Abstract
One‐lung ventilation (OLV), a common ventilation technique, is associated with perioperative lung injury, tightly connected with inflammatory responses. Dexmedetomidine has shown positive anti‐inflammatory effects in lung tissues in pre‐clinical models. This study investigated the efficacy of dexmedetomidine for suppressing inflammatory responses in patients requiring OLV. We searched PubMed, MEDLINE, Embase, Scopus, Ovid, and Cochrane Library for randomized controlled trials focusing on dexmedetomidine’s anti‐inflammatory effects on patients requiring OLV without any limitation on the year of publication or languages. 20 clinical trials were assessed with 870 patients in the dexmedetomidine group and 844 in the control group. Our meta‐analysis investigated the anti‐inflammatory property of dexmedetomidine perioperatively [T1 (30‐min OLV), T2 (90‐min OLV), T3 (end of surgery) and T4 (postoperative day 1)], demonstrating that dexmedetomidine’s intraoperative administration resulted in a significant reduction in serum concentration of interleukin‐6, tumor necrosis factor‐α and other inflammatory cytokines perioperatively. By calculating specific I 2 index, significant heterogeneity was observed on all occasions, with I 2 index ranging from 95% to 99%. For IL‐6 changes, sensitivity analysis showed that the exclusion of a single study led to a significant decrease of heterogeneity (96%–0%; p < 0.00001). Besides, pulmonary oxygenation was ameliorated in the dexmedetomidine group comparing with the control group. In conclusion, perioperative administration of dexmedetomidine can attenuate OLV induced inflammation, ameliorate pulmonary oxygenation, and may be conducive to a decreased occurrence of postoperative complications and better prognosis. However, the results should be prudently interpreted due to the evidence of heterogeneity and the limited number of studies.
Keywords: anti‐inflammatory agents, dexmedetomidine, inflammation, one‐lung ventilation
1. INTRODUCTION
One‐lung ventilation (OLV), aiming for double‐lung isolation and reduction of operative lung injury, has become a commonly used ventilation technique in thoracic surgeries.1 However, it also results in multiple complications, including hypoxaemia,2 lung injury,3 acute respiratory distress syndrome4 and death.5 Ventilator‐induced lung injury (VILI) is one of the most serious potential complications of one‐lung ventilation6 and has raised extensive concern on finding its preventative measures. In a recent review, the pathology of VILI was characterized as pulmonary infiltration, hyaline membrane formation, increased vascular endothelium permeability, pulmonary oedema and hypoxia.7 Inflammatory cell infiltration could result in damage to the isolated lung and extrapulmonary organs by activating pro‐inflammatory and pro‐injurious cytokine cascade.4, 8, 9 Moreover, being the main cause of both short‐ and long‐term morbidity, elevated systematic inflammation may induce peritoneal membrane fibrosis and angiogenesis, diabetes mellitus, metabolic syndrome, non‐alcoholic fatty liver disease and even cardiovascular disease,10, 11 which makes anesthetics that possesses anti‐inflammatory properties a promising potential approach to suppress inflammatory responses by reducing the release of lipid mediators derived from arachidonic acid, relevant proteins and gaseous molecules.
Dexmedetomidine, a highly selective α‐2 adrenergic receptor agonist, has an outstanding property of allowing easily controllable analgesia and sedation without respiratory depression, which prompts widespread use in the intensive care unit. Over the past five years, increasing numbers of clinical studies12, 13, 14, 15 have demonstrated the anti‐inflammatory effects of dexmedetomidine. Dexmedetomidine’s promising anti‐inflammatory property with minimal effects on respiration makes it the potential anaesthetic for patients requiring OLV.
The potential anti‐inflammatory effects of dexmedetomidine are thought to be conferred, at least partly, by agonizing the α‐2 adrenergic receptor. With the administration of yohimbine,16, 17 an α‐2 agonist blockade, the effect was attenuated. However, the specific signalling pathway that dexmedetomidine took a role in was still obscure. Zhu et al.18 demonstrated that the extracellular signal‐regulated kinase 1/2 (ERK1/2) pathway contributed to the anti‐inflammatory effect in a latest study. Chen et al.16 suggested that mechanical stretch may induce the release of toll‐like receptor (TLR4), thus activating nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), which regulates the genes encoding a broad range of inflammatory molecules.
Various pre‐clinical studies16, 17, 18, 19, 20, 21, 22, 23 showed the anti‐inflammatory property of dexmedetomidine in reducing VILI (Table 1). VILI was usually induced by high tidal volume ventilation (tidal volume 6–20 mL/kg; respiratory rate 50–80 breaths/min) in these pre‐clinical models. Significant pathology changes, such as structural changes of alveolar wall, alveolar perihelial death, inflammatory cell infiltration, and increased concentration of inflammatory cytokines (interleukin (IL)‑6), tumour necrosis factor‑α (TNF‑α), etc.), were observed in the control groups. In the dexmedetomidine groups, histopathology damage was significantly alleviated, along with a decreased expression of inflammatory cytokines, wet‐to‐dry ratio, and myeloperoxidase activity.
TABLE 1.
Main characteristics of pre‐clinical studies
| Author | Model | Ventilation strategy | DEX setting | Inflammatory mediator | MPO activation | W/D ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tidal volume | Respiration rate/min | Ventilation time | Other settings | |||||||
| Zhu 2020 | Rat | 20 ml/kg | 50 | 4 h | – |
Bolus (10 μg/kg for 15 min) followed by continuous infusion (10 μg/kg/h) |
IL‐6 IL‐1β TNF‐α |
↓ ↓ ↓ |
↓ | ↓ |
| Li 2020 | Rat | 8 ml/kg | 60–80 | 2 h |
I:E = 1:3 FiO2=45% |
1.0 or 5.0 μg/kg/h continuous infusion |
IL‐8 TNF‐α |
↓ ↓ |
– | – |
| Chen 2018 | Rat | 20 ml/kg | 50 | 4 h | – | Bolus (10 μg/kg for 15 min), followed by continuous infusion (10 μg/kg/h) |
IL‐1β TNF‐α IL‐6 |
↓ ↓ ↓ |
↓ | ↓ |
| Wang 2018 | Rat | Right lung OLV 10 ml/kg | 55 | 2 h | FiO2 = 100% | 5 μg/kg/h continuous infusion for 50 min |
IL‐6 IL‐10 TNF‐α |
↓ ↓ ↓ |
↓ | – |
| Heil 2016 | Rat | 6 ml/kg | 80 | 1 h |
FIO2 = 1.0 (first 5 min) 0.4 (next 1 h) |
Bolus (1 μg/kg for 10 min) followed by continuous infusion (0.5 μg/kg/h) |
TNF‐α IL‐6 |
↓ ↓ |
– | – |
| Chen 2013 | Dog | 20 ml/kg | 15 | 4 h | =50% | Bolus (0.5, 1, 2 μg/kg for 10 min) followed by continuous infusion (0.5, 1.0, 2.0 μg/kg/h) |
NF‐kB TNF‐α iNOS |
↓ ↓ ↓ |
↓ |
No statistical significance |
| Yang 2010 | Rat | 20 ml/kg | 50 | 4 h | FiO2 = 21% | Bolus (1 mg/kg for 10 min) followed by continuous infusion (0.5 mg/kg/h) |
NO PGE2 MIP‐2 IL‐1β |
↓ ↓ ↓ ↓ |
↓ | ↓ |
| Yang 2008 | Rat | 20 ml/kg | 50 | 4 h | FiO2 = 21% | Bolus (1 μg/kg for 10 min) followed by continuous infusion (0.5, 2.5, 5.0 μg/kg/h) |
NO PGE2 TNF‐α IL‐1β IL‐6 MIP‐2 |
↓ ↓ ↓ ↓ ↓ ↓ |
– | – |
Abbreviations: IL‐1β interleukin‐1β; IL‐6 interleukin‐6; IL‐8 interleukin‐8; iNOS inducible nitric oxide synthase; MIP‐2 macrophage inflammatory protein‐2; NF‐kB nuclear factor kappa‐light‐chain‐enhancer of activated B cells; NO nitric oxide; PGE2 prostaglandin E2; TNF‐α tumor necrosis factor‐α.
Although pre‐clinical studies had shown promising results, clinical trials are needed to evaluate dexmedetomidine’s anti‐inflammatory effects. Nevertheless, there has been no systematic review nor meta‐analysis concerning this topic. Therefore, we conducted this systematic review to investigate dexmedetomidine’s impact on inflammatory responses on patients requiring OLV.
2. RESULTS
2.1. Article selection
Of the 1218 potential articles in the initial search, 20 RCTs24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 met the inclusion criteria. The screening and selection process is demonstrated in Figure 1. After removing 407 duplicate, 616 non‐clinical articles and 43 full‐text inaccessible articles, 152 articles remained. Among those, 137 articles were excluded based on exclusion criteria.
FIGURE 1.
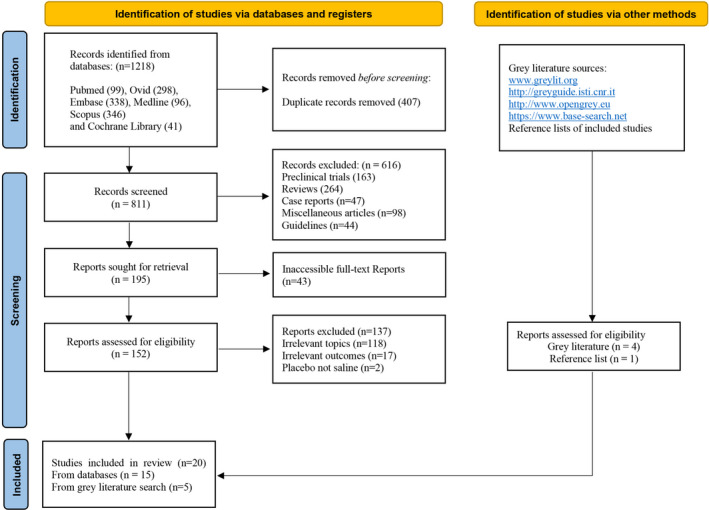
Complete Search Strategy
2.2. Major statistics of included studies
Major characteristics of included studies are presented in Table S1a,b. A total of 1714 patients (870 in the dexmedetomidine group, 844 in the control group) undergoing surgeries with OLV were included in this meta‐analysis, with an average age of [MD, 58.35; 95%CI, (42.56, 74.15)]. Among those, three trials applied dexmedetomidine only before induction, while the others applied dexmedetomidine through surgery, with initial doses ranging from 0.3 to 1 μg/kg [MD, 0.67; 95%CI, (0.118, 1.220)] and maintenance doses of 0.3–0.5 μg/kg/h [MD, 0.4; 95%CI, (0.187, 0.613)]. Considering the diversity in surgery types, the duration of surgery and OLV varies from 117 to 223 min [MD, 163.78; 95%CI, (83.657, 243.906)] and from 58 to 169.33 min [MD, 108.204; 95%CI, (50.621, 165.787)] respectively. Supplementary anaesthetics administrated during the surgery include sevoflurane, propofol, midazolam, etomidate, fentanyl, sufentanil, remifentanil, vecuronium, rocuronium, atracurium, cisatracurium, and penehyclidine hydrochloride.
2.3. Quality assessment
Amongst the 20 included clinical trials, 16 studies were classified as high quality and four as low quality. Only four studies were considered as low risk‐of‐bias, three as high risk‐of‐bias, and 13 remained unclear. The complete summary of methodological quality assessment and risk of bias assessment is demonstrated in Supplementary Material a and b, respectively.
2.4. Inflammatory cytokine changes: TNF‑α
A total of 17 trials investigated perioperative changes of TNF‐α from baseline at four timepoints, all resulting in significant decrease (Figure 2). Compared with control groups, the administration of dexmedetomidine in observation groups lead to a most effective validity of inflammation suppression at the end of surgery [MD, −14.41; 95% CI, (−19.76 to 9.07)] pg/mL; p < 0.00001], which is in consistent with the TNF‐α variation trends in several studies. It is noticeable that serious heterogeneity was observed on all occasions [T1, I 2 = 97%, p < 0.00001; T2, I 2 = 97%, p < 0.00001; T3, I 2 = 99%, p < 0.00001); T4, I 2 = 98%, p < 0.00001)]. Thus, subgroup analysis was performed to identify potential sources of heterogeneity.
FIGURE 2.
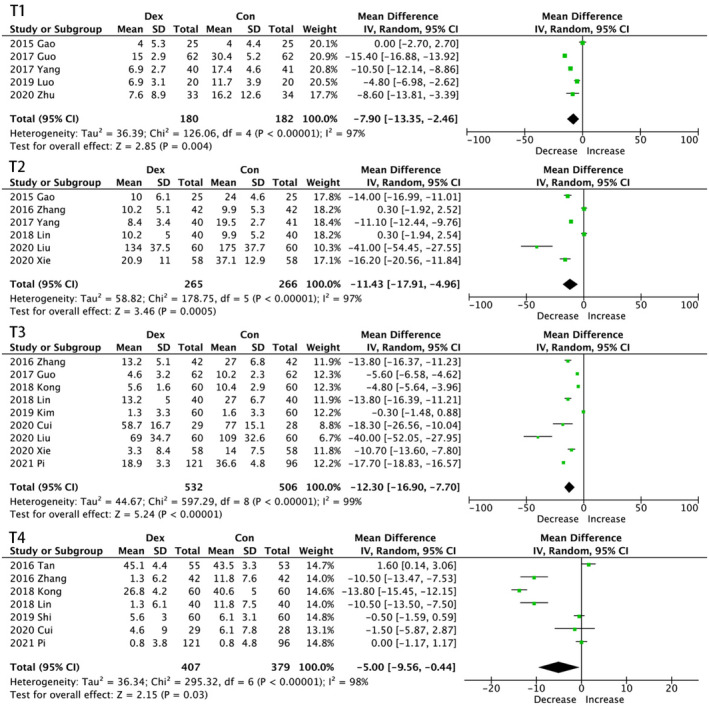
Forest graph showing the effect of dexmedetomidine on the change from baseline in serum tumour necrosis factor (TNF)‑α levels. (A) at the end of surgery, (B) 30 min after one lung ventilation (OLV) and (C) the first postoperative day. CI indicates confidence interval, SD, standard deviations
In subgroup analysis, as shown in Table 2, only combined infusion mode resulted in statistical significance on all occasions, while bolus injection of dexmedetomidine even led to an increase in the concentration of TNF‑α. Trials performed on non‐lung associated surgeries showed no significance at T2. Nevertheless, those conducted on lung associated surgeries had no statistical significance at T4 either. The concentration of TNF‑α dropped in groups with a balanced sex ratio at T1 and T2, but not in other subgroups. At T4, surgery duration had no decreasing effect on TNF‑α levels. All subgroups had a significant decrease in the concentration of TNF‑α at T3.
TABLE 2.
Subgroup analysis of TNF‐α
| Subgroups | Studies with | Number of studies | MD & 95%CI | Between subgroup significance |
|---|---|---|---|---|
| TNF‐α | ||||
| T1 | Balanced sex ratio | 2 | −5.31 [−15.60, 4.98] | Z = 1.01, p = 0.31 |
| Unbalanced sex ratio | 3 | −9.67 [−17.64, −1.69] | Z = 2.38, p = 0.02 | |
| Combined infusion | 4 | −7.21 [−15.09, 0.66] | Z = 1.79, p = 0.07 | |
| Bolus | 1 | −10.50 [−12.14, −8.86] | Z = 12.56, p < 0.00001 | |
| Surgery duration above average | 1 | −4.80 [−6.98, −2.62] | Z = 4.31, p < 0.0001 | |
| Surgery duration below average | 4 | −8.70 [−15.01, −2.38] | Z = 2.70, p = 0.007 | |
| Non‐lung‐associated surgery | 4 | −7.75 [−13.93, −1.57] | Z = 2.46, p = 0.01 | |
| Lung‐associated surgery | 1 | −8.60 [−13.81, −3.39] | Z = 3.23, p = 0001 | |
| OLV duration above average | 1 | −4.80 [−6.98, −2.62] | Z = 4.31, p < 0.0001 | |
| OLV duration below average | 4 | −8.70 [−15.01, −2.38] | Z = 2.70, p = 0.007 | |
| T2 | Balanced sex ratio | 4 | −4.41 [−12.71, 3.90] | Z = 1.04, p = 0.30 |
| Unbalanced sex ratio | 2 | −27.75 [−52.00, −3.50] | Z = 2.24, p = 0.02 | |
| Combined infusion | 4 | −11.26 [−20.20, −2.31] | Z = 2.47, p = 0.01 | |
| Bolus | 2 | −12.23 [−15.00, −9.46] | Z = 8.65, p < 0.00001 | |
| Surgery duration above average | 2 | 0.30 [−1.94, 2.54] | Z = 0.37, p = 0.71 | |
| Surgery duration below average | 4 | −20.35 [−28.70, −12.00] | Z = 4.78, p < 0.00001 | |
| Non‐lung‐associated surgery | 3 | −7.84 [−24.01, −8.33] | Z = 0.95, p = 0.34 | |
| Lung‐associated surgery | 3 | −16.22 [−30.27, −2.16] | Z = 2.26, p = 0.02 | |
| OLV duration above average | 2 | 0.30 [−1.94, 2.54] | Z = 0.37, p = 0.71 | |
| OLV duration below average | 4 | −20.35 [−28.70, −12.00] | Z = 4.78, p < 0.00001 | |
| T3 | Balanced sex ratio | 4 | −15.27 [−25.35, −5.20] | Z = 2.97, p = 0.003 |
| Unbalanced sex ratio | 5 | −10.99 [−16.89, 5.09] | Z = 3.65, p = 0.0003 | |
| Surgery duration above average | 2 | −13.80 [−16.39, −11.21] | Z = 14.82, p < 0.00001 | |
| Surgery duration below average | 7 | −11.86 [−17.19, −6.54] | Z = 4.36, p < 0.0001 | |
| Non‐lung‐associated surgery | 2 | −12.33 [−15.36, −9.29] | Z = 7.96, p < 0.00001 | |
| Lung‐associated surgery | 7 | −12.38 [−17.76, −6.99] | Z = 4.50, p < 0.00001 | |
| OLV duration above average | 2 | −13.80 [−16.39, −11.21] | Z = 14.82, p < 0.00001 | |
| OLV duration below average | 7 | −11.86 [−17.19, −6.54] | Z = 4.36, p < 0.0001 | |
| T4 | Balanced sex ratio | 5 | −15.27 [−25.35, −5.20] | Z = 6.87, p < 0.00001 |
| Unbalanced sex ratio | 2 | −10.99 [−16.89, 5.09] | Z = 0.98, p = 0.33 | |
| Combined infusion | 5 | −8.90 [−15.05, −2.75] | Z = 2.06, p = 0.04 | |
| Continuous infusion | 1 | −0.50 [−1.59, 0.59] | Z = 0.90, p = 0.37 | |
| Bolus | 1 | 1.60 [0.14, 3.06] | Z = 2.14, p = 0.03 | |
| Surgery duration above average | 3 | −7.07 [−14.84, 0.70] | Z = 1.78, p = 0.07 | |
| Surgery duration below average | 4 | −3.45 [−10.81, 3.91] | Z = 0.92, p = 0.36 | |
| OLV duration above average | 2 | −5.40 [−15.20, 4.40] | Z = 1.08, p = 0.28 | |
| OLV duration below average | 5 | −4.85 [−11.36, 1.67] | Z = 1.46, p = 0.14 | |
| Non‐lung‐associated surgery | 6 | −5.86 [−11.57, −0.16] | Z = 2.01, p = 0.04 | |
| Lung‐associated surgery | 1 | −0.00 [−1.17, 1.17] | Z = 0.00, p = 1.00 |
Abbreviations: TNF‐α, tumour necrosis factor‐α; OLV, one lung ventilation.
2.5. Inflammatory cytokine changes: IL‐6
Dexmedetomidine administration also resulted in statistically significant reductions in serum concentration of IL‐6 [MD, −4.94, 95%CI (−8.54, −1.33) pg/mL, p < 0.00001 at T1 (Figure 3); MD, −9.86, 95%CI (−16.79, −2.93) pg/mL, p < 0.00001 at T2; MD, −14.41, 95%CI (−19.76, −9.07) pg/mL, p < 0.00001 at T3; MD, −14.48, 95%CI (−20.76, −8.3) pg/mL, p < 0.00001 at T4]. Unlike the impact on TNF‐α, the anti‐inflammatory effect on IL‐6 was gradually enhanced until 24 h after surgery. The subgroup was conducted due to evident heterogeneity.
FIGURE 3.
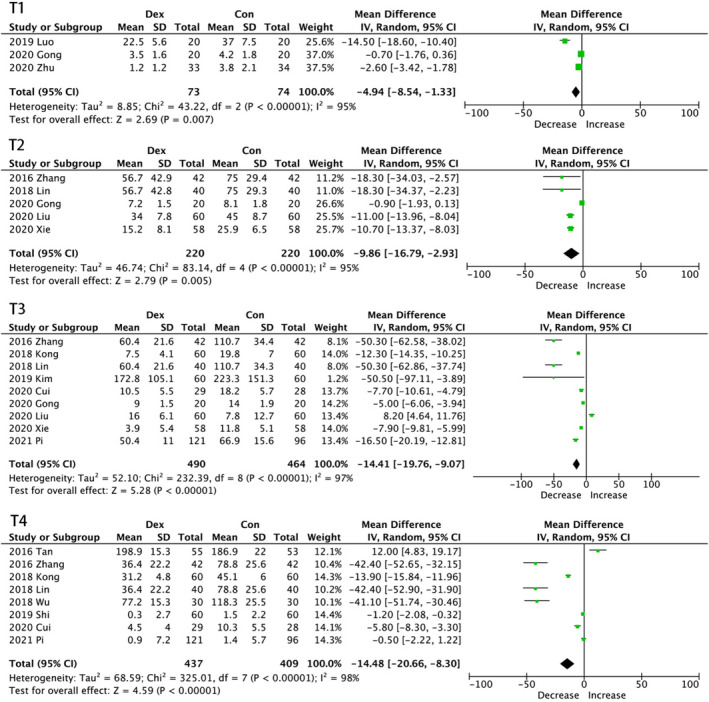
Forest graph showing the effect of dexmedetomidine on the change from baseline in serum interleukin (IL)‑6 levels. (A) 30 min after one lung ventilation (OLV), (B) at the end of surgery and (C) the first postoperative day. CI indicates confidence interval, SD, standard deviations
In the subgroup analysis, as shown in Table 3, administration mode, sex ratio, surgery duration, OLV duration, and surgery types impacted the results. Only combined infusion led to a significant decrease in IL‐6 concentrations at all time points, while the single‐dose group had an increase in IL‐6 concentrations at T4, but no significant difference was seen in the continuous infusion group, which is consistent with subgroup analysis on TNF‑α at T3. Although no statistical significance was found in the balanced sex ratio group at T3, more occasions showed that an unbalanced sex ratio led to statistical insignificance. When surgery duration was below average, IL‐6 concentration decreased at T1. A significant decrease was observed in trials in which OLV duration was above average on all occasions except postoperative day 1. However, at T3, only the sex ratio and infusion mode affected the outcome; the other subgroups all had a significant decrease in IL‐6 concentration.
TABLE 3.
Subgroup analysis of IL‐6
| Subgroups | Studies with | Number of studies | MD & 95%CI | Between subgroup significance |
|---|---|---|---|---|
| IL‐6 | ||||
| T1 | Surgery duration above average | 1 | −14.50 [−18.60, −10.40] | Z = 6.93, p < 0.00001 |
| Surgery duration below average | 2 | −1.68 [−3.54, 0.18] | Z = 1.77, p < 0.08 | |
| Non‐lung‐associated surgery | 2 | −2.60 [−3.42, −1.78] | Z = 1.08, p < 0.28 | |
| Lung‐associated surgery | 1 | −7.45 [−20.97, 6.07] | Z = 6.24, p < 0.00001 | |
| OLV duration above average | 1 | −14.50 [−18.60, −10.40] | Z = 6.93, p < 0.00001 | |
| OLV duration below average | 2 | −1.68 [−3.54, 0.18] | Z = 1.77, p < 0.08 | |
| T2 | Balanced sex ratio | 2 | −11.47 [−14.33, −8.61] | Z = 7.86, p < 0.00001 |
| Unbalanced sex ratio | 2 | −5.72 [−15.32, 3.88] | Z = 1.17, p = 0.24 | |
| Combined infusion | 3 | −11.06 [−13.01, −9.11] | Z = 11.10, p < 0.00001 | |
| Continuous infusion | 1 | −0.90 [−1.93, 0.13] | Z = 1.72, p = 0.09 | |
| Surgery duration above average | 2 | −10.69 [−24.78, 3.40] | Z = 1.49, p = 0.14 | |
| Surgery duration below average | 2 | −10.83 [−12.82, −8.85] | Z = 10.71, p < 0.00001 | |
| Non‐lung‐associated surgery | 2 | −7.99 [−16.85, 0.86] | Z = 1.17, p = 0.24 | |
| Lung‐associated surgery | 2 | −11.24 [−14.15, −8.33] | Z = 7.58, p < 0.00001 | |
| OLV duration above average | 1 | −18.30 [−34.37, −2.23] | Z = 3.19, p = 0.001 | |
| OLV duration below average | 3 | −7.45 [−15.17, 0.27] | Z = 1.89, p = 0.06 | |
| T3 | Balanced sex ratio | 4 | −20.73 [−78.05, 36.60] | Z = 1.65, p = 0.10 |
| Unbalanced sex ratio | 5 | −8.18 [−11.55, −4.80] | Z = 5.18, p < 0.00001 | |
| Combined infusion | 7 | −11.33 [−19.06, −3.60] | Z = 4.50, p < 0.00001 | |
| Continuous infusion | 2 | −5.00 [−6.06, −3.94] | Z = 0.98, p = 0.33 | |
| Surgery duration above average | 2 | −50.30 [−62.86, −37.74] | Z = 11.22, p < 0.00001 | |
| Surgery duration below average | 7 | −5.12 [−9.74, −0.50] | Z = 3.13, p = 0.002 | |
| Non‐lung‐associated surgery | 3 | −6.34 [−9.17, −3.50] | Z = 3.97, p < 0.0001 | |
| Lung‐associated surgery | 6 | −13.52 [−25.26, −1.77] | Z = 3.13, p < 0.002 | |
| OLV duration above average | 2 | −50.30 [−62.86, −37.74] | Z = 11.22, p < 0.00001 | |
| OLV duration below average | 7 | −5.12 [−9.74, −0.50] | Z = 3.13, p = 0.002 | |
| T4 | Balanced sex ratio | 3 | −41.98 [−48.02, −35.94] | Z = 13.63, p < 0.00001 |
| Unbalanced sex ratio | 5 | −2.59 [−8.07, 2.89] | Z = 0.93, p = 0.35 | |
| Combined infusion | 5 | −18.97 [−28.03, −9.92] | Z = 4.11, p < 0.00001 | |
| Continuous infusion | 2 | −20.78 [−59.88, 18.31] | Z = 1.04, p = 0.30 | |
| Bolus | 1 | 12.00 [4.83, 19.17] | Z = 3.28, p = 0.001 | |
| Surgery duration above average | 2 | −21.47 [−61.84, −18.90] | Z = 1.04, p = 0.30 | |
| Surgery duration below average | 6 | −13.74 [−22.22, −5.25] | Z = 3.17, p = 0.002 | |
| OLV duration above average | 3 | −27.92 [−60.42, 4.58] | Z = 1.68, p = 0.09 | |
| OLV duration below average | 5 | −9.01 [−17.41, −0.62] | Z = 2.10, p = 0.04 |
Abbreviations: IL‐6, interleukin‐6; OLV, one lung ventilation.
2.6. Other inflammatory mediators
A significant decrease in IL‐8 level from baseline at T2 was observed [MD, −13.64; 95% CI (−25.13, −2.15) pg/mL; p < 0.00001] in four studies,24, 25, 26, 43 while there was no significant difference found in serum concentration of IL‐10 at T2 (Figure S1).
As for other inflammatory cytokines, the data were insufficient to conduct a meta‐analysis. But we observed a significant reduction of interleukin (IL)‐1β27 at T2 [MD, −1.42; 95% CI (−1.65, −1.19) pg/mL; p < 0.00001], which was in consistence with the anti‐inflammatory effect of dexmedetomidine. However, the only significant difference found in monocyte chemoattractant protein‐1 (MCP‐1)30 was observed in the control group one hour after of end of one‐lung ventilation.
2.7. PaO2 and oxygen index
Seven trials27, 31, 32, 34, 37, 38, 39 evaluated the effect dexmedetomidine had on oxygenation at 30 min after OLV. As shown in Figure S2, significant increases were observed of both PaO2 and oxygen index in the dexmedetomidine group [PaO2: MD, 8.55, 95%CI (0.79, 16.30), p < 0.00001; OI: MD, 61.90, 95%CI (43.78, 80.01)], p < 0.00001)].
2.8. Sensitivity analysis
Influence analysis was performed by selecting a specific study and observing the changes in heterogeneity. After evaluating the impact of each study on the outcomes at all time points, we found that after deleting the study conducted by Gong et al.34, the heterogeneity on IL‐6 at T2 was reduced from 96% to 0%. Luo’s study42 affected the overall heterogeneity by 8% on IL‐8 at T1.
3. DISCUSSION
This meta‐analysis evaluated the value of perioperative administration of dexmedetomidine. From 20 RCTs, it demonstrated that, as a perioperative adjuvant anaesthetic, dexmedetomidine significantly inhibited serum concentration of IL‐6, IL‐8, and TNF‑α as well as ameliorated pulmonary oxygenation in patients undergoing thoracic surgeries with OLV, which was in conformity with the pre‐clinical trials. By subgroup analysis, bolus injection combined with continuous infusion was the only administration mode conferring the anti‐inflammatory effects perioperatively, and single injection mode even resulted in an increased level of both IL‐6 and TNF‑α postoperatively. These data postulated that dexmedetomidine could attenuate perioperative pulmonary inflammation induced by OLV and preserve pulmonary oxygenation function.
The sensitivity analysis revealed that continuous infusion of dexmedetomidine might account for the heterogeneity reduction on IL‐6 at T1. Though high heterogeneity was observed in every forest plot, by subgroup analysis and sensitivity analysis, we managed to find that unbalanced sex ratio and diverse administration mode were the potential factors. In addition, we noticed that the radioimmunoassay technique was only applied by Tan et al, while all other studies used enzyme‐linked immunosorbent assay technique (ELISA) to identify the serum concentration of inflammatory cytokines, which might account for the prominent data of IL‐6 and TNF‑α at T4. Moreover, all ELISA kits, except for the ones used in these two studies, came from different manufactures. It is known that reference interval could be different due to different manufactures. These deductions might give a reasonable explanation for the evident heterogeneity on all occasions.
To specialize in OLV induced pulmonary inflammation, we only assessed clinical trials that administered dexmedetomidine perioperatively on OLV patients in all aspects. In Li’s study,13 a meta‐analysis conducted in 2015, only pre‐operative administration of dexmedetomidine was reviewed; in Wang’s meta‐analysis,44 they did not cover all RCTs that had patients undergoing surgeries with OLV; and Flanders et al.45 put little emphasis on analyzing pre‐existing clinical trials as well as the interrelation between animal studies and clinical trials. Compared with all the existing meta‐analyses in this field, our review is the first one that synthesized the available data from all existing clinical trials on the effect of perioperative use of dexmedetomidine on inflammation induced by OLV. Our results are aligned with the previous systematic reviews13, 44, 45 that dexmedetomidine attenuated immune responses by inhibiting the release of IL‐6, IL‐8, and TNF‑α, and is beneficial to patients’ recovery and outcomes.
Though high heterogeneity was observed in forest plots at all timepoints, we analyzed several potential heterogeneity resources through subgroup analysis, which may be ascribed to dexmedetomidine administration mode, unbalanced sex ratio, and OLV duration.
For patients undergoing surgeries with OLV, perioperative administration of dexmedetomidine may exert anti‐inflammatory effects, which could mitigate VILI, preserve patients’ pulmonary function, and facilitate postoperative recovery.
3.1. Limitations
We acknowledged that there are five aspects of serious limitations.
First, there are limited studies that could meet our inclusion criteria, thus resulting in insufficient number of trials assessed on several occasions, which may undermine the credibility of our results. There are few studies regarding the concentration of IL‐1β, MCP‐1, and CRP, which makes it impossible to draw conclusive conclusions in this regard.
Second, all studies included were performed in Eastern countries, thus making extrapolation of our conclusions to Western populations questionable.
Third, though we managed to explain the obvious heterogeneity by performing subgroup analysis and sensitivity analysis, no exact reason was determined. Apart from the potential contributing factors, various centre settings, populations enrolled, and different anaesthetics used during the perioperative period could also interfere with the manifestation of dexmedetomidine’s effects.
Forth, there is no available study concerning the association between long‐term clinical outcome and perioperative immunosuppression, thus requiring further studies to evaluate this aspect.
Lastly, the processes of randomization, allocation concealment and blinding were inadequate in these studies,29, 30, 31, 32, 33, 34, 35, 38, 39, 40, 41, 42, 43 which indeed would cause selection bias and diagnostic bias.
4. CONCLUSION
This meta‐analysis demonstrated that perioperative administration of dexmedetomidine could attenuate inflammation induced by OLV and ameliorate pulmonary oxygenation, which may contribute to better clinical outcomes. More adequately powered and appropriately designed clinical trials are needed to further refine our results.
5. METHODS
This study conformed to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement (PRISMA) in reporting this systematic review and meta‐analysis.46 A detailed PRISMA guidelines checklist was presented in Appendix S1. This study was registered in The International Prospective Register of Systematic Reviews (PROSPERO registration number CRD42021223923).
5.1. Search strategy
We searched PubMed, MEDLINE, Embase, Scopus, Ovid, and Cochrane Library without any limitation on the year of publication or languages to find randomized‐controlled trials (RCT) assessing the anti‐inflammatory property of dexmedetomidine on patients requiring OLV. Four websites were used as grey literature search engine (Figure 1). The latest search was done on May 5, 2021. In addition, the reference lists of included studies were manually screened to identify additional articles not found during the search of databases and websites. Detailed keywords and syntaxes used for each database and grey literature website were described in Appendix S2.
5.2. Study selection
Two authors (Yun‐Xiao Bai and Jie‐Han Zhang) independently screened and evaluated the qualifications of the title and abstract. As for abstracts whose detailed information was insufficient to determine eligibility, a full‐text article was searched. The two authors are very consistent in including the selected articles (κ = 0.77).
5.3. Inclusion and exclusion criteria
The patient, intervention, comparison, outcomes, study design (PICOS) strategy was applied to identify the eligible studies of this systematic review and meta‐analysis.47
Inclusion criteria: (1) clinical trials of adult patients undergoing surgeries with OLV wherein dexmedetomidine was administrated perioperatively; (2) the anti‐inflammatory effect of dexmedetomidine was compared with normal saline/placebo; (3) trials reporting serum concentration changes of the inflammatory mediator (including at least IL‐6 or TNF‐α); and (4) randomized controlled trials.
Exclusion criteria: (1) trials without full‐text access; (2) trials with no available data.
5.4. Data extraction
The retrieved data were as follows: first author, year of publication, surgery characteristics, participants’ characteristics, allocation, OLV duration, anaesthesia strategy, supplementary anaesthetics, and outcome indicators. Data were collected at T1 (30‐minute OLV), T2 (90‐minute OLV or closet time point to 90‐minute OLV), T3 (end of surgery), and T4 (postoperative day).
5.5. Outcomes
The primary outcome of this review was serum concentration changes from baseline of IL‑6 and TNF‑α following dexmedetomidine application. The secondary outcome was changes from baseline of other inflammatory mediators (i.e., IL‐8, IL‐1β, MCP‐1, and CRP) and oxygenation indicators such as partial pressure of oxygen in arterial blood gas (PaO2) and oxygen index (OI) in response to dexmedetomidine application.
5.6. Quality assessment
The risk‐of‐bias was assessed with the Cochrane Collaborations’ tool.48 Studies that had more than four high risks in the risk‐of‐bias assessment were considered as high quality. The consistency between the two authors on the overall quality assessment was almost perfect (κ = 0.95).
5.7. Statistical analyses
Meta‐analysis was performed using Review Manager 5.4.1 (The Cochrane Collaboration) under random‐effects (REM) models. Both primary and secondary outcomes were both continuous variables. The standard mean difference (SMD) and 95% confidential intervals (95% CI) were calculated from the changes of mean and standard deviations (SD). For comparing cytokines and indicators with the baseline, Meanchange, SDchange were calculated as follows: Meanchange Meanfinal–Meanbaseline; . Because the variables are moderately correlated, the value of correlation was imputed as 0.5.49, 50 For articles that reported medians with interquartile ranges, Mean = (First quartile + Median + Third quartile)/3, and SD were calculated via inverse cumulative distribution function (ICDF).51 For articles with data only shown in graphs, Getdata Graph Digitizer v2.5 was used to capture data.52
I2 index was reported for statistical heterogeneity between studies. Significant heterogeneity was determined among studies with I 2 > 50% and p‐value less than 0.05. Subgroup analysis was conducted to determine the impact of surgery type, administration route, the timing of administration, dosage concentration, duration of OLV on inflammatory cytokine, and oxygenation indicator changes. Influence analysis was performed to assess the impact of some specific trials on overall effect size. Statistical significance was set at p < 0.05 (2‐sided). Mean difference (MD) and 95% CIs were reported for all comparisons.
CONFLICTS OF INTEREST
None.
Supporting information
Fig S1
Fig S2
Table S1
Appendix S1
Appendix S2
Bai Y‐X, Zhang J‐H, Zhao B‐C, Liu K‐X, Bai Y‐W. Dexmedetomidine attenuates one‐lung ventilation associated lung injury by suppressing inflammatory responses: A systematic review and meta‐analysis. Clin Exp Pharmacol Physiol. 2021;48:1203–1214. 10.1111/1440-1681.13525
Y.‐X. Bai, J.‐H. Zhang and B.‐C. Zhao contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Fischer GW, Cohen E. An update on anesthesia for thoracoscopic surgery. Curr Opin Anaesthesiol. 2010;23(1):7–11. [DOI] [PubMed] [Google Scholar]
- 2.Karzai W, Schwarzkopf K. Hypoxemia during one‐lung ventilation: prediction, prevention, and treatment. Anesthesiology. 2009;110(6):1402–1411. [DOI] [PubMed] [Google Scholar]
- 3.Lohser J, Slinger P. Lung injury after one‐lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg. 2015;121(2):302–318. [DOI] [PubMed] [Google Scholar]
- 4.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):109–116. [DOI] [PubMed] [Google Scholar]
- 5.Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. Br J Anaesth. 2014;113(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreyfuss D, Saumon G. Ventilator‐induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323. [DOI] [PubMed] [Google Scholar]
- 7.Slutsky AS, Ranieri VM. Ventilator‐induced lung injury. N Engl J Med. 2013;369(22):2126–2136. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c‐fos m‐RNA expression in an isolated rat lung model. J Clin Invest. 1997;99(5):944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. [DOI] [PubMed] [Google Scholar]
- 10.Morvaridzadeh M, Fazelian S, Agah S, et al. Effect of ginger (Zingiber officinale) on inflammatory markers: a systematic review and meta‐analysis of randomized controlled trials. Cytokine. 2020;135: 155224. [DOI] [PubMed] [Google Scholar]
- 11.Farsi F, Heshmati J, Keshtkar A, et al. Can coenzyme Q10 supplementation effectively reduce human tumor necrosis factor‐α and interleukin‐6 levels in chronic inflammatory diseases? A systematic review and meta‐analysis of randomized controlled trials. Pharmacol Res. 2019;148:104290. [DOI] [PubMed] [Google Scholar]
- 12.Tang C, Huang X, Kang F, et al. Intranasal dexmedetomidine on stress hormones, inflammatory markers, and postoperative analgesia after functional endoscopic sinus surgery. Mediators Inflamm. 2015;2015:939431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li BO, Li Y, Tian S, et al. Anti‐inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta‐analysis. Sci Rep. 2015;5:12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Yang Y, Yu C, et al. Dexmedetomidine analgesia effects in patients undergoing dental implant surgery and its impact on postoperative inflammatory and oxidative stress. Oxid Med Cell Longev. 2015;2015:186736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao N, Tang B. Organ‐protective effects and the underlying mechanism of dexmedetomidine. Mediators Inflamm. 2020;2020:6136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Sun X, Yang X, et al. Dexmedetomidine reduces ventilator‐induced lung injury (VILI) by inhibiting Toll‐like receptor 4 (TLR4)/nuclear factor (NF)‐kappaB signaling pathway. Bosn J Basic Med Sci. 2018;18(2):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C‐L, Tsai P‐S, Huang C‐J. Effects of dexmedetomidine on regulating pulmonary inflammation in a rat model of ventilator‐induced lung injury. Acta Anaesthesiol Taiwanica. 2008;46(4):151–159. [DOI] [PubMed] [Google Scholar]
- 18.Zhu CH, Yu J, Wang BQ, Nie Y, Wang L, Shan SQ. Dexmedetomidine reduces ventilator‐induced lung injury via ERK1/2 pathway activation. Mol Med Rep. 2020;22(6):5378–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Han J, Zhang D, Cao S, Su C. Effects of dexmedetomidine on oxidative stress and inflammatory response in lungs during mechanical ventilation in COPD rats. Exp Ther Med. 2020;19(2):1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Zhang Z, Chen K, Zhang F, Peng M, Wang Y. Dexmedetomidine regulates inflammatory molecules contributing to ventilator‐induced lung injury in dogs. J Surg Res. 2014;187(1):211–218. [DOI] [PubMed] [Google Scholar]
- 21.Yang CL, Chen CH, Tsai PS, Wang TY, Huang CJ. Protective effects of dexmedetomidine‐ketamine combination against ventilator‐induced lung injury in endotoxemia rats. J Surg Res. 2011;167(2):e273–e281. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yi X, Jiang L, et al. Protective effects of dexmedetomidine on lung in rats with one‐lung ventilation. Exp Ther Med. 2019;17(1):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros Heil LB, Santos CL, Santos RS, et al. The effects of short‐term propofol and dexmedetomidine on lung mechanics, histology, and biological markers in experimental obesity. Anesth Analg. 2016;122(4):1015–1023. [DOI] [PubMed] [Google Scholar]
- 24.Kong L, Lu XH. Effect of dexmedetomidine on perioperative inflammatory response and cellular immune in patients undergoing radical operation of thoracoscopic lung cancer. Zhonghua Yi Xue Za Zhi. 2018;98(36):2929–2932. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y, Jiang W, Zhao L, Wu Y, Xie H. Effect of dexmedetomidine on perioperative inflammation and lung protection in elderly patients undergoing radical resection of lung cancer. Int J Clin Exp Pathol. 2020;13(10):2544–2553. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu GC, Sun K, Fu HG, Dong TL, Yuan F. Effects of dexmedetomidine on injury of lungs and CHOP protein expression in elderly patients with lung cancer during one‐lung ventilation. Zhonghua Yi Xue Za Zhi. 2020;100(1):37–41. [DOI] [PubMed] [Google Scholar]
- 27.Guo YB, Xu JD, Ji XX, Zhang JX, Liang JX, Zhou GB. Protective effect of dexmedetomidine against perioperative inflammation and on pulmonary function in patients undergoing radical resection of lung cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(12):1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Li JB, Lu Z. Clinical application and effect of dexmedetomidine in combination with continuous positive airway pressure on one‐lung ventilation in lung surgery of elder patients. Pak J Pharm Sci. 2018;31(6(Special)):2879–2883. [PubMed] [Google Scholar]
- 29.Cui J, Gao M, Huang H, Huang X, Zeng Q. Dexmedetomidine improves lung function by promoting inflammation resolution in patients undergoing totally thoracoscopic cardiac surgery. Oxid Med Cell Longev. 2020;2020:8638301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CY, Lu YF, Wang ML, et al. Effects of dexmedetomidine infusion on inflammatory responses and injury of lung tidal volume changes during one‐lung ventilation in thoracoscopic surgery: a randomized controlled trial. Mediators Inflamm. 2018;2018:2575910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Zhang S, Li B, Sun M, Zhang J. Paravertebral dexmedetomidine as an adjuvant to ropivacaine protects against independent lung injury during one‐lung ventilation: a preliminary randomized clinical trial. BMC Anesthesiol. 2018;18(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Z‐G, Mi W‐D. Application of dexmedetomidine for lung injury in elderly patients undergoing one‐lung ventilation. Arch Med Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng J, Lv Q, Yao J, Wang S, Yang K. Effect of dexmedetomidine on postoperative lung injury during one‐lung ventilation in thoracoscopic surgery. Biomed Res Int. 2020;2020:4976205. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Gong Z, Long X, Wei H, et al. Dexmedetomidine combined with protective lung ventilation strategy provides lung protection in patients undergoing radical resection of esophageal cancer with one‐lung ventilation. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(7):1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao S, Wang Y, Zhao J, Su A. Effects of dexmedetomidine pretreatment on heme oxygenase‐1 expression and oxidative stress during one‐lung ventilation. Int J Clin Exp Pathol. 2015;8(3):3144–3149. [PMC free article] [PubMed] [Google Scholar]
- 36.Tan WF, Guo B, Ma H, Li XQ, Fang B, Lv HW. Changes in postoperative night bispectral index of patients undergoing thoracic surgery with different types of anaesthesia management: a randomized controlled trial. Clin Exp Pharmacol Physiol. 2016;43(3):304–311. [DOI] [PubMed] [Google Scholar]
- 37.Shen Q, Xu G, Liu J, et al. Dexmedetomidine alleviates non‐ventilation associated lung injury via modulating immunology phenotypes of macrophages. Life Sci. 2020;259:118249. [DOI] [PubMed] [Google Scholar]
- 38.Zhu L, Zhang Y, Zhang Z, Ding X, Gong C, Qian Y. Activation of PI3K/Akt/HIF‐1α signaling is involved in lung protection of dexmedetomidine in patients undergoing video‐assisted thoracoscopic surgery: a pilot study. Drug Des Devel Ther. 2020;14:5155–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Q, Wu X. Influence of the dexmedetomidine combined with parecoxib sodium on inflammatory factor. J Hainan Med Univ. 23(20):2834‐2837, 41. [Google Scholar]
- 40.Kim JA, Ahn HJ, Yang M, Lee SH, Jeong H, Seong BG. Intraoperative use of dexmedetomidine for the prevention of emergence agitation and postoperative delirium in thoracic surgery: a randomized‐controlled trial. Can J Anaesth. 2019;66(4):371–379. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JC, Meng RX, Han WL, Du LZ. Effect of dexmedetomidine combined with continuous positive airway pressure on oxidative stress and inflammatory responses in elderly patients undergoing chest operation. Med J Wuhan Univ. 2016;37(01):97–100. [Google Scholar]
- 42.Luo JJ, Liang X, Pi LH, Huang Y, Xie FY, Yang FR. Effects of dexmedetomidine with sevoflurane on lung injury in patients with one‐lung ventilation. Chongqing Med. 2019;48(11):1844–1847+1851. [Google Scholar]
- 43.Pi Y, Yang Y. Application value of conventional anesthesia combined with dexmedetomidine in thoracoscopic radical resection of lung cancer. Int J Clin Exp Med. 2021;14(2):1301–1308. [Google Scholar]
- 44.Wang K, Wu M, Xu J, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta‐analysis. Br J Anaesth. 2019;123(6):777–794. [DOI] [PubMed] [Google Scholar]
- 45.Flanders CA, Rocke AS, Edwardson SA, Baillie JK, Walsh TS. The effect of dexmedetomidine and clonidine on the inflammatory response in critical illness: a systematic review of animal and human studies. Crit Care. 2019;23(1):402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 47.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 (Updated March 2011). Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 49.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768. [DOI] [PubMed] [Google Scholar]
- 50.Kiss R, Schedler S, Muehlbauer T. Associations between types of balance performance in healthy individuals across the lifespan: a systematic review and meta‐analysis. Front Physiol. 2018;9:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.GetData Graph Digitizer. https://getdata‐graph‐digitizer.com/index.php
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Appendix S1
Appendix S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


