Abstract
Introduction:
Anger is an important clinical feature of posttraumatic stress disorder (PTSD) that can hamper recovery. We recently reported that intermittent theta burst stimulation (iTBS) demonstrated preliminary efficacy to reduce symptoms of posttraumatic stress disorder and major depression; here we performed a secondary analysis testing whether iTBS reduced symptoms of anger over the course of iTBS treatment and compared to sham stimulation.
Materials and Methods:
Fifty veterans with chronic PTSD received 10 daily sessions of sham-controlled, double-blind iTBS (1800 pulses/session, once per weekday) targeting the right dorsolateral prefrontal cortex (intent-to-treat=25 per group). Participants who completed the double-blind phase were offered another 10 sessions of unblinded iTBS. Participants completed the Dimensions of Anger Reactions scale at pre-iTBS baseline, treatment midpoints and endpoints of the blinded and unblinded phases, and at one-month after the last stimulation session. Correlations between anger, PTSD, depression and sleep were also explored.
Results:
After the first week, during the double-blind phase, participants randomized to active stimulation reported significantly reduced anger compared to sham stimulation (p=.04). Participants initially randomized to sham appeared to catch-up to the point they no longer differed from those initially randomized to active iTBS when they received iTBS during the unblinded phase (p=.14). Anger reduction was maintained at one-month after iTBS in participants initially randomized to active stimulation (i.e., total of four weeks of iTBS).
Conclusions:
This secondary analysis suggests that iTBS might reduce anger in veterans with PTSD. Future studies focused on more granular level anger outcomes and effects of number of stimulation sessions are needed.
Keywords: posttraumatic stress disorder, transcranial magnetic stimulation, anger
1. Introduction
Posttraumatic stress disorder (PTSD) is a common and frequently chronic psychiatric disorder that can occur after exposure to a traumatic event [1]. PTSD is associated with significant psychiatric comorbidity including depression [2]. Besides commonly recognized PTSD symptoms such as re-experiencing, hypervigilance, and avoidance, many patients also suffer from debilitating anger [3, 4]. Despite increasing recognition of the prevalence of anger in individuals with trauma-, anxiety-, and mood-related disorders generally [5], the high incidence of anger in PTSD is especially well-documented, and may be particularly prominent in combat-related PTSD [6]. Moreover, recent research indicates that anger could be considered a separate PTSD-related symptom domain [4, 6, 7]. This differs from previous diagnostic conceptions of anger as a symptom of hyperarousal.
Although anger might temporarily be beneficial (e.g., in avoiding trauma-based fear [8]), more enduring expressions of anger result in a host of negative consequences on interpersonal relationships and occupational functioning [9]. PTSD-related anger is associated both with a more severe PTSD course [10] and poorer overall treatment outcomes [8]. Accordingly, addressing anger is recommended as part of PTSD treatment [3, 11]. Yet, little is known about effective treatments in this population, and treatments targeting anger specifically are early in development. The few controlled studies targeting anger specifically have small sample sizes [12, 13] or no comparison group [14-16].
Recent advances in the areas of neuroscience, psychiatry, and technology have set the stage to explore non-invasive neuromodulation treatments for PTSD [17]. Currently the most explored technique is repetitive transcranial magnetic stimulation (rTMS). rTMS is an established treatment for pharmacoresistant major depression [18, 19], with increasing evidence of efficacy in PTSD [20, 21]. However, the beneficial effects of rTMS appear to be mixed and may depend upon stimulation parameters and other factors. While a recent trial using standard rTMS settings observed no difference between active and sham stimulation in veterans with depression and PTSD [22], we and others have demonstrated that rTMS using alternative frequency parameters can reduce symptoms in veterans with PTSD and comorbid depression [23-25]. Yet, implementation of rTMS can be hampered by logistical concerns, as rTMS sessions take up to 45 minutes, frequently delivered daily.
We therefore recently evaluated the efficacy of theta burst stimulation (TBS), a novel TMS protocol, for PTSD [26]. TBS uses short bursts of low intensity, high (50 Hz) frequency stimulation delivered in “bursts” at 5 Hz, separated by 200 ms. TBS can be applied in intermittent (iTBS) or continuous fashion associated with putatively long-term potentiation and long-term depression-like activity, respectively [27]. TBS is of great interest since it is much faster to deliver vs standard rTMS (3-10 minutes vs. 45 minutes), and was found noninferior to rTMS in depression [28].
In previous reports, we described that iTBS was able to improve social and occupational functioning as well as reduce PTSD and depressive symptoms [26], with outcomes at one year strongly favoring active stimulation [29]. Here, we report a follow-up analysis of the effects of iTBS regimen on self-reported anger and related clinical features. The assessment of anger was initially not a focus of our clinical trial and was as such not included in earlier reports. Yet given the importance of anger on functioning and a lack of clear treatments, we felt it of interest to explore the effects of iTBS on anger in this sample. We hypothesized anger would decrease from pre to post iTBS compared to sham. We further examined whether any anger improvement was related to reduction in PTSD and depression symptom severity, as well as improved quality of sleep, since sleep disruption is common in PTSD [30, 31].
2. Materials and Methods
2.1. Participants
Full details for the parent study, including the CONSORT diagram and stratified randomization methods can be found in Philip et al. [26]. In brief, fifty veterans with PTSD were included in the intent-to-treat sample (25 active, 25 sham), defined as randomized participants who received at least one iTBS session. Table 1 describes demographic and clinical variables. Inclusion criteria consisted of age between 18-70 years, trauma exposure based on the Life Events Checklist [32], a DSM-5 diagnosis of PTSD as assessed by SCID-5 [33]. Ongoing treatment (i.e., medications and/or psychotherapy) was allowed to continue unchanged during study participation and were stable for at least six weeks prior to start of study. Standard TMS safety criteria were used for exclusion (e.g., implanted devices, metal above the upper thoracic spinal cord); also, pregnancy, lifetime of moderate or severe traumatic brain injury, neurological conditions including lifetime history of seizure disorder, tumors, stroke or aneurysm, unstable medical conditions, primary psychotic disorder, bipolar I, current moderate or severe substance use disorders, or active suicidality. The Providence VA Medical Center Institutional Review Board granted approval before the start of any study procedures and institutional guidelines were followed throughout the study.
Table 1.
Demographic and clinical features intent-to-treat samples.
| Active TBS (n=25) | Sham TBS (n=25) | p valuea | |
|---|---|---|---|
| Demographic Variables | |||
| Age (years, SD) | 48.6 (12.8) | 53.2 (11.6) | 0.19 |
| Sex, N (%) female | 5 (20) | 3 (12) | 0.44 |
| Race, n (%)b | 0.35 | ||
| Caucasian/White | 22 (88) | 20 (80) | |
| African American | 0 (0) | 2 (8) | |
| American Indian/Alaskan Native | 1 (4) | 0 (0) | |
| Multiracial | 2 (8) | 1 (4) | |
| Ethnicity, n (%)b | 0.37 | ||
| Hispanic origin | 0 (0) | 2 (8) | |
| Not of Hispanic origin | 23 (92) | 23 (92) | |
| Education, n (%)b | 0.15 | ||
| Less than high school | 1 (4) | 1 (4) | |
| High school or equivalent | 2 (8) | 5 (20) | |
| Some college | 11 (44) | 7 (28) | |
| Trade or vocational degree | 3 (12) | 0 (0) | |
| Bachelor’s degree | 2 (8) | 8 (32) | |
| Advanced degree and/or education beyond college | 2 (8) | 3 (12) | |
| Employment status, n (%)b | 0.80 | ||
| Full time | 6 (24) | 4 (16) | |
| Part time | 1 (4) | 2 (8) | |
| Unemployed | 10 (40) | 9 (36) | |
| Retired | 6 (24) | 9 (36) | |
| Service connected disability, n (%) approved for mental health | 14 (56) | 17 (68) | |
| Military History | |||
| Branch, n (%)b | 0.61 | ||
| Army | 8 (32) | 8 (32) | |
| Navy | 8 (32) | 6 (24) | |
| Marines | 2 (8) | 1 (4) | |
| Air Force | 0 (0) | 3 (12) | |
| Clinical Variables (mean, SD) | |||
| PTSD CAPS-5 Score | 47.4 (10.6) | 47.9 (10.0) | 0.85 |
| PTSD PCL-5 Score | 49.4 (9.4) | 50.0 (11.4) | 0.85 |
| Psychiatric Comorbidity, n (%) | 47.4 (10.6) | 47.9 (10.0) | 0.80 |
| Major Depressive Disorderc | 25 (100) | 25 (100) | 1.00 |
| Bipolar II, most recent episode depressed | 2 (8) | 3 (12) | 0.64 |
| Substance Use Disorder (Mild) | 16 (64) | 11 (44) | 0.16 |
| Opioid use disorder | 6 (24) | 5 (20) | 0.73 |
| Prior brain stimulation, n (%) | |||
| Transcranial Magnetic Stimulation | 0 (0) | 2 (8) | 0.15 |
| Electroconvulsive Therapy | 2 (8) | 1 (4) | 0.55 |
| Mild traumatic brain injury, n (%) | 7 (28) | 5 (20) | 0.51 |
| Psychiatric History, n (%) | |||
| Suicide attempt(s) | 6 (24) | 6 (24) | 1.00 |
| Inpatient hospitalization(s) | 13 (52) | 15 (64) | 0.57 |
| DAR (mean, SD) | |||
| Baseline | 11.5 (6.3) | 14.1 (5.6) | 0.13 |
| Midpoint double blind | 9.1 (6.3) | 13.2 (5.5) | 0.02 |
| Endpoint double blind | 9.4 (6.6) | 12.7 (5.9) | 0.07 |
| Midpoint open label | 8.8 (6.8) | 11.6 (6.5) | 0.14 |
| Endpoint open label | 7.8 (6.8) | 9.3 (6.5) | 0.41 |
| Follow-up | 8.2 (6.5) | 10.9 (6.9) | 0.17 |
| PSQI (mean, SD) | |||
| Baseline | 14.0 (2.9) | 13.8 (4.7) | 0.86 |
| Midpoint double blind | 11.7 (4.0) | 12.8 (4.3) | 0.34 |
| Endpoint double blind | 11.3 (3.9) | 12.6 (4.8) | 0.31 |
| Midpoint open label | 10.6 (3.7) | 12.1 (4.9) | 0.26 |
| Endpoint open label | 10.2 (3.6) | 11.1 (5.1) | 0.54 |
| Follow-up | 9.5 (3.3) | 10.2 (4.8) | 0.58 |
Abbreviations: SD, Standard deviation; PTSD, posttraumatic stress disorder; CAPS-5, Clinician Administered PTSD Scale for DSM-5; PCL-5, PTSD Checklist for DSM-5. DAR, Dimensions of Anger Reactions scale; PSQI, Pittsburgh Sleep Quality Index.
p values represent between group contrast at baseline
Total < 100% due to participant non-response
Score of ≥ 14 on the 30-Item Inventory of Depressive Symptomology, Self-Report (IDS-SR)
p values represent independent samples t-test between groups.
2.2. TBS procedures
Participants were randomized by a study member uninvolved with iTBS delivery, 1:1 to active and sham groups stratified by sex and PTSD symptom severity using the PTSD Checklist for DSM-5 [PCL; 34]. Device calibration was based on the active motor threshold and defined by the stimulator output required to induce contralateral hand movement >50% of the time. Participants received 10 daily sessions of sham-controlled iTBS targeting the right DLPFC; the empiric and neurobiological rationale behind right sided stimulation is fully described in Philip et al. [26]. Briefly, we chose to apply iTBS over the right DLPFC based on recent meta-analyses that indicated greater PTSD symptom reduction with right-sided stimulation [20, 21]. iTBS was delivered for 1800 pulses/session at 80% active motor threshold, following prior sham-controlled TBS studies [35], using a Magstim Rapid 2+1 system (Magstim, UK). The coil was placed over EEG coordinate F4 based on scalp measurements done at every session, following [36]. All participants were offered another ten sessions of unblinded stimulation after completing the double-blind phase. This design allowed exploration of the effects of number of iTBS sessions on outcome variables. Out of the 25 participants assigned to active and sham iTBS each, 24 and 21 participants continued with the unblinded phase respectively. Participants were asked about which treatment group they thought they belonged to after completion of the double-blind phase. Blinding was successful, as participants could not accurately guess group assignment (X2=1.43, p=.49). To maintain the blind, study staff not involved with iTBS delivery placed the appropriate active or sham coil prior to all participant appointments.
2.3. Assessments
Anger severity was measured using the Dimensions of Anger Reactions scale [DAR; 37, 38], which includes seven self-report items, here each item was scored on a 0-4 point scale, resulting in a 0-28 range of total scores per Shea et al. [13]. The DAR assesses anger associated with stress and psychological adjustment problems, and correlates highly with trait and state anger as measured with the State Trait Anger Expression Inventory [37, 39]. The brevity of the DAR balances the necessity for an anger-specific measure while keeping participant burden low in a multiscale assessment study.
Other assessments included self-reported PTSD symptoms using the PCL-5. Self-reported depression symptoms were measured using the Inventory of Depressive Symptomatology, Self-Report [IDS-SR; 40]; results from these have been reported previously. In this secondary analysis we also included the Pittsburgh Sleep Quality Index [PSQI; 41], which was administered to assess quality of sleep across seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction). Participants completed the DAR, PCL-5, IDS-SR, and PSQI at six time points; baseline, midpoint and endpoint of both double-blind and unblinded phases, and again at one-month follow-up. See Figure 1 for a timeline of assessments administered during double-blind and unblinded phases.
Figure 1.
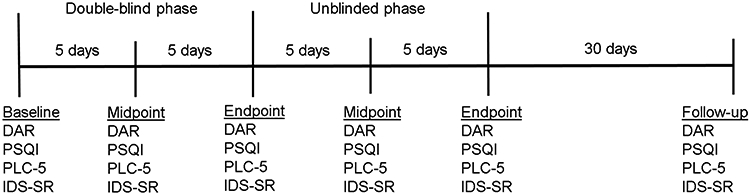
Timeline of assessments administered during double-blind and unblinded phases.
2.4. Statistical Analyses
To examine whether anger decreased over time data were analyzed using mixed-design repeated measures ANOVA with specific time points as within-subjects variable and Group (active or sham) as between-subjects factor (SPSS v24, Armonk, NY USA). The same approach was taken to analyze changes in quality of sleep over time. Data on change in PTSD and depression scores have been previously reported in Philip et al. [26] and will not be repeated here. Follow-up t-tests were performed where indicated. Double-blind and unblinded phases were analyzed separately because groups differed in the number of iTBS sessions completed, i.e. those initially randomized to active stimulation received a total of four weeks of iTBS (two weeks double-blind plus two weeks unblinded) and those initially randomized to sham received two weeks of iTBS (unblinded only). For the same reason we utilized paired sample t-tests in active iTBS and sham groups separately to test for changes in self-reported anger from end of unblinded phase to one-month follow-up. Pearson correlations tested the relationship between self-reported anger, PTSD and depressive symptoms, and quality of sleep. Missing data were addressed using multiple (n=20) imputations [42]. A two-sided alpha level of 0.05 was applied to determine significance in all analyses. Greenhouse-Geisser or Huynh-Feldt corrections were performed in case of significant sphericity.
3. Results
3.1. Anger reduction over time
3.1.1. Double-blind phase
There was no statistically significant group difference in baseline DAR score, t(48)=1.55, p=.13, see Table 2. During the double-blind phase, the repeated measures ANOVA with Time (three time points: baseline, midpoint and endpoint double-blind) as within-subjects variable and Group as between subjects factor revealed a significant main effect of Time, F(1.61,77.07)=5.56, p=0.01, and a significant main effect of Group, F(1,48)=4.53, p=.04. The Time*Group interaction was not significant, F(1.61,77.01)=0.88, p=.39. This demonstrates that self-reported anger was lower in the active iTBS group compared to the sham group across the three double-blind time points and that anger reduced significantly over time. Figure 2 illustrates DAR symptoms over time.
Table 2.
Anger and sleep quality scores for intent-to-treat samples across time points.
| Active TBS (n=25) | Sham TBS (n=25) | p value a | |
|---|---|---|---|
| DAR (mean, SD) | |||
| Baseline | 11.50 (6.3) | 14.10 (5.6) | 0.13 |
| Midpoint double blind | 9.06 (6.3) | 13.22 (5.5) | 0.02b |
| Endpoint double blind | 9.43 (6.6) | 12.74 (5.9) | 0.07 |
| Midpoint open label | 8.77 (6.8) | 11.61 (6.5) | 0.14 |
| Endpoint open label | 7.76 (6.8) | 9.33 (6.5) | 0.41 |
| Follow-up | 8.18 (6.5) | 10.88 (6.9) | 0.17 |
| PSQI (mean, SD) | |||
| Baseline | 14.04 (2.9) | 13.84 (4.7) | 0.86 |
| Midpoint double blind | 11.67 (4.0) | 12.83 (4.3) | 0.34 |
| Endpoint double blind | 11.33 (3.9) | 12.63 (4.8) | 0.31 |
| Midpoint open label | 10.58 (3.7) | 12.05 (4.9) | 0.26 |
| Endpoint open label | 10.22 (3.6) | 11.05 (5.1) | 0.54 |
| Follow-up | 9.52 (3.3) | 10.24 (4.8) | 0.58 |
Abbreviations: SD, Standard deviation; DAR, Dimensions of Anger Reactions scale; PSQI, Pittsburgh Sleep Quality Index.
p values represent independent samples t-test between group differences.
denotes p<0.05
Figure 2.
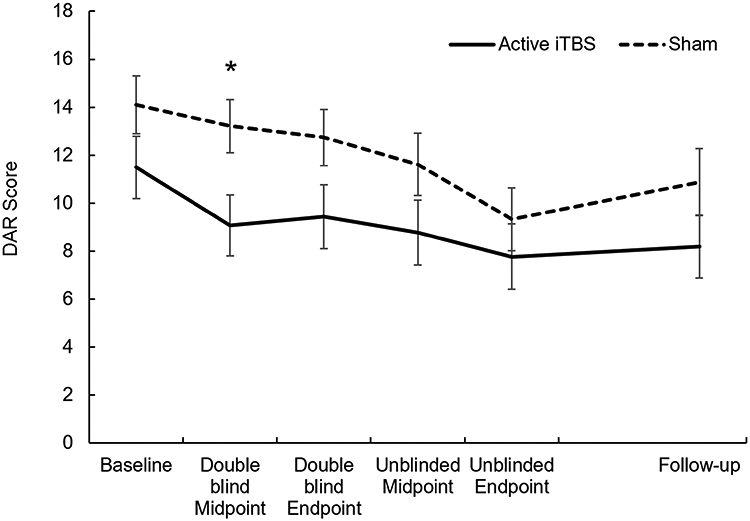
Average reported anger (DAR score) at each time point for individuals who received four weeks of iTBS – those initially randomized to active iTBS during double-blind phase (Active iTBS) – and those who received two weeks of iTBS – those initially randomized to sham stimulation (Sham). Error bars represent standard error. * p<.05 between groups.
Even though the Time*Group interaction was not significant, Figure 2 indicates an early reduction in anger in the first week from baseline to midpoint for the active iTBS group. Moreover, we previously observed in Philip et al. [26] that iTBS might have a beneficial effect in the first week after starting stimulation. Such a rapid reduction in symptoms would not be detectible by the Time*Group interaction when all three double-blind time points were included. Given the novelty of using iTBS for PTSD and anger, we subsequently examined the main effect of Group for each of the three time points using t-tests. This revealed that, compared to sham, those receiving iTBS reported significantly less anger during midpoint of the double-blind phase, t(48)=2.47, p=.02 only. Groups did not differ significantly on anger at baseline, t(48)=1.55, p=.13, or at the end of the double-blind phase, t(48)=1.86, p=.07. These results hint towards a reduction in anger for participants randomized to active iTBS vs. sham after the first week of stimulation, although these results should be interpreted with caution considering that the Time*Group interaction did not meet statistical significance at α <0.05.
3.1.2. Unblinded phase
During the unblinded phase, the repeated measures ANOVA with Time (three time points: endpoint double-blind which served as the “baseline” for this phase, midpoint unblinded, and endpoint unblinded) as within-subjects variable and Group (initial randomized to active iTBS vs sham) as between subjects factor resulted in a significant main effect of Time only, F(2,96)=10.08, p<.001. The main effect of Group and the Time*Group interaction were not significant, F(1,48)=2.22, p=.14 and F(2,96)=1.22, p=.30, respectively. This implies that although there was still a significant reduction in anger over time, the groups no longer differed in reported anger across the three time points in this unblinded phase, see Figure 2. This suggests that the sham group ‘caught up’ with the active iTBS group once these individuals received active iTBS during this phase.
3.1.3. Maintenance of anger reduction during one-month follow-up
In participants originally randomized to active iTBS (i.e., who had received a total of four weeks of active stimulation), anger ratings at one-month follow-up remain unchanged from the end of the unblinded phase, t(24)=−0.46, p=.65. For individuals initially randomized to sham (i.e., received two weeks of active stimulation) anger symptoms significantly increased (worsened) at the one month follow-up visit, t(24)=−2.12, p=.045. However, a mixed-design repeated measures ANOVA with end of unblinded phase and one month-follow-up as within-subjects variables and Group as between-subject factor did not reveal a significant interaction, F(1,48)=7.87, p=.35
3.2. Quality of Sleep
For the double-blind phase, the repeated measures ANOVA with Time (baseline, mid-, endpoint double-blind) as within-subjects variable and Group as between-subjects factor revealed a significant main effect of Time, F(1.97,94.51)=13.44, p<.001. The main effect of Group and the Time*Group interaction were not significant, both p>.1. Similarly, for the unblinded phase, repeated measures ANOVA with Time (endpoint double-blind, mid-, endpoint unblinded) as within-subjects variable and Group as between-subjects factor resulted in a significant main effect of Time, F(2.47)=5.70, p=.006, but no significant main effect of Group or interaction (both p>.1). A paired-sample t-test, across both groups, from endpoint unblinded phase to one-month follow-up demonstrated a continued improvement in sleep quality throughout the one month, t(49)=2.33, p=.02. See also Table 2.
3.3. Association with symptoms of PTSD and depression, and quality of sleep
Next, we explored whether a reduction in anger from baseline to end-of-treatment (at endpoint of unblinded phase) was associated with changes in PTSD and depressive symptoms and sleep quality over this same timeframe separately for individuals who received four weeks of active iTBS and two weeks of active iTBS. Data of one participant in the sham group was removed due to outlier status (SD ≥ 3 on the PCL and PSQI difference measures and high Cooks’ distance and leverage values).
For those who received four weeks of iTBS, there was a significant positive correlation between the reductions in anger and PTSD symptom severity, r(23)=0.40, p=.04, as well as between reductions in anger and depression severity, r(23)=0.43, p=.03. There was a non-significant correlation between anger reduction on the DAR and improved sleep quality on the PSQI, r(23)=0.37, p=.07. In individuals who received two weeks of iTBS, there was a near, but non-significant correlation between reductions in PTSD symptoms and anger (r(22)=0.40, p=.051). In this sample, the reduction in anger did not correlate with improvements in depression or sleep quality (both p>.1). See Figure 3 for scatterplots reflecting the relationship between changes from baseline to end-of-treatment for different variables.
Figure 3.
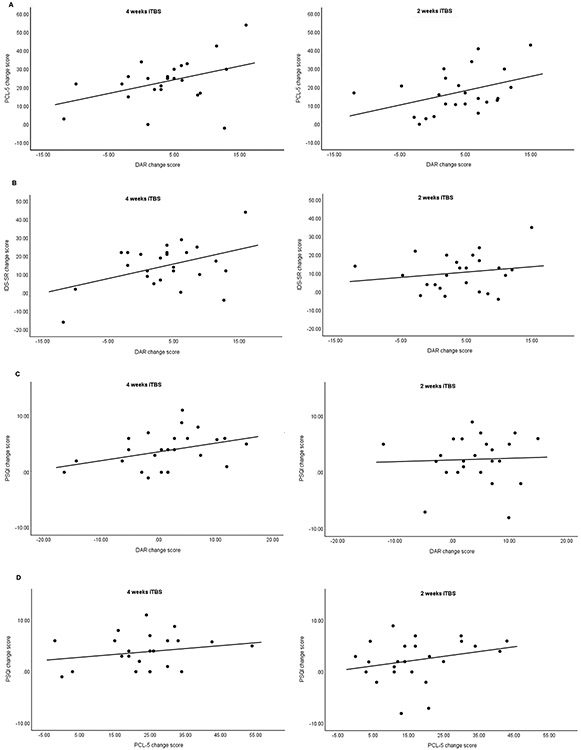
Scatterplots reflecting the relationship between changes in anger from baseline to end-of-treatment (at endpoint of unblinded phase) and changes in PTSD symptoms (A), depressive symptoms (B), and sleep quality (C), as well as between changes in PTSD symptoms baseline to end-of-treatment improvements and changes in sleep quality (D) for those who received four or two weeks of iTBS.
Fisher r-to-z transformation demonstrated that the differences between the correlation coefficients for those who received four or two weeks of active iTBS did not differ for any of the correlations, all p>.05.
4. Discussion
Our results indicate that iTBS targeting the right DLPFC may reduce self-reported anger in veterans with PTSD. We observed a reduction in anger scores from pretreatment baseline over the course of the double-blind phase across both groups, as well as a significant difference in self-reported anger for active iTBS compared to sham across the double-blind phase. Yet follow-up group comparisons showed that only at double-blind midpoint iTBS was associated with significantly less anger compared to sham, and this group difference was, at p=.07, no longer significant at double-blind endpoint. Self-reported anger further improved over the course of the unblinded phase across both groups, and during which all participants now received active iTBS. Although preliminary, these findings are relevant, as anger can be significant and debilitating in this patient population, warranting specific treatment [3, 6].
Anger reductions appeared to occur early - after only five sessions of iTBS in those initially randomized to active stimulation. This early effect of iTBS is consistent with our previous finding that iTBS improved clinical symptoms during the first week of exposure to active stimulation [26]. Importantly, reduction in anger remained stable over the second week of active stimulation and throughout the additional two weeks of unblinded iTBS and one-month follow-up period.
Observed crossover effects are consistent with the above finding: self-rated anger did not change in participants originally randomized to sham during double-blind phase, but appeared to go down once they received active stimulation during the unblinded phase. Furthermore, the reduction in anger in those who received active stimulation during the unblinded phase was not maintained during the one-month follow-up period, as indicated by a significant increase in reported anger from end-of-treatment to follow-up in this group. These results suggest that number of treatment sessions might be an important variable for enduring benefit in anger reduction that underscores the need for future anger-specific studies in iTBS using longer treatment protocols, and in non-invasive brain stimulation more broadly.
PTSD-related anger may mediate relationships between various symptom domains in PTSD [43]. Thus, an exploratory focus of this study was to evaluate the relationship between changes in anger on the one hand and changes in PTSD and depressive symptoms as well as sleep quality on the other hand. In individuals who received four weeks of active iTBS, reduction in anger was related to self-reported reduction in both PTSD and depressive symptom severity. These findings were attenuated or lost in those who were originally randomized to sham and received two weeks of active iTBS (but note, correlation coefficients for those who received four or two weeks of active iTBS did not significantly differ). These results are broadly supportive of the interconnectedness of anger, PTSD, and depression [4, 6, 7].
Of note, improvement in quality of sleep was not significantly correlated with anger reduction in this sample. This is interesting because not only are anger and sleep disruptions common occurrences in PTSD [30, 31, 44], prior research also demonstrates a relationship between inability to control anger and sleep quality [45]. Moreover, sleep deficiencies negatively impact prefrontal cortex supported executive functioning [46] including the regulation of emotions [47, 48]. It could therefore be expected that DLPFC-targeted iTBS would also improve sleep quality in PTSD when anger diminishes. It is likely that our study was underpowered to detect these effects, even though sleep quality did improve over the course of the study, including throughout the one-month follow-up. Moreover, a two or four-week treatment duration might have been too short to observe specific iTBS effects on sleep.
Enthusiasm for findings should be tempered by likely expectation effects related to unblinded stimulation. How and whether these nonspecific effects specifically pertain to anger (as opposed to other clinical symptoms) is uncertain, and one-year clinical outcomes demonstrated clear superiority of active stimulation over sham [29]. Likewise, we cannot rule out that the early reduction in anger was moderated or mediated by improvements in other clinical domains, and thus whether there was an independent effect of iTBS on anger reduction. Other limitations include a modestly sized and demographically homogeneous veteran sample, and the use of a relatively brief anger measure. While the DAR was used in this study to minimize participant burden, it does not provide information on specific anger-related behaviors and cognitions, such as those assessed by the State-Trait Anger Expressive Inventory 2 [49]. Although we did not explicitly recruit based on anger symptoms, observed baseline scores were comparable to previously detected scores using the 5-item DAR-5 which included a trauma-exposed subsample [50]. Furthermore, Cash et al. [51] reported, again based on the DAR-5, that a pre-post treatment reduction of 4.5 or greater represents a reliable treatment change. Such a 4.5 points or greater reduction from baseline to end of treatment (unblinded phase) was only met by those who received two weeks of active iTBS, i.e. initially randomized to sham. Nonetheless caution should be exercised here when interpreting these numbers. Not only did we include a modestly sized clinical sample not explicitly recruited on anger, what would constitute a clinically significant reduction may be different on the 7-item version of the DAR used here. As such, future studies might want to incorporate more faceted anger assessments to further examine the effects of non-invasive brain stimulation, and different TMS protocols in particular, on anger in PTSD. Furthermore, assessment of biological variables, such as neuroimaging and other modalities, may provide important biological elements of observed findings. Finally, it should be recognized that participants were on current medications and/or psychotherapy (stable for >6 weeks prior to participation) and as such any observed effects may be adjunctive.
5. Conclusion
Our findings provide preliminary evidence that iTBS might be helpful in reducing self-reported anger in veterans with PTSD, along with improvement in other symptomatology as reported previously [26]. Moreover, the degree in anger reduction from start to end-of-treatment was associated with the degree of PTSD and depressive symptom severity reduction, but not sleep quality. This study provides a first step in testing the effects of non-invasive brain stimulation for PTSD-related anger. Future studies using non-invasive brain stimulation techniques should focus in more detail on anger as well as use clinician-assessed anger measurements or assessments that encompass more facets of anger, as well as potential other brain regions implicated in anger, to address this important and often overlooked element of clinical care.
Source(s) of Financial Support:
This study was supported by U.S. Veterans Affairs grants I21 RX002032, I01 RX002450 and the Center for Neurorestoration and Neurotechnology (N2864-C) from the U.S. Department of Veterans Affairs, Rehabilitation Research and Development Service, Providence, RI. Effort on this project was also supported by grant P20 GM130452 (Drs. van ‘t Wout-Frank, Greenberg, and Philip).
Footnotes
Conflicts of Interest: In the past three years, Dr. Philip has been an unpaid scientific advisory board member for Neuronetics. The other authors report no biomedical conflicts of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N. Engl. J. Med 2017; 376: 2459–2469. [DOI] [PubMed] [Google Scholar]
- 2.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of anxiety disorders. 2011; 25: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakupcak M, Conybeare D, Phelps L, Hunt S, Holmes HA, Felker B et al. Anger, hostility, and aggression among Iraq and Afghanistan war veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007; 20: 945–954. [DOI] [PubMed] [Google Scholar]
- 4.Orth U, Wieland E. Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: A meta-analysis. Journal of consulting and clinical psychology. 2006; 74: 698. [DOI] [PubMed] [Google Scholar]
- 5.Cassiello-Robbins C, Barlow DH. Anger: The unrecognized emotion in emotional disorders. Clin. Psychol 2016; 23: 66–85. [Google Scholar]
- 6.Olatunji BO, Ciesielski BG, Tolin DF. Fear and loathing: A meta-analytic review of the specificity of anger in PTSD. Behav. Ther 2010; 41: 93–105. [DOI] [PubMed] [Google Scholar]
- 7.McHugh T, Forbes D, Bates G, Hopwood M, Creamer M. Anger in PTSD: is there a need for a concept of PTSD-related posttraumatic anger? Clin. Psychol. Rev 2012; 32: 93–104. [DOI] [PubMed] [Google Scholar]
- 8.Foa EB, Riggs DS, Massie ED, Yarczower M. The impact of fear activation and anger on the efficacy of exposure treatment for posttraumatic stress disorder. Behav. Ther 1995; 26: 487–499. [Google Scholar]
- 9.Blum MD, Kelly EM, Meyer M, Carlson CR, Hodson W. An assessment of the treatment needs of Vietnam-era veterans. Psychiatr. Serv 1984; 35: 691–696. [DOI] [PubMed] [Google Scholar]
- 10.Jayasinghe N, Giosan C, Evans S, Spielman L, Difede J. Anger and posttraumatic stress disorder in disaster relief workers exposed to the 9/11/01 World Trade Center Disaster: One-year follow-up study. J. Nerv. Ment. Dis 2008; 196: 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novaco RW, Chemtob CM. Anger and combat-related posttraumatic stress disorder. J. Trauma. Stress 2002; 15: 123–132. [DOI] [PubMed] [Google Scholar]
- 12.Chemtob CM, Novaco RW, Hamada RS, Gross DM. Cognitive-behavioral treatment for severe anger in posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 1997; 65: 184. [DOI] [PubMed] [Google Scholar]
- 13.Shea MT, Lambert J, Reddy MK. A randomized pilot study of anger treatment for Iraq and Afghanistan veterans. Behav. Res. Ther 2013; 51: 607–613. [DOI] [PubMed] [Google Scholar]
- 14.Gerlock AA. Veterans' responses to anger management intervention. Issues Ment. Health Nurs 1994; 15: 393–408. [DOI] [PubMed] [Google Scholar]
- 15.Linkh DJ, Sonnek SM. An application of cognitive-behavioral anger management training in a military/occupational setting: Efficacy and demographic factors. Military medicine. 2003; 168: 475–478. [PubMed] [Google Scholar]
- 16.Morland LA, Greene CJ, Rosen CS, Foy D, Reilly P, Shore J et al. Telemedicine for anger management therapy in a rural population of combat veterans with posttraumatic stress disorder: a randomized noninferiority trial. J. Clin. Psychiatry 2010; 71: 855–863. [DOI] [PubMed] [Google Scholar]
- 17.Koek RJ, Roach J, Athanasiou N, van t Wout-Frank M, Philip NS. Neuromodulatory treatments for post-traumatic stress disorder (PTSD). Prog. Neuropsychopharmacol. Biol. Psychiatry 2019. [DOI] [PubMed] [Google Scholar]
- 18.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry 2010; 67: 507–516. [DOI] [PubMed] [Google Scholar]
- 19.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 2007; 62: 1208–1216. [DOI] [PubMed] [Google Scholar]
- 20.Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014; 7: 151–157. [DOI] [PubMed] [Google Scholar]
- 21.Berlim MT, Van den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. Can J Psychiatry. 2014; 59: 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yesavage JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA psychiatry. 2018; 75: 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carpenter LL, Conelea C, Tyrka AR, Welch ES, Greenberg BD, Price LH et al. 5 Hz Repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J. Affect. Disord 2018; 235: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philip NS, Ridout SJ, Albright SE, Sanchez G, Carpenter LL. 5-Hz transcranial magnetic stimulation for comorbid posttraumatic stress disorder and major depression. J. Trauma. Stress 2016; 29: 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012; 5: 38–43. [DOI] [PubMed] [Google Scholar]
- 26.Philip NS, Barredo J, Aiken E, Larson V, Jones RN, Shea MT et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am. J. Psychiatry 2019; 176: 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005; 45: 201–206. [DOI] [PubMed] [Google Scholar]
- 28.Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018; 391: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 29.Petrosino NJ, van’t Wout-Frank M, Aiken E, Swearingen HR, Barredo J, Zandvakili A et al. One-year clinical outcomes following theta burst stimulation for post-traumatic stress disorder. Neuropsychopharmacology. 2019: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. J. Clin. Psychiatry 2007. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007; 44: 660–669. [DOI] [PubMed] [Google Scholar]
- 32.Weathers F, Blake D, Schnurr P, Kaloupek D, Marx B, Keane T. The life events checklist for DSM-5 (LEC-5). Instrument available from the National Center for PTSD at www.ptsd.va.gov. 2013. [Google Scholar]
- 33.First MB. Structured clinical interview for the DSM (SCID). The encyclopedia of clinical psychology. 2014: 1–6. [Google Scholar]
- 34.Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P. The PTSD Checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov. 2013. [Google Scholar]
- 35.Li C-T, Chen M-H, Juan C-H, Huang H-H, Chen L-F, Hsieh J-C et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014; 137: 2088–2098. [DOI] [PubMed] [Google Scholar]
- 36.Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009; 2: 50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forbes D, Hawthorne G, Elliott P, McHugh T, Biddle D, Creamer M et al. A concise measure of anger in combat-related posttraumatic stress disorder. J Trauma Stress. 2004; 17: 249–256. [DOI] [PubMed] [Google Scholar]
- 38.Novaco R Dimensions of anger reactions. Irvine, CA: University of California. 1975; 639. [Google Scholar]
- 39.Spielberger C State-trait anger expression inventory research edition. Professional manual. Odessa, FL: Psychological Assessment Resources. 1988. [Google Scholar]
- 40.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol. Med 1996; 26: 477–486. [DOI] [PubMed] [Google Scholar]
- 41.Buysse DJ, Reynolds III CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 42.Ware JH, Harrington D, Hunter DJ, D'Agostino RB Sr. Missing data. N. Engl. J. Med 2012: 1353–1354.22475612 [Google Scholar]
- 43.Claycomb M, Roley ME, Contractor AA, Armour C, Dranger P, Wang L et al. The relationship between negative expressivity, anger, and PTSD symptom clusters. Psychiatry Res. 2016; 243: 1–4. [DOI] [PubMed] [Google Scholar]
- 44.Wang S-J, Rushiti F, Sejdiu X, Pacolli S, Gashi B, Salihu F et al. Survivors of war in northern Kosovo (III): The role of anger and hatred in pain and PTSD and their interactive effects on career outcome, quality of sleep and suicide ideation. Conflict and health. 2012; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hisler G, Krizan Z. Anger tendencies and sleep: Poor anger control is associated with objectively measured sleep disruption. J Res Pers. 2017; 71: 17–26. [Google Scholar]
- 46.Van Dongen H, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003; 26: 117–126. [DOI] [PubMed] [Google Scholar]
- 47.Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cogn. Emot 2013; 27: 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med. Rev 2017; 31: 6–16. [DOI] [PubMed] [Google Scholar]
- 49.Spielberger CD. State-Trait anger expression inventory. The Corsini Encyclopedia of Psychology. 2010: 1-1. [Google Scholar]
- 50.Forbes D, Alkemade N, Mitchell D, Elhai JD, McHugh T, Bates G et al. Utility of the Dimensions of Anger Reactions–5 (DAR-5) scale as a brief anger measure. Depress. Anxiety 2014; 31: 166–173. [DOI] [PubMed] [Google Scholar]
- 51.Cash R, Varker T, McHugh T, Metcalf O, Howard A, Lloyd D et al. Effectiveness of an anger intervention for military members with PTSD: a clinical case series. Military medicine. 2018; 183: e286–e290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


