Abstract
Background
Levothyroxine (L-T4) is used as a standard-of-care treatment in patients with hypothyroidism. L-T4 is absorbed throughout the small intestine, and consistent drug absorption is required for successful treatment. Patients with Hashimoto’s disease (autoimmune thyroiditis) often have food and medication sensitivities, as well as comorbid gastrointestinal (GI) disorders detrimental to L-T4 absorption.
Case Presentation
This case report describes a 51-year-old female patient with long-standing Hashimoto’s disease and multiple sensitivities to food chemicals and medications. The patient suffered from GI symptoms and poor thyroid-stimulating hormone (TSH) control. She was switched to compounded thyroxine/triiodothyronine medication due to her multiple allergies, with only mild improvement in thyroid function and no symptom resolution. She was subsequently diagnosed with gastroparesis and small intestinal bacterial overgrowth (SIBO). Because of further worsening of her GI symptoms and weight loss, she was switched to levothyroxine sodium oral solution (Tirosint®-SOL), which contains only three ingredients—levothyroxine, water, and glycerol—which was well tolerated and led to normalization of TSH levels.
Conclusion
Malabsorption of L-T4 is often seen in patients with Hashimoto’s disease-related hypothyroidism and comorbid GI conditions, such as gastroparesis and SIBO. L-T4 tablets and a compounded oral suspension were inefficiently absorbed, leading to suboptimal TSH control. Switching to levothyroxine sodium oral solution resulted in sustained TSH control with subsequent resolution of symptoms. No side effects or reactions to the medication were observed, despite the patient’s multiple allergies and sensitivities to food chemicals and medications.
Keywords: levothyroxine malabsorption, small intestinal bacterial overgrowth, hypothyroidism, Hashimoto’s disease, levothyroxine sodium oral solution, L-T4 oral solution, gastroparesis
Introduction
Thyroid hormones modulate protein synthesis, energy metabolism, and the sensitivity of tissues to other hormones. Thyroid hormones produced in the thyroid gland consist of 80–90% thyroxine (T4) and 10–20% triiodothyronine (T3) under physiological conditions. Levothyroxine (L-T4), a synthetic hormone structurally identical to T4, is used as a standard-of-care treatment for conditions related to hypothyroidism.1
L-T4 tablets contain L-T4 sodium salt compressed into a tablet with various excipients, such as lactose monohydrate, cornstarch, carboxymethyl starch, gelatin, croscarmellose sodium, magnesium stearate, citric acid, microcrystalline cellulose, talc, calcium phosphate dibasic dihydrate, and others.2 Tablet and capsule forms of L-T4 are absorbed throughout the small intestine, primarily at the jejunum and upper ileum,3 and successful treatment relies on drug delivery to the small intestine with consistent full daily absorption. Gastroparesis, a delayed gastric emptying, not only delays delivery of L-T4 to the upper ileum4 but has been associated in some studies with small intestinal bacterial overgrowth (SIBO) syndrome5 and L-T4 malabsorption.3 Gastrointestinal malabsorption of oral L-T4 is a frequent phenomenon accounting for many cases of refractory hypothyroidism.6
Additionally, patients with Hashimoto’s disease (autoimmune thyroiditis) often have sensitivities to foods, dyes, and other additives7,8 possibly due to altered immune function.
This case report demonstrates the utility of levothyroxine sodium oral solution (Tirosint®-SOL) in a patient with altered gastric motility, small intestinal absorption, and multiple sensitivities to food chemicals, dyes, and additives.
Case Presentation
A 51-year-old female with long-standing Hashimoto’s disease, chronic urticaria, mast cell activation disorder, hypertension, and diabetes mellitus type 2 (well controlled, with glycosylated hemoglobin A1C ranging from 5.1% to 6.0%), treated with metformin 1000 mg orally, twice daily, and liraglutide (Victoza®) 1.8 mg daily, presented to the clinic complaining of recurrent hives, skin dryness, fatigue, weight gain, intermittent constipation and diarrhea, cold intolerance, brain fog, difficulty with word finding, swelling in her lower extremities, and daytime somnolence. The patient was also suffering from multiple chemical sensitivities, including 76 food allergies, medication sensitivities, and reactions to food dyes and additives. She was very careful with her food and medication choices due to multiple symptoms. Written informed consent for publication of this case report was obtained from the patient. No institutional approval was required to publish this case report.
Physical examination demonstrated an otherwise healthy woman with a temperature of 36.6°C, pulse 92 beats per minute, blood pressure 136/88 mmHg, respiratory rate 18 respirations per minute, O2 saturation 98%, height 1.57 m, weight 92 kg, body mass index 37.3 kg/m2. She was in no acute distress, with no palpable thyroid nodules on physical examination and no visible urticaria. She had hypoactive bowel sounds on physical examination and minimal tenderness to palpation in the left lower quadrant. She had a family history of papillary thyroid cancer in a sister. Multiple bilateral nodules had previously been biopsied and found to be benign. She had previously tried and failed generic L-T4 and transitioned to L-T4 sodium tablets (Synthroid®) at 125 µg daily. Because of the variability in her gastrointestinal (GI) function, she had discontinued metformin for several weeks without resolution of the GI symptoms.
Table 1 presents her thyroid-stimulating hormone (TSH), free T3, and free T4 values at presentation. She was aiming for TSH optimization and resolution of symptoms. For six months, she was put on increasing doses of L-T4 ranging from 112 µg to 137 µg daily. Because of multiple drug sensitivities and intolerances to additives, she was transitioned to a compounded T4/T3 prescription without additives (sustained-release L-T4/T3 at doses ranging from 125 to 200 µg L-T4 and 7.5–20 µg T3). However, her dose was escalated three times over the course of three months with only mild improvement in thyroid function. At 24 weeks, she experienced worsening GI symptoms, including projectile vomiting with undigested food and pill capsules, weight loss, and 10–20 bowel movements daily. Metformin and liraglutide were discontinued.
Table 1.
Thyroid Timeline Before Administration of L-T4 Oral Solution
| Treatment/Action Taken/Presentation | Initial Presentation | 6 Weeks | 12 Weeks | 18 Weeks | 24 Weeks |
|---|---|---|---|---|---|
| L-T4 Tablet (Synthroid®) 125 µg | T4/T3 Compounded Medication | T4/T3 Compound at Escalated Dosing | T4/T3 Compound at Escalated Dosing | Started with Worsening GI Issues, Gastroparesis, and Vomiting | |
| TSH, mIU/L (NR 0.45–4.50 mIU/L) | 5.30 | 4.65 | 6.50 | 2.60 | 7.42 |
| Free T4, pmol/L (NR 10.55–22.78 pmol/L) | 16.60 | 16.60 | 18.53 | 24.07 | 19.82 |
| Free T3 pmol/L (NR 3.39–6.16 pmol/L) | Not evaluated | 4.47 | 5.24 | 4.93 | 5.24 |
Abbreviations: GI, gastrointestinal; L-T4, levothyroxine; NR, normal range; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Upper endoscopy revealed gastritis and a hiatal hernia. Gastric emptying examination demonstrated 20% gastric retention at 4 hours; she was diagnosed with gastroparesis. She was placed on high-dose probiotics and dicyclomine hydrochloride (Bentyl®) and initiated on a gastroparesis diet.
A timeline of treatments administered is shown in Table 1.
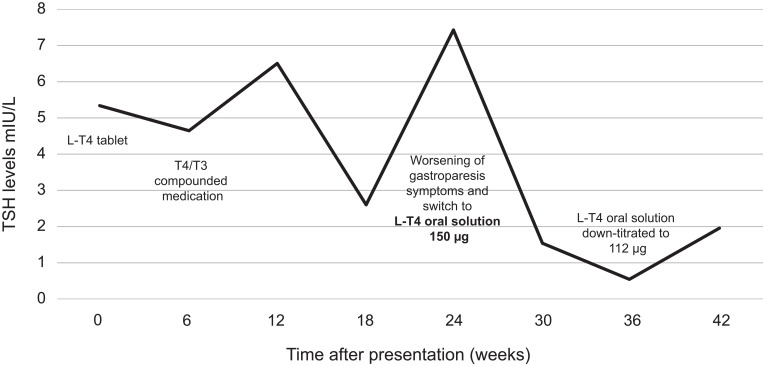
Because of continued bloating and abdominal pain, she was treated with one cycle of rifaximin (Xifaxan®) after she was also diagnosed with SIBO, confirmed by a GI examination including clinical history, physical examination, and the gold standard SIBO breath test. At 24 weeks, due to further worsening symptoms and thyroid levels, she was switched to L-T4 oral solution at 150 µg (Figure 1).
Figure 1.
Thyroid-stimulating hormone timeline.
Abbreviations: L-T4, levothyroxine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
The medication was well tolerated, led to a resolution of the thyroid symptoms, and was successfully down-titrated to 112 µg daily dose based on her body weight. She did not experience any side effects or reaction to the medication despite her multiple chemical sensitivities. TSH control was successfully sustained as shown at the latest monitoring six weeks following down-titration of L-T4 oral solution (Table 2).
Table 2.
Thyroid Timeline After Administration of L-T4 Oral Solution
| Treatment/Action Taken | 24 Weeks | 30 Weeks | 36 Weeks | 42 Weeks | ||
|---|---|---|---|---|---|---|
| L-T4 Oral Solution 150 µg | L-T4 Oral Solution 150 µg | L-T4 Oral Solution 112 µg | ||||
| TSH, mIU/L (NR 0.45–4.50 mIU/L) | Switched to L-T4 oral solution 150 µg | 1.55 | 1 treatment cycle of rifaximin for SIBO | 0.56 | L-T4 oral solution down-titrated to 112 µg | 1.95 |
| Free T4, pmol/L (NR 10.55–22.78 pmol/L) | 27.93 | 29.99 | 20.33 | |||
| Free T3, pmol/L (NR 3.39–6.16 pmol/L) | 4.93 | 5.39 | 4.77 | |||
Abbreviations: L-T4, levothyroxine; NR, normal range; SIBO, small intestinal bacterial overgrowth; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Discussion
Gastroparesis is characterized by nausea, vomiting, bloating, postprandial fullness, and abdominal pain in the presence of impaired gastric emptying.9 SIBO may present with symptoms such as bloating, distention, and abdominal discomfort in the presence of abnormal or increased levels of bacteria in the proximal GI tract.9 A relationship between SIBO and gastroparesis has been observed,5,10 as well as an association between SIBO and hypothyroidism,11,12 typically caused by Hashimoto’s disease.13 It has been reported that significantly more patients with overt hypothyroidism presented with SIBO, compared with healthy subjects without thyroid disease or SIBO (p < 0.001).11 As altered GI motility is a risk factor for SIBO and is common in patients with hypothyroidism, patients with chronic GI symptoms and hypothyroidism should be assessed for the presence of SIBO.12
SIBO is characterized by abnormally high levels of bacteria in the small intestine,9 which may lead to malabsorption and a subsequent suboptimal response of a narrow therapeutic index medication, such as L-T4,11 absorbed in the small intestine.3 Malabsorption of L-T4 has been recognized as an important medical problem in patients with hypothyroidism.3 An expert consensus report noted that GI disorders, including gastroparesis, can limit L-T4 absorption,3 which can also be impacted by the abnormally high bacterial levels seen in SIBO syndrome.11
Treatment with L-T4 tablets can result in poorly controlled TSH levels due to several factors, including gastric disorders, malabsorption, and/or concomitant drugs affecting gastric pH and absorption.1 Dissolution is considered a contributing factor to the bioequivalence differences seen with L-T4 tablet formulations as they contain various excipients, including dye additives, that require gastric dissolution, which can hinder L-T4 absorption, bioavailability, and subsequent therapeutic efficacy.14,15 Because the L-T4 oral solution is free of excipients, it does not need a gastric phase of dissolution required by solid tablets, leading to a more consistent absorption.14
A study assessing factors affecting L-T4 efficacy and tolerability in 925 patients with hypothyroidism treated with L-T4 monotherapy identified several factors affecting the absorption and efficacy of L-T4 tablet formulations,16 including GI comorbidities, allergies to tablet excipients, prescription or over-the-counter medications, and dietary supplements.16 Approximately half of the patients had one or more comorbid conditions, which could affect L-T4 absorption, and for those with GI conditions and disorders significantly more patients reported difficulty controlling their hypothyroid symptoms vs patients without comorbid GI conditions (p < 0.01).16 The frequency of L-T4 dose changes, the need for unexpectedly high doses, and the failure of L-T4 to control the symptoms of hypothyroidism associated with tablets are known to correlate with the presence of GI comorbidities.17 In an L-T4 malabsorption case of a patient with gastroparesis, changing from L-T4 sodium tablets to a gelatin capsule formulation seemed to correct thyroid function assays.18 In addition, an L-T4 oral solution was shown to be more effective than the tablet formulations in controlling TSH levels in patients with hypothyroidism suffering from gastric disorders, malabsorption, or drug interference, and also in patients without malabsorptive disorders.1
A systematic review and meta-analysis of the use of liquid solution L-T4 retrieved data on 141 patients and showed that patients with suboptimal TSH on tablet L-T4 significantly improved TSH by switching to a liquid L-T4 formulation at an unchanged dose. Patients switched to the liquid formulation mainly due to malabsorption related to various factors, including concomitant use of multiple drugs, calcium and/or iron supplements, and bariatric surgery.19 Despite the limited number of studies, the availability of novel preparations of L-T4, a drug widely diffused worldwide, widens the treatment options for patients with different needs and comorbidities, offering the possibility for enhanced performance in those with all causes of gastric acid secretion impairment as per pharmacokinetic and in vitro evidence.20
L-T4 oral solution contains only L-T4, glycerol, and water,21 is free of problematic excipients, and does not require a gastric phase of dissolution prior to absorption. It is thus more readily absorbed than tablets,22 being less sensitive to the factors known to reduce the absorption of tablet L-T4.1
Conclusion
This case demonstrated the utility of L-T4 oral solution as a highly absorbable and effective treatment option for Hashimoto’s disease patients with hypothyroidism and gastroparesis and/or SIBO, where standard L-T4 tablets and a compounded oral suspension were inefficiently absorbed leading to suboptimal TSH control.
Following the switch to L-T4 oral solution, TSH control was optimized, with subsequent resolution of symptoms, and the patient did not experience any side effects or reaction to the medication despite her multiple chemical allergies and sensitivities.
Levothyroxine sodium oral solution is an appropriate treatment for patients with hypothyroidism and GI conditions, including SIBO, and may be the gold standard for patients with GI conditions and food, chemical, and medication sensitivity.
Acknowledgments
The author wishes to acknowledge Diane Kwiatkoski and Chrysi Petraki of IQVIA, Parsippany, New Jersey, USA, for assistance in the preparation of this manuscript.
Funding Statement
Funding for preparation of this manuscript was provided by IBSA Pharma Inc., Parsippany, New Jersey, USA.
Abbreviations
GI, gastrointestinal; L-T4, levothyroxine; SIBO, small intestinal bacterial overgrowth.
Data Sharing Statement
The data that support this case report are available from the author upon reasonable request.
Ethical Approval
Written informed consent for publication of this case report was obtained from the patient.
No institutional approval was required to publish this case report.
Consent for Publication
The patient has provided informed consent for publication of the case. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Disclosure
The author declares that she has no conflicts of interest.
References
- 1.Fallahi P, Ferrari SM, Ruffilli I, et al. Advancements in the treatment of hypothyroidism with L-T4 liquid formulation or soft gel capsule: an update. Expert Opin Drug Deliv. 2017;14(5):647–655. doi: 10.1080/17425247.2016.1227782 [DOI] [PubMed] [Google Scholar]
- 2.Nagy EV, Perros P, Papini E, Katko M, Hegedus L. New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid. 2021;31(2):193–201. doi: 10.1089/thy.2020.0515 [DOI] [PubMed] [Google Scholar]
- 3.Centanni M, Benvenga S, Sachmechi I. Diagnosis and management of treatment-refractory hypothyroidism: an expert consensus report. J Endocrinol Invest. 2017;40(12):1289–1301. doi: 10.1007/s40618-017-0706-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fass R, McCallum RW, Parkman HP. Treatment challenges in the management of gastroparesis-related GERD. Gastroenterol Hepatol (N Y). 2009;5(10):4–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Brechmann T, Sperlbaum A, Schmiegel W. Levothyroxine therapy and impaired clearance are the strongest contributors to small intestinal bacterial overgrowth: results of a retrospective cohort study. World J Gastroenterol. 2017;23(5):842–852. doi: 10.3748/wjg.v23.i5.842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal malabsorption of thyroxine. Endocr Rev. 2019;40(1):118–136. [DOI] [PubMed] [Google Scholar]
- 7.Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto’s thyroiditis. Cureus. 2019;11(4):e4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burek CL, Talor MV. Environmental triggers of autoimmune thyroiditis. J Autoimmun. 2009;33(3–4):183–189. doi: 10.1016/j.jaut.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke JO. SIBO in gastroparesis: sci-fi or science fact? Dig Dis Sci. 2014;59(3):510–512. doi: 10.1007/s10620-013-3011-4 [DOI] [PubMed] [Google Scholar]
- 10.George NS, Sankineni A, Parkman HP. Small intestinal bacterial overgrowth in gastroparesis. Dig Dis Sci. 2014;59(3):645–652. doi: 10.1007/s10620-012-2426-7 [DOI] [PubMed] [Google Scholar]
- 11.Lauritano EC, Bilotta AL, Gabrielli M, et al. Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab. 2007;92(11):4180–4184. doi: 10.1210/jc.2007-0606 [DOI] [PubMed] [Google Scholar]
- 12.Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18(3):307–309. doi: 10.4103/2230-8210.131155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017;390(10101):1550–1562. doi: 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72(1):105–110. doi: 10.1016/j.ejpb.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 15.Synthroid® (levothyroxine sodium) tablets, for oral use. US prescribing information. USA: AbbVie Inc; 2018. [Google Scholar]
- 16.McMillan M, Rotenberg KS, Vora K, et al. Comorbidities, concomitant medications, and diet as factors affecting levothyroxine therapy: results of the CONTROL surveillance project. Drugs R D. 2016;16(1):53–68. doi: 10.1007/s40268-015-0116-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruchala M, Szczepanek-Parulska E, Zybek A. The influence of lactose intolerance and other gastro-intestinal tract disorders on L-thyroxine absorption. Endokrynol Pol. 2012;63(4):318–323. [PubMed] [Google Scholar]
- 18.Reardon DP, Yoo PS. Levothyroxine tablet malabsorption associated with gastroparesis corrected with gelatin capsule formulation. Case Rep Endocrinol. 2016;2016:1316724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virili C, Giovanella L, Fallahi P, et al. Levothyroxine therapy: changes of TSH levels by switching patients from tablet to liquid formulation. A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2018;9:10. doi: 10.3389/fendo.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virili C, Trimboli P, Centanni M. Novel thyroxine formulations: a further step toward precision medicine. Endocrine. 2019;66(1):87–94. doi: 10.1007/s12020-019-02049-x [DOI] [PubMed] [Google Scholar]
- 21.Tirosint®-SOL (levothyroxine sodium) oral solution. US prescribing information. Switzerland: Institut Biochimique SA (IBSA); 2000. [Google Scholar]
- 22.Yue CS, Scarsi C, Ducharme MP. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vs. other available dosage forms. Arzneimittelforschung. 2012;62(12):631–636. doi: 10.1055/s-0032-1329951 [DOI] [PubMed] [Google Scholar]