Abstract
Lactobacilli recovered from the blood, cerebrospinal fluid, respiratory tract, and gut of 20 hospitalized immunocompromised septic patients were analyzed. Biochemical carbohydrate fermentation and total soluble cell protein profiles were used to identify the species. Hydrogen peroxide production was measured. Susceptibility to 19 antibiotics was tested by a diffusion method, and the MICs of benzylpenicillin, amoxicillin, imipenem, erythromycin, vancomycin, gentamicin, and levofloxacin were determined. A small number of species produced H2O2, and antibiotic susceptibilities were species related. Eighteen (90%) of the isolates were L. rhamnosus, one was L. paracasei subsp. paracasei, and one was L. crispatus. L. rhamnosus, L. paracasei subsp. paracasei isolates, and the type strains were neither H2O2 producers nor vancomycin susceptible (MICs, ≥256 μg/ml). L. crispatus, as well as most of the type strains of lactobacilli which belong to the L. acidophilus group, was an H2O2 producer and vancomycin susceptible (MICs, <4 μg/ml).
Lactobacilli are ubiquitous and widespread commensal bacteria in the human and animal microflora. They are widely used by humans: as adjuvants against gastrointestinal disorders, as dietary supplements, and as biological food processors in view of their fermentative properties (1, 15).
Severe lactobacillus infections occur as endocarditis in patients with valve defects and as local or disseminated infections in neutropenic patients with sepsis receiving broad-spectrum antibiotics. The conditions of the patients in the latter group are usually leukemia with chemotherapy, an immunocompromised state due to organ transplantation, or AIDS (1, 2, 5, 13, 15, 22, 23, 25, 31). The current widespread use of glycopeptides and broad-spectrum cephalosporins for the management of sepsis may increase the rate of occurrence of lactobacillus infections among immunocompromised patients (5, 13).
Recent phylogenetic analyses have resulted in taxonomic changes in this genus (6, 26, 30, 34, 36). For example, in 1989, Lactobacillus casei subsp. rhamnosus was elevated to species status as L. rhamnosus sp. nov., and all other members of the L. casei subspecies except L. casei subsp. casei were grouped in a separate species, L. paracasei sp. nov. (7). Further changes are under way (9, 10, 30). Since 1980, the members of the L. acidophilus group have been divided into two subgroups, the L. acidophilus subgroup and the L. gasseri subgroup, and the two subgroups are divided into six species (14, 24).
Identification of Lactobacillus species to the species level is not possible on a routine basis. Commercially available carbohydrate fermentation tests fail to identify various Lactobacillus species. However, highly standardized whole-cell protein patterns obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) have proved useful for Lactobacillus species identification (27, 32). The potential value of other tests, such as hybridization with oligonucleotide probes, is under investigation (32).
Few studies of lactobacilli from normal and diseased vaginal and intestinal mucosae report the species involved (11, 29). In vitro H2O2 production by lactobacilli from the vaginal microflora has recently been surveyed. H2O2-producing lactobacilli predominate in the normal vagina but are seldom found in the vaginas of patients with bacterial vaginosis (11). H2O2 is known to inhibit the growth of some bacteria and may be involved in the control of the normal microflora.
Vancomycin and teicoplanin are active against most gram-positive bacteria. However, various species (L. rhamnosus, L. casei, and L. plantarum) are intrinsically resistant to glycopeptides (21, 35). In contrast, most of the lactobacilli from the vaginal flora that we have tested were susceptible to these antibiotics (unpublished data). Our aim was to characterize the lactobacilli isolated from 20 patients with sepsis. The clinical status, carbohydrate fermentation profiles, whole-cell protein patterns, ability to produce H2O2, and antibiotic susceptibilities of the strains were analyzed.
MATERIALS AND METHODS
Patients and controls.
The 20 patients were immunocompromised adults and children hospitalized in St-Louis Hospital between 1993 and 1995 (Table 1).
TABLE 1.
Characteristics of 20 immunocompromised patients infected with a Lactobacillus
| Strain no.a | Age (yr)/ sexb | Underlying conditionc | Anti-mitotic drug | Duration (wk) of granulo-cytopenia | Other sign(s) | Antibiotic(s) administered
|
Origin of isolates (no. of samples) | Time of death (days) after isolation of Lacto-bacillus | |
|---|---|---|---|---|---|---|---|---|---|
| Glyco-peptide | Othersd | ||||||||
| 1132 | 35/M | Lymphoma, HBMT | 8 | GVHe | IMP, PEF, MTR | Stools | |||
| 1199 | 12/M | AA | 150 | + | CAZ, CIP | Blood (4), catheter, throat | 7 | ||
| 1200 | 50/F | AML | + | 4 | Diarrhea | + | CAZ, CIP, RIF, POL (p.o.f) | Stools | |
| 1201 | 55/M | PCM, ABMT | Diarrhea | Sputum | |||||
| 1202 | 11/F | AA, CBT | 2 | + | PIP, AM, OFL (p.o.) | Blood (14), CSF | |||
| 1273 | 7/M | AML, ABMT | 2 | + | CAZ, AMX, CIP, POL (p.o.) | Blood (4; relapsed) | |||
| 1274 | 24/M | AML | + | 4 | + | CAZ, OFLO, AM, POL (p.o.) | Throat | ||
| 1284 | 4/F | ALL | + | 2 | + (p.o.) | AM, FOS | Stools | ||
| 1285 | 9/M | Relasped AML | + | 1 | + | CAZ, AM, TMP-SMZ | Blood (1) | ||
| 1286 | 35/F | AML | + | 1 | Diarrhea | CAZ, NET | Stools | ||
| 1287 | 65/M | Gut carcinoma, surgery | <1 | Septic shock | CTX, AM, MTR | Blood (1) | 1 | ||
| 1288 | 16/M | Relapsed ALL | + | 2 | Mucositis | + (po) | CAZ, NET, POL (p.o.) | Blood (2) | |
| 1290 | 29/M | Kidney transplantation | 8 | Hepatitis, diarrhea | + (po) | CAZ, TMP-SMZ, CIP | Blood (2) | 1 | |
| 1292 | 6/M | AL, CBT | 2 | GVH, diarrhea | + (po) | IMP, MTR (p.o.), TMP-SMZ | Stools | ||
| 1293 | 2/F | AML, HBMT | 3 | GVH, mucositis | + | CAZ, AM, POL (p.o.) | Blood (5) | ||
| 1294 | 80/M | Diabetes, liver failure | Blood (1) | ||||||
| 1295 | 34/M | AIDS | 8 | TMP-SMZ | Blood (1) | ||||
| 1296 | 10/M | AML | + | 1 | + | CAZ, AM | Blood (1) | ||
| 1297 | 52/M | PCM, ABMT | 8 | Septic shock | + | PIP, CIP, GM | Lower respiratory tract | ||
| 1298 | 29/M | AIDS | 1 | Shock, diarrhea | TMP-SMZ | Blood (3) | 1 | ||
All strains except strains 1294 and 1295 were L. rhamnosus.
M, male; F, female.
AA, aplastic aplasia; ABMT, autologous bone marrow transplantation; AL, acute leukemia (ALL, lymphoblastic; AML, myeloid); HBMT, homologous bone marrow transplantation; CBT, cord blood transplantation; PCM, plasma cell myeloma.
AM, amikacin; AMX, amoxicillin; CAZ, ceftazidime; CIP, ciprofloxacin; CTX, cefotaxime; FOS, fosfomycin; GM, gentamicin; IMP, imipenem; MTR, metronidazole; NET, netilmicin; OFL, ofloxacin; PEF, pefloxacin; PIP, piperacillin; POL, polymyxin B; RIF, rifampin; TMP-SMZ, trimethoprim-sulfamethoxazole.
GVH, graft-versus-host disease.
p.o., per os.
Strains.
Lactobacilli were isolated from diverse samples taken in the course of the biological management of sepsis and were then stored at −80°C in the laboratory and freeze-dried in the Bacterial Strains Collection at the Institute Pasteur (CIP). No strain was an obligate anaerobe.
Type strains.
The following CIP type strains were tested in parallel as references: L. rhamnosus CIP A 157T, L. paracasei subsp. paracasei CIP 103918T, and L. jensenii CIP 69.17T. The following six strains of the L. acidophilus group were tested in parallel as references: four strains from the L. acidophilus subgroup (L. acidophilus CIP 76.13T, L. crispatus CIP 102990T, L. gallinarum CIP 103611T, and L. amylovorus CIP 102989T) and two strains from the L. gasseri subgroup (L. gasseri CIP 102991T and L. johnsonii CIP 103620T).
Fermentation profile.
The API 50 CH test kit and API CHL medium (bioMérieux, La Balme les Grottes, France) were used to test the abilities of the strains to ferment 49 carbohydrates. An 18-h culture in de Man-Rogosa-Sharpe (MRS) broth was centrifuged, and 200 μl of the sediment was introduced into API CHL medium. These samples were then tested with the API strips according to the manufacturer’s instructions, the top of the cupule was covered with mineral oil, and the results were read after 24 h of incubation at 37°C under aerobic conditions.
The species identifications obtained from the biochemical profiles were confirmed with identification software (bioMérieux). Carbohydrate fermentations gave either the species identification (noted as good or doubtful) or inconclusive results.
Whole-cell protein profile.
An 18-h culture on an MRS agar slope was harvested, and the proteins were extracted by disruption with glass microbeads. The samples were then subjected to gel electrophoresis (SDS-PAGE) and the proteins in the gel were stained with Coomassie blue.
Computerized normalization of densitometric scans of the gels and numerical analysis were done as described by Kersters and De Ley (27). Clusters were identified by unweighted average pair group method analysis with Gelcompar software (Applied Maths, Ghent, Belgium). The similarities between the protein patterns of the isolates and the type strains were scored as the Pearson product moment correlation coefficient. In agreement with previous assays, isolates and type strains with more than 82% similarity were clustered, and their assignment to the same species or the same L. acidophilus subgroup was considered (10, 28, 32).
H2O2 production.
According to the qualitative method of Eschenbach et al. (11) lactobacilli were streaked onto a 20-ml MRS agar plate containing 5 mg of 3,3′,5,5′-tetramethylbenzidine (TMB; T2885; Sigma), a benzidine-like chromogenic substrate of peroxidase, and 0.20 mg of horseradish peroxidase (P6782; Sigma) (11). Peroxidase generates O2 from any H2O2 produced by the lactobacilli, and the TMB stains the colonies blue in the presence of O2. After 48 h of incubation under 5% CO2 in air, colonies that produce H2O2 on MRS agar thus appear dark blue. Nonproducers are colorless. The media were used within 48 h after preparation. All strains were tested twice. The Lactobacillus type strains were used as quality controls.
Agar diffusion method for determination of antibiotic susceptibility patterns.
Nineteen antibiotics were tested: benzylpenicillin, amoxicillin, cephalothin, streptomycin (10 IU), kanamycin (30 IU), gentamicin (10 IU), vancomycin, teicoplain, erythromycin, azithromycin, pristinamycin, lincomycin, rifampin, tetracycline, chloramphenicol, pefloxacin, sparfloxacin, fosfomycin, and fusidic acid (disks; Sanofi Diagnostics Pasteur, Marnes la Coquette, France). The instructions of the Comité Français de l’Antibiogramme related to streptococci were followed (34a).
Fifty microliters of the pellet of an overnight culture was diluted in MRS broth to about 107 CFU/ml. Mueller-Hinton agar plates containing 5% sheep blood (bioMérieux, Marcy l’Etoile, France) were flooded with this suspension in order to give confluent colonies and air dried for 15 min, and the disks impregnated with antibiotics were positioned on the plates. After 36 h of incubation at 37°C in air containing 5% CO2, the diameters of the bacteria-free zones were measured.
MICs.
Benzylpenicillin, amoxicillin, imipenem, gentamicin, erythromycin, vancomycin, and levofloxacin MICs were tested by the E-test method (Biomedical Diagnostics, Marne La Vallée, France). The inoculum, agar plates, and incubation conditions for the MIC determinations were as described above for the susceptibility assay. A large, 12-cm square Mueller-Hinton agar plate was flooded with this suspension, air dried for 15 min, and overlaid with the seven E-test antibiotic strips. After 36 h of incubation at 37°C in air containing 5% CO2, the elliptical zones of growth inhibition were examined and the MICs were interpreted as the value on the E-test strip scale where the inhibition zone intersected the edge of the strip. Staphylococcus aureus ATCC 25923 was tested simultaneously on Mueller-Hinton agar, with an overnight culture diluted in Mueller-Hinton broth.
Both MICs and susceptibility patterns were determined for benzylpenicillin, amoxicillin, vancomycin, gentamicin, and erythromycin. Only the MICs of imipenem and levofloxacin were measured.
RESULTS
Patients.
The 20 patients (15 males and 5 females; age range, 2 to 80 years; median age, 27 years) had severe underlying conditions, as indicated in Table 1. Seven patients had undergone autologous, homologous bone marrow or cord blood transplantation, one had undergone a kidney transplantation, seven were receiving antimitotic chemotherapy, two had septic shock, and two had AIDS (Table 1). Eighteen patients were granulocytopenic. Sixteen patients were treated with several systemic antibiotics, and nine were treated with oral antibiotics.
Lactobacilli were isolated from the blood of 12 patients: 7 with persistent lactobacillus septicemia (a lactobacillus was also isolated from cerebrospinal fluid samples from one of these patients) and 5 for whom only one blood sample was cultured. Lactobacilli were isolated from the respiratory tracts of two patients and from the stools or throats of six patients during treatment for total bacterial decontamination.
Antibiotic regimens were designed according to lactobacillus susceptibility in vitro. Four patients died over the next few days, and one had a lactobacillemia relapse.
Carbohydrate fermentation.
Table 2 summarizes the results of the API CH 50 test.
TABLE 2.
Species of Lactobacillus isolates from 20 septic patients according to fermentation properties and performance by SDS-PAGE and result of respective H2O2 production
| Isolatea | Total no. of isolates | Identification | No. of isolates identified by fermentation | SDS-PAGE
|
No. of isolates positive for H2O2 production | |
|---|---|---|---|---|---|---|
| No. of isolates identified | % Similarityb | |||||
| L. rhamnosus | 18 | L. rhamnosus | 18 | 18 | 84–98 | 0 |
| 1295 | 1 | L. paracasei subsp. paracasei | 1 | 1 | 87 | 0 |
| 1294 | 1 | L. acidophilus group | 1c | 1 | 84, 94d | 1 |
| Total | 20 | 20 | 20 | 1 | ||
See L. rhamnosus strain numbers in Table 1. All isolates are L. rhamnosus except isolates 1294 and 1295.
Percent similarity of the protein pattern with that of the type strain of the species.
L. crispatus according to fermentation pattern.
Percent similarity with L. crispatusT and L. gasseriT, respectively.
Eighteen isolates were L. rhamnosus (see Table 1 for strain numbers) one was L. paracasei subsp. paracasei (strain 1295), and one was L. crispatus (strain 1294).
Cluster analysis of whole-cell protein profiles.
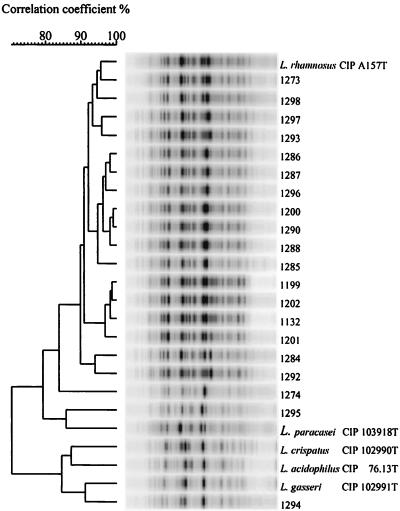
The results of cluster analysis of the protein profiles of the strains from patients and the type strains are presented in Fig. 1.
FIG. 1.
Identification of Lactobacillus isolates from patients according to whole-cell protein patterns. Point traces were used for the calculation of the correlation coefficient (percent similarity) between individual traces. The clinical origin of each numbered strain is given in Table 1. The dendrogram was built by the unweighted average pair group method.
The 18 L. rhamnosus strains fell into one cluster; 17 had more than 90% similarity with L. rhamnosusT and 1 (strain 1274) with 84% similarity with L. rhamnosusT. The protein profile of L. paracasei subsp. paracasei shared 86% similarity with that of L. paracasei subsp. paracaseiT. The protein profile of the lactobacillus identified as L. crispatus by its fermentation profile (strain 1294) fell into a cluster that included all six type strains of the species from the L. acidophilus group (results for three members of this group, L. acidophilusT, L. crispatusT, and L. gasseriT, are presented in Fig. 1) and shared 94% similarity with L. gasseriT but only 84% similarity with L. crispatusT (Table 2; Fig. 1).
H2O2 production by lactobacilli.
The findings from studies of H2O2 production by the lactobacilli tested are presented in Table 2.
Only one isolate, isolate 1294, either L. crispatus or L. gasseri, generated H2O2. Among the type strains studied, the only H2O2 producers were the members of the L. acidophilus group, but not L. acidophilusT itself, and L. jenseniiT (data not shown).
Antibiotic susceptibility patterns determined by disc diffusion method.
The mean diameters of the zones of inhibition (± standard deviations) were as follows: amoxicillin, 22.5 (± 6.4) mm; streptomycin, 12.1 (± 5.4) mm; kanamycin, 10.1 (±5.3) mm; gentamicin, 17.6 (±5.6) mm; erythromycin, 31.0 (±7.8) mm; azithromycin, 27.4 (±8.2) mm; pristinamycin, 27.7 (±5.5) mm; lincomycin, 22.6 (±10.5) mm; rifampin, 30.2 (±10) mm; tetracycline, 24.0 (±3) mm; and chloramphenicol, 23.0 (±4.8) mm. Fosfomycin and fusidic acid did not give inhibition zones for any strain tested. These results were similar to those for members of the normal vaginal flora studied simultaneously (data not shown).
For the strains from patients, the mean diameter of the zone of inhibition (±standard deviation) were as follows: benzylpenicillin, 22.5 (±6.4) mm; cephalothin, 13.4 (±9) mm; and vancomycin, no growth inhibition except for the strain from one patient (strain 1294). The diameters of the zones of inhibition for these antibiotics were significantly larger with normal vaginal lactobacillus. The findings for teicoplanin were similar to those for vancomycin. Among the quinolones, the mean (± standard deviation) diameter-for pefloxacin was 12.7 (±4) mm, and that for sparfloxacin was 24.3 (±5.2) mm. Both of these diameters are significantly larger than those found for normal vaginal lactobacilli (data not shown).
MIC results.
The ranges of the MICs of benzylpenicillin, imipenem, and vancomycin were narrow: the MICs at which 50 and 90% of strains are inhibited were 0.5 and 2 μg/ml, respectively, for benzylpenicillin, 1 and 2 μg/ml, respectively, for imipenem, and ≥256 and ≥256 μg/ml, respectively, for vancomycin. For 19 (95%) isolates, all 18 L. rhamnosus strains and 1 L. paracasei strain), vancomycin MICs were ≥256 μg/ml.
MICs were generally similar for type strains and isolates from the same species. The range of MICs for L. rhamnosus and L. paracasei subsp. paracasei (19 isolates and 2 type strains) and for the L. acidophilus group (1 isolate and 6 type strains) are presented in Table 3. The results for vancomycin and levofloxacin are noteworthy. The MICs of vancomycin for L. rhamnosus and L. paracasei subsp. paracasei of all origins were ≥256 μg/ml, and the MICs for the L. acidophilus group were ≤2 μg/ml. The MICs of levofloxacin for L. rhamnosus and L. paracasei subsp. paracasei were ≤4 μg/ml, and those for the L. acidophilus group were all ≥32 μg/ml.
TABLE 3.
Distribution of MICs according to Lactobacillus speciesa
| Antibiotic | Species | No. of isolates for which the MIC (mg/liter) was as follows:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 | ||
| Benzylpenicillin | L. acidophilus group | 2 | 2 | 2 | 1 | |||||||
| L. rhamnosus and L. paracasei | 5 | 9 | 1 | 5 | 1 | |||||||
| Amoxicillin | L. acidophilus group | 7 | ||||||||||
| L. rhamnosus and L. paracasei | 5 | 8 | 6 | 2 | ||||||||
| Imipenem | L. acidophilus group | 3 | 3 | 1 | ||||||||
| L. rhamnosus and L. paracasei | 1 | 2 | 7 | 9 | 2 | |||||||
| Vancomycin | L. acidophilus group | 5 | 2 | |||||||||
| L. rhamnosus and L. paracasei | 21b | |||||||||||
| Gentamicin | L. acidophilus group | 1 | 6 | |||||||||
| L. rhamnosus and L. paracasei | 6 | 9 | 3 | 3 | ||||||||
| Erythromycin | L. acidophilus group | 1 | 6 | |||||||||
| L. rhamnosus and L. paracasei | 4 | 12 | 4 | 1 | ||||||||
| Levofloxacin | L. acidophilus group | 7 | ||||||||||
| L. rhamnosus and L. paracasei | 3 | 14 | 1 | 3 | ||||||||
Seven strains were from the L. acidophilus group and 21 strains were from the L. rhamnosus and L. paracasei subsp. paracasei groups (19 and 2 strains respectively).
MICs, ≥256 μg/ml.
DISCUSSION
The prevalence of L. rhamnosus in patients (up to 90%) is consistent with recent reports of disseminated lactobacillus infections in immunocompromised hosts (5, 15, 22, 25, 33). Our patients had severe underlying conditions (acute leukemia, neoplasia, organ transplantation, or AIDS) and received various systemic and oral antibiotics, including glycopeptides for 13 of them (65%) (Table 1).
Both fermentation profiles and protein profiles were reliable for the identification to the species level of all the isolates except the isolate belonging to the L. acidophilus group (Table 2; Fig. 1).
H2O2 was produced by one isolate, either L. crispatus or L. gasseri, by five of the six type strains of the L. acidophilus group, and by L. jenseniiT (Table 2). It was also produced by 80% of the normal vaginal isolates that we studied simultaneously (data not shown), in agreement with previous data (11).
The disk diffusion method and the E test gave concordant results. However, previous studies of cephalosporins determined that the MICs were much higher by the E test than by the dilution method with agar (8, 20).
The median MICs of benzylpenicillin and amoxicillin for L. rhamnosus and L. paracasei subsp. paracasei were two times higher than those for the L. acidophilus group, and those of imipenem were four times higher (Table 3).
The MICs at which 90% of strains are inhibited for erythromycin and gentamicin were similar for all lactobacilli (0.06 and 4 μg/ml, respectively) (Table 3). A synergistic bactericidal effect between the penicillins and gentamicin has been demonstrated previously (3, 16).
The MICs of vancomycin are species related: the MICs for L. rhamnosus and L. paracasei subsp. paracasei were ≥256 μg/ml, whereas those for the L. acidophilus group were ≤2 μg/ml (Table 3). L. caseiT and L. rhamnosusT have cell wall peptigoglycan precursors that end in a depsipeptide d-alanine–d-lactate instead of the dipeptide d-alanine–d-alanine, the target for vancomycin activity (4, 18).
The activities of the oral antibiotics used for the treatment of urinary tract infections were assessed with lactobacilli. The high levels of resistance to fosfomycin, norfloxacin, ofloxacin, and ciprofloxacin have been described before (17, 20). L. rhamnosus had low-level resistance to pefloxacin and sparfloxacin and was susceptible to levofloxacin. These are the first tests of the in vitro susceptibility of L. rhamnosus to levofloxacin to be published: the low levofloxacin MICs for L. rhamnosus are in contrast to the high-level resistance of the L. acidophilus group (Table 3). Most studies of the antibiotic susceptibilities of lactobacilli do not report on those for the Lactobacillus species. Thus, our results might help in the determination of the antibiotic susceptibilities of the Lactobacillus species.
Sixty-five percent of our patients were on oral or parenteral glycopeptides (Table 1). In studies of oral glycopeptide treatment human volunteers had increased levels of fecal carriage of vancomycin-resistant enterococci and lactobacilli (37). The pathogenicity of L. rhamnosus selected by the use of glycopeptides may be due to many factors such as its exploitation of mucosal defects and its colonization properties. Seventy-five percent of our patients had digestive tract mucosal alterations as a consequence of various conditions: mucositis following antimitotic chemotherapy, digestive graft-versus-host disease after bone marrow transplantation, protracted diarrhea with AIDS, septic shock, or gut carcinoma (Table 1). In liver transplant patients, biliary anastomosis is an independent risk factor for lactobacillus bacteremia (31). Platelet aggregation and endothelial cell binding have been demonstrated with L. rhamnosus strains responsible for endocarditis (19).
The L. rhamnosus isolates from patients with clinical infections and milk products studied by Klein et al. (28) were unrelated according to their total soluble protein patterns. The comparison of L. rhamnosus isolates from patients with bacteremia and of the probiotic strain of L. rhamnosus GG performed by Saxelin et al. (33) showed various fermentation patterns. Our L. rhamnosus isolates belonged to various clusters according to their randomly amplified polymorphic DNA patterns (12).
Every lactobacillus isolate from immunocompromised patients with sepsis was identified to the species level according to its fermentation profile (Table 2). However, in a simultaneous study of normal vaginal isolates, fermentation profiles gave no identification for 40% of the isolates, and determination of the whole-cell protein pattern was necessary for their identification (data not shown).
The prevalence of the various Lactobacillus species is significantly different in immunocompromised patients with disseminated infections and the normal vaginal microflora (data not shown). H2O2 production and glycopeptide MICs are species related: H2O2 production and susceptibility to glycopeptides are common characteristics of the L. acidophilus group. These characteristics are not found in L. rhamnosus or L. paracasei subsp. paracasei. Identification of Lactobacillus species to the species level will help to elucidate the conditions for the emergence of infections by different species and will prompt study into species-related properties.
REFERENCES
- 1.Aguirre M, Collins M D. Lactic acid bacteria and human clinical infection. J Appl Bacteriol. 1993;75:95–107. doi: 10.1111/j.1365-2672.1993.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 2.Antony S J, Stratton C W, Dummer J S. Lactobacillus bacteremia: description of the clinical course in adult patients without endocarditis. Clin Infect Dis. 1996;23:773–778. doi: 10.1093/clinids/23.4.773. [DOI] [PubMed] [Google Scholar]
- 3.Bayer A S, Chow A W, Conception N, Guze L B. Susceptibility of 40 lactobacilli to six antimicrobial agents with broad gram-positive anaerobic spectra. Antimicrob Agents Chemother. 1978;14:720–722. doi: 10.1128/aac.14.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billot-Klein D, Gutmann L, Sable S, Guittet E, van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VanB type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococccus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994;176:2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomarat M, Espinouse D. Lactobacillus rhamnosus septicemia in patients with prolonged aplasia receiving ceftazidime-vancomycin. Eur J Clin Microbiol Infect Dis. 1991;10:44. doi: 10.1007/BF01967099. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 6.Collins M D, Rodriguez U, Ash C, Aguirre M, Farrow J A E, Martinez-Murcia A, Williams A M, Wallbanks S. Phylogenic analysis of the genus Lactobacillus and related lactic acid bacteria as determined by reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol Lett. 1991;77:5–12. [Google Scholar]
- 7.Collins M D, Phillips B A, Zanoni P. Deoxyribonucleic acid homology studies of Lactobacillus casei, Lactobacillus paracasei sp. nov., subsp. paracasei and subsp. tolerans, and Lactobacillus rhamnosus sp. nov. Int J Syst Bacteriol. 1989;39:105–108. [Google Scholar]
- 8.Croco J L, Erwin M E, Jennings J M, Putnam L R, Jones R N. Evaluation of the E-test for antimicrobial spectrum and potency determinations of anaerobes associated with bacterial vaginosis and peritonitis. Diagn Microbiol Infect Dis. 1994;20:213–219. doi: 10.1016/0732-8893(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Dellaglio F, Dicks L M T, du Toit M, Torriani S. Designation of ATCC 334 in place of ATCC 393 (NCDO 161) as the neotype strain of Lactobacillus casei subsp. casei and rejection of the name Lactobacillus paracasei (Collins et al., 1989): request for an opinion. Int J Syst Bacteriol. 1991;41:340–342. [Google Scholar]
- 10.Dicks L M T, Du Plessis E M, Dellaglio F, Lauer E. Reclassification of Lactobacillus casei subsp. casei ATCC 393 and Lactobacillus rhamnosus ATCC 15820 as Lactobacillus zeae nom. rev., designation of ATCC 334 as the neotype of L. casei subsp casei, and rejection of the name Lactobacillus paracasei. Int J Syst Bacteriol. 1996;46:337–340. doi: 10.1099/00207713-46-1-337. [DOI] [PubMed] [Google Scholar]
- 11.Eschenbach D A, Davick P R, Williams B L, Kleibanoff S J, Young-Smith K, Critchlow C M, Holmes K K. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felten A, Barreau C, Taillez P, Bizet C, Philippon A. Programm and abstracts of the 21st International Congress on Microbial Ecology and Disease. Paris, France: Institut Pasteur; 1996. Phenotypic and genotypic relationship between Lactobacillus rhamnosus clinical isolates from immunocompromised patients, abstr. 84; p. 60. [Google Scholar]
- 13.Fruchart C, Salah A, Gray C, Martin E, Stamatoullas A, Bonmarchand G, Lemeland J F, Tilly H. Lactobacillus species as emerging pathogens in neutropenic patients. Eur J Clin Microbiol Infect Dis. 1997;16:681–684. doi: 10.1007/BF01708560. [DOI] [PubMed] [Google Scholar]
- 14.Fujisawa T, Benno Y, Yaeshima T, Mitsuoka T. Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov., and synonymy of Lactobacillus acidophilus group A3 (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981) Int J Syst Bacteriol. 1992;42:487–491. doi: 10.1099/00207713-42-3-487. [DOI] [PubMed] [Google Scholar]
- 15.Gasser F. Safety of lactic acid bacteria and their occurrence in human clinical infections. Bull Inst Pasteur. 1994;92:45–67. [Google Scholar]
- 16.Griffiths J K, Daly J S, Dodge R A. Two cases of endocarditis due to Lactobacillus species: antimicrobial susceptibility, review, and discussion of therapy. Clin Infect Dis. 1992;15:250–255. doi: 10.1093/clinids/15.2.250. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton-Miller J M T, Shah S. Susceptibility patterns of vaginal lactobacilli to eleven oral antibiotics. J Antimicrob Chemother. 1994;33:1059–1060. doi: 10.1093/jac/33.5.1059. [DOI] [PubMed] [Google Scholar]
- 18.Handwerger S, Pucci M J, Volk K J, Liu J, Lee M S. Vancomycin resistant Leuconostoc mesenteroides and Lactobacillus casei synthesize cytoplasmic peptidoglycan precursors that terminate in lactate. J Bacteriol. 1994;176:260–264. doi: 10.1128/jb.176.1.260-264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harty D W S, Oakey H J, Patrikakis M, Hume E B H, Knox K W. Pathogenic potential of lactobacilli. Int J Food Microbiol. 1994;24:179–189. doi: 10.1016/0168-1605(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 20.Herra C M, Cafferkey M T, Keane C T. The in-vitro susceptibilities of vaginal lactobacilli to four broad spectrum antibiotics, as determined by the agar dilution and E-test methods. J Antimicrob Chemother. 1995;35:775–783. doi: 10.1093/jac/35.6.775. [DOI] [PubMed] [Google Scholar]
- 21.Holliman R E, Bone G P. Vancomycin resistance of clinical isolates of lactobacilli. J Infect. 1988;16:279–283. doi: 10.1016/s0163-4453(88)97676-1. [DOI] [PubMed] [Google Scholar]
- 22.Horwitch C A, Furseth H A, Larson A M, Jones T J, Olliffe J F, Spach D H. Lactobacillemia in three patients with AIDS. Clin Infect Dis. 1995;21:1460–1462. doi: 10.1093/clinids/21.6.1460. [DOI] [PubMed] [Google Scholar]
- 23.Husni R N, Gordon S M, Washington J A, Longworth D L. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis. 1997;25:1048–1055. doi: 10.1086/516109. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J L, Phelps C F, Cummins C S, London J L, Gasser F. Taxonomy of the Lactobacillus acidophilus group. Int J Syst Bacteriol. 1980;30:53–68. [Google Scholar]
- 25.Kalima P, Masterton R G, Roddie P H, Thomas A E. Lactobacillus rhamnosus infection in a child following bone marrow transplant. J Infect. 1996;32:165–167. doi: 10.1016/s0163-4453(96)91622-9. [DOI] [PubMed] [Google Scholar]
- 26.Kandler O, Weiss N. Bergey’s manual of systematic bacteriology. 8th ed. Baltimore, Md: The Williams & Wilkins Co.; 1986. Regular, nonsporing gram-positive rods; pp. 1208–1234. [Google Scholar]
- 27.Kersters K, De Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975;87:333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- 28.Klein G, Hack B, Hanstein S, Zimmermann K, Reuter G. Intraspecies characterization of clinical isolates and biotechnologically used strains of Lactobacillus rhamnosus by analysis of the total soluble cytoplasmic proteins with silver staining. Int J Food Microbiol. 1995;25:263–275. doi: 10.1016/0168-1605(94)00141-r. [DOI] [PubMed] [Google Scholar]
- 29.Molin G, Jeppsson B, Johansson M L, Ahrné S, Nobaek S, Stahl M, Bengmark S. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol. 1993;74:314–323. doi: 10.1111/j.1365-2672.1993.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 30.Mori K, Yamazaki K, Ishiyama T, Katsumata M, Kobayashi K, Kawai Y, Inoue N, Shinano H. Comparative sequence analyses of the genes coding for 16S rRNA of Lactobacillus casei related taxa. Int J Syst Bacteriol. 1997;47:54–57. doi: 10.1099/00207713-47-1-54. [DOI] [PubMed] [Google Scholar]
- 31.Patel R, Cockerill F R, Porayko M K, Osmon D R, Ilstrup D M, Keating M R. Lactobacillemia in liver transplant patients. Clin Infect Dis. 1994;18:207–212. doi: 10.1093/clinids/18.2.207. [DOI] [PubMed] [Google Scholar]
- 32.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer K H. Identification and classification of Lactobacillus acidophilus, L. gasseri and L. johnsonii strains by SDS-PAGE and rRNA targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–517. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 33.Saxelin M, Chuang N H, Chassy B, Rautelin H, Makela P H, Salminen S, Gorbach S L. Lactobacilli and bacteremia in southern Finland, 1989–1992. Clin Infect Dis. 1996;22:564–566. doi: 10.1093/clinids/22.3.564. [DOI] [PubMed] [Google Scholar]
- 34.Shleifer K H, Kandler O. Phylogenetic relationships of lactic bacteria. In: Wood B J B, Holzabfel W H, editors. The genera of lactic acid bacteria. 2. The lactic acid bacteria. Glasgow, Scotland: Blackie Academic & Professional Publishers; 1995. pp. 7–18. [Google Scholar]
- 34a.Société Française de Microbiologie, Comité de l’Antibiogramme. Communiqué 1998. Pathol. Biol. 46:I-XVI.
- 35.Swenson J M, Facklam R R, Thornsberry C. Anntimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species. Antimicrob Agents Chemother. 1990;34:543–547. doi: 10.1128/aac.34.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandamme P, Pot B, Gillis M, De Vos P, Kersters K, Swings J. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438. doi: 10.1128/mr.60.2.407-438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van der Auwera P, Pensart N, Korten V, Murray B E, Leclercq R. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide resistant enterococci. J Infect Dis. 1996;173:1129–1136. doi: 10.1093/infdis/173.5.1129. [DOI] [PubMed] [Google Scholar]