Abstract
Blast exposures are a hallmark of contemporary military conflicts. We need improved preclinical models of blast traumatic brain injury for translation of pharmaceutical and therapeutic protocols. Compared with rodents, the ferret brain is larger, has substantial sulci, gyri, a higher white to gray matter ratio, and the hippocampus in a ventral position; these attributes facilitate comparison with the human brain. In this study, ferrets received compressed air shock waves and subsequent evaluation of glia and forms of tau following survival of up to 12 weeks. Immunohistochemistry and Western blot demonstrated altered distributions of astrogliosis and tau expression after blast exposure. Many aspects of the astrogliosis corresponded to human pathology: increased subpial reactivity, gliosis at gray-white matter interfaces, and extensive outlining of blood vessels. MRI analysis showed numerous hypointensities occurring in the 12-week survival animals, appearing to correspond to luminal expansions of blood vessels. Changes in forms of tau, including phosphorylated tau, and the isoforms 3R and 4R were noted using immunohistochemistry and Western blot in specific regions of the cerebral cortex. Of particular interest were the 3R and 4R isoforms, which modified their ratio after blast. Our data strongly support the ferret as an animal model with highly translational features to study blast injury.
Keywords: Aquaporin, Astrocyte, Blood vessels, Phosphorylated tau, Pia, Tau isoforms
INTRODUCTION
The effects of exposure to blast in people are cumulative and persistent (1, 2). Blasts can cause damage that involves blood vessels, brain parenchyma, and white matter among other structures (3). In order to successfully improve treatments, appropriate animal models are essential (4–6). The ferret is an excellent model to study blast injury, with specific advantages relative to the lissencephalic rodent. The gyrencephalic ferret brain contains substantial amounts of white matter, sulci and gyri, and possesses a hippocampus predominantly in a ventral position (7). These more complex anatomical features are similar to those of humans, making the ferret an important model to study with strong translational capability.
Multiple questions remain regarding the precise neuropathology that occurs following blast exposure. For many of those serving in the military, blast exposure is a frequent occurrence that leads to persistent symptoms including headache, anxiety, problems with sleep, memory impairment, and prolonged pain, among others (8–11). Overall, we need clarity in understanding the neuropathologic effects of blast exposure. It is also not obvious whether people subjected to blast are likely to develop premature dementia or chronic traumatic encephalopathy (CTE), although several reports find blast exposure leads to neuropathology typical of this problem (12, 13).
One of the important components of CTE is stated to be the aberrant expression of tau and phosphorylated tau (14–18). Tau is reported to stabilize microtubules, as well as playing a role in axonal transport and cell morphology (19, 20). Tau phosphorylation occurs as a normal process on multiple sites of the tau molecule, but in several conditions including blast injury, can become abnormally phosphorylated, or hyperphosphorylated, resulting in impaired function that may occur after traumatic brain injury (TBI) (12, 21, 22). An animal model expressing distinct forms of tau and phosphorylated tau in response to brain injury would be an important resource to the research community. A study of combat-related blast injuries, however, reported that the predominant neuropathologic feature is astrogliosis, with only sporadic evidence of tauopathy (11). Therefore, an animal model demonstrating persistent astrogliosis neuroanatomically similar to the human distribution would also be a strong adjunct to current experimental models.
Alternative splicing of tau results in 6 isoforms in the human brain (23). The microtubule binding domains of tau consist primarily of the 3R and 4R isoforms (24, 25). The human 3R and 4R isoforms of tau show expression in roughly equal amounts, but the ratio differs in a number of disease conditions (20, 25, 26). Again, an animal model normally expressing these 2 tau isoforms critical to neurogenerative diseases would be of strong benefit to study the effect of various injuries. To use the ferret as a scalable model for TBI and blast exposure would be important to evaluate isoform expression and response to injury.
The studies presented here use blast injury with ferrets and assess a survival period including 1, 4, or 12 weeks. Overall, we found that alterations in expression of forms of tau and reactivity of glial cells increased with survival time. Ferrets display a distribution of increased astrogliois similar in many respects to the human pattern of those exposed to blast. This includes overall increases in generalized white matter, white-gray matter interface regions, the subpial region and surrounding blood vessels. Furthermore, significantly different increases and decreases in several forms of tau are present after injury in ferrets in specific neocortical regions and exhibit patterns that relate to those found in people.
MATERIALS AND METHODS
Subjects
We obtained 39 male ferrets (Mustela putorius furo) aged 6–10 months from Marshall Bioresources (North Rose, NY). They were housed in pairs using a 12-hour light/dark cycle with continuous access to food and water. The animals were randomly assigned to a group of either healthy control animals (15 animals) or those that received a shock wave injury (24 animals). Following a 1-, 4-, or 12-week survival, the brains of each group were analyzed using either immunohistochemistry or Western blots. All animals were treated in accord with the guidelines of the Uniformed Services University of the Health Sciences and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and all procedures were approved by the University Institutional Animal Care and Use Committee. Table 1 summarizes the specifics of animal use.
TABLE 1.
Number of Animals Used in Each Type of Experiment
| Survival Time | Control | Single Blast | 4× Blast |
|---|---|---|---|
| 1 day | 1 | 0 | |
| 1 week | 1 | 0 | |
| 4 weeks | 3 WB/4 IHC/(2 MRI) | 1 | 12 (4 MRI) |
| 12 weeks | 4 WB/4 IHC/(4 MRI) | 0 | 9 (2 MRI) |
| Total | 15 | 3 | 21 |
IHC, immunohistochemistry; MRI, magnetic resonance imaging; WB, Western blot.
The brains used for MRI were also used for IHC.
Shock Wave Exposure
Animals were anesthetized with isoflurane (2%–5% in oxygen) for 10 minutes with a heating pad set to low under the induction box. Each animal was removed from anesthesia and positioned prone within the blast tube in a mesh hammock in the center of the shock tube with the head forward; the mesh wrapped around the body leaving the head exposed. The animals remained anesthetized while exposed to the shock wave, which was delivered using an Advanced Blast Simulator (ABS), pressurized with compressed air (27, 28). Briefly, the driver section of the ABS was sealed with Valmex MEHATOP F1, FR 1400-type IV (7270-5246; Low & Bonar Martinsville, Martinsville, VA) and pressurized with air. Upon rupture of the Valmex, a blast wave traveled though the ABS that mimicked a Friedlander wave curve (29) (Supplementary Data Fig. S1). The mean positive pressure for recorded shocks was 21.29 ± 0.76 psi (mean ± standard deviation, coefficient of variation [COV]: 3.56%), negative pressure was –4.29 ± 0.24 psi (COV 5.50%), the duration of the positive phase was 7.21 ± 0.311 ms (COV 4.31%), the impulse (positive phase pressure × duration) was 0.0648 ± 0.196 psi x s, and the velocity was 510.31 ± 6.74 m/s (COV 1.32%). Each animal was then removed from the tube and placed back into the anesthesia induction box for another 10 minutes (2%–5% isoflurane delivered in oxygen) and the series of steps for shock wave exposure repeated a total of 4 times. Three animals received only one shock wave exposure. Following the final blast, we transferred the animal to a heating pad to recover. Once ambulatory (∼3–6 minutes), the animal returned to their home cage. Control/sham animals received identical treatments but no shock waves.
Tissue Analysis
At the end of the survival period, each animal was deeply anesthetized with isoflurane inhalation (5% in oxygen) and given an IP injection of Euthasol (0.22 mL/kg). On cessation of reflexes, each brain was collected for Western blot analysis or for immunohistochemistry. For Western blot analysis, brains were blocked into regions of interest: prefrontal cortex ([PFC], specifically the orbital gyrus), the medial frontal cortex ([FCM], specifically the anterior portion of the cingulate gyrus), the lateral temporal cortex ([TCL], specifically the posterior ectosylvian gyrus and the hippocampus), and the lateral part of the occipital cortex ([OCL], specifically the inferior portion of the lateral gyrus) and immediately frozen. For reference to the ferret sulci and gyri see Hutchinson et al (30) and https://scalablebrainatlas.incf.org/ferret/HSRetal17 (last accessed December 31, 2020). For immunohistochemistry, the animals were perfused transcardially with 1 L ice-cold 0.1 M phosphate-buffered saline (PBS) (pH 7.4) containing 53.1 mg of heparin (Sigma-Aldrich, St. Louis, MO) followed by 1 L of ice-cold 4% paraformaldehyde solution in PBS (Santa Cruz Biotechnology, Dallas, TX). The brains were postfixed in 4% paraformaldehyde, and then transferred to a storage solution containing 0.04% sodium azide in PBS (PBS-NaN3). Coronal sections (50 µm) were cut using a vibratome (Leica VT1000; Leica, Wetzlar, Germany) and stored in PBS-NaN3 at 4°C until processed with the subsequent immunofluorescence staining.
Immunohistochemistry
To assess changes after a blast injury, we conducted immunohistochemistry procedures as described previously (7, 31). We used the following primary antibodies: chicken anti-glial fibrillary acidic protein (GFAP) (1:500; Abcam, Cambridge, UK: ab4674; RRID: AB 304558), mouse anti-3R tau isoform (1:2000; Sigma, 05-803; RRID: AB 310013), mouse anti-4R tau isoform (1:100; Sigma, MABN1185) mouse anti-AT8 phosphorylated tau (1:100; Thermo Fisher Scientific, Waltham, MA: MN1020B, RRID: AB_223648), phosphorylated tau antisera CP13 and PHF1 (1:1000, both gifts from Dr. P. Davies); anti-aquaporin 4 (1:200; Abcam ab9512; RRID; AB_307299), and rabbit anti-IBa1 (1:1000, Wako, Richmond, VA: 019-19741; RRID: AB_839504). Sections underwent antigen retrieval (1× citrate buffer [Thermo Fisher Scientific] in an 80°C water bath for 20 minutes), followed by blocking with 3% normal goat serum in PBS-T for 3 hours and overnight incubation with the primary antibody. Immunoreactivity was revealed by incubation with either AlexaFluor 555- or 488-conjugated secondary antibody (1:500) and nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Waltham, MA: D21490, 1:2000). Sections were mounted with Mowiol 4-88 medium (Polysciences, Warrington, PA).
Imaging and Analysis
We acquired fluorescent images at different magnifications using a Zeiss Axio Observer.Z1 microscope with an Apotome using Zen 2012 software (Blue edition) version 1.1.2.0 (Carl Zeiss Microscopy). To quantify aspects of the immunoreactivity, 3–4 coronal sections at specific regions from 3 to 4 animals in each group were analyzed and averaged for each ferret and compared between groups by an operator blinded to the treatment. To evaluate the astrocytic density in the white matter or subpial region, we assessed the area occupied by GFAP immunoreactivity by adapting a procedure in Fiji (http://imagej.net, last accessed December 31, 2020) used to measure the total area immunoreacted in histologic samples (adapted from https://www.youtube.com/watch?v=nLfVSWcxMKw&list=TLPQMDUw NjIwMjAhHiT0TuG0XA&index=2). For the white matter measurements, we selected a region just lateral to the juncture of the corpus callosum with the corona radiata at the level of the head of the caudate nucleus; for the subpial region we selected a site in the same rostrocaudal location or in the region of the PFC. For the white matter we outlined a region 100 µm2, corresponding to 510 pixels2 of a z-stack through a 50-µm-thick section, with images taken at 1.25 µm. For the subpial region, we selected an oval of 200 µm2, corresponding to 930 pixels2, with the same z parameters.
For measuring microglial density, we used the temporal neocortex lateral to the hippocampus. Images were exported to Fiji (32), converted to 8-bit, background subtracted (rolling ball radius 50.0 pixels), and autothresholded (triangle thresholding method; Zack et al [33]). The percent area labeled was averaged with each animal and then compared between groups. For each measurement we used 3–4 sections taken from at least 3 to 4 animals in each group.
Western Blot
Forty microgram protein equivalents were added to 5 µL Nupage LDS sample buffer (4×) and 2 µL Nupage reducing agent (10×) and heated for 10 minutes at 70°C. Samples were loaded on a Nupage Novex 10% Bis-Tris gel (Invitrogen-NP0315BOX) with 1× Nupage MOPS SDS Running Buffer (Invitrogen-NP0001) and electrophoresis performed at 200 V for 50 minutes. Proteins were transferred onto a nitrocellulose membrane (Invitrogen-IB301002) using the iBlot2 dry blotting system (Thermo Fisher Scientific) for 7 minutes and then blocked (PBS/T 0.1% for 1–2 hour at room temperature). Membranes were then incubated with primary antibodies overnight (12–14 hours, 4°C) with gentle agitation. The following antibodies were used: anti-phosphorylated tau (CP13, a gift from Dr. P. Davies), anti-3R tau isoform (monoclonal mouse 1:1000, Sigma, 05-803; RRID: AB 310013), anti-4R tau isoform (monoclonal mouse 1:1000, Sigma, MABN1185), HT7 total tau (monoclonal mouse 1:000, Thermo Fisher Scientific, RRID: AB_2314654). All primary antibodies were diluted in 0.1% PBS/T. After overnight incubation, the membranes were washed 4× 10 minutes (0.1% PBS/T), and then incubated with horseradish peroxidase-conjugated secondary antibodies against an appropriate species (1:2000 dilution; Invitrogen) in blocking buffer (1 hour, RT) and then washed again 4× 10 minutes. Protein bands were visualized using Clarity Max Western ECL Substrate (Bio-Rad, Hercules, CA) for 5 minutes.
Magnetic Resonance Imaging
A subset of the brains described in this study, collected 4 or 12 weeks following repetitive blast, as well as controls, were imaged using high-resolution MRI to determine the potential presence of abnormalities consistent with the observed histologic findings. Brain specimens were imaged following perfusion fixation and tissue rehydration and prior to histologic processing as described above.
Ex vivo MRI was acquired using a Bruker 7 T microimaging system with a 30-mm linear RF volume coil and Paravision 5.1 software. High-resolution MRI with voxel dimensions of 50 microns isotropic was collected using a 3D fast low angle (FLASH) MRI pulse sequence. High-resolution MRI volumes were used to calculate minimum intensity projections (MIPs) generated in ImageJ using a moving window of 20 slices or 1-mm thickness as a means to visualize the vasculature qualitatively (34). The data obtained from the MIPs was quantified using a procedure similar to the one described above for histology by using Fiji to measure the area occupied by the presumptive blood vessels in each MIP. For each brain analyzed, 7 rostral to caudal equally spaced MIP images were quantified to represent regions from the occipital to PFC.
Statistics
Randomization and blinding procedures were employed for the analysis of histologic data, which are presented as mean ± standard error of the mean. We compared the percent area labeled across groups using a 1-way or 2-way analysis of variance, followed by the Tukey or Sidak post hoc multiple comparisons test. When 2 groups were involved, we used an unpaired t-test. Statistical analyses were performed using GraphPad Prism (GraphPad Software, www.graphpad.com). A value of p < 0.05 was considered statistically significant.
RESULTS
Blast Injury Alters Glial Immunoreactivity
Astrocytes
Astrocytosis is a process commonly associated with blast injury, including human cases. Although our analysis focused on blast injuries delivering 4 blast shock waves in succession, we initially assessed brains where a single blast was delivered and the animal survived for a shorter period of time (see the Table 1 for specifics). Figure 1 illustrates that a single blast with a short, 1-week exposure (weeks post-injury [WPI]) or with a longer survival time (4 WPI) leads to increases of GFAP, especially surrounding blood vessels. With longer survival times and an increased number of blasts, however, the astrocytic reactivity intensifies, which led us to concentrate our analysis on an increased number of blasts with longer survival time. It is possible that animals receiving 4 blasts and a short survival time would also show strong increases in GFAP reactivity, but our results so far, and those presented below, suggest that longer survival times lead to increased immunostaining for GFAP.
FIGURE 1.
Examples of coronal sections in the parietal cortex taken from animals receiving single or multiple blasts. Compared with a control animal (A), those receiving a single blast and surviving for 1 or 4 weeks show evident astrocytic reactivity (B, C), which is especially evident surrounding blood vessels (red arrows), but reduced from the animal with multiple blasts surviving 4 weeks post injury (D). By 4 weeks of survival with 4 blast injuries in one day, the GFAP immunoreactivity has increased dramatically in the white matter, subpial region and surrounding blood vessels. Also shown are higher-power images showing examples of GFAP immunoreactivity surrounding blood vessels in control (E) and animals receiving a single blast and surviving for 1 week post injury (F) or 4 weeks post injury (G) or an example of an animal receiving 4 blasts and surviving for 4 weeks post injury (H). WPI, weeks post injury. Scale = 200 µm for upper and lower scale bars.
Figure 2 shows a generalized increase in astrocytic reactivity in the white matter in an animal that survived for 4 WPI after 4 exposures (Fig. 2B) when compared with a section from a control animal (Fig. 2A). Several features can be seen at higher power in Figure 2C–G, including increased astrocyte immunoreactivity in the subpial plate (Fig. 2D, E), at the fundus of sulci (Fig. 2F, G), at gray-white matter junctions and surrounding penetrating blood vessels (Fig. 2C, E). Additional examples of GFAP reactivity surrounding blood vessels in control and injured animals can be seen in Supplementary Data Figure S2.
FIGURE 2.
GFAP immunoreactivity in control and blast-injured animals. (A) Example of a coronal section through the level of the caudate/putamen and internal capsule showing the normal (control) immunoreactivity for GFAP (astrocytes). Slight staining can be seen in the white matter, in the septal region and ventrally surrounding blood vessels. (B) A coronal section in a similar region in an animal that received 4 blast injuries in one day and survived for 4 weeks (WPI). Substantially more immunoreactivity can be seen in the white matter and surrounding blood vessels. (C) Displays a higher-power view with strong reactivity in the white matter where several sites show clusters of immunoreactive cells. Increased reactivity can also be seen in the subpial region and at gray-white matter interfaces. (D, E) Panels show the subpial region at higher power where strongly reactive astrocytes are evident in the section obtained from the animal with blast injury (E), whereas the same region in a control animal (D) shows lightly stained astrocytes with few intense processes. (F, G) Images taken from the fundus of the coronal sulcus and indicate that for the blast-injured animal (G), the immunoreactivity is substantially more extensive than for the control animal (F). Scale bars: C = 100 µm, D and E = 50 µm, F and G = 200 µm.
To better evaluate changes, we quantified the increase in GFAP reactivity in several brain regions, comparing control animals with those surviving blast injuries for 4 and 12 WPI. In general, the astrocytic reactivity further increases with longer survival times, but even after 4 weeks of survival the GFAP increase is substantial. Compared with the area occupied by GFAP reactivity in control animals, immunoreactive astrocytes were significantly increased in the region of white matter indicated in Figure 3A after 12 weeks of survival post injury (Fig. 3B, C, F). The same region in the animals surviving for 4 WPI was increased, but did not reach significance. Comparing the region just deep to the pia in ferrets that survived 12 WPI with the control animals, we found significant increases in the area occupied by GFAP immunoreactivity (Fig. 3D, E, G).
FIGURE 3.
Quantification of the immunoreactive density in a region occupied by GFAP immunoreactive astrocytes in the subpial and white matter region of the frontoparietal cortex after 4 shock wave (blast) exposures. (A) Indicates the region that measurements were taken; the black oval surrounded by the red oval, indicates the site of the pial samples and the black square surrounded by the red square indicates the site of the white matter samples. To assess overall area of GFAP fluorescence, 3–4 sections were measured and averaged for each brain; 3–4 brains were used in each condition. (B, C) Images of GFAP immunoreactivity in the white matter taken from a control (B) and a 12 weeks post injury animal (C). The control animals used here survived for 12 weeks post injury. For the white matter measurements, the animals surviving for 12 weeks showed a significant increase compared with the control (F, one way ANOVA followed by a Tukey multiple comparison test, *p = 0.03). The animals surviving for 4 weeks also showed increases but they were not significant. (D, E) Examples of GFAP immunoreactivity in the subpial region for a control (D) and 12-week survival animal (E). The animals surviving for 12 weeks showed significantly greater area occupied by GFAP reactivity compared with the control (G, unpaired t test, *p = 0.02).
Blood Vessels and Astrocytes Show Morphologic Changes After Blast Injury
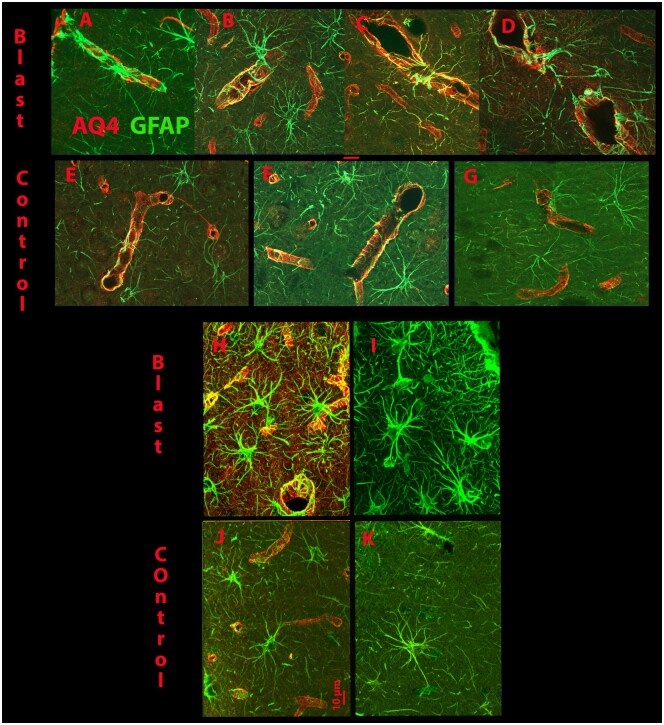
Figure 4 shows views of astrocytes and blood vessels in control and blast-injured brains (12-week survival). The blood vessels are visible though the immunoreactivity of aquaporin 4 (AQ4), which reveals the end feet of astrocytes that surround the vasculature. The astrocytes revealed with GFAP immunoreactivity can be seen strongly integrated with the blood vessels as revealed by AQ4 (Fig. 4A–G, H, J). In the animals receiving a blast injury with 12-week survival, the astrocytic processes are more prominent and appear thicker and more extensive. Those in the control brains, also show astrocytic processes which are less thick. In Figure 4H, I, subpial astrocytes can be seen that show morphology with processes thicker and more extensive than in the control sections (Fig. 4J, K). It may be that additional fine astrocytic processes are actually present in the control brain, but less visible with the tools used here.
FIGURE 4.
Examples of immunoreactivity for GFAP and Aquaporin 4 (AQ4) in the prefrontal cortex. (A–D) The top row shows examples of merged immunoreactivity of GFAP and AQ4 surrounding blood vessels in the prefrontal cortex in animals that received 4 blast injuries and survived for 12 weeks. The control animals were also in the 12-week survival group. (E–G) Panels show similar examples taken from control animals. The examples taken from the injured animals (A–D) show increased GFAP reactivity surrounding the blood vessels and integrating with the AQ4 reactivity compared with the images taken from the control animals (E–G). (H, I) Views of subpial astrocytes that are more engorged with thicker processes in injured animals (Blast) compared with the control animals (J, K). Also seen is greater integration of the astrocytes (GFAP) with AQ4 reactivity in H compared with J. The red scale bar = 10 µm.
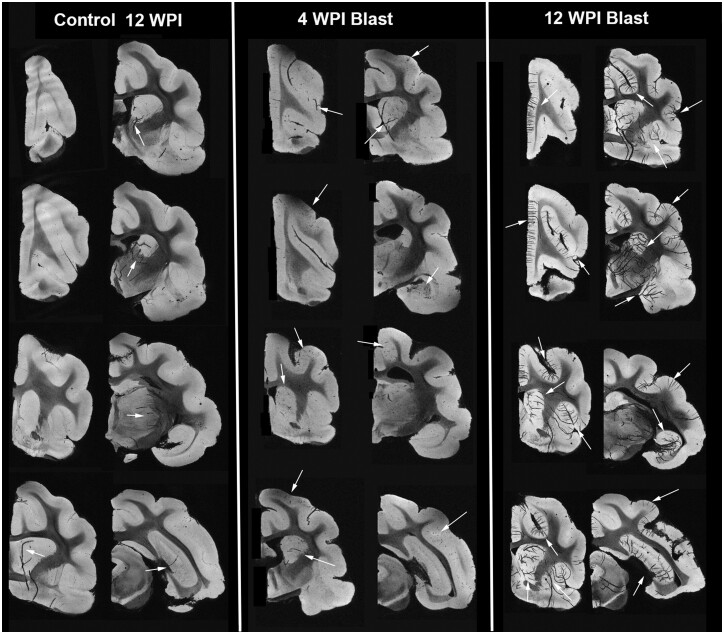
Several of the brains of control and blast-injured animals received MRI. While no gross anatomical abnormalities were observed in the brains that received blast injury, we found prominent vessel-shaped hypointensities in the brains of animals surviving for 12 WPI (Figs. 5 and 6). The high-resolution gradient echo MRI protocol used in this study is sensitive to tissue susceptibility differences and in combination with MIP revealed patterns of vascular anatomy. The most likely interpretation of these hypointensities is from T2* effects of paramagnetic deoxyhemoglobin from red blood cells trapped in the vessels or from hemosiderin deposited in the tissue near damaged vessels. The latter is consistent with the more punctate appearance of hypointensities in the cortex (Fig. 5, white arrows) (34). For normal control brains, the most evident vascular features were large and found in the deeper structures, while for the blast-injured brains, patterns of cortical vascular abnormalities were evident either as clusters of small punctate hypointensities or larger features consistent with cortical arteriole and venule anatomy. While large subcortical blood vessels were often conspicuous in both control and blast brains, the appearance of neocortical vascular hypointensities were more commonly observed in blast-injured brains. The finding of large hypointense vessels in deep structures across all groups suggests that incomplete clearing of red blood cells during perfusion may participate in this irregularity. For example, Figure 5 shows examples of obvious large subcortical blood vessels in deep structures of a control animal. Interestingly, only blast-injured brains (Fig. 5, 4-week postblast) demonstrated cortical hypointensities in the absence of deep vessel involvement, which suggests vascular damage without perfusion artifact. We should also emphasize that the number of animals imaged with a 12-week survival post injury was low (n = 2), so different patterns might emerge with a larger number of subjects. We also evaluated the immunohistology cut from a brain that was imaged after a 12-week survival. Figure 6 indicates with asterisks comparable sites in a histologic section (Figs. 5 and 6A) and the MRI from the same brain (Figs. 5 and 6B), with a higher-power view in Figure 6C. The MRI is imaged to include a much thicker region than revealed in the histology, so they do not look identical. We quantified the data obtained in the MRI using a tool in Fiji that measured the total area of selected regions, in this case, we assessed the area occupied by the black regions revealed by the hypointensities in control (n = 6), 4-week survival (n = 4), and 12 week-survival (n = 2) images. Using this method, the animals surviving 12 WPI showed significant differences from both the 4-week survival and control animals, despite the low number of 12-week survivals (Fig. 6D). We did not see any differences, however, between the control and 4-week survival animals.
FIGURE 5.
High-resolution MR volumes of 1-mm-thickness obtained from control and injured brains that survived for 12 or 4 WPI (weeks post injury); representative rostral to caudal images are shown. Although a few hypointensities can be seen in the control sections (arrows) 12-week survival period, they are not prevalent. In the injured brains shown in the middle 2 columns from 4 WPI brains, limited hypointensities are visible (arrows) that appear to correspond to blood vessels. For the injured brains surviving for 12 WPI (right 2 columns) multiple hypointensities are strongly evident (arrows).
FIGURE 6.
Comparison of MRI with immunohistology and quantification. (A) Example of Aquaporin 4 reactivity in the brain of an animal receiving 4 shock wave injuries and surviving for 12 weeks. (B) Minimum intensity projection (MIP) MRI taken from the same brain indicating regions of hypointensities that appear to correspond to blood vessels. (C) A higher-power view of the boxed in region in (A). The asterisks indicate corresponding sites in the MRI and the histology. Panels A and B do not show identical features as the MIP MRI shown in B is much thicker (∼1 mm) than the histology image (50 µm). Panel C shows quantification of the area occupied by the presumptive blood vessels in the control (n = 6) 12-week survival period, 4 week post injury survival (n = 4), and 12 week post injury survival (n = 2) MRIs. The area occupied by the presumptive blood vessels was significantly increased in the animals surviving 12 weeks post injury compared with the control (***p = 0.0009) and 4-week survival (***p < 0.0008) animals. One-way ANOVA followed by a Tukey post hoc comparison.
Microglia
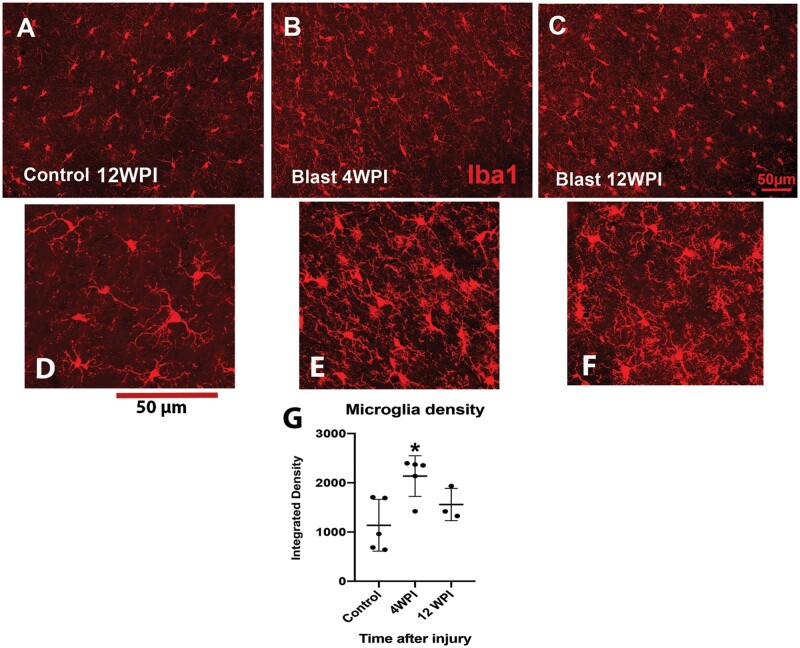
To evaluate microglial immunoreactivity in conjunction with neuroinflammation after injury we used the antibody Iba1. The density of fluorescence in the temporal neocortex adjacent to the hippocampus increased significantly after a 4-week survival, but in contrast to the astrocytic changes that consistently increased with survival time, the microglial density remained elevated at 12 weeks, but was no longer significantly higher than controls (Fig. 7). Evaluating the morphology of the microglia at higher power (Fig. 7D–F), the processes after injury appear thicker and less clearly ramified than in the control animals.
FIGURE 7.
Examples of immunoreactivity for microglia using Iba1. We obtained density measurements in the posterior sigmoid gyrus and found that fluorescent density was increased significantly in animals that survived 4 weeks post injury (WPI) (B) and although the density remained slightly increased at 12 WPI (C), it was not significantly different from the control density obtained at 12 WPI (A). At least 3 sections per animal were obtained and averaged, and 3–5 animals used per group. WPI, weeks post injury (D). We used a 1-way ANOVA followed by a Tukey post hoc analysis. *p < 0.05.
Measurements of Tau-Related Proteins Differentially Alter After Blast Injury Depending on Neocortical Region
Tau and Isoforms
Several reports indicate that forms of tau change after neurotrauma, but this finding is not consistent across all studies (11, 15). To evaluate potential alterations in forms of tau after blast injury we studied the distribution of total tau, phosphorylated tau (pTau), and the 3R and 4R tau isoforms, using both immunohistochemistry and Western blot.
We selected 4 representative cortical locations in injured and control animals. These included the PFC (specifically the orbital gyrus), the medial FCM (specifically the anterior portion of the cingulate gyrus), the lateral TCL (specifically the posterior ectosylvian gyrus and the hippocampus), and the lateral part of the OCL (specifically the inferior portion of the lateral gyrus). These represent rostral (PFC) and caudal (OCL), as well as medial (FCM) and lateral (TCL) portions of the cerebral cortex. We conducted Western blots on animals that survived for 4 WPI but unlike the GFAP immunoreactivity, the 4-week surviving animals did not show significant changes in protein expression compared with control animals (data not shown). Therefore, all of the Western blots shown here were carried out using tissue from animals that survived for 12 weeks. We first assessed total tau (HT7) and observed a significant decrease in HT7 expression in the PFC. We next assessed the levels of phosphorylated tau (CP13), since this type of tau expression is often representative of abnormal changes in tau conformation (Fig. 8B). In this case, 3 regions (OCL, TCL, FCM) showed significant increases in pTau (CP13), whereas the PFC demonstrated only a slight increase in protein expression, compared with control animals.
FIGURE 8.
Quantification of Western blots for (A) HT7 (total tau), (B) CP13 (phosphorylated tau), and the tau isoforms (E) 3R and (F) 4R. Several regions of the cerebral cortex were analyzed for measurement of HT7, CP13, 3R, and 4R using Western blot with GAPDH as a loading control. (A) For HT7 (4 blasts surviving for 12 weeks) the PFC (prefrontal cortex) showed a significant decrease. (B) When assessing the protein expression of CP13 (pTau) all regions showed a significant increase, except the PFC, which only showed a slight increase. The arrows point to the molecular weight for HT7 or CP13. Four brains were used for each analysis, a 2-way ANOVA was followed by a Sidak post hoc test. **p < 0.01, ***p < 0.001. (C, D) Examples of the relative levels of the 3R and 4R isoforms of tau. In control brains obtained at 12 weeks post injury (C), there are no significant differences between the expression levels of each. (D) After injury (4 blasts, survival for 12 weeks) significant differences emerge for the relative level of expression for 3R and 4R isoforms. This occurs for the TCL and PFC, which now show significant differences between control and injured levels. A 2-way ANOVA was followed by Sidak post hoc test. ****p < 0.0001. The same regions of the cerebral cortex were analyzed for measurement of 3R or 4R. (E) For 3R, significant increases were observed for the TCL (temporal cortex lateral) and the prefrontal cortex (PFC). A slight, but not significant decrease was observed for the OCL (occipital cortex lateral) after a blast injury (4 blasts surviving for 12 weeks). (F) For 4R, we observed a significant increase for the FCM (frontal cortex medial) and a decrease for the PFC (prefrontal cortex). The arrows point to the molecular weight for 3R or 4R. OCL, occipital cortex lateral, TCL, temporal cortex lateral, FCM, frontal cortex medial. Four brains were used for each analysis, a 2-way ANOVA was followed by a Sidak post hoc test. *p < 0.05; **p < 0.01.
We also assessed the expression levels of the tau isoforms 3R and 4R; these isoforms are of particular interest, because although normally present in roughly equal levels in people, the relative ratio of expression often alters in neurodegenerative diseases (20, 25, 26). Figure 8C reveals that in control ferret cortex, relative levels of expression for 3R and 4R are similar in the different regions we assessed, as in normal human cortex. Figure 8E, F shows the protein levels for the 3R and 4R isoforms of tau in control animals compared with those receiving 4 blast injuries and surviving for 12 weeks. We find that for 3R, after injury, protein expression increases significantly in the TCL (contains the hippocampus) and the PFC. The same increases do not occur for the 4R isoform, but a significant increase in the FCM and a decrease in the PFC occurs. This can also be seen in Figure 8D that plots the changes in 3R and 4R expression levels after blast injury.
Immunohistochemistry for Tau and Isoforms
pTau
We next assessed a selection of the regions of cerebral cortex showing changes in protein expression for antibodies directed against pTau (AT8, CP13, PHF) and the 3R and 4R isoforms. pTau immunoreactivity increased in the hippocampus of animals injured with 4 blasts and surviving for either 4 (Fig. 9A, B) or 12 weeks (Fig. 9C, D), corresponding to the increase in pTau expression detected in the Western blots for this region. The patterns of immunoreactivity were slightly different for each antibody, with CP13 showing greater label in the cells of the superficial layers after blast injury (Fig. 9D, arrows), whereas AT8 revealed increases in the axons running between the dentate gyrus and hippocampus proper (Fig. 9B, arrows).
FIGURE 9.

Examples of immunoreactivity for pTau in the hippocampus of control and injured brains. Shown here are representative sections from control and animals injured with 4 blasts and surviving for 12 weeks, using either AT8 (A, B) or CP13 (C, D) as the antibody. The injured brain shows increased reactivity with both antibodies, but in slightly different regions depending on the antibody. AT8 (A, B) reveals increased reactivity in the axons projecting between the dentate gyrus and hippocampus proper (arrows) and CP13 (C, D) reveals increased numbers of labeled cells in the upper layers of the hippocampus (arrows). (E, F) Examples of immunoreactivity against AT8. This shows immunoreactivity in the temporal cortex for control obtained at 4 weeks post injury (E) and blast (F) injured brains. Although the number of cells and density of labeled axons appears greater in the inured brain (F), a substantial amount of immunoreactivity is present in the control brain as well.
In general, we observed substantial immunoreactivity for AT8 in both the injured or control cerebral cortex, but with overall increased immunoreactivity in the brains subjected to blast. Figure 9E, F shows increased AT8 immunoreactivity in the TCL in a control brain. In this image, more labeled cells and axons can be seen after 4 blasts and survival for 4 weeks, but substantial immunoreactivity can also be seen in the control cortex, both in neurons and in axons. Using another form of antibody against pTau, PHF, immunoreactive axons traveling between the dentate gyrus and hippocampus proper are also seen in the injured brains (Supplementary Data Fig. S3). It is worth noting that each antibody recognizes slightly different epitopes of phosphorylated tau: AT8 labels Serine 202 and Threonine 205, CP13 labels Serine 202, and PHF1 labels Serine 396 and Serine 404. In none of the animals did we see aggregated pTau in neuronal somas (neurofibrillary tangles) or astrocyte cytoplasm (astrocytic tangles), as is encountered in human neurodegenerative conditions.
3R and 4R
Immunoreactivity against 3R showed a strong pattern in the hippocampus, revealing axons traveling between cells in the dentate gyrus and hippocampus proper (Fig. 10A, B). The label shows more intensely in the injured brain both in the axons and cell bodies, as observed in the Western blot. Figure 10 also shows increased 3R immunoreactivity in layer 2 of the PFC from the animal receiving blast injury and surviving for 12 weeks (Fig. 10C, D), another region of strong protein expression in the Western blots. We also found increased reactivity for 3R in other regions of the cerebral cortex, including the lateral temporal region surrounding the hippocampus; cells that populate layer 2 are the most immunoreactive (Fig. 11).
FIGURE 10.
3R immunoreactivity in the hippocampus and prefrontal cortex. (A, B) In the hippocampus, label for 3R is present in both the control and injured brains, but reactivity for axons running between the dentate gyrus and hippocampus proper is clearly increased in the injured brain (arrows). A greater number of cells and processes are also obvious in the injured hippocampus proper (asterisks). (C, D) In the prefrontal cortex, more labeled cells are present in layer two of the injured brain (D), compared with the control (C). The red horizonal lines in the bottom 2 images represent the boundary between layer 1 and 2. The red scale bar equals 500 µm in the top 2 panels (A, B, hippocampus) and 100 µm in the bottom 2 panels (C, D, prefrontal cortex).
FIGURE 11.

3R label in the temporal cortex. These are examples of 3R immunoreactivity in layer 2 of the temporal cortex of control (A) and blast-injured (B) brains. The boxed in area is shown at higher power in the upper right of each image. Increased numbers of cells can be seen in the injured brain. WPI, weeks post injury.
A strong pattern of 3R label additionally occurred in a region deep to the pyriform cortex and belonging to the olfactory system. Figure 12 shows a swatch of immunoreactivity in the anterior part of the anterior commissure surrounding the olfactory portion of the lateral ventricle in 4 brains. It is present in both the control (Fig. 12A, C) and blast-injured brains (Fig. 12B, D: 4-week survival), but substantially stronger in the injured brains. That portion of the lateral ventricle also appears enlarged in the injured animals (Fig. 12B, D).
FIGURE 12.

3R Immunoreactivity surrounding the anterior pole of the lateral ventricle. Examples of 3R labeling in 2 control (A, C, on the left) and 2 injured brains (B, D, on the right) showing increased reactivity in the injured brains accompanying enlarged openings in the anterior process of the lateral ventricle.
DISCUSSION
We report here that blast injury in the gyrencephalic ferret results in specific alterations in astroglial reactivity, including astrogliosis in subpial cortex, surrounding blood vessels, and within white matter. Modifications in various forms of tau were also seen, revealing that shock wave injury brings about increases of pTau in ferrets, which varied according to neocortical region. Also, the isoforms of tau, 3R and 4R, show changes in their protein expression and relative ratio, mimicking some of the changes seen in humans after blast; alterations in blood vessel morphology were additionally observed after blast injury. These findings suggest the ferret is an important animal to study for the neuropathological effects of explosive blast.
Changes in Glial Reactivity
Astrocytes
In healthy adult ferret brain, using GFAP immunoreactivity, astrocytes are sparingly visible throughout the telencephalon, with light immunoreactivity in the subpial region, the white matter, and surrounding blood vessels. After several blast exposures, the astrocytes increase in immunoreactivity in multiple regions. These include the subpial plate, throughout the white matter, at the fundus of sulci, at gray-white matter junctions, and heavily surrounding blood vessels. Strong GFAP immunoreactivity was apparent at 4 weeks after 4 blast injuries, however, more subtle immunoreactivity appeared at one week with exposure to a single blast injury (Fig. 1). The GFAP immunoreactivity exhibited some variability at 4 WPI, but as indicated by our quantitative analysis, the density of astrocytes augmented with longer survival times (up to 12 weeks) (Fig. 3). A recent report studying postmortem brains of military servicemen that experienced blast exposures found increases in GFAP reactivity that range from mild to strong, with variable exposures to blast injury, other forms of trauma, and the time of survival (11). These are similar to the types of increases that we see here in ferrets surviving single or multiple exposures to blast. Shively and colleagues also report increased astrogliosis in specific neocortical sites, including in the subpial plate, at gray/white matter junctions, and surrounding blood vessels (11). This pattern of immunoreactivity is similar, although not identical, to the pattern occurring in ferrets after blast injury. Especially concordant with the human pattern of astrogliosis in the ferret model is the increase surrounding blood vessels over time, the density of immunoreactivity in the subpial plate, and generalized increases in white matter. The ferret astrocyte immunolabel is strong in the fundus of sulci, also obvious in the human pattern. Other work shows increased astrogliosis after blast injury in different gyrencephalic animals (primates, swine), but so far, the pattern reported does not directly mimic that seen in people (35–38). We also note that the overall pattern of astrocytic reactivity differed in our hands when ferrets received a CCI injury (31), suggesting the observed distribution of astrogliosis in the current study is specific to blast exposure.
The astrocytes we observe after blast injury also display thicker and more conspicuous processes, which are morphologically different from astrocytes in control brains (Figs. 3 and 4). This response to blast exposure is typical of reactive astrocytes and most likely a result of increased GFAP as well as other markers (39). We did not assess other elements that might be present with increased astroglial reactivity, but the change in morphology and overall density is clear. It is also interesting that GFAP and aquaporin processes surrounding blood vessels are more evident after injury and that many blood vessels are enlarged. Astrocytes participate in the regulation of blood flow, as well as well as water transport through their interaction with capillaries, which might explain the change in morphology after injury (40–42). Accordingly, hypointensities evident in MRI after a 12-week survival post injury appear to correspond with penetrating blood vessels (Figs. 5 and 6). Recent studies report that vascular pathology, although not identical to that seen here, may be especially evident after blast injury and persist for lengthy periods post trauma (5, 42).
Whether astrocytes proliferate, migrate, or demonstrate hypertrophied processes after an injury is also not completely clear. A report by Bardehle et al considers that a subset of astrocytes related to blood vessels proliferate, while others do not (43). Several studies report that in most conditions, astrocytes do not migrate, but are restricted to specific territories (39, 43–45). Another factor to consider is that many astrocytes in homeostatic adult conditions do not express detectable levels of GFAP and do so only under conditions of stress or injury (46, 47). It is also likely that different injuries and pathology may result in different astrocytic reactions (48). Nevertheless, further exploration of the extent and mechanisms of the reactive astrocytes induced by blast would be useful.
A study of the biomechanics and biophysics of blast injury suggests that large deformations occur when different densities of tissue are impacted by pressure waves (49). Since the ferret possesses a brain with substantial sulci and gyri, as does the human, this may account for the pattern of astrogliosis that we observe, especially at the gray-white interface and the fundus of sulci. In addition, Gupta and Przekwas suggest that micro interfaces are also particularly subject to damage, which may explain the increased immunoreactivity for astrocytes surrounding blood vessels and at the gray-white interface.
Microglia
Modifications in microglial immunoreactivity also occur after blast injury. The changes observed in the ferret were not as dramatic as the astrocytic alterations, but showed initial increases in overall densities, which resolved with longer survival times. It is interesting that the microglial reactivity decreased slightly over time, while astrogliosis remained elevated or increased. In general, the microglial changes were less intense in the ferret after blast exposure than those seen after CCI (31). This could be due to several reasons, possibly because microglial reactivity is often related to disruption of the blood brain barrier, which clearly occurred in the CCI, but is not obvious after blast (50). In other species, blast injury did not lead to chronic increases in microglial activation, suggesting there may be differences in the glial response to different types and intensity of injury (51–54).
Changes in Tau
Total Tau and Phosphorylated Tau
Substantial alterations in both expression of protein levels and immunoreactivity of tau and related proteins occurred after blast exposure (Figs. 8–12). Measurements of total tau (HT7) only showed a significant change in the prefrontal region, which displayed a significant decrease. pTau exhibited significant increases in all the neocortical regions studied with Western blot, except the PFC, which only showed a slight increase. The Western blot changes in pTau correlated with the immunohistochemistry; the regions showing increases in protein expression also having increased immunoreactivity. The level of pTau is very low in all the control cortical regions, but shows increases after a survival of 12 weeks post blast injury. Phosphorylation of tau is a routine process and occurs under normal conditions in various states and at various sites of the tau protein (19). Although phosphorylation may contribute to the abnormal accumulation of tau that form inclusions under several disease conditions, tau phosphorylation is not the only cause of abnormal inclusions, which may involve several additional mechanisms including ubiquitination, glycation, and glycosylation, among others (19).
We do not have strong evidence of specific tau inclusions (tangles, etc.) in neurons after exposure to blast in the ferret; however, it is clear that the significant increase in pTau after blast relative to that in control animals is indicative of injury. These types of increases agree with other occurrences of tau pathology that evolve after TBI (22, 55). Several clinical studies report that TBI-induced tau histopathology occurs in the upper layers of the cerebral cortex, as we see here in ferrets, compared with pathology manifest in Alzheimer disease (depending on the cytoarchitectonic area), mainly in the pyramidal cells of layers 3 and 5 (19, 56). The mechanism of TBI is relevant, and changes in tau may correlate more directly with repetitive TBI (10, 57, 58). Accumulation of tau is also a gradual process, and if our studies were extended over even longer periods of time, we may find increases or changes in tau not evident after a relatively short survival time.
The immunoreactivity of pTau differed slightly depending on the antibody we used. Each antibody is directed against distinct epitopes and domains of the tau molecule, so this is not necessarily unusual. The AT8 antibody tended to show more overall labeling than CP13 and PHF1 (Fig. 9). AT8 labels phosphorylation of a serine (202) and a threonine (205), which may have resulted in more reactivity, whereas CP13 labels only the serine (202). Substantial AT8 immunoreactivity appeared in the control ferret brains as well. PHF1 on the other hand labels 2 serines (404 and 396) in slightly different loci from the AT8 and CP13 serine, which may result in less overall immunostaining. Increases in the phosphorylation sites revealed by all of these antibodies (AT8, CP13, and PHF1), however, are reported to be associated with Alzheimer disease (59).
A report using Western blot to evaluate the specificity of various pTau antibodies noted that AT8 showed a relatively high level of nonspecificity in their assay, whereas PHF1 and CP13 showed high specificity (60). Another recent study using a different assay (flow cytometry in an HEK binding assay) found that AT8 and PHF1 showed highly specific binding (61). These findings may help to explain the different patterns observed immunohistochemically with different pTau antibodies.
It is also not definitive that people display tau pathology when exposed specifically to blast injury. Several clinical studies report that TBI results in CTE-like symptoms and accumulations of tau pathology. Shively et al observed accumulations of pTau in humans, but only in chronic cases (11). A recent report using Tau PET ligand found that veterans exposed to blast, but not other types of injury, demonstrated accumulation of tau (13). A different recent study of veterans, however, found no evidence of tau pathology, although only 2 of these subjects were exposed to blast (62). Still a different study reported that 5 out of 10 veterans exposed to blast exhibited evidence of tauopathy (63). As a result, it is likely that additional work will be necessary to define tau pathology evoked by blast injury in people. Using multiple types of injury, various animal studies report mixed results in tau pathology, but only a few have used blast (8, 14, 21, 22, 63).
Delivery of anesthesia can induce increases in pTau in animals and people (64–66). Several factors appear to influence its expression, which include the amount of exposure, type of anesthesia, time after exposure, and temperature of the recipient. In the case of isofluorane (the anesthesia used here) pTau expression diminishes substantially over time. It is also strongly affected by colder body temperature of the animal and reduces substantially when body temperature returns to normal (64). In our experiments, the ferrets received isofluorane in 10-minute bouts (during the blast procedure), were maintained in a thermally controlled situation and survived for long periods of time after delivery of anesthesia. In general, experiments showing increases in pTau expression evaluate much shorter survival times and deliver the anesthetic for longer periods of time than used in this study (64). In sum, this suggests that our observations of increased pTau at the time points we conducted either immunohistochemistry or Western blot were most likely not affected by anesthesia. There is another point to consider, however. All animals received isofluorane anesthesia for a short time prior to their death, which was followed by pentobarbital. But since all animals in all groups received the same anesthetics in all conditions, it is also unlikely that our overall conclusions should differ.
3R and 4R Isoforms of tau
There are 6 isoforms of tau, and each plays a distinct role during development as well as in the mature brain (19, 25, 67). Alternative splicing produces different isoforms; alternative splicing of exon 10 produces isoforms 3R and 4R, which contain either 3 or 4 microtubule binding sites (23, 25). These sites are the primary microtubule binding regions and central to neurodegenerative diseases, as they change their ratio with disease progression (19, 20, 25). In humans, 3R and 4R isoforms occur in roughly equal amounts, but in several neurodegenerative diseases, changes in the 3R to 4R ratio occur, as does their phosphorylation. For many diseases, the 4R isoform increases, although in Alzheimer disease or primary age-related tauopathy, both 3R and 4R are involved (68–70), but in Pick disease, a 3R involvement occurs (71). We show here that ferrets also express these 2 isoforms in roughly equal amounts in several neocortical areas and their level of expression changes after blast injury (Fig. 8). It is interesting that the PFC shows specific changes for Tau isoform expression. In this region, total tau shows a reduction, while 3R increases significantly, and 4R decreases. Of note, Pick disease shows frontotemporal involvement, with several symptoms that may mimic TBI, such as mood disturbances (72). Few studies investigate changes in 3R and 4R after TBI, but pathologic alterations have been seen (58, 73). In this regard, a recent study by Cherry et al shows that in people with CTE, pTau plus the 3R and 4R isoforms either increase with disease progression (pTau) or show changes in their expression ratio (3R and 4R) (74). They conclude that CTE is a mixed 3R-4R tauopathy.
A functional study in Drosophila suggests that different phenotypes occur with manipulation of 3R or 4R. Sealey et al report that the 3R isoform relates more to axonal transport and locomotion, while 4R pathology relates more to learning and memory, although the same altered function may not occur in the 3R-4R changes we see in ferrets (75). It is not clear how to interpret the decrease in total tau and 4R in PFC accompanied by the increase in 3R, along with a few more subtle changes. The generalized increase in several cortical areas of pTau appears consistent with redistribution of axonal tau to the somatodendritic compartment, which may be associated with neuronal malfunction. However, more changes of tau isoforms and phosphorylation within regions cannot yet be specifically interpreted relative to pathological processes.
Summary
Using a gyrencephalic animal to evaluate the effects of blast injuries shows that the ferret displays features mimicking several of the histopathologic findings that occur in people after similar injury. We find overall increases in astrogliosis that remain elevated for many weeks to months after blast exposure, and may reflect the pattern observed postmortem in human military TBI cases associated with blast. The alterations in astrocytes are likely to correlate with changes in the morphology of blood vessels. Various forms of tau protein expression also show changes, but only after a 12-week survival. We did not check at very early time points after the injury, however. The 3R and 4R isoforms of tau, which normally occur in roughly equal ratio in control animals, and in human brain, show changes in their relative levels of expression in several neocortical areas after blast injury. These changes observed with Western blot are confirmed immunohistochemically. Our study should be instructive to those investigating human pathology as we note that changes in tau expression vary across neocortical regions and may be informative for interpreting such changes in human cases. Overall, our findings corroborate that ferrets are an important animal to study TBI and will provide a useful model to test treatments for blast injury.
Future Directions
It would be helpful to include in the future, more levels of blast injury with short and long survival times. It would be useful to know if animals that survive for longer periods show increasing levels of abnormal tau with cellular inclusions. It is also possible that other markers associated with neuronal degeneration after blast are present that were not analyzed here. While high-resolution MRI detected likely vascular pathology, other advanced MRI methods may be sensitive to more subtle pathologies, such as gliosis. We also decided to limit our experiments to male ferrets because female ferrets remain in estrous when they reach maturity unless they become pregnant; if they are allowed to remain in this condition, they develop persistent issues with anemia which could confound our data (76). Therefore, for these experiments, we included only male ferrets, but will determine the best way to include females at a later time. We were also limited in the number of control animals and hope that in future studies more animals overall will be available. A complete behavioral assessment of the effects of controlled explosive injury on these gyrencephalic animals is ongoing and will add greatly to our understanding of how this injury affects the overall health of animals and people.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank CNRM for core facility support enabling this work, specifically the Advanced Blast Simulator (with Yeonho Kim and Amanda Fu) and the translational imaging facility. They also thank Dr. Aviva Symes for helpful comments on the manuscript.
This study was supported by the Center for Neuroscience and Regenerative Medicine—CNRM-70-8956, USU-PAT-74-3439, and the Congressionally Directed Medical Research Program W81XWH-13-2-0018.
The authors have no duality or conflicts of interest to declare.
Supplementary Data can be found at http://academic.oup.com/jnen.
REFERENCES
- 1.LaValle CR, Carr WS, Egnoto MJ, et al. Neurocognitive performance deficits related to immediate and acute blast overpressure exposure. Front Neurol 2019;10:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Wilson CM, Mendelev N, et al. Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. J Neurotrauma 2020;37:1221–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryden DW, Tilghman JI, Hinds SR 2nd. Blast-related traumatic brain injury: Current concepts and research considerations. J Exp Neurosci 2019;13:117906951987221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein LE, McKee AC, Stanton PK. Considerations for animal models of blast-related traumatic brain injury and chronic traumatic encephalopathy. Alzheimers Res Ther 2014;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gama Sosa MA, De Gasperi R, Janssen PL, et al. Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol Commun 2014;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elder GA, Stone JR, Ahlers ST. Effects of low-level blast exposure on the nervous system: Is there really a controversy? Front Neurol 2014;5:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwerin SC, Hutchinson EB, Radomski KL, et al. Establishing the ferret as a gyrencephalic animal model of traumatic brain injury: Optimization of controlled cortical impact procedures. J Neurosci Methods 2017;285:82–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012;4:134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald CL, Johnson AM, Nelson EC, et al. Functional status after blast-plus-impact complex concussive traumatic brain injury in evacuated United States military personnel. J Neurotrauma 2014;31:889–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee AC, Robinson ME. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement 2014;10:S242–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shively SB, Horkayne-Szakaly I, Jones RV, et al. Characterisation of interface astroglial scarring in the human brain after blast exposure: A post-mortem case series. Lancet Neurol 2016;15:944–53 [DOI] [PubMed] [Google Scholar]
- 12.McKee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol 2015;127:45–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson ME, McKee AC, Salat DH, et al. Positron emission tomography of tau in Iraq and Afghanistan Veterans with blast neurotrauma. Neuroimage Clin 2019;21:101651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulbe JR, Hall ED. Chronic traumatic encephalopathy-integration of canonical traumatic brain injury secondary injury mechanisms with tau pathology. Prog Neurobiol 2017;158:15–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iverson GL, Gardner AJ, Shultz SR, et al. Chronic traumatic encephalopathy neuropathology might not be inexorably progressive or unique to repetitive neurotrauma. Brain 2019;142:3672–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: A potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med 2011;30:179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015;66:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 2000;33:95–130. [DOI] [PubMed] [Google Scholar]
- 20.Goedert M, Eisenberg DS, Crowther RA. Propagation of Tau aggregates and neurodegeneration. Annu Rev Neurosci 2017;40:189–210 [DOI] [PubMed] [Google Scholar]
- 21.Ojo JO, Mouzon BC, Crawford F. Repetitive head trauma, chronic traumatic encephalopathy and tau: Challenges in translating from mice to men. Exp Neurol 2016;275:389–404 [DOI] [PubMed] [Google Scholar]
- 22.Washington PM, Villapol S, Burns MP. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 2016;275:381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goedert M, Spillantini MG, Jakes R, et al. Multiple isoforms of human microtubule-associated protein tau: Sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 1989;3:519–26 [DOI] [PubMed] [Google Scholar]
- 24.Iqbal K, Liu F, Gong CX, et al. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 2009;118:53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gotz J, Halliday G, Nisbet RM. Molecular pathogenesis of the tauopathies. Annu Rev Pathol Mech Dis 2019;14:239–61 [DOI] [PubMed] [Google Scholar]
- 26.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: Correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 1990;9:4225–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vu PA, Tucker LB, Liu J, et al. Transient disruption of mouse home cage activities and assessment of orexin immunoreactivity following concussive- or blast-induced brain injury. Brain Res 2018;1700:138–51 [DOI] [PubMed] [Google Scholar]
- 28.Ritzel DV, Parks SA, Roseveare J, et al. Experimental blast simulation for injury studies. RTO Human Factors and Medicine Panel Symposium 2011. Halifax, NS: NATO Science and Technology Organization 2011
- 29.Friedlander FG. The diffraction of sound pulses; diffraction by a semi-infinite plane. Proc R Soc Lond A Math Phys Sci 1946;186:322–44 [DOI] [PubMed] [Google Scholar]
- 30.Hutchinson EB, Schwerin SC, Radomski KL, et al. Population based MRI and DTI templates of the adult ferret brain and tools for voxelwise analysis. Neuroimage 2017;152:575–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwerin SC, Chatterjee M, Imam-Fulani AO, et al. Progression of histopathological and behavioral abnormalities following mild traumatic brain injury in the male ferret. J Neuro Res 2018;96:556–72 [DOI] [PubMed] [Google Scholar]
- 32.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods 2012;9:676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zack GW, Rogers WE, Latt SA. Automatic measurement of sister chromatid exchange frequency. J Histochem Cytochem 1977;25:741–53 [DOI] [PubMed] [Google Scholar]
- 34.Griffin AD, Turtzo LC, Parikh GY, et al. Traumatic microbleeds suggest vascular injury and predict disability in traumatic brain injury. Brain 2019;142:3550–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallakuri S, Desai A, Feng K, et al. Neuronal injury and glial changes are hallmarks of open field blast exposure in swine frontal lobe. PLoS One 2017;12:e0169239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodrich JA, Kim JH, Situ R, et al. Neuronal and glial changes in the brain resulting from explosive blast in an experimental model. Acta Neuropathol Commun 2016;4:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu J, Ng KC, Ling G, et al. Effect of blast exposure on the brain structure and cognition in Macaca fascicularis. J Neurotrauma 2012;29:1434–54 [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Goodrich JA, Situ R, et al. Periventricular white matter alterations from explosive blast in a large animal model: Mild traumatic brain injury or “subconcussive” injury? J Neuropathol Exp Neurol 2020;79:605–17 [DOI] [PubMed] [Google Scholar]
- 39.Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 2016;1862:483–91 [DOI] [PubMed] [Google Scholar]
- 40.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 2007;10:1369–76 [DOI] [PubMed] [Google Scholar]
- 41.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci USA 2011;108:17815–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gama Sosa MA, De Gasperi R, Perez Garcia GS, et al. Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol Commun 2019;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bardehle S, Kruger M, Buggenthin F, et al. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 2013;16:580–6 [DOI] [PubMed] [Google Scholar]
- 44.Ge WP, Miyawaki A, Gage FH, et al. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012;484:376–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai HH, Li H, Fuentealba LC, et al. et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 2012;337:358–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolte C, Matyash M, Pivneva T, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: A tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 2001;33:72–86 [PubMed] [Google Scholar]
- 47.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol 2011;93:421–43 [DOI] [PubMed] [Google Scholar]
- 48.Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. J Neurosci 2012;32:6391–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta RK, Przekwas A. Mathematical models of blast-induced TBI: Current status, challenges, and prospects. Front Neurol 2013;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma 2014;31:1180–93 [DOI] [PubMed] [Google Scholar]
- 51.Gama Sosa MA, De Gasperi R, Perez Garcia GS, et al. et al. Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI. Acta Neuropathol Commun 2017;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elder GA, Ehrlich ME, Gandy S. Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci Lett 2019;707:134294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Garcia G, Gama Sosa MA, De Gasperi R, et al. Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behav Brain Res 2018;340:117–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loane DJ, Kumar A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp Neurol 2016;275:316–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 2004;190:192–203 [DOI] [PubMed] [Google Scholar]
- 56.Hof PR, Cox K, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer's disease: I. Superior frontal and inferior temporal cortex. J Comp Neurol 1990;301:44–54 [DOI] [PubMed] [Google Scholar]
- 57.McKee AC, Alosco ML, Huber BR. Repetitive head impacts and chronic traumatic encephalopathy. Neurosurg Clin N Am 2016;27:529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simic G, Babic Leko M, Wray S, et al. Tau protein hyperphosphorylation and aggregation in Alzheimer's disease and other tauopathies, and possible neuroprotective Strategies. Biomolecules 2016;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petry FR, Pelletier J, Bretteville A, et al. Specificity of anti-tau antibodies when analyzing mice models of Alzheimer's disease: Problems and solutions. PLoS One 2014;9:e94251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li D, Cho YK. High specificity of widely used phospho-tau antibodies validated using a quantitative whole-cell based assay. J Neurochem 2020;152:122–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tripathy A, Shade A, Erskine B, et al. No evidence of increased chronic traumatic encephalopathy pathology or neurodegenerative proteinopathy in former military service members: A preliminary study. J Alzheimers Dis 2019;67:1277–89 [DOI] [PubMed] [Google Scholar]
- 63.Dickstein DL, De Gasperi R, Gama Sosa MA, et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol Psychiatry 2020. [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittington RA, Bretteville A, Dickler MF, et al. Anesthesia and tau pathology. Prog Neuropsychopharmacol Biol Psychiatry 2013;47:147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong Y, Wu X, Xu Z, et al. Anesthetic isoflurane increases phosphorylated tau levels mediated by caspase activation and Abeta generation. PLoS One 2012;7:e39386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Run X, Liang Z, Zhang L, et al. Anesthesia induces phosphorylation of tau. J Alzheimers Dis 2009;16:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kosik KS, Orecchio LD, Bakalis S, et al. Developmentally regulated expression of specific tau sequences. Neuron 1989;2:1389–97 [DOI] [PubMed] [Google Scholar]
- 68.Espinoza M, de Silva R, Dickson DW, et al. Differential incorporation of tau isoforms in Alzheimer's disease. J Alzheimers Dis 2008;14:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santa-Maria I, Haggiagi A, Liu X, et al. The MAPT H1 haplotype is associated with tangle-predominant dementia. Acta Neuropathol 2012;124:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol 2014;128:755–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Irwin DJ, Brettschneider J, McMillan CT, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 2016;79:272–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constantinidis J, Richard J, Tissot R. Pick's disease. Histological and clinical correlations. Eur Neurol 1974;11:208–17 [DOI] [PubMed] [Google Scholar]
- 73.Arena JD, Smith DH, Lee EB, et al. Tau immunophenotypes in chronic traumatic encephalopathy recapitulate those of ageing and Alzheimer's disease. Brain 2020;143:1572–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cherry JD, Kim SH, Stein TD, et al. Evolution of neuronal and glial tau isoforms in chronic traumatic encephalopathy. Brain Pathol 2020;30:913–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sealey MA, Vourkou E, Cowan CM, et al. Distinct phenotypes of three-repeat and four-repeat human tau in a transgenic model of tauopathy. Neurobiol Dis 2017;105:74–83 [DOI] [PubMed] [Google Scholar]
- 76.Fox JG. Biology and Diseases of the Ferret. Hoboken, NJ: Wiley Blackwell, 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.