Abstract
Zinc dynamics are essential for oocyte meiotic maturation, egg activation, and preimplantation embryo development. During fertilisation and egg activation, the egg releases billions of zinc atoms (Zn2+) in an exocytotic event termed the ‘zinc spark’. We hypothesised that this zinc transport and exocytosis is dependent upon the intracellular trafficking of cortical granules (CG) which requires myosin-actin-dependent motors. Treatment of mature mouse and human eggs with ML-7, a myosin light chain kinase inhibitor (MLCK), resulted in an 80% reduction in zinc spark intensity compared to untreated controls when activated with ionomycin. Moreover, CG migration towards the plasma membrane was significantly decreased in ML-7-treated eggs compared with controls when activated parthenogenetically with ionomycin. In sperm-induced fertilisation via intracytoplasmic sperm injection (ICSI), ML-7-treated mouse eggs exhibited decreased labile zinc intensity and cortical CG staining. Collectively, these data demonstrate that ML-7 treatment impairs zinc release from both murine and human eggs after activation, demonstrating that zinc exocytosis requires myosin light chain kinase activity. Further, these results provide additional support that zinc is likely stored and released from CGs. These data underscore the importance of intracellular zinc trafficking as a crucial component of egg maturation necessary for egg activation and early embryo development.
Keywords: egg, cortical granule, exocytosis, fertilisation, zinc spark, myosin light chain kinase, zona pellucida, polyspermy
Introduction
Mammalian egg activation at fertilisation is characterised by completion of meiosis with extrusion of the second polar body, cortical granule (CG) exocytosis resulting in zona pellucida (ZP) hardening, pronucleus (PN) formation and maternal mRNA recruitment (Miao and Williams, 2012; Krauchunas and Wolfner, 2013). The release of intracellular calcium stores and the appearance of ‘calcium waves’ within the mammalian egg drive these essential events of egg activation. More recently, studies have described a rapid exocytosis of zinc from the egg during fertilisation and activation (Suzuki et al., 2010; Kim et al., 2011; Que et al., 2015; Tokuhiro and Dean, 2018). Prior to activation, labile pools of zinc are localised at the egg cortex in the hemisphere opposite the meiotic spindle, and this zinc is released upon fertilisation or parthenogenetic activation (Kim et al., 2011). The rapid release of zinc appears as a ‘zinc spark’ during dynamic live-cell fluorescence imaging and occurs in the mouse, cow, non-human primate and human (Kim et al., 2010; Kim et al., 2011; Duncan et al., 2016; Zhang et al., 2016; Que et al., 2019). This loss of zinc at the time of fertilisation is necessary and sufficient for triggering the cell cycle and exit from metaphase II arrest, and in fact, egg activation can be induced by the use of a zinc chelator alone (Suzuki et al., 2010; Bernhardt et al., 2011; Duncan et al., 2016). The amount of zinc released at the time of fertilisation/activation correlates with embryo development, with larger spark amplitudes associated with higher-quality blastocyst-stage embryos (Zhang et al., 2016).
Despite the importance of zinc exocytosis during fertilisation and activation, the mechanisms governing its release have not been fully elucidated. During the meiotic transition of the oocyte from the prophase I arrest to the metaphase II arrest (MII), intracellular labile zinc changes from a symmetrical to a polarised distribution (Que et al., 2015). This shift in zinc localisation pattern mirrors that observed for CGs (Que et al., 2017). Because labile zinc pools and ovastacin-loaded CGs traffic identically within the maturing oocyte, and both are effluxed upon egg activation (Tokuhiro and Dean, 2018), we hypothesised that vesicle trafficking of CGs through the myosin-actin motor system is necessary for the zinc spark (Tokuhiro and Dean, 2018). To test this hypothesis, we perturbed this trafficking system using ML-7, an antagonist of myosin light chain kinase (MLCK) that inhibits cortical actin localisation and lateral CG movements in the mouse egg cortex (Matson et al., 2006). MLCK is a Ca2+ calmodulin (CaM)-dependent enzyme in non-muscle cells that regulates myosin II via phosphorylation of Ser-19 or Ser-19/Ther-18 within the myosin light chain (Bresnick, 1999; Green et al., 1999; Ducibella and Fissore, 2008). MLCK activity is stimulated at fertilisation by the increase in intracellular calcium, resulting in the activation of a myosin motor involved in CG translocation (Matson et al., 2006; Que et al., 2017; Tokuhiro and Dean, 2018; Vogt et al., 2019). ML-7 has been widely applied to study MLCK function and does not decrease the activity of Ca2+ CaM protein kinase II. Instead, ML-7 robustly inhibits the formation of the second polar body and CG exocytosis (Matson et al., 2006).
In this study, we used ML-7 to inhibit MLCK-dependent organelle trafficking in mouse and human eggs to determine whether CG exocytosis is obligatory for the zinc efflux observed during parthenogenetic (mouse and human) or sperm-induced (mouse) egg activation (Tadros and Lipshitz, 2009). We demonstrate that MLCK activity is required for both CG exocytosis and zinc release in both the mouse and human during egg activation. These findings provide new mechanistic insight into how inorganic signalling through zinc mediates the oocyte-to-egg transition in the absence of canonical (e.g. genomic and proteomic) cues.
Materials and Methods
Animals
All experiments were performed using male (5–6-month-old) and female (6–8-week-old) CD-1 mice. Mice were housed in polypropylene cages and provided food and water ad libitum. Animals were kept on a 14 h light: 10 h dark cycle (6 a.m.–8 p.m.). Temperature and humidity were kept constant. Animals were fed Teklad Global irradiated 2916 chow (Madison, WI, USA), which does not contain soybean or alfalfa meal to minimise exposure to phytoestrogens (Xiao et al., 2015). Animals used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Northwestern University, and all procedures were carried out under an IACUC-approved protocol.
Egg collection
To collect MII eggs, female mice were injected with 5 IU of pregnant mare serum gonadotropin (PMSG; Sigma). At 46–48 h after PMSG injection, these same mice were injected with 5 IU human chorionic gonadotropin (hCG; Sigma), and 13–14 h post hCG, MII eggs were collected from the oviducts (Navarrete et al., 2016). Eggs were retrieved into HEPES-buffered Tyrode-lactate solution (TL-HEPES, Millipore, Burlington, MA) supplemented with 5% heat-treated fetal calf serum (FCS; Gibco BRL, Grand Island, NY) followed by treatment with 0.1% bovine testes hyaluronidase for 3–5 min to remove cumulus cells (Yoon et al., 2008).
Human samples
De-identified immature human oocytes were obtained from women age 35–39 years old undergoing treatment for infertility at Northwestern Memorial Hospital through the Northwestern University Reproductive Tissue Library. All experiments using human oocytes were approved by Northwestern University’s Institutional Review Board (IRB). Participants recruited to the study were scheduled to undergo egg retrieval; after obtaining written informed consent, immature oocytes were collected from among the oocytes retrieved. These immature oocytes would have otherwise been discarded in routine practice, as only eggs at the MII stage within 2 h of retrieval are used clinically for insemination. De-identified immature oocytes (germinal vesicle [GV] stage) were obtained ~7–9 h post-retrieval. Oocytes were assessed for quality and stage by light microscopy (Combelles et al., 2002; Tadros and Lipshitz, 2009). For in vitro maturation, oocytes were placed in SAGE oocyte maturation medium (Cooper Surgical Fertility Companies, Denmark) pre-equilibrated in 4-well plates at 37°C in 5% CO2. Oocytes that were at the GV-intact stage upon arrival in the lab were in vitro matured for ~24 h to reach MII (Escrich et al., 2012; Duncan et al., 2016). Thus, all human oocytes were used for experimentation ~ 36 h after ovum retrieval. All human egg activation studies were done using ionomycin, and no federal funds were used for these experiments.
Imaging of CGs
The distribution of CGs and tubulin was investigated in MII eggs. To detect CGs, MII eggs were exposed to acidic Tyrode’s solution (Sigma-Aldrich, pH 2.5) to remove the ZP and fixed in 3.8% paraformaldehyde (Electron Microscopy Science, Hatfield, PA) in Dulbecco’s PBS (DPBS, Thermo Fisher Scientific, Waltham, MA) for 30 min at room temperature. Fixed eggs were washed in blocking solution containing 3 mg/ml BSA, 100 mM glycine and 0.01% Tween 20 in DPBS before permeabilisation with 0.1% Triton-X 100 in DPBS for 15 min at room temperature. Eggs were washed three times in blocking solution and incubated with rhodamine-conjugated Lens culinaris agglutinin (LCA, Vector Laboratories, Burlingame, CA) for 1 h at room temperature to label CGs (de Paola et al., 2015). After staining, eggs were washed three times in blocking solution and mounted with 4′,6-diamidino-2-phenylindole (DAPI) mounting solution (Vector Laboratories) then examined by confocal microscopy (Leica, Wetzlar, Germany). Fluorescence intensity was analysed by ImageJ, and intensity histograms along regions of interest were assembled to visualise detailed localisation within the cortical region (Zhang et al., 2016).
Fluorescence imaging of calcium and zinc during parthenogenetic egg activation
Imaging of intracellular calcium ions ([Ca2+]i) and extracellular zinc ions (Zn2+) during egg activation was performed as previously described (Zhang et al., 2016). Fluorescent indicators (FluoZin-3), Ca-ionomycin (ionomycin), and pluronic acid were obtained from Life Technologies (Grand Island, NY) (Zhang et al., 2016). Intracellular calcium was measured using the plasma-membrane-permeable Ca2+ sensitive dye Fura-2-acetoxymethyl ester (Fura 2-AM, Molecular Probes; Invitrogen, Carlsbad, CA) (Lee et al., 2016), and extracellular zinc was measured using a membrane-impermeable Zn2+ selective indicator FluoZin-3 in the culture media. The MLCK inhibitor ML-7 (MilliporeSigma, Burlington, MA) was dissolved in dimethyl sulfoxide (DMSO), aliquoted and stored at −80°C until use. On the day of the experiment, ML-7 was added to TL-HEPES containing 0.1% polyvinyl alcohol (PVA; Sigma, St. Louis, MO) with Fura 2-AM for 30 min before Ca2+ and Zn2+ monitoring. The final concentration of ML-7 was 15 μM for mouse and 30 μM for human eggs (Green et al., 1999; Matson et al., 2006). The treatment of ML-7 and Fura 2-AM was performed in a 4-well dish (Thermo Scientific Nunclon, Rochester, NY) at room temperature. Eggs were then placed in a new 360-μl drop of 20 μM FluoZin-3 in Ca2+-free HEPES-buffered CZB (Chatot, Ziomek and Bavister: hCZB) medium in a coverslip-bottom imaging dish (MatTek Corp, Ashland, MA) (Yoon et al., 2008). Ca2+ and Zn2+ were monitored simultaneously using an inverted Nikon Eclipse Ti microscope (Nikon, Japan) outfitted with fluorescence measurement capabilities. Eggs were activated with 40 μl of 10× ionomycin stock (25 μM) solution introduced to the imaging drop 1 min after the start of monitoring. Fura 2-AM dye was excited between 340 and 380 nm, and FluoZin-3 was excited at 488 nm by a filter wheel (Lambda 10-3, Sutter Instrument), and fluorescence was captured every 4 s (Zhang et al., 2016). Emitted light was collected by a CoolSnapES2 CCD camera (Photometrics) (Zhang et al., 2016). Acquisition of fluorescence ratios was analysed by ImageJ (NIH). Importantly, all human egg activation studies were done using ionomycin, and no federal funds were used for these experiments.
Intracellular labile zinc imaging
Live cell imaging of labile zinc within activated eggs was performed using the membrane-permeable zinc-specific fluorophore ZincBY-1 (Kong et al., 2015; Que et al., 2015). ZincBY-1 was prepared as a 50-μM stock solution in DMSO and diluted 1:1000 in TL-HEPES. For staining intracellular labile zinc, eggs were incubated in 50 nM ZincBY-1 for 30 min. Eggs were placed in a 360-μl drop in Ca2+-free hCZB medium in a coverslip-bottom imaging dish (MatTek Corp, Ashland, MA) (Yoon et al., 2008). Eggs were examined on an SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL) with a 20× objective, and HeNe (543 nm), Ar (488 nm) and near-UV (405 nm) laser lines. Eggs were activated with 40 μl of 10× ionomycin stock (25 μM for mouse, 50 μM for human) solution introduced to the imaging drop 1 min after the start of monitoring.
Mouse intracytoplasmic sperm injection
After treatment with hyaluronidase to remove cumulus cells, sperm microinjection was performed using a piezo micropipette-driven unit (Prime Tech LTD, PMM-150FU) with mercury (Sigma-Aldrich, USA) following protocols previously described (Kurokawa and Fissore, 2003). All manipulation was carried out using hCZB medium at 37°C under light mineral oil at room temperature. For injection, spermatozoa from the cauda epididymis were collected and placed in 500 μl of HTF (human tubal fluid, Millipore Sigma) media. After 10 min incubation at 37°C (sperm swim-out), epididymis tissue was removed. Sperm heads were then separated from the tails using sonication (B2500A-MTH, VWR, Radnor, PA) and microinjected using a piezo micropipette by applying pulses of different intensities to penetrate first the ZP (higher intensity) and then plasma membrane (lower intensity) of the egg. After plasma membrane penetration, the sperm head was released into the ooplasm and the pipette carefully withdrawn from the egg (Hachem et al., 2017).
Mouse in vitro fertilisation
Spermatozoa from the cauda epididymis were collected and placed in 1 ml of HTF (human tubal fluid, Millipore Sigma, Burlington, MA) media. After a 10-min incubation at 37°C (sperm swim-out), epididymis tissue was removed, and the sperm were capacitated in a 5% CO2 incubator at 37°C for 1 h. Cumulus oocyte complexes were placed into a well with 1 ml of HTF media previously equilibrated in an incubator with 5% CO2 at 37°C. Then, 20–30 eggs (untreated control eggs or eggs treated with 15 μM ML-7) were loaded per fertilisation well, and inseminated with capacitated sperm to a final concentration of 1 × 106 cells/ml (Navarrete et al., 2016). In the ML-7-treated cohort, ML-7 was added to HTF media at a final concentration of 15 μM for the mouse eggs (Matson et al., 2006). Pronuclei formation was scored 5 h after insemination. Fertilised oocytes were identified by the presence of two or multiple PNs. Embryos were then fixed in 3.8% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in PBS for 1 h at room temperature. The eggs were mounted in Vectashield Mounting Media with DAPI (Vector Laboratories, Burlingame, CA, USA) and examined on an SP5 confocal microscope with a ×40 oil-immersion objective and near-UV laser line (405 nm) for fluorescent imaging.
Statistical analysis
Statistical analysis was performed using Prism software (GraphPad Prism 5, La, Jolla, CA). Data were tested for normal distribution with the D’ Agostino & Pearson omnibus normality test and Sharpiro–Wilk normality test. Normal distribution data were compared between two groups using Student’s t tests and non-normal distribution data were analysed Mann–Whitney test. Non-normally distributed data (CG distance from the plasma membrane and human egg CG and ZincBY1 fluorescence intensities) were analysed using the Kruskal–Wallis test. Data are presented as the mean ± SEM for normally distributed data and as the median with first and third quartile for non-normal distribution data. Statistically significant differences were indicated by P values < 0.05.
Results
ML-7 inhibits CG localisation to the cortex
To determine that ML-7 was functional in our system, we used an IVF assay to screen for polyspermy and PN formation. ML-7 treatment should inhibit CG exocytosis and thereby increase polyspermy due to the lack of ZP hardening. As expected, ML-7-treated mouse eggs fertilised through IVF demonstrated a significantly increased percentage of polyspermy as evidenced by zygotes with greater than three PN (control eggs: 0/9 multiple PN; vs. ML-7-treated eggs: 8/12 multiple PN; Supplementary Fig. S1A and B). These results confirmed the function of this inhibitor which was subsequently used throughout this study in both mouse and human eggs.
To further confirm this finding that ML-7 inhibits CG exocytosis during egg activation, eggs were fixed and stained for CGs using fluorescently labelled LCA after ML-7 treatment and activation with ionomycin. As anticipated, activation with ionomycin led to a shift in the localisation of CGs to the outer cortex (Fig. 1A), while CGs remained dispersed throughout the sub-cortical region of non-activated MII eggs. The precise subcortical localisation of CGs was established using LCA-based fluorescence intensity histograms assembled from vector lines bisecting the plasma membrane (PM) of representative eggs (yellow lines drawn through egg PMs in Fig. 1B). In ionomycin-activated controls, CGs were highly enriched at the PM, where they formed a sharp and continuous half-ring around the egg (Fig. 1A and B); whereas in non-activated MII and ML-7-treated activated eggs, CGs were observed in a more disperse region throughout the PM and subcortical cytoplasm and were observed in a discontinuous punctate pattern (Fig. 1A and B). Furthermore, the average distance of CGs from the PM varied between ML-7-treated and untreated eggs after activation with ionomycin, but did not vary between non-activated eggs and ML-7-treated eggs after activation (non-activated MII eggs: median = 2.410, 25% = 1.74, 75% = 5.25 n = 25; Control activated eggs: median = 0.866, 25% = 0.4865, 75% = 1.44, n = 25; ML-7-treated activated eggs: median = 2.980, 25% = 1.245, 75% = 5.205, n = 25; P < 0.0001, Fig. 1C). Thus, CG distribution in ML-7-treated eggs after activation is most similar to that in unactivated eggs. These findings confirm that ML-7 treatment inhibits CG trafficking to the PM during egg activation with ionomycin.
Figure 1.
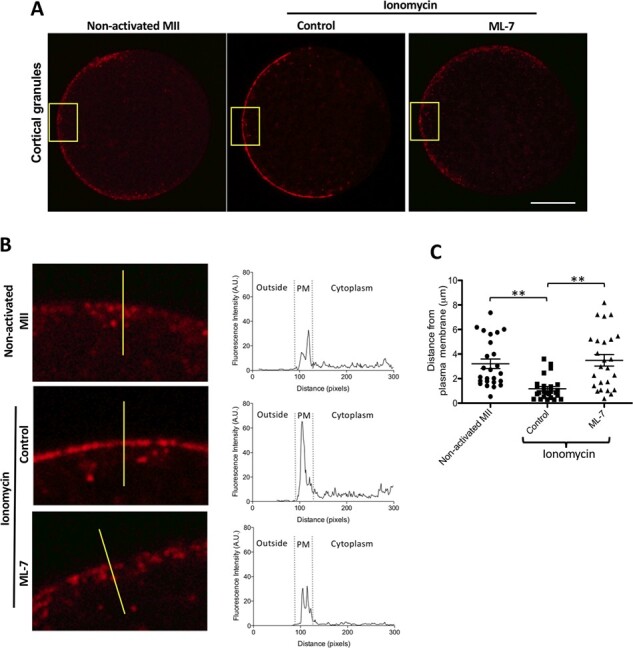
ML-7 inhibits CG localisation to the outer cortex after activation of mouse eggs with ionomycin. (A) The cellular distribution of CGs (LCA) in representative non-activated, ionomycin-activated (control) and ML-7 treated and ionomycin-activated mouse MII eggs. Scale bar, 20 μm. (B) Highly magnified images of the PM and adjacent cortex from eggs in A (yellow square) with the distribution of their fluorescence mapped in histograms composed from vector lines bisecting their PM, from outside the cell on the left towards the cytoplasm on the right, (represented by yellow lines in the left panels of B). (C) A dot graph quantifying the distances of CGs (punctate fluorescent intensities) from the PM (n = 25). Data were presented as the median of at least three independent experiments (** P < 0.001).
ML-7 reduces zinc mobilisation and exocytosis in ionomycin-activated mouse eggs
To determine whether MLCK inhibition additionally disrupts the zinc spark, we treated murine eggs with ML-7 and activated them with ionomycin during live fluorescent imaging to visualise labile zinc. Zinc release was imaged using extracellular FluoZin-3 in the culture media surrounding eggs during activation, either in the presence or absence of ML-7. Intracytoplasmic calcium was imaged simultaneously in a different channel using Fura-2AM fluorescence (Fig. 2A). The intensity of extracellular zinc sparks upon activation was prominent in control eggs compared to those treated with ML-7. Activated ML-7-treated eggs demonstrated a 79% reduction in the zinc fluorescence amplitude compared to controls (Fig. 2A–C, Supplementary Movie S1). This reduction of the zinc spark, however, occurred without any differences in calcium amplitude between the treatment groups, demonstrating that the effect of ML-7 is specific to Zn2+ exocytosis and does not perturb intracellular calcium transients within the egg (Fig. 2B and C). To examine the effect of ML-7 treatment on intracellular zinc, we examined the spatial distribution and change in labile zinc intensity throughout egg activation using the intracellular probe ZincBY1 (Fig. 2D, Supplementary Fig. S2A) (Que et al., 2015). Upon activation with ionomycin, ML-7-treated eggs demonstrated a reduced change in the intensity (ΔF) of ZincBY1 signal at the PM relative to control eggs (Fig. 2D). We visualised zinc using fluorescence intensity histograms that bisected the PM of representative eggs (yellow lines across PMs in Fig. 2E). This analysis revealed that control eggs have a high amplitude of ZincBY1 intensity concentrated at the PM, which is then reduced by ~ 18% after activation. ML-7-treated activated eggs also showed a reduction in PM-associated ZincBY1 intensity; however, this reduction was considerably less than in the controls (Fig. 2E). Upon quantification, ML-7-treated eggs exhibited 51% less change in ZincBY-1 signal (ΔF) upon activation compared with controls (∆F, Control activated eggs: 0.177 ± 0.02047 vs ML-7-treated activated eggs: 0.088 ± 0.0086, Fig. 2F and G). In conclusion, in non-activated eggs, there is strong ZincBY1 fluorescent signal at the cortex of the MII egg near the PM, which upon activation is dramatically reduced; however, if eggs are treated with ML-7, this reduction does not occur. Together, these data demonstrate that the ionomycin-induced zinc spark is sensitive to MLCK inhibitors for both the intracellular zinc mobilisation to the PM and zinc exocytosis from the PM (zinc spark) in mouse eggs.
Figure 2.
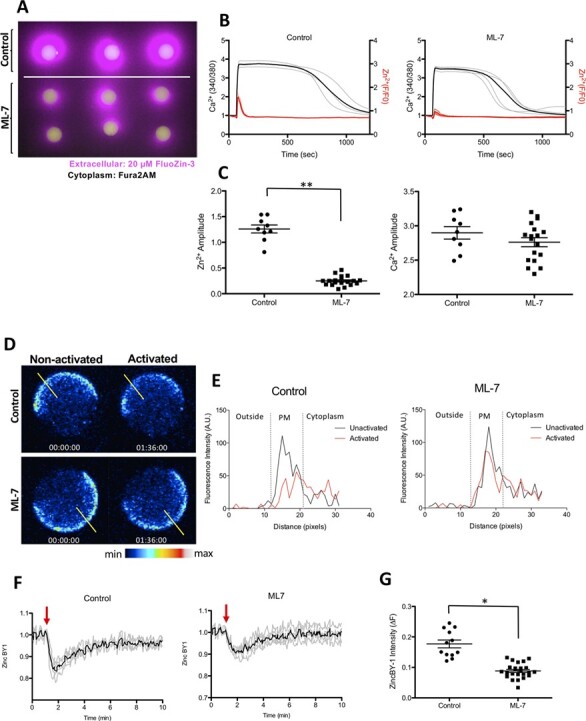
ML-7 treatment blunts the zinc spark in ionomycin-activated mouse eggs and reduces zinc localisation to the cortex. (A) The moment of the zinc spark (detected by 20 μM Fluozin-3) in control and ML-7-treated mouse MII eggs in response to activation by 2.5 μM ionomycin. (B) Zinc and calcium profiles in control and ML-7-treated mouse eggs. Representative time traces of normalised zinc fluorescence (red, F/F0) and calcium fluorescence (black. 340/380) are shown for control and ML-7-treated mouse MII eggs. (C) The amplitudes of the zinc spark and calcium rise in control and ML-7 treated eggs. (D) A representative of the time course montage of the intensity of intracellular labile zinc located in the cortex of 5 μM ionomycin-activated control and ML-7-treated mouse eggs. The LUT colour scale is displayed at the bottom. (E) Histograms of the fluorescence intensity zincBY1 in eggs across their PMs. Yellow lines were drawn through the eggs, and pixel intensities were quantified, with outside the cell, PM and cytoplasm regions noted. (F) Representative ZincBY-1 intensity profiles of ionomycin-induced control and ML-7 treated eggs. (G) A dot graph quantifying the change in ZincBY-1 intensity (ΔF) across activation; ML-7 treated eggs exhibited 51% less reduction in their ZincBY1 staining after activation compared with control eggs. Red arrows signify the addition of ionomycin. Data were presented as the mean (mean ± standard error or the mean) of at least three independent experiments. (* P < 0.05, ** P < 0.01).
ML-7 treatment reduces zinc localisation in the cortex and reduces zinc spark amplitude after ionomycin-induced activation in human eggs
Next, we translated these murine findings to human eggs. As observed in the mouse, egg activation via multiple parthenogenetic means (Saunders et al., 2002) induces the zinc spark in human MII eggs (Duncan et al., 2016). Thus, we tested whether ML-7 treatment combined with ionomycin activation would affect CG trafficking and reduce the zinc spark intensity of human eggs. First, human eggs were stained with LCA to observe the distribution of CGs before and after activation with ionomycin. Human unactivated control MII eggs had a clear staining pattern forming a continuous layer with visible punctate CGs; in contrast, activated control eggs exhibited a discontinuous staining pattern of CGs along the PM (Fig. 3A). Compared with non-activated eggs, ML-7-treated eggs had a reduced abundance of CGs at the PM after activation, but in contrast to control activated eggs, they maintained a mostly continuous ring around the PM. To observe the specific localisation of CGs around the PM and the subcortical cytoplasm, vector lines bisecting the PM were utilised to create fluorescence histograms for representative eggs (yellow lines, Fig. 3B). Non-activated MII eggs demonstrated a high CG intensity at the PM, which was significantly abrogated in untreated activated egg. ML-7 mitigated this observed decrease following activation (Fig. 3B). To quantify these results, we created donut-shaped regions of interest (ROI) circumscribing the full PM to measure the total intensity of CGs between different treatment groups (Fig. 3C). The total intensity (A.U.) of CGs decreased in both activated groups; however, ML-7-treated activated eggs had significantly less reduction in CG fluorescent intensity than control activated eggs (non-activated MII eggs: median = 25.08, 25% = 17.03, 75% = 33.319, n = 2; control activated MII eggs: median = 6.585, 25% = 6.199, 75% = 7.6895, n = 5; ML-7-treated activated MII eggs: median = 9.163, 25% = 7.992, 75% = 11.806, n = 5; * P < 0.05, ** P < 0.001, Fig. 3D). These data suggest that similar to our murine results, ML-7 inhibits CG mobilisation and exocytosis from the PM in human eggs activated with ionomycin.
Figure 3.
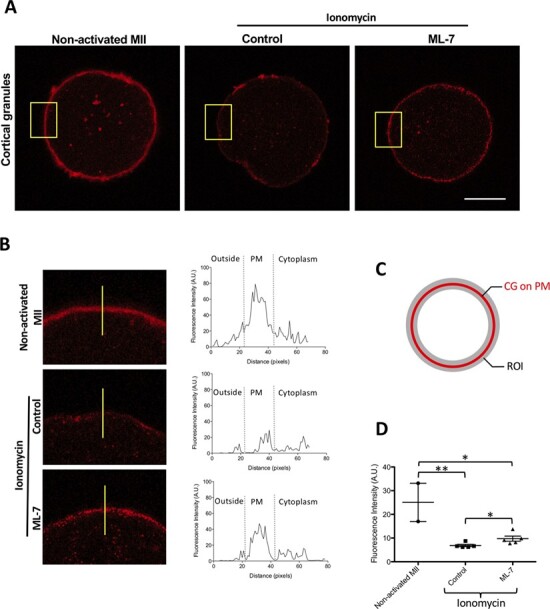
CG staining decreases in the cortex after activation of human eggs with ionomycin. (A) The cellular distribution of CGs (LCA) in a non-activated egg, an ionomycin-activated control egg and a ML-7-treated and ionomycin-activated egg. Scale bar, 50 μm. (B) A zoomed area of the PM and surrounding areas (yellow square) and fluorescence intensity on a line crossing the cell from outside the cytoplasm (represented yellow line, left panel). The distribution of CGs was examined in non-activated and control and ML-7-treated activated MII eggs. Intensity profiles of LCA staining perpendicularly across the PM and into the cytosol of eggs (right panel in (B). (C) The ROI to measurement of total intensity of CGs in PM. (D) The fluorescence intensity of CGs was measured in non-activated, activated control and activated ML-7 eggs. Data were presented as the median. (* P < 0.05, ** P < 0.001).
To determine whether Zn2+ exocytosis is similarly reduced in humans when CG exocytosis is perturbed, eggs were activated with ionomycin and zinc sparks were visualised. After activation, the ML-7 treated group had a 67% reduction in zinc spark amplitude compared to untreated control eggs (untreated activated eggs: 1.298 ± 0.1467, n = 7; vs. ML-7-treated activated eggs: 0.4336 ± 0.0589, n = 9; Fig. 4A–C, Supplementary Fig. S3A and B, Supplementary Movie S2). Similar to results in the mouse, calcium transients were not significantly affected, demonstrating that the influence of ML-7 on zinc exocytosis is downstream of Ca-CAM binding events. We next used ZincBY1 to follow intracellular zinc dynamics in activated human eggs. As occurred in the mouse, ML-7 treatment inhibited the release of cortical intracellular zinc following human egg activation compared to control activated eggs (Fig. 4D, Supplementary Fig. S2B, Supplementary Movie S3). This is as expected, because if ML-7 prevents zinc exocytosis from occurring during egg activation, it would cause less reduction in intracellular zinc compared with untreated control eggs. Fluorescence intensity histograms were used to visualise the precise localisation of zinc in representative eggs across activation. In non-activated eggs, labile zinc localised near the PM and within the cortex of the egg in both control and ML-7-treated conditions. However, upon the addition of ionomycin, control eggs exhibited a far more prominent reduction in ZincBY1 intensity (A.U.) near their PM than did ML-7-treated eggs, which retained a relatively reduced but still dominant peak of intensity at the PM even after activation (Fig. 4E). Similarly, the relative intensity of ZincBY1 only declined in control activated eggs, and not in ML-7-treated eggs, upon activation, which we quantified based on a donut-shaped ROI circumscribing the full PM to measure the total intensity of ZincBY1 between different treatment groups (∆F, control activated eggs: median = 0.2049, 25% = 1.1031, 75% 0.3066, n = 2 vs ML-7 treated activated eggs: median = 0.04024, 25% = 0.0326, 75% = 0.0612, Fig. 4D, F and G). Despite the small sample size per group, our results collectively suggest that trafficking of CGs loaded with labile zinc is sensitive to MLCK inhibition in the human egg, similar to that observed in the mouse.
Figure 4.
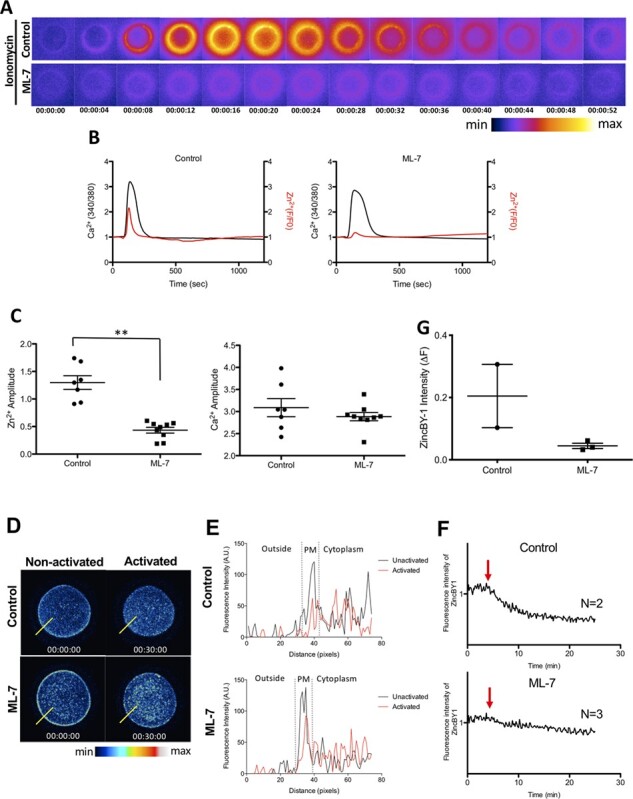
The zinc spark is blunted, and labile zinc localisation to the cortex is reduced, in ML-7-treated human eggs after activation by ionomycin. (A) A time-lapse montage demonstrating representative zinc sparks (detected by 20 μM Fluozin-3) in control and ML-7-treated human MII eggs in response to activation by 5 μM ionomycin. The LUT colour scale is displayed at the bottom. (B) Representative time traces of normalised zinc fluorescence (red, F/F0) and calcium fluorescence (black, 340/380) are shown for control and ML-7-treated human MII eggs. (C) A dot graph quantifying the amplitudes of the zinc sparks and calcium transients in control and ML-7-treated eggs; ML-7-treated eggs demonstrated a 67% reduction in their zinc spark amplitude compared with controls (** P < 0.001; non-treated activated eggs: 1.298 ± 0.1467, n = 7; vs. ML-7-treated activated eggs: 0.4336 ± 0.0589, n = 9). (D) Representative images of ZincBY-1 staining in human eggs, demonstrating their intracellular zinc intensity and location between control and ML-7-treated eggs, both before and after activation by ionomycin. The LUT colour scale is displayed at the bottom. (E) A representative histogram of ZincBY1 fluorescence intensity across the PM of human eggs after activation in control versus ML-7 treatment groups (assembled using the yellow lines drawn through eggs in D). (F) Representative time traces of normalised labile zinc fluorescence in the cortical region of activated human eggs. Red arrows signify the addition time of ionomycin. (G) A dot graph quantifying the change in ZincBY-1 intensity (ΔF) across activation. Data were presented as the median. (* P < 0.05, ** P < 0.001).
ML-7 reduces zinc exocytosis in mouse eggs fertilised through intracytoplasmic sperm injection
To determine how MLCK inhibition through ML-7 affects sperm-induced egg activation, we performed ICSI on untreated and ML-7-treated mouse MII eggs. Due to ethical considerations and legal restrictions concerning the fertilisation of human eggs for research, only mouse eggs were used for all fertilisation experiments (Tingen et al., 2010). Upon ICSI of ML-7-treated mouse eggs, we observed a 25% reduction in the zinc amplitude measured by FluoZin-3 compared with untreated eggs. However, no significant changes were observed between groups in calcium amplitude measured by Fura-2AM (Fig. 5A and B). These data suggest that ML-7-mediated inhibition of zinc exocytosis is not restricted to parthenogenetic activation but is also conserved in sperm-mediated fertilisation.
Figure 5.
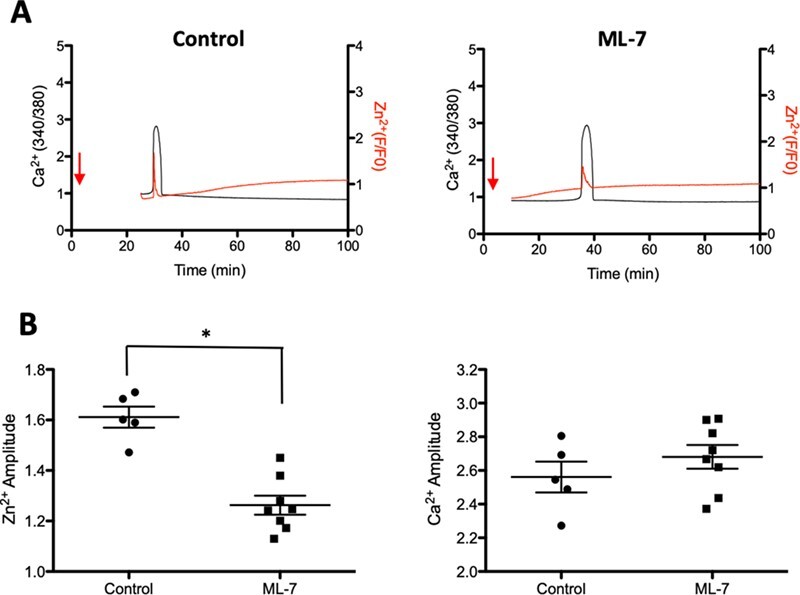
ML-7 treated mouse eggs have a reduced zinc spark upon ICSI. (A) Representative time traces of the zinc spark (F/F0) and calcium transients of mouse eggs upon fertilisation via ICSI, of ML-7 treated or control eggs. Red arrows signify the injection of sperm. (B) Dot graphs quantifying the amplitudes of the intensity of calcium and zinc sparks after ICSI between ML-7-treated (N = 5) and control groups (N = 8). ML-7-treated eggs had a 25% reduced zinc spark amplitude after fertilisation via ICSI compared with controls. Data were presented as the mean (mean ± standard error or the mean). (* P < 0.05).
Discussion
Zinc sparks are known to originate from a system of peripheral vesicles that are enriched in labile zinc. In mouse eggs, these vesicles have an average diameter of ~250 nm and contain on average one million Zn2+ ions. Recent studies (Tokuhiro and Dean, 2018) reveal that labile zinc pools in mouse eggs colocalise with ovastacin, a well-established CG marker. In this study, we tested whether CG exocytosis is at the heart of the zinc spark mechanism in human eggs. Initially, we confirmed that MLCK inhibition, through treatment with its inhibitor ML-7, was associated with decreased CG localisation to the PM upon activation with ionomycin in the mouse egg. Then using ML-7 as a tool to inhibit CG exocytosis, we investigated the effects of MLCK inhibition upon the zinc spark using ionomycin as an activator. We found reduced zinc exocytosis in ML-7-treated eggs compared with untreated ones, in both mouse and human eggs. Of note, this blockade in zinc exocytosis occurred in the presence of normal calcium transients, demonstrating MLCK to be dispensable for intracellular calcium movement, but obligatory for CG and zinc migration and exocytosis. These findings were not limited to parthenogenesis, because similar zinc and calcium findings were obtained when murine eggs were fertilised through ICSI. Based on these results, we conclude that MLCK is necessary for CG-based zinc trafficking and exocytosis during fertilisation/activation in the mouse and human.
Although we were unable to simultaneously track both labile Zn2+ and CGs during live fluorescent imaging (LCA labelling was performed on eggs post-fixation), the symmetry between the reduction of the zinc spark with the subsequent inhibition of CG localisation to the outer cortex strongly suggests that the Zn2+ necessary for the zinc spark is housed within CGs, consistent with previous reports by Tokuhiro et al. and Que et al. (Que et al., 2015; Tokuhiro and Dean, 2018). A further limitation to the current study is our inability to fertilise human MII eggs, per ethical considerations and legal restrictions against the utilisation of human embryos for basic science research. All human MII eggs used in this study originated from women aged 35–39 years of age, who were undergoing IVF for infertility. Therefore, the human samples tested in this study might not accurately represent eggs from younger individuals or those without a history or diagnosis of infertility. Despite these limitations, the similarities between the parthenogenetic activation of murine and human eggs suggest similar inhibition of the zinc spark would have occurred in the case of human ICSI, had it been performed. These results increase confidence in the utility of the zinc spark as a biomarker of developmental competence, perhaps in conjunction with ICSI to screen for and select the best eggs for embryo transfer following fertilisation.
The results of these studies shed new light on the possible mechanisms through which the zinc spark acts as a predictor of fertilisation quality and embryogenesis potential. As we have shown previously, the amplitude of the zinc spark correlates well with developmental outcomes after fertilisation in mouse eggs, posing as a useful biomarker for fertilisation studies (Matson et al., 2006; Que et al., 2015; Zhang et al., 2016; Tokuhiro and Dean, 2018). The current study, is the first to demonstrate that a targeted perturbation of MLCK, previously shown to inhibit CG exocytosis, also results in a significant reduction of the zinc spark (Matson et al., 2006; Que et al., 2015; Tokuhiro and Dean, 2018; Vogt et al., 2019). Collectively, these data provide strong corroboration for the identification of CGs as the primary zinc-containing vesicle from which the zinc sparks originate, and for the first time, we have shown that the mechanism is conserved in humans.
The role of Zn2+ following fertilisation is less defined than the periods leading up to and during fertilisation. The effects of excess zinc upon a fertilised egg after incomplete CG/Zn2+ exocytosis are still poorly understood. We have postulated that labile Zn2+ fluxes act as ‘switch mechanisms’ regulating the oocyte’s cell cycle and meiotic progression throughout transcriptional quiescence. Specifically, intracellular changes in zinc concentration around a threshold value may control the egg’s ability to end its second meiotic arrest at MII and resume meiosis, ultimately progressing into mitosis after fertilisation (Bernhardt et al., 2012). One plausible mechanism is that the endogenous meiosis inhibitors of the egg rely upon cytosolic zinc levels exceeding a threshold value before the inhibition function is manifest. A top candidate for this hypothesis is Emi2, a meiosis inhibitor and antagonist of the Anaphase Promoting Complex/Cyclosome (APC/C), with known zinc-binding regions (Bernhardt et al., 2012). Whether or not Emi2 function differs between varying zinc spark amplitudes or degrees of CG exocytosis has never been directly studied and presents an interesting hypothesis through which temporal fluctuations in Zn2+ localisation might regulate meiotic arrest. If this is observed to occur, the zinc spark could neatly serve as both a biomarker for the block to polyspermy and a powerful predictor of meiotic resumption in the activated egg.
Cortical reactions of the fertilised murine egg are also known to be perturbed in a variety of artificial settings, including delayed fertilisation of MII eggs (Miao et al., 2009; Ajduk et al., 2011; Bernhardt et al., 2012; Lord and Aitken, 2013). The effects of delayed fertilisation (post-ovulatory age) have been relatively understudied compared to the increased biological age of an organism. However, post-ovulatory aged eggs have decreased MLCK activity, such that ML-7-treated ‘young’ eggs (with no delay in fertilisation) phenocopy the age-dependent fertilisation defects, including defects in fertilisation cone formation (Mackenzie et al., 2016). Delayed fertilisation often results in polyspermy, consistent with the existence of a natural time-dependent diminishing of the egg’s ability to surmount an effective modification to the ZP. This encompasses multiple processes which occur in preparation for fertilisation, including the loading and exocytosis of intracellular stores of ZP-modifying ovastacin into CGs, which are first trafficked and anchored to the PM (Austin and Braden, 1953; Yanagimachi and Chang, 1961; Ajduk et al., 2011; Bernhardt et al., 2012; Vogt et al., 2019). Given the importance of vesicle trafficking for the zinc spark, and the use of the zinc spark in the prediction of embryonic development, the ability to reduce or rescue zinc and CG migration and exocytosis from the egg could raise innovative opportunities to enhance egg quality for IVF, as well as provide targets for non-hormonal contraceptive strategies.
Conclusions
We found that ML-7-treated mouse eggs have a decreased zinc spark intensity compared to untreated control eggs, regardless of whether parthenogenetic activation (ionomycin) or fertilisation (ICSI) is performed. CGs in ML-7-treated eggs did not migrate towards the egg PM at the time of egg activation, and this was coincident with maintenance of labile zinc at the cortex. Blocking CG exocytosis with ML-7 led to a simultaneous decrease in zinc exocytosis, as indicated by lower zinc spark amplitudes. A dramatically reduced zinc spark was also observed in experiments with human eggs, suggesting that CGs are also the zinc-enriched structure responsible and necessary for the zinc spark in humans. Collectively, these data stress the importance of MLCK function for CG zinc intracellular trafficking and exocytosis, as measures to assess in the prediction of developmental competency in both mouse and human eggs. Further investigations are needed to elucidate the exact mechanisms through which the Zn2+ efflux impacts cell-cycle arrest of the MII egg and predicts embryogenesis, as well as to further observe and characterise zinc dynamics post-fertilisation.
Supplementary Material
Acknowledgements
We thank Dr Mary Ellen Pavone, the Director of the Northwestern Tissue Library, as well as the embryology team at Northwestern Memorial Hospital, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility (Dr John Zhang, Karen Horan, and Jaclyn Lambe-Steinmiller), for assistance with the collection of de-identified immature human oocytes. We also thank Megan Elizabeth Connolly and Rafael Confino for their clinical research coordination efforts and regulatory and compliance support.
Authors’ roles
All experimentation and writing were performed by H.C.L. with contributions in data analysis and writing of the manuscript by M.E.E. F.E.D. additionally contributed to the writing of this manuscript and the experimental design. T.V.O. and T.K.W. were the co-principal investigators for this work and oversaw the design and execution of the experimentation, as well as the writing of the manuscript.
Funding
Thomas J. Watkins Endowment Research grant (TKW) from Ferring Pharmaceuticals Inc.; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, P50HD076188 to T.K.W.). Due to legal restrictions, no funds from the NICHD, or any other federal source, were used for the human egg activation experiments. Instead, the human work reported in this manuscript was completely funded through a research grant from Ferringx Pharmaceuticals.
Conflict of interest
There are no conflicts of interest, for any of the contributing authors towards the completion of this work and the submission of this manuscript.
References
- Ajduk A, Ilozue T, Windsor S, Yu Y, Seres KB, Bomphrey RJ, Tom BD, Swann K, Thomas A, Graham C et al. Rhythmic actomyosin-driven contractions induced by sperm entry predict mammalian embryo viability. Nat Commun 2011;2:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR, Braden AW. An investigation of polyspermy in the rat and rabbit. Aust J Biol Sci 1953;6:674–692. [PubMed] [Google Scholar]
- Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Zinc requirement during meiosis I-meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod 2011;84:526–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 2012;86:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick AR. Molecular mechanisms of nonmuscle myosin-II regulation. Curr Opin Cell Biol 1999;11:26–33. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod 2002;17:1006–1016. [DOI] [PubMed] [Google Scholar]
- de Paola M, Bello OD, Michaut MA. Cortical granule exocytosis is mediated by alpha-SNAP and N-ethilmaleimide sensitive factor in mouse oocytes. PLoS One 2015;10:e0135679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducibella T, Fissore R. The roles of Ca2+, downstream protein kinases, and oscillatory signaling in regulating fertilization and the activation of development. Dev Biol 2008;315:257–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Que EL, Zhang N, Feinberg EC, O'Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep 2016;6:24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrich L, Grau N, de los Santos MJ, Romero JL, Pellicer A, Escriba MJ. The dynamics of in vitro maturation of germinal vesicle oocytes. Fertil Steril 2012;98:1147–1151. [DOI] [PubMed] [Google Scholar]
- Green KM, Kim JH, Wang WH, Day BN, Prather RS. Effect of myosin light chain kinase, protein kinase A, and protein kinase C inhibition on porcine oocyte activation. Biol Reprod 1999;61:111–119. [DOI] [PubMed] [Google Scholar]
- Hachem A, Godwin J, Ruas M, Lee HC, Ferrer Buitrago M, Ardestani G, Bassett A, Fox S, Navarrete F, de Sutter P et al. PLCzeta is the physiological trigger of the Ca(2+) oscillations that induce embryogenesis in mammals but conception can occur in its absence. Development 2017;144:2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Bernhardt ML, Kong BY, Ahn RW, Vogt S, Woodruff TK, O'Halloran TV. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem Biol 2011;6:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010;6:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong BY, Duncan FE, Que EL, Xu Y, Vogt S, O'Halloran TV, Woodruff TK. The inorganic anatomy of the mammalian preimplantation embryo and the requirement of zinc during the first mitotic divisions. Dev Dyn 2015;244:935–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas AR, Wolfner MF. Molecular changes during egg activation. Curr Top Dev Biol 2013;102:267–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Fissore R. ICSI-generated mouse zygotes exhibit altered calcium oscillations, inositol 1,4,5-trisphosphate receptor-1 down-regulation, and embryo development. Mol Hum Reprod 2003;9:523–533. [DOI] [PubMed] [Google Scholar]
- Lee HC, Yoon SY, Lykke-Hartmann K, Fissore RA, Carvacho I. TRPV3 channels mediate Ca(2)(+) influx induced by 2-APB in mouse eggs. Cell Calcium 2016;59:21–31. [DOI] [PubMed] [Google Scholar]
- Lord T, Aitken RJ. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013;146:R217–R227. [DOI] [PubMed] [Google Scholar]
- Mackenzie AC, Kyle DD, McGinnis LA, Lee HJ, Aldana N, Robinson DN, Evans JP. Cortical mechanics and myosin-II abnormalities associated with post-ovulatory aging: implications for functional defects in aged eggs. Mol Hum Reprod 2016;22:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson S, Markoulaki S, Ducibella T. Antagonists of myosin light chain kinase and of myosin II inhibit specific events of egg activation in fertilized mouse eggs. Biol Reprod 2006;74:169–176. [DOI] [PubMed] [Google Scholar]
- Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update 2009;15:573–585. [DOI] [PubMed] [Google Scholar]
- Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev 2012;79:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete FA, Alvau A, Lee HC, Levin LR, Buck J, Leon PM, Santi CM, Krapf D, Mager J, Fissore RA et al. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci Rep 2016;6:33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que EL, Bleher R, Duncan FE, Kong BY, Gleber SC, Vogt S, Chen S, Garwin SA, Bayer AR, Dravid VP et al. Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nat Chem 2015;7:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que EL, Duncan FE, Bayer AR, Philips SJ, Roth EW, Bleher R, Gleber SC, Vogt S, Woodruff TK, O’Halloran TV. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr Biol 2017;9:135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que EL, Duncan FE, Lee HC, Hornick JE, Vogt S, Fissore RA, O'Halloran TV, Woodruff TK. Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation. Theriogenology 2019;127:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders C, Larman M, Parrington J, Cox L, Royse J, Blayney L, Swann K, Lai F. PLC zeta: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 2002;129:3533–3544. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC. Full-term mouse development by abolishing Zn2+-dependent metaphase II arrest without Ca2+ release. Development 2010;137:2659–2669. [DOI] [PubMed] [Google Scholar]
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development 2009;136:3033–3042. [DOI] [PubMed] [Google Scholar]
- Tingen CM, Kim AM, Wu PH, Woodruff TK. Sex and sensitivity: the continued need for sex-based biomedical research and implementation. Womens Health (Lond) 2010;6:511–516. [DOI] [PubMed] [Google Scholar]
- Tokuhiro K, Dean J. Glycan-independent gamete recognition triggers egg zinc sparks and ZP2 cleavage to prevent polyspermy. Dev Cell 2018;46:627, e625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt EJ, Tokuhiro K, Guo M, Dale R, Yang G, Movilla MJ, Shroff H, Dean J. Anchoring cortical granules in the cortex ensures trafficking to the plasma membrane for post-fertilization exocytosis. Nat Commun 2019;10:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction 2015;150:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R, Chang MC. Fertilizable life of golden hamster ova and their morphological changes at the time of losing fertilizability. J Exp Zool 1961;148:185–203. [DOI] [PubMed] [Google Scholar]
- Yoon S-YY, Jellerette T, Salicioni AM, Lee HC, Yoo M-SS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J Clin Invest 2008;118:3671–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Duncan FE, Que EL, O'Halloran TV, Woodruff TK. The fertilization-induced zinc spark is a novel biomarker of mouse embryo quality and early development. Sci Rep 2016;6:22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


