Abstract
Aims and objectives
To explore the role of health beliefs in affecting patients’ chronic diabetic complication (CDC) screening.
Background
Patients’ adherence to the guideline‐recommended CDC screening was far from optimal. While many demographic and clinical characteristics were documented to influence patients’ adherence, psychological profiles, such as health beliefs, were not well studied before. It is crucial to understand how health beliefs affect patients’ CDC screening behaviour and thus to provide implications for future intervention programmes.
Design
A cross‐sectional study was conducted.
Methods
785 type 2 diabetes were enrolled from the community health centre in Wuhou District, Chengdu, China. Structured questionnaires were used to collect data regarding the demographic and clinical information, knowledge about CDC, health belief model constructs and CDC screening behaviour. Mediation analysis was performed to explore the mechanisms of health belief model constructs on CDC screening behaviour. The study methods were compliant with the STROBE checklist.
Results
Knowledge had a significant indirect effect on CDC screening behaviour through perceived susceptibility, perceived benefits, perceived barriers and self‐efficiency. Cues to action exerted both significant direct and indirect effects on CDC screening behaviour. The indirect effects of cues to action were exerted through perceived susceptibility, perceived barriers and self‐efficiency.
Conclusion
Health beliefs played vital roles in mediating the effects of knowledge and cues to action on patients’ CDC screening behaviour. Health beliefs should be assessed and modified through creative educational methods. Strategies aimed at increasing cues to action are also expected to facilitate patients’ CDC screening behaviour.
Relevance to clinical practices
The study contributes to the exploration of how health beliefs affect patients’ CDC screening behaviour. The results could be used to inspire future community‐based intervention programmes.
Keywords: chronic diabetic complication, complication screening, health belief model, path analysis, type 2 diabetes
What does this paper contribute to wider global clinical community?
The paper argues the vital role of health beliefs in affecting patients’ chronic diabetic complication screening.
The study strongly recommends the inclusion of cues to action when applying the health belief model.
Future educational programmes could benefit from a creative educational strategy that aims at strengthening patients’ health beliefs.
1. INTRODUCTION
Diabetes is a public health concern across the world given its high and increasing prevalence (Cho et al., 2018; Federation, 2017). Patients with diabetes will confront higher risk of death and disability if they developed chronic diabetic complications (CDC), namely cardiovascular disease, diabetic retinopathy (DR), diabetic nephropathy (DN) and diabetic foot (DF), which were usually asymptomatic at their early stages (Klein, 2007; Nakamura et al., 2017; Tsai et al., 2018; Zhang et al., 2017). Thus, annually or biennially CDC screening has been recommended globally and proved to be a cost‐effective way to reduce the morbidity and mortality of diabetes (Ang et al., 2017; Farmer et al., 2014; Wong et al., 2018). However, the low compliance rate of CDC screening remains a challenge for diabetes management in clinical practice. Patients with diabetes usually did not participate in CDC screening as recommended. According to the published literatures, the participation rate of annually or biennially DR screening was 52.9–82.8% in developed countries such as the USA (An et al., 2018), the UK (Scanlon, 2017) and Australia (Foreman et al., 2018), whereas less than 50% of diabetic patients in developing countries have underwent DR screening after diabetes diagnosis (Byun et al., 2013; Rani et al., 2007). The participation rate of DN screening and DF screening was even lower, varied from 6.5%–40.5% (Byun et al., 2013; Webb et al., 2015). In China, the largest developing country in the world, the compliance rate of CDC screening was also unsatisfactory. Existing evidences revealed that the highest participation rate (75%) of DN screening was documented in an urban community health centre in Beijing, the capital city of China (Zhao et al., 2015), while the figure in rural areas in Guangzhou was as low as 33.3% (Wang et al., 2010). And the screening rate of other chronic diabetic complications has rarely surpassed 50% (Liu et al., 2010; Luo & Huang, 2019; Wang et al., 2010). Thus, to develop effective intervention measures for diabetic patients, it is of great importance to understand the factors that predict better CDC screening compliance.
2. BACKGROUND
Known evidences were that patients’ compliance with CDC screening was positively associated with demographic profiles such as older age (Kreft et al., 2018), favourable economic conditions (An et al., 2018) and higher education level (Byun et al., 2013). Clinical variables including longer diabetes duration (Byun et al., 2013), severe disease condition (Kreft et al., 2018), well‐controlled blood glucose (An et al., 2018) and better knowledge/awareness of diabetic complications (Kashim et al., 2018; Lake et al., 2017) were also documented to promote patients’ participation in CDC screening. Nevertheless, most of these documented factors were unmodifiable or hardly modifiable when applied to intervention programmes.
It is well acknowledged that people's attitudes or beliefs towards a disease or a health behaviour, which was highly modifiable, would influence the extent to which will they adopt the health behaviour (Cummings et al., 1978). The health belief model (HBM), a well‐established and widely used psychological theory, explained how health beliefs influenced peoples’ health behaviours and why some people adopted certain health behaviour, while others failed (Janz & Becker, 1984). The model posits that people's health behaviour was determined by five health belief constructs (perceived susceptibility to a disease, perceived severity of a disease, perceived benefits of a health behaviour, perceived barriers to adopt the behaviour, self‐efficiency) and cues to action that may trigger the health behaviour (Janz, & Becker, 1984; Rosenstock et al., 1988). In addition, amendatory factors including demographic profiles, knowledge and previous experience of a disease may also influence patients’ health behaviours through their health beliefs (Rosenstock et al., 1988). Considerable evidences have proved the effectiveness of the HBM in predicting people's preventive health behaviours such as cancer screening (Annan et al., 2019), vaccination intaking (Cheung & Mak, 2016) and health lifestyle adoption (Chen et al., 2013). Scholars have also implemented the HBM in explaining self‐care behaviour (Dehghani‐Tafti et al., 2015; Vazini & Barati, 2014), self‐monitoring of blood glucose (Gucciardi et al., 2013) and the CDC screening behaviour among diabetic patients (Hsieh et al., 2016; Sheppler et al., 2014). However, studies are still needed to better illustrate the usefulness of the model. One fact should be noted was that some of the constructs, especially cues to action and self‐efficiency, which has been proved to influence health behaviour tremendously, were not always included in published studies (Jones et al., 2014; Poss, 2001). In most cases, the model was only partially or additively applied. In addition, the majority of these studies have only tested the direct effects of the model constructs on health behaviour, while the indirect relationships among these variables were not delineated. There is need to figure out the mechanisms through which health belief constructs affect patients’ health behaviour with the comprehensive use of the HBM.
Therefore, this study was designed to apply the overall HBM to explain CDC screening behaviour in type 2 diabetes and to clarify the direct and indirect relationships among the HBM constructs.
3. METHODS
3.1. Study design and sample selection
This cross‐sectional study was conducted in the community health centre in Wuhou District, Chengdu, China, from May–November 2019. Registered type 2 diabetes who settled a health record and received diabetes management in the study centre were recruited and interviewed using a convenience sampling method. When eligible patients attended to the study centre for daily care, they were recruited by the researchers after a brief introduction of the study. In detail, patients met the following inclusion criteria were included: documented diagnosis of type 2 diabetes; aged over 35; residents lived in the community for more than 6 months; received diabetes management in the community health centre; and voluntary participation in the study. Mentally incapable patients were excluded as they were unable to give consent and fulfil the survey. The strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed in the elaboration of the study (supplementary file 2).
The sample size was calculated based on the proportion of patients who received diabetic peripheral nerve disease (DPN) screening in China, which was documented to be lower than diabetic retinopathy (DR) and diabetic nephropathy (DN) screening according to the published literatures (Liu et al., 2010; Luo et al., 2019; Wang et al., 2010; Zhao et al., 2015). Assuming 32% of the participants have undertook DPN screening (Liu et al., 2010), 638 patients would be recruited with a sampling error of 0.2. The final sample size should be 766 patients to allow a no‐response rate of 20%.
3.2. Questionnaires
Since there was no existing questionnaire fit the measurement requirements of this study, researchers developed the survey instrument by drawing inspiration from existing HBM questionnaires that have been successfully used in breast cancer screening (Champion, 1984), diabetes regimen management (Becker & Janz, 1985) and influenza vaccine intake (Santos et al., 2017). Studies focused on exploring the influencing factors of CDC screening have also inspired the questionnaire preparation (Kashim et al., 2018; Liu Y. & Swearingen, 2017). To confirm the content validity, the questionnaires were reviewed by a group of experts, which engaged 2 endocrinologists, 3 diabetes nursing specialist, 1 general practitioner and 2 researchers with expertise of questionnaire development. All experts had more than ten years of working experience and obtained at least a deputy senior title in the field of diabetes care. They reviewed each question and responses listed in the questionnaire, judged the necessity and appropriateness of each item from irrelevant/weak relevant, relevant to strongly relevant. Then, the content validity index (CVI) was calculated according to the experts’ responses (Lynn, 1986). The internal consistency of the questionnaires (Cronbach's α coefficient or KR 20 coefficient) was calculated using the data of 30 patients, which were collected in the pilot study before the formal investigation. All scales were reliable with a CVI above 0.9 and an internal consistency coefficient above 0.7.
3.2.1. Demographic and clinical information
Demographic variables were as follows: gender, age, nationality, marital status, way of living, education levels, occupation status, household monthly income per capita, health insurance status, smoking and alcohol drinking. Clinical variables were as follows: family history of diabetes, duration of diabetes, diabetes therapy, current fast blood glucose level, the latest HbA1c level, presence of diabetes‐related comorbidities/complications (hypertension, hyperlipidaemia, cardiovascular disease, cerebrovascular disease, diabetic nephropathy, diabetic retinopathy, lower extremity vascular disease and peripheral neuropathy).
3.2.2. Knowledge about CDC
A 15‐item scale was designed to access patients’ knowledge about CDC. These items involved statements describing the common chronic complications of diabetes, basic characteristics of these complications and the useful measures to prevent them. Patients were required to response ‘yes’/ ‘no or uncertain’ to each statement. A sample question was ‘Diabetic patients have higher possibilities to get hypertension than non‐diabetes’. One point was given to a correct response, whereas zero point was given to an incorrect response. Total score (0–15) of the 15 items was calculated to reflect patients’ overall knowledge. Higher scores indicate better knowledge. The content validity index of the scale was 0.96 through the expert consultation. The internal consistency of the scale was reliable with a KR‐20 value of 0.90. The full version of the scale is available online in supplement materials.
3.2.3. The HBM Scales for CDC Screening
This was a 25‐item Likert five‐point scale with six sub‐scales to reflect the main constructs of the HBM. In detail, perceived susceptibility contained 3 items to rate patients’ self‐evaluated chances of getting chronic diabetic complications (e.g. I am susceptible to chronic diabetic complications for my diabetes was not well‐controlled). Perceived severity included 7 items that measured patients’ perception of the detrimental effects of chronic diabetic complications on their well‐being (e.g. My vision would be impaired and even deprived if I get diabetic retinopathy). Perceived benefit was determined by a single item described as ‘regular screening would help me figure out chronic diabetic complications in their early stages’. Perceived barriers used 8 items to measure patients’ perception of the possible obstacles they may encounter in complication screening (e.g. The expenses of diabetic complication screening are expensive, I can't get myself screened as recommended). Two items measured patients’ confidence in their own ability to obtain chronic complication screening, namely self‐efficiency (e.g. I can seek for complication screening regularly according to the doctors’ advice). Cues to action (4 items) were factors that may stimulate patients’ undertaking of complication screening (e.g. I have been recommended to undertake chronic diabetic complication screening by health workers). Patients responded to each item from strongly disagree (1 point)–strongly agree (5 points). Each construct was indicated by the mean score of items in each sub‐scale. The content validity index of the sub‐scales ranges from 0.9–1.0. Cronbach's α values of the sub‐scales range from 0.73–0.91. The full version of the scale is available online in supplement materials.
3.2.4. CDC Screening Behavior Scale
Patients were asked to rate how frequent had they participated in the following guideline‐recommended clinical tests that related to CDC screening: (1) serum lipids test, ECG test and HbA1c test, which were related to cardiovascular risk assessment; (2) diabetic nephropathy (DN) screening through UACR or eGFR test; (3) diabetic retinopathy (DR) screening through fundus examination; (4) diabetic peripheral neuropathy (DPN) screening through simple clinical examinations (the ankle reflex, pinprick sensation, temperature sensation, vibration perception and pressure sensation) or nerve conduction study; and (5) lower extremity atherosclerotic disease (LEAD) screening through ABI and/or TBI test. The responses to these questions and the scoring criteria were as follows: never have screened or uncertain (1 point), only screened once after diabetes diagnosis (2 points), once every two years or longer (3 points), and annually or more frequent (4 points). Mean score of these 8 items represented patients’ complication screening behaviour, which higher scores mean better screening practice. The scale was reliable with a content validity index of 0.97 and Cronbach's α value of 0.87.
3.3. Statistical analysis
Data analysis was conducted in SPSS version 20.0. Descriptive statistics were performed to present the characteristics of the participants. Pearson's correlation analysis was performed primarily to explore the relationship between the dependent variable and the study variables. Using the PROCESS macro developed by Hayes (Montoya & Hayes, 2017; Preacher & Hayes, 2008), we analysed a parallel multiple mediator model based on the theoretical hypothesis of the HBM. The theoretical framework is presented in Figure 1, and the casual effect of the independent variable X (Knowledge about chronic diabetic complications or cues to action) can be apportioned into its direct effect on the dependent variable Y (CDC screening behaviour) and the indirect effects on Y through five mediators: M1 (perceived susceptibility), M2 (perceived severity), M3 (perceived benefits), M4 (perceived barriers) and M5 (self‐efficiency). Figure 1a showed the direct effect of X on Y (c´) and the indirect effects of X on Y through the five mediators. In Figure 1a, a1 represented the effect of X on M1, b1 represented the effect of M1 on Y, the specific indirect effect of X on Y through M1 was estimated as a1b1, and so on. The total indirect effect was the sum of the five specific indirect effects. Figure 1b showed the total effect of X on Y (c). The total effect was the sum of the direct effect and the indirect effects through mediators, which could be calculated using the formula c= c´+ a1b1 + a2b2 +a3b3 + a4b4 + a5b5. The inference for the direct and indirect effects and the contrast of each specific indirect effect were proceeded by relying on the estimates of the bootstrap standard errors for hypothesis testing or the bootstrap confidence interval construction in PROCESS macro. This study employed Model 4 in Hayes’ PROCESS macro to generate the bias‐corrected bootstrap 95% CI when proceeding with the inference for each effect, with 5000 bootstrapping samples. If the bootstrap 95% CI for an effect did not cross zero, it was considered statistically significant. Meanwhile, patients’ demographic and clinical characteristics were included as control variables in the model.
FIGURE 1.
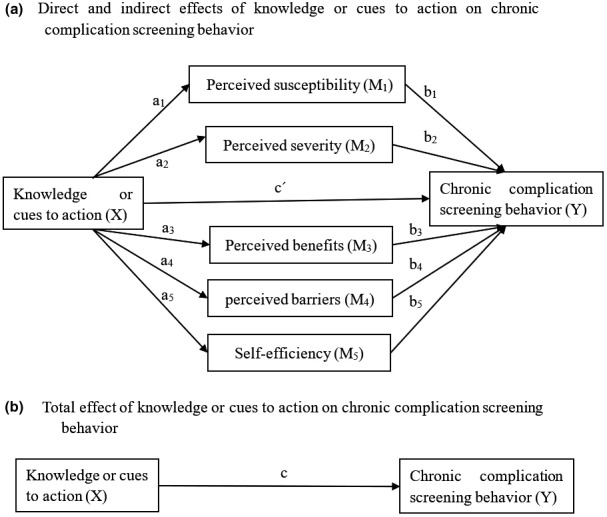
Conceptual diagram of the proposed parallel multiple mediator model
3.4. Ethical consideration
The study was pre‐registered (ChiCTR 1900023577). Ethical approval for the study was granted by the Clinical Trial and Biomedical Ethics Committee of West China Hospital of Sichuan University (No. 2019–234). All participants were informed that the completion of the study was voluntary and implied consent. They were also well informed about their right to not participate/ withdraw from the study. Patients’ anonymity was maintained during the whole study period.
4. RESULTS
4.1. Characteristics of the participants
This study enrolled 785 patients totally (male/female, 375/410; Han nationality/minority nationality, 779/6; with spouse/without spouse, 712/73). Participants’ mean age was 68.76±10.85, more than 80% of them aged over 60 and have retired. Over 60% of participants had completed senior high school or more education. Regarding their clinical characteristics, more than seventy per cent of participants had five years or longer diabetes duration, while diabetes family history was reported in one‐fifth of participants. The vast majority of participants (73%) were treated with oral hypoglycaemic therapy, and nearly 60% of participants reported well‐controlled fast glucose or HbA1c. More than four‐fifth of participants declared diabetes‐related comorbidities or complications. Detailed information is presented in Table 1.
TABLE 1.
Demographic and clinical characteristics of the participants (n = 785)
| Variables | n (%) | Variables | n (%) |
|---|---|---|---|
| Gender | Marital status | ||
| Male | 375 (47.77) | With spouse | 712 (90.70) |
| Female | 410 (52.23) | Without spouse | 73 (9.30) |
| Nationality | Occupation status | ||
| Han nationality | 779 (99.2) | Not retired | 132 (16.82) |
| Minority nationality | 6 (0.8) | Retired | 653 (83.18) |
| Age | Way of living | ||
| ≤59 | 153 (19.49) | Live alone | 29 (3.69) |
| 60–79 | 500 (63.69) | Live with non‐spouse | 61 (7.77) |
| >80 | 132 (16.82) | Live with spouse and non‐spouse | 260 (33.12) |
| Live with spouse only | 435 (55.42) | ||
| Education level | Household monthly income per capita | ||
| Middle school education or less | 313 (39.88) | ≤1800 RMB | 58 (7.38) |
| Senior high school education | 231 (29.42) | 1801–2800 RMB | 220 (28.03) |
| Diploma education | 131 (16.69) | 2801–5000 RMB | 375 (47.77) |
| Bachelor degree or above | 110 (14.01) | >5000 RMB | 132 (16.82) |
| Insurance status | Smoking | ||
| Basic medical insurance (BMI) | 33 (4.20) | Yes | 110 (14.01) |
| BMI+special outpatient subsidy for diabetes (SOSD) | 369 (47.01) | No | 675 (85.99) |
| BMI+SOSD+supplementary Medical insurance | 364 (46.37) |
Alcohol drinking Yes |
86 (10.96) |
| Free medical care | 19 (2.42) | No | 699 (89.04) |
| Diabetes family history | Duration of diabetes | ||
| Yes | 165 (21.02) | <5 years | 226 (28.79) |
| No | 606 (77.20) | 5–10 years | 331 (42.17) |
| Not sure | 14 (1.78) | >10 years | 228 (29.04) |
| Current fast glucose level | The latest HbA1c level | ||
| ≤6.99 mmol/L | 470 (59.87) | ≤6.99% | 452 (57.58) |
| ≥7.0 mmol/L | 315 (40.13) | ≥7.0% | 252 (32.10) |
| Not clear | 81 (10.32) | ||
| Diabetes therapy | Presence of diabetes‐related Comorbidities/ complications | ||
| Lifestyle adjustment | 12 (1.53) | None | 103 (13.13) |
| Oral hypoglycaemic therapy | 576 (73.37) | Hypertension or/and hyperlipidaemia | 198 (25.22) |
| Injection of insulin | 24 (3.06) | Other chronic complications† | 49 (6.24) |
| Oral hypoglycaemic therapy+Injection of insulin | 173 (22.04) | Hypertension or/and hyperlipidaemia +other chronic complications | 435 (55.41) |
Other chronic complications: cardiovascular disease, cerebrovascular disease, diabetic nephropathy, diabetic retinopathy, lower extremity vascular disease and peripheral neuropathy.
4.2. Correlations analysis of the study variables
As shown in Table 2, the dependent variable (CDC screening behaviour) was significantly (p < .01) correlated with the independent variables (knowledge and cues to action), and all mediating variables (perceived susceptibility, perceived severity, perceived benefits, perceived barriers, self‐efficiency) were significantly (p < .01) correlated with the dependent and independent variables.
TABLE 2.
Correlations between the study variables (n = 785)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Chronic complication screening behaviour | 1 | |||||||
| 2. Knowledge | 0.411** | 1 | ||||||
| 3. Perceived susceptibility | 0.520** | 0.480** | 1 | |||||
| 4. Perceived severity | 0.447** | 0.676** | 0.536** | 1 | ||||
| 5. Perceived benefits | 0.494** | 0.475** | 0.496** | 0.601** | 1 | |||
| 7. Perceived barriers | −0.498** | −0.366** | −0.227** | −0.394** | −0.463** | 1 | ||
| 8. Self‐efficiency | 0.454** | 0.349** | 0.293** | 0.426** | 0.498** | −0.560** | 1 | |
| 9. Cues to action | 0.506** | 0.464** | 0.515** | 0.480** | 0.493** | −0.420** | 0.370** | 1 |
p < .01
4.3. Mediation analysis
Figure 2 showed the relationship between knowledge, the five mediators and CDC screening behaviour, and Table 3 presented the total, direct and indirect effects of knowledge on CDC screening behaviour and the associated 95% bootstrap confidence interval. The analysis yielded a significant total effect (effect size = 0.251, 95% Boot CI = 0.188–0.316) and indirect effect (effect size = 0.267, 95% Boot CI = 0.211–0.326). The indirect effect of knowledge on CDC screening behaviour was significantly exerted through perceived susceptibility (effect size = 0.100, 95% Boot CI=0.073–0.132), perceived benefits (effect size = 0.033, 95% Boot CI = 0.006–0.062), perceived barriers (effect size = 0.085, 95% Boot CI = 0.058–0.116) and self‐efficiency (effect size = 0.020, 95% Boot CI=0.004–0.039). The contrast of each specific indirect effect showed that the specific indirect effect of perceived susceptibility and perceived barriers was significantly higher than that of perceived benefits (Contrast 2 = 0.066,95% Boot CI = 0.024–0.111, Contrast 8 = −0.052, 95% Boot CI = −0.095‐‐0.011) and self‐efficiency (Contrast 4 = 0.079,95% Boot CI = 0.046–0.116, Contrast 10 = 0.065, 95% Boot CI=0.030–0.102), while the specific indirect effect of perceived susceptibility and perceived barriers showed no significant difference (Contrast 3 = 0.015,95% Boot CI = −0.025–0.056).
FIGURE 2.
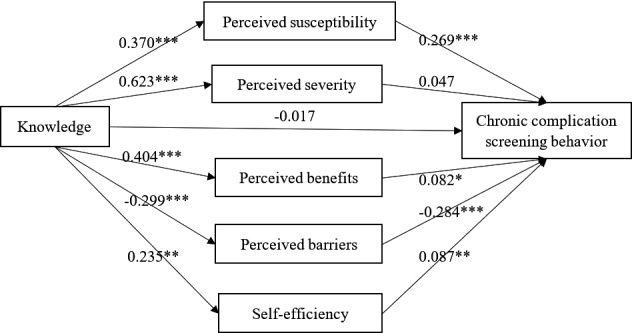
The parallel multiple mediator model of the relationship between knowledge and chronic complication screening behaviour. *p < .05; **p < .01; ***p < .001
TABLE 3.
Direct effect and indirect effects of knowledge on chronic complication screening behaviour
| Effect size | Boot SE | Bootstrap 95% CI | % of total effect | ||
|---|---|---|---|---|---|
| BootLLCI | BootULCI | ||||
| Total effect | 0.251 | 0.033 | 0.188 | 0.316 | ‐ |
| Direct effect | −0.017 | 0.033 | −0.083 | 0.048 | −6.70% |
| Total indirect effect | 0.267 | 0.029 | 0.211 | 0.326 | 106.70% |
| Indirect effect through perceived susceptibility | 0.100 | 0.015 | 0.073 | 0.132 | 39.73% |
| Indirect effect through perceived severity | 0.029 | 0.024 | −0.020 | 0.077 | 11.68% |
| Indirect effect through perceived benefits | 0.033 | 0.014 | 0.006 | 0.062 | 13.24% |
| Indirect effect through perceived barriers | 0.085 | 0.015 | 0.058 | 0.116 | 33.91% |
| Indirect effect through self‐efficiency | 0.020 | 0.009 | 0.004 | 0.039 | 8.14% |
| Contrast 1: perceived susceptibility‐perceived severity | 0.070 | 0.031 | 0.014 | 0.132 | |
| Contrast 2: perceived susceptibility‐perceived benefits | 0.066 | 0.022 | 0.024 | 0.111 | |
| Contrast 3: perceived susceptibility‐perceived barriers | 0.015 | 0.021 | −0.025 | 0.056 | |
| Contrast 4: perceived susceptibility‐self‐efficiency | 0.079 | 0.018 | 0.046 | 0.116 | |
| Contrast 5: perceived severity‐perceived benefits | −0.004 | 0.032 | −0.068 | 0.059 | |
| Contrast 6: perceived severity‐perceived barriers | −0.056 | 0.028 | −0.112 | −0.002 | |
| Contrast 7: perceived severity‐self‐efficiency | 0.009 | 0.027 | −0.045 | 0.059 | |
| Contrast 8: perceived benefits‐perceived barriers | −0.052 | 0.021 | −0.095 | −0.011 | |
| Contrast 9: perceived benefits‐self‐efficiency | 0.013 | 0.018 | −0.023 | 0.047 | |
| Contrast 10: perceived barriers‐self‐efficiency | 0.065 | 0.018 | 0.030 | 0.102 | |
Control variables included all demographic and clinical variables. Nationality, occupation status, duration of diabetes, the latest HbA1c level, presence of diabetes‐related comorbidities or complications were found to be significant covariates.
Abbreviation: CI, confidence interval.
Figure 3 showed the relationship between cues to action, the five mediators and CDC screening behaviour, and Table 4 presented the total, direct and indirect effects of cues to action on CDC screening behaviour and the associated 95% bootstrap confidence interval. Cues to action was found to have a significant total (effect size = 0.368, 95% Boot CI = 0.300–0.435), direct (effect size = 0.113, 95% Boot CI = 0.045–0.184) and indirect effect (effect size = 0.255, 95% Boot CI = 0.207–0.306) on CDC screening behaviour. The specific indirect effect through perceived susceptibility (effect size = 0.092, 95% Boot CI = 0.061–0.124), perceived barriers (effect size = 0.101, 95% Boot CI = 0.070–0.136) and self‐efficiency (effect size = 0.025, 95% Boot CI = 0.005–0.048) were significant. When contracting each specific indirect effect, perceived susceptibility and perceived barriers again showed significantly higher effect size than self‐efficiency (Contrast 4 = 0.067,95% Boot CI = 0.029–0.105, Contrast 10 = 0.076, 95% Boot CI = 0.037‐‐0.118). Meanwhile, the specific indirect effect of perceived susceptibility and perceived barriers did not have significant difference (Contrast 3 = −0.009, 95% Boot CI = −0.053–0.036).
FIGURE 3.
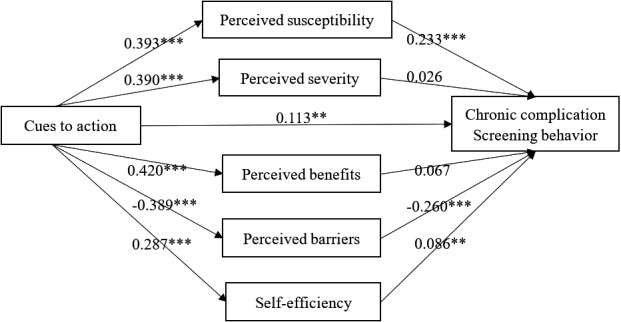
The parallel multiple mediator model of the relationship between cues to action and chronic complication screening behaviour. *p < .05; **p < .01; ***p < .001
TABLE 4.
Direct effect and indirect effects of cues to action on chronic complication screening behaviour
| Effect size | Boot SE | Bootstrap 95% CI | % of total effect | ||
|---|---|---|---|---|---|
| BootLLCI | BootULCI | ||||
| Total effect | 0.368 | 0.035 | 0.300 | 0.435 | ‐ |
| Direct effect | 0.113 | 0.035 | 0.045 | 0.184 | 30.63% |
| Total indirect effect | 0.255 | 0.025 | 0.207 | 0.306 | 69.37% |
| Indirect effect through perceived susceptibility | 0.092 | 0.016 | 0.061 | 0.124 | 24.87% |
| Indirect effect through perceived severity | 0.010 | 0.014 | −0.018 | 0.038 | 2.77% |
| Indirect effect through perceived benefits | 0.028 | 0.015 | −0.001 | 0.057 | 7.64% |
| Indirect effect through perceived barriers | 0.101 | 0.017 | 0.070 | 0.136 | 27.43% |
| Indirect effect through self‐efficiency | 0.025 | 0.011 | 0.005 | 0.048 | 6.66% |
| Contrast 1: perceived susceptibility‐perceived severity | 0.081 | 0.023 | 0.037 | 0.129 | |
| Contrast 2: perceived susceptibility‐perceived benefits | 0.063 | 0.024 | 0.019 | 0.111 | |
| Contrast 3: perceived susceptibility‐perceived barriers | −0.009 | 0.022 | −0.053 | 0.036 | |
| Contrast 4: perceived susceptibility‐self‐efficiency | 0.067 | 0.020 | 0.029 | 0.105 | |
| Contrast 5: perceived severity‐perceived benefits | −0.018 | 0.024 | −0.064 | 0.027 | |
| Contrast 6: perceived severity‐perceived barriers | −0.091 | 0.022 | −0.135 | −0.049 | |
| Contrast 7: perceived severity‐self‐efficiency | −0.014 | 0.019 | −0.052 | 0.020 | |
| Contrast 8: perceived benefits‐perceived barriers | −0.073 | 0.024 | −0.121 | −0.028 | |
| Contrast 9: perceived benefits‐self‐efficiency | 0.004 | 0.020 | −0.036 | 0.042 | |
| Contrast 10: perceived barriers‐self‐efficiency | 0.076 | 0.021 | 0.037 | 0.118 | |
Control variables included all demographic and clinical variables. Nationality, occupation status, duration of diabetes, the latest HbA1c level, presence of diabetes‐related comorbidities or complications were found to be significant covariates.
Abbreviation: CI, confidence interval.
5. DISCUSSION
Guided by the HBM, this study tested health beliefs as mediators of the relationship between knowledge/cues to action and CDC screening behaviour among patients with type 2 diabetes, which was rarely studied before. Findings from this study supported the theoretical hypothesis of the HBM, that is the model conceptualised health belief constructs mediated the effects of knowledge and cues to action on CDC screening behaviour.
Numerous studies have identified knowledge as a significant predictor of patients’ preventive health behaviour (Liu Y. & Swearingen, 2017; Srinivasan et al., 2017; Sun et al., 2006). However, non‐significant association between knowledge and health behaviour has also been reported in some studies (Adejoh, 2014; Almadi & Alghamdi, 2019) and cast doubt on the effectiveness of knowledge in producing health behaviour change. This study found that knowledge completely exerted its effect on patients’ CDC screening behaviour through their health beliefs (total indirect effects = 0.267, Boot 95% CI did not cross zero), especially through their perceived susceptibility (specific indirect effect = 0.100), perceived benefits (specific indirect effect = 0.033), perceived barriers (specific indirect effect = 0.085) and self‐efficiency (specific indirect effect = 0.020). Similar to our results, Annan et al also reported that knowledge significantly exerted its effect on patients’ cervical cancer screening practice through perceived severity indirectly (Annan et al., 2019). These results implied that disease‐related knowledge is a necessary but not sufficient factor to motivate patients’ health behaviour. Only when enhanced knowledge has transformed into strong health beliefs will they adopt the health behaviour. Future researchers are recommended to assess and modify patients’ health beliefs as knowledge itself could not stimulate CDC screening behaviour solely.
Previously, perceived susceptibility, perceived barriers, perceived benefits and self‐efficiency were commonly cited as significant predictors of patients’ health behaviour, while perceived severity was not in many cases (Carpenter, 2010; Nancy K. Janz & Marshall H. Becker, 1984). Contrasts of each specific indirect effect in this study also yielded similar results. All health belief constructs, except perceived severity, significantly mediated the effect of knowledge on CDC screening behaviour. And the mediation effects of perceived susceptibility and perceived barriers were significantly greater than the other health belief constructs. This result may result from the fact that traditional health education programmes have already informed our patients about the severe adverse outcomes of CDC and the benefits of regular screening through repeated emphasis, but such programmes seem to be inefficient to arouse patients’ perceived susceptibility of CDC as they usually feel asymptomatic before irreversible organ dysfunction, or eliminate their perceived barriers to adopt CDC screening. Creative health educational strategies are necessary to address this problem. For instance, peer education dominated by patients who have already suffered from CDC due to the neglect of regular screening could be employed to educate the newly diagnosed patients to arouse their alertness. Mass media education integrated with telehealth services may also be a promising way to help patients intuitively understand the progress of CDC and detailed information about CDC screening.
This study also tested the mechanism through which cues to action affects CDC screening behaviour. Apart from the significant direct effect (direct effect = 0.113), our analysis showed that cues to action had significant indirect effects on CDC screening behaviour through health beliefs (total indirect effect = 0.255). Cues to action is the most underdeveloped and rarely measured constructs of the HBM, and many reviewers even failed to provide an overview of its effectiveness due to the lack of studies that included it (Carpenter, 2010; Janz & Becker, 1984). Limited evidences have proved the effectiveness of cues to action in predicting patients’ health behaviour such as breast self‐examination (Dewi et al., 2019), antihypertensive medication adherence (Yue et al., 2015) and diabetes self‐management (Cerkoney, & Hart, 1980). But, information on how cues to action affected patients’ health behaviours are lacked. Only one study in the fields of tuberculosis treatment has explored the indirect effects of cues to action on patients’ treatment adherence (Tola et al., 2017). The authors combined the original health belief constructs (perceived susceptibility and perceived severity were summed up as perceived threat, perceived barriers minus perceived benefits were also calculated to substitute the original constructs) to perform further analysis. They reported that cues to action had a significant indirect effect on patients’ tuberculosis treatment adherence through perceived barriers‐benefits, while no significant indirect effect was found through perceived susceptibility +severity or self‐efficiency. Different from this study, we used the original constructs for data analysis and clarified both the direct and indirect effects of cues to action on patients’ CDC screening behaviour. Although discrepancies existed, our results, together with the study mentioned above, suggested the necessity of including cues to action in future studies given it has been found to significantly influence health behaviour in multiple pathways. When design intervention programmes, strategies tailed to place more cues to action will trigger health behaviour change directly or indirectly through modifying patients’ health beliefs. Anyway, the observed mechanisms of cues to action on CDC screening behaviour need to be studied in depth.
Regarding the contrasts of the specific indirect effects, perceived susceptibility and perceived barriers again showed significantly greater effect size. However, the specific indirect effect of cues to action through perceived benefits was not significant. This was a little different from that of knowledge. The results suggested that knowledge could optimise patients’ CDC screening behaviour by increasing their perceived benefits, while cues to action could not. Future studies are required to figure out the underlying reasons for this discrepancy. Nevertheless, our findings, coupled with some previous studies (Hsieh et al., 2016; Sheppler et al., 2014), all tend to demonstrate that perceived barriers and perceived susceptibility may be the most effective factors that can influence patients’ CDC screening behaviour both directly and indirectly. Intervention programmes tailed to eliminate patients’ perceived barriers and strengthen their perceived susceptibility are expected to gain success. Despite peer education and mass media education we have elaborated above, some other strategies could also be considered. First, future CDC screening programmes should be better integrated with the routine diabetes management service to decrease patients’ extra time cost and thus to facilitate their engagement. Second, reminder system and family member involvement in diabetes management, which were useful ways to increase cues to action, could be used to avoid unintended absence of scheduled screening. Lastly, since some of patients’ perceived barriers, such as the expensive finical cost, are beyond the capacity of primary healthcare providers to resolve, it is ideal that the expense of CDC screening could be covered by the special outpatient medical subsidy for diabetes on a medical insurance policy level.
This study has several limitations. One limitation was the cross‐sectional design, which only provides a glimpse of the participants at a specific point of time. Another limitation was that the study was conducted in an economically developed urban community, and this may restrict the generalisability of the results. The study also has some strengths. Compared to many previous studies that only included some of the HBM constructs, this study measured all the model conceptualised constructs and tested the model proposed relationships between these constructs. Findings from this study may contribute to a deeper understanding of the HBM and consequently provide some practical inspirations for future researchers and healthcare providers. Additionally, the data analysis method employed in this study, the parallel multiple mediator model, has tried the best to respect the complex casual process of the study variables in the real world, so the results were close to the reality to a great extent.
6. CONCLUSION
In conclusion, this study found that knowledge only indirectly affected patients’ CDC screening behaviour through perceived susceptibility, perceived benefits, perceived barriers and self‐efficiency. Cues to action affected patients’ CDC screening behaviour both directly and indirectly through perceived susceptibility, perceived barriers and self‐efficiency. Perceived susceptibility and perceived barriers played vital roles in mediating the effects of knowledge and cues to action on CDC screening behaviour.
7. RELEVANCE TO CLINICAL PRACTICES
The study contributes to a better understanding of the mechanism through which health beliefs affect CDC screening behaviour. Findings from this study have implications for primary care providers. First, health beliefs are recommended to be measured as process index in community health education programmes. Second, educational strategies should be innovated to better strengthening patients’ health beliefs as we have elaborated hereinbefore. Meanwhile, care coordination and care management are expected to help patients better engage themselves in CDC screening with more cues to actions and less perceived barriers. Our findings also have implications for researchers, that is, comprehensive application of the HBM, which include the overall constructs are recommended in future studies.
CONFLICT OF INTEREST
All authors declared no conflicts of interests.
AUTHOR CONTRIBUTIONS
Study proposal: Suzhen Liu, Lingjun Jiang. Data collection: Lingjun Jiang, Hang Li, Lingna Xie, Yuan Jiang. Data analysis and drafting of the manuscript: Lingjun Jiang. Approval of the manuscript: Suzhen Liu. Reading and approval of the final manuscript: All authors.
Supporting information
Table S1‐S2
Table S3
ACKNOWLEDGEMENTS
The authors would like to thank all the medical staffs who have assisted us a lot in patient recruitment and data collection. The author would also want to thank all the patients for their participation in this study.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
REFERENCES
- Adejoh, S. O. (2014). Diabetes knowledge, health belief, and diabetes management among the igala. Nigeria. Sage Open, 4(2), 215824401453996. 10.1177/2158244014539966 [DOI] [Google Scholar]
- Almadi, M. A. & Alghamdi, F. (2019). The gap between knowledge and undergoing colorectal cancer screening using the Health Belief Model: A national survey. Saudi Journal of Gastroenterology, 25(1), 27–39. 10.4103/sjg.SJG_455_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, J., Niu, F., Turpcu, A., Rajput, Y., & Cheetham, T. C. (2018). Adherence to the American Diabetes Association retinal screening guidelines for population with diabetes in the United States. Ophthalmic Epidemiology, 25(3), 257–265. 10.1080/09286586.2018.1424344 [DOI] [PubMed] [Google Scholar]
- Ang, G. Y., Yap, C. W., & Saxena, N. (2017). Effectiveness of diabetes foot screening in primary care in preventing lower extremity amputations. Annals of the Academy of Medicine, Singapore, 46(11), 417–423. [PubMed] [Google Scholar]
- Annan, F. M., Oppong Asante, K., & Kugbey, N. (2019). Perceived seriousness mediates the influence of cervical cancer knowledge on screening practices among female university students in Ghana. BMC Womens Health, 19(1), 140–148. 10.1186/s12905-019-0842-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, M. H. & Janz, N. K. (1985). The health belief model applied to understanding diabetes regimen compliance. The Diabetes Educator, 3, 41–47. [Google Scholar]
- Byun, S. H., Ma, S. H., Jun, J. K., Jung, K. W., & Park, B. (2013). Screening for diabetic retinopathy and nephropathy in patients with diabetes: a nationwide survey in Korea. PLoS One, 8(5), e62991. 10.1371/journal.pone.0062991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, C. J. (2010). A meta‐analysis of the effectiveness of health belief model variables in predicting behavior. Health Communication, 25(8), 661–669. 10.1080/10410236.2010.521906 [DOI] [PubMed] [Google Scholar]
- Cerkoney, A. K. & Hart, L. K. (1980). The relationship between the health belief model and compliance of persons with diabetes mellitus. Diabetes Care, 3(5), 594–598. [DOI] [PubMed] [Google Scholar]
- Champion, V. L. (1984). Instrument development for health belief model constructs. Advances in Nursing Science, 6(3), 73–85. 10.1097/00012272-198404000-00011 [DOI] [PubMed] [Google Scholar]
- Chen, J., Liao, Y., Li, Z., Tian, Y. E., Yang, S., He, C., Tu, D., & Sun, X. (2013). Determinants of salt‐restriction‐spoon using behavior in China: application of the health belief model. PLoS One, 8(12), e83262. 10.1371/journal.pone.0083262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, K. W. & Mak, Y. W. (2016). Association between psychological flexibility and health beliefs in the uptake of influenza vaccination among people with chronic respiratory diseases in hong kong. International Journal of Environmental Research and Public Health, 13(2), 155–169. 10.3390/ijerph13020155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, N. H., Shaw, J. E., Karuranga, S., Huang, Y., da Rocha Fernandes, J. D., Ohlrogge, A. W., & Malanda, B. (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice, 138, 271–281. 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- Cummings, K. M., Jette, A. M., & Rosenstock, I. M. (1978). Construct validation of the health belief model. Health Educ Monogr, 6(4), 394–405. 10.1177/109019817800600406 [DOI] [PubMed] [Google Scholar]
- Dehghani‐Tafti, A., Mazloomy Mahmoodabad, S. S., Morowatisharifabad, M. A., Afkhami Ardakani, M., Rezaeipandari, H., & Lotfi, M. H. (2015). Determinants of self‐care in diabetic patients based on health belief model. Global Journal of Health Science, 7(5), 33–42. 10.5539/gjhs.v7n5p33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi, T. K., Massar, K., Ruiter, R. A. C., & Leonardi, T. (2019). Determinants of breast self‐examination practice among women in Surabaya, Indonesia: An application of the health belief model. BMC Public Health, 19(1), 1581–1589. 10.1186/s12889-019-7951-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, A. J., Stevens, R., Hirst, J., Lung, T., Oke, J., Clarke, P., Glasziou, P., Neil, A., Dunger, D., M Colhoun, H., Pugh, C., Wong, G., Perera, R., & Shine, B. (2014). Optimal strategies for identifying kidney disease in diabetes: Properties of screening tests, progression of renal dysfunction and impact of treatment ‐ Systematic review and modelling of progression and cost‐effectiveness. Health Technology Assessment, 18(14), 1–128. 10.3310/hta18140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedration, I. D.The 8th edition of the diabetes atlas. 2017. [EB / OL]. [2020‐03‐26]. http://www.diabetesatlas.org
- Foreman, J., Keel, S., & Dirani, M. (2018). Adherence to diabetic eye examination guidelines in Australia: the national eye health survey. Medical Journal of Australia, 208(2), 97. [DOI] [PubMed] [Google Scholar]
- Gucciardi, E., Fortugno, M., Senchuk, A., Beanlands, H., McCay, E., & Peel, E. E. (2013). Self‐monitoring of blood glucose in black caribbean and south Asian Canadians with non‐insulin treated type 2 diabetes mellitus: A qualitative study of patients’ perspectives. BMC Endocrine Disorders, 13, 46. 10.1186/1472-6823-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, Y. L., Lee, F. H., Chen, C. L., Chang, M. F., & Han, P. H. (2016). Factors influencing intention to receive examination of diabetes complications. Asian Nursing Research, 10(4), 289–294. 10.1016/j.anr.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Janz, N. K. & Becker, M. H. (1984). The health belief model: a decade later. Health Education Quarterly, 11(1), 1–47. 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- Jones, C. J., Smith, H., & Llewellyn, C. (2014). Evaluating the effectiveness of health belief model interventions in improving adherence: A systematic review. Health Psychology Review, 8(3), 253–269. 10.1080/17437199.2013.802623 [DOI] [PubMed] [Google Scholar]
- Kashim, R. M., Newton, P., & Ojo, O. (2018). Diabetic retinopathy screening: A systematic review on patients’ non‐attendance. International Journal of Environmental Research and Public Health, 15(1), 157–169. 10.3390/ijerph15010157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, B. E. (2007). Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiology, 14(4), 179–183. 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- Kreft, D., McGuinness, M. B., Doblhammer, G., & Finger, R. P. (2018). Diabetic retinopathy screening in incident diabetes mellitus type 2 in Germany between 2004 and 2013 ‐ A prospective cohort study based on health claims data. PLoS One, 13(4), e0195426. 10.1371/journal.pone.0195426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake, A. J., Browne, J. L., Rees, G., & Speight, J. (2017). What factors influence uptake of retinal screening among young adults with type 2 diabetes? A qualitative study informed by the theoretical domains framework. Journal of Diabetes and Its Complications, 31(6), 997–1006. 10.1016/j.jdiacomp.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Liu, F., Bao, Y., Hu, R., Zhang, X., Li, H., Zhu, D., Li, Y., Yan, L., Li, Y., Lu, J., Li, Q., Zhao, Z., Ji, Q., & Jia, W. (2010). Screening and prevalence of peripheral neuropathy in type 2 diabetic outpatients: a randomized multicentre survey in 12 city hospitals of China. Diabetes/Metabolism Research and Reviews, 26(6), 481–489. 10.1002/dmrr.1107 [DOI] [PubMed] [Google Scholar]
- Liu, Y. & Swearingen, R. (2017). Diabetic eye screening: Knowledge and perspectives from providers and patients. Current Diabetes Reports, 17(10), 94–102. 10.1007/s11892-017-0911-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, X. & Huang, Y. (2019). Status quo of contracted family doctor service in Wuhou district, Chengdu. Chinese General Practice, 22(13), 1559–1565. 10.12114/j.issn.1007-9572.2019.00.219 [DOI] [Google Scholar]
- Lynn, M. R. (1986). Determination and quantification of content validity. Nursing Research, 35(6), 382–385. [PubMed] [Google Scholar]
- Montoya, A. K. & Hayes, A. F. (2017). Two‐condition within‐participant statistical mediation analysis: A path‐analytic framework. Psychological Methods, 22(1), 6–27. 10.1037/met0000086 [DOI] [PubMed] [Google Scholar]
- Nakamura, J., Kamiya, H., Haneda, M., Inagaki, N., Tanizawa, Y., Araki, E., Ueki, K., & Nakayama, T. (2017). Causes of death in Japanese patients with diabetes based on the results of a survey of 45,708 cases during 2001–2010: report of the committee on causes of death in diabetes mellitus. Journal of Diabetes Investigation, 8(3), 397–410. 10.1111/jdi.12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss, J. E. (2001). Developing a new model for cross‐cultural research: Synthesizing the health belief model and the theory of reasoned action. Journal of Diabetes Investigation, 23(4), 1–15. 10.1097/00012272-200106000-00002 [DOI] [PubMed] [Google Scholar]
- Preacher, K. J. & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. 10.3758/brm.40.3.879 [DOI] [PubMed] [Google Scholar]
- Rani, P. K., Raman, R., Sharma, V., Mahuli, S. V., Tarigopala, A., Sudhir, R. R., Kumaramanickavel, G., & Sharma, T. (2007). Analysis of a comprehensive diabetic retinopathy screening model for rural and urban diabetics in developing countries. British Journal of Ophthalmology, 91(11), 1425–1429. 10.1136/bjo.2007.120659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock, I. M., Strecher, V. J., & Becker, M. H. (1988). Social learning theory and the health belief model. Health Education Quarterly, 15(2), 175–183. 10.1177/109019818801500203 [DOI] [PubMed] [Google Scholar]
- Santos, A. J., Kislaya, I., Machado, A., & Nunes, B. (2017). Beliefs and attitudes towards the influenza vaccine in high‐risk individuals. Epidemiology and Infection, 145(9), 1786–1796. 10.1017/S0950268817000814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon, P. H. (2017). The English national screening programme for diabetic retinopathy 2003–2016. Acta Diabetologica, 54(6), 515–525. 10.1007/s00592-017-0974-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppler, C. R., Lambert, W. E., Gardiner, S. K., Becker, T. M., & Mansberger, S. L. (2014). Predicting adherence to diabetic eye examinations: Development of the compliance with annual diabetic eye exams survey. Ophthalmology, 121(6), 1212–1219. 10.1016/j.ophtha.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, N. K., John, D., Rebekah, G., Kujur, E. S., Paul, P., & John, S. S. (2017). Diabetes and diabetic retinopathy: Knowledge, attitude, practice (KAP) among diabetic patients in a tertiary eye care centre. Journal of Clinical and Diagnostic Research, 11(7), Nc01–Nc07. 10.7860/jcdr/2017/27027.10174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X., Guo, Y., Wang, S., & Sun, J. (2006). Predicting iron‐fortified soy sauce consumption intention: Application of the theory of planned behavior and health belief model. Journal of Nutrition Education and Behavior, 38(5), 276–285. 10.1016/j.jneb.2006.04.144 [DOI] [PubMed] [Google Scholar]
- Tola, H. H., Karimi, M., & Yekaninejad, M. S. (2017). Effects of sociodemographic characteristics and patients’ health beliefs on tuberculosis treatment adherence in Ethiopia: A structural equation modelling approach. Infect Dis Poverty, 6(1), 167–177. 10.1186/s40249-017-0380-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, Y. C., Lee, C. S., Chiu, Y. W., Lee, J. J., Lee, S. C., Hsu, Y. L., & Kuo, M. C. (2018). Angiopoietin‐2, renal deterioration, major adverse cardiovascular events and all‐cause mortality in patients with diabetic nephropathy. Kidney and Blood Pressure Research, 43(2), 545–554. 10.1159/000488826 [DOI] [PubMed] [Google Scholar]
- Vazini, H. & Barati, M. (2014). The health belief model and self‐care behaviors among type 2 diabetic patients. Iranian Journal of Diabetes and Obesity, 6(3), 107–113. [Google Scholar]
- Wang, D., Ding, X., He, M., Yan, L., Kuang, J., Geng, Q., & Congdon, N. (2010). Use of eye care services among diabetic patients in urban and rural china. Ophthalmology, 117(9), 1755–1762. 10.1016/j.ophtha.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Webb, E. M., Rheeder, P., & Van Zyl, D. G. (2015). Diabetes care and complications in primary care in the Tshwane district of South Africa. Primary Care Diabetes, 9(2), 147–154. 10.1016/j.pcd.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Wong, T. Y., Sun, J., Kawasaki, R., Ruamviboonsuk, P., Gupta, N., Lansingh, V. C., Maia, M., Mathenge, W., Moreker, S., Muqit, M. M. K., Resnikoff, S., Verdaguer, J., Zhao, P., Ferris, F., Aiello, L. P., & Taylor, H. R. (2018). Guidelines on diabetic eye care: The international council of ophthalmology recommendations for screening, follow‐up, referral, and treatment based on resource settings. Ophthalmology, 04(007), 1–14. 10.1016/j.ophtha.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Yue, Z., Li, C., Weilin, Q., & Bin, W. (2015). Application of the health belief model to improve the understanding of antihypertensive medication adherence among Chinese patients. Patient Education and Counseling, 98(5), 669–673. 10.1016/j.pec.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Zhang, P., Lu, J., Jing, Y., Tang, S., Zhu, D., & Bi, Y. (2017). Global epidemiology of diabetic foot ulceration: A systematic review and meta‐analysis (dagger). Annals of Medicine, 49(2), 106–116. 10.1080/07853890.2016.1231932 [DOI] [PubMed] [Google Scholar]
- Zhao, W., Zhang, J., Xu, S., & Xiao, J. (2015). Relationship between screening rates of microvascular complications and the level of diabetic knowledge in type 2 diabetic patients from Beijing community clinics. Chinese Journal of Diabetes, 08, 478–481. 10.3760/cma.j.issn.1674-5809.2015.08.005 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Table S3


