Rheumatology key message
Use of rIL-18BP in refractory sJIA was well tolerated with reduced MAS frequency and severity.
Sir, Patients with systemic JIA (sJIA) are at high risk for macrophage activation syndrome (MAS) [1]. IL-18 is a proinflammatory cytokine elevated in sJIA and Adult Onset Stills Disease, and may represent a pathogenic link between sJIA and MAS [2, 3]. IL-18 is counterbalanced by its high-affinity endogenous antagonist, IL-18 binding protein (IL-18BP) [4]. Based on this, many authors suggested using exogenous IL-18BP as a novel therapeutic approach for inflammatory diseases [4, 5]. A recent Phase II trial of recombinant IL-18BP (rIL-18BP-Tadekinig alfa) showed promising results for Adult Onset Stills Disease [6]. Here, we report the first use of rIL-18BP in a patient with refractory sJIA and recurrent MAS.
We report a 6-year-old mixed race male diagnosed at age 14 months with sJIA. His subsequent course was complicated by recurrent MAS episodes requiring pulse (30 mg/kg/day) steroids for 3 days followed by daily prednisolone at 2 mg/kg/day, and ciclosporin 5–7 mg/kg/day in order to control episodes. He failed to achieve remission despite numerous non-biologic and biologic medications including anakinra, canakinumab, IVIG, tocilizumab and rituximab. Despite this, he continued to have frequent flares and recurrent MAS with any attempts to wean steroids. Fourteen months into his illness, he was diagnosed with interstitial lung disease in the setting of persistent tachypnoea and erythematous finger clubbing. His biopsy subsequently showed features of pulmonary alveolar proteinosis and lipoid pneumonia.
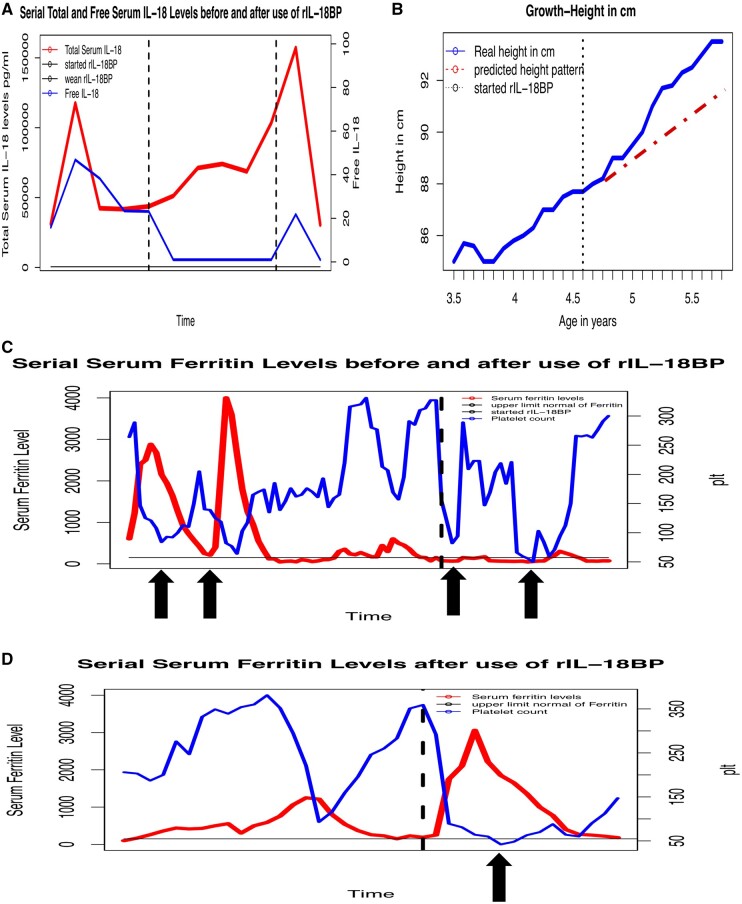
Laboratory findings during disease flares and MAS episodes showed classic features including persistently elevated ferritin levels and total serum IL-18 levels up to 117 346 pg/ml. Corresponding free IL-18 levels during disease flares and MAS episodes were elevated up to 46.82 pg/ml (most healthy individuals have undetectable levels but can be up to 5 pg/ml [2]) (Fig. 1A).
Given the patient’s persistently elevated total and free IL-18, he was started on rIL-18BP (tadekinig alfa) under a compassionate-use investigational new drug authorization, 2 mg/kg subcutaneously every 48 h, with continuation of previous therapy. Immediately prior to the start of rIL-18BP, his total IL-18 was 43 724 pg/ml and free IL-18 was 23.17 pg/ml. His free IL-18 levels were undetectable 24 h after the first dose (Fig. 1A).
Fig. 1.
rIL-18BP use in a patient with severe sJIA and MAS
(A) Total and Free IL-18 levels over time. Vertical lines: start (left) and missed dose (right) of rIL-18BP therapy. (B) Linear growth change over time and after start of rIL-18BP. Actual height shown in blue. Vertical line: start of rIL-18BP treatment, red line: predicted height based on pre-treatment growth velocity. (C and D) Serial platelet counts (blue) and serum ferritin levels (red) before and after rIL-18BP treatment and MAS flare after missed dose. Vertical black line indicates start of treatment (C) and change in rIL-18BP dosing interval (D). Arrows point to MAS episodes. sJIA: systemic JIA; MAS: macrophage activation syndrome.
Over the first year of treatment, he slowly improved clinically, and oral steroids were tapered from 2 mg/kg/day to 0.75 mg/kg/day while continuing other medications (ciclosporin, anakinra (switched from canakinumab later in the course), monthly IVIG). He showed signs of stable disease including increased growth velocity from 1.75 cm/year before initiation of rIL-18BP to 5.75 cm/year after rIL-18BP (Fig. 1B). As detailed below, MAS episodes after initiation of rIL-18BP were controlled with IV methylprednisolone only, without the need for increase in oral steroids or ciclosporin.
He was diagnosed with MAS triggered by parainfluenza-3, 4 weeks after starting rIL-18BP. He was febrile but otherwise appeared clinically well despite typical laboratory findings of MAS (Fig. 1C). Remarkably, his ferritin level remained normal. Several months later he developed another MAS episode triggered by gastroenteritis. This episode similarly only required three IV steroid pulses, and was again noted that ferritin levels did not exceed 250 ng/ml (Fig. 1C).
One year later, he developed persistent productive cough with mild hypoxia (95–96%). Although extensive infectious evaluation was negative, he was suspected to have pneumonia and improved after broad-spectrum antibiotics. With concerns about rIL-18BP contributing to lung infections, an attempted medication wean was started by decreasing frequency of injection. The patient did not receive a dose for four days (first missed dose) and promptly developed a severe MAS episode, which required six doses of IV steroid pulses, increasing baseline ciclosporin, anakinra and daily steroid dose. Notably, this episode showed marked elevation of ferritin level for the first time since initiation of rIL-18BP (Fig. 1D). It is likely that this was triggered by lung infection and exacerbated by missing one dose of rIL-18BP. In addition, free IL-18 levels, previously undetectable, increased to 21.8 pg/ml at the time of MAS onset. He has since resumed rIL-18BP at 3 mg/kg every 48 h with clinical stabilization, decrease in free IL-18 to undetectable levels, and allowing restart of a steroid taper.
In summary, use of IL-18BP was associated with stabilization of disease course and reduced MAS severity, but also notable improvement of linear growth with steroid wean. Levels of free IL-18 were undetectable shortly after initiation of rIL-18BP, although total serum IL-18 levels remained elevated, consistent with other studies and suggesting that free IL-18 might be a more accurate biomarker for disease activity [2, 7]. The hypothesis of balance between IL-18 and IL-18BP is also well supported by mouse models of MAS [7, 8]. This is interesting in light of our clinical experience, where the first missed rIL-18BP disrupted that balance, and was followed by immediate development of MAS. This is also in agreement with recent findings that IL-18 both distinguishes and promotes MAS [7]. This report raises hope for treating diseases marked by excessive free IL-18 such as sJIA, and preventing progression to MAS.
Acknowledgements
S.Y., A.A.G. and G.S.S. conceived and designed the study. S.Y. obtained patient samples and analysed biospecimens. K.S., E.S., A.S., A.A.G. and G.S.S. conducted the rIL-18BP compassionate use study, including collection of patient data. S.W.C., C.G. and C.G.-G. performed free IL-18 testing. S.Y., A.A.G. and G.S.S. analysed the data. S.Y. wrote the first draft of the manuscript. All authors contributed to and approved the final version of the manuscript.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: S.W.C. is a consultant for AB2Bio; C.G.-G.’s salary is supported by an unrestricted grant from AB2 Bio. C.G. has received grants from Pfizer, Roche, AB2 Bio, consultant and speaker’s fees from Roche, Pfizer, BMS, Merck, Sanofi, Regeneron, Eli Lilly, Novartis, AB2 Bio, and possesses shares in AB2 Bio. A.S. and E.S. are AB2 Bio employees; A.A.G. has received grant/research support from: NovImmune, AB2Bio and is a consultant for Novartis and Juno; G.S.S. is a consultant for Novartis.
References
- 1.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med 2015;66:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard C, Rech J, Brown M et al. Elevated serum levels of free interleukin-18 in adult-onset Still's disease. Rheumatology 2016;55:2237–47. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu M, Nakagishi Y, Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine 2013;61:345–8. [DOI] [PubMed] [Google Scholar]
- 4.Novick D, Elbirt D, Miller G et al. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J Autoimmun 2010;34:121–6. [DOI] [PubMed] [Google Scholar]
- 5.Canna SW, Girard C, Malle L et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 2017;139:1698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabay C, Fautrel B, Rech J et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Ann Rheum Dis 2018;77:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss ES, Girard-Guyonvarc'h C, Holzinger D et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 2018;131:1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard-Guyonvarc'h C, Palomo J, Martin P et al. Unopposed IL-18 signaling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood 2018;131:1430–41. [DOI] [PubMed] [Google Scholar]