Abstract
Context
Experimental studies suggest that magnesium levels in pregnant women may affect the length of gestation, as magnesium affects the activity of smooth muscle in the uterus. Little is known about the association between magnesium levels or supplementation and the rate of preterm birth.
Objective
The aim of this systematic review was to summarize the data on magnesium soil levels and preterm birth rates from ecological, observational, and interventional studies.
Data Sources
Soil magnesium levels were obtained from US Geological Survey data, and preterm birth rates were acquired from the March of Dimes Foundation. Relevant epidemiological and clinical studies published until April 2019 in peer-reviewed journals were retrieved from PubMed, Google Scholar, and related reference lists.
Study Selection
Original studies published in English, conducted in humans, and in which magnesium (dietary/supplemental intake or biomarkers) was an exposure and preterm birth was an outcome were included.
Data Extraction
Eleven studies were included in the systematic review. Meta-analysis was performed on 6 studies. Overall relative risk (RR) and corresponding 95%CIs for risk of preterm birth in relation to magnesium supplementation were estimated by a random-effects model.
Results
The ecological study revealed an inverse correlation between magnesium content in soil and rates of preterm birth across the United States (r = −0.68; P < 0.001). Findings from 11 observational studies generally support an inverse association between serum magnesium levels and rates of preterm birth. Of the 6 eligible randomized controlled trials, which included 3068 pregnant women aged 20 to 35 years and 352 preterm infants, the pooled RR was 0.58 (95%CI, 0.35–0.96) for women in the magnesium supplementation group compared with women in the control group.
Conclusions
Accumulated evidence from ecological, observational, and interventional studies consistently indicates that adequate magnesium intake during pregnancy may help reduce the incidence of preterm birth.
Keywords: magnesium supplementation, preterm birth, serum magnesium, soil magnesium
INTRODUCTION
Preterm birth, defined as birth before 37 weeks of pregnancy, is the leading cause of infant morbidity and mortality.1 In the United States, it is estimated that more than 1 in 10 infants were born prematurely in 2016.1 Preterm birth is associated with many long-term complications in survivors, including cerebral palsy and delayed development as well as impaired vision and hearing,1,2 which may lead to early childhood disabilities.3 In addition, costs associated with preterm birth in the United States are approximately $26.2 billion annually. These costs are attributed, in part, to medical care, special education, and loss of labor.4 Therefore, it is imperative to identify modifiable risk factors to develop strategies that could potentially reduce the rate of preterm birth.
Magnesium is the second most abundant mineral in the human body and serves as a cofactor for numerous enzymatic reactions.5 It is essential for muscle contraction, neurological function, energy metabolism, and synthesis of nucleic acids and proteins.6 Studies indicate that magnesium deficiency is associated with hyperactivity of the muscle cells in the uterus, which may consequently increase the risk of spontaneous abortion, preeclampsia, and preterm birth.7,8 Magnesium deficiency has also been linked to endothelial dysfunction,9 which has been shown to present disproportionately in women who deliver preterm.10 Of note, magnesium sulfate, a calcium antagonist, has been used for decades as a tocolytic agent to prevent preterm delivery. However, as its efficacy has not been validated, reversing magnesium status through nutritional support (both dietary and supplemental) may be an alternative method in obstetric care.
Magnesium in the environment, especially in soil, may contribute to magnesium status in humans, which is usually a reflection of dietary magnesium intake.11 Since soil harbors living plants, the availability of magnesium in the soil may affect the magnesium content in vegetables and grains,12 which are major dietary sources of magnesium.11 According to the US Geological Survey, soil magnesium levels vary widely across the mainland United States, which may result in geographic variations in magnesium intake.13 The US Department of Agriculture reported most Americans prefer eating local produce.14 Another potential pathway of magnesium transport from soil to individual dietary intake is through drinking water, given that the mineral content of soil may greatly influence the minerals present in drinking water.15 Furthermore, data from 2016 show that preterm birth rates vary across the United States and are disproportionately higher in the Southeast region.16 Differing levels of magnesium intake among pregnant women may be associated with regional disparities in rates of preterm birth. Thus, presumably, the regional distribution of magnesium content in soil may be associated with geographic variations in preterm birth rates through magnesium intake.
Clinically, findings from observational studies are generally consistent that preterm birth is more likely to occur in women who have hypomagnesemia status during the middle stage of their pregnancy.17–27 However, data from randomized controlled trials (RCTs) on magnesium supplementation are conflicting. Some studies,28–31 but not all,32–33 report that magnesium supplementation might reduce rates of preterm birth. A recent Cochrane review on the association between magnesium supplementation and various pregnancy-related outcomes concluded that magnesium supplementation might not be necessary to prevent preterm birth,34 though preterm birth was a secondary outcome in the review and most primary studies (6 of 7) included were conducted in the 1990s.
The objective of this study is to quantitatively and qualitatively summarize the data on magnesium and preterm birth from ecological, observational, and interventional studies. The PRISMA checklist served as a guideline during the systematic review and meta-analysis (see Appendix S1 in the Supporting Information online).
METHODS
Ecological study
The magnesium levels in soil were obtained from the US Geological Survey Publications Warehouse. From 2007 to 2010, soil samples collected from 4857 locations (1 per 1600 km2) in the contiguous United States were analyzed for magnesium content using inductively coupled plasma–atomic emission spectrometry.35 The median concentration of soil magnesium for each state was calculated. Data on soil magnesium content in Alaska and Hawaii are missing. The rates of preterm birth were obtained from the 2017 Premature Birth Report Card issued by the March of Dimes Foundation.36 This report provides the rate of preterm births in 2016 at the state level. The Spearman correlation coefficient was computed to determine the relation between magnesium content in soil and the preterm birth rates in 48 US states.
Systematic review and meta-analysis
A systematic review was carried out to examine the association between magnesium biomarkers and rates of preterm birth in observational studies, whereas a meta-analysis was conducted to assess the effect of magnesium supplementation on preterm birth. Because the processes of study selection, quality assessment, and data extraction and management were consistent for both the meta-analysis and the systematic review, the descriptions have been combined in the sections below.
Study selection.
PubMed was searched for relevant studies published up to April 2019. The search terms include “magnesium intake” OR “magnesium status” OR “magnesium supplementation” AND “premature labo(u)r” OR “premature delivery” OR “premature birth” OR “preterm labo(u)r” OR “preterm delivery” OR “preterm birth.” Using the same search terms, additional studies were identified by hand searching Embase, Google Scholar, and the reference lists of relevant articles. Detailed search strategies for PubMed and Embase are available in Table S1 in the Supporting Information online.
Table 1 summarizes the PICOS criteria for eligibility of studies. The following inclusion criteria were applied: human studies; original articles; published in English; and epidemiological studies in which magnesium (dietary intake or biomarkers) was an exposure and preterm birth was an outcome. There were no restrictions on study design, ie, both RCTs and observational studies were eligible. Of note, magnesium sulfate is a tocolytic agent and has been used to prevent preterm birth in the hospital setting.37 This meta-analysis excluded studies that examined the pharmacological effect of magnesium and focused only on studies that examined the nutritional impact.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Pregnant women | |
| Exposure (or Intervention)/Comparators |
|
Administration of intravenous or intramuscular magnesium sulfate to prolong labor |
| Outcome | Preterm birth (preferably spontaneous preterm birth) | |
| Study design | Human studies; original studies (observational studies or randomized controlled trials); published in English | Systematic reviews, meta-analyses, protocols |
Abbreviation: RDA, Recommended Dietary Allowance.
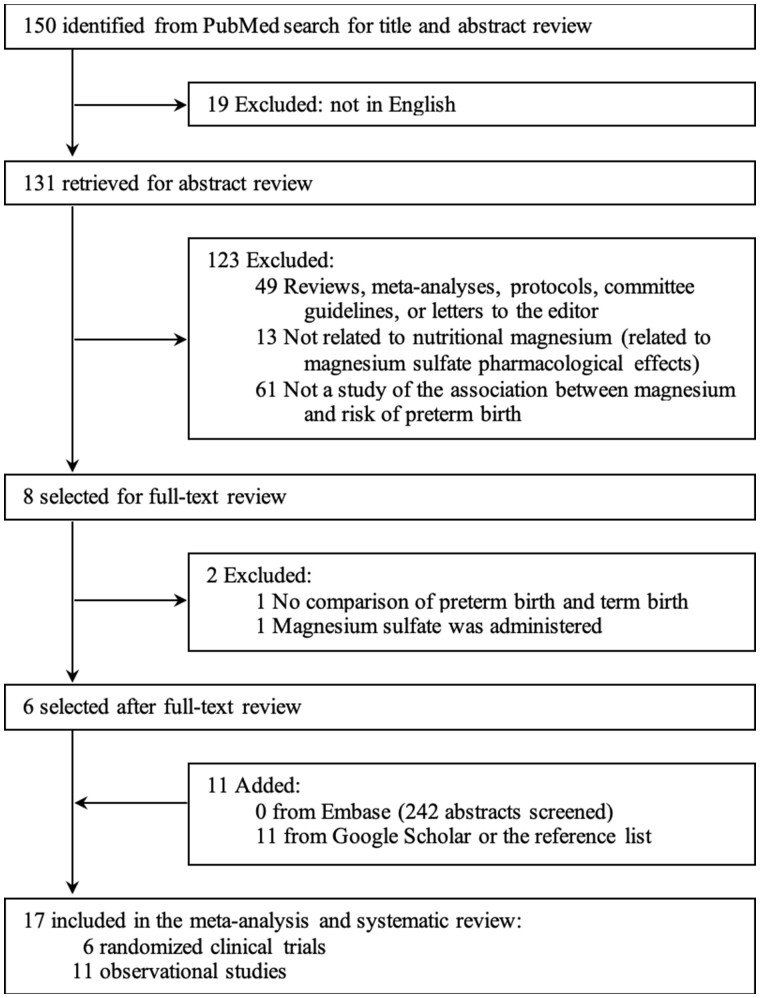
The initial PubMed search generated 150 abstracts, of which 144 were excluded by screening because they were not published in English, were not original studies (eg, reviews, meta-analyses, committee guidelines, or letters to the editor), or were not related to magnesium or the risk of preterm birth. The full texts of the 6 remaining studies were independently reviewed by 2 authors (Y.Z. and P.X.) and were all included. Eleven additional studies were retrieved from Google Scholar or from the reference lists of relevant articles (Figure 1).
Figure 1.
Flow chart of the literature search process.
Data extraction and quality assessment
Data were extracted using tables created specifically to record data. The following were documented for all studies: first author’s name, year of publication, country in which the study was conducted, characteristics of participants, outcomes assessed, and major findings. In addition, information about the intervention was recorded for RCTs, while information about exposure and adjusted covariates was extracted for observational studies.
Two authors (Y.Z. and P.X.) independently conducted the quality assessment using 3 validated instruments. The quality of RCTs was evaluated using the tool from the Cochrane handbook, which addresses the risk for various sources of bias, including selection bias (1 point for random sequence generation and 1 point for allocation concealment), performance bias (1 point for blinding of participants and personnel), detection bias (1 point for blinding of outcome assessment), attrition bias (1 point if there were no incomplete outcome data), reporting bias (1 point if there was no selective reporting), and others (1 point if there were no other types of bias).38 The total score ranged from 0 (lowest quality) to 7 (highest quality).
For prospective cohort studies, the Newcastle-Ottawa Scale was used to assess the quality of the studies in 3 categories: selection (4 points), comparability (1 point), and exposure (3 points).39 The total score ranged from 0 (lowest quality) to 8 (highest quality).
Lastly, for cross-sectional studies, the 14-item scale developed by the National Institutes of Health was used to assess the internal validity of the studies.40 This system, which can be used to assess the quality of both cross-sectional studies and prospective cohort studies, consisted of 14 rating criteria (Y for positive ratings, N for negative ratings, and NA for not applicable). The criteria addressed the following: research question (1 question), study population (2 questions), study recruitment (1 question), sample size (1 question), exposures (5 questions), outcome assessment (2 questions), follow-up (1 question), and statistical analyses (1 question). At the end, the numbers of Y, N, and NA responses were counted.
Statistical analysis in meta-analysis
Because the relative risks (RRs) for the risk of preterm birth relative to magnesium supplementation status were not directly reported in most of the RCTs included, they were derived from the original data by creating a dataset that included the number of participants and events in both the magnesium supplementation group (treatment group) and the control group, using the intention-to-treat principle. The RR and 95%CIs were then calculated for each study, and a pooled RR and 95%CI were estimated using a random-effects model. Cochran’s Q test was used to test the heterogeneity among studies, and I2 was calculated to quantify the inconsistency across studies. The Egger asymmetry test was used to assess publication bias. To test the robustness of the findings, sensitivity analysis was performed by excluding 1 primary study at a time from the pooled analysis.
All analyses were performed using STATA statistical software (version 13.0, STATA Corp, College Station, TX). P ≤ 0.10 was considered statistically significant for evaluation of heterogeneity and publication bias. P ≤ 0.05 was considered statistically significant for all other tests.
RESULTS
Evidence from the ecological study
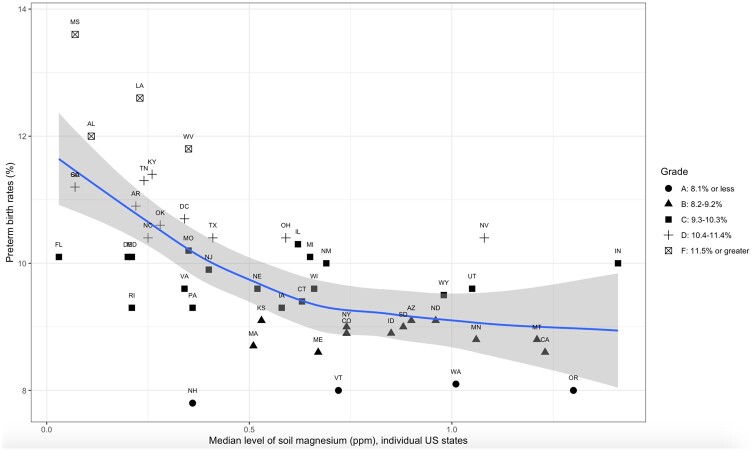
The Spearman correlation coefficient (rs) between magnesium content in soil and the preterm birth rate in the United States was −0.68 (P < 0.001). As shown in Figure 2,36 nonparametric regression analysis also revealed an inverse correlation.
Figure 2.
Association between median levels of magnesium in soil and preterm birth rates in 49 US states. The x-axis indicates the soil magnesium concentration (ppm), which was measured using inductively coupled plasma–atomic emission spectrometry. The y-axis denotes state-level preterm birth rates (%) in 2016. The symbols represent data for each state (designated by the 2-letter abbreviation for each US state name) and were categorized by the grade of preterm birth rates. The solid line is the LOESS (locally estimated scatterplot smoothing) regression line, which models the association of interest, and the shaded area stands for its 95% confidence band. The term grade is explained as follows: “Grade ranges were established in 2015 based on standard deviations of final 2014 state and District of Columbia preterm birth rates away from the March of Dimes goal of 8.1% by 2020. Grades were determined using the following scoring formula: (preterm birth rate of each jurisdiction −8.1%)/standard deviation of final 2014 state and District of Columbia preterm birth rates.”36
Evidence from observational studies
Tables S2 and S3 in the Supporting Information online show the results of quality assessment of all observational studies. The systematic review consisted of 2 prospective cohort studies and 9 cross-sectional studies (Table 217–27). The prospective cohort studies included a total of 310 individuals (68 cases of preterm birth), aged between 20 and 35 years. The study participants were recruited in the middle of the second trimester or early in third trimester and were followed until delivery. Cross-sectional studies included a total of 1397 individuals at delivery (620 in the preterm birth group), aged between 16 and 40 years.
Table 2.
Characteristics of the 11 observational studies included in the systematic review
| Reference | Country | No. of participants | Age of participants (years) | Duration of follow-up | Exposure assessment | Exposure categories | Adjusted covariates | No. of incident preterm births | Major findings | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective cohort studies | ||||||||||
| Enaruna et al (2013)17 | Nigeria | 160 | < 20, 20–35, > 35 | 2nd trimester (wk 24–26 of gestation) to 1 wk post delivery |
|
|
Age, parity, social class, and BMI | 24 | Preterm birth was 19% more likely in the hypomagnesemia group vs the normal group (P = 0.03) | |
| Shaikh et al (2012)18 | Pakistan | 150 | 20–35 | 2nd or 3rd trimester to delivery (not clear) |
|
|
Age | 44 | Incidence was significantly higher in the hypomagnesemia group (40%) vs the normal group (18.7%) (P < 0.01) | |
| Cross-sectional studies | ||||||||||
| Mahmoud et al (2016)25 | Iraq | 180 |
|
NA |
|
|
NA | 100 | Serum Mg level was lower in women who gave birth prematurely (P = 0.03) | |
| Jenabi et al (2017)23 | Iran | 64 |
|
NA |
|
|
NA | 32 |
|
|
| Okunade et al (2014)26 | Nigeria | 200 |
|
NA |
|
|
NA | 100 | RR = 1.83 (95%CI, 1.39–2.63) in group with low serum Mg (< 1.6 mg/dL) | |
| Bhat & Waheed (2012)21 | India | 200 |
|
NA |
|
|
NA | 100 | Serum Mg level was lower in the preterm birth group (P < 0.001) | |
| Begum & Das (2010)20 | Bangladesh | 160 |
|
NA | “Special magnesium analysis kit” |
|
NA | 80 | Serum Mg level was lower in the preterm birth group (P < 0.001) | |
| Shahid et al (2010)27 | Bangladesh | 200 | 16-40 | NA | Analysis was performed using a Xylidyl blue colorimetric method |
|
NA | 100 |
|
|
| Hantoushzadeh et al (2007)22 | Iran | 84 | NA | NA | Unknown |
|
NA | 42 | Serum Mg level was lower in the preterm birth group (P < 0.001) | |
| Kamal et al (2003)24 | India | 100 | NA | NA | Analysis was performed using a colorimetric method (Mg reagent kit; Ranbaxy Diagnostics, Mumbai, India) |
|
NA | 50 | Serum Mg level was lower in the preterm birth group (P < 0.001) | |
| Arikan et al (1999)19 | Austria | 209 |
|
Started at ≤ 18 wk of gestation |
|
NA | 16 | Serum Mg level did not differ significantly between preterm birth group and term birth group (P value not reported) | ||
Abbreviations: BMI, body mass index; Mg, magnesium; NA, not available; RR, relative risk.
Gestational age.
Two prospective cohort studies recruited 150 women (75 in the hypomagnesemia or normal group) and 160 women (80 in the hypomagnesemia or normal group), respectively, who had begun the second trimester of pregnancy and for whom serum magnesium concentrations were measured at baseline.17,18 Both studies found, after controlling for potential confounding variables (eg, age, parity, social class, and body mass index), that the rate of preterm birth in the hypomagnesemia group was significantly higher than that in the normal magnesium group.
Of the 9 cross-sectional studies,19–27 the 8 conducted in developing countries (ie, India, Bangladesh, Iran, Iraq, and Nigeria), consistently demonstrated lower serum magnesium levels in the preterm birth group than in the full-term birth group (P < 0.05). One study conducted in Nigeria showed that the rate of preterm birth in the hypomagnesemia group was 1.83 times higher than that in the control group.26 The authors noted that no statistically significant differences were identified with regard to age, previous pregnancy, or socioeconomic status between the preterm and term groups. Their findings were also supported by a study conducted in Bangladesh, which showed a 220% increase in risk of preterm birth among women with hypomagnesemia compared with those who had normal magnesium levels (serum magnesium above 1.9 mg/dL).27
However, 1 study from Austria, conducted in women who had not yet reached the 18th week of pregnancy and who did not consume any magnesium supplementation, demonstrated no significant difference in serum magnesium concentrations between preterm birth and full-term birth groups.19 Serum magnesium concentrations were measured at baseline and every 4 to 6 weeks thereafter. Of note, nearly one-third of the study population was excluded from the final analysis because of various medical considerations.
Evidence from clinical trials
The meta-analysis included 6 independent RCTs that examined the effects of magnesium supplementation on the rate of preterm birth (Table 329–33,41). The dataset comprised 3068 individuals (352 preterm cases) between the ages of 20 and 35 years. One trial, from Iran, was conducted recently (in 2016), while the other 5, all from Western countries, were conducted between 1981 and 1994. The study populations varied in size, ranging from 54 to 1766. On average, interventions were started between the end of the first trimester and the middle of second trimester of pregnancy. Overall, the quality of these trials was good, ie, all received more than 4 points when assessed with the 7-point tool from the Cochrane handbook38 (see Table S4 in the Supporting Information online).
Table 3.
Characteristics of the 6 randomized clinical trials included in the meta-analysis
| Reference | Country | Group | No. of participants | No. lost to follow-up | Age of participants (years) | Intervention | Duration of intervention | No. of incident preterm births | Major findings |
|---|---|---|---|---|---|---|---|---|---|
| Zarean & Tarjan (2017)29 | Iran | C1 | 60 | 0 | 29.8±5.1 |
|
Started between wk 12 and wk 14 of gestation | 15 | Preterm birth was significantly less frequent in group C (P = 0.04) |
| C2 | 60 | 0 | 29.7±6.2 |
|
16 | ||||
| T | 60 | 0 | 29.4±5.7 |
|
6 | ||||
| Zarcone et al (1994)30 | Italy | T | 50 | 4 | 18–28 | 15 mmol Mg hydrochlorate aspartate per day | Started from wk 12 of gestation | 1 | Difference in incidence of premature labor appears to be significant (P value not available) |
| C | 50 | 5 | 18–28 | 15 mmol aspartic acid per day | 4 | ||||
| Martin et al (1992)32 | USA | T | 27 | 5 | 24.2 | Mg gluconate tablet (216 mg, equivalent to 8.9 mmol elemental Mg) given daily | NA | 16 | No association between treatment and the risk of preterm labor (RR = 1.21; 95%CI, 0.80–1.83) |
| C | 27 | 2 | 26.5 | Placebo tablet | 15 | ||||
| Sibai et al (1989)33 | USA | T | 200 | 15 | 20–35 | Mg aspartate hydrochloride tablet (365 mg, equivalent to 15 mmol elemental Mg) given daily | Wk 13 to wk 24 of gestation | 13 | No difference in frequency of preterm labor (RR not available) |
| C | 200 | 11 | 20–35 | 14 | |||||
| Spätling & Spätling (1988)31 | Switzerland | T | 278 | 61 | 20.3–37.9 | 15 mmol Mg aspartate hydrochloride per day | Before wk 16 of gestation | 12 | Decreased frequency of preterm delivery in the treatment group vs the control group (P < 0.05) |
| C | 290 | 70 | 19.5–34.6 | 13.5 mmol aspartic acid per day | 26 | ||||
| Kuti et al (1981)41 | Hungary | 1 | 637 | No supplementation | 88 | Group supplemented with highest dose of Mg showed greatest reduction in frequency of preterm birth | |||
| 2 | 592 | 30–150 g Mg citrate total (elemental Mg, 143–716 mmol) | Unknown | 88 | |||||
| 3 | 424 | 151–250 g Mg citrate total (elemental Mg, 721–1193 mmol) | Unknown | 33 | |||||
| 4 | 113 | 251–400 g Mg citrate total (elemental Mg, 1198–1909 mmol) | Unknown | 5 |
Abbreviations: C, control; Mg, magnesium; NA, not available; RCT, randomized controlled trial; RR, relative risk; T, treatment.
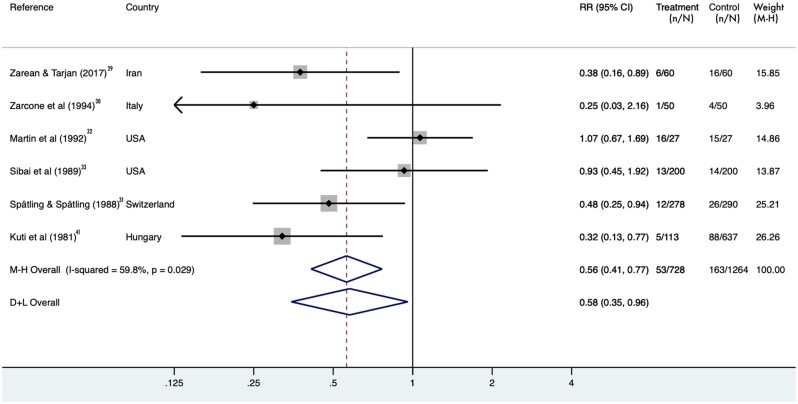
After pooling data from the 6 identified RCTs, magnesium supplementation was significantly associated with a reduced rate of preterm birth. When intention-to-treat analysis was performed, the pooled RR was 0.58 (95%CI, 0.35–0.96), indicating a beneficial effect of magnesium supplementation on preterm birth, though there was significant heterogeneity among studies (I2 = 60%; P = 0.03) (Figure 329–33,41). However, results from the Egger test showed no small-study effect (P = 0.62). By excluding 1 study at a time, the pooled RRs ranged from 0.50 (95%CI, 0.32–0.75) after excluding Sibai et al33 to 0.66 (95%CI, 0.40–1.09) after excluding Kuti et al.33,41 In addition, the pooled RR was recalculated by using per-protocol analysis. The result was attenuated and became marginally significant (RR = 0.58; 95%CI, 0.32–1.05; P = 0.073).
Figure 3.
Pooled relative risks (RRs) and 95%CIs of incident preterm birth using intention-to-treat analysis. A random-effects model was used to estimate the pooled RRs. The solid diamonds indicate the RRs from individual randomized controlled trials; RRs were calculated by comparing magnesium supplementation with placebo. The size of the shaded square is proportional to the percent weight of each study. The horizontal lines represent 95%CIs, and the arrows indicate the truncated ends. The hollow diamonds indicate the pooled RRs. Abbreviations: D+L, DerSimonian and Laird; M-H, Mantel-Haenszel.
DISCUSSION
Main findings
In the present study, data from ecological, observational, and interventional studies were synthesized through meta-analysis and systematic review. The accumulated evidence supports a significant inverse association between magnesium levels and preterm birth rates.
Although the underlying mechanisms between magnesium levels and preterm birth are not fully understood, it is well known that the relation between these 2 is bidirectional. During pregnancy, low magnesium levels may be caused by fetal needs, changes in maternal tissue, or increased renal loss; thus, low magnesium levels should not be mistaken for dilution effects alone.8 By contrast, higher serum magnesium in pregnant women has been associated with lower rates of preeclampsia, hypertension, and gestational diabetes.6 The present review of the cross-sectional studies suggests that the serum magnesium concentration might be a useful predictor of the onset of preterm birth. Serum magnesium levels were compared between women who delivered preterm (cases) and women who delivered term (control). Most studies indicated that serum magnesium levels were significantly lower among the cases (differences ranging from 0.17 to 0.54 mg/dL), though not necessarily reaching a state of deficiency. The exact serum magnesium levels of the cases vary across studies, which is presumably attributable to diverse food cultures, environmental factors, or bioavailability.
In addition, results from the 2 prospective studies in the present review, although limited, generally show that magnesium deficiency, as measured by serum magnesium levels, may lead to a higher rate of preterm birth.17,18 Notably, the cutoff points used to define magnesium deficiency status were inconsistent between these 2 studies. Although the normal range of serum magnesium levels defined by the laboratory kit (manufactured in the USA) is 1.6 to 3.0 mg/dL, this cutoff point set in the United States may not be appropriate for other populations, especially relatively disadvantaged populations that may have a suboptimal nutrition status. Thus, in the study conducted in Nigeria, the cutoff point was 1.25 mg/dL (calculated using the formula of “mean minus 2 × standard deviation”).17 Prospective cohort studies in general provide stronger evidence than cross-sectional studies, as the exposure, magnesium, precedes the outcome, preterm birth.42 They are also ideal to study magnesium status measured by biomarkers such as serum magnesium levels since RCTs are not feasible.
Previously, magnesium adequacy has been linked to a reduced rate of several pregnancy-related outcomes, including preeclampsia, intrauterine growth restriction, and preterm birth.8,43 This meta-analysis of RCTs provides evidence to help elucidate the relation between magnesium and preterm birth. The pooled analysis demonstrates a lower rate of preterm birth among women who took antenatal magnesium supplementation. It is possible that decreased magnesium levels during pregnancy may be resolved by oral supplementation.8 Nevertheless, the effects of dietary magnesium intake on the outcome of preterm birth could not be determined because the source of dietary magnesium may have been different from that of supplemental magnesium. Thus, the conclusions about magnesium consumption are based solely on prophylactic supplementation. Notably, the findings from the ecological study included in this systematic review may shed light on the understanding of how dietary magnesium impacts preterm birth rates, since the mineral content of soil has been strongly linked to dietary mineral intake and consequent human health.44 Heavy metal accumulation in soil, coupled with soil erosion, can lead to a soil magnesium deficit, which in turn can lead to magnesium deficit in plants.12,44 For example, a dramatic decrease in the magnesium levels in wheat has been noted since the 1960s.44 Because humans consume magnesium mainly from leafy greens and whole grains, it is believed that the depletion of magnesium in the soil may lead to magnesium deficiency in the human body.44 This ecological study included in this systematic review shows a significant inverse correlation between soil magnesium content and the rate of preterm birth, which provides additional evidence in support of a link between magnesium status and preterm birth, though the ecological data are subject to confounding.
Strengths and limitations
To the best of knowledge, this is the first review to comprehensively assess the current literature on the overall association between magnesium status and supplementation and the risk of preterm birth reported in ecological, observational, and interventional studies. Nevertheless, the current review is subject to several limitations.
Meta-analysis
The meta-analysis of 6 RCTs showed a beneficial effect of magnesium supplementation on the rate of preterm birth. A stratified analysis to test any potential effect modifiers was not performed because of the limited number of primary studies. Notably, although the design of RCTs can largely reduce various biases by randomization, there is no guarantee that the RCTs in this meta-analysis were immune to postrandomization biases, such as noncompliance and censoring, which were not well addressed in the primary studies. However, the findings from the per-protocol analysis were generally consistent with those from intention-to-treat analysis, which may, at least partially, relieve this concern. In addition, further examination of the racial differences in relation to magnesium and preterm birth was not performed because studies were sporadic in certain regions.
Furthermore, in one RCT,41 there was insufficient information to determine whether the preterm cases were spontaneous preterm births or induced preterm births (the outcome variable is usually delivery before 36 weeks). This can be problematic because the latter can result from a variety of maternal, fetal, and placental complications. However, in this meta-analysis, an effort was made to consistently include the studies that reported spontaneous preterm birth only, which is different from the previous Cochrane review.34
Systematic review
Previous studies suggest that various factors such as age, socioeconomic status, diet, and parity may confound the association between magnesium status and the risk of preterm birth.45 Since the cross-sectional studies in this systematic review failed to adjust for these factors, they may be prone to confounding.
The cross-sectional design of some studies represents another major limitation. The lack of temporal association limited the ability to establish any causal relationship between serum magnesium levels and the risk of preterm birth. In addition, these studies measured serum magnesium levels at different times during pregnancy. For example, 2 studies measured serum magnesium levels at delivery, while 3 other studies measured serum magnesium levels before delivery. Of note, the serum magnesium concentration tends to decrease over the course of pregnancy,17,19,22 likely reaching its lowest point as delivery approaches. Differences in the time at which the magnesium concentration is measured may confound the results to some extent.
In all studies, serum magnesium concentrations were measured as the proxy for magnesium levels in the body, which can lead to inaccurate measurements, given that less than 1% of magnesium is in the serum.11 Nevertheless, because intracellular magnesium levels can be technically difficult and expensive to measure,11,46 serum magnesium concentrations are commonly used to evaluate the magnesium status in the human body.11 Additionally, hypomagnesemia status was not defined consistently across studies, and cutoff values in some studies were not in concordance with the value commonly used to establish magnesium deficiency (< 1.8 mg/dL). This can create a problem because women at risk of magnesium deficiency may be confused about which serum magnesium level is considered adequate to avoid adverse pregnancy-related outcomes, including preterm birth. However, as discussed above, the exact cutoff for magnesium deficiency should be established by taking into consideration a variety of environmental and genetic factors that can contribute to wide variations in average serum magnesium concentrations among populations.
Interpretation
To date, 2 formats of magnesium therapy have been used to prolong pregnancy.7 The first is the injection of magnesium sulfate (tocolytic agent) during labor, which is a short-term pharmacological method for the prevention of preterm birth.47 It is a common practice in obstetrics, and its effect has been examined in 5 large-scale prospective clinical trials.37,48–51 However, a review of current clinical trials suggests that magnesium sulfate may not be efficient in preventing preterm birth or providing neuroprotection to the fetus, as suggested previously.52 The second format is long-term supplementation with magnesium, the purpose of which is to reverse magnesium deficiency. The nutritional effect of magnesium was less noticeable than that of magnesium sulfate, but it is relatively inexpensive and can potentially be used to reach broader at-risk populations. Currently, it is recommended that women over 18 years of age consume 310 to 320 mg of magnesium per day. However, fewer than half of the women in the United States meet the Recommended Dietary Allowance for magnesium,53–55 let alone the pregnant population. Since magnesium deficiency may be asymptomatic in the early stages,6 it might be of clinical importance to advise pregnant women to consume more magnesium-rich foods prior to pregnancy or during the early stages of pregnancy.
CONCLUSION
Accumulated evidence from ecological, observational, and interventional studies consistently supports an inverse association between higher magnesium levels or magnesium supplementation during pregnancy and a lower incidence of preterm birth. This review further supports the dietary recommendation of foods rich in magnesium, such as whole grains, nuts, and seeds, for pregnant women. For those pregnant women with low magnesium status, supplementation may be considered. Additional, well-designed, large-scale RCTs are needed to confirm the findings from this review and to establish causal inference.
Supplementary Material
Acknowledgments
Author contributions. K.H. conceptualized the project and designed the study; Y.Z. and P.X. performed the literature searches and collected, presented, and interpreted the data; Y.Z. wrote the first draft of the manuscript; P.X., C.C., L.L., M.S., A.R., and K.H. critically revised the draft and contributed intellectual content. All the authors approved the final version of the manuscript.
Funding/support. This study was supported in part by 2 grants from the US National Institutes of Health (R01DK116603 and R01AG056111). The funding sources had no involvement in the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.
Declaration of interest: The authors have no relevant interests to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA 2009 checklist
Table S1 Search terms in PubMed and Embase
Table S2 Quality assessment of the 2 prospective cohort studies included in the systematic review
Table S3 Quality assessment of the 9 cross-sectional studies included in the systematic review
Table S4 Quality assessment of the 6 randomized clinical trials included in the meta-analysis
References
- 1.Centers for Disease Control and Prevention. Preterm birth. Centers for Disease Control and Prevention website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm. Accessed July 22, 2018.
- 2.Bloomfield FH. How is maternal nutrition related to preterm birth? Annu Rev Nutr. 2011;31:235–261. [DOI] [PubMed] [Google Scholar]
- 3.Alves JG, de Araujo CA, Pontes IE, et al. The BRAzil MAGnesium (BRAMAG) trial: a randomized clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity. BMC Pregnancy Childbirth. 2014;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Societal Costs of Preterm Birth: Causes, Consequences, and Prevention. In: Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- 5.Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem.1991;28:19–26. [DOI] [PubMed] [Google Scholar]
- 6.Dalton LM, Ní Fhloinn DM, Gaydadzhieva GT, et al. Magnesium in pregnancy. Nutr Rev. 2016;74:549–557. [DOI] [PubMed] [Google Scholar]
- 7.Durlach J, Pages N, Bac P, et al. New data on the importance of gestational Mg deficiency. Magnes Res. 2004;17:116–125. [PubMed] [Google Scholar]
- 8.Spätling L, Classen H-G, Kisters K, et al. Supplementation of magnesium in pregnancy. J Preg Child Health. 2017;4:302. [Google Scholar]
- 9.Kostov K, Halacheva L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int J Mol Sci. 2018;19:E1724. doi:10.3390/ijms19061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Scholl TO. Maternal biomarkers of endothelial dysfunction and preterm delivery. PLoS One. 2014;9:e85716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health, Office of Dietary Supplements. Magnesium fact sheet for health professionals. National Institutes of Health, Office of Dietary Supplements website. https://ods.od.nih.gov/factsheets/Magnesium-HealthProfessional/. Updated March 2, 2018. Accessed July 22, 2018.
- 12.Guo W, Nazim H, Liang Z, et al. Magnesium deficiency in plants: an urgent problem. Crop J. 2016;4:83–91. [Google Scholar]
- 13.Newby PK, Noel SE, Grant R, et al. Race and region are associated with nutrient intakes among black and white men in the United States. J Nutr. 2011;141:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tropp D. Why Local Food Matters: the Rising Importance of Locally-Grown Food in the U.S. Food System. A National Perspective. Washington, DC: US Department of Agriculture; 2014. https://www.ams.usda.gov/sites/default/files/media/Why%20Local%20Food%20MattersThe%20Rising%20Importance%20of%20Locally%20Grown%20Food%20in%20the%20U.S.%20Food%20System.pdf. Published March 2014. Accessed January 31, 2019.
- 15.World Health Organization. Hardness in drinking-water. https://www.who.int/water_sanitation_health/dwq/chemicals/hardness.pdf. Published 2011. Accessed January 31, 2019.
- 16.Centers for Disease Control and Prevention, National Center for Health Statistics. Percentage of babies born preterm by state: 2016. https://www.cdc.gov/nchs/pressroom/sosmap/preterm_births/preterm.htm. Updated April 28, 2020. Accessed July 31, 2018.
- 17.Enaruna NO, Ande A, Okpere EE. Clinical significance of low serum magnesium in pregnant women attending the University of Benin Teaching Hospital. Niger J Clin Pract. 2013;16:448–453. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh K, Baloch GH, Abbas T, et al. Magnesium associated complications in pregnant women. World Appl Sci J. 2012;17:1074–1078. [Google Scholar]
- 19.Arikan GM, Panzitt T, Gucer F, et al. Course of maternal serum magnesium levels in low-risk gestations and in preterm labor and delivery. Fetal Diagn Ther. 1999;14:332–336. [DOI] [PubMed] [Google Scholar]
- 20.Begum AA, Das TR. Low serum magnesium in preterm labour. J Bangladesh Coll Phys Surg. 2010;28:86–91. doi:10.3329/jbcps.v28i2.5368 [Google Scholar]
- 21.Bhat S, Waheed A. Serum magnesium levels in preterm labour. Sri Lanka J Obstet Gynaecol. 2012;34:37–44. [Google Scholar]
- 22.Hantoushzadeh S, Jafarabadi M, Khazardoust S. Serum magnesium levels, muscle cramps, and preterm labor. Int J Gynaecol Obstet. 2007;98:153–154. [DOI] [PubMed] [Google Scholar]
- 23.Jenabi E, Poorolajal J, Fereidooni B, et al. The association between maternal serum magnesium level and pregnancy outcomes. J Postgrad Med Inst. 2017;31:77–81. [Google Scholar]
- 24.Kamal S, Sharan A, Kumar U, et al. Serum magnesium level in preterm labour. Indian J Pathol Microbiol. 2003;46:271–273. [PubMed] [Google Scholar]
- 25.Mahmoud SA, Saleh IM, Khalaf HH. The correlation between maternal hypomagnesemia and preterm labour. Int J Reprod Contracept Obstet Gynecol. 2016;5:2571–2575. [Google Scholar]
- 26.Okunade KS, Oluwole AA, Adegbesan-Omilabu MA. A study on the association between low maternal serum magnesium level and preterm labour. Adv Med. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahid A, Hosna A, Tahmina H. Hypomagnesaemia in pregnancy: a predictor of preterm labour. J Dhaka Med Coll. 2010;19:51–57. [Google Scholar]
- 28.Arikan G, Panzitt T, Gücer F, et al. Oral magnesium supplementation and the prevention of preterm labor. Am J Obstet Gynecol. 1997;176:PS45. doi:10.1016/S0002-9378(97)80192-5. [Google Scholar]
- 29.Zarean E, Tarjan A. Effect of magnesium supplement on pregnancy outcomes: a randomized control trial. Adv Biomed Res. 2017;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarcone R, Cardone G, Bellini P. Role of magnesium in pregnancy. Panminerva Med. 1994;36:168–170. [PubMed] [Google Scholar]
- 31.Spätling L, Spätling G. Magnesium supplementation in pregnancy. A double-blind study. BJOG. 1988;95:120–125. PMID: 3349001 [PubMed] [Google Scholar]
- 32.Martin RW, Perry KG Jr, Hess LW, et al. Oral magnesium and the prevention of preterm labor in a high-risk group of patients. Am J Obstet Gynecol. 1992;166(1 pt 1):144–147. [DOI] [PubMed] [Google Scholar]
- 33.Sibai BM, Villar MA, Bray E. Magnesium supplementation during pregnancy: a double-blind randomized controlled clinical trial. Am J Obstet Gynecol. 1989;161:115–119. [DOI] [PubMed] [Google Scholar]
- 34.Makrides M, Crosby DD, Bain E, et al. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014(4):CD000937. doi:10.1002/14651858.CD000937.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DB, Cannon WF, Woodruff LG, et al. Geochemical and Mineralogical Data for Soils of the Conterminous United States. Reston, VA: US Geological Survey; 2013. Data series 801. https://pubs.usgs.gov/ds/801/pdf/ds801.pdf. Published 2013. Accessed: •••.
- 36.March of Dimes Foundation. March of Dimes 2017 premature birth report card. https://www.marchofdimes.org/materials/PrematureBirthReportCard-United-States-2017.pdf. Published 2017. Accessed July 20, 2018.
- 37.Crowther CA, Hiller JE, Doyle LW, et al. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. http://handbook.cochrane.org/. Accessed July 20, 2018.
- 39.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2012. Accessed October 12, 2018.
- 40.National Heart, Lung, and Blood Institute. Quality assessment tool for observational cohort and cross-sectional studies. National Heart, Lung, and Blood Institute website. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed October 12, 2018.
- 41.Kuti V, Balázs M, Morvay F, et al. Effect of maternal magnesium supply on spontaneous abortion and premature birth and on intrauterine foetal development: experimental epidemiological study. Magnes-Bull. 1981;3:73–79. [Google Scholar]
- 42.Mann CJ. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman A, Desai N, Rochelson B, et al. Maternal magnesium supplementation reduces intrauterine growth restriction and suppresses inflammation in a rat model. Am J Obstet Gynecol. 2013;208:383.e1–383.e7. doi:10.1016/j.ajog.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 44.DiNicolantonio JJ, O’Keefe JH, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Heart. 2018;5:e000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eunice Kennedy Shriver National Institute of Child Health and Human Development. What are the risk factors for preterm labor and birth? https://www.nichd.nih.gov/health/topics/preterm/conditioninfo/who_risk. Updated January 21, 2017. Accessed July 21, 2018.
- 46.Fox CH, Timm EA Jr, Smith SJ, et al. A method for measuring intracellular free magnesium concentration in platelets using flow cytometry. Magnes Res. 2007;20:200–207. PMID: 17972463 [PubMed] [Google Scholar]
- 47.Committee Opinion no. 652: magnesium sulfate use in obstetrics. Obstet Gynecol. 2016;127:e52–e53. doi:10.1097/AOG.000000000000126 [DOI] [PubMed] [Google Scholar]
- 48.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG. 2007;114:289–299. doi:10.1111/j.1471-0528.2006.01165.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marret S, Marpeau L, Benichou J. Benefit of magnesium sulfate given before very preterm birth to protect infant brain. Pediatrics. 2008;121:225–226. [DOI] [PubMed] [Google Scholar]
- 50.Mittendorf R, Dambrosia J, Pryde PG, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol. 2002;186:1111–1118. [DOI] [PubMed] [Google Scholar]
- 51.Rouse DJ. Magnesium sulfate for the prevention of cerebral palsy. Am J Obstet Gynecol. 2009;200:610–612. [DOI] [PubMed] [Google Scholar]
- 52.Mercer BM, Merlino AA, Society for Maternal-Fetal Medicine. Magnesium sulfate for preterm labor and preterm birth. Obstet Gynecol. 2009;114:650–668. [DOI] [PubMed] [Google Scholar]
- 53.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–2882. [DOI] [PubMed] [Google Scholar]
- 54.Rosanoff A, Weaver CM, Rude RK. Suboptimal magnesium status in the United States: are the health consequences underestimated? Nutr Rev. 2012;70:153–164. [DOI] [PubMed] [Google Scholar]
- 55.DietaryGuidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington, DC: US Department of Agriculture, Agricultural Research Service; 2015. https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. Published February 2015. Accessed October 22, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.