Abstract
Background
Recently, the concept of persistent postsurgical opioid use has been described for patients undergoing cancer surgery. Our hypothesis was based on the premise that patients with oral tongue cancer require high dosages of opioids before, during, and after surgery, and thus a large percentage of patients might develop persistent postsurgical opioid use.
Methods
After institutional review board approval, we conducted a retrospective study that included a cohort of patients with oral tongue cancers who underwent curative-intent surgery in our institution. Multivariable logistic regression models were fit to study the association of the characteristics of several patients with persistent (six months after surgery) and chronic (12 months after surgery) postoperative opioid use.
Results
A total of 362 patients with oral tongue malignancies were included in the study. The rate of persistent use of opioids after surgery was 31%. Multivariate analysis showed that patients taking opioids before surgery and those receiving adjuvant therapy were 2.9 and 1.78 times more likely to use opioids six months after surgery. Fifteen percent of the patients were taking opioids 12 months after surgery. After adjusting for clinically relevant covariates, patients complaining of moderate tongue pain before surgery and those taking opioids preoperatively had at least three times higher risk of still using these analgesics one year after surgery.
Conclusions
Patients with oral tongue cancers have a high risk of developing persistent and chronic postsurgical opioid use.
Keywords: neoplasm, tongue, surgery, opioid
Introduction
In the United States, three out of four patients with cancer receive opioids for cancer pain management [1,2]. In patients with head and neck cancers, opioids are frequently prescribed (55.5–83.1%) in the early phases of oncological treatments, and in 39.3% they are given in high dosages [3,4]. This evidence supports previous studies indicating that patients with head and neck cancers have greater odds of receiving opioids during their cancer treatment [5–7].
Opioids are also given perioperatively to patients with head and neck cancers. Pang et al. have demonstrated that more than a third of the patients scheduled for oral cancer surgery are taking opioids before the operation [8]. Patients with head and neck cancers also require large amounts of opioids during extensive surgical procedures such as neck explorations and reconstructive operations. In this regard, Patino et al. showed that the median intraoperative use of intravenous fentanyl equivalents was 1,081 μg [9]. Previous studies have demonstrated that a high preoperative and intraoperative consumption of opioids can lead to increased use of these analgesics immediately after surgery [10,11]. For instance, Pang et al. reported that at the time of hospital discharge the majority of patients undergoing oral cancer surgery had received >200 mg of oral morphine equivalents (OME) in total [8].
Recently, the concept of persistent postsurgical opioid use has been described for patients undergoing nonpalliative cancer surgery [12]. This entity is defined as the use of opioids between 90 to 180 days after surgery and in a large cohort of mixed cancer patients; its rate ranged from 4.5% to 58.9% [12]. The unintended consequences of persistent opioid use include tolerance, misuse, and lower levels of quality of life [4]. Therefore, it has been suggested that the understanding of the patterns of opioid use after surgery should be one of the initial steps to avoid persistent opioid use, misuse, and diversion [13]. Although previous studies have investigated factors associated with chronic opioid use and the use of opioids 181–265 days postsurgery in a mixed population of patients with oropharynx and oral cavity cancers, the literature is almost nonexistent for those with oral tongue cancers.
This study was designed to investigate the prevalence and factors associated with persistent postoperative (six months after surgery) and chronic (12 months after surgery) opioid use after oral tongue cancer surgery. We consider these outcomes clinically relevant because approximately 25% of the hospitalized patients with malignant tongue neoplasms show clinical signs of opioid tolerance or dependence [14]. The hypothesis of our work is that patients with oral tongue cancer have a high risk for persistent and chronic opioid use after surgery. Our hypothesis was based on the following premises: patients with oral tongue cancer might require high dosages of opioids before, during, and after surgery. These patients are also exposed to adjuvant therapies associated with painful syndromes (i.e., radiotherapy- and chemoradiotherapy-induced pain), and a wide variation in opioid prescription practice exists among oral cancer surgeons and other health providers [6,15].
Methods
Study Design and Patient Population
After institutional review board (#PA16-1033) approval, we performed a retrospective study that included a cohort of patients with oral tongue cancers who underwent curative-intent surgery at MD Anderson Cancer Center from January 2004 to January 2018. Our inclusion criteria were limited to patients aged 18 or older who had scheduled surgery. Patients with oral cancers located outside the tongue, those with repeated oral procedures, and those with missing information regarding our primary or secondary outcomes were excluded from the study.
Data Collection
All data points were collected from electronic medical records and stored in our perioperative REDCap database containing information from patients with oral cancers who were treated in our institution from 2000 to 2018. Demographic, tumor-related, clinical, and perioperative information included age, body mass index (BMI), gender, history of tobacco and alcohol use, history of preoperative pain, preoperative pain intensity using a verbal numeric rating score (VNRS; 0 = no pain, 10 = worse pain ever) at the time of anesthesia assessment, and pre- and in-hospital opioid consumption. We also collected data regarding the history of neoadjuvant or adjuvant therapy, type of surgery (glossectomy with or without neck dissection, and with or without muscle flap use for reconstruction), pathological tumor and node staging, and evidence of perineural invasion or extracapsular extension. We estimated the risk for opioid abuse using the opioid risk tool [16]. Preoperative opioid use was calculated in OME and defined as any opioid use before surgery. Also, we calculated the postoperative in-hospital OME, the OME between 90 and 180 days after surgery, and that between 181 and 365 days after the operation.
Outcomes
The primary outcome of our study was persistent opioid use, defined as any active opioid consumption reported by the patient during follow-up clinical visits between 90 and 180 days after surgery [12]. Chronic opioid use was our secondary outcome and was defined as any opioid consumption reported by the patient during follow-up clinical visits between 181 and 365 days after surgery [12].
Statistical Analysis
Patient demographics, perioperative factors, and study outcomes were summarized through descriptive statistics. The chi-square and Fisher exact tests were used to assess the relationship between two categorical variables, and the Wilcoxon rank-sum test was used to assess the association between categorical and continuous variables. Multivariable logistic regression models were fit to study the association of several patients’ characteristics with the primary and secondary outcomes in adjusted analysis. A backward stepwise model selection based on Akaike information criteria with variables that were statistically significant at the univariate level (alpha = 0.20) was applied to build parsimonious models with most relevant factors. A P value <0.05 was considered statistically significant. Statistical analyses were conducted in R, version 3.4.2.
Results
Patient Demographics and Perioperative Factors
A total of 362 patients were included in the study (Figure 1, Table 1). The median age of our patients was 58 years, 58% of them were male, and 47% were smokers. The most common type of surgical procedure was glossectomy with neck dissection (84%). Few patients (14%) received neo-adjuvant therapy, and about half of them were treated with adjuvant chemotherapy and/or radiation (46%). In terms of tumor-related variables, the large majority of our patients had pT1-2 (77%) and pN0-1 (75%). Of note, perineural invasion was present in about a third (30%) of the subjects included in the analysis.
Figure 1.
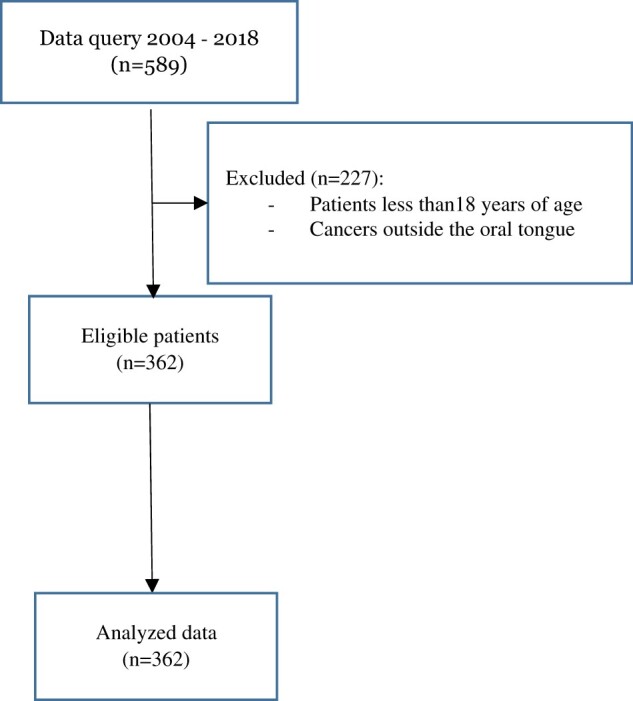
Consort flow diagram.
Table 1.
Patients’ demographics and clinical variables
| Patient Characteristics | N = 362 | |
|---|---|---|
| Age, mean (SD), y | 56.8 (14.08) | |
| BMI, mean (SD), kg/m2 | 28.08 (6.51) | |
| Gender, No. (%) | Female | 151 (41.71) |
| Male | 211 (58.29) | |
| Tobacco use, No. (%) | No | 192 (53.04) |
| Yes | 170 (46.96) | |
| Alcohol abuse, No. (%) | No | 278 (76.8) |
| Yes | 84 (23.2) | |
| History of chronic pain, No. (%) | No | 167 (46.13) |
| Yes | 195 (53.87) | |
| Preoperative pain intensity, No. (%) | 0 | 236 (65.19) |
| 1–5 | 60 (16.57) | |
| 6–10 | 28 (7.73) | |
| Unknown | 38 (10.5) | |
| Preoperative opioid use, mean (SD), mg | 28.29 (74.93) | |
| Neoadjuvant therapy, No. (%) | No | 310 (85.64) |
| Yes | 52 (14.36) | |
| Adjuvant therapy, No. (%) | No | 197 (54.42) |
| Yes | 165 (45.58) | |
| No | 58 (16.02) | |
| Yes | 304 (83.98) | |
| Neck reconstruction, No. (%) | No | 206 (56.91) |
| Yes | 156 (43.09) | |
| pT staging, No. (%) | 1 | 164 (45.3) |
| 2 | 116 (32.04) | |
| 3 | 44 (12.15) | |
| 4 | 38 (10.5) | |
| pN staging, No. (%) | 0 | 233 (64.36) |
| 1 | 35 (9.67) | |
| 2 | 54 (14.92) | |
| 3 | 32 (8.84) | |
| 4 | 8 (2.21) | |
| Perineural invasion, No. (%) | No | 253 (69.89) |
| Yes | 109 (30.11) | |
| Extracapsular extension, No. (%) | No | 287 (79.28) |
| Yes | 73 (20.17) | |
| Unknown | 2 (0.55) |
BMI = body mass index.
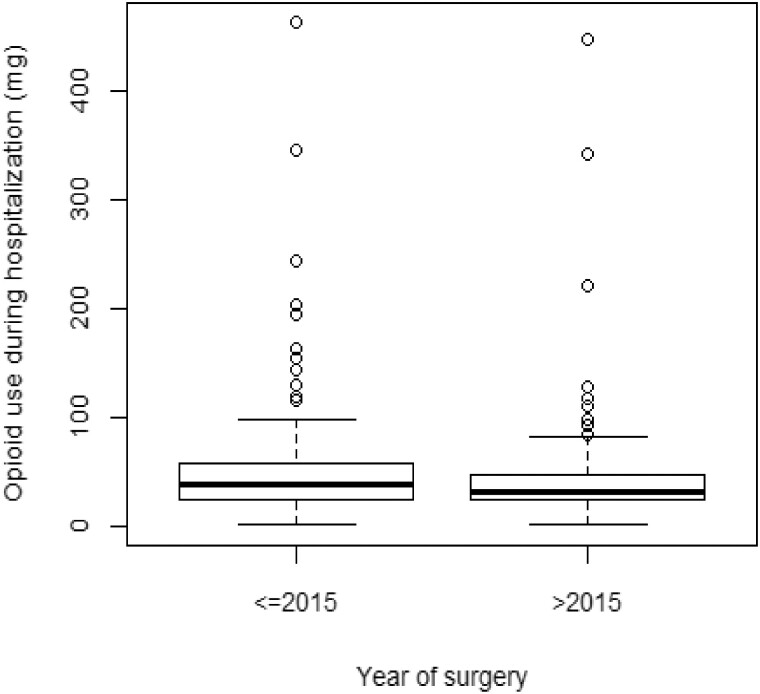
Nearly half of the patients (54%) reported oral pain since their cancer diagnosis, and 35% complained of pain at the preoperative anesthesia or surgical assessment. Of those reporting pain immediately before surgery, most patients complained of moderate to severe discomfort (55%). Forty-one percent of the patients were taking opioids before surgery (median [range] = 0 [0–750] mg). The median (range) in-hospital postoperative OME was 35.99 (1–462) mg. However, we observed a significant reduction in the median OME in patients hospitalized between 2015 and 2017 (29.79 mg, P < 0.001) in comparison with those who had surgery between 2004 and 2014 (41.33 mg) (figure 2). In terms of recurrence status, 57 (16%) and 69 (19%) patients showed cancer recurrence six and 12 months after surgery.
Figure 2.
Opioid morphine equivalence during hospitalization. The figure illustrates changes in OME before and after 2015. Patients treated after 2015 received significantly less opioid perioperatively.
Persistent Opioid Use After Surgery
The rate of persistent use of opioids after surgery was 31%, and the median (range) OME at this time was 45 (5–354) mg. The most commonly prescribed opioid was oral hydrocodone (46%), followed by oral tramadol (11%) and oral oxycodone (5%). Other opioids included codeine (3%), fentanyl (3%), hydromorphone (3%), methadone (4%), morphine (3%), oxymorphone (2%), and their combinations (20%). As tumor recurrence can be a source of pain, we estimated the rate of persistent opioid use in patients with and without recurrent tumors. Briefly, 37.5% (N = 42) of those taking opioids six months after surgery had recurrence tumors. According to the preoperative opioid risk tool, the proportion of patients with moderate–high opioid abuse risk was not statistically different between those taking (9.2%) or not taking (14.3%) opioids six months after surgery (Table 2).
Table 2.
Patients with and without persistent opioid use and with and without chronic opioid use
| Persistent Opioid Use |
Chronic Opioid Use |
||||||
|---|---|---|---|---|---|---|---|
| Patient Characteristics | No N = 250 | Yes N = 112 | P Value | No N = 306 | Yes N = 56 | P Value | |
| Age, mean (SD), y | 56.46 (14.4) | 58.68 (12.11) | 0.414 | 56.46 (14.4) | 58.68 (12.11) | 0.414 | |
| BMI, mean (SD), kg/m2 | 28.04 (6.46) | 28.31 (6.82) | 28.04 (6.46) | 28.31 (6.82) | |||
| Gender, No. (%) | Female | 108 (43.2) | 43 (38.39) | 0.458 | 133 (43.46) | 18 (32.14) | 0.152 |
| Male | 142 (56.8) | 69 (61.61) | 173 (56.54) | 38 (67.86) | |||
| Tobacco use, No. (%) | No | 142 (56.8) | 50 (44.64) | 0.043 | 169 (55.23) | 23 (41.07) | 0.071 |
| Yes | 108 (43.2) | 62 (55.36) | 137 (44.77) | 33 (58.93) | |||
| Alcohol use, No. (%) | No | 191 (76.4) | 87 (77.68) | 0.895 | 234 (76.47) | 44 (78.57) | 0.865 |
| Yes | 59 (23.6) | 25 (22.32) | 72 (23.53) | 12 (21.43) | |||
| ORT, No. (%) | 0.256 | 0.386 | |||||
| Low | Risk | 223 (85.7) | 98 (90.8) | 279 (90.33) | 53 (9.34) | ||
| Moderate/high | Risk | 32 (14.3) | 9 (9.2) | 27 (9.67) | 3 (5.66) | ||
| History of tongue pain, No. (%) | No | 132 (52.8) | 35 (31.25) | <0.001 | 152 (49.67) | 15 (26.79) | 0.003 |
| Yes | 118 (47.2) | 77 (68.75) | 154 (50.33) | 41 (73.21) | |||
| Preoperative pain intensity, No. (%) | 0 | 171 (68.4) | 65 (58.04) | 0.096 | 207 (67.65) | 29 (51.79) | 0.01 |
| 1–5 | 37 (14.8) | 23 (20.54) | 45 (14.71) | 15 (26.79) | |||
| 6–10 | 16 (6.4) | 12 (10.71) | 20 (6.54) | 8 (14.29) | |||
| NA | 26 (10.4) | 12 (10.71) | 34 (11.11) | 4 (7.14) | |||
| Preoperative VRS, mean (SD) | 1.03 (2.15) | 1.68 (2.65) | 0.023 | 1.06 (2.24) | 2.1 (2.64) | 0.001 | |
| Preoperative opioid use, No. (%) | No | 169 (67.6) | 43 (38.39) | <0.001 | 194 (63.4) | 18 (32.14) | <0.001 |
| Yes | 81 (32.4) | 69 (61.61) | 112 (36.6) | 38 (67.86) | |||
| Preoperative MEDD, No. (%) | 18.51 (50.46) | 50.14 (108.86) | <0.001 | 21.51 (52.07) | 65.38 (141.99) | <0.001 | |
| Neoadjuvant therapy, No. (%) | No | 217 (86.8) | 93 (83.04) | 0.434 | 263 (85.95) | 47 (83.93) | 0.85 |
| Yes | 33 (13.2) | 19 (16.96) | 43 (14.05) | 9 (16.07) | |||
| Adjuvant therapy, No. (%) | No | 152 (60.8) | 45 (40.18) | <0.001 | 176 (57.52) | 21 (37.5) | 0.009 |
| Yes | 98 (39.2) | 67 (59.82) | 130 (42.48) | 35 (62.5) | |||
| Neck dissection, No. (%) | No | 47 (18.8) | 11 (9.82) | 0.046 | 53 (17.32) | 5 (8.93) | 0.169 |
| Yes | 203 (81.2) | 101 (90.18) | 253 (82.68) | 51 (91.07) | |||
| Reconstructive surgery, No. (%) | No | 144 (57.6) | 62 (55.36) | 0.777 | 175 (57.19) | 31 (55.36) | 0.914 |
| Yes | 106 (42.4) | 50 (44.64) | 131 (42.81) | 25 (44.64) | |||
| pT staging, No. (%) | 1 + 2 | 196 (78.4) | 84 (75) | 0.563 | 236 (77.12) | 44 (78.57) | 0.949 |
| 3 + 4 | 54 (21.6) | 28 (25) | 70 (22.88) | 12 (21.43) | |||
| pN staging, No. (%) | 0 | 163 (65.2) | 70 (62.5) | 0.635 | 197 (64.38) | 36 (64.29) | 0.915 |
| 1 + 2 | 62 (24.8) | 27 (24.11) | 76 (24.84) | 13 (23.21) | |||
| 3 + 4 | 25 (10) | 15 (13.39) | 33 (10.78) | 7 (12.5) | |||
| PNI, No. (%) | No | 179 (71.6) | 74 (66.07) | 0.349 | 218 (71.24) | 35 (62.5) | 0.249 |
| Yes | 71 (28.4) | 38 (33.93) | 88 (28.76) | 21 (37.5) | |||
| ECS, No. (%) | No | 201 (80.4) | 86 (76.79) | 0.43 | 243 (79.41) | 44 (78.57) | 0.958 |
| Yes | 47 (18.8) | 26 (23.21) | 61 (19.93) | 12 (21.43) | |||
| NA | 2 (0.8) | 0 (0) | 2 (0.65) | 0 (0 | |||
| Recurrence, No. (%) | No | 193 (77.2) | 70 (62.5) | 0.006 | 225 (73.5) | 35 (62.5) | 0.127 |
| Yes | 57 (22.8) | 42 (37.5) | 81 (26.5) | 21 (37.5) | |||
| Surgery year, No. (%) | ≤2015 | 162 (64.8) | 76 (67.86) | 0.655 | 203 (66.34) | 35 (62.5) | 0.687 |
| >2015 | 88 (35.2) | 36 (32.14) | 103 (33.66) | 21 (37.5) | |||
BMI = body mass index; ECS = extracapsular spread; MEDD = morphine equivalent daily dose; ORT = Opioid Risk Tool; PNI = perineural invasion; VRS = verbal rating scale.
As shown in Table 2, univariate analysis indicated that tobacco use (P = 0.04), preoperative pain (P < 0.001), preoperative opioid use (P < 0.001), adjuvant therapy (P < 0.001), and neck dissection (P = 0.046) were the categorical covariates most strongly related to persistent use of opioids after surgery. In other words, compared with nonpersistent opioid users after surgery, a significantly higher percentage of patients in the persistent use group were exposed to tobacco (55% vs 43%), had a history of chronic pain (69% vs 47%), were taking opioids (62% vs 32%), had received adjuvant therapy (60% vs 39%), or had had a neck dissection (90% vs 81%). In addition, the consumption of opioids during hospitalization was significantly higher in the persistent opioid use group (median [range] OME = 41.25 [4–447] mg) than in those not taking opioids six months after surgery (median [range] OME = 32.75 [1–462] mg, P = 0.002). Results from the backward model selection (Table 3) demonstrated that patients taking opioids before surgery and those receiving adjuvant therapy were 2.9 and 1.78 times more likely to use opioids six months after surgery. In the results from the backward model selection, including preoperative morphine use and preoperative pain level as continuous variables, we observed that adjuvant therapy (odds ratio [OR] = 1.785, 95% confidence interval [CI] = 1.101–2.895, P = 0.019) and preoperative pain intensity (OR = 1.834, 95% CI = 1.099–2.895, P = 0.02) were associated with persistent postoperative opioid use. The amount of preoperative morphine use was not associated with opioid consumption six months after surgery (OR = 1.003, 95% CI = 1–1.007, P = 0.086). Among patients with persistent use of opioids after surgery, 37.5% recurred by six months after surgery, and among patients with no persistent use of opioids after surgery, 22.8% recurred six months postoperatively (P = 0.006).
Table 3.
Multivariate analysis for persistent postoperative opioid use
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Intercept | 0.205 | 0.138–0.303 | <0.001 | |
| Adjuvant therapy | Yes vs no | 1.781 | 1.102–2.877 | 0.018 |
| Preoperative morphine use | Yes vs no | 2.9 | 1.796–4.683 | <0.001 |
CI = confidence interval.
Chronic Opioid Use After Surgery
Fifteen percent of the patients included in the analysis were taking opioids 12 months after surgery. The median (range) OME was 45 (5–615) mg, and oral hydrocodone (52%) was the most commonly prescribed drug. Codeine (4%), fentanyl (2%), hydromorphone (5%), morphine (7%), oxycodone (7%), tramadol (13%), and their combinations (10%) were prescribed less frequently. Our analysis demonstrates that 22 (39%) patients of those still taking opioids 12 months after surgery had recurrent tumors.
Our univariate analysis (Table 2) demonstrated that, compared with nonchronic opioid users, those taking opioids one year after surgery had significantly more pain before surgery (48% vs 32%), demonstrated a higher rate of preoperative opioid use (68% vs 37%), and received adjuvant therapy (62% and 42%) in a larger proportion. In addition, the median (range) OME during hospitalization was significantly higher in the chronic opioid (41.25 [5–447] mg) group than in those who were not taking opioids one year after surgery (32.75 [1–462] mg, P = 0.035). The multivariate analysis demonstrated that patients complaining of moderate tongue pain (VNRS: 4–6) before surgery and those taking opioids preoperatively had at least three times higher risk of still using these analgesics one year after surgery (Table 4). Results from the backward model selection, including preoperative morphine use (OR = 1.004, 95% CI = 1.00–1.007, P = 0.032) and preoperative pain intensity (OR = 1.128, 95% CI = 1.003–1.268, P = 0.044) as continuous variables, demonstrated that both were independent prognostic factors of chronic opioid use. There was no statistically significant association between recurrence by 12 months after surgery and chronic use of opioids after surgery (P = 0.127).
Table 4.
Multivariate analysis for persistent chronic opioid use
| Variable | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Intercept | 0.067 | 0.036–0.124 | <0.001 | |
| Adjuvant therapy | Yes vs no | 1.659 | 0.865–3.179 | 0.127 |
| Preoperative morphine use | Yes vs no | 2.947 | 1.514–5.734 | 0.001 |
| Preoperative pain level | 1–5 vs 0 | 1.827 | 0.882–3.783 | 0.105 |
| 6–10 vs 0 | 2.165 | 0.833–5.63 | 0.113 |
CI = confidence interval.
Discussion
This is the first study investigating the incidence rate of persistent and chronic opioid use after oral tongue cancer surgery. Briefly, close to one-third (31%) of the patients included in the analysis were taking opioids six months after surgery. Our results are in agreement with those previously published by Saraswathula et al. using a mixed cohort of elderly patients with head and neck cancers [17]. In that study, the prevalence of persistent postoperative opioid use was 33% [17]. Saraswathula’s and our rates of persistent postoperative opioid use are higher than those recently reported by Lee et al. in an opioid-naïve cohort of patients with melanoma (11%), colorectal (15.1%), hepato-pancreato-biliary (4.5%), and thoracic (10.5%) cancers [12]. This suggests that patients with oral tongue cancers have a higher risk of persistent postoperative opioid use in comparison with other cancers. The relevance of our findings lies in evidence suggesting that for some cancers, such as lung malignancies, persistent postoperative opioid use is an independent predictor of shorter survival [18]. Also, the importance of our work is based on the fact that the incidence of oral tongue squamous cell carcinoma is rising, especially in younger patients [19]. Thus, an increasing number of young patients could be at risk of developing chronic opioid use or abuse after oral cancer surgery.
Our study also supports the findings of previous investigations indicating that a large proportion of patients (23–50%) scheduled to have surgery for nonmetastatic cancers are receiving opioids preoperatively [12,17]. The rate of preoperative opioid use in our work was lower than that reported in Saraswathula’s study (50%), which can be explained by methodological differences including the type and location of cancers and patient population [17]. The importance of investigating the prevalence of preoperative opioid use in our patient population was based on studies indicating that it can lead to persistent opioid use [17,20]. In support of that evidence, we observed that the preoperative use of opioids was strongly associated (OR = 2.9) with persistent consumption of these analgesics six months after surgery. However, this was not the case in Lee’s study, where there was no evidence of new persistent opioid use after excluding non-opioid-naïve patients from the overall population of patients [12]. It is worth mentioning that Lee’s work included a mixed population of non–head and neck cancer patients, which could account for the differences in findings among the studies [12].
A third important finding is that slightly over one-third of the patients taking opioids six months after surgery developed recurrence. Tumor recurrence per se can be the source of pain. However, our findings highlight the fact that in a large percentage of tongue cancer survivors, persistent postoperative opioid use has causes other than tumor recurrence, which is in line with another observation demonstrating that the administration of adjuvant therapies was an independent predictor of persistent opioid use. This finding has also been demonstrated in patients with other malignancies, including those with head and neck cancers [12,17]. Patients with oral tongue cancers may receive chemoradiation therapy after surgery to improve their survival [21,22]. Unfortunately, chemotherapy (i.e., cisplatin) and radiation may be associated with significant pain, such as severe neuropathies (4–71%), mucositis (26–41%), and dysphagia (12%), for which patients are treated with opioids, although with limited efficacy at the doses found in this study [6,21–26]. Although Saraswathula et al. found an association only between adjuvant radiotherapy and persistent postoperative opioid use and not with chemotherapy, Shah et al. reported that patients with chemotherapy-induced peripheral neuropathies were twice as likely to receive opioids five years after treatment compared with those without the neuropathy [17,23].
The prevalence of chronic (one year after surgery) opioid use in our study was double that reported by McDermott et al. (7%) in a cohort of oral cavity and oropharynx cancers [3]. However, it was lower than the prevalence rates previously published in studies by Silver et al. and Bollig et al., who indicated that 23% and 45.9% of patients with oropharyngeal cancer were taking opioids chronically [20,27]. Presurgical opioid use was an independent risk factor for chronic opioid use in our study and in McDermott’s work [3]. Interestingly, McDermott et al. indicated that a high dose (100 mg morphine equivalent) of opioid before treatment initiation was also a risk factor for long-term consumption [3]. Other studies have demonstrated that factors including the surgery itself, tumor staging, alcohol use, age, and psychiatric disorders are also associated with chronic opioid use in patients with oral cavity and oropharynx cancers [4]. There are several differences between our study and the works of McDermott et al, Silver et al. and Bollig et al. [3,20,27]. First, we included only oral tongue cancer patients who had undergone surgery, whereas Silver et al. and McDermott et al. analyzed data from subjects with oropharyngeal cancer who had primarily undergone chemoradiation [20]. And second, Silver et al. and Bollig et al. defined chronic opioid use as taking any active opioid three months after treatment completion [20,27]. These differences can explain the discrepancies in results among studies.
About half of the patients included in our work presented with pain between the time of diagnosis and surgery. This is in agreement with a previous study indicating that 60% of patients with head and neck cancers complain of pain at the time of diagnosis [11]. We also observed that pain intensity before surgery was a predictor of chronic opioid use. This can be explained by the predominant neuropathic pain component present in head and neck cancers [11].
Our study has at least two important limitations. First, this was a retrospective study. The presence of confounding due to unknown and unmeasured variables, such as duration of opioid use before surgery and severity of complications related to chemoradiation (i.e., neuropathies), was not included in the study. Second, changes in the pattern of opioid prescription might also have influenced our findings. This speculation is based on the fact that after 2015 we observed a significant decrease in opioid consumption. It is possible to speculate that several factors might have influenced a change in prescription practice including a more aggressive use of multimodal analgesic techniques, as recommended by the Enhanced Recovery After Surgery guidelines. Although it is worth noting that guidelines for head and neck oncology patients were published after 2015 [28]. And third, our database does not capture data on surgical procedures that might have occurred outside our institution and that could have extended the duration of opioid use.
In conclusion, patients with oral tongue cancers who experience significant pain before treatment initiation, those treated with opioids preoperatively, and those who are receiving adjuvant therapies have a higher risk of developing persistent and chronic postoperative opioid use. A high level of perioperative opioid consumption before, during, and immediately after surgery and a high opioid prescription rate at the time of hospital discharge are important risk factors for long-term opioid use. This is evidence of the need to develop perioperative strategies to adequately treat perioperative pain in patients with oral tongue cancers [29,30].
Conflicts of interest: The authors have no conflicts of interest.
References
- 1.Kolodny A, Frieden TR. Ten steps the federal government should take now to reverse the opioid addiction epidemic. JAMA 2017;318(16):1537–8. [DOI] [PubMed] [Google Scholar]
- 2.Mercadante S, Prestia G, Ranieri M, Giarratano A, Casuccio A. Opioid use and effectiveness of its prescription at discharge in an acute pain relief and palliative care unit. Supportive Care Cancer 2013;21(7):1853–9. [DOI] [PubMed] [Google Scholar]
- 3.McDermott JD, Eguchi M, Stokes WA, et al. Short- and long-term opioid use in patients with oral and oropharynx cancer. Otolaryngol Head Neck Surg 2019;160(3):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry M, Alias A, Frenkiel S, et al. Contribution of psychiatric diagnoses to extent of opioid prescription in the first year post-head and neck cancer diagnosis: A longitudinal study. Psycho-Oncol 2019;28(1):107–15. [DOI] [PubMed] [Google Scholar]
- 5.Sethi RKV, Panth N, Puram SV, Varvares MA. Opioid prescription patterns among patients with head and neck cancer. JAMA Otolaryngoly Head Neck Surg 2018;144(4):382–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cramer JD, Johnson JT, Nilsen ML. Pain in head and neck cancer survivors: Prevalence, predictors, and quality-of-life impact. Otolaryngoly Head Neck Surg 2018;159(5):853–8. [DOI] [PubMed] [Google Scholar]
- 7.Haumann J, Geurts JW, van Kuijk SMJ, Kremer B, Joosten EA, van den Beuken-van Everdingen M. Methadone is superior to fentanyl in treating neuropathic pain in patients with head-and-neck cancer. Eur J Cancer 2016;65:121–9. [DOI] [PubMed] [Google Scholar]
- 8.Pang J, Tringale KR, Tapia VJ, et al. Opioid prescribing practices in patients undergoing surgery for oral cavity cancer. Laryngoscope 2018;128(10):2361–66. [DOI] [PubMed] [Google Scholar]
- 9.Patino MA, Ramirez RE, Perez CA, et al. The impact of intraoperative opioid use on survival after oral cancer surgery. Oral Oncol. 2017;74:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Lavand'homme P, Steyaert A. Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol 2017;31(4):487–98. [DOI] [PubMed] [Google Scholar]
- 11.Buchakjian MR, Davis AB, Sciegienka SJ, Pagedar NA, Sperry SM. Longitudinal perioperative pain assessment in head and neck cancer surgery. Ann Otol Rhinol Laryngol 2017;126(9):646–53. [DOI] [PubMed] [Google Scholar]
- 12.Lee J-J, Hu HM, Edelman AL, et al. New persistent opioid use among patients with cancer after curative-intent surgery. J Clin Oncol 2017;35(36):4042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharasch ED, Brunt LM. Perioperative opioids and public health. Anesthesiology 2016;124(4):960–5. [DOI] [PubMed] [Google Scholar]
- 14.Nalliah RP, Shroff D, Stein K, et al. Opioid abuse and dependence in those hospitalized due to head and neck cancer. J Oral Maxillofac Surg 2018;76(12):2525–31. [DOI] [PubMed] [Google Scholar]
- 15.Svider PF, Arianpour K, Guo E, et al. Opioid prescribing patterns among otolaryngologists: Crucial insights among the Medicare population. Laryngoscope 2018;128(7):1576–81. [DOI] [PubMed] [Google Scholar]
- 16.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the Opioid Risk Tool. Pain Med 2005;6(6):432–42. [DOI] [PubMed] [Google Scholar]
- 17.Saraswathula A, Chen MM, Mudumbai SC, Whittemore AS, Divi V. Persistent postoperative opioid use in older head and neck cancer patients. Otolaryngol Head Neck Surg 2019;160(3):380–7. [DOI] [PubMed] [Google Scholar]
- 18.Nelson DB, Cata JP, Niu J, et al. Persistent opioid use is associated with worse survival after lobectomy for stage I non-small cell lung cancer. Pain. 2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Campbell BR, Netterville JL, Sinard RJ, et al. Early onset oral tongue cancer in the United States: A literature review. Oral Oncol. 2018:89:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver N, Dourado J, Hitchcock K, et al. Chronic opioid use in patients undergoing treatment for oropharyngeal cancer. Laryngoscope 2019;129(9):2087–93. [DOI] [PubMed] [Google Scholar]
- 21.Bernier J, Domenge C, Ozsahin M, et al. ; European Organization for Research and Treatment of Cancer Trial . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350(19):1945–52. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JS, Pajak TF, Forastiere AA, et al. ; Radiation Therapy Oncology Group I. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350(19):1937–44. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Hoffman EM, Mauermann ML, et al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry 2018;89(6):636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol 2015;14(2):162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackiewicz J, Rybarczyk-Kasiuchnicz A, Łasińska I, et al. The comparison of acute toxicity in 2 treatment courses. Medicine 2017;96(51):e9151.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfieri S, Ripamonti CI, Marceglia S, et al. Temporal course and predictive factors of analgesic opioid requirement for chemoradiation-induced oral mucositis in oropharyngeal cancer. Head Neck 2016;38(S1):E1521–7. [DOI] [PubMed] [Google Scholar]
- 27.Bollig CA, Jorgensen JB. Effect of treatment modality on chronic opioid use in patients with T1/T2 oropharyngeal cancer. Head Neck 2019;41(4):892–8. [DOI] [PubMed] [Google Scholar]
- 28.Coyle MJ, Main B, Hughes C, et al. Enhanced Recovery After Surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol 2016;41(2):118–26. [DOI] [PubMed] [Google Scholar]
- 29.Militsakh O, Lydiatt W, Lydiatt D, et al. Development of multimodal analgesia pathways in outpatient thyroid and parathyroid surgery and association with postoperative opioid prescription patterns. JAMA Otolaryngol Head Neck Surg 2018;144(11):1023–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dort JC, Farwell DG, Findlay M, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction. JAMA Otolaryngol Head Neck Surg 2017;143(3):292–303. [DOI] [PubMed] [Google Scholar]