Abstract
Recent evidence indicates that both cardiovascular fitness and gross motor skill performance are related to enhanced neurocognitive functioning in children by influencing brain structure and functioning. This study investigates the role of white matter microstructure in the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning in healthy children. In total 92 children (mean age 9.1 years, range 8.0–10.7) were included in this study. Cardiovascular fitness and gross motor skill performance were assessed using performance‐based tests. Neurocognitive functioning was assessed using computerized tests (working memory, inhibition, interference control, information processing, and attention). Diffusion tensor imaging was used in combination with tract‐based spatial statistics to assess white matter microstructure as defined by fractional anisotropy (FA), axial and radial diffusivity (AD, RD). The results revealed positive associations of both cardiovascular fitness and gross motor skills with neurocognitive functioning. Information processing and motor response inhibition were associated with FA in a cluster located in the corpus callosum. Within this cluster, higher cardiovascular fitness and better gross motor skills were both associated with greater FA, greater AD, and lower RD. No mediating role was found for FA in the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning. The results indicate that cardiovascular fitness and gross motor skills are related to neurocognitive functioning as well as white matter microstructure in children. However, this study provides no evidence for a mediating role of white matter microstructure in these relationships.
Keywords: cross‐sectional design, DTI, neurocognitive functioning, preadolescents, white matter microstructure
Cardiovascular fitness and gross motor skills were both related to neurocognitive functioning and white matter microstructure. No evidence was found for a mediating role of white matter microstructure in the relationship between both cardiovascular fitness and gross motor skills with neurocognitive functioning.
Significance.
This is the first study to investigate the role of white matter microstructure in the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning in children. This study shows that cardiovascular fitness and gross motor skills relate to white matter microstructure, while no evidence was found for a mediating role of white matter microstructure in the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning. This study adds to the cumulating evidence that physical activity exposure in childhood contributes to brain development, potentially through changes in cardiovascular fitness and gross motor skill development.
1. INTRODUCTION
The prevalence of a sedentary lifestyle among children is rapidly increasing (Gabel et al., 2016; Tremblay et al., 2011). This trend is especially worrisome in light of evidence indicating that physical activity leads to a wide range of physical benefits, such as reduced risks for cardiovascular disease and obesity, and increased bone health (Janssen & LeBlanc, 2010). Moreover, recent evidence also indicates that engagement in physical activity is related to enhanced neurocognitive functioning in children, in particular concerning executive functioning (de Greeff et al., 2018; Donnelly et al., 2016; Singh et al., 2018; Vazou et al., 2019). Executive functions, such as working memory and inhibition, facilitate goal directed behavior (Collins & Koechlin, 2012; Salthouse, 2005) and are important predictors of behavioral functioning, academic achievement, and even social well‐being, health, wealth, and quality of life (Diamond & Lee, 2011). Physical activity induced changes in brain structure and functioning are thought to be instrumental in the beneficial effects of physical activity on behavioral measures of neurocognitive functioning.
A recent systematic review and meta‐analysis concerning the effects of physical activity on brain structure and neurophysiological functioning support the idea that physical activity indeed has beneficial effects on neurophysiological functioning in children, while little is known about the effects of physical activity on brain structure in children (Meijer, Königs, Vermeulen, et al., 2020; Valkenborghs et al., 2019). Of the few studies into the effects of physical activity on brain structure in children, all focused on white matter microstructure (Chaddock‐Heyman et al., 2018; Hyde et al., 2009; Krafft et al., 2014; Schaeffer et al., 2014). White matter microstructure facilitates the structural connectivity of the brain to allow functional integration of processes originating from specialized brain areas (Park & Friston, 2013). Diffusion tensor imaging (DTI) allows the assessment white matter microstructure by quantification of water molecule diffusion characteristics (Le Bihan et al., 2001). The most commonly used DTI measure is fractional anisotropy (FA), which is considered to be higher in tightly bundled, structurally compact fibers with high integrity (Beaulieu, 2002). Other DTI parameters are axial diffusivity (AD) and radial diffusivity (RD), possible markers for axonal density (Song et al., 2003) and myelination (Song et al., 2002), respectively. Although DTI parameters are measures of water diffusion and thus indirect measures of structural connectivity (Jones et al., 2013), a profile of higher FA and AD and lower RD is thought to be compatible with higher white matter integrity.
Developmental studies demonstrate age‐related increases in FA in most white matter regions until the late adolescence (Lebel et al., 2008; Peters et al., 2012). Hence, white matter microstructure may be particularly sensitive for physical activity during the window of strong brain development in childhood and adolescence. This idea is supported by a recent study indicating that physical activity (sports participation and outdoor play time) is associated with higher FA in healthy preadolescent children (Rodriguez‐Ayllon, Derks, et al., 2020). Moreover, cardiovascular fitness (Esteban‐Cornejo et al., 2019) and motor skills (Langevin et al., 2014) seems to be also associated with white matter microstructure in children. Cardiovascular fitness and motor skills are strongly related to physical activity exposure (Aires et al., 2010; Stodden et al., 2008) and physical activity is considered as an important determinant of cardiovascular fitness levels and motor skill development during childhood and adolescence (Aires et al., 2010; Ortega et al., 2008; Sallis et al., 1997; Stodden et al., 2008). Taken together, this suggests that cardiovascular fitness and gross motor skills are related to white matter microstructure in children and that this relationship may be influenced by the exposure to physical activity.
Most research concerning the relationship between physical activity and white matter microstructure has focused on cardiovascular fitness (Meijer, Königs, Vermeulen, et al., 2020). The level of cardiovascular fitness is determined by long‐term physical activity at moderate to high intensity (Rowland, 2007) in combination with physical maturation (Haywood & Getchell, 2019) and genetic factors (Malina et al., 2004). Long‐term physical activity is associated with an increased release of neurotrophic factors (e.g., brain‐derived neurotrophic factor and neural growth factor) and with both increased neural blood vessel formation and neurogenesis (Colcombe et al., 2006; Dishman et al., 2006; Swain et al., 2003). These neural mechanisms are known to promote plasticity in the structure and function of brain areas that support neurocognitive functioning (Vaynman & Gomez‐Pinilla, 2006). Accordingly, cross‐sectional studies using DTI indicate that higher levels of cardiovascular fitness are related to higher FA in healthy and obese adults and elderly (Johnson et al., 2012; Marks et al., 2011; Opel et al., 2019). More specifically, positive associations were found for cardiovascular fitness and FA (Johnson et al., 2012; Marks et al., 2011; Opel et al., 2019), while a negative relation was found with RD and no association between cardiovascular fitness and AD (Johnson et al., 2012). Very few studies have been conducted in children (Meijer, Königs, Vermeulen, et al., 2020). However, the available studies show that higher cardiovascular fitness is related to greater FA in healthy preadolescent children (Chaddock‐Heyman et al., 2014) and greater FA and lower AD and RD in adolescents (Ruotsalainen et al., 2020). In contrast, the study by Rodriguez‐Ayllon, Esteban‐Cornejo, et al. (2020) did not find a significant relationship between cardiovascular fitness and FA in obese children. In addition, long‐term intervention studies indicate effects of aerobic exercise programs on white matter microstructure in healthy children (Chaddock‐Heyman et al., 2018), obese children (Krafft et al., 2014), and deaf children (Xiong et al., 2018). Results indicate increased FA (Chaddock‐Heyman et al., 2018; Krafft et al., 2014), as well as decreased FA (Xiong et al., 2018) and RD (Chaddock‐Heyman et al., 2018) and no changes in AD (Chaddock‐Heyman et al., 2018; Krafft et al., 2014). These findings support a causal role of physical activity, targeting cardiovascular fitness, in white matter microstructure.
An alternative or complementary pathway for physical activity induced changes in brain structure is motor skill development (Voss, 2016). Development of complex motor skills is thought to enhance axonal arborization and increase cell density in white matter structures that are involved in motor functioning (Jones et al., 1999). Indeed, a study in child musicians has indicated that better fine motor skills are related to enhanced FA in specific white matter tracts compared to children who did not play music instruments (Bengtsson et al., 2005). Furthermore, intervention studies in adults indicate that gross motor skill training (practice juggling or balancing) leads to increased FA (Scholz et al., 2009; Taubert et al., 2010). Learning of novel complex motor skills, which require swift responses and tax concentration, appeals to both motor and cognitive functioning and enhanced functional connectivity is observed among the brain structures involved (Desmond et al., 1997; Diamond, 2000). In line with this finding gross motor skills, requiring control over large body muscles involved in balance, limb, and trunk movements, seem closely related to executive functioning in children (van der Fels et al., 2015, 2019). Taken together, the literature indicates that both cardiovascular fitness and gross motor skills may contribute to the changes in white matter microstructure in children that could benefit neurocognitive functioning.
To gain more knowledge on the role of white matter microstructure in the relationship between cardiovascular fitness and neurocognitive functioning, and between gross motor skills and neurocognitive functioning, it is important to determine whether induced white matter changes indeed go hand in hand with better neurocognitive functioning. Among the already small number of studies focusing on the relationship between cardiovascular fitness and white matter microstructure, and between motor skills and white matter microstructure, there are only four studies that also included measurements of neurocognitive functioning. One study in adults, showed that white matter microstructure, as assessed by FA, mediates the association between cardiovascular fitness (walking endurance) and cognitive performance (Opel et al., 2019). Another study in adolescents showed that FA of the corpus callosum and the right superior corona radiata mediates the association between of aerobic fitness with working memory (Ruotsalainen et al., 2020). The other two studies were conducted in obese and deaf children, respectively, and showed that the beneficial effects of aerobic exercise programs on FA were paralleled by improvements in executive functioning (Krafft et al., 2014; Xiong et al., 2018). Although these findings support the idea that white matter microstructure is involved in the relation between cardiovascular fitness and neurocognitive functioning, the findings are based on only four studies, with only two studies in small samples of children, all from clinical populations. Therefore, the generalizability of these findings to healthy children remains unknown. Furthermore, all of the existing studies focused on cardiovascular fitness, with no work being performed on motor skill development.
The present study aims to investigate the relationships of both cardiovascular fitness and gross motor skills with (a) neurocognitive functioning and (b) white matter microstructure and (c) whether white matter microstructure mediates the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning. White matter microstructure as defined by FA, AD, and RD was assessed using DTI in combination with tract‐based spatial statistics and neurocognitive functioning was assessed using a comprehensive and well‐defined set of neurocognitive function measures targeting executive functions. Based on the existing literature, it was hypothesized that higher levels of cardiovascular fitness and gross motor skills are be associated with higher levels of neurocognitive functioning and higher FA (presumably accompanied by a profile of higher AD and lower RD). Furthermore, the relation between cardiovascular fitness or gross motor skills and neurocognitive functioning is expected to be mediated by white matter microstructure.
2. METHODS
2.1. Participants
The current MRI study is part of a larger randomized controlled trial (“Learning by Moving”) that was executed at 22 primary schools in the Netherlands (891 children; mean age = 9.2 years old, SD = 0.7). For more information about this trial, see De Bruijn et al., 2020; van der Fels, Hartman, et al., 2020; Meijer, Königs, van der Fels, et al., 2020 and the Netherlands Trial register [NTR5341]. From the larger study sample, a sample was recruited for the current study of children aged 8 years or older without contraindications for MRI. Inclusion was guided by an inclusion protocol in order to balance the representation of sex, school grade (grade 3 or 4), and scanning site (Amsterdam and Groningen, the Netherlands) in the study sample. Consequently, a sample of 93 children was included in the current study. Parents and/or guardians gave written consent for participation of their child. The inclusion protocol and deviations from this protocol can be found in Table A1 of the Appendix.
2.2. Measures
2.2.1. Cardiovascular fitness
Cardiovascular fitness was assessed with the 20 m Shuttle Run Test (20 m SRT; Adam et al., 1993). During this test, children run back and forth on a 20 m track, and need to reach the other side of the track at or before an auditory signal. The timing of the auditory signal is initially set at a required average speed of 8 km/h, and is manipulated each minute to increase the required speed by 0.5 km/h at a time. The test was terminated when a child failed to reach the required distance in time on two consecutive crossings of the track. Based on the last trajectory that was successfully completed, the maximal oxygen uptake (VO2max in ml kg−1 min−1) was estimated by using the following formula: (31.025 + (3.238 × velocity) − (3.248 × age) + (0.1536 × age × velocity); Leger et al., 1988).
2.2.2. Gross motor skills
Gross motor skills were assessed using three subtests (jumping sideways, moving sideways, and backward balancing) of the Körper Koordinationstest für Kinder (KTK; Kiphard & Schilling, 2007). Additionally, one item of the Bruininks‐Oseretsky Test of Motor Proficiency, second Edition (BOT‐2) was used to include a measure for ball skills (Bruininks, 2005). Both motor skill test batteries have shown to be reliable and valid for primary school children (Bruininks, 2005; Deitz et al., 2007; Kiphard & Schilling, 2007).
2.2.3. Neurocognitive functioning measures
A set of neurocognitive functioning measures tapping into core domains of executive function (i.e., working memory, motor inhibition, and interference control) and lower‐level neurocognitive functions (information processing and attention) was used. All neurocognitive tasks and corresponding outcome measures are listed in Table 2. All measures have established psychometric properties and have been used in previous research (Königs et al., 2015; Verbruggen & Logan, 2009; Verburgh et al., 2014; Wechsler, 1991). In addition, full scale IQ was estimated by a two‐subtest short form (Information and Block Design) of the Wechsler Intelligence Scale for Children III (WISC‐III; Wechsler, 1991). All neurocognitive functioning measures are comprehensively described in previous work (Meijer, Königs, de Bruijn et al., 2020).
TABLE 2.
Baseline characteristics
| Total sample (n = 92) | |
|---|---|
| Number of girls, n (%) | 46 (50%) |
| Age in years, M (SD) | 9.12 (0.62) |
| BMI in kg/m2, M (SD) | 16.81 (2.24) |
| Normal weight, n (%)a | 77 (84%) |
| Overweight, n (%)a | 13 (14%) |
| Obesity, n (%)a | 2 (2%) |
| Grade three, n (%) | 47 (51%) |
| IQ, M (SD) | 101.29 (15.33) |
| SES, M (SD)b | 4.60 (1.04) |
| Cardiovascular fitness (VO2max, ml kg−1 min−1), M (SD) | 48.89 (4.37) |
| Gross motor skills, M (SD)c | 0.00 (1.00) |
Abbreviations: BMI, body‐mass index; M, mean; SD, standard deviation; SES, socioeconomic status.
According to the reference values by Cole and Lobstein (2012).
The average level of parental education ranged from 0 (no education) to 7 (post‐doctoral education).
Component scores derived from four motor skills subtests including: Jumping Sideways, Moving Sideways, Backwards Balancing, and Ball Skills.
Additional information was collected by parent questionnaires to assess demographic information (sex, age, socioeconomic status [SES]). SES was defined as the average level of parental education ranging from 0 (no education) to 7 (post‐doctoral education; Statistics Netherlands, 2006). Participation in sports was defined as parent‐reported weekly participation in organized sports expressed in minutes, not including physical education, transport to school, and playing outside (Ooijendijk et al., 2007, Table 1).
TABLE 1.
Description and operationalization of neurocognitive functioning measures
| Task | Measures | Description | Dependent variable | |
|---|---|---|---|---|
| ANT | Computerized task in which target stimuli consisting of an arrow pointing left or right are presented on a computer screen. Children are instructed to respond as quickly as possible to the direction of a target stimulus by pressing the corresponding button. The ex‐Gaussian model was used to extract the influence of extreme slow responses (tau) on information processing speed (Fan et al., 2002; Lacouture & Cousineau, 2008; Rueda et al., 2004) | Information processing | The speed of responding to target appearance | Mean reaction time (ms) on neutral trials |
| Tau | Lapses of attention | The average of the exponential component of the fitted ex‐Gaussian curve, reflecting the influence of extremely slow responses (lapses of attention) on information processing | ||
| Alerting attention | The speed of achieving an alert state | The difference in mean reaction time (ms) between central cue trials and no cue trials | ||
| The accuracy of achieving an alert state | The difference in percentage of correct responses on central cue trials and no cue trials | |||
| Spatial attention | The speed of spatially orienting to information | The difference in mean reaction time (ms) between spatial cue trials and central cue trials | ||
| The accuracy of spatially orienting to information | The difference in the percentage of correct responses on spatial cue trials and central cue trials | |||
| Interference control | The speed of suppressing irrelevant information | The difference in mean reaction time (ms) between incongruent trials and congruent trials | ||
| The accuracy of suppressing irrelevant information | The difference in the percentage of correct responses on incongruent trials and congruent trials | |||
| DS | Children are required to repeat a sequence of numbers presented auditorily in the order of presentation (forward condition) or reversed order (backward condition (WISC‐III; Wechsler, 1991)) | Verbal short‐term memory | The ability to hold verbal information in short‐term memory | The product of the number of correct responses and the highest span reached in the forward condition (Kessels et al., 2000). |
| Verbal working memory | The ability to manipulate verbal information in working memory | The product of the number of correct responses and the highest span reached in the backward condition (Kessels et al., 2000) | ||
| GT | A sequence of yellow dots is presented on a four by four digital grid. Children are required to repeat the sequence in the order of presentation (forward) or reversed order (backward) by clicking on the relevant locations in the grid (Nutley et al., 2009) | Visuospatial short‐term memory | The ability to hold visuospatial information in short‐term memory | The product of the number of correct responses and the highest span reached in the forward condition (Kessels et al., 2000) |
| Visuospatial working memory | The ability to manipulate visuospatial information in working memory | The product of the number of correct responses and the highest span reached in the backward condition (Kessels et al., 2000) | ||
| SST | A computerized task involved Go trials and Stop trials. Go trials consist of an airplane either pointing to the right or left side. Stop trials are identical to Go trials but with a stop signal superimposed on the airplane. Children are instructed to respond as quickly as possible to Go trials by pressing the corresponding button, and to inhibit the motor response when the stop signal is presented (Logan, 1994) | Motor inhibition efficiency | The latency of an inhibitory process | The mean reaction time (ms) calculated for correct responses on go trials subtracted by the average stop signal delay time (ms) |
Abbreviations: ANT, attention network test; DS, digit span; GT, grid task; SST, stop signal task.
2.3. MRI acquisition
MRI was performed on two 3 Tesla whole‐body units, a GE Discovery MR750 3T (location VU Medical Center Amsterdam) and a Phillips Intera 3T (location University Medical Center Groningen), using a 32‐channel head coil. Two‐dimensional echo‐planar diffusion tensor images were acquired using 5 volumes without diffusion weighting and 30 (GE) or 32 (Philips) volumes with non‐collinear diffusion gradients (b‐value = 750 s/mm2) in 56–58 transverse oblique slices of 2.5 mm thickness (angulated parallel to the line connecting the pituitary to the fastigium of the fourth ventricle), covering the whole brain (GE TR/TE = 6,000/76 ms; Phillips TR/TE = 6,000/57 ms). The acquired in‐plane resolution was 2.5 × 2.5 mm, reconstructed to 1 × 1 mm. Parallel imaging was applied with an acceleration factor of 2. In addition, reference scans with reversed phase‐encode blips were acquired to correct for echo‐planar imaging distortions.
2.4. Preprocessing
2.4.1. Behavioral data
Preprocessing steps and statistical analysis of the behavioral data were performed using IBM SPSS Statistics version 25.0 (SPSS IBM, New York, U.S.A) and R for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria). Outliers (z ≤ −3.29 or ≥ 3.29) were winsorized, that is, replaced with a value one unit greater than the next non‐outlier value (Field, 2013). To determine if data were normally distributed, histograms and values of skewness and kurtosis were inspected. Van der Waerden transformations were used to correct deviations from the normal distribution. All neurocognitive functioning measures were recoded with higher scores indicating better performance. To reduce the number of gross motor skills and neurocognitive functioning measures and to enhance their reliability, principal component analysis with varimax rotation was performed on all baseline gross motor skills and neurocognitive functioning measures separately. These analyses used the group of children included in the larger “Learning by Moving” cross‐sectional study (n = 814). For more information of this procedure, see Meijer, Königs, de Bruijn, et al. (2020).
2.4.2. DTI
All processing of MRI images was performed using the Functional MRI of the Brain (FMRIB) Software Library (FSL) version 5.0.11 (Jenkinson et al., 2012). Pre‐processing of DTI included estimation and correction of susceptibility induced distortions using topup, and correction of eddy currents and head motion using FSL eddy, including detection and imputation of outlier slices (average number of imputed slices: 21 [range 0–71] per subject; out of the total number of ~2,000 slices Andersson et al., 2003; Smith et al., 2004). Estimation of the diffusion tensor model was performed on brain‐extracted images (FSL bet; Smith, 2002) to create maps of FA, AD, and RD. The quality of the tensor fit was visually inspected on the sum of squared error maps (Behrens et al., 2003). Voxel‐wise statistical analysis of the DTI data was performed using TBSS (Smith et al., 2006). First, the FA maps of all subjects were aligned to the most typical subject, as determined by non‐linear registrations of FA maps between all children. Next, the DTI maps were transformed to MNI152 space via the most typical subject using non‐linear transformation, after which a white matter skeleton (FA > 0.30) was computed to reduce the influence of imperfect registration and inter‐subject variability in white matter tract anatomy. The DTI maps were projected onto the skeleton in standard space.
2.5. Procedure
For all participants, the described assessments were collected within a period of 2 weeks. The 20 m SRT was conducted during a physical education lesson at the primary schools and was administered by trained test leaders under the guidance of physical education teachers in groups of up to 15 children. Gross motor skills were individually assessed in circuit form during a separate physical education lesson. All tests were administered in a random order. The neurocognitive assessment was individually executed during the school day by trained examiners using standardized protocols, and tasks were administered in a fixed order. To limit the influence of fatigue and distraction, the neurocognitive tasks were administered in two sessions performed on separate days, with a duration of 30–35 min per session. MRI scanning took place at the VU University Medical Centre in Amsterdam (GE; n = 48), or at the University Medical Center in Groningen (Philips; n = 44). Prior to the MRI scan, children were made familiar with the MRI procedure using a mock scanner in which the environment of the MRI scanner (including scanner sounds) was recreated. The MRI scanning protocol was part of a larger protocol which comprises structural and functional sequences in the following order: T1, DTI, resting‐state fMRI and active‐state fMRI, and lasted approximately 35 min. Head movements were minimized by inserting small pillows between the head coil and the child's head. During the structural MRI scans (T1 and DTI), children watched a cartoon distracting them from the scanning environment. In between the scans, participants were reminded to keep still when necessary. Scans of poor quality due to head motion during scanning were directly repeated. Children received a small present and a copy of their structural T1‐weighted scan. This study was approved by the ethical board of the Vrije Universiteit Amsterdam (Faculty of Behavioural and Movement Sciences, approval number VCWE‐S‐15‐00197).
2.6. Statistical analysis
Statistical analysis on behavioral data was performed in IBM SPSS Statistics (SPSS IBM, New York, USA). First, we investigated the associations between cardiovascular fitness and the neurocognitive functioning components, and gross motor skills and the neurocognitive functioning components. These relations were examined using linear regression analyses in which the neurocognitive functioning components (resulting from the principal component analysis) were used as dependent variables and either cardiovascular fitness or gross motor skills were included as predictor. Demographic variables (sex, grade [3 or 4], age, BMI, and SES) were selected in each model as covariates using a stepwise backward selection approach, providing a data‐driven selection of relevant covariates for each dependent variable.
Second, we investigated the role of white matter microstructure in the observed (significant) relationships between cardiovascular fitness and the neurocognitive functioning components, and gross motor skills and the neurocognitive functioning components. Therefore, we performed spatial regressions between the relevant neurocognitive functioning component scores and FA in the white matter skeleton using one‐sided permutation testing in FSL Randomise, where threshold‐free cluster enhancement and p values as corrected for family‐wise error accounted for multiple testing (Winkler et al., 2014). White matter regions showing a significant relationship between one or more of the neurocognitive functioning components and FA, were selected as region of interest (ROI) for further analysis. Within these ROIs, we investigated the relation between cardiovascular fitness and FA, and gross motor skills and FA by performing spatial regression using two‐sided permutation testing with FSL Randomise. If cardiovascular fitness and/or gross motor skills were significantly associated with FA, we further explored the identity of this effect by extracting the AD and RD values in these ROIs. To control for possible effects of demographic variables, the covariates: sex, age, BMI, and SES were added to the models. Only significant covariates were retained in the final model. To account for possible differences between scanning sites, we added Scanning Site in all final models.
Third, the role of FA in the relation between cardiovascular fitness and neurocognitive functioning, and between gross motor skills a neurocognitive functioning was tested using mediation analysis. The mediation analysis was performed using the PROCESS SPSS macro developed by Hayes (2017). All indirect effects were tested using 5,000 bootstrap samples and bias‐corrected bootstrap confidence intervals. For all analyses, the level of significance was set at 0.05.
3. RESULTS
3.1. Participants
For the analyses concerning the relation between cardiovascular fitness and FA and between gross motor skills and FA, participating children were excluded if they did not attend the cardiovascular fitness measurement (n = 1) or gross motor skill measurement (n = 0), leaving a total 92 children in the analysis. An overview of the included, scanned, and analyzed children can be found in Table A1 of the Appendix. Table 2 shows the demographic characteristics of the total sample of participating children (n = 92). The prevalence of overweight (14%) and obesity (2%) observed in our sample parallel recent figures observed in the Dutch pediatric population (Cole & Lobstein, 2012; Volksgezondheid en zorg, 2018).
3.2. Neurocognitive functioning components
Principal component analysis of all the neurocognitive functioning measures using data of the larger study group (n = 856) extracted a total of six components, together explaining 70% of the total variance (see Table A2 of the Appendix for Eigenvalues and factor loadings). Based on the variables with the strongest contributions, the neurocognitive functioning components were labeled as follows: (a) Information Processing and Control (information processing, lapses of attention and motor inhibition), (b) Interference Control (speed of interference control and accuracy of interference control), (c) Attention Accuracy (accuracy of alerting attention and accuracy of spatial attention), (d) Visuospatial Working Memory (visuospatial short‐term memory and visuospatial working memory), (e) Verbal Working Memory (verbal short‐term memory and verbal working memory), and (6) Attention Efficiency (speed of alerting attention and speed of spatial attention).
3.3. Associations between both cardiovascular fitness or gross motor skills and neurocognitive functioning components
Results of the analyses focusing on the relation between cardiovascular fitness and neurocognitive functioning components are displayed in Table 3. The results revealed a significant and positive relation between cardiovascular fitness and Information Processing and Control (R 2 = 0.173, p = 0.007). No meaningful associations were found between cardiovascular fitness and any of the other neurocognitive functioning components: Interference Control, Attention Accuracy, Visuospatial Working Memory, Verbal Working Memory, and Attention Efficiency.
TABLE 3.
Results of linear regression analysis relating cardiovascular fitness to neurocognitive functioning components
| Neurocognitive functioning component | Covariatesa | B (SD) | 95% CI | p value | R 2 |
|---|---|---|---|---|---|
| Information Processing and Control | Grade | 0.061 (0.022) | 0.017–0.105 | 0.007 | 0.173 |
| Interference Control | – | 0.027 (0.024) | −0.021–0.075 | 0.266 | 0.014 |
| Attention Accuracy | BMI | −0.024 (0.023) | −0.069–0.021 | 0.297 | 0.066 |
| Visuospatial Working Memory | – | 0.046 (0.026) | −0.006–0.098 | 0.083 | 0.033 |
| Verbal Working Memory | SES | 0.018 (0.023) | −0.027–0.064 | 0.422 | 0.072 |
| Attention Efficiency | – | 0.031 (0.022) | −0.014–0.075 | 0.172 | 0.021 |
Abbreviation: SES, socioeconomic status.
Covariates significantly related to the neurocognitive functioning component.
Results of the analysis focusing on the relation between gross motor skills and neurocognitive functioning components are displayed in Table 4. The results revealed significant and positive relations between gross motor skills and Information Processing and Control (R 2 = 0.188, p < 0.001) and Visuospatial Working Memory (R 2 = 0.046, p = 0.038). No meaningful associations were found between gross motor skills and any of the other component scores: Interference Control, Attention Accuracy, Verbal Working Memory, and Attention Efficiency.
TABLE 4.
Results of linear regression analysis relating gross motor skills to neurocognitive functioning components
| Neurocognitive functioning component | Covariatesa | B (SD) | 95% CI | p value | R 2 |
|---|---|---|---|---|---|
| Information Processing and Control | – | 0.437 (0.095) | 0.248–0.626 | <0.001 | 0.188 |
| Interference Control | – | 0.084 (0.105) | −0.124–0.292 | 0.425 | 0.007 |
| Attention Accuracy | BMI | −0.084 (0.095) | −0.272–0.105 | 0.380 | 0.063 |
| Visuospatial Working Memory | – | 0.236 (0.112) | 0.013–0.459 | 0.038 | 0.046 |
| Verbal Working Memory | SES | 0.095 (0.097) | −0.098–0.289 | 0.330 | 0.074 |
| Attention Efficiency | – | 0.081 (0.098) | −0.113–0.275 | 0.407 | 0.008 |
Abbreviation: SES, socioeconomic status.
Covariates significantly related to the neurocognitive functioning component.
3.4. Associations between neurocognitive functioning components and FA
Next, we employed spatial regression between FA in the white matter skeleton and the neurocognitive functioning components that were related to either cardiovascular fitness (Information Processing and Control) or gross motor skills (Information Processing and Control and Visuospatial Working Memory). The results of these analyses revealed a large cluster of white matter tracts with a significant positive relation between FA and Information Processing and Control (p < 0.05; Figure 1). Higher FA in the white matter tracts of the cluster was associated with better Information Processing and Control. The cluster was selected as ROI for further analysis focusing on the relationship between cardiovascular fitness and FA and between gross motor skills and FA. No significant associations were found between FA and Visuospatial Working Memory.
FIGURE 1.
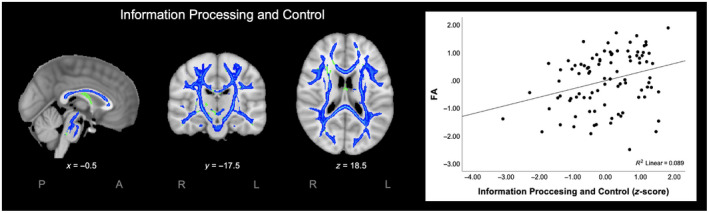
Significant voxel‐wise associations for Information Processing and Control (left) and the significant association between the mean fractional anisotropy (FA) and Information Processing and Control (z‐score) (right). Significant associations (blue) are displayed on the mean FA skeleton (green; threshold: 0.3) using threshold‐free cluster enhancement correction in TBSS (p < 0.05). The results are overlaid on a MNI152 1 mm T1 brain and for visualization purposes, significant associations between FA and Information Processing and Control were thickened
3.5. Associations between both cardiovascular fitness and gross motor skills and FA, AD, and RD
First, we investigated the relation between cardiovascular fitness and FA and between gross motor skills and FA. This was analyzed within the selected ROI of white matter tracts with relevance for Information Processing and Control. The results revealed significant positive relations between cardiovascular fitness and FA (Figure 2a) as well as between gross motor skills and FA (Figure 2b). These findings indicate that higher cardiovascular fitness and better gross motor skills were both associated with greater FA, particularly in the corpus callosum. More specifically, higher cardiovascular fitness was associated with greater FA, particularly in the left and right splenium and the left body of the corpus callosum. Better gross motor skills were associated with greater FA in the left splenium and the right posterior corona radiata.
FIGURE 2.
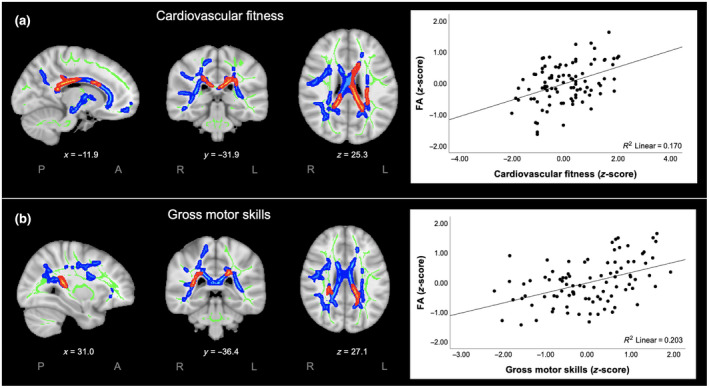
Significant voxel‐wise associations for cardiovascular fitness (a) and gross motor skills (b; both left) and the significant associations between fractional anisotropy (FA) (z‐score) and (a) cardiovascular fitness (z‐score) and (b) gross motor skills (z‐score; both right). Significant voxel‐wise associations are displayed (red) within the ROI of white matter tracts with relevance for Information Processing and Control (blue), displayed on the mean FA skeleton (green; threshold: 0.3) using threshold‐free cluster enhancement correction in TBSS (p < 0.05). The results are overlaid on a MNI152 1 mm T1 brain and for visualization purposes, significant associations between cardiovascular fitness or gross motor skills and Information Processing and Control were thickened
Subsequently, we further explored the origin of the observed relations between cardiovascular fitness and FA and between gross motor skills and FA, by extracting AD and RD from the selected ROI. For both cardiovascular fitness and gross motor skills, results indicated significant positive associations with AD and negative associations with RD (Table 5), suggesting that better cardiovascular fitness and gross motor skills are associated with greater axonal fiber density and/or diameter (AD) and a greater degree of myelination (RD) in the white matter microstructure of the ROIs. Of the covariates: Scanning Site, sex, age, BMI, and SES, only Scanning Site showed a significant association with FA, hence all models included Scanning Site as covariate.
TABLE 5.
Results of linear regression analysis relating DTI parameters to cardiovascular fitness and gross motor skills
| Cardiovascular fitnessa | Mean (SD) | B | 95% CI | p value | R 2 |
|---|---|---|---|---|---|
| FA | 0.576 (0.065) | 0.004 (0.001) | 0.002–0.006 | <0.001 | 0.667 |
| ADc | 153.1 (8.5) | 0.362 (0.131) | 0.102–0.622 | 0.007 | 0.604 |
| RDc | 54.1 (6.8) | −0.395 (0.117) | −0.627–−0.163 | 0.001 | 0.504 |
| Gross motor skillsb | Mean (SD) | B | 95% CI | p value | R 2 |
|---|---|---|---|---|---|
| FA | 0.395 (0.631) | 0.019 (0.004) | 0.069–0.100 | <0.001 | 0.574 |
| ADc | 138.5 (7.5) | 1.219 (0.600) | 0.027–2.411 | 0.045 | 0.458 |
| RDc | 57.9 (5.4) | −1.959 (0.469) | −2.891–−1.027 | <0.001 | 0.359 |
Corrected for Scanning Site and Grade.
Corrected for Scanning Site.
10−5 mm2/s.
Last, a mediation model (Figure 3) was used to investigate the potentially mediating role of FA (within the ROI of white matter tracts with relevance for Information Processing and Control) in the relations between (a) cardiovascular fitness and Information Processing and Control and (b) gross motor skills and Information Processing and Control. With regard to cardiovascular fitness (Figure 3), we found positive relations with Information Processing and Control (p = 0.009) and FA (p < 0.001). There was no evidence for a mediating role of FA in the relationship between cardiovascular fitness and Information Processing and Control (95% confidence interval = −0.002 to 0.040). With regard to gross motor skills, we also found positive relations with Information Processing and Control (p < 0.001) and FA (p < 0.001). However, there was no evidence for a mediating role of FA in the relationship between gross motor skills and Information Processing and Control. The mediation models were controlled for Scanning Site (both) and/or Grade (cardiovascular fitness model only).
FIGURE 3.
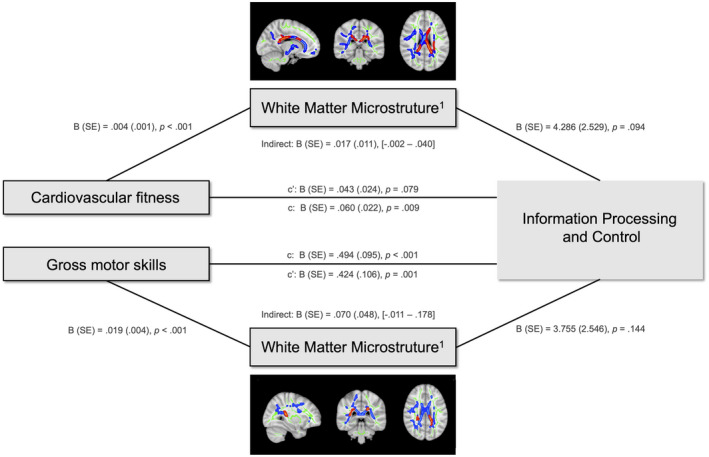
Mediation model Note. Mediation model testing the mediating role of white matter microstructure (FA) in the relation between cardiovascular fitness or gross motor skills and Information Processing and Control. 1FA in the cluster of white matter tracts associated with the neurocognitive component Information Processing and Control and cardiovascular fitness; 2FA in the cluster of white matter tracts associated with the neurocognitive component Information Processing and Control and motor skills. c’ represents the direct effect and c the total effect of Cardiovascular fitness/Gross motor skills on Information Processing and Control.
4. DISCUSSION
This study is the first study that investigated the role of white matter microstructure in the relationship of both cardiovascular fitness and gross motor skills with neurocognitive functioning. The results show that both cardiovascular fitness and gross motor skills are related to neurocognitive functioning and white matter microstructure which in turn was shown to be related to neurocognitive functioning. However, no evidence was found for a mediating role of white matter microstructure in the relationship between both cardiovascular fitness and gross motor skills with neurocognitive functioning. These findings support the idea that physical activity may induce changes in white matter microstructure that benefit neurocognitive functioning in childhood.
The results of the current study in a sample of healthy children revealed positive relations between cardiovascular fitness and neurocognitive functioning as well as between gross motor skills and neurocognitive functioning. More specifically, we found that higher levels of cardiovascular fitness and better gross motor skills were associated with better neurocognitive functioning as measured in terms of the speed and variability of information processing and motor response inhibition (Information Processing and Control). In addition, better gross motor skills were also related to better visuospatial working memory. These results are partly in line with earlier studies and recent meta‐analyses, which also found associations between cardiovascular fitness and interference control and attention (de Greeff et al., 2018; Donnelly et al., 2016). Although the relationship between gross motor skills and neurocognitive functioning in children has thus far not received much attention, earlier research indicated positive associations with visuospatial working memory and attention (van der Fels et al., 2015). We argue that one compelling explanation for the discrepant findings between our and previous studies with regard to the specific neurocognitive functions that are related to cardiovascular fitness or gross motor skills, may stem from the fact that the current study corrected for processing speed in the assessment of executive functions (also see Meijer, Königs, de Bruijn, et al., 2020). In the current study, analysis carefully corrected for processing speed and revealed no evidence for relations with interference control, contrasting earlier findings that did not correct for processing speed (de Greeff et al., 2018; Donnelly et al., 2016). However, our findings did replicate the relations between cardiovascular fitness and information processing and motor response inhibition (Donnelly et al., 2016) and the relationship between gross motor skills and visual working memory (van der Fels et al., 2015; van der Fels, de Bruijn et al., 2020).
Analyses focusing on white matter microstructure parameters (FA, AD, and RD) revealed that higher cardiovascular fitness and better gross motor skills were related to higher FA, predominantly in the corpus callosum. More specifically, the ROI’s for cardiovascular fitness were located in the bilateral splenium and the left body of the corpus callosum and for gross motor skills in both the left splenium and the right posterior corona radiata which connects the cerebral cortex and the brain stem and communicates motor and sensory information. Our findings concerning cardiovascular fitness and gross motor skills were both located in the splenium, which is part of the forceps major, a white matter tract which connects temporal, parietal, and occipital cortical regions and is involved in the communication of somatosensory information (Caminiti et al., 2009; Hofer & Frahm, 2006; Wakana et al., 2004). The fact that our findings were obtained in the splenium might reflect functional importance of the splenium in the relation between physical activity and neurocognitive functioning. Alternatively, local anatomical properties of the splenium (e.g., density of the white matter fibers) may increase the sensitivity of this structure to detect relations between white matter microstructure and cardiovascular fitness and gross motor skills (Hofer & Frahm, 2006). The profile of relations with DTI parameters (positive relations to FA and negative relations to RD) suggests that higher cardiovascular fitness and better gross motor skills are related to more tightly packed bundles and structurally compact fibers (FA) and increased myelination (RD). Furthermore, we observed higher AD which might reflect greater axonal fiber density and/or diameter.
Our findings concerning the relation between both cardiovascular fitness and gross motor skills with white matter microstructure are in line with earlier work in healthy and clinical samples of children, adolescents, adults and elderly, indicating that higher cardiovascular fitness and better gross motor skills are related to increased FA in presumably the corpus callosum and corona radiata (Bengtsson et al., 2005; Chaddock‐Heyman et al., 2014, 2018; Johnson et al., 2012; Krogsrud et al., 2018; Marks et al., 2011; Opel et al., 2019). However, our results concerning cardiovascular fitness are in contrast with the results of a recent cross‐sectional study which indicated no significant relationship between physical fitness and FA (Rodriguez‐Ayllon, Esteban‐Cornejo, et al., 2020). An important difference between their and our study is that Rodriguez‐Ayllon and colleagues included only obese children, which may indicate that the association between cardiovascular fitness and white matter microstructure may differ between healthy and clinical populations. However, it is currently unknown whether the proposed underlying mechanisms linking cardiovascular fitness to white matter microstructure may act similarly in obese children (or other clinical groups) and healthy children (Meijer, Königs, Vermeulen, et al., 2020). Future research may further clarify the possible differences between healthy and clinical populations.
Lastly, we investigated the potential mediating role of white matter in the observed relation between cardiovascular fitness and neurocognitive functioning and between gross motor skills and neurocognitive functioning. We found neither evidence for a mediating role of white matter microstructure in the relationship between cardiovascular fitness and neurocognitive functioning, nor between gross motor skills and neurocognitive functioning. These findings contrast with earlier studies of Opel et al. (2019) and Ruotsalainen et al. (2020) in adults and adolescents, which both indicated a mediating role of FA in the relationship between cardiovascular fitness and executive functioning. These contradictory results could be explained by the idea that white matter plasticity is age dependent and that during adolescence and young adulthood white matter microstructure is more sensitive for physical activity‐induced effects than during preadolescence. However, we did find strong relationships for both cardiovascular fitness and gross motor skills with FA in the same brain structures. Furthermore, the mediation effect of FA in the relationship between cardiovascular fitness and neurocognitive functioning and between gross motor skills and neurocognitive functioning just escaped conventional levels of significance. Thus, with a larger sample size and greater statistical power, the mediation analyses might have revealed significant mediating effects.
Our findings should be interpreted within the knowledge that cardiovascular fitness and gross motor skill are related constructs (Reeves et al., 1999; Stodden et al., 2009). However, an exploratory analysis showed that cardiovascular fitness and gross motor skills were only modestly related in our sample (r = 0.45, p < 0.001, shared variance: 20%). When repeating our analyses concerning the association with neurocognitive functioning and include both cardiovascular fitness and gross motor skills within the same model, we replicated the reported association between gross motor skills and the neurocognitive component Information Processing and Control (p < 0.001). Hence, the potential contributions of cardiovascular fitness and gross motor skill do not have to be mutually exclusive and may be complementary in their influence on brain structure and neurocognitive functioning. Future research may further clarify the relative contributions of aspects of physical activity of potential changes in brain structure.
Our study has some important strengths, such as the relatively large sample size of healthy children and the use of a well‐defined set of neurocognitive function measures. In addition, the current study is the first study to investigate the role of white matter microstructure in the two proposed pathways through which physical activity is thought to induce changes in neurocognitive functioning. This study also has some limitations. We included only children from 8 to 10 years old, restricting the generalizability of our findings to other stages of childhood development. Due to the rapid proliferation of executive functioning during childhood, it is highly possible that the effects of cardiovascular fitness and gross motor skills depend on the child's developmental stage (Haywood & Getchell, 2019). Due to practical reasons, we scanned at two locations with scanners from different vendors. Accordingly, we have matched scanning protocols and included scanning site as covariate in all analyses. All DTI images were acquired with isotropic resolution, which were interpolated during reconstruction only in‐plane. This led to anisotropic voxels as input for tensor estimation. According to Dyrby et al. (2014) the effect of interpolation is unlikely to impact the results. Last, our findings are based on a cross‐sectional design, which prevents us to draw causal conclusions. Future research should determine the effects of physical activity on brain structure and neurocognitive functioning using a randomized controlled trial design to test the potential of physical activity programs to improve healthy brain development.
In conclusion, the present study shows that cardiovascular fitness and gross motor skills are associated with enhanced performance in a specific set of neurocognitive functions (i.e., relating to the speed and variability of information processing and motor response inhibition) as well as enhanced FA in a cluster of white matter tracts with overlapping relevance for neurocognitive functions (i.e., predominantly in the corpus callosum and corona radiata). Although white matter microstructure could not be indicated as a mediator in the relationship in both cardiovascular fitness and gross motor skills with neurocognitive functioning, our findings do support the idea that cardiovascular fitness and gross motor skill are related to white matter microstructure which in turn was shown to be related to neurocognitive functioning in healthy children. Although more research is needed to substantiate these results, they might indicate that physical activity exposure contributes to brain development and children's neurocognitive functioning through cardiovascular fitness and gross motor skills.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, C.V., R.J.B., E.H., and J.O.; Methodology, P.J.W.P. and M.K.; Software, M.K.; Formal Analysis, A.M., P.J.W.P., and M.K.; Investigation, A.M.; Writing – Original Draft, A.M. and M.K.; Writing – Review & Editing, A.M., P.J.W.P., J.S., C.V., R.J.B., E.H., J.O., and M.K.; Visualization, A.M.; Supervision, J.O. and M.K.; Funding Acquisition, E.H.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.24851.
Supporting information
TABLE A1 Inclusion protocol of DTI measurements separated for Sex and Scanning Site
TABLE A2 Results of principal component analysis on the neurocognitive functioning measures
Transparent Peer Review Report
Transparent Science Questionnaire for Authors
ACKNOWLEDGMENTS
The authors thank all participating children and their parents and Ton Schweigmann for his help with performing the MRI scans. The authors also want to acknowledge the financial support provided by the Netherlands Initiative for Education Research under Grant 405‐15‐410 and Dutch Brain Foundation under Grant GH 2015‐3‐01. The funding resources were not involved in conduction of the research and preparation of the manuscript.
Meijer A, Pouwels PJW, Smith J, et al. The relationship between white matter microstructure, cardiovascular fitness, gross motor skills, and neurocognitive functioning in children. J Neurosci Res. 2021;99:2201–2215. 10.1002/jnr.24851
Edited by Jeremy Hogeveen and Cristina Ghiani. Reviewed by Maria Rodriguez‐Ayllon, Christopher Watson, and Alena Svatkova.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adam, C., Klissouras, V., Ravazzolo, M., Renson, R., Tuxworth, W., Kemper, H. C. G., van Mechelen, W., Hlobil, H., Beunen, G., & Levarlet‐Joye, H. (1993). Eurofit tests of physical fitness (2nd edition) (2nd ed.). Strasbourgh: Council of Europe. Committee for the development of sport. [Google Scholar]
- Aires, L., Andersen, L. B., Mendonça, D., Martins, C., Silva, G., & Mota, J. (2010). A 3‐year longitudinal analysis of changes in fitness, physical activity, fatness and screen time. Acta Paediatrica, 99(1), 140–144. [DOI] [PubMed] [Google Scholar]
- Andersson, J. L., Skare, S., & Ashburner, J. (2003). How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. Neuroimage, 20(2), 870–888. [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Behrens, T., Woolrich, M. W., Jenkinson, M., Johansen‐Berg, H., Nunes, R. G., Clare, S., Matthews, P. M., Brady, J. M., & Smith, S. M. (2003). Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magnetic Resonance in Medicine, 50(5), 1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Bengtsson, S. L., Nagy, Z., Skare, S., Forsman, L., Forssberg, H., & Ullén, F. (2005). Extensive piano practicing has regionally specific effects on white matter development. Nature Neuroscience, 8(9), 1148. [DOI] [PubMed] [Google Scholar]
- Bruininks, B. D. (2005). Bruininks‐Oseretsky test of motor proficiency: BOT‐2. NCS Pearson/AGS. [Google Scholar]
- Caminiti, R., Ghaziri, H., Galuske, R., Hof, P. R., & Innocenti, G. M. (2009). Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proceedings of the National Academy of Sciences of the United States of America, 106(46), 19551–19556. 10.1073/pnas.0907655106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock‐Heyman, L., Erickson, K. I., Holtrop, J. L., Voss, M. W., Pontifex, M. B., Raine, L. B., Hillman, C. H., & Kramer, A. F. (2014). Aerobic fitness is associated with greater white matter integrity in children. Frontiers in Human Neuroscience, 8, 584. 10.3389/fnhum.2014.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock‐Heyman, L., Erickson, K. I., Kienzler, C., Drollette, E. S., Raine, L. B., Kao, S.‐C., Bensken, J., Weisshappel, R., Castelli, D. M., Hillman, C. H., & Kramer, A. F. (2018). Physical activity increases white matter microstructure in children. Frontiers in Neuroscience, 12, 950. 10.3389/fnins.2018.00950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., Elavsky, S., Marquez, D. X., Hu, L., & Kramer, A. F. (2006). Aerobic exercise training increases brain volume in aging humans. Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(11), 1166–1170. 10.1093/gerona/61.11.1166 [DOI] [PubMed] [Google Scholar]
- Cole, T. J., & Lobstein, T. (2012). Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatric Obesity, 7(4), 284–294. 10.1111/j.2047-6310.2012.00064.x [DOI] [PubMed] [Google Scholar]
- Collins, A., & Koechlin, E. (2012). Reasoning, learning, and creativity: Frontal lobe function and human decision‐making. PLoS Biology, 10(3), e1001293. 10.1371/journal.pbio.1001293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruijn, A. G. M., Kostons, D. D. N. M., Van Der Fels, I. M. J., Visscher, C., Oosterlaan, J., Hartman, E., & Bosker, R. J. (2020). Effects of aerobic and cognitively‐engaging physical activity on academic skills: A cluster randomized controlled trial. Journal of Sports Sciences, 38(15), 1806–1817. 10.1080/02640414.2020.1756680 [DOI] [PubMed] [Google Scholar]
- de Greeff, J. W., Bosker, R. J., Oosterlaan, J., Visscher, C., & Hartman, E. (2018). Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta‐analysis. Journal of Science and Medicine in Sport, 21(5), 501–507. 10.1016/j.jsams.2017.09.595 [DOI] [PubMed] [Google Scholar]
- Deitz, J. C., Kartin, D., & Kopp, K. (2007). Review of the Bruininks‐Oseretsky test of motor proficiency, (BOT‐2). Physical & Occupational Therapy in Pediatrics, 27(4), 87–102. [PubMed] [Google Scholar]
- Desmond, J. E., Gabrieli, J. D., Wagner, A. D., Ginier, B. L., & Glover, G. H. (1997). Lobular patterns of cerebellar activation in verbal working‐memory and finger‐tapping tasks as revealed by functional MRI. Journal of Neuroscience, 17(24), 9675–9685. 10.1523/JNEUROSCI.17-24-09675.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A. (2000). Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development, 71(1), 44–56. 10.1111/1467-8624.00117 [DOI] [PubMed] [Google Scholar]
- Diamond, A., & Lee, K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333(6045), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman, R. K., Berthoud, H.‐R., Booth, F. W., Cotman, C. W., Edgerton, V. R., Fleshner, M. R., Gandevia, S. C., Gomez‐Pinilla, F., Greenwood, B. N., Hillman, C. H., Kramer, A. F., Levin, B. E., Moran, T. H., Russo‐Neustadt, A. A., Salamone, J. D., Van Hoomissen, J. D., Wade, C. E., York, D. A., & Zigmond, M. J. (2006). Neurobiology of exercise. Obesity, 14(3), 345–356. 10.1038/oby.2006.46 [DOI] [PubMed] [Google Scholar]
- Donnelly, J. E., Hillman, C. H., Castelli, D., Etnier, J. L., Lee, S., Tomporowski, P., & Szabo‐Reed, A. N. (2016). Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Medicine and Science in Sports and Exercise, 48(6), 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrby, T. B., Lundell, H., Burke, M. W., Reislev, N. L., Paulson, O. B., Ptito, M., & Siebner, H. R. (2014). Interpolation of diffusion weighted imaging datasets. Neuroimage, 103, 202–213. 10.1016/j.neuroimage.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Esteban‐Cornejo, I., Rodriguez‐Ayllon, M., Verdejo‐Roman, J., Cadenas‐Sanchez, C., Mora‐Gonzalez, J., Chaddock‐Heyman, L., Raine, L. B., Stillman, C. M., Kramer, A. F., Erickson, K. I., Catena, A., Ortega, F. B., & Hillman, C. H. (2019). Physical fitness, white matter volume and academic performance in children: findings from the ActiveBrains and FITKids2 projects. Frontiers in Psychology, 10, 208. 10.3389/fpsyg.2019.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J., McCandliss, B. D., Sommer, T., Raz, A., & Posner, M. I. (2002). Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience, 14(3), 340–347. 10.1162/089892902317361886 [DOI] [PubMed] [Google Scholar]
- Field, A. (2013). Discovering statistics using IBM SPSS statistics. Sage. [Google Scholar]
- Gabel, L., Ridgers, N. D., Della Gatta, P. A., Arundell, L., Cerin, E., Robinson, S., Daly, R. M., Dunstan, D. W., & Salmon, J. (2016). Associations of sedentary time patterns and TV viewing time with inflammatory and endothelial function biomarkers in children. Pediatric Obesity, 11(3), 194–201. 10.1111/ijpo.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2017). Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach. Guilford Publications. [Google Scholar]
- Haywood, K. M., & Getchell, N. (2019). Life span motor development. Human Kinetics. [Google Scholar]
- Hofer, S., & Frahm, J. (2006). Topography of the human corpus callosum revisited—Comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage, 32(3), 989–994. 10.1016/j.neuroimage.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Hyde, K. L., Lerch, J., Norton, A., Forgeard, M., Winner, E., Evans, A. C., & Schlaug, G. (2009). Musical training shapes structural brain development. Journal of Neuroscience, 29(10), 3019–3025. 10.1523/JNEUROSCI.5118-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen, I., & LeBlanc, A. G. (2010). Systematic review of the health benefits of physical activity and fitness in school‐aged children and youth. International Journal of Behavioral Nutrition and Physical Activity, 7(1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M., Beckmann, C. F., Behrens, T. E., Woolrich, M. W., & Smith, S. M. (2012). Fsl. Neuroimage, 62(2), 782–790. [DOI] [PubMed] [Google Scholar]
- Johnson, N. F., Kim, C., Clasey, J. L., Bailey, A., & Gold, B. T. (2012). Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage, 59(2), 1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. K., Knösche, T. R., & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. Neuroimage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Jones, T. A., Chu, C. J., Grande, L. A., & Gregory, A. D. (1999). Motor skills training enhances lesion‐induced structural plasticity in the motor cortex of adult rats. Journal of Neuroscience, 19(22), 10153–10163. 10.1523/JNEUROSCI.19-22-10153.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels, R. P., Van Zandvoort, M. J., Postma, A., Kappelle, L. J., & De Haan, E. H. (2000). The Corsi block‐tapping task: Standardization and normative data. Applied Neuropsychology, 7(4), 252–258. 10.1207/S15324826AN0704_8 [DOI] [PubMed] [Google Scholar]
- Kiphard, E. J., & Schilling, F. (2007). Körperkoordinationstest für kinder: KTK. Beltz‐Test. [PubMed] [Google Scholar]
- Konigs, M., Heij, H. A., van der Sluijs, J. A., Vermeulen, R. J., Goslings, J. C., Luitse, J. S. K., Poll‐The, B. T., Beelen, A., van der Wees, M., Kemps, R. J. J. K., Catsman‐Berrevoets, C. E., & Oosterlaan, J. (2015). Pediatric traumatic brain injury and attention deficit. Pediatrics, 136(3), 534–541. 10.1542/peds.2015-0437 [DOI] [PubMed] [Google Scholar]
- Krafft, C. E., Schaeffer, D. J., Schwarz, N. F., Chi, L., Weinberger, A. L., Pierce, J. E., Rodrigue, A. L., Allison, J. D., Yanasak, N. E., Liu, T., Davis, C. L., & McDowell, J. E. (2014). Improved frontoparietal white matter integrity in overweight children is associated with attendance at an after‐school exercise program. Developmental Neuroscience, 36(1), 1–9. 10.1159/000356219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsrud, S. K., Fjell, A. M., Tamnes, C. K., Grydeland, H., Due‐Tønnessen, P., Bjørnerud, A., Sampaio‐Baptista, C., Andersson, J., Johansen‐Berg, H., & Walhovd, K. B. (2018). Development of white matter microstructure in relation to verbal and visuospatial working memory—A longitudinal study. PLoS ONE, 13(4), e0195540. 10.1371/journal.pone.0195540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture, Y., & Cousineau, D. (2008). How to use MATLAB to fit the ex‐Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology, 4(1), 35–45. 10.20982/tqmp.04.1.p035 [DOI] [Google Scholar]
- Langevin, L. M., MacMaster, F. P., Crawford, S., Lebel, C., & Dewey, D. (2014). Common white matter microstructure alterations in pediatric motor and attention disorders. Journal of Pediatrics, 164(5), 1157–1164.e1151. 10.1016/j.jpeds.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Le Bihan, D., Mangin, J. F., Poupon, C., Clark, C. A., Pappata, S., Molko, N., & Chabriat, H. (2001). Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging, 13(4), 534–546. 10.1002/jmri.1076 [DOI] [PubMed] [Google Scholar]
- Lebel, C., Walker, L., Leemans, A., Phillips, L., & Beaulieu, C. (2008). Microstructural maturation of the human brain from childhood to adulthood. Neuroimage, 40(3), 1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Leger, L. A., Mercier, D., Gadoury, C., & Lambert, J. (1988). The multistage 20 metre shuttle run test for aerobic fitness. Journal of Sports Sciences, 6(2), 93–101. 10.1080/02640418808729800 [DOI] [PubMed] [Google Scholar]
- Logan, G. D. (1994). On the ability to inhibit thought and action: A users' guide to the stop signal paradigm. San Diego, CA: Academic Press. [Google Scholar]
- Malina, R. M., Bouchard, C., & Bar‐Or, O. (2004). Growth, maturation, and physical activity. Human Kinetics. [Google Scholar]
- Marks, B., Katz, L., Styner, M., & Smith, J. (2011). Aerobic fitness and obesity: Relationship to cerebral white matter integrity in the brain of active and sedentary older adults. British Journal of Sports Medicine, 45(15), 1208–1215. 10.1136/bjsm.2009.068114 [DOI] [PubMed] [Google Scholar]
- Meijer, A., Königs, M., de Bruijn, A. G. M., Visscher, C., Bosker, R. J., Hartman, E., & Oosterlaan, J. (2020). Cardiovascular fitness and executive functioning in primary school‐aged children. Developmental Science, 24(2), e13019. 10.1111/desc.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer, A., Königs, M., van der Fels, I. M. J., Visscher, C., Bosker, R. J., Hartman, E., & Oosterlaan, J. (2020). The effects of aerobic versus cognitively demanding exercise interventions on executive functioning in school‐aged children: A cluster‐randomized controlled trial. Journal of Sport & Exercise Psychology, 43(1), 1–13. 10.1123/jsep.2020-0034 [DOI] [PubMed] [Google Scholar]
- Meijer, A., Königs, M., Vermeulen, G. T., Visscher, C., Bosker, R. J., Hartman, E., & Oosterlaan, J. (2020). The effects of physical activity on brain structure and neurophysiological functioning in children: A systematic review and meta‐analysis. Developmental Cognitive Neuroscience, 45, 100828. 10.1016/j.dcn.2020.100828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutley, S. B., Söderqvist, S., Bryde, S., Humphreys, K., & Klingberg, T. (2009). Measuring working memory capacity with greater precision in the lower capacity ranges. Developmental Neuropsychology, 35(1), 81–95. 10.1080/87565640903325741 [DOI] [PubMed] [Google Scholar]
- Ooijendijk, W., Wendel‐Vos, W., & De Vries, S. (2007). Consensus Vragenlijsten Sport en Bewegen. TNO Kwaliteit van Leven. [Google Scholar]
- Opel, N., Martin, S., Meinert, S., Redlich, R., Enneking, V., Richter, M., Goltermann, J., Johnen, A., Dannlowski, U., & Repple, J. (2019). White matter microstructure mediates the association between physical fitness and cognition in healthy, young adults. Scientific Reports, 9(1), 1–9. 10.1038/s41598-019-49301-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, F. B., Ruiz, J. R., Hurtig‐Wennlöf, A., & Sjöström, M. (2008). Physically active adolescents are more likely to have a healthier cardiovascular fitness level independently of their adiposity status. The European youth heart study. Revista Espanola de Cardiologia, 61(2), 123–129. [PubMed] [Google Scholar]
- Park, H.‐J., & Friston, K. (2013). Structural and functional brain networks: From connections to cognition. Science, 342(6158), 1238411. 10.1126/science.1238411 [DOI] [PubMed] [Google Scholar]
- Peters, B. D., Szeszko, P. R., Radua, J., Ikuta, T., Gruner, P., DeRosse, P., Zhang, J.‐P., Giorgio, A., Qiu, D., Tapert, S. F., Brauer, J., Asato, M. R., Khong, P. L., James, A. C., Gallego, J. A., & Malhotra, A. K. (2012). White matter development in adolescence: Diffusion tensor imaging and meta‐analytic results. Schizophrenia Bulletin, 38(6), 1308–1317. 10.1093/schbul/sbs054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves, L., Broeder, C. E., Kennedy‐Honeycutt, L., East, C., & Matney, L. (1999). Relationship of fitness and gross motor skills for five‐to six‐yr.‐old children. Perceptual and Motor Skills, 89(3), 739–747. 10.2466/pms.1999.89.3.739 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Ayllon, M., Derks, I. P. M., van den Dries, M. A., Esteban‐Cornejo, I., Labrecque, J. A., Yang‐Huang, J., Raat, H., Vernooij, M. W., White, T., Ortega, F. B., Tiemeier, H., & Muetzel, R. L. (2020). Associations of physical activity and screen time with white matter microstructure in children from the general population. Neuroimage, 205, 116258. 10.1016/j.neuroimage.2019.116258 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Ayllon, M., Esteban‐Cornejo, I., Verdejo‐Román, J., Muetzel, R. L., Mora‐Gonzalez, J., Cadenas‐Sanchez, C., Plaza‐Florido, A., Molina‐Garcia, P., Kramer, A. F., Catena, A., & Ortega, F. B. (2020). Physical fitness and white matter microstructure in children with overweight or obesity: The ActiveBrains project. Scientific Reports, 10(1), 12469. 10.1038/s41598-020-67996-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland, T. W. (2007). Evolution of maximal oxygen uptake in children. In Tomkinson G. R. & Olds T. S. (Eds.), Pediatric fitness. Secular trends and geographic variability (Vol. 50, pp. 200–209). Basel: Karger Publishers. [DOI] [PubMed] [Google Scholar]
- Rueda, M. R., Fan, J., McCandliss, B. D., Halparin, J. D., Gruber, D. B., Lercari, L. P., & Posner, M. I. (2004). Development of attentional networks in childhood. Neuropsychologia, 42(8), 1029–1040. 10.1016/j.neuropsychologia.2003.12.012 [DOI] [PubMed] [Google Scholar]
- Ruotsalainen, I., Gorbach, T., Perkola, J., Renvall, V., Syväoja, H. J., Tammelin, T. H., Karvanen, J., & Parviainen, T. (2020). Physical activity, aerobic fitness, and brain white matter: Their role for executive functions in adolescence. Developmental Cognitive Neuroscience, 42, 100765. 10.1016/j.dcn.2020.100765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis, J. F., McKenzie, T. L., Alcaraz, J. E., Kolody, B., Faucette, N., & Hovell, M. F. (1997). The effects of a 2‐year physical education program (SPARK) on physical activity and fitness in elementary school students. Sports, Play and Active Recreation for Kids. American Journal of Public Health, 87(8), 1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse, T. A. (2005). Relations between cognitive abilities and measures of executive functioning. Neuropsychology, 19(4), 532–545. 10.1037/0894-4105.19.4.532 [DOI] [PubMed] [Google Scholar]
- Schaeffer, D. J., Krafft, C. E., Schwarz, N. F., Chi, L., Rodrigue, A. L., Pierce, J. E., Allison, J. D., Yanasak, N. E., Liu, T., Davis, C. L., & McDowell, J. E. (2014). An 8‐month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology, 51(8), 728–733. 10.1111/psyp.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, J., Klein, M. C., Behrens, T. E., & Johansen‐Berg, H. (2009). Training induces changes in white‐matter architecture. Nature Neuroscience, 12(11), 1370–1371. 10.1038/nn.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A. S., Saliasi, E., van den Berg, V., Uijtdewilligen, L., de Groot, R. H. M., Jolles, J., Andersen, L. B., Bailey, R., Chang, Y.‐K., Diamond, A., Ericsson, I., Etnier, J. L., Fedewa, A. L., Hillman, C. H., McMorris, T., Pesce, C., Pühse, U., Tomporowski, P. D., & Chinapaw, M. J. M. (2018). Effects of physical activity interventions on cognitive and academic performance in children and adolescents: A novel combination of a systematic review and recommendations from an expert panel. British Journal of Sports Medicine, 53(10), 640–647. 10.1136/bjsports-2017-098136 [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–155. 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M., Jenkinson, M., Johansen‐Berg, H., Rueckert, D., Nichols, T. E., Mackay, C. E., Watkins, K. E., Ciccarelli, O., Cader, M. Z., Matthews, P. M., & Behrens, T. E. J. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage, 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen‐Berg, H., Bannister, P. R., De Luca, M., Drobnjak, I., Flitney, D. E., Niazy, R. K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J. M., & Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Song, S.‐K., Sun, S.‐W., Ju, W.‐K., Lin, S.‐J., Cross, A. H., & Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage, 20(3), 1714–1722. 10.1016/j.neuroimage.2003.07.005 [DOI] [PubMed] [Google Scholar]
- Song, S.‐K., Sun, S.‐W., Ramsbottom, M. J., Chang, C., Russell, J., & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage, 17(3), 1429–1436. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Statistics Netherlands . (2006). Standaard onderwijsindeling. www.cbs.nl/nl‐NL/menu/methoden/classificaties/overzicht/soi/2006/default.htm [Google Scholar]
- Stodden, D. F., Goodway, J. D., Langendorfer, S. J., Roberton, M. A., Rudisill, M. E., Garcia, C., & Garcia, L. E. (2008). A developmental perspective on the role of motor skill competence in physical activity: An emergent relationship. Quest, 60(2), 290–306. 10.1080/00336297.2008.10483582 [DOI] [Google Scholar]
- Stodden, D., Langendorfer, S., & Roberton, M. A. (2009). The association between motor skill competence and physical fitness in young adults. Research Quarterly for Exercise and Sport, 80(2), 223–229. 10.1080/02701367.2009.10599556 [DOI] [PubMed] [Google Scholar]
- Swain, R. A., Harris, A. B., Wiener, E. C., Dutka, M. V., Morris, H. D., Theien, B. E., Konda, S., Engberg, K., Lauterbur, P. C., & Greenough, W. T. (2003). Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience, 117(4), 1037–1046. 10.1016/S0306-4522(02)00664-4 [DOI] [PubMed] [Google Scholar]
- Taubert, M., Draganski, B., Anwander, A., Müller, K., Horstmann, A., Villringer, A., & Ragert, P. (2010). Dynamic properties of human brain structure: Learning‐related changes in cortical areas and associated fiber connections. Journal of Neuroscience, 30(35), 11670–11677. 10.1523/JNEUROSCI.2567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay, M. S., LeBlanc, A. G., Kho, M. E., Saunders, T. J., Larouche, R., Colley, R. C., Goldfield, G., & Gorber, S. (2011). Systematic review of sedentary behaviour and health indicators in school‐aged children and youth. International Journal of Behavioral Nutrition and Physical Activity, 8(1), 98. 10.1186/1479-5868-8-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenborghs, S. R., Noetel, M., Hillman, C. H., Nilsson, M., Smith, J. J., Ortega, F. B., & Lubans, D. R. (2019). The impact of physical activity on brain structure and function in youth: A systematic review. Pediatrics, 144(4), e20184032. 10.1542/peds.2018-4032 [DOI] [PubMed] [Google Scholar]
- van der Fels, I. M. J., de Bruijn, A. G. M., Renken, R. J., Königs, M., Meijer, A., Oosterlaan, J., Kostons, D. D. N. M., Visscher, C., Bosker, R. J., Smith, J., & Hartman, E. (2020). Relationships between gross motor skills, cardiovascular fitness, and visuospatial working memory‐related brain activation in 8‐ to 10‐year‐old children. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 842–858. 10.3758/s13415-020-00805-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fels, I. M. J., Hartman, E., Bosker, R. J., de Greeff, J. W., de Bruijn, A. G. M., Meijer, A., Oosterlaan, J., Smith, J., & Visscher, C. (2020). Effects of aerobic exercise and cognitively engaging exercise on cardiorespiratory fitness and motor skills in primary school children: A cluster randomized controlled trial. Journal of Sports Sciences, 38(17), 1975–1983. 10.1080/02640414.2020.1765464 [DOI] [PubMed] [Google Scholar]
- van der Fels, I. M. J., Smith, J., de Bruijn, A. G. M., Bosker, R. J., Königs, M., Oosterlaan, J., Visscher, C., & Hartman, E. (2019). Relations between gross motor skills and executive functions, controlling for the role of information processing and lapses of attention in 8–10 year old children. PLoS ONE, 14(10), e0224219. 10.1371/journal.pone.0224219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fels, I. M. J., te Wierike, S. C., Hartman, E., Elferink‐Gemser, M. T., Smith, J., & Visscher, C. (2015). The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: A systematic review. Journal of Science and Medicine in Sport, 18(6), 697–703. 10.1016/j.jsams.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Vaynman, S., & Gomez‐Pinilla, F. (2006). Revenge of the “sit”: How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. Journal of Neuroscience Research, 84(4), 699–715. 10.1002/jnr.20979 [DOI] [PubMed] [Google Scholar]
- Vazou, S., Pesce, C., Lakes, K., & Smiley‐Oyen, A. (2019). More than one road leads to Rome: A narrative review and meta‐analysis of physical activity intervention effects on cognition in youth. International Journal of Sport and Exercise Psychology, 17(2), 153–178. 10.1080/1612197X.2016.1223423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen, F., & Logan, G. D. (2009). Models of response inhibition in the stop‐signal and stop‐change paradigms. Neuroscience & Biobehavioral Reviews, 33(5), 647–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgh, L., Scherder, E. J. A., van Lange, P. A. M., & Oosterlaan, J. (2014). Executive functioning in highly talented soccer players. PLoS ONE, 9(3), e91254. 10.1371/journal.pone.0091254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volksgezondheid en zorg . (2018). Huidige situatie overgewicht kinderen. https://www.volksgezondheidenzorg.info/onderwerp/overgewicht/cijfers‐context/huidige‐situatie#node‐overgewicht‐kinderen [Google Scholar]
- Voss, M. W. (2016). Chapter 9—The chronic exercise‐cognition interaction: fMRI research. In McMorris T. (Ed.), Exercise‐cognition interaction (pp. 187–209). Academic Press. [Google Scholar]
- Wakana, S., Jiang, H., Nagae‐Poetscher, L. M., Van Zijl, P. C., & Mori, S. (2004). Fiber tract–based atlas of human white matter anatomy. Radiology, 230(1), 77–87. 10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (1991). WISC‐III: Wechsler intelligence scale for children: Manual. Psychological Corporation. [Google Scholar]
- Winkler, A. M., Ridgway, G. R., Webster, M. A., Smith, S. M., & Nichols, T. E. (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., Zhu, L.‐N., Dong, X.‐x., Wang, W., Yan, J., & Chen, A.‐G. (2018). Aerobic exercise intervention alters executive function and white matter integrity in deaf children: A randomized controlled study. Neural Plasticity, 2018, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE A1 Inclusion protocol of DTI measurements separated for Sex and Scanning Site
TABLE A2 Results of principal component analysis on the neurocognitive functioning measures
Transparent Peer Review Report
Transparent Science Questionnaire for Authors
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


