google Scholar]
- Sohn, J. A., Gebhardt, T., Ammer, C., Bauhus, J., Häberle, K.‐H., Matyssek, R., & Grams, T. E. (2013). Mitigation of drought by thinning: Short‐term and long‐term effects on growth and physiological performance of Norway spruce (Picea abies). Forest Ecology and Management, 308, 188–197. 10.1016/j.foreco.2013.07.048 [DOI] [Google Scholar]
- Sohn, J. A., Hartig, F., Kohler, M., Huss, J., & Bauhus, J. (2016). Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecological Applications, 26(7), 2190–2205. [DOI] [PubMed] [Google Scholar]
- Sohn, J. A., Saha, S., & Bauhus, J. (2016). Potential of forest thinning to mitigate drought stress: A meta‐analysis. Forest Ecology and Management, 380, 261–273. 10.1016/j.foreco.2016.07.046 [DOI] [Google Scholar]
- Staelens, J., De Schrijver, A., Verheyen, K., & Verhoest, N. E. C. (2008). Rainfall partitioning into throughfall, stemflow, and interception within a single beech (Fagus sylvatica L.) canopy: Influence of foliation, rain event characteristics, and meteorology. Hydrological Processes, 22(1), 33–45. 10.1002/hyp.6610 [DOI] [Google Scholar]
- Stängle, S. M., & Dormann, C. F. (2018). Modelling the variation of bark thickness within and between European silver fir (Abies alba Mill.) trees in southwest Germany. Forestry, 91(3), 283–294. [Google Scholar]
- Stängle, S. M., Weiskittel, A. R., Dormann, C. F., & Brüchert, F. (2016). Measurement and prediction of bark thickness in Picea abies: Assessment of accuracy, precision, and sample size requirements. Canadian Journal of Forest Research, 46(1), 39–47. [Google Scholar]
- Stuart‐Haëntjens, E., De Boeck, H. J., Lemoine, N. P., Mänd, P., Kröel‐Dulay, G., Schmidt, I. K., Jentsch, A., Stampfli, A., Anderegg, W. R. L., Bahn, M., Kreyling, J., Wohlgemuth, T., Lloret, F., Classen, A. T., Gough, C. M., & Smith, M. D. (2018). Mean annual precipitation predicts primary production resistance and resilience to extreme drought. Science of the Total Environment, 636, 360–366. 10.1016/j.scitotenv.2018.04.290 [DOI] [PubMed] [Google Scholar]
- Teets, A., Fraver, S., Weiskittel, A. R., & Hollinger, D. Y. (2018). Quantifying climate–growth relationships at the stand level in a mature mixed‐species conifer forest. Global Change Biology, 24(8), 3587–3602. 10.1111/gcb.14120 [DOI] [PubMed] [Google Scholar]
- Temperli, C., Bugmann, H., & Elkin, C. (2012). Adaptive management for competing forest goods and services under climate change. Ecological Applications, 22(8), 2065–2077. 10.1890/12-0210.1 [DOI] [PubMed] [Google Scholar]
- Temperli, C., Bugmann, H., & Elkin, C. (2013). Cross‐scale interactions among bark beetles, climate change, and wind disturbances: A landscape modeling approach. Ecological Monographs, 83(3), 383–402. 10.1890/12-1503.1 [DOI] [Google Scholar]
- Tinner, W., Colombaroli, D., Heiri, O., Henne, P. D., Steinacher, M., Untenecker, J., Vescovi, E., Allen, J. R., Carraro, G., & Conedera, M. (2013). The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecological Monographs, 83(4), 419–439. [Google Scholar]
- van Dijk, A. I. J. M. , & Bruijnzeel, L. A. (2001). Modelling rainfall interception by vegetation of variable density using an adapted analytical model. Part 1. Model description. Journal of Hydrology, 247(3), 230–238. 10.1016/S0022-1694(01)00392-4 [DOI] [Google Scholar]
- Vicente‐Serrano, S. M., Beguería, S., & López‐Moreno, J. I. (2010). A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. Journal of Climate, 23(7), 1696–1718. 10.1175/2009JCLI2909.1 [DOI] [Google Scholar]
- Vincent, G., & Kantor, J. (1971). Das frühzeitige Tannensterben, seine Ursachen und Vorbeugung. Centralblatt Für Gesamte Forstwesen, 88, 101–115. [Google Scholar]
- Vitali, V., Büntgen, U., & Bauhus, J. (2017). Silver fir and Douglas fir are more tolerant to extreme droughts than Norway spruce in south‐western Germany. Global Change Biology, 23(12), 5108–5119. 10.1111/gcb.13774 [DOI] [PubMed] [Google Scholar]
- Vitali, V., Büntgen, U., & Bauhus, J. (2018). Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia, 48, 1–9. 10.1016/j.dendro.2018.01.001 [DOI] [Google Scholar]
- Vitali, V., Forrester, D. I., & Bauhus, J. (2018). Know your neighbours: Drought response of Norway spruce, silver fir and douglas fir in mixed forests depends on species identity and diversity of tree neighbourhoods. Ecosystems, 21(6), 1215–1229. 10.1007/s10021-017-0214-0 [DOI] [Google Scholar]
- Vitasse, Y., Bottero, A., Cailleret, M., Bigler, C., Fonti, P., Gessler, A., Lévesque, M., Rohner, B., Weber, P., Rigling, A., & Wohlgemuth, T. (2019). Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Global Change Biology, 25(11), 3781–3792. 10.1111/gcb.14803 [DOI] [PubMed] [Google Scholar]
- Vitasse, Y., Bottero, A., Rebetez, M., Conedera, M., Augustin, S., Brang, P., & Tinner, W. (2019). What is the potential of silver fir to thrive under warmer and drier climate? European Journal of Forest Research, 138(4), 547–560. 10.1007/s10342-019-01192-4 [DOI] [Google Scholar]
- Wang, Y., & Jarvis, P. (1990). Description and validation of an array model—MAESTRO. Agricultural and Forest Meteorology, 51(3–4), 257–280. 10.1016/0168-1923(90)90112-J [DOI] [Google Scholar]
- Weise, U. (1995). Zuwachs‐und Jungwuchsentwicklung in Versuchen zur natürlichen Verjüngung von Fichten‐Tannen (Buchen)‐Beständen in Baden‐Württemberg. Mitteilungen der Forstlichen Versuchs‐ und Forschungsanstalt Baden‐Württemberg (Vol. 192, pp. 1–75). Forstliche Versuchs‐und Forschungsans. [Google Scholar]
- Wiedemann, E. (1927). Untersuchungen über das Tannensterben. Forstwissenschaftliches Centralblatt, 49(21), 759–780. 10.1007/BF01774334 [DOI] [Google Scholar]
- Yousefpour, R., Temperli, C., Jacobsen, J. B., Thorsen, B. J., Meilby, H., Lexer, M. J., Lindner, M., Bugmann, H., Borges, J. G., Palma, J. H. N., Ray, D., Zimmermann, N. E., Delzon, S., Kremer, A., Kramer, K., Reyer, C. P. O., Lasch‐Born, P., Garcia‐Gonzalo, J., & Hanewinkel, M. (2017). A framework for modeling adaptive forest management and decision making under climate change. Ecology and Society, 22(4). 10.5751/ES-09614-220440 [DOI] [Google Scholar]
- Yue, C., Kohnle, U., Hanewinkel, M., & Klädtke, J. (2011). Extracting environmentally driven growth trends from diameter increment series based on a multiplicative decomposition model. Canadian Journal of Forest Research, 41(8), 1577–1589. 10.1139/x11-056 [DOI] [Google Scholar]
- Zweifel, R., Etzold, S., Sterck, F., Gessler, A., Anfodillo, T., Mencuccini, M., von Arx, G. , Lazzarin, M., Haeni, M., Feichtinger, L., Meusburger, K., Knuesel, S., Walthert, L., Salmon, Y., Bose, A. K., Schoenbeck, L., Hug, C., De Girardi, N., Giuggiola, A., … Rigling, A. (2020). Determinants of legacy effects in pine trees – Implications from an irrigation‐stop experiment. New Phytologist, 227(4), 1081–1096. 10.1111/nph.16582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


