Abstract
Owing to the rise of ART and mounting reports of epigenetic modification associated with them, an understanding of optimal embryo culture conditions and reliable indicators of embryo quality are highly sought after. There is a growing body of evidence that mechanical biomarkers can rival embryo morphology as an early indicator of developmental potential and that biomimetic mechanical cues can promote healthy development in preimplantation embryos. This review will summarize studies that investigate the role of mechanics as both indicators and promoters of mammalian preimplantation embryo development and evaluate their potential for improving future embryo culture systems.
Keywords: preimplantation, embryo, zona pellucida, mechanobiology, shear stress, substrate stiffness, microfluidics, biophysics, IVF, hatching
Introduction
ART is responsible for roughly 1.7% of infants born every year in the USA and since 1978 over 8 million babies have been born through IVF worldwide (Kamel, 2013; Harper et al., 2018). Despite being a remarkable accomplishment, the metric of live births for a determination of embryo culture efficacy is parochial as babies born through ART have higher rates of epigenetic disorders and other abnormalities including Beckwith–Wiedemann syndrome (BWS) (DeBaun et al., 2003; Maher et al., 2003; Sutcliffe et al., 2006; Doornbos et al., 2007), Angelman syndrome (De Rycke et al., 2002; Ludwig et al., 2005; Doornbos et al., 2007) and Silver–Russell syndrome (Kagami et al., 2007; Chiba et al., 2013; Cocchi et al., 2013). For example, the prevalence of BWS in ART is reported to be around 4.6%, while the background rate in the USA is around 0.8% (DeBaun et al., 2003). Such epigenetic alterations, commonly linked with suboptimal embryo culture conditions, are unsurprising in ART as preimplantation embryos undergo sensitive but critical epigenetic reprogramming, especially in imprint genes (Ventura-Juncá et al., 2015). This creates a window of opportunity for environmental conditions to alter the reprogramming process and impact development.
Over the past few decades, embryo culture media, incubation/observation systems and oxygen level controls have been drastically improved. However, the persistence of the abovementioned issues leads researchers to study other potential contributing factors. It has been found that mechanical cues influence gene expression during late embryogenesis (Farge, 2003; Desprat et al., 2008; Desmond et al., 2014). Eroshkin and Zaraisky (2017) have recently published a thorough review on this topic. Moreover, there is now a growing body of evidence that mechanics is also relevant in preimplantation embryo and oocyte development and may have the potential to augment clinical embryo culture (Matsuura et al., 2010; Kolahi et al., 2012). These findings have led to the development of novel embryo culture platforms taking those biomechanical cues into consideration. On the other hand, the biomechanical properties of embryos, such as stiffness, viscosity and cavity pressure, have been utilized as ‘mechanical biomarkers’ for evaluating their quality. These works clearly demonstrate the importance of understanding the role of these biophysical properties in early embryo development.
It is well known that mammalian cells can perceive mechanical cues and output biochemical responses, a process termed mechanotransduction (Sun et al., 2012a). It has heavily contributed to an understanding of many processes by which cells interact with their surroundings including stem cell differentiation, cancer metastasis, bone remodeling and cell migration (Duncan and Turner, 1995; McGrail et al., 2014; Mao et al., 2016; Hadden et al., 2017). We and others have also extensively characterized the mechanosensitivity of embryonic stem cells (Chowdhury et al., 2010; Keung et al., 2012; Sun et al., 2012b, 2014). However, the means by which embryos respond to various mechanical stimuli, including substrate mechanics and shear stresses, might be very different from adherent mammalian cells. This is because a preimplantation embryo is contained within a thin and relatively stiff, glycoprotein shell called the zona pellucida (ZP) and thus cannot adhere to the tissue with which it comes in contact (Croxatto, 2002). In contrast to the mechanosensing of most adherent mammalian cells, integrin-based matrix stiffness or shear stress sensing may not play a dominant role in preimplantation embryos. Also, the embryo’s migration through the oviduct is completely dependent on external forces as it remains still during in vitro culture conditions (Croxatto, 2002). These unique features have been largely overlooked in current mechanobiology research and, therefore, more fundamental studies are needed to elucidate the mechanisms for the mechanosensitivity of preimplantation embryos. A thorough understanding of the connection between the mechanical properties of the embryos and their microenvironment and the development of preimplantation embryos will not only lead to novel ways to assess the quality of embryos cultured in vitro, but also guide the design of the next generation embryo culture platform to improve the overall success rate of IVF and reduce the occurrence of related epigenetic disorders.
In this review, we first discussed the current characterizations of the physical environment of the fallopian tubes, and then discussed the two potential applications of biomechanical cues, i.e., assessing and improving the embryo quality. We further discussed current available technologies in promoting embryo culture quality and pinpointed the future directions for both fundamental studies and technology development. In our view, the essential next step is to elucidate the unique mechanosensation mechanisms for embryos and develop integrated devices for simultaneous control and monitoring the biophysical and biochemical microenvironment.
Physical environment of the fallopian tubes
Studies of the fallopian tube mechanical environment will provide a basis for the optimization of in vitro culture. Though the dynamics of tubal transport have received attention in recent studies (Croxatto et al., 1978; Pandey and Chaube, 2010; Narla et al., 2019), the resulting mechanics that describe the deformation and provide mechanobiological insights have not been thoroughly evaluated. In this section, we review studies that investigate the dynamics and mechanics of tubal transport and further assess how this information can be used to guide the design of embryo culture systems.
In humans, tubal transport is reported to take roughly 80 h (Croxatto et al., 1978). During this time, there are a variety of factors that influence the ovulated oocyte and developing embryo after fertilization. In many aspects, the conditions within the fallopian tubes are spatially distinct. As a preimplantation embryo passes through the fallopian tube, it encounters changes in nutrients, cytokines, hormones, growth factors and pH (Feuer and Rinaudo, 2012). Though the uterine wall is known to act as a peristaltic pump that pushes the embryo through the fallopian tube, mechanical conditions fluctuate within the fallopian tubes as well (Pauerstein, 1979).
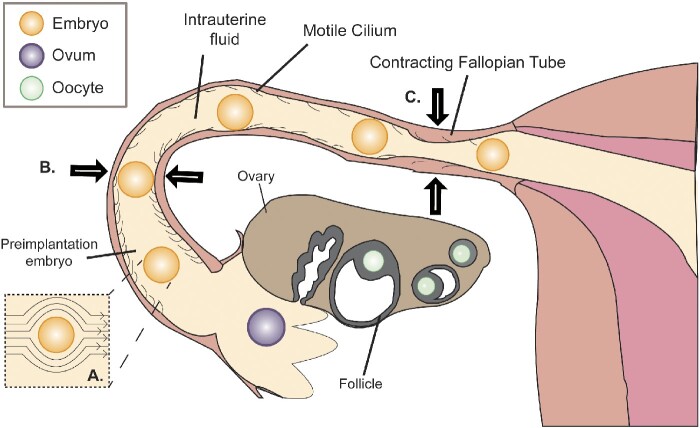
The ampulla is the largest section of the fallopian tube in humans and ranges from 5 to 8 cm in length (Fig. 1) (Pauerstein, 1979). Despite spanning only 70% of the length of the fallopian tube, the ampulla houses the embryo for over 90% of the duration of tubal transport (Croxatto and Ortiz, 1975; Croxatto et al., 1978). Interestingly, fertilization is reported to take place in the ampullary–isthmic junction; yet for some reason, the developing embryo spends the next 72 h in the ampulla (Croxatto et al., 1978). One theory that could explain this is the partial obstruction of the isthmus by isthmic toning and dense mucus; both of which are later assuaged by the effects of progesterone and the downregulation of α-adrenergic receptors (Jansen, 1978). While an embryo is trapped within the ampulla, it experiences flow from episodic contractions of oviductal smooth muscles and the beating of motile cilia (Halbert et al., 1976; Talo and Pulkkinen, 1982). The episodic contractions act as peristaltic waves that propagate in both axial directions, therefore it is likely that the embryo is being mixed back and forth rather than continuously pushed against any obstruction. Although a developing embryo spends far less time in the isthmus than the ampulla, there is value in studying the mechanical conditions in both sections. The ampullary–isthmic junction and the uterotubal junction are the two sites within the fallopian tubes where sustained tonic contractions are localized (Ezzati et al., 2014). Therefore, the isthmus likely serves as the venue for the transient arrest of gametes or an embryo, during which the episodic contractions can mix the tubal passengers.
Figure 1.
Illustration of the major contributors of the mechanical conditions during tubal transport. (A) Depiction of intrauterine fluid flow profile around an embryo resulting in shear stress. (B) The embryo is shown to be lightly squeezed by the soft but tight-fitting isthmus. (C) Fallopian tubes undergoing peristaltic contractions resulting in the flow of intrauterine fluid.
Notably, the lumen diameter of the isthmus is relatively small with some sections being roughly equal to the diameter of a human embryo (Fig. 1). This may cause additional shear stress and pressure (Pauerstein, 1979). In addition, the substrate stiffness that a preimplantation embryo would encounter in vivo is between 101 and 103 Pa (Halbert et al., 1976; Talo and Pulkkinen, 1982), in sharp contrast to in vitro embryo culture conditions (∼109 Pa). It should be acknowledged that fallopian tubes also vary by person. Certain factors, such as smoking, age, hormonal imbalances and sexually transmitted diseases, all of which are known to increase the risk of infertility and ectopic pregnancy, influence the mechanical conditions of the fallopian tubes as well (Feuer and Rinaudo, 2012). Though much is known about how factors like cotinine and progesterone can attenuate the contractile strength of the fallopian tubes, it is unclear how this ultimately affects tubal transport as the relative strength of each method of actuation is not well-defined. It has been suggested that motile cilia alone are responsible for propelling an embryo through the fallopian tube, however, there have been reported cases of individuals with primary ciliary dyskinesia successfully achieving pregnancies (Halbert et al., 1976; Afzelius et al., 1978; Pedersen, 1983; Lurie et al., 1989). Therefore, more research is required on what the individual contributions are from each type of actuator before reliable conclusions can be made on the effect of any individual risk factor on tubal motility.
Complementing experimental investigations, mathematical models have been developed to aid in the understanding of oviductal fluid mechanics (Eytan et al., 2001; Pandey and Chaube, 2010). As there are innumerable technological and ethical boundaries that impede in vivo tubal transport experimentation in humans and many relevant parameters, such as oviduct dimensions and flow rate, are different within animal models, modeling and simulation are very useful tools in understanding flow conditions in the fallopian tubes. Recently, Narla et al. (2019) developed a mathematical model to investigate the effects that electro-osmosis can have on a viscoelastic (Jeffrey) fluid in a tapered channel designed to mimic the uterotubal junction. The findings of the authors suggest that electro-osmosis within the uterus may be strong enough to generate retrograde flows (Narla et al., 2019). Models such as this have great potential to demystify how data from animal fertility models can be extrapolated to the fallopian tubes. Despite ethical considerations in testing computer modeling and simulation results against human tubal transport via experimentation, validation with biomimetic and artificial oviduct systems is quite feasible. This emphasizes the need for engineering systems that can mimic in vivo conditions using biomaterials.
Mechanical biomarkers in oocyte and embryo development
Emerging evidence shows that some mechanical properties of the oocyte and embryo, including stiffness and thickness of oocyte ZP, viscosity of oocyte cytoplasm, and cavity pressure in the embryo, can be utilized as ‘mechanical biomarkers’ for evaluating their quality. While conventionally the change in these mechanical properties might be viewed as consequences of undesirable biochemical events, it is also possible that the changes in mechanical properties directly impact the development of oocytes and embryos. This section reviews current knowledge in this aspect and calls for more in-depth studies to establish the independent roles of mechanical and biochemical cues in embryo development.
Fluctuations in the mechanical properties of the oocyte ZP
Because the ZP is a thin shell that protects an embryo, the mechanical stiffness of ZP provides vital information on how well it can protect against stresses. It is well established that, upon fertilization, the ZP experiences a ‘hardening’, which is caused by cortical granule exocytosis (Boccaccio et al., 2012). Despite being called a ‘hardening’, the phenomenon was initially not at all regarded as a mechanical change, but an increase resistance to biochemical dissolution (Braden et al., 1954). However, it has been demonstrated with atomic force microscopy that this ‘hardening’ also results in an increase in elastic modulus values across many species of embryos (Fig. 2A). For example, measurements in mouse and bovine embryos show a significant increase in elastic modulus after fertilization (Papi et al., 2012). This stiffness increase is currently attributed to an increased amount of inter-filament cross-links within the ZP (Green, 1997; Boccaccio et al., 2012). It has been reported that inadequate mechanical hardening, caused by insufficient cortical granule release in the oocyte, is an indicator of low developmental potential in the zygote (Yanez et al., 2016).
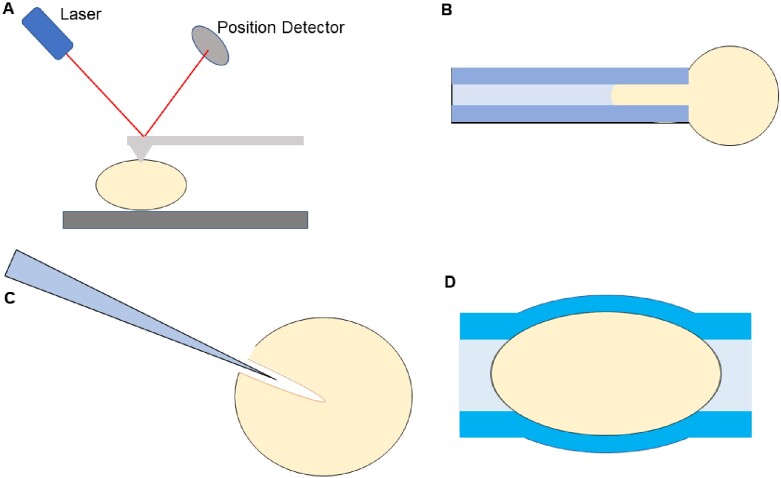
Figure 2.
Various methods of sampling potential mechanical biomarkers in preimplantation embryos. (A) Depiction of atomic force microscopy to determine the changing stiffness values of the zona pellucida (ZP) during preimplantation development. (B) Simple schematic of an automated micropipette aspirator to gauge viscoelastic properties of embryos. (C) Schematic portraying the persistence of the microinjection funnel left by a micropipette. This technique was used to make measurements of oocyte cytoplasmic viscosity. (D) Deformable hydrogel assay used for measuring the internal pressure within preimplantation embryos.
It is important to note that the biochemical and mechanical ‘hardening’ are not necessarily tethered. In fact, during IVF, bovine oocytes have a high incidence of polyspermy that is thought to be due to the lack of both exposures to oviduct-specific glycoprotein-heparin complexes and any increases in proteolysis resistance. Despite missing the hallmarks of biochemical ‘hardening’, mechanical stiffness increases significantly in bovine oocytes after fertilization (Papi et al., 2010, 2012). Therefore, further research is required to understand what assessments can be made from the mechanical stiffness of the ZP.
Variation in ZP stiffness neither begins nor ends at fertilization. There is a significant softening of the ZP in mature oocytes that is believed to be conducive to fertilization (Murayama et al., 2013). After fertilization and the associated ZP ‘hardening’, the stiffness of the ZP is found to decrease as the embryo develops (Murayama et al., 2006). A collection of the measured elastic modulus values can be found in Table I. Interestingly, embryos are known to become less sensitive to shear-induced apoptosis as they develop, but it is unclear if the decrease in mechanosensitivity is at all coupled with the softening of the ZP (Xie et al., 2006).
Table I.
Stiffness of the mouse ZP measured at various stages of embryo development.
| Stage of development | Elastic modulus of ZP (kPa) | Sample size (n) |
|---|---|---|
| Germinal vesicle | 22.8 ± 10.4 | 30 |
| Metaphase-II | 8.26 ± 5.22 | 74 |
| Pronuclear | 22.3 ± 10.5 | 66 |
| 2-cell | 13.8 ± 3.54 | 41 |
| 4-cell | 12.6 ± 3.34 | 19 |
| 8-cell | 5.97 ± 4.97 | 6 |
| Morulae | 1.88 ± 1.34 | 8 |
| Early blastocyst | 3.39 ± 1.86 | 4 |
ZP, zona pellucida.
Values were recorded from a microtactile sensor experiment performed by Murayama et al (2006).
Mechanical characterization of the ZP is beginning to show potential as a marker of oocyte/embryo quality. In fact, Yanez et al. (2016) were recently able to establish a link between the viscoelastic properties of the ZP and the developmental potential of two pronuclear (2PN) stage human zygotes. Upon verification of their predictive mechanical markers, the authors identified differences in the transcriptomes of viable and nonviable zygotes predicted by a modified Zener model (Fig. 2B). This is a remarkable finding that demonstrates the potential of mechanical biomarkers to give an early indication of oocyte and embryo quality just hours after fertilization. The thickness of the ZP has also been linked to embryo quality (Balakier et al., 2012; Zhou et al., 2014). Experiments performed by Balakier et al. (2012) demonstrate a strong correlation between the grade of human embryos, according to a modified Veeck classification system, and the thickness of their ZP on day 3 of development. The authors noted that the grade 1 embryos had a thinner ZP than the grade 2-5 embryos, the grade 2 embryos had a thinner ZP than the grade 3–5 embryos and the ZP thickness values of the grade 3 embryos were indistinguishable from those assigned grade 4–5. This is strong evidence that there is a connection between ZP thickness and embryo quality, though the authors state categorically their findings suggest ZP thickness has no impact on implantation or pregnancy outcomes. Instead, the authors suggest that the ZP thickness is regulated by lytic factors within the embryo that cause ZP thinning. This is corroborated by prior studies that demonstrate trophectodermal projections induce ZP thinning/lysis in bovine, equine, hamster and human embryos by acting as carriers of key molecules into the ZP (Gonzales et al., 1996; Seshagiri et al., 2009).
Despite the controversy on the impact of ZP thickness in implantation and pregnancy, there is strong evidence that ZP thickness affects oocyte maturation in mouse oocytes (Zhou et al., 2014). Zhou et al. (2014) demonstrated that, among oocytes at the germinal vesicle (GV) stage, ZP thickness values 5 µm ≤ ZP < 8 µm correlated with the highest maturation potential. Furthermore, when the authors used acidic Tyrode solution to manually digest GV stage oocyte ZPs ≥ 8 µm and generate oocytes 5 µm ≤ ZP < 8 µm, they found increased maturation rates compared to untreated groups of oocytes with ZP thickness values < 8 µm. Also, Zhou et al. (2014) found the maturation rates of the treated group matched well with their observations in the untreated group with the same ZP thickness range. This finding suggests that there may be an optimal ZP thickness range for oocyte maturation, rather than the ZP thickness simply being a trivial byproduct of intrinsic oocyte activity. However, Zhou et al. (2014) do corroborate Balakier et al. (2012) with the finding that manually digesting the ZP does not impact embryo development.
Mechanical properties of the cytoplasm
The mechanical properties of the preimplantation embryo are not only dependent on the ZP but also the body within. The mechanical properties of the oolemma and oocyte have also been demonstrated to give an early indication of the quality of embryos. Research has shown that mechanical characteristics of the cytoplasm and oolemma of an oocyte are reliable markers of maturity and aging (Ebner et al., 2003; Krause et al., 2016; Yanez et al., 2016). Although the cytoplasm is more challenging to access than the ZP in oocytes, assaying certain mechanical properties can be done in parallel to procedures such as ICSI. Such is the case in a study performed by Krause et al. (2016), where they observed the formation and persistence of the injection funnel formed with a pipet 2–4 min after injection (Fig. 2C). They found that the cytoplasm of mature metaphase-II oocytes is much more viscous, and distinct viscosity also exists among these mature oocytes, indicating cell cycle is not the sole variant during the dynamics of cytoplasmic viscosity.
Measuring for mechanical biomarkers offers the advantage of testing an oocyte or embryo with a very low impact on quality. This is much unlike testing for polyspermy or resistance to dissolution by biochemical reagents. Remarkably, one can perform the ICSI procedure and inadvertently gauge the cytoplasmic viscosity by leaving an injection funnel. However, more research must be done on what can be inferred from these cytoplasmic biomarkers and how reliable they are in predicting quality. For example, it is critical to elucidate the transcriptional differences in oocytes with different viscosities.
Cavity pressure in embryos
ZP hatching is an event that it is vital to the implantation process. The timing is critical as late hatching can cause the window of uterine receptivity to be missed and early hatching can lead to apoptosis or ectopic pregnancy. ZP hatching has long been analyzed from the perspective of enzymatic activity, however Leonavicius et al. (2018) developed a hydrogel deformation assay to assess the role of mechanics in the hatching process of mouse blastocysts (Fig. 2D). The authors demonstrated a link between the hatching probability and the cavity pressure within embryonic day (E)3.5 embryos (Leonavicius et al., 2018). From this link, they developed a mechanical model of hatching probability. Furthermore, they compared the individual contribution of ZP enzymatic degradation and cavity pressure in the hatching process by culturing embryos in medium containing either 0.5 mg/ml collagenase I to erode the ZP or 150 mM sucrose to reduce the internal pressure. Their results showed the sucrose-treated group being 3 times less likely to hatch while the collagenase group being 3.8 times more likely to hatch, demonstrating that cavity pressure is a key player in ZP hatching. Interestingly, they also showed that cryopreservation decreases cavity pressure within mouse embryos and found that the consequence is around a 10-h delay in ZP hatching. The authors suggest that cavity pressure may serve as a reliable biomarker for embryo development, especially following the thawing of cryopreserved embryos.
Cavity pressure also seems to be a sensitive biomarker of embryo quality that is worthy of further research. This method of testing cavity pressure appears to be a promising method of assaying embryo quality as it is a completely external measurement that is minimally invasive.
Mechanotransduction in early development
As previously mentioned, the fallopian tubes provide a dynamic environment that applies a variety of forces on a developing embryo. Mechanical cues are present during in vitro embryo culture; however, they are much different both in magnitude and duration than expected in vivo and the consequences of this are not well defined. Also, it remains unclear if and how mechanical stimuli can impact key events within the embryo such as the maternal-to-zygotic transition, compaction, and polarization. This section reviews studies on mechanotransduction in early embryos and how the mechanical conditions of in vitro embryo culture may affect development.
Shear stress
Although embryos cultured in vitro are typically left still, handling an embryo with a thin pipet will inevitably cause excessive shear stress to the embryo. This is especially of concern when an embryo that has hatched from its ZP is being transferred for implantation. Such concerns have led researchers to investigate the effects of prolonged handling on embryo development. This section will summarize the studies that investigate embryonic responses to shear stress.
As previously mentioned in this review, large and prolonged shear stress on preimplantation embryos housed within the ZP can induce apoptosis in mouse embryos (Xie et al., 2006). Xie et al. (2006) found that this apoptosis was the consequence of increased phosphorylation of the mitogen-activated protein kinase (MAPK) 8/9 (formerly known as SAPK/JNK1/2), which reached a 350% increase after 12 h of exposure to 1.2 dyn/cm2 of shear stress in E3.5 blastocysts. The authors also suggest that the trophectoderm is responsible for sensing this shear stress as they witnessed a sudden increase in expression of the proto-oncogene FOS localized in the area when shear stress (1.2 dyn/cm2) was applied (Xie et al., 2006).
Although the trophectoderm was the site of increased phosphorylation of MAPK 8/9, the method of shear stress reaching those cells is unclear in experiments where the ZP was intact. The authors speculated that the shear contacts the trophectoderm through the ZP in one or more of the following ways: physical transduction, percolation of fluid or cytoplasmic projections of the trophectoderm that extend through the ZP (Xie et al., 2006).
Despite this being an interesting finding that demonstrates embryo mechanosensitivity within the ZP, the shear stress used by the authors is too large to be physiologically relevant. The authors did a separate experiment where they demonstrated upregulation of phosphorylated MAPK 8/9 and induction of FOS in a dose-dependent manner in response to pipetting (Xie et al., 2007). Although the shear stress was not measured, they used mouth pipetting for 30 s in each cycle and ensured that the same person performed all pipetting to establish a baseline. It was found that E4.5 embryos show significant upregulation after nine titrations whereas the E3.5 embryos show significant upregulation at about 15 titrations. The authors suggest this difference in sensitivity is related to the embryo being hatched from the ZP at E4.5, but not at E3.5. This study has obvious implications for the potential side effects of transferring blastocysts after ZP hatching and highlights the potential benefit of earlier embryo transfer.
It is important to note that gentle and continuous flows over embryos are generally considered beneficial (Talo and Pulkkinen, 1982; Pedersen, 1983; Lurie et al., 1989; Matsuura et al., 2010). For instance, Matsuura et al. tested the effects of a tilting embryo culture system (TECS) that applied shear stress of 0.007 dyn/cm2 to both mouse embryos at the 2-cell stage and human embryos in the 3- to 11-cell stage, resulting in an increased cell division rate in both species (Matsuura et al., 2010). Future studies will be needed to decouple the effects of augmenting the retention of the autocrine/paracrine growth factors and applying shear stress, and investigate changes in gene expression that result from these gentle flows and assess their potential to improve the health of offspring.
Substrate stiffness
The elastic modulus of a substrate has been reported to influence significant cellular behavior such as cancer cell migration and stem cell differentiation (McGrail et al., 2014; Wen et al., 2014). Recent evidence also shows that the pluripotency maintenance of embryonic stem cells (Chowdhury et al., 2010; Sun et al., 2012b) and trophoblast cell functions (Ma et al., 2020) can be regulated by substrate stiffness in vitro. Embryo culture is typically carried out in a stiff polystyrene petri dish (∼3 GPa). This polystyrene is 106 times stiffer than the uterine epithelium that the embryo would contact in vivo (Thie et al., 1998; Mathews and Hamilton, 2016). Kolahi et al. (2012) found that the blastocyst and hatching frequency in mouse embryos both increase when mouse zygotes are cultured on soft (∼1 kPa) collagen substrates. The authors state that projections of the trophectoderm may be responsible for the sensitivity to substrate stiffness in the mouse embryos (Gonzales et al., 1996). This conclusion is supported by the observation that the main differences are seen around the time these projections should be able to protrude through the ZP. Alternatively, the authors also suggest that the soft collagen’s shape conformation to the stiffer ZP may also yield developmental benefits (Fig. 3A). In parallel with embryo development, the ZP is becoming softer and it is important to note that even in static conditions the deformation profile of an embryo is likely changing because of the softening of its protective ZP.
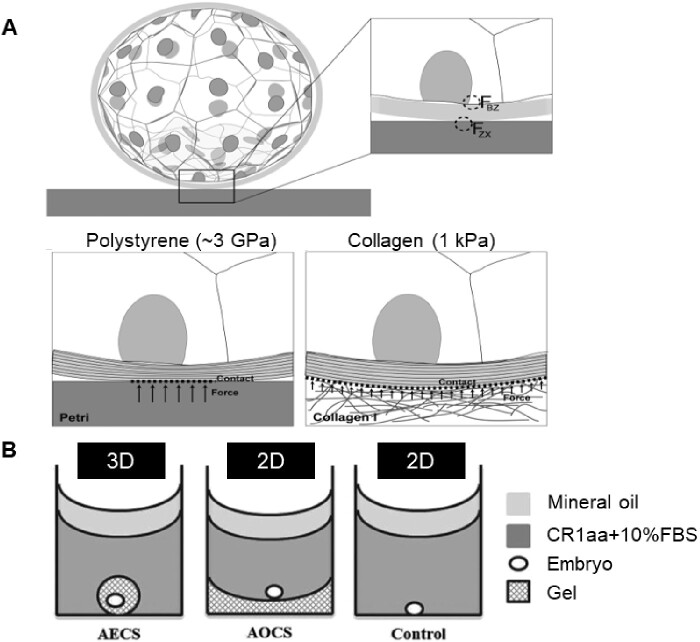
Figure 3.
Culturing embryos on substrates with different mechanical properties. (A) Schematic of the possible force distribution and resulting deformation of the ZP when in contact with a stiff polystyrene (3 GPa) (left) and a soft Collagen I (1 kPa) substrate (right). FZX is defined as the net force operating on the ZP because of the extra-embryonic environment; FBZ indicates the net force acting on the blastomeres and caused by the ZP. Adapted from Kolahi et al. (2012). (B) A schematic illustrating the 3D hydrogel culture systems for bovine embryos post-hatching, including the alginate encapsulation culture system (AECS), the alginate overlay culture system (AOCS) and the polystyrene control. CR1aa, Charles Rosenkrans medium + amino acids; FBS, fetal bovine serum. Reprinted with permissions from Zhao et al. (2015).
Despite the many studies that remove the ZP to investigate its role, few studies question how the surrounding mechanical environment comes into play after the embryo hatches from the ZP and is directly exposed to the surrounding matrix. Real-time RT-PCR experiments by Kolahi et al. (2012) show a 5-fold increase in cdx2 expression when zona-free embryos were cultured on collagen I gel rather than standard polystyrene dishes. This contrasts with the relative cdx2 expression levels in embryos with their zonas still intact, where no statistically significant difference was seen between embryos cultured on the two substrates. Although in this experiment the embryos’ ZPs were removed with acid Tyrode’s solution, the results still seem to warrant some skepticism about the effects of substrate stiffness on hatched embryos. This was, in part, addressed by Zhao et al. (2015) in a more recent study on architecture maintenance in hatched bovine embryos. They used a 3D alginate culture system to study the effects of architecture maintenance in hatched bovine embryos as compared to a 2D alginate substrate and control (Fig. 3B). Their findings suggest that 3D alginate culture systems support cell proliferation, cell survival, embryo elongation, and differentiation of hatched bovine blastocysts more so than 2D or standard polystyrene petri dishes. The authors state these findings imply the necessity of soft 3D matrix support to maintain the appropriate embryo architecture during elongation.
Another interesting study on the topic of architecture maintenance in early development was the investigation of the effect of a soft (low shear modulus), 3D alginate matrix on mouse follicle development (West et al., 2007). The authors aimed to test the hypothesis that stiffer environments prevent the follicle from expanding and thus restrict oocyte maturation. They found that their alginate hydrogel does promote the release of higher quality oocytes, by their assessment of meiotic resumption after 12 days of culture. They also found that changing the surrounding stiffness of the follicle altered the profile of hormonal secretions to favor estrogen instead of progesterone and androgen. Although this study does not pertain to oocyte/embryo mechanosensitivity, it gives relevant insight into the design of in vitro follicle culture systems, which can promote healthy oocyte development and preserve the reproductive potential for women receiving cancer therapy (West et al., 2007).
The role of the ZP in dampening stress
The ZP serves as the interface between the embryo and its surrounding environment during travel through the fallopian tubes. Thus, a thorough understanding of mechanotransduction in embryos and oocytes would require a detailed analysis of the ZP. This section will summarize what is known about the role of the ZP in transducing/dampening stresses and how this function can be utilized to benefit ART.
The ZP is an acellular, glycoprotein shell that is about 15 µm thick in humans (Murayama et al., 2013). The embryo is completely encapsulated in the ZP until it eventually hatches during the late blastocyst stage. A study by Bronson and McLaren (1970) found that transferring 8-cell stage mouse embryos in the oviduct of pseudopregnant female mice without their ZP resulted in the death of nearly all the embryos transferred. However, the live fetus rate was over 70% when embryos were transferred with intact ZPs and when cultured in vitro, while embryos that were cultured in vitro with no ZP and were transferred at the blastocyst stage had a live fetus rate of about 50%. Furthermore, there was nearly no difference in the blastocyst survival rate between embryos with and without their ZPs. From this finding, they concluded that the ZP was instrumental in the survival of the embryo in vivo, but not in vitro (Bronson and McLaren, 1970).
The findings of Bronson and McLaren provided strong evidence of the ZP acting as a protective layer for developing embryos but highlighted the mystery of from what the ZP is protecting the embryo. The ZP is thought to protect the embryo in many ways including filtering out bacteria, viruses, and certain luminal molecules (Modliński, 1970; Rankin and Dean, 1996). It has also been suggested that the ZP protects the developing embryo from shear stress (Xie et al., 2006). By applying high shear stress (1.2 dyn/cm2) to mouse embryos with a programmed rotating wall vessel, Xie et al. (2006) demonstrated shear-induced apoptosis in both E3.5 and E2.5 embryos with and without their ZPs (Fig. 4A). Their findings corroborated Bronson and McLaren on two points: first, E2.5 embryos were far more sensitive than E3.5 embryos when their ZPs were removed; and second, that removing the ZP is lethal if the embryo is placed in dynamic conditions. It should be noted that the magnitude of shear stress used by Xie et al. (2006) was much higher than what is estimated for in vivo conditions. However, similar apoptotic markers in E4.5 mouse embryos were found when excessive pipetting is used (Xie et al., 2007), validating that ZP damping of stress is at least relevant in vitro. Thus, an understanding of why and how the ZP regulates mechanical stresses affecting an embryo has a high potential to benefit ART as it provides a new metric in assessing ZP quality. Modifying substrate stiffness of in vitro culture systems can have many developmental benefits. Once again, the mechanisms are not clear, but the two studies discussed in this section show impressive results and leave a strong foundation for future studies.
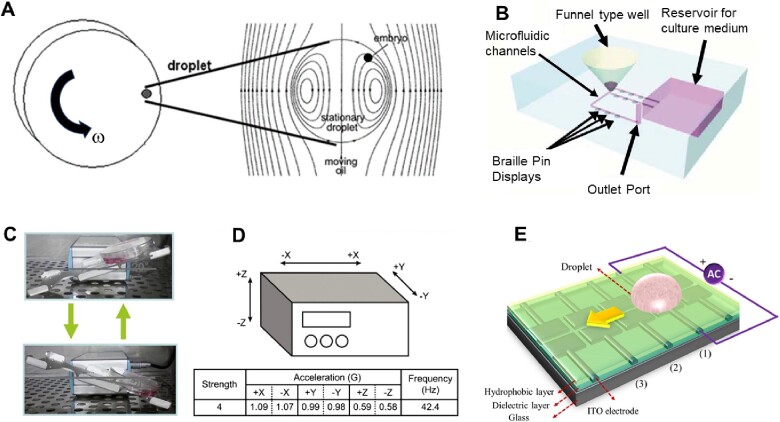
Figure 4.
Dynamic embryo culture systems designed to generate flow on a preimplantation embryo. (A) The braille pin actuated microfluidic embryo culture device. Adapted from Heo et al. (2010). (B) The tilting embryo culture system. Adapted from Hara et al. (2013). (C) Schematic of the microvibration stage used to create dynamic embryo culture conditions in experiments performed by Hur et al. (2013). Adapted from Hur et al. (2013). (D) Schematic of the digitalized microfluidic device powered by electrowetting on the dielectric. Adapted from Huang et al. (2015). (E) Rotating vessel used to generate shear stresses in an embryo culture droplet. ITO, indium tin oxide. Adapted from Xie et al. (2006).
In addition to the aforementioned mechanical forces in the microenvironment, embryos also experience hydraulic pressure and can generate tensile forces by myosin-mediated contractility. While cadherin-mediated differential adhesion was believed to regulate the cell sorting process, recent works using high-resolution in toto imaging revealed the role of these forces in the early morphogenetic events. The formation of the first inner cells was found to be driven by myosin II-mediated tensile forces, which led to apical constrictions and positioning of inner cells (Samarage et al., 2015). Niwayama et al. further demonstrated that the oriented cell division process was governed by the interplay between the apical domain orientation and cell shape (Niwayama et al., 2019). In another study, the same group discovered that during mouse blastocyst development the increasing lumenal pressure leads to a concomitant increase in cell cortical tension and tissue stiffness of the trophectoderm (Chan et al., 2019). Importantly, the authors demonstrated that such hydraulic pressure in the blastocoel regulated the blastocyst size and the first cell-fate decisions. Together, these exciting works highlight the importance of biomechanical forces in early morphogenetic events and cell fate patterning, and it is of great interest to understand how external stimuli can regulate these intrinsic biomechanical events.
Engineering systems to modulate the biophysical microenvironment for IVF
As there are multiple reports which demonstrate the benefits of gentle surrounding fluid-flow, it is desirable to incorporate it into embryo culture systems (Bavister, 1995; Wheeler et al., 2002). The drawbacks, as previously mentioned, are that large shear stresses applied for long durations of time are lethal to preimplantation embryos via apoptosis and large volumes will dilute autocrine/paracrine factors in the medium. It is not clear what other effects this shear stress may have on preimplantation embryos, aside from apoptosis, so typically lower shear stresses (≪1.2 dyn/cm2) are selected as the safe option. The magnitude of shear stress acting on a human embryo during tubal transport is challenging to define, despite reports of average velocities ∼0.1 µm/s and maximum velocities around 8.6 µm/s (Greenwald, 1961; Anand and Guha, 1978; Halbert et al., 1989), because it is unclear how much can be extrapolated from animal models or even oviductal tissue samples of nonpregnant women. It is imperative that researchers develop controllable, reliable and precise engineering systems to establish a safe range of shear stress and time durations. This section reviews some of these systems and assesses their potential for clinical usage.
The first dynamic microfluidic embryo culture system was designed to solve a serious dilemma in embryo culture at the time, which was whether medium should be changed during culture (Suh et al., 2003; Smith and Takayama, 2017). It was known that in vivo conditions would provide varying pH and nutrients for the embryo as it develops, but to accomplish this in vitro requires excessive handling of the embryos and stresses imposed by sudden changes in pH, temperature, and osmolarity. Wheeler et al. (2002) designed a microfluidic device that allowed for a gradual change in medium that would produce gentle flow and shear stress. This system showed a very consistent increase in the development rate of mouse embryos compared to a static control in a standard microdroplet. This study does produce impressive results; however, it alters shear stress and nutrient availability simultaneously, making it difficult to isolate the effect of either component individually. Though it is not clear to what the improvements should be attributed, this early study highlights the potential benefits of dynamics and biomimicry in embryo culture.
Considering the innumerable animal models with dynamic embryo conditions and scientific publications that suggest developmental benefits of gentle and continuous flows on an embryo during culture (Halbert et al., 1989; Bavister, 1995; Wheeler et al., 2002), the intuitive next step is to decouple the different effects of fluid-flow on developing embryos. Impressively, Heo et al. (2010) developed a microfunnel culture system capable of applying shear stress to preimplantation embryos while still retaining autocrine factors. This system holds embryos inside a funnel-shaped well with a microfluidic channel connected to a small hole in the bottom (Fig. 4B). When a flow is applied, the passing fluid creates a vortex inside the well. Interestingly, the flow is generated using computer-controlled, piezoelectric pin actuators from a braille display to deform the polydimethylsiloxane microfluidic channels and create a pulsatile flow. To demonstrate the benefits of their system, Heo et al. cultured mouse zygotes for 96 h and found that the trophectoderm cell number, implantation rate, and pregnancy rates were all significantly greater in the treatment group than the control. This design greatly assuages the usual burdens associated with microfluidics by not requiring any pumps or tubing and has potential for application in clinical settings owing to its simple collection system and pump-free operation.
Naruse et al. studied the effects of gentle shear stress on embryos on a system with embryo velocities approximated by microsphere experiments (Matsuura et al., 2010; Hara et al., 2013). They developed a TECS that applies roughly 0.7 dynes/mm2 to an embryo during culture (Fig. 4C). This system is very attractive for clinical embryo culture owing to its simplicity and compatibility with standard embryo culture procedures. The results of their experiment show that, compared to control, for different numbers of embryos per 50 µl droplet the most improvement is seen at the lowest embryo densities while almost no benefit is seen at the higher densities. This suggests that it is the increased accessibility of the autocrine/paracrine signals that is driving the improvement. Similarly, there have been several reports of improved embryo development using a microvibration stage to culture porcine, mouse and human embryos (Mizobe et al., 2010; Isachenko et al., 2011; Hur et al., 2013). For instance, Hur et al. (2013) cultured human and mouse single-cell zygotes using a microvibration stage and observed that there is no significant difference between the control group and experimental group when observed on day 3 of culture, despite there being a significant difference in development on day 5, as also seen in the TECS experiment (Fig. 4D). Notably, Mizobe et al. (2010) found that porcine embryos exposed to mechanical vibration showed an improved rate of blastocyst formation contingent upon treatment during oocyte maturation. Despite this trend, mechanical vibration neither improved oocyte maturation rates in any case nor did it show significant improvement when started after oocyte maturation. These results highlight that the mechanosensitivity of embryos may depend on the development stages.
More recently, a digital microfluidic system for embryo culture was developed (Huang et al., 2015). This system utilizes the electrowetting phenomenon to move a microculture droplet through a microfluidic channel, producing a flow with seemingly safe shear stress (Fig. 4E). The advantages of this system are that it is programmable, repeatable, precise, and can operate with relatively low energy. However, the effect of the electric field on the preimplantation embryo is still unclear. The system used by the authors has an electric potential of 60 VRMS. It has been demonstrated that sufficient levels of electrical stimulation (1.36 kV/cm for 40–60 µs) can facilitate fertilization in human oocytes (Zhang et al., 1999), but there is also evidence that exposure to electric pulses (>1 kV/cm for 30 µs) increase levels of reactive oxygen species in porcine embryos (Koo et al., 2008). Therefore, caution should be taken in using culture systems that involve electric fields, which would be present in the microdroplets.
Together, current technology development focuses on creating a controllable fluid microenvironment for embryos cultured in vitro using either microvibration systems or microfluidic systems. As pointed out above, in addition to shear stresses, the mechanical/biophysical properties of substrates also strongly influence the quality of embryos cultured in vitro. Thus, various biomaterial systems should also be evaluated for improving the embryo culture in vitro.
Conclusions and further questions
The average age of first-time mothers in the USA is increasing steadily, however female fertility decreases with age (Mathews and Hamilton, 2016). Thus, there is a potentially growing need for ART and a commensurate increase in the percentage of the population born through these methods. Here we reviewed two main aspects from which biomechanical factors contribute to the ART field. First, mechanical phenotyping can improve embryo/oocyte screening both before and after freezing, allowing clinicians to avoid wasting time and resources on non-viable embryos/oocytes. Unlike ZP dissolution and preimplantation genetic screening, mechanical phenotyping can be inexpensive, minimally invasive, and fast. Second, an in depth understanding of the role of biomechanical cues in early stage embryo development will guide the design of novel in vitro embryo culture platforms that precisely control the biomechanical environment (e.g., shear stresses and compression forces), and ultimately improve the quality of embryos. In addition to those direct contributions, mechanics also adds value to the study of embryo development and has provided different perspectives on phenomena such as hatching, compaction, and ZP hardening.
As attractive as the inclusion of mechanical phenotyping and mechanotransduction in embryo culture may seem, further research should be performed prior to making any definite conclusions on what they can offer. The data available on mechanical biomarkers and mechanotransduction is mostly empirical and should be corroborated by mechanistic studies to understand the transcriptional changes and epigenetic modifications associated with biomechanical cues. In addition, most current studies focused on blastocyst formation and live birth rates. Although those are important milestones, long-term studies should be carried out to assess the health of the offspring, as ART is associated with the development of epigenetic disorders (Feuer and Rinaudo, 2012; Ventura-Juncá et al., 2015). Another factor that limits the current studies is the lack of biomechanical characterization of human fallopian tubes with a high spatiotemporal resolution. Without those data, it will be difficult to design engineering systems that properly mimic the in vivo microenvironment, and particularly in a dynamic manner.
From our perspective, the future research in the field of biomechanics of embryo development should prioritize three directions. The first direction is to tease out the unique molecular mechanisms for the mechanotransduction in embryos and depict the functional connections between the forces and morphogenesis of early development. The benefits of understanding how external forces affect embryos and oocytes also apply to tubal transport. Conditions such as polycystic ovary syndrome and pelvic inflammatory disease are known to affect the mechanical conditions that an oocyte or embryo would experience in the fallopian tubes, so characterizations of how these mechanical alterations affect fertility can allow physicians to make more informed decisions. The second direction is to expand the biomaterials available for embryo culture. While there are numerous studies using various functional biomaterials and scaffolds for the culture of embryonic stem cells or embryonic tissues (He, 2017), it is surprising that the ART field has not fully benefited from those recent advances. The impact from extracellular matrices should not be underestimated even if embryos are non-adherent. The third direction is to leverage the current microfabrication technologies, including lithography, 3D printing, and laser cutting, to fabricate integrated microdevices with biosensing components to monitor levels of O2, pH and growth factors in real-time, as well as relevant biophysical cues (see recent review by (Gu et al., 2020)). Altogether, the next-generation embryo culture systems need to be designed based on mechanobiology principles and allow full control of the biophysical and biochemical microenvironment of embryos.
Data availability
All the data reported were obtained from published literature and no new datasets were generated or analyzed in this work.
Authors’ roles
J.H. and Y.S. designed the review, J.H. performed literature retrieval, prepared the figures, and wrote the manuscripts, X.M., W.C. and Y.S. edited and approved the final manuscript.
Funding
This work is supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institute of Health (1R21HD098686 to Y.S. and W.C.).
Conflict of interest
The authors declare no competing interests.
References
- Afzelius BA, Camner P, Mossberg B. On the function of cilia in the female reproductive tract. Fertil Steril 1978;29:72–74. [DOI] [PubMed] [Google Scholar]
- Anand S, Guha SK. Mechanics of transport of the ovum in the oviduct. Med Biol Eng Comput 1978;16:256–261. [DOI] [PubMed] [Google Scholar]
- Balakier H, Sojecki A, Motamedi G, Bashar S, Mandel R, Librach C. Is the zona pellucida thickness of human embryos influenced by women's age and hormonal levels? Fertil Steril 2012;98:77–83. [DOI] [PubMed] [Google Scholar]
- Bavister BD. Culture of preimplantation embryos: facts and artifacts. Hum Reprod Update 1995;1:91–148. [DOI] [PubMed] [Google Scholar]
- Boccaccio A, Frassanito MC, Lamberti L, Brunelli R, Maulucci G, Monaci M, Papi M, Pappalettere C, Parasassi T, Sylla L et al. Nanoscale characterization of the biomechanical hardening of bovine zona pellucida. J R Soc Interface 2012;9:2871–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden AW, Austin CR, David HA. The reaction of zona pellucida to sperm penetration. Aust J Biol Sci 1954;7:391–409. [DOI] [PubMed] [Google Scholar]
- Bronson RA, McLaren A. Transfer to the mouse oviduct of eggs with and without the zona pellucida. J Reprod Fertil 1970;22:129–137. [DOI] [PubMed] [Google Scholar]
- Chan CJ, Costanzo M, Ruiz-Herrero T, Mönke G, Petrie RJ, Bergert M, Diz-Muñoz A, Mahadevan L, Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature 2019;571:112–116. [DOI] [PubMed] [Google Scholar]
- Chiba H, Hiura H, Okae H, Miyauchi N, Sato F, Sato A, Arima T. DNA methylation errors in imprinting disorders and assisted reproductive technology. Pediatr Int 2013;55:542–549. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Li Y, Poh Y-C, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions. Plos One 2010;5:e15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi G, Marsico C, Cosentino A, Spadoni C, Rocca A, De Crescenzo A, Riccio A. Silver-Russell syndrome due to paternal H19/IGF2 hypomethylation in a twin girl born after in vitro fertilization. Am J Med Genet A 2013;161:2652–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxatto HB. Physiology of gamete and embryo transport through the fallopian tube. Reprod Biomed Online 2002;4:160–169. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Ortiz ME. Egg transport in the fallopian tube. Gynecol Obstet Invest 1975;6:215–225. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Ortiz ME, Díaz S, Hess R, Balmaceda J, Croxatto HD. Studies on the duration of egg transport by the human oviduct: II. Ovum location at various intervals following luteinizing hormone peak. Am J Obstet Gynecol 1978;132:629–634. [DOI] [PubMed] [Google Scholar]
- De Rycke M, Liebaers I, Van Steirteghem A. Epigenetic risks related to assisted reproductive technologies: risk analysis and epigenetic inheritance. Hum Reprod 2002;17:2487–2494. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003;72:156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond ME, Knepper JE, DiBenedetto AJ, Malaugh E, Callejo S, Carretero R, Alonso MI, Gato A. Focal adhesion kinase as a mechanotransducer during rapid brain growth of the chick embryo. Int J Dev Biol 2014;58:35–43. [DOI] [PubMed] [Google Scholar]
- Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell 2008;15:470–477. [DOI] [PubMed] [Google Scholar]
- Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod 2007;22:2476–2480. [DOI] [PubMed] [Google Scholar]
- Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 1995;57:344–358. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Sommergruber M, Puchner M, Wiesinger R, Tews G. Developmental competence of oocytes showing increased cytoplasmic viscosity. Hum Reprod 2003;18:1294–1298. [DOI] [PubMed] [Google Scholar]
- Eroshkin FM, Zaraisky AG. Mechano-sensitive regulation of gene expression during the embryonic development. Genesis 2017;55:e23026. [DOI] [PubMed] [Google Scholar]
- Eytan O, Jaffa AJ, Elad D. Peristaltic flow in a tapered channel: application to embryo transport within the uterine cavity. Med Eng Phys 2001;23:475–484. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Djahanbakhch O, Arian S, Carr BR. Tubal transport of gametes and embryos: a review of physiology and pathophysiology. J Assist Reprod Genet 2014;31:1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol 2003;13:1365–1377. [DOI] [PubMed] [Google Scholar]
- Feuer S, Rinaudo P. Preimplantation stress and development. Birth Defect Res C 2012;96:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales DS, Jones JM, Pinyopummintr T, Carnevale EM, Ginther OJ, Shapiro SS, Bavister BD. Trophectoderm projections: a potential means for locomotion, attachment and implantation of bovine, equine and human blastocysts. Hum Reprod 1996;11:2739–2745. [DOI] [PubMed] [Google Scholar]
- Green DP. Three-dimensional structure of the zona pellucida. Rev Reprod 1997;2:147–156. [DOI] [PubMed] [Google Scholar]
- Greenwald GS. A study of the transport of ova through the rabbit oviduct. Fertil Steril 1961;12:80–95. [DOI] [PubMed] [Google Scholar]
- Gu Z, Guo J, Wang H, Wen Y, Gu Q. Bioengineered microenvironment to culture early embryos. Cell Prolif 2020;53:e12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, Taylor-Weiner H, Wen JH, Lee AR, Bieback K et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci USA 2017;114:5647–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert SA, Becker DR, Szal SE. Ovum transport in the rat oviductal ampulla in the absence of muscle contractility. Biol Reprod 1989;40:1131–1136. [DOI] [PubMed] [Google Scholar]
- Halbert SA, Tam PY, Blandau RJ. Egg transport in the rabbit oviduct: the roles of cilia and muscle. Science 1976;191:1052–1053. [DOI] [PubMed] [Google Scholar]
- Hara T, Matsuura K, Kodama T, Sato K, Kikkawa Y, Muneto T, Tanaka J, Naruse K. A tilting embryo culture system increases the number of high-grade human blastocysts with high implantation competence. Reprod Biomed Online 2013;26:260–268. [DOI] [PubMed] [Google Scholar]
- Harper JC, Aittomaki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I et al. ; on behalf of the European Society of Human Reproduction and Embryology and European Society of Human Genetics. Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur J Hum Genet 2018;26:12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X. Microscale biomaterials with bioinspired complexity of early embryo development and in the ovary for tissue engineering and regenerative medicine. ACS Biomater Sci Eng 2017;3:2692–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD. Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates. Hum Reprod 2010;25:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-Y, Shen H-H, Tien C-H, Li C-J, Fan S-K, Liu C-H, Hsu W-S, Yao D-J. Digital microfluidic dynamic culture of mammalian embryos on an electrowetting on dielectric (EWOD) chip. PLoS One 2015;10:e0124196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur YS, Park JH, Ryu EK, Park SJ, Lee JH, Lee SH, Yoon J, Yoon SH, Hur CY, Lee WD et al. Effect of micro-vibration culture system on embryo development. J Assist Reprod Genet 2013;30:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isachenko V, Maettner R, Sterzik K, Strehler E, Kreinberg R, Hancke K, Roth S, Isachenko E. In-vitro culture of human embryos with mechanical micro-vibration increases implantation rates. Reprod Biomed Online 2011;22:536–544. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Fallopian tube isthmic mucus and ovum transport. Science 1978;201:349–351. [DOI] [PubMed] [Google Scholar]
- Kagami M, Nagai T, Fukami M, Yamazawa K, Ogata T. Silver-Russell syndrome in a girl born after in vitro fertilization: partial hypermethylation at the differentially methylated region of PEG1/MEST. J Assist Reprod Genet 2007;24:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel RM. Assisted reproductive technology after the birth of louise brown. J Reprod Infertil 2013;14:96–109. [PMC free article] [PubMed] [Google Scholar]
- Keung AJ, Asuri P, Kumar S, Schaffer DV. Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integr Biol 2012;4:1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahi K, Donjacour A, Liu X, Lin W, Simbulan RK, Bloise E, Maltepe E, Rinaudo P. Effect of substrate stiffness on early mouse embryo development. PLoS One 2012;7:e41717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo OJ, Jang G, Kwon DK, Kang JT, Kwon OS, Park HJ, Kang SK, Lee BC. Electrical activation induces reactive oxygen species in porcine embryos. Theriogenology 2008;70:1111–1118. [DOI] [PubMed] [Google Scholar]
- Krause I, Pohler U, Grosse S, Shebl O, Petek E, Chandra A, Ebner T. Characterization of the injection funnel during intracytoplasmic sperm injection reflects cytoplasmic maturity of the oocyte. Fertil Steril 2016;106:1101–1106. [DOI] [PubMed] [Google Scholar]
- Leonavicius K, Royer C, Preece C, Davies B, Biggins JS, Srinivas S. Mechanics of mouse blastocyst hatching revealed by a hydrogel-based microdeformation assay. Proc Natl Acad Sci USA 2018;115:10375–10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Katalinic A, Gross S, Sutcliffe A, Varon R, Horsthemke B. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet 2005;42:289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie M, Tur-Kaspa I, Weill S, Katz I, Rabinovici J, Goldenberg S. Ciliary ultrastructure of respiratory and fallopian tube epithelium in a sterile woman with Kartagener's syndrome. A quantitative estimation. Chest 1989;95:578–581. [DOI] [PubMed] [Google Scholar]
- Ma Z, Sagrillo-Fagundes L, Mok S, Vaillancourt C, Moraes C. Mechanobiological regulation of placental trophoblast fusion and function through extracellular matrix rigidity. Sci Rep 2020;10:5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod 2003;18:2508–2511. [DOI] [PubMed] [Google Scholar]
- Mao AS, Shin JW, Mooney DJ. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 2016;98:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. 2016. NCHS Data Brief;2016;232:1–8. [PubMed]
- Matsuura K, Hayashi N, Kuroda Y, Takiue C, Hirata R, Takenami M, Aoi Y, Yoshioka N, Habara T, Mukaida T et al. Improved development of mouse and human embryos using a tilting embryo culture system. Reprod Biomed Online 2010;20:358–364. [DOI] [PubMed] [Google Scholar]
- McGrail DJ, Kieu QM, Dawson MR. The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho-ROCK pathway. J Cell Sci 2014;127:2621–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobe Y, Yoshida M, Miyoshi K. Enhancement of cytoplasmic maturation of in vitro-matured pig oocytes by mechanical vibration. J Reprod Dev 2010;56:285–290. [DOI] [PubMed] [Google Scholar]
- Modliński JA. The role of the zona pellucida in the development of mouse eggs in vivo. J Embryol Exp Morphol 1970;23:539–547. [PubMed] [Google Scholar]
- Murayama Y, Mizuno J, Kamakura H, Fueta Y, Nakamura H, Akaishi K, Anzai K, Watanabe A, Inui H, Omata S. Mouse zona pellucida dynamically changes its elasticity during oocyte maturation, fertilization and early embryo development. Hum Cell 2006;19:119–125. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Yoshida K, Takahashi H, Mizuno J, Akaishi K, Inui H. Softening of the mouse zona pellucida during oocyte maturation. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2013, pp. 6834–6837. [DOI] [PubMed]
- Narla VK, Tripathi D, Anwar Bég O. Electro-osmosis modulated viscoelastic embryo transport in uterine hydrodynamics: mathematical modeling. J Biomech Eng 2019;141:021003. [DOI] [PubMed] [Google Scholar]
- Niwayama R, Moghe P, Liu Y-J, Fabrèges D, Buchholz F, Piel M, Hiiragi T. A tug-of-war between cell shape and polarity controls division orientation to ensure robust patterning in the mouse blastocyst. Dev Cell 2019;51:564–574.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SK, Chaube MK. Peristaltic transport of a visco-elastic fluid in a tube of non-uniform cross section. Math Comput Model 2010;52:501–514. [Google Scholar]
- Papi M, Brunelli R, Familiari G, Frassanito MC, Lamberti L, Maulucci G, Monaci M, Pappalettere C, Parasassi T, Relucenti M et al. Whole-depth change in bovine zona pellucida biomechanics after fertilization: how relevant in hindering polyspermy? PLoS One 2012;7:e45696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papi M, Brunelli R, Sylla L, Parasassi T, Monaci M, Maulucci G, Missori M, Arcovito G, Ursini F, De Spirito M. Mechanical properties of zona pellucida hardening. Eur Biophys J 2010;39:987–992. [DOI] [PubMed] [Google Scholar]
- Pauerstein CEC. Morphology of the fallopian tube. The Biology of the Fluids of the Genital Tract. North Holland: Elsevier, 1979. [Google Scholar]
- Pedersen H. Absence of dynein arms in endometrial cilia: cause of infertility? Acta Obstet Gynecol Scand 1983;62:625–627. [DOI] [PubMed] [Google Scholar]
- Rankin T, Dean J. The molecular genetics of the zona pellucida: mouse mutations and infertility. Mol Hum Reprod 1996;2:889–894. [DOI] [PubMed] [Google Scholar]
- Samarage CR, White Melanie D, Álvarez Yanina D, Fierro-González Juan C, Henon Y, Jesudason Edwin C, Bissiere S, Fouras A, Plachta N. Cortical tension allocates the first inner cells of the mammalian embryo. Dev Cell 2015;34:435–447. [DOI] [PubMed] [Google Scholar]
- Seshagiri PB, Sen Roy S, Sireesha G, Rao RP. Cellular and molecular regulation of mammalian blastocyst hatching. J Reprod Immunol 2009;83:79–84. [DOI] [PubMed] [Google Scholar]
- Smith GD, Takayama S. Application of microfluidic technologies to human assisted reproduction. Mol Hum Reprod 2017;23:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh RS, Phadke N, Ohl DA, Takayama S, Smith GD. Rethinking gamete/embryo isolation and culture with microfluidics. Hum Reprod Update 2003;9:451–461. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen CS, Fu J. Forcing stem cells to behave: a biophysical perspective of the cellular microenvironment. Annu Rev Biophys 2012a;41:519–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Villa-Diaz LG, Lam RH, Chen W, Krebsbach PH, Fu J. Mechanics regulates fate decisions of human embryonic stem cells. Plos One 2012b;7:e37178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yong KMA, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 2014;13:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe AG, Peters CJ, Bowdin S, Temple K, Reardon W, Wilson L, Clayton-Smith J, Brueton LA, Bannister W, Maher ER. Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Hum Reprod 2006;21:1009–1011. [DOI] [PubMed] [Google Scholar]
- Talo A, Pulkkinen MO. Electrical activity in the human oviduct during the menstrual cycle. Am J Obstet Gynecol 1982;142:135–147. [DOI] [PubMed] [Google Scholar]
- Thie M, Röspel R, Dettmann W, Benoit M, Ludwig M, Gaub HE, Denker HW. Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum Reprod 1998;13:3211–3219. [DOI] [PubMed] [Google Scholar]
- Ventura-Juncá P, Irarrázaval I, Rolle AJ, Gutiérrez JI, Moreno RD, Santos MJ. In vitro fertilization (IVF) in mammals: epigenetic and developmental alterations. Scientific and bioethical implications for IVF in humans. Biol Res 2015;48:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat Mater 2014;13:979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007;28:4439–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MB, Beebe DJ, Walters EM, Raty S. Microfluidic technology for in vitro embryo production. 2nd Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology Proceedings (Cat No02EX578). 2002, pp. 104–108.
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev 2007;74:1287–1294. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee D. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod 2006;75:45–55. [DOI] [PubMed] [Google Scholar]
- Yanez LZ, Han J, Behr BB, Pera RAR, Camarillo DB. Human oocyte developmental potential is predicted by mechanical properties within hours after fertilization. Nat Commun 2016;7:10809–10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wang CW, Blaszcyzk A, Grifo JA, Ozil J, Haberman E, Adler A, Krey LC. Electrical activation and in vitro development of human oocytes that fail to fertilize after intracytoplasmic sperm injection. Fertil Steril 1999;72:509–512. [DOI] [PubMed] [Google Scholar]
- Zhao S, Liu Z-X, Gao H, Wu Y, Fang Y, Wu S-S, Li M-J, Bai J-H, Liu Y, Evans A et al. A three-dimensional culture system using alginate hydrogel prolongs hatched cattle embryo development in vitro. Theriogenology 2015;84:184–192. [DOI] [PubMed] [Google Scholar]
- Zhou H-X, Ma Y-Z, Liu Y-L, Chen Y, Zhou C-J, Wu S-N, Shen J-P, Liang C-G. Assessment of mouse germinal vesicle stage oocyte quality by evaluating the cumulus layer, zona pellucida, and perivitelline space. PLoS One 2014;9:e105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data reported were obtained from published literature and no new datasets were generated or analyzed in this work.