Abstract
Objectives
To examine left ventricular assist devices (LVAD) patient outcomes across a range of center surgical volumes.
Background
To qualify for reimbursement, Centers for Medicare and Medicaid Services (CMS) standards require centers to implant ≥10 LVADs or total artificial hearts over a 3-year period. The impact of center LVAD surgical volumes on patient outcomes has not been thoroughly scrutinized.
Methods
Center volume was provided for 7,416 patients undergoing LVAD implant who were enrolled into INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support). Center LVAD volume was categorized as very low (≤10 implants/year, n=617 patients), low (11–30 implants/year, n=2561), medium (31–50 implants/year, n=2458), and high volume (>50 implants/year, n=1750). The main outcome of interest was patient survival based on center volume, derived from Kaplan-Meier and multivariable Cox regression.
Results
Overall survival was associated with center volume (p=0.003): 71±1.8% (very low volume), 81±0.8% (low volume), 83±0.8% (medium volume), and 79±1.0% (high volume) at 1 year. Compared with medium volume centers, the 90-day mortality was higher in very low volume (odds ratio (OR) 1.35 (p=0.04)) and high volume (OR 1.28 (p=0.018)) VAD centers. The adjusted hazard ratio (HR, [95% CI]) for mortality was 1.32 [1.11–1.56], 1.07 [0.95–1.21], and 1.17 [1.03–1.30] for very low, low, and high volume centers, respectively. Center volume did not predict mortality (p=0.25) in INTERMACS profile 1-2 patients (n=3688).
Conclusion
Center volume correlates with post-VAD survival, with worse survival noted at very low volume centers. These findings would suggest that current U.S. VAD center standards warrant reconsideration.
Keywords: LVAD, mortality, risks, volume
Introduction
Various studies have demonstrated associations between mortality and surgical volumes following various cardiac operations1–6. In an analysis of first generation LVADs implanted for destination therapy, Lietz et al found a correlation with mortality and lower center surgical volume, but center volume was not an independent predictor of death when other risks were considered7. Using the U.S. National Inpatient Sample, Shah et al demonstrated that patients undergoing mechanical circulatory support implant at low volume centers (<22 LVADs/year) had inferior inpatient survival compared with patients undergoing LVAD surgery at higher volume centers.6 In contrast, a study of 88 academic medical centers in the U.S. found no association between hospital LVAD surgical volume and inpatient mortality, but operative survival was greatest when the LVAD operation was performed by the highest volume surgeons.5 During the derivation of the HeartMate II Risk Score, it was noted that center volume was an independent correlate of operative mortality in multivariable analysis8. Aside from age, center volume (hazard ratio 1.6) was also the only predictor of longer term survival after HeartMate II implant.8 Granular analyses restricted to approved continuous flow devices are lacking and the influence of center volume on long term outcomes is not clear.
As more centers are opening for mechanical circulatory support (MCS) implant, the impact (or lack thereof) of center volume on patient short- and long-term outcomes warrants study. Using data from patients entered into the Interagency Registry of Assisted Circulatory Support (INTERMACS), we compared patient outcomes based on centers’ VAD volume.
Methods
The full INTERMACS cohort consisted of 14,014 patients who underwent primary continuous flow (CF) LVAD or biventricular assist device (BiVAD) implant between 2009–2015. Patients receiving total artificial heart support or isolated right ventricular support were excluded.9 Center volume data were provided by INTERMACS administrators for 7,416 patients undergoing LVAD or BiVAD implant between the years 2012–2014. Center volume was defined as the number of durable LVAD implants performed at the center in the same calendar year as the patient’s LVAD implant. Patients undergoing right ventricular assist device (RVAD) support simultaneous with the LVAD operation were counted as a single event. To maintain center anonymity during analysis, center MCS volumes were subdivided by INTERMACS administrators prior to data release into the following thresholds of yearly implants: ≤10 (very low volume), 11–20, 21–30, 31–50 (medium volume), and >50 (high volume). After further data analysis, survival and adjusted survival were deemed equivalent between centers implanting 11–20 VADs and 21–30 VAD per year; these patient groups were consolidated into one group (11–30 VADs per year) termed “low volume”.
Preoperative clinical characteristics, demographics and frequencies of preoperative vasoactive medication use, hemodialysis, ventilator support, and application of extracorporeal membrane oxygenation (ECMO) and/or intra-aortic balloon pump support (IABP) were compared between the entire INTERMACS cohort (n=14,014) and the center volume sample (n=7,416). Then, these same variables were compared between thresholds of center volumes (very low, low, medium, and high volume).
To examine survival in patients who were critically ill at the time of LVAD implant, we did a sub-analysis of center volume and outcomes exclusive to patients categorized as INTERMACS Profiles 1-2. Prior studies have shown much heterogeneity in assigning Profile 1 vs 2 in critically ill patients, so the consolidated grouping herein was felt appropriate.10
Outcomes of Interest
The main clinical outcome of interest was the difference in adjusted survival by center LVAD volume. Early mortality (defined as death within 90 days of MCS implant) was a secondary outcome. The 90-day time frame for early mortality was chosen because prior INTERMACS analyses have shown this to be the postoperative time frame with the highest hazard for adverse outcome following LVAD, after which the hazard declines11.
Statistical Analysis
SAS version 9.3 (Cary, North Carolina) and SPSS version 24 (Chicago, IL) statistical software were used for analyses. Categorical variables were tallied as frequencies and were compared with Fisher’s exact or Pearson’s X2 tests for >2×2 comparisons. Continuous variables were assessed for normality using histograms and are reported as mean ± standard error or median [25th, 75th], as appropriate, unless otherwise specified. Possible differences between groups were assessed by Student’s t or Mann-Whitney testing, as appropriate.
Odds ratios for 90-day mortality were generated with logistic regression. Kaplan-Meier survival estimates were calculated at each center volume threshold, censoring patients at the time of transplant or explant for recovery. For all survival analyses, differences between center volume groups were compared with log rank testing and then pairwise comparisons between center volume groups were made. To account for any bias in Kaplan-Meier estimates due to censoring for transplant, a separate analysis was performed only on those patients who did not undergo transplant.
Mortality hazard ratios based on center volume for the whole cohort were calculated with Cox regression modelling. Mortality comparisons were adjusted for known clinical risks. Simultaneous Cox modelling included the following covariates: advanced patient age (age >69 years), sex, prior cardiac surgery, bridge to transplant listed status, INTERMACS Profile 1-2 status, preoperative creatinine and albumin, preoperative ventilator support (within 48 hours), and concomitant surgery and/or implant of RVAD support at time of durable LVAD8,11–13. Device type (axial vs centrifugal flow) was also forced into the model. Due to a large amount of missing data (>20%), social measures (substance use, education level), vasopressor use, and the temporary circulatory support modifier were not included in data analysis. Hazard ratios (HR) [95% confidence interval] are provided. For all analyses, including the multivariable regression candidate variable exit criteria, a p ≤0.05 was considered significant.
This study, including the manuscript, was approved by the Data Access, Analysis, and Publication Committee of INTERMACS. Patient consent for INTERMACS data collection is obtained at enrolling centers per local Review Board requirements.
Results
Table 1 shows the preoperative demographics, characteristics, and laboratory values for the entire INTERMACS cohort and those in the 2012–2014 INTERMACS sample. The 2012–2014 sample was similar to the full INTERMACS CF-LVAD cohort. Survival at 1 year was 80±0.4% in the full INTERMACS CF-LVAD cohort and 81±0.5% in the 2012–2014 sample.
Table 1.
Characteristics in the total INTERMACS Cf-LVAD/BiVAD sample and in the 2012–2014 cohort.
| INTERMACS Total CF-LVAD Sample (n=14014) | 2012–2014 INTERMACS Cohort (n=7416) | |
|---|---|---|
|
| ||
| Age Group, years | ||
| <50 | 3536 (26%) | 1834 (25%) |
| 50–59 | 3863 (28%) | 1967 (27%) |
| 60–69 | 4595 (33%) | 2488 (34%) |
| ≥70 years | 1920 (14%) | 1127 (15%) |
|
| ||
| Male, n(%) | 11011 (79%) | 5834 (79%) |
|
| ||
| Ischemic myopathy, n(%) | 6447 (46%) | 3466 (47%) |
|
| ||
| Congenital Heart, n(%) | 70 (0.5%) | 36 (0.5%) |
|
| ||
| Prior cardiac surgery, n(%) | 4755 (34%) | 2472 (33%) |
|
| ||
| BTT listed, n(%) | 3881 (28%) | 1799 (25%) |
|
| ||
| INTERMACS Profile | ||
| 1 | 2064 (15%) | 1055 (14%) |
| 2 | 5190 (37%) | 2633 (36%) |
| 3 | 4217 (30%) | 2331 (32%) |
| 4–7 | 2483 (18%) | 1356 (18%) |
|
| ||
| Preoperative ECMO | 355 (2.5%) | 188 (2.5%) |
| IABP | 3487 (25%) | 1626 (22%) |
|
| ||
| Preoperative vasopressor † | 982 (7.3%) | 513 (7.2%) |
|
| ||
| Ventilator support 48 hours preop., n(%) | 840 (6.0%) | 382 (5.2%) |
|
| ||
| Renal replacement, n(%)‡ | 317 (2.3%) | 147 (2.0%) |
|
| ||
| Creatinine, mg/dL | 1.41 ± 0.06 | 1.39 ± 0.01 |
|
| ||
| INR | 1.2 [1.1, 1.4] | 1.2 [1.1, 1.4] |
|
| ||
| Albumin, g/dL | 3.40 ± 0.01 | 3.41 ± 0.01 |
|
| ||
| LVAD only, n(%) | 13563 (96.8%) | 2554 (95.8%) |
| BiVAD (simultaneous) | 451 (3.2%) | 34 (4.3%) |
|
| ||
| Continuous flow, n(%) | ||
| Cf-Af | 12051 (86%) | 6004 (81%) |
| Cf-Cf | 1963 (14%) | 1412 (19%) |
|
| ||
| Bypass Time, min | 96.2 ± 0.4 | 95.3 ± 0.6 |
|
| ||
| Concomitant surgery, n(%) | 5620 (40%) | 3054 (41%) |
|
| ||
| Total Months Support | 11.7 [4.9, 24.8] | 13.9 [7.2, 23.9] |
|
| ||
| 90 day Death, n(%) | 1339 (10%) | 705 (10%) |
|
| ||
| Survival, 1 year | 80 ± 0.4% | 81 ± 0.5% |
Mean ± standard error of mean, median [25th, 75th] or n(%) shown.
Defined as epinephrine or norepinephrine preop.
defined as dialysis or ultrafiltration administered within 48 hours prior to operation.
Abbreviations: BTT= bridge to transplant. Cf-Af= continuous flow, axial flow, Cf-Cf= continuous flow, centrifugal flow. ECMO= extracorporeal membrane oxygenation. IABP= intraaortic balloon pump. LVAD= left ventricular assist device. BiVAD= biventricular assist device.
In the 2012–2014 INTERMACS sample (n=7416), 8.3% (n=617) of implants were from very low volume centers, 34.5% (n=2561) from low volume centers, 33.5% (n=2488) from medium volume centers, and 23.6% (n=1750) were from high volume centers. Table 2 shows the baseline characteristics of patients by center volume category. Very low volume centers implanted more advanced age (≥70 years) patients and a destination therapy indication was more common. Very low volume centers were less likely to implant a balloon pump preoperatively, had longer bypass times intraoperatively, and were less likely to insert right ventricular assist (RVAD) support intraoperatively. High volume centers implanted more patients with an INTERMACS 1 Profile preoperatively but the frequency of ECMO and preoperative ventilator use was not greater. Patients at high volume centers were less likely to be of advanced age and had higher baseline serum creatinine and albumin levels.
Table 2.
Characteristics of patients by center volume of CF-LVAD implants in 2012–2014.
| Center Volume | |||||
|---|---|---|---|---|---|
| ≤10 (n=617) | 11–30 (n=2561) | 31–50 (n=2488) | >50 (n=1750) | p value | |
|
| |||||
| Age Group, years | <0.001 | ||||
| <50 | 113 (18%) | 622 (24%) | 647 (26%) | 452 (26%) | |
| 50–59 | 131 (21%) | 672 (26%) | 682 (27%) | 482 (28%) | |
| 60–69 | 207 (34%) | 822 (32%) | 851 (34%) | 608 (35%) | |
| ≥70 years | 166 (27%) | 445 (17%) | 308 (12%) | 208 (12%) | |
|
| |||||
| Male, n(%) | 487 (79%) | 2039 (80%) | 1951 (78%) | 1357 (78%) | 0.57 |
|
| |||||
| Ischemic myopathy, n(%) | 321 (53%) | 1202 (47%) | 1122 (46%) | 821 (47%) | 0.021 |
|
| |||||
| Congenital Heart, n(%) | 3 (0.6%) | 13 (0.5%) | 9 (0.4%) | 11 (0.6%) | 0.32 |
|
| |||||
| Prior cardiac surgery, n(%) | 216 (35%) | 824 (32%) | 860 (35%) | 572 (33%) | 0.23 |
|
| |||||
| BTT listed, n(%) | 95 (15%) | 617 (24%) | 604 (24%) | 483 (28%) | <0.001 |
|
| |||||
| INTERMACS Profile | <0.001 | ||||
| 1 | 80 (13%) | 311 (12%) | 356 (15%) | 308 (18%) | |
| 2 | 204 (33%) | 979 (38%) | 841 (34%) | 609 (35%) | |
| 3 | 205 (33%) | 802 (31%) | 801 (33%) | 523 (30%) | |
| 4–7 | 125 (20%) | 463 (18%) | 460 (19%) | 308 (18%) | |
|
| |||||
| Support 48 hours preop: ECMO, n(%) | 14 (2.3%) | 53 (2.1%) | 70 (2.8%) | 51 (2.9%) | 0.24 |
| IABP, n(%) | 97 (16%) | 560 (22%) | 582 (23%) | 387 (22%) | 0.001 |
| Ventilator, n(%) | 36 (5.8%) | 111 (4.3%) | 143 (5.7%) | 92 (5.3%) | 0.12 |
| Vasopressor†, n(%) | 32 (5.3%) | 142 (5.8%) | 210 (8.8%) | 129 (7.8%) | <0.001 |
| Dialysis, n(%)‡ | 16 (2.6%) | 34 (1.3%) | 50 (2.0%) | 47 (2.7%) | 0.010 |
|
| |||||
| Creatinine, mg/dL | 1.36±0.02 | 1.39±0.01 | 1.36±0.02 | 1.44±0.02 | 0.006 |
|
| |||||
| INR | 1.30±0.02 | 1.31±0.01 | 1.31±0.01 | 1.32±0.01 | 0.65 |
|
| |||||
| Albumin, g/dL | 3.35±0.03 | 3.42±0.01 | 3.40±0.01 | 3.46±0.02 | 0.003 |
|
| |||||
| LVAD only, n(%) | 609 (98.7%) | 2494 (97.4%) | 2408 (96.8%) | 1686 (96.3%) | 0.014 |
| BiVAD (simultaneous) | 8 (1.3%) | 67 (2.6%) | 80 (3.2%) | 64 (3.7%) | |
|
| |||||
| Continuous flow, n(%) | |||||
| Cf-Af | 582 (94%) | 2153 (84%) | 1935 (78%) | 1334 (76%) | <0.001 |
| Cf-Cf | 35 (5.7%) | 408 (16%) | 553 (22%) | 416 (24%) | |
|
| |||||
| Bypass Time, min | 107±2.1 | 97±1.0 | 96±1.0 | 87±1.1 | <0.001 |
|
| |||||
| Concomitant surgery, n(%) | 256 (42%) | 968 (38%) | 1017 (41%) | 799 (46%) | <0.001 |
|
| |||||
| Total months support | 15 [7.1,25] | 13 [6.9, 23] | 15 [8.0,25.0] | 13 [6.7, 23] | 0.002 |
Mean ± standard error of mean, median [25th, 75th] or n(%) shown.
Defined as epinephrine or norepinephrine preop.
defined as dialysis or ultrafiltration administered within 48 hours prior to operation.
Abbreviations: BTT= bridge to transplant. Cf-Af= continuous flow, axial flow, Cf-Cf= continuous flow, centrifugal flow. ECMO= extracorporeal membrane oxygenation. IABP= intraaortic balloon pump. LVAD= left ventricular assist device. BiVAD= biventricular assist device.
Survival Based on 2012–2014 Center Volumes
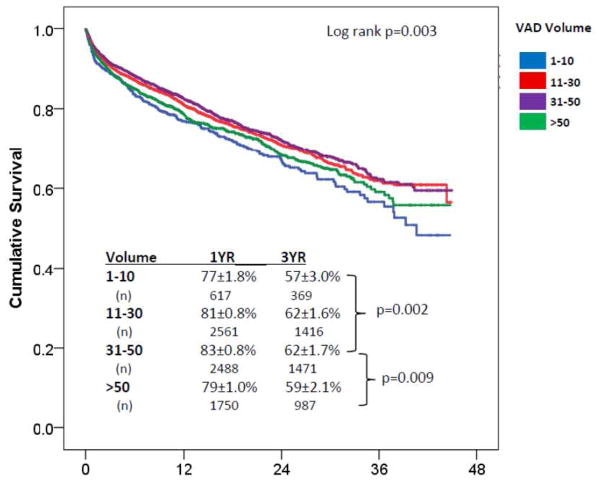
The median duration of support for the 2012–2014 sample was 415 [211, 718] days (mean 486 days). Using medium volume (n=31–50 VADs/year) centers as a reference, early mortality (within 90 days, n=708 total) occurred in 11.5% (OR 1.35 [1.01–1.80], p=0.04), 10% (OR 1.11 [0.92–1.3], p=0.29), 9.1% (reference), and 11.7% (OR 1.28 [1.04, 1.58], p=0.018) of very low, low, medium and high volume centers (p=0.029). Unadjusted overall survival (figure 1) was also associated with center volume (p=0.003) and 1 year survivals are as follows: 77±1.8% (very low volume), 81±0.8% (low volume), 83±0.8% (medium volume), and 79±1.0% (high volume). On pairwise comparison of center volumes (figure 1), overall survival was worse in the high vs. medium volume centers (p=0.009, HR 1.17[1.04–1.32]). There was no difference in survival between high volume (>50 VADs a year) and very low volume centers (p=0.25). Very low volume centers (<10 VADs/year) had worse survival than medium volume centers (HR 1.29 [1.1–1.52], p=0.002). To account for the competing outcome of transplant, survival was examined in the 5601 patients who did not undergo cardiac transplant during the period of study. Significant survival differences persisted (p=0.036) with 1 year survivals as follows: 74±1.9% (very low volume), 75±1.0% (low volume), 78±1.0% (medium volume), and 73±1.0% (high volume).
Figure 1. Survival in INTERMACS patients (years 2012–2014) by Center VAD Volume.
Significant differences were noted on pairwise comparison between medium volume (31–50 VADs/yr) and high volume (>50 VADs/yr) and medium volume and very low volume (<10 VADs/yr) centers.
After adjusting for known correlates of risk (see methods),8,11–13 center volume remained predictive of adverse outcome (table 3, p=0.005). Compared with medium volume centers, the adjusted hazard ratio for mortality was 32% (adjusted HR=1.32 [1.11–1.56], p=0.001) higher for patients implanted at very low volume centers and 17% (adjusted HR=1.17 [1.03–1.30], p=0.016) higher for patients implanted at high volume centers. There was no difference in survival between medium volume centers and low volume centers. Upon restricting the analysis to patients who did not undergo transplant, center volume remained a significant predictor of outcome (p=0.024). Compared with medium volume centers, very low volume centers had an adjusted patient mortality of 1.27 [1.07–1.50] (p=0.006) and high volume centers had an adjusted mortality of 1.16 [1.02–1.31] (p=0.025).
Table 3.
Predictors of mortality on multivariable analysis.
| Hazard Ratio Mortality [95% CI] | p value | |
|---|---|---|
|
| ||
| Center Volume | 0.005 | |
| ≤10 | 1.32 [1.11–1.56] | 0.001* |
| 11–30 | 1.07 [0.95–1.21] | 0.25* |
| 31–50 | -- | Reference |
| >50 | 1.17 [1.03–1.30] | 0.016* |
|
| ||
| Age >69 years | 1.64 [1.46–1.84] | <0.001 |
|
| ||
| BTT (listed) | 0.69 [0.59–0.80] | <0.001 |
|
| ||
| Male sex | 0.84 [0.75–0.94] | 0.003 |
|
| ||
| Previous cardiac surgery | 1.42 [1.29–1.57] | <0.001 |
|
| ||
| Preop. Ventilator support | 1.08 [0.88–1.32] | 0.48 |
|
| ||
| Patient Profile 1 or 2 | 1.21 [1.10–1.34] | <0.001 |
|
| ||
| Creatinine, mg/dL | 1.18 [1.13–1.23] | <0.001 |
|
| ||
| Albumin, g/L | 0.88 [0.82–0.95] | 0.001 |
|
| ||
| CF-AF | 0.82 [0.71–0.96] | 0.012 |
|
| ||
| Concomitant surgery | 1.25 [1.13,1.38] | <0.001 |
|
| ||
| BiVAD | 2.22 [1.78–2.78] | <0.001 |
p value is compared with medium volume. All variables were added in simultaneously into Cox modelling.
Survival Restricted to Patient Profiles 1-2
There were 3688 patients categorized as either INTERMACS profile 1 (cardiogenic shock) or 2 (progressive hemodynamic decline despite inotropes) preoperatively. Baseline characteristics and demographics of these patients grouped by center volume are available online (Table 1S). High volume centers implanted Profile 1-2 patients who had a sicker phenotype, inclusive of a greater frequency of preoperative cardiac arrests, higher serum creatinine, and greater needs for preoperative renal replacement therapy and IABP support. Higher volume centers had shorter cardiopulmonary bypass times but employed more BiVAD support then other groups. Very low volume centers had the lowest percentage of patients on BiVAD support and the longest cardiopulmonary bypass times.
There were 459 early (within 90 days) deaths overall in Profile 1-2 patients. No significant differences in 90-day mortality were observed among very low (15%, n=42), low (11%, n=144), medium (12%, n=140), or high volume (15%, n=133) centers (p=0.057). A significant difference existed in overall survival of Profile 1-2 patients based on center volume (p=0.037, figure 2). On pairwise comparison between groups, there was no difference in survival between very low volume centers (HR=1.2 [0.95–1.54]) and medium volume centers. However, patients implanted at higher volume centers had higher unadjusted mortality than patients implanted at medium (HR 1.2 [1.1–1.5]) volume centers. After accounting for known correlates of risk, center volume was not predictive of mortality in INTERMACS Profile 1-2 patients (p=0.25). The adjusted hazard for mortality in high volume (HR 1.17 [0.99–1.38], p=0.06) and very low volume centers (HR 1.17 [0.92–1.50, p=0.21) was not significantly higher than that of medium volume centers.
Figure 2. Survival in Profile 1–2 Patients Based on Center Volume.
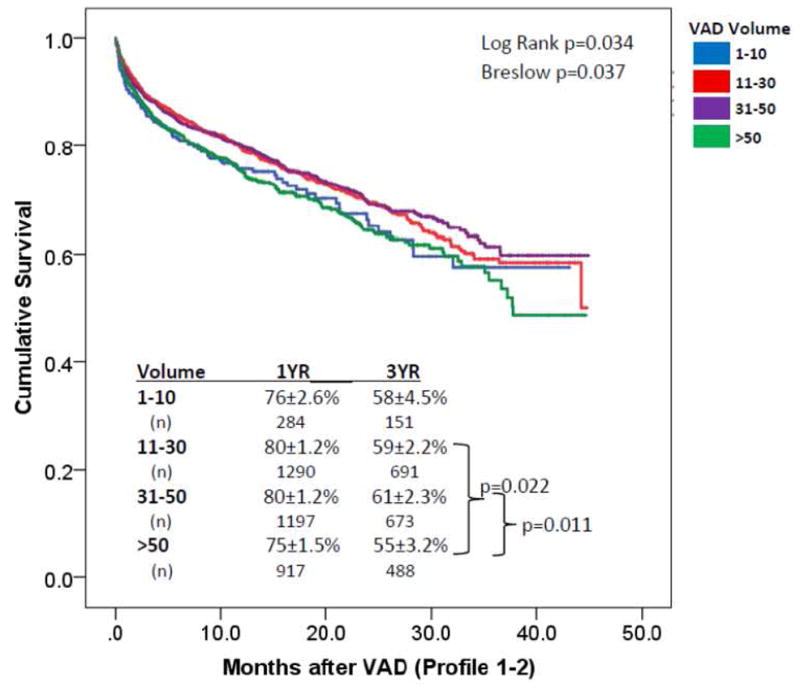
Survival was examined in Profile 1–2 patients. The Breslow p value favors the early period of the curve (operative interval). Significant differences in survival were noted in pairwise comparisons between high volume (>50 VADs/yr) centers and both medium (31–50 VADs/yr) and low volume (11–30 VADs/yr) centers. No differences were noted between very low volume (<10 VADs/yr) and medium volume centers.
Causes of Death by Center Volume
Table 4 shows the causes of death in the INTERMACS sample according to center volume (Pearson p >0.05). Very low volume centers had higher frequencies of death from infection and from pulmonary and noncardiac causes. High volume centers had more multisystem organ failure and few reported deaths due to right ventricular failure.
Table 4. Causes of Death by Center Volume.
Overall p value >0.05
| Center Volume | ||||
|---|---|---|---|---|
| ≤10 (n=195) | 11–30 (n=630) | 31–50 (n=603) | >50 (n=469) | |
|
| ||||
| Device Malfunction | 4 (2.1%) | 20 (3.2%) | 18 (3.0%) | 11 (2.3%) |
|
| ||||
| Hemolysis | 2 (1.0%) | 3 (0.5%) | 4 (0.7%) | 2 (0.4%) |
|
| ||||
| Neurological dysfunction | 31 (16%) | 97 (15%) | 128 (21%) | 97 (21%) |
|
| ||||
| Major Bleed | 4 (2.1%) | 19 (3.0%) | 18 (3.0%) | 8 (1.7%) |
|
| ||||
| Infection | 15 (7.7%) | 37 (5.9%) | 30 (5.0%) | 21 (4.5%) |
|
| ||||
| RV failure | 8 (4.1%) | 25 (4.0%) | 20 (3.3%) | 8 (1.7%) |
|
| ||||
| MSOF | 27 (14%) | 110 (18%) | 119 (20%) | 101 (22%) |
|
| ||||
| Myocardial infarction | 0 (0%) | 4 (0.6%) | 3 (0.5%) | 2 (0.4%) |
|
| ||||
| Endstage heart failure | 12 (6.2%) | 31 (4.9%) | 36 (6.0%) | 42 (9.0%) |
|
| ||||
| Cardiovascular: other | 9 (4.6%) | 26 (4.1%) | 19 (3.2%) | 19 (4.1%) |
|
| ||||
| Arrhythmia | 3 (1.5%) | 15 (2.4%) | 13 (2.2%) | 14 (0.7%) |
|
| ||||
| Sudden Cardiac Death | 5 (2.6%) | 24 (3.8%) | 23 (3.8%) | 21 (4.5%) |
|
| ||||
| Non-CNS embolism | 2 (1.0%) | 5 (0.8%) | 1 (0.2%) | 1 (0.2%) |
|
| ||||
| Pulmonary failure | 19 (9.7%) | 42 (6.7%) | 38 (6.3%) | 29 (6.2%) |
|
| ||||
| Renal failure | 1 (0.5%) | 2 (0.3%) | 2 (0.3%) | 4 (0.9%) |
|
| ||||
| Hepatic failure | 0 (0%) | 3 (0.5%) | 3 (0.5%) | 2 (0.4%) |
|
| ||||
| GI dysfunction | 0 (0%) | 8 (1.3%) | 1 (0.2%) | 4 (0.9%) |
|
| ||||
| Cancer | 2 (1.0%) | 11 (1.7%) | 4 (0.7%) | 5 (1.1%) |
|
| ||||
| Psychiatric | 0 (0%) | 1 (0.2%) | 2 (0.3%) | 2 (0.4%) |
|
| ||||
| NonCardiovascular: Other | 19 (9.7%) | 46 (7.3%) | 29 (4.8%) | 27 (5.8%) |
|
| ||||
| Trauma/accident | 3 (1.5%) | 5 (0.8%) | 3 (0.2%) | 2 (0.4%) |
|
| ||||
| Withdrawal Care | 29 (15%) | 96 (15%) | 89 (15%) | 47 (10%) |
Discussion
While several studies have identified preoperative risk factors for mortality after LVAD implant, the impact of center surgical experience on overall patient survival has not been thoroughly examined. In this analysis of 7,416 patients enrolled into INTERMACS, we found a bimodal risk of adverse outcomes associated with center volume: very low and high volume centers have lower average survivals than centers that perform 30–50 VADs a year. The increased mortality association persisted even after adjusting for known correlates of LVAD candidate operative risk.
Surgical volume has been associated with patient outcomes in several studies, including that of patients undergoing general cardiac procedures. In a study by Birkmeyer et al, patients undergoing cardiac bypass surgery at high volume centers had an operative mortality of 4.8% compared with 6.1% at very low volume centers1. In a separate analysis by Gonzalez et al, patients undergoing aortic valve replacement at very low volume centers were 12% more likely to have a major complication than those at high volume hospitals, and patients were 57% more likely to die if a complication occurred.4 Similar to these prior studies, yet from a different patient population, we found an increase in mortality in patients undergoing LVAD implant at centers performing <10 implants per year. Aside from advanced age, patients implanted at very low volume centers did not present with a greater frequency of high risk features. After controlling for known correlates of LVAD mortality, 8,11 adjusted mortality remained 32% higher at very low volume LVAD centers compared with medium volume centers.
This study was not designed to determine why outcomes are worse at very low volume VAD centers but the data from this analysis and others foster hypotheses. Ninety-day mortality in very low volume INTERMACS centers was 35% higher than medium volume centers. The data herein support findings from Shat et al, who showed that U.S. centers performing <23 LVADs/year had 50% higher inpatient mortality than higher volume centers.6 Very low volume centers in INTERMACS increased the overall period of operative risk with the addition of concomitant procedures (42%) and with extended times on cardiopulmonary bypass. Others have shown that concomitant procedures increase LVAD operative mortality11,14–16. Aside for addressing mechanical aortic valves, data demonstrating consistent benefit from mitral and tricuspid valve interventions and concomitant coronary bypass are lacking.14,15,17,18 At low volume centers, omitting additional procedures, thereby reducing procedural complexity, may be one means of improving operative outcomes.
Unmeasured factors, such as surgical experience, perioperative management, the quality and frequency of outpatient follow-up, the identification and management of LVAD related complications, and variations in patient management protocols (e.g. anticoagulation and blood pressure) could also be hypothesized to play a role in the increased incidence of death at very low volume LVAD centers. Certainly, more studies are needed to better understand the causes of increased mortality at low volume centers. Until then, it begs the question if stricter center volume minimums are necessary for ensuring good patient outcomes after LVAD. In the U.S., the Centers for Medical and Medicaid Services (CMS) center volume standards are currently set at 10 VADs or total artificial hearts (TAHs) over a 3 year period19. This INTERMACS data and data from Shah et al6 would suggest that CMS VAD volume minimums are set too low.
An unexpected finding from this analysis was the higher risk-adjusted mortality among patients implanted at high volume centers compared to medium volume centers. In fact, survival in the high volume cohort was no better than in the very low volume cohort. The reasons for this are not entirely clear. One could hypothesize that referral bias plays a role in mortality differences, with larger volume institutions having a sicker mix of patients, some of whom may have already been declined for surgery at lower volume centers. This hypothesis is supported by the greater proportion of patients categorized as INTERMACS Profile 1 or 2, on BiVAD support, and/or with preoperative renal dysfunction in the high center volume group, with a high percentage of deaths from multisystem organ failure and/or progressive heart failure. Within the INTERMACS Profile 1-2 sample, patients at high volume centers were older, had more preoperative cardiac arrests and worse renal function than other groups. These factors could account for the increased operative mortality [HR 1.28] observed in high volume INTERMACS centers. After adjusting for known LVAD mortality risk correlates, overall mortality remained 17% higher in high volume centers compared with centers implanting 31–50 VADs a year. While trends were noted, high volume centers did not demonstrate a significantly higher operative and/or adjusted overall mortality in the sickest of VAD patients- the INTERMACS Profile 1-2 group.
While many risk models and risk factors for LVAD mortality have been devised, predicting mortality after LVAD implant remains imperfect.7,8,11–13 Thus, it remains possible that, despite multivariable adjustments, high patient urgency due to referral bias may be the basis for the inferior outcomes at high volume centers. However, since the survival curves continue to separate after the perioperative period, it is also important to consider other causes for the increased mortality seen at higher volume centers. Perhaps volumes of patients on LVAD support are so large at these centers that differences exist in the quality of outpatient management and/or the management of device complications. Alternatively, patient selection may play a role, and mortality at high volume centers could be the result of the progression of other medical comorbidities during long-term support. These questions cannot be answered from the study herein but warrant investigation. While simultaneously acknowledging the presence of unmeasured factors possibly leading to a higher risk LVAD candidate phenotype at higher volume centers, one could still argue that measures to improve outcomes at any VAD center with higher than risk-predicted mortality are warranted.
Limitations
There are several limitations that must be acknowledged. We were only provided center volume for the 2012–2014 years. While the 2012–2014 cohort was similar to the entire INTERMACS sample, this represents a limited INTERMACS analysis. Further, we were unable to investigate if fluctuations in center LVAD volume impacted patient outcomes, or if outcomes varied based on the presence or absence of transplant capabilities or an academic affiliation. Surgeon volume has been shown to be inversely related to operative mortality for cardiac (including LVAD) and vascular procedures3,5. Many LVAD centers have more than one LVAD surgeon and the impact of individual surgeon experience, rather than overall center volume, was not analyzed. This is particularly true for larger volume centers, which may have more surgeons (inclusive of trainees) who have individually less surgical experience. It is possible that an individual very low- or high-volume center had disproportionally high mortality, skewing group results. In the spirit of INTERMACS and collaborative research, this data is not provided for analysis. Finally, multiple comparisons were performed in several analyses herein, increasing the possibility of false positive covariate correlations with VAD outcomes.
In conclusion, patient undergoing LVAD implant at very low (<10 implants per year) and high volume U.S. centers (>50 implants per year) having inferior outcome to patients having surgery at centers performing 31–50 VADs a year. While the higher mortality observed at higher volume centers may be due to referral bias, these findings support the need for regular performance improvement evaluations, comparing individual center outcomes to risk-adjusted national averages. Coincidently, strategies enacted to improve average LVAD patient survival in those centers that underperform are warranted. Finally, INTERMACS data would suggest that current U.S. VAD center standards (10 VAD/TAHs over 3 years) imposed by CMS are too lenient and warrant reconsideration19.
Supplementary Material
Clinical Perspective.
Center surgical experience has been shown to impact patient outcome for many cardiac and noncardiac surgeries. Using a large national database of LVAD patients, we show that patients undergoing LVAD implant at very low volume centers (≤10 LVADs a year) have inferior outcome to those implanted at centers performing 30–50 LVADs a year. In addition, patients implanted at high volume centers (>50 VADs a year) have similar operative mortality but worse long term survival then patients implanted at lower volume centers. These results highlight the need for development of national performance evaluations for LVAD centers and reconsideration of current U.S. LVAD center implant minimums.
Translational outlook.
These data identify another facet impacting variability in patient outcomes after LVAD implant with important implications for U.S. health care expenditures. The findings may provide data for devising healthcare performance reimbursement goals and for guiding targeted improvements in individual LVAD center outcomes.
Acknowledgments
Funding: Data collection for this work was funding in whole or in part with the Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268201100025C.
Footnotes
Disclosures: Dr Cowger received travel support (<$5,000) from St. Jude/Abbott. Drs. Cowger, Salerno, Shah, Dardas, Stulak, Maltais, Pagani, Aaronson, and Mokadam receive institutional research funds from HeartWare and St. Jude. Dr. Shah also receives research support from Haemonetic. Dr. Dardas has an ISHLT research award and Dr. Mokadam receives support from Syncardia. Dr. Mokadam is a consultant for HeartWare and St. Jude.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 2.Hannan EL, Kilburn H, Jr, Bernard H, O’Donnell JF, Lukacik G, Shields EP. Coronary artery bypass surgery: the relationship between inhospital mortality rate and surgical volume after controlling for clinical risk factors. Med Care. 1991;29(11):1094–1107. [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez AA, Dimick JB, Birkmeyer JD, Ghaferi AA. Understanding the volume-outcome effect in cardiovascular surgery: the role of failure to rescue. JAMA Surg. 2014;149(2):119–123. doi: 10.1001/jamasurg.2013.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis KF, Hohmann SF, Doukky R, Levine D, Johnson T. The Impact of Hospital and Surgeon Volume on In-Hospital Mortality of Ventricular Assist Device Recipients. Journal of Cardiac Failure. 2016;22(3):226–231. doi: 10.1016/j.cardfail.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Shah N, Chothani A, Agarwal V, et al. Impact of Annual Hospital Volume on Outcomes after Left Ventricular Assist Device (LVAD) Implantation in the Contemporary Era. Journal of Cardiac Failure. 2016;22(3):232–237. doi: 10.1016/j.cardfail.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Lietz K, Long JW, Kfoury AG, et al. Impact of center volume on outcomes of left ventricular assist device implantation as destination therapy: analysis of the Thoratec HeartMate Registry, 1998 to 2005. Circulation. Heart failure. 2009;2(1):3–10. doi: 10.1161/CIRCHEARTFAILURE.108.796128. [DOI] [PubMed] [Google Scholar]
- 8.Cowger JA, Castle L, Aaronson KD, et al. The HeartMate II Risk Score: An Adjusted Score for Evaluation of All Continuous-Flow Left Ventricular Assist Devices. ASAIO J. 2016;62(3):281–285. doi: 10.1097/MAT.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed October 1, 2016];Interagency Registry for Mechanically Assisted Circulatory Support: About Us. http://www.uab.edu/medicine/intermacs/about-us.
- 10.Cowger J, Shah P, Stulak J, et al. INTERMACS profiles and modifiers: Heterogeneity of patient classification and the impact of modifiers on predicting patient outcome. J Heart Lung Transplant. 2016;35(4):440–448. doi: 10.1016/j.healun.2015.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34(12):1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Ravichandran AK, Cowger J. Left ventricular assist device patient selection: do risk scores help? J Thorac Dis. 2015;7(12):2080–2087. doi: 10.3978/j.issn.2072-1439.2015.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle AJ, Ascheim DD, Russo MJ, et al. Clinical outcomes for continuous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J Heart Lung Transplant. 2011;30(4):402–407. doi: 10.1016/j.healun.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Robertson JO, Naftel DC, Myers SL, et al. Concomitant aortic valve procedures in patients undergoing implantation of continuous-flow left ventricular assist devices: An INTERMACS database analysis. J Heart Lung Transplant. 2015;34(6):797–805. doi: 10.1016/j.healun.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John R, Naka Y, Park SJ, et al. Impact of concurrent surgical valve procedures in patients receiving continuous-flow devices. J Thorac Cardiovasc Surg. 2014;147(2):581–589. doi: 10.1016/j.jtcvs.2013.10.024. discussion 589. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJBY, Naftel DC, Kirklin JK, Moazami N, Myers SL, Strueber M, Dickinson MG, Starling RC. Impact of Concomitant Cardiovascular Surgeries at the Time of CF-LVAD Implantation: An INTERMACS Analysis. J Heart and Lung Transplant. 2015;34(4S):S163–164. [Google Scholar]
- 17.Robertson JO, Grau-Sepulveda MV, Okada S, et al. Concomitant tricuspid valve surgery during implantation of continuous-flow left ventricular assist devices: a Society of Thoracic Surgeons database analysis. J Heart Lung Transplant. 2014;33(6):609–617. doi: 10.1016/j.healun.2014.01.861. [DOI] [PubMed] [Google Scholar]
- 18.Stulak JM, Tchantchaleishvili V, Haglund NA, et al. Uncorrected pre-operative mitral valve regurgitation is not associated with adverse outcomes after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant. 2015;34(5):718–723. doi: 10.1016/j.healun.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Services CfMM. [Accessed October 13, 2016];Decision Memo for Ventricular Assist Devices as Destination Therapy. https://www.cms.gov/medicare-coverage-database/details/nca-decision6memo.aspx?NCAId=187&ver=16&NcaName=Ventricular+Assist+Devices+as+Destination+Therapy+(1st+Recon)&bc=BEAAAAAAEAAA&&fromdb=true.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.