Abstract
Objective
Some children with chronic pain struggle with fear of pain, avoidance behaviors, and associated disability; however, movement adaptations in the context of chronic pain in childhood is virtually unknown. Variability in adaptive movement responses previously observed between individuals might be largely explained by the presence of problematic psychological drivers (eg, fear, avoidance). The goals of this study were to quantify the variability of gait and examine relationships among pain, fear, avoidance, function (perceived and objective), and gait variability.
Methods
This study used a cross-sectional design. Eligible patients were between 8 and 17 years of age and had musculoskeletal, neuropathic, or headache pain that was not due to acute trauma (eg, active sprain) or any specific or systemic disease. Participants completed the Numeric Pain Rating Scale, Fear of Pain Questionnaire (FOPQ), Functional Disability Inventory, and 6-Minute Walk Test and received kinematic gait analysis. Relationships were analyzed among these measures, and the self-report and functional measures were examined to determine whether they predicted gait variability (GaitSD).
Results
The 16 participants who were evaluated (13.8 [SD = 2.2] years of age; 13 female) had high Numeric Pain Rating Scale scores (6.2 [SD = 2.1]), FOPQ-Fear scores (25.9 [SD = 12.1]), FOPQ-Avoidance scores (22.8 [SD = 10.2]), and Functional Disability Inventory scores (28.6 [SD = 9.4]) and low 6-Minute Walk Test distance (437.1 m [SD = 144.6]). Participants had greater GaitSD than age-predicted norms. Fear was related to self-selected GaitSD, and avoidance was related to both self-selected and standardized GaitSD. Avoidance predicted 43% and 47% of the variability in self-selected and standardized GaitSD, respectively.
Conclusion
GaitSD was significantly related to both fear of pain and avoidance behaviors, suggesting the interplay of these psychological drivers with movement. FOPQ-Avoidance was robust in accounting for GaitSD.
Impact
This study offers preliminary evidence in understanding movement adaptations associated with adolescents with chronic pain. They may lend to more directed interventions.
Keywords: Graded Exposure in Vivo, Pain-Related Fear, Gait, Avoidance, Pediatric Pain
Introduction
The typical response to acute musculoskeletal pain is a movement adaptation that protects the injured body part while still allowing function.1,2 Once the pain resolves, normal movement patterns resume,3 with individuals remaining fully integrated in society. Unfortunately for some individuals, pain is accompanied by heightened fear and generalized avoidance of movements that could potentially be associated with pain.4,5 This maladaptive response pattern perpetuates movement adaptations after the initial injury has healed and can lead to functional disability and poor quality of life as described in the Fear Avoidance Model of Chronic Pain.4 Butera and colleagues6 recently extended the fear avoidance model to integrate movement as a key factor in understanding chronic pain. This newer model suggests that feedback to the nervous system from adapted movement contributes to the pain experience. How this feedback is processed affects subsequent movement. Thus, if altered movement is promoted due to psychological, sensory, or neuromuscular activation, it will persist, preventing recovery.6 As evidence, studies have demonstrated clear relationships between pain-related fear and movement in adults with subacute low back pain7 and experimentally induced back pain,3,8,9 and pediatric patients with complex regional pain syndrome10 (CRPS) and joint hypermobility.11
Much of the current understanding of movement adaptations in response to chronic pain comes from studying adults; however, it is estimated that at least 11% to 38%12–14 (median rates) of children and adolescents suffer from some form of chronic pain. Similar to adults, a significant portion of children with chronic pain struggle with fear of movement, avoidance behaviors, and associated disability5,10,15–17; however, movement adaptations in the context of chronic pain in childhood is virtually unknown. Recent research has investigated movement adaptations18 and biomechanical responses to exercise19 in adolescents with juvenile fibromyalgia. The results of these investigations suggest movement differences exist between those with juvenile fibromyalgia compared with healthy controls18 and that a combination of cognitive behavioral therapy and neuromuscular training resulted in improved strength and movement control.19 This work is important and has begun to highlight the value of measuring movement related to chronic pain during a “critical period of physical development.”16p467
These studies in juvenile fibromyalgia provide initial evidence regarding altered movement in pediatric chronic pain, but none to our knowledge have examined movement adaptations in relation to potential psychological drivers highlighted in the fear avoidance model. It may be that the variability in adaptive movement responses observed between individuals may be largely explained by the presence of these problematic psychological drivers (fear, avoidance).1,2 Given the limited data on movement adaptations in pediatric patients with chronic pain, the goals of the current study were to quantify the variability of gait, and examine the relations between gait variability (GaitSD) and pain, fear, avoidance, and function. The hypotheses were that participants would have greater kinematic variability of gait due to their chronic pain condition and that greater kinematic variability of gait would be associated with higher pain, fear, avoidance, and lower function.
Methods
Participants
This study was part of a larger pilot trial of graded exposure therapy jointly delivered by a physical therapist and psychologist (NCT01974791).20 The initial funding for the study did not include sufficient support for the biomechanical assessment. The second pilot grant funded a more comprehensive assessment battery. The first 14 participants recruited did not undergo biomechanical testing. To be eligible for the larger study, patients had to have persistent pain and fear and be referred by a medical, physical therapy, or psychology provider. Inclusion criteria were (1) ages 8 to 17 years; (2) pain-related fear (score >40 on the Fear of Pain Questionnaire [FOPQ]21 or clinician determination if scores were below the cut-off, which was thoroughly screened by the study team; (3) musculoskeletal, neuropathic, or headache pain that was not due to acute trauma (active sprain or fracture) or any specific or systemic disease (unless the pain and associated disability was disproportionate to underlying disease process); and (4) functional limitations (score >12 on the Functional Disability Inventory [FDI]).22 Additional inclusion criteria specific to this study were that participants had to be ambulatory without an assistive device. This trial was run pragmatically, and as a pilot, investigators had the opportunity to examine the degree of fit of patients enrolled who did not meet strict cut-off scores. As indicated, patients who did not meet score criteria were thoroughly screened to ensure that the evaluating clinician felt the patient had sufficient levels of fear avoidance and functional disability to benefit from the treatment. Exclusion criteria were (1) significant cognitive impairment based on medical record review, (2) serious psychopathology (eg, suicidality) based on medical record review, (3) acute trauma (<6 weeks), (4) biomechanical deficit that would limit ability to safely engage in exposure activities (eg, severe muscle atrophy) based on physical therapist evaluation, (5) and making gains in current physical therapist or no prior physical therapist treatment when clearly indicated. During the study, patients were instructed to not seek new treatments for pain. For each patient referred to graded exposure therapy (GEXP), eligibility for enrollment was determined by a screening evaluation with a pain psychologist (PI or GEXP psychologist) and the GEXP physical therapist. Institutional review board approval was obtained from a large northeast children’s hospital and the university where this portion of the study took place.
Procedures
Prior to initiating any testing, a parent and the patient met with the research coordinator to review study procedures, sign consent and assent forms, and complete questionnaires. Biomechanical testing was completed on the same day.
Measures of Pain, Fear, Avoidance, and Function
Numeric Pain Rating Scale (NPRS)
The NPRS scale was used to measure the average subjective intensity of pain experienced in the past week.23 The NPRS is an 11-point scale from 0 to 10 with 0 = no pain and 10 = worst pain imaginable.23 Moderate and severe pain were considered ratings of ≥4 and ≥7, respectively.24
Fear of Pain Questionnaire
We used the FOPQ to collect data on pain-related fear and avoidance patterns.21 For this study, the self-reported questionnaire was provided to the child and consisted of 24 statements, such as “feelings of pain are scary for me” and “I walk around in constant fear of hurting.” Items were rated on a 5-point Likert scale, ranging from 0 to 4 (0 = strongly disagree, 4 = strongly agree). The FOPQ-C contains 2 subscales: Fear of Pain (11 items) and Avoidance of Activities (13 items). The total score (out of 96) is derived by summing items, with higher scores indicating greater pain related fear and avoidance of activities. Moderate and severe fear and avoidant patterns were derived from tertile analyses derived from the sample for each subscale score. FOPQ-Fear scores ≥20 and ≥30.2 and FOPQ-Avoidance scores ≥15 and ≥25 were considered moderate and severe, respectively. The FOPQ has established reliability for both subscales and validity for use in this population.21
Functional Disability Inventory
We used the FDI to measure the physical functioning and disability of the participants.22 The FDI is a 15-item self-report inventory used to assess the perceived difficulty with performance of daily activities in home, school, recreational, and social domains. Participants were asked to rate how much difficulty they had completing various tasks “in the past few days …” with activities such as “doing chores at home” or “being in school all day.” Items were rated on a 5-point Likert scale, ranging from 0 to 4 (0 = no trouble, 4 = impossible), and the sum of responses created a total score out of 60, with higher scores indicating greater pain-related disability. Moderate and severe disabilities were considered scores ≥13 and ≥30, respectively.22 The FDI has established validity and reliability within this population.22
Six-Minute Walk Test (6MWT)
The 6MWT was completed by following a standard protocol to assess physical function.25,26 One hundred feet was measured in a level, open hallway. Two Xs were taped on the floor to indicate the beginning and end of 100 feet. Participants were instructed to walk back and forth around the 2 Xs, as fast as they were comfortable, without an assistive device for 6 minutes. Breaks were allowed if requested. The experimenter tracked the number of laps to calculate the overall distance walked during the 6 minutes. Distance was recorded in feet and converted to meters. The 6MWT has been validated for use among healthy children.25
Biomechanical Testing
Gait Analysis
Gait trials were collected during 2 walking conditions: (1) self-selected pace, and (2) standardized pace (1.2 m/s).19,27,28 For the self-selected pace condition, participants were instructed to walk at their normal pace with no corrective feedback. During the standardized pace condition, participants were given feedback to either walk slightly faster or slower depending on their self-selected pace. Participants’ pace was determined by the time required to walk a known (3 m) distance. This approach has been validated for use among adolescents with fibromyalgia.18 All testing was completed in a research laboratory in which the participants walked on the floor along a 5-meter path.
Trunk and limb kinematics were recorded via 41 strategically placed retro-reflective markers at 100 Hz using a 10-camera, real-time, high-speed, 3-D motion analysis system (The Motion Monitor, Innovative Sports Training Inc., Chicago, IL, USA) and stored offline for subsequent analysis. Kinematic data were low-pass filtered at 6 Hz using a second-order Butterworth filter. Custom software (The Motion Monitor) was used to determine the start and end of the gait cycle for both the left and right lower extremities. The gait cycle was defined as beginning with the heel strike of 1 leg and ending with the subsequent heel strike of the same leg.29,30 These data were averaged across trials and time normalized to 101 data points to allow for comparison across 100% of the gait cycle. Custom written MATLAB (Mathworks, Natick, MA, USA) software was used to calculate position and angle for the lower extremity joints of interest. Analysis focused on sagittal and frontal planes for the hip, knee, ankle, and foot due to the feasibility of most clinical settings to quantify these motions. For each trial under both self-selected and standard pace conditions, peak joint angles during the first 60% of the gait cycle and stride length were extracted.19,31
Because gait has been reported to be antalgic in nonmusculoskeletal diseases32 and asymmetrical in musculoskeletal conditions both involving33 and not involving the lower extremities,34–36 GaitSD37 was calculated to analyze gait kinematic variability for all participants. Previous research suggests that due to the complexity of gait, analyzing multiple segments would better represent gait than focusing specifically on individual segments.38 GaitSD produces a single value (SD) expressed in degrees, which represents the variability across multiple joints of the lower extremity, with higher values signaling greater variability. In the present study, GaitSD was composed of 12 kinematic variables: bilateral hip flexion, hip abduction, hip rotation, knee flexion, ankle dorsiflexion, and foot eversion. All complete strides were used to compute GaitSD, and the goal was to obtain at least 637 full strides per person. All kinematic gait variables were computed for both self-selected and standard paced trials.
Statistical Analysis
Graphical representations of gait kinematics represent the mean performance (solid/dashed lines) and the standard error (± shaded area) for each joint across the gait cycle.
IBM SPSS version 25 was used for all statistical analyses. Variable distributions were examined for normality using the Kolmogrov-Smirnov test, and all variables were normally distributed. To quantify GaitSD, the overall mean and SD of GaitSD was calculated. To determine if there was an effect of pace, the effect sizes of GaitSD, stride length, and peak joint angles during the gait cycle were estimated. Cohen d was used, and interpretation of the size of the effect was as follows: d = .20 was considered small, d = .50 was considered medium, and d = .80 was considered large.
Pearson product moment correlations were used to examine the relationships of GaitSD with NPRS, FOPQ-fear, FOPQ-avoidance, FDI, and the 6MWT. Conventional magnitudes of r = .10, .30, and .50 were used to evaluate these relationships and refer to effect sizes of small, medium, and large, respectively. A stepwise multiple regression analysis was used to determine which psychological measures or physical characteristics could explain the variance in GaitSD. The dependent variable was GaitSD under both conditions of self-selected and standard pace. The independent variables considered for inclusion were only variables with a medium effect or larger (r ≥ .30).
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Sample Characteristics
Twenty participants with various chronic pain complaints consented to participate in this study (Tab. 1). Of those who consented, 3 withdrew during the baseline period, and one became ineligible due to crutch use at initial testing. The sample included data from 13 females and 3 males aged a mean of 13.6 (± 2.4) years old. On average, participants had pain for 16.0 months, and their primary pain complaint was located in the lower quarter (n = 7), abdomen (n = 4), diffuse/affecting multiple areas (n = 2), spine (n = 2), or upper quarter (n = 1). Participants reported moderate to severe pain severity (moderate: 31.2%; severe: 56%), moderate to high fear of pain (moderate: 13%; high: 47%), high avoidance (moderate: 0%; high: 67%), and moderate to severe disability (moderate: 56%; severe: 44%). Four participants had FOPQ scores lower than 40; however, as indicated, these patients were thoroughly screened by the evaluating clinician to ensure the patient had sufficient levels of fear avoidance and functional disability to benefit from treatment. Specific diagnoses are included in Table 1. Group mean data are included for NPRS, FOPQ, FDI, and the 6MWT (Tab. 1).
Table 1.
Participant Characteristicsa
| Characteristic | Mean (SD) | Range/Percent |
|---|---|---|
| Age (y) | 13.8 (2.2) | (10–17) |
| Sex | 13 female | (81.0%) |
| 3 male | (19.0%) | |
| Height (cm) | 158.3 (11.8) | (135.3–185.4) |
| Weight (kg) | 50.3 (10.6) | (31.8–72.6) |
| Body mass index | 19.6 (2.3) | (8% to 89%) |
| Pain duration (mo) | 16.0 (11.3) | (1b–36) |
| Diagnosis | ||
| Spine | Neck pain (1) | (12.5%) |
| Chronic fatigue and back pain (1) | ||
| Upper quarter | Shoulder CRPS (1) | (6.0%) |
| Lower quarter | Hip pain (1) | (44.0%) |
| Neuropathy (1) | ||
| CRPS (3) | ||
| Pain amplification LE (2) | ||
| Abdomen | Abdominal pain (3) | (25.0%) |
| Abdominal/pelvic or endometriosis (1) | ||
| Diffuse or multiple areas | Fibromyalgia, general weakness, headaches, lupus, POTS (2) | (12.5%) |
| Typical NPRS | 6.2 (2.1) | (2–9) |
| FOPQ Fear | 25.9 (12.1) | (3–44) |
| Avoidance | 22.8 (10.2) | (6–39) |
| FDI | 28.6 (9.4) | (16–47) |
| 6MWT (m) | 437.1 (144.6) | (159.0–631.8) |
aCRPS = chronic regional pain syndrome; FDI = Functional Disability Inventory; FOPQ = Fear of Pain Questionnaire; LE = lower extremity; 6MWT = Six-Minute Walk Test; NPRS = Numeric Pain Rating Scale; POTS = postural orthostatic tachycardia syndrome.
bPatients referred to the tertiary care chronic pain clinic have undergone evaluations that suggest they have a chronic primary pain condition; thus, duration is less relevant. Patients referred to the study were thoroughly screened by the team psychologist and physical therapist to ensure suitability.
Gait Analysis
Graphical representations of kinematic gait analysis for the participants with kinematic data are included in Figure 1. Due to system errors, 1 participant did not have kinematic data for the standard pace condition, and 1 participant did not have any kinematic data collected. Across the whole sample, only peak knee flexion angle (Fig. 1; Tab. 2) was found to differ between conditions (self: 24.9 degrees ± 7.7, standard: 31.8 degrees ± 6.5, d = 0.96 [95% CI = 0.16–1.70]). For all other assessed joints, minimal kinematic differences existed between self-selected or standardized pace conditions across the gait cycle. Participants demonstrated slightly less variable gait patterns (GaitSDself = 2.80 degrees ± .73; GaitSDstandard = 3.19 ± .55) and shorter stride length (self = 1.08 m ± .27; standard = 1.21 m ± .24) during the self-selected condition than during the standard pace condition with the effect of pace moderate in size, but due to variance in this sample the effects were not statistically significant (GaitSD: d = 0.60 [95% CI = −0.16 to 1.33]; Stride: d = 0.51 [95% CI −0.25 to 1.23]) (Tab. 2).
Figure 1.
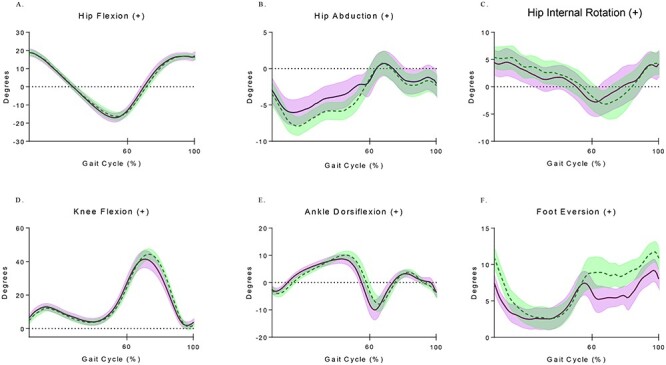
Time series plots of lower extremity joint angle kinematics across the entire sample for both the self-selected (dashed) and standard (solid) pace conditions. The mean range of motion (solid/dashed lines) and the standard error (± shaded area) for each joint are shown across the gait cycle.
Table 2.
Descriptive Statistics (Means [SD]) of Kinetics and Peak Kinematics Values During the First 60% of Stance During Self-Selected and Standard Pace Conditionsa
|
Self-Selected Pace Mean (SD) |
Standard Pace Mean (SD) |
d (CI 95% ) | |
|---|---|---|---|
| n = 15 | n = 14 | ||
| GaitSD (°) | 2.80 (0.73) | 3.19 (0.55) | 0.60 (−0.16 to 1.33) |
| Stride length (m) | 1.08 (0.27) | 1.21 (0.24) | 0.51 (−0.25 to 1.23) |
| Hip flexion (°) | 19.11 (5.92) | 21.31 (4.57) | 0.41 (−0.33 to 1.14) |
| Hip extension (°) | 18.14 (7.75) | 19.00 (7.54) | 0.11 (−0.62 to 0.84) |
| Hip abduction (°) | −0.64 (4.25) | 1.08 (3.67) | 0.43 (−0.32 to 1.16) |
| Hip rotation (°) | 9.18 (7.82) | 9.18 (7.85) | 0.00 (−0.73 to 0.73) |
| Knee flexion (°) | 24.93 (7.66) | 31.75 (6.49) | 0.96 (0.16–1.70) |
| Ankle dorsiflexion (°) | 11.44 (4.29) | 11.41 (3.62) | −0.01 (−0.74 to 0.72) |
| Foot eversion (°) | 14.00 (5.20) | 13.73 (6.85) | −0.04 (−0.77 to 0.69) |
aCohen d ≥ 0.50 are bold. GaitSD = gait variability
bEffect size considered significant (CI95%).
As a subanalysis, there was specific interest in what the predicted GaitSD would be, based on age, using the regression model developed by Sangeux et al.37 Therefore, the mean age of participants in this study was inserted into the regression equation (GaitSD = 9.65 × age−0.623) and estimated GaitSD to be 1.88 degrees for healthy 13.8-year-old children.
Correlation and Regression Analysis
On determining that kinematic data were unavailable for 2 participants due to system errors, correlation analysis with GaitSD proceeded a priori with 15 participants in Gaitself and 14 participants in Gaitstandard. Correlational analyses revealed GaitSDself was strongly associated with both FOPQ-Fear (r = .60) and FOPQ-Avoidance (r = .66), but was not associated with NPRS, FDI, or the 6MWT (Tab. 3). Interestingly, GaitSDstandard was associated only with FOPQ-Avoidance (r = .69) and not with FOPQ-Fear, NPRS, FDI, or the 6MWT (Tab. 3). Subanalysis of the effect of standardized pace revealed that participants with high fear had relatively similar GaitSD to those with lower fear (high: 3.28 degrees; low: 3.11 degrees), and participants with higher avoidance had substantially greater GaitSD (high: 3.52 degrees; low: 2.95 degrees) than those with lower avoidance. The FDI was strongly associated with NPRS (r = .63) and FOPQ-Avoidance (r = .49) (Tab. 3). The 6MWT was not significantly related to any of the other variables. Stepwise multiple regression was used to determine if any of the psychological variables could predict GaitSD in both walking conditions. FOPQ-Avoidance predicted 43% of the variance in GaitSDself (F1,13 = 9.91, P = .008) and 47% of the variance in GaitSDstandard (F1,12 = 10.71, P = .007). No other variable tested entered the prediction model.
Table 3.
Pearson Product Moment Correlations (n = 16)a
| GaitSD standard b | NPRS | FOPQ-Fear | FOPQ-Avoid | FDI | 6MWT c | |
|---|---|---|---|---|---|---|
| GaitSDselfd | .70e | .14 | .60f | .66e | .19 | −.26 |
| GaitSDstandardb | .22 | .36 | .69e | .22 | −.13 | |
| NPRS | .32 | .34 | .63e | .16 | ||
| FOPQ-Fear | .64e | .47 | −.24 | |||
| FOPQ-Avoid | .49f | −.21 | ||||
| FDI | −.07 |
aFDI = Functional Disability Inventory; FOPQ = Fear of Pain Questionnaire; GaitSD = gait variability; 6MWT = Six-Minute Walk Test; NPRS = Numeric Pain Rating Scale.
bGaitSDstandard: n = 14 system dysfunction for 2 participants.
c6MWT: n = 14 missing data for 2 participants.
dGaitSDself: n = 15 system dysfunction for 1 participant.
eLarge effect size r ≥ .50.
fMedium effect size, r ≥ .30.
Discussion
Understanding movement adaptations is essential to fully assessing and addressing the pain experience in those with chronic pain. To gain insight into the psychological drivers of movement in the context of chronic pain, gait kinematics and variability were quantified, and relationships with pain, fear and avoidance beliefs, perceived disability, and function were examined. Participants had high levels of pain, fear, avoidance, and disability and performed relatively poorly on the 6MWT (Tab. 1).
Although the kinematic variables were generally mediated by pace (ie, walking at a faster pace yielded increased range of motion), the sample demonstrated significant variability in gait. Looking specifically at the shape of the kinematic waveforms, the data reflect relatively normal patterns of movement. However, because gait patterns have been shown to mature quite early in life,39 the question of GaitSD as a way to quantify movement adaptations in children with chronic pain was of particular interest. Previous research using GaitSD established a strong negative correlation with age (r = −0.71) and reported that kinematic variability in gait may not change after age 16 years in normal healthy children.37 Further, Gouelle et al40 reported that in normal development, age and normalized base of support had a linear relationship with GaitSD. Although some methodological differences existed, both studies reported age to be most strongly associated with GaitSD.37,40 The subanalysis using the GaitSD regression equation37 predicted GaitSD to be 1.88 degrees for healthy 13.8-year-old children. The participants in this sample had a GaitSD of 2.80 degrees for the self-paced condition and a GaitSD of 3.19 degrees for the standard-paced condition. These values greatly exceed the age-predicted norms and approach-predicted norms for children between 6 and 7.5 years. Though the variability was increased in both conditions, it may be more appropriate to only compare the GaitSDself condition with the age-predicted value because this equation was derived from participants walking at a self-selected pace only.37 An artificially imposed pace, by virtue of being less natural for the participant, may explain why the variability is higher during the GaitSDstandard condition. Conflicting evidence is available regarding the impact of walking speed on GaitSD and may be related to the population studied.41–43 Assuming the participants in the present study were developing normally prior to pain onset, chronic pain seems to have disrupted their motor control strategy during gait. Maintaining a variable movement pattern may be a factor that predisposes children to persistent chronic pain into adulthood.4,6 The ability to identify and quantify these movement adaptations could allow for combined therapeutic approaches to positively affect movement control19 and persistent chronic pain.
Participants were expected to exhibit greater variability in movement due to persistent pain. This hypothesis was based on the idea that children with chronic pain may have disrupted sensory feedback and are constantly searching for a consistent, pain-free way to move. It is known that variation occurs in motor control as a result of pain,1 particularly to complete a task without loss of function.3 For example, in experimental pain conditions, previous authors have reported directional changes in knee extension force to complete a knee extension movement.44 Other authors report that there is substantial inter-subject kinematic variability in experimental pain models.45,46 Clinical studies of patients with CRPS of the upper extremity have also found slower, more variable movements compared with healthy controls47 in addition to finding impairments in the unaffected limb of patients with CRPS.47 In the short term, these movement adaptations are thought to be protective, but when they persist they can become detrimental.6 The kinematic variability seen in the present study is likely the result of persistent movement adaptations and fear avoidance.
The current findings build on existing literature examining the relationships between fear and movement in individuals with chronic pain,1,3,7–11,18 and this work was extended to include avoidance, pain, and function. The findings that GaitSDself was significantly related to both fear of pain and avoidance behaviors (r = 0.60 and r = 0.66, respectively) and that no other variable approached this magnitude suggest the impact these psychological drivers have on movement. These results are consistent with others who found that fear plays a role in motor variability in adults with experimental low back pain,3 upper extremity motor control in a child with CRPS,10 and in lower extremity biomechanics in individuals with juvenile fibromyalgia.18 The results do contradict those of Lamoth et al,8 who reported that fear of pain was not likely to be a major factor in adaptations in gait; however, their study focused on acute experimental low back pain in which fear may not have as great an effect.
Considering function, it was surprising to find the lack of relationship between 6MWT and FDI and that these measures had such weak relationships with GaitSD. The negative relationships found between the 6MWT and both GaitSD and FOPQ were expected. These findings suggest that greater GaitSD and fear and avoidant behaviors were not conducive to function. The weak relationships of 6MWT with all variables may be due to the sample size and relative lack of variability across participants in this measure. The FDI was found to have weak relationships with GaitSD and moderate relationships with fear and avoidance. Similar to above, the small within-group variation in both GaitSD and FDI scores likely explains the minimal relationship. That GaitSD had strong relationships with fear and avoidance may attest to the impact of these psychological factors on both perceived functional disability and movement control and highlight the importance of beginning to understand the role of movement in the fear avoidance model. As for other variables, it was surprising to find that only avoidance was related to GaitSDstandard (r = 0.69). The subanalysis further investigating standardized pace found relatively similar GaitSD between participants with high and low fear, whereas substantially greater GaitSD was found between participants with higher vs lower avoidance. So in addition to physical deconditioning that is often present in individuals with avoidant behaviors,48–50 when individuals avoid activity and movement, their motor control may effectively become deconditioned. The regression models from this study support this finding and suggest that avoiding movement plays a larger than expected role in perpetuating adverse movement adaptations.
The results of this study must be considered within the context of several limitations. The sample size was small with a diverse set of pain conditions. Future studies are needed to confirm the utility of GaitSD within a diverse pain population and the predictive value of regression model derived from this study in a larger sample. GaitSD is also a relatively new approach to gait analysis, and more research is needed around the reliability and validity of the measure. For example, it is not known whether more variability is actually a bad thing. However, clinically, if someone presents with an antalgic gait, clinicians recognize this variability of movement as a compensatory strategy and work to promote “normal” gait to prevent persistent pain or dysfunction. This study focused on pain, fear, avoidance, and perceived disability but did not consider other factors such as motivation or depression. These variables were selected to be consistent with the fear avoidance model; however, it is possible that other factors could have predicted additional variance in GaitSD beyond that which avoidance predicted. Physical contributions such as strength and balance, though not the focus of this study, may have contributed to the regression model in this study and should be considered in future studies. As for other variables of interest, the 6MWT, a common test used in physical therapy, was selected as a way to capture overall physical functioning. Compared with the age reference norm of approximately 660 m,26 participants in the present study walked approximately one-third less distance. Thus, it seemed that that the 6MWT accurately represented function in this sample, though it was expected to correlate with pain, avoidance, disability, or GaitSD. It may be that similar to GaitSDstandard, gait speed may have affected these relationships. Alternatively, it may be that although the 6MWT was used as an overall measure of function, the smaller sample may have affected the estimates of variance, thus making significant associations more difficult to detect.
Little is known about movement adaptations in a pediatric population with chronic pain, or if these adaptations are influenced by one’s psyche. This study found that movement adaptations during walking exist in adolescents with chronic pain who report pain-related fear avoidance. These adaptations in the form of highly variable gait demonstrate a lack of motor control during a task that should be remarkably consistent for adolescents. Additionally, this study found FOPQ-Avoidance can be used to predict GaitSD and provide biomechanical data to support the idea that functional disability stems from fear of movement and avoidant behaviors.5 The results of this study serve as preliminary evidence in understanding movement adaptations associated with adolescents with chronic pain and may lead to more directed interventions.
Author Contributions
Concept/idea/research design: J.A. Beebe, M. Hogan, C. Ploski, L. Simons
Writing: J.A. Beebe, L.E. Simons
Data collection: J.A. Beebe, C. Kronman, F. Mahmud, M. Basch
Data analysis: J.A. Beebe, C. Kronman
Project management: J.A. Beebe, C. Kronman, F. Mahmud, L.E. Simons
Fund procurement: L.E. Simons
Providing participants: C. Kronman, L. Simons
Providing facilities/equipment: J.A. Beebe
Providing institutional liaisons: F. Mahmud, L.E. Simons
Clerical/secretarial support: C. Kronman, F. Mahmud, L.E. Simons
Consultation (including review of manuscript before submitting): J.A. Beebe, C. Kronman, M. Basch, M. Hogan, E. Li, C. Ploski
Funding
This study was funded by grants from the American Pain Society/Sharon S. Keller Chronic Pain Research Grant; Deborah Munroe Noonan Memorial Research Fund; National Institute of Arthritis and Musculoskeletal and Skin Diseases (R21 AR072921) awarded to L.E.S.
Ethics Approval
Institutional Review Board approval was obtained from Boston Children’s Hospital and Simmons University, where this portion of the study took place.
Clinical Trial Registration
This study protocol is registered in the Clinical Trials Registry of the National Institutes of Health (ClinicalTrials.gov identifier NCT01974791).
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1.Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90–S98. [DOI] [PubMed] [Google Scholar]
- 2.Hodges PW, Smeets RJ. Interaction between pain, movement, and physical activity: short-term benefits, long-term consequences, and targets for treatment. Clin J Pain. 2015;31:97–107. [DOI] [PubMed] [Google Scholar]
- 3.Moseley GL, Hodges PW. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci. 2006;120:474–476. [DOI] [PubMed] [Google Scholar]
- 4.Vlaeyen JWS, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain. 2012;153:1144–1147. [DOI] [PubMed] [Google Scholar]
- 5.Simons LE, Kaczynski KJ. The fear avoidance model of chronic pain: examination for pediatric application. J Pain. 2012;13:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butera KA, Fox EJ, George SZ. Toward a transformed understanding: from pain and movement to pain with movement. Phys Ther. 2016;96:1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas JS, France CR. Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain. Spine. 2007;32:E460–E466. [DOI] [PubMed] [Google Scholar]
- 8.Lamoth CJC, Daffertshofer A, Meijer OG, Lorimer Moseley G, Wuisman PIJM, Beek PJ. Effects of experimentally induced pain and fear of pain on trunk coordination and back muscle activity during walking. Clin Biomech (Bristol, Avon). 2004;19:551–563. [DOI] [PubMed] [Google Scholar]
- 9.Trost Z, France CR, Sullivan MJ, Thomas JS. Pain-related fear predicts reduced spinal motion following experimental back injury. Pain. 2012;153:1015–1021. [DOI] [PubMed] [Google Scholar]
- 10.Osumi M, Sumitani M, Otake Y, Morioka S. Fear of movement modulates the feedforward motor control of the affected limb in complex regional pain syndrome (CRPS): a single-case study. Med Hypotheses. 2018;110:114–119. [DOI] [PubMed] [Google Scholar]
- 11.van Meulenbroek T, Huijnen IPJ, Wiertz CMH, Verbunt JA. Pain-related fear and its disabling impact in hypermobile adolescents with chronic musculoskeletal pain. J Orthop Sports Phys Ther. 2017;47:1–24. [DOI] [PubMed] [Google Scholar]
- 12.Huguet A, Miró J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9:226–236. [DOI] [PubMed] [Google Scholar]
- 13.King S, Chambers CT, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. [DOI] [PubMed] [Google Scholar]
- 14.Gobina I, Villberg J, Välimaa R, et al. Prevalence of self-reported chronic pain among adolescents: evidence from 42 countries and regions. Eur J Pain Lond Engl. 2017;47:775–771. [DOI] [PubMed] [Google Scholar]
- 15.Gauntlett-Gilbert J, Eccleston C. Disability in adolescents with chronic pain: patterns and predictors across different domains of functioning. Pain. 2007;131:132–141. [DOI] [PubMed] [Google Scholar]
- 16.Clinch J, Eccleston C. Chronic musculoskeletal pain in children: assessment and management. Rheumatology (Oxford). 2009;48:466–474. [DOI] [PubMed] [Google Scholar]
- 17.Stommen NC, Verbunt JA, Gorter SL, Goossens ME. Physical activity and disability among adolescents and young adults with non-specific musculoskeletal pain. Disabil Rehabil. 2012;34:1438–1443. [DOI] [PubMed] [Google Scholar]
- 18.Sil S, Thomas S, DiCesare C, et al. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis Care Res. 2015;67:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran ST, Thomas S, DiCesare C, et al. A pilot study of biomechanical assessment before and after an integrative training program for adolescents with juvenile fibromyalgia. Pediatr Rheumatol Online J. 2016;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons L, Vlaeyen JWS, Declercq L, et al. Avoid or engage? Outcomes of graded exposure in youth with chronic pain using a sequential replicated single-case randomized design. Pain. 2020;161:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The fear of pain questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12:677–686. [DOI] [PubMed] [Google Scholar]
- 22.Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the functional disability inventory among a multicenter sample of youth with chronic pain. Pain. 2011;152:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the numerical rating scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. [DOI] [PubMed] [Google Scholar]
- 24.Boonstra AM, Stewart RE, Köke AJA, et al. Cut-off points for mild, moderate, and severe pain on the numeric rating scale for pain in patients with chronic musculoskeletal pain: variability and influence of sex and catastrophizing. Front Psychol. 2016;7:1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li AM, Yin J, Yu CCW, et al. The six-minute walk test in healthy children: reliability and validity. Eur Respir J. 2005;25:1057–1060. [DOI] [PubMed] [Google Scholar]
- 26.Li AM, Yin J, Au JT, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. Am J Respir Crit Care Med. 2007;176:174–180. [DOI] [PubMed] [Google Scholar]
- 27.Oberg T, Karsznia A, Oberg K. Basic gait parameters: reference data for normal subjects, 10-79 years of age. J Rehabil Res Dev. 1993;30:210–223. [PubMed] [Google Scholar]
- 28.Kwon JW, Son SM, Lee NK. Changes of kinematic parameters of lower extremities with gait speed: a 3D motion analysis study. J Phys Ther Sci. 2015;27:477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene BR, McGrath D, O’Neill R, O’Donovan KJ, Burns A, Caulfield B. An adaptive gyroscope-based algorithm for temporal gait analysis. Med Biol Eng Comput. 2010;48:1251–1260. [DOI] [PubMed] [Google Scholar]
- 30.Bötzel K, Marti FM, Rodríguez MÁC, Plate A, Vicente AO. Gait recording with inertial sensors—how to determine initial and terminal contact. J Biomech. 2016;49:332–337. [DOI] [PubMed] [Google Scholar]
- 31.Ferber R, McClay Davis I, Williams DS, Laughton C. A comparison of within- and between-day reliability of discrete 3D lower extremity variables in runners. J Orthop Res. 2002;20:1139–1145. [DOI] [PubMed] [Google Scholar]
- 32.Naranje S, Kelly DMD, Sawyer JR. A systematic approach to the evaluation of a limping child. Am Fam Physician. 2015;92:12. [PubMed] [Google Scholar]
- 33.de Kruijf M, Verlinden VJA, Huygen FJPM, et al. Chronic joint pain in the lower body is associated with gait differences independent from radiographic osteoarthritis. Gait Posture. 2015;42:354–359. [DOI] [PubMed] [Google Scholar]
- 34.Barzilay Y, Segal G, Lotan R, et al. Patients with chronic non-specific low back pain who reported reduction in pain and improvement in function also demonstrated an improvement in gait pattern. Eur Spine J. 2016;25:2761–2766. [DOI] [PubMed] [Google Scholar]
- 35.Koch C, Hänsel F. Chronic non-specific low back pain and motor control during gait. Front Psychol. 2018;9:2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirmizi M, Simsek IE, Elvan A, Akcali O, Angin S. Gait speed and gait asymmetry in individuals with chronic idiopathic neck pain. Musculoskelet Sci Pract. 2019;41:23–27. [DOI] [PubMed] [Google Scholar]
- 37.Sangeux M, Passmore E, Graham HK, Tirosh O. The gait standard deviation, a single measure of kinematic variability. Gait Posture. 2016;46:194–200. [DOI] [PubMed] [Google Scholar]
- 38.Ebrahimi S, Kamali F, Razeghi M, Haghpanah SA. Comparison of the trunk-pelvis and lower extremities sagittal plane inter-segmental coordination and variability during walking in persons with and without chronic low back pain. Hum Mov Sci. 2017;52:55–66. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland D, Olshen R. The Development of Mature Walking . London: Mac Keith Press; 1988. [Google Scholar]
- 40.Gouelle A, Leroux J, Bredin J, Mégrot F. Changes in gait variability from first steps to adulthood: normative data for the gait variability index. J Mot Behav. 2016;48:249–255. [DOI] [PubMed] [Google Scholar]
- 41.Dingwell JB, Salinas MM, Cusumano JP. Increased gait variability may not imply impaired stride-to-stride control of walking in healthy older adults. Gait Posture. 2017;55:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moissenet F, Leboeuf F, Armand S. Lower limb sagittal gait kinematics can be predicted based on walking speed, gender. Sci Rep. 2019;9:9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brændvik SM, Goihl T, Braaten RS, Vereijken B. The effect of increased gait speed on asymmetry and variability in children with cerebral palsy. Front Neurol. 2020;10:1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tucker KJ, Hodges PW. Changes in motor unit recruitment strategy during pain alters force direction. Eur J Pain Lond Engl. 2010;14:932–938. [DOI] [PubMed] [Google Scholar]
- 45.Murray GM, Peck CC. Orofacial pain and jaw muscle activity: a new model. J Orofac Pain. 2007;21:263–278 discussion 279–288. [PubMed] [Google Scholar]
- 46.Sae-Lee D, Whittle T, Peck CC, Forte ARC, Klineberg IJ, Murray GM. Experimental jaw-muscle pain has a differential effect on different jaw movement tasks. J Orofac Pain. 2008;22:15–29. [PubMed] [Google Scholar]
- 47.Schilder JCM, Schouten AC, Perez RSGM, et al. Motor control in complex regional pain syndrome: a kinematic analysis. Pain. 2012;153:805–812. [DOI] [PubMed] [Google Scholar]
- 48.Turk DC, Robinson JP, Burwinkle T. Prevalence of fear of pain and activity in patients with fibromyalgia syndrome. J Pain. 2004;5:483–490. [DOI] [PubMed] [Google Scholar]
- 49.Nijs J, Roussel N, van Oosterwijck J, et al. Fear of movement and avoidance behaviour toward physical activity in chronic-fatigue syndrome and fibromyalgia: state of the art and implications for clinical practice. Clin Rheumatol. 2013;32:1121–1129. [DOI] [PubMed] [Google Scholar]
- 50.Gatchel RJ, Neblett R, Kishino N, Ray CT. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther. 2016;46:38–43. [DOI] [PubMed] [Google Scholar]


