Abstract
Sleep disturbances (SDs) are among the most distressing and commonly reported symptoms in posttraumatic stress disorder (PTSD). Despite increased attention on sleep in clinical PTSD research, SDs remain difficult to treat. In Phase 2 trials, 3,4‐methylenedioxymethamphetamine (MDMA)–assisted psychotherapy has been shown to greatly improve PTSD symptoms. We hypothesized that MDMA‐assisted psychotherapy would improve self‐reported sleep quality (SQ) in individuals with PTSD and be associated with declining PTSD symptoms. Participants in four studies (n = 63) were randomized to receive 2–3 sessions of active MDMA (75–125 mg; n = 47) or placebo/control MDMA (0–40 mg, n = 16) during all‐day psychotherapy sessions. The PSQI was used to assess change in SQ from baseline to the primary endpoint, 1–2 months after the blinded sessions. Additionally, PSQI scores were measured at treatment exit (TE) and 12‐month follow‐up. Symptoms of PTSD were measured using the CAPS‐IV. At the primary endpoint, CAPS‐IV total severity scores dropped more after active MDMA than after placebo/control (−34.0 vs. −12.4), p = .003. Participants in the active dose group showed more improvement in SQ compared to those in the control group (PSQI total score ΔM = −3.5 vs. 0.6), p = .003. Compared to baseline, SQ had improved at TE, p < .001, with further significant gains reported at 12‐month follow‐up (TE to 12‐months ΔM = −1.0), p = .030. Data from these randomized controlled double‐blind studies provide evidence for the beneficial effects of MDMA‐assisted psychotherapy in treating SDs in individuals with PTSD.
Sleep Quality Improvements After MDMA‐Assisted Psychotherapy for the Treatment of Posttraumatic Stress Disorder
The associations between sleep disturbance and posttraumatic stress disorder (PTSD) are complex and multidirectional, with evidence supporting a causal association between traumatic stress and sleep disturbance (Harvey et al., 2003). Adding to this complexity, sleep disturbance is not a unitary construct, and different aspects of sleep disturbance are likely related in different ways to the risk, pathophysiology, maintenance of, and recovery from, PTSD (Richards et al., 2020). Trauma‐exposed individuals report disturbed sleep at increased rates compared to the general population, including difficulty initiating and maintaining sleep, atypical sleep‐disruptive behaviors, recurrent nightmares, and frequent awakenings, and sleep disturbance can be seen as a core feature of PTSD (Spoormaker & Montgomery, 2008). Sleep disturbances may precipitate and perpetuate PTSD symptoms, creating a vicious cycle (Germain et al., 2017; van Liempt, 2012). Disturbed sleep due to nightmares increases the risk for PTSD, and PTSD, in turn, leads to increased sleep fragmentation, frequent nightmares, and decreased growth hormone secretion, which may then compromise fear extinction, synaptic plasticity, and degree of recovery (van Liempt et al., 2013; van Liempt et al., 2011).
Among mental health disorders, PTSD is unique in that sleep problems represent two of the diagnostic criteria listed in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5; American Psychiatric Association, 2013). Recurrent nightmares are part of the intrusion symptom cluster, and insomnia is a component of the cluster of symptoms categorized as alterations in arousal and reactivity. Both subjective reports and objective data on sleep quality and continuity suggest that most individuals with PTSD also suffer from at least one type of sleep problem (Germain, 2013; Krakow, Germain, et al., 2001; Krakow et al., 2004). In addition, PTSD patients who report significant sleep disturbance also report higher levels of substance use, more severe health‐related complaints, depression, and suicidality (Clum et al., 2001; Krakow et al., 2000; Nishith et al., 2001; Saladin et al., 1995; Vermetten et al., 2018).
Improvements in sleep quality have been associated with clinically relevant reductions in the severity and impact of PTSD symptoms (Germain, 2013), highlighting the importance of specifically targeting sleep as part of a multifaceted approach to PTSD treatment. Primary therapies that specifically target PTSD, such as cognitive behavioral therapy (CBT) for PTSD, are often insufficient to completely improve sleep disturbances despite reductions in other PTSD‐attributable symptoms. Concomitant sleep problems can affect the efficacy of first‐line treatments and have been shown to negatively impact psychiatric treatment resistance (Germain, 2013). Currently, CBT for insomnia (CBT‐I) is the only recommended psychotherapy intervention for sleep disturbances among PTSD‐diagnosed veterans; however, previous research has shown that up to 40% of research participants drop out of CBT‐I treatment (Miller et al., 2019), and El‐Solh et al. (2019) reported that among individuals who complete treatment, fewer than half experience clinical improvement in insomnia at 6‐month follow‐up.
Among the available pharmacological approaches to sleep disturbance, prazosin is thought to attenuate central arousal during sleep. Despite conflicting results, this pharmacological approach is still being recommended (Germain et al., 2017; Zhang et al., 2020), as is doxazosin and terazosin (Pallesen et al., 2020; Smith & Koola, 2016). Targeting the endocannabinoid system also has yielded promising results (Jetly et al., 2015; Krediet et al., 2020). However, evidence of the underutilization of (Seal et al., 2010) and high attrition rates for (DeViva, 2014) standard‐of‐care PTSD treatment, coupled with findings that SD often persists following trauma‐focused treatment, demonstrate that there is a need for novel interventions that can target both sleep disturbance and PTSD (Germain et al., 2017).
Randomized, placebo‐controlled trials investigating the efficacy of 3,4‐methylenedioxymethamphetamine (MDMA)–assisted psychotherapy for individuals with chronic, treatment‐resistant PTSD have demonstrated statistically significant improvements in PTSD symptoms as measured by standard symptom scales (Mithoefer et al., 2013, 2011, 2018, 2019; Oehen et al., 2013; Ot'alora et al., 2018), including the Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV; Weathers et al., 2001). The pharmacological profile of MDMA is unique and distinct from psychostimulants (Bershad et al., 2016), and it has been reported to decrease feelings of fear while maintaining a clear‐headed, alert state of consciousness (Greer & Tolbert, 1998). The effects of MDMA typically last 3–6 hr and are characterized by feelings of euphoria, increased well‐being, and sociability, along with slight changes in perception and anxiety (Feduccia et al., 2018; Kamilar‐Britt & Bedi, 2015; Kirkpatrick et al., 2014; Liechti et al., 2001).
Long‐term follow‐up data collected an average of 45 months after the completion of the first MDMA‐assisted psychotherapy trial for PTSD showed that participants, on average, maintained statistically and clinically significant gains in PTSD symptom relief (Mithoefer et al., 2011, 2013). The study authors found that 63% of participants reported improved sleep at long‐term follow‐up, as measured using a single item on the CAPS‐IV. Subsequent Phase 2 studies replicated the promising safety and efficacy findings for MDMA‐assisted psychotherapy in individuals with PTSD (Mithoefer et al., 2018, 2019; Ot'alora et al., 2018). In four of six Phase 2 randomized, placebo‐controlled trials investigating the safety and efficacy of MDMA‐assisted psychotherapy for the treatment of PTSD, investigators included the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989) as a secondary outcome measure to more fully elucidate the changes in sleep observed in participants in the first study (Mithoefer et al., 2011).
For the present study, we conducted secondary analyses using pooled data collected in the four previously discussed randomized, placebo‐controlled trials to explore sleep quality and the associations between sleep quality and PTSD symptom severity. We hypothesized that reductions in PSQI scores at primary endpoint, 1 to 2 sessions after blinded sessions, would be correlated with reductions in CAPS‐IV total scores such that improvements in sleep quality would be associated with overall posttreatment PTSD symptom reductions. Similarly, we sought to understand whether a clinically meaningful (i.e., decrease of 3 points or more) drop in PSQI scores at primary endpoint (TE) would be associated with larger reductions in CAPS‐IV scores at TE and 12‐month follow‐up.
Method
Participants and Procedure
Four randomized, double‐blind Phase 2 studies with similar study designs took place at four sites. The sites were located in the United States (MP‐8, MP‐12), Canada (MP‐4), and Israel (MP‐9). Three sites were private practices, and one site was a government‐funded psychiatric hospital. Data were collected from January 2012 to March 2017. Studies were approved by the Western‐Copernicus Institutional Review Board (Research Triangle Park or Cary, NC; (MP‐8, MP‐12), IRB Services/Chesapeake, Aurora ON (MP‐4), and the Helsinki Committee of Beer Yaakov Hospital (MP‐9). The first two Phase 2 trials investigating MDMA‐assisted psychotherapy for PTSD did not include the PSQI; data from these studies were not included herein. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Participants were recruited through referrals by health professionals, Internet advertisements, and by word of mouth. Studies enrolled civilians, veterans, and first responders aged 18 and older with previous inadequate response to at least one pharmacotherapy and/or psychotherapy. The CONSORT flow diagram for studies is presented in Figure 1. The studies restricted enrollment to individuals with PTSD symptoms that had lasted at least 6 months and a CAPS‐IV total severity score of 50 or higher for MP‐8, MP‐12, MP‐9, and 60 or higher for MP‐4 (Weathers, 2004). Volunteers with anxiety disorders and/or depression were enrolled but excluded if they reported past or current psychotic disorders, bipolar disorder I, borderline personality disorder, eating disorders with active purging, or substance abuse disorder within 60 days (MP‐8, MP‐12, MP‐9) or 6 months (MP‐4) of screening. Major medical conditions except for controlled hypertension or adequately treated hypothyroidism were excluded. In addition, pregnant or lactating women, women who were not using effective contraception, and individuals who weighed under 48 kg were excluded.
Figure 1.
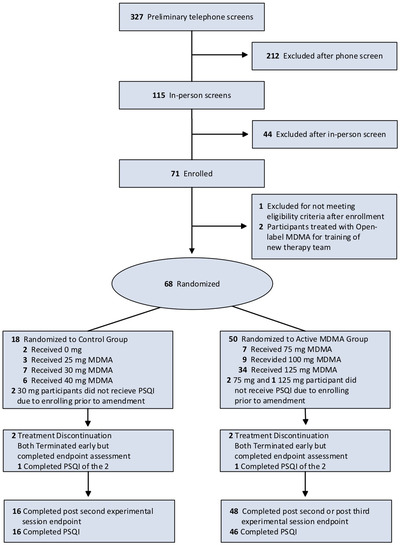
CONSORT Flow Diagram
All psychiatric medications were tapered and discontinued prior to participation, with the exception of anxiolytics and sedative‐hypnotics used as needed between experimental sessions, gabapentin for pain management, and attention‐deficit/hyperactivity disorder medications, which were halted on the day of an experimental session but could be resumed 10 days after each experimental session. All participants confirmed comprehension of study procedures and provided written informed consent.
Participants in randomized, controlled, double‐blind studies underwent 8‐hr blinded sessions with either active‐dose MDMA (i.e., 75 mg, 100 mg, or 125 mg initial dose, followed by an optional supplemental half dose) or a control dose (0 mg, 25 mg, 30 mg, or 40 mg MDMA, followed by an optional supplemental half‐dose; (Mithoefer et al., 2018, 2019; Ot'alora et al., 2018b). Therapists performed manualized psychotherapy (Mithoefer, 2017) throughout the study. Prior to the experimental sessions, participants met with a co‐therapy team during three 90‐min preparatory therapy sessions without drug administration. Subsequently, there were two blinded experimental sessions, spaced 3–5 weeks apart, each followed by three 90‐min integrative nondrug sessions. The MDMA was synthesized by David Nichols at Purdue University (MP‐8, MP‐12), or Lipomed A.G. in Arlesheim, Switzerland (MP‐4, MP‐9), and encapsulated with lactose to create equivalent‐weight gelatin capsules across dose groups.
Therapy occurred in a designated space with a sofa or futon and aesthetically pleasing surroundings. The approach was largely nondirective, wherein each participant was encouraged to spend periods of time focusing attention on their inner experience, using eyeshades and headphones if they were comfortable doing so, alternating with periods of time interacting with the co‐therapy team to process emotions and memories that arose. Participants remained at the study site overnight and were supported by a trained night attendant until an integration session with the therapists the following day.
Participants and investigators were unblinded to group assignment after completing the primary endpoint, 1–2 months after the second blinded experimental session. Full‐dose participants (i.e., 100 mg, 125 mg) had one additional open‐label session except those enrolled in MP‐9, which did not include a third session. Participants assigned to receive 0–75 mg MDMA crossed over to receive two (MP‐9) or three (MP‐4, MP‐8, MP‐12) open‐label MDMA sessions (i.e., 100–125 mg) in the open‐label crossover segments. Participants completed the CAPS‐IV and PSQI at baseline, the primary endpoint, at TE (i.e., 2 months after the third active MDMA session for MP4, MP8, MP12, and 2 months after two active MDMA sessions for MP‐9), and at a 12‐month follow‐up assessment. Because the PSQI was added as a study amendment in MP‐8, only 76.9% (20 of 26) of participants completed the PSQI. Among the remaining participants from the other three studies, all but one participant completed the measure at least once after baseline. Details of the study methods and secondary measures can be found elsewhere (Mithoefer et al., 2018, 2019; Ot'alora, et al., 2018).
Measures
Sleep Quality
The 19‐item PSQI was used to assess participants’ sleep quality. The PSQI is a self‐report questionnaire used to assess past‐month disturbances related to seven clinically relevant components of sleep quality. Each component is rated on a scale of 0 (not in the past month) to 3 (3 or more times in a week), with a composite score derived from the frequencies of each type of disturbance (range: 0–21). A PSQI global score is then calculated as the sum of these seven component scores. A cutoff score of 5 has been shown to discriminate between good and poor sleepers. The PSQI has demonstrated high internal consistency, (Cronbach's α = .83) good test–retest reliability (.85–.87; (Backhaus et al., 2002), and a diagnostic sensitivity and specificity of 89.6% and 86.5%, respectively (κ = .75, p < .001) in distinguishing good and poor sleepers (Buysse et al., 1989). In the present sample, Cronbach's alpha for the PSQI was .63 at baseline, .71 at the primary endpoint, .69 at treatment exit, and .80 at long‐term follow‐up.
Although a PSQI global score of 5 or higher has been associated with clinically significant sleep disturbances in civilians, mean PSQI global scores in samples of civilians and military veterans with PTSD can range from 10 to 15 (Germain et al., 2004; Krakow, Hollifield, et al., 2001; Krakow, Melendrez, et al., 2001; Mellman et al., 1995; Ulmer et al., 2011). For this reason, we chose to adopt the clinically meaningful difference utilized by the authors of the instrument, who have conducted sleep‐focused PTSD trials and determined that a 3‐point drop represents a clinically meaningful difference (Buysse et al., 2011; Germain et al., 2006; Vermetten et al., 2018).
PTSD Symptoms
The CAPS‐IV is a 23‐item, semistructured interview that is used to assess the frequency and intensity of individual DSM‐IV PTSD symptoms on separate 5‐point (i.e., 0–4) Likert scales that can be summed to create a 9‐point (range: 0–8) severity score for each symptom. Total symptom severity scores range from 0 to 136, with higher values signifying higher levels of symptom severity. The CAPS‐IV assigns diagnosis as being present or absent. Test–retest reliabilities have been reported to range from .77 to .96 for the three symptom clusters and from .90 to .98 for the 17‐item core symptom scale (Blake et al., 1995). The CAPS‐IV has demonstrated high internal consistency, with alpha values for the three symptom clusters ranging from .85 to .87 and an alpha value of .94 reported for the total score (Blake et al., 1995). In all four studies, the CAPS‐IV was assessed by an independent rater who was not present during any of the psychotherapy sessions.
Data Analysis
Data from the four studies were pooled into two treatment groups (control: 0–40 mg; active: 75–125 mg) for a modified intent‐to‐treat set that included data from all participants who completed at least one blinded experimental session and at least one follow‐up assessment. One participant completed the CAPS‐IV at the primary endpoint but did not complete the PSQI at baseline and was therefore omitted from the analyses. Data aggregation was based on the designation of doses as control or active within individual study protocols and by inspection of posttreatment CAPS‐IV scores, which indicated grouping individual doses (Mithoefer et al., 2019). For the primary analyses, we conducted independent samples t tests, with an alpha value of 0.05 (two‐tailed), to evaluate the change in CAPS‐IV total scores and PSQI total scores from baseline to the primary endpoint for treatment groups. Between‐group effect sizes were calculated with Hedges’ g. We analyzed PSQI subscale scores in a similar manner. Because the pilot studies were not powered to detect statistical significance, no power analyses were performed prior to the individual Phase 2 trials or for this exploratory analysis.
As participants from both groups had received active doses of MDMA in blinded or open‐label stages, a mixed‐effect repeated model (MMRM) was performed. We utilized an unstructured variance/covariance structure to impose as few assumptions on the data as possible. Restricted maximum likelihood was used for the estimation of the parameters. Outcome scores from all participants were aggregated at three time points and analyzed via MMRM. The base model included time (baseline, TE, 12‐month follow‐up), baseline CAPS‐IV score, and study as fixed effects, and participant was specified as a random effect. A test for an interaction of dose versus visit failed to reject the null hypothesis. To explore whether a clinically significant drop of 3 points or more on the PSQI at the primary endpoint impacted CAPS‐IV scores at TE and 12‐month follow‐up, we performed a subgroup analysis. In an MMRM, the base model included PSQI group (3‐point or more drop or drop of 3 points or less), time (baseline, TE, 12‐month follow‐up), baseline CAPS‐IV scores, and study as fixed effects, and participant was entered as a random effect. To assess the associations among age, PTSD duration, sex, race, and prestudy self‐reported “ecstasy” use (i.e., substances assumed to contain MDMA), these covariates were added to the base model one by one. The base model included a fixed effect for the visit, and each candidate predictor was fitted in the model one by one. A prespecified cutoff of p ≤ .01 was used to choose candidate predictors to be included in a full model. Among the candidate predictors, age and race met the cutoff and were added to the full model. In the full model, race did not meet the prespecified criteria of p < .05. The Akaike information criterion calculated to assess model fit showed a 22.2‐point drop from the base model to the model that included age and baseline PSQI score. Pearson's correlations (two‐tailed) were calculated to assess the association between change in CAPS‐IV and PSQI scores from baseline to TE. A chi‐square test was performed to test the associations among categorical variables between groups. All analyses were performed using SPSS (Version 20), and SAS (Version 9.3) was employed for the MMRM.
Results
Sample Characteristics
Table 1 displays the demographic and baseline characteristics of the sample. Demographic characteristics were approximately the same between treatment groups. The mean participant age was 40.80 years (SD = 11.49), and the sample included 34 male (54.0%) and 29 female participants (46.0%). The sample was largely White/Caucasian 85.7%, with other races and ethnicities including Latino/Hispanic (3.2%), Native American (3.2%), Middle Eastern (1.6%), and other/biracial (6.4%). The mean duration of PTSD was 225.30 months (SD = 208.66), with various trauma types reported. The most commonly experienced traumatic event was combat (71.4%), followed by sexual assault (46.0%), child abuse (30.2%), accidents (15.9%), and “other” (69.8%). Many participants had a lifetime history of suicidal ideation (87.3%) and/or behavior (30.2%). Regarding prior ecstasy use, 28.6% of participants reported trying ecstasy one or more times prior to enrolling in the study.
Table 1.
Demographic and Baseline Characteristics
| Control (n = 16) | Active MDMA (n = 47) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | % | M | SD | n | % | M | SD |
| Age (years) | 40.31 | 10.24 | 40.97 | 11.99 | ||||
| Sex | ||||||||
| Male | 10 | 62.5 | 24 | 51.1 | ||||
| Female | 6 | 37.5 | 23 | 48.9 | ||||
| Race or ethnicity | ||||||||
| White/Caucasian | 14 | 87.5 | 40 | 85.1 | ||||
| Latino/Hispanic | 0 | 0 | 2 | 4.3 | ||||
| Native American | 1 | 6.3 | 1 | 2.1 | ||||
| Middle Eastern | 1 | 6.3 | 0 | 0 | ||||
| Other/Biracial | 0 | 0 | 4 | 8.5 | ||||
| Trauma type | ||||||||
| Combat | 16 | 100.0 | 29 | 61.7 | ||||
| Sexual assault | 6 | 37.5 | 23 | 48.9 | ||||
| Child abuse | 4 | 25.0 | 15 | 31.9 | ||||
| Accident | 3 | 18.8 | 7 | 14.9 | ||||
| Other | 4 | 25.0 | 40 | 85.1 | ||||
| PTSD duration (months) | 154.88 | 136.11 | 249.28 | 224.34 | ||||
| Prior ecstasy use | ||||||||
| Yes | 3 | 18.8 | 15 | 31.9 | ||||
| No | 13 | 81.3 | 32 | 68.1 | ||||
| Prestudy sedatives and hypnoticsa | ||||||||
| Diphenhydramine | 0 | 0 | 3 | 6.4 | ||||
| Eszopiclone | 0 | 0 | 5 | 10.6 | ||||
| Melatonin | 1 | 6.3 | 3 | 6.4 | ||||
| Incarnata flower | 0 | 0 | 1 | 2.1 | ||||
| Temazepam | 0 | 0 | 1 | 2.1 | ||||
| Zolpidem | 1 | 6.3 | 8 | 17.0 | ||||
| Zolpidem tartrate | 1 | 6.3 | 5 | 10.6 | ||||
| Zopiclone | 2 | 12.5 | 1 | 2.1 | ||||
| Lifetime C‐SSRSb | ||||||||
| Positive Ideation | 12 | 75.0 | 43 | 91.5 | ||||
| Serious Ideation | 3 | 18.8 | 20 | 42.6 | ||||
| Positive Behavior | 5 | 31.3 | 14 | 29.8 | ||||
Note. PTSD = posttraumatic stress disorder; C‐SSRS = Columbia Suicide Severity Rating Scale. aWorld Health Organization Drug Dictionary ACT3 term (i.e., Sedatives and Hypnotics) and preferred drug name. bLifetime accounts for all suicidal ideation and behavior prior to the study, according to participant recall and medical records. According to the C‐SSRS scoring guide, scores of 4–5 on items related to suicidal ideation are considered serious ideation, and scores of 1–3 are considered positive behavior or ideation.
Blinded Endpoint
At the primary endpoint (Table 2), there was a significant treatment effect on change in CAPS‐IV total scores (active group: M = −34.0, SD = 26.46; control group: M = −12.4, SD = 16.38), p = .003, and PSQI scores (active group: M = −3.53, SD = 5.03; control group: M = 0.56, SD = 3.05), p = .003, indicating that active doses of MDMA improved outcomes (Figure 2). More participants in the active group (53.2%), χ2(df, 1, N = 61) = 8.87, p = .003, reported a PSQI score drop of 3 points or more than in the control group (12.5%). It is worth noting that sleep‐specific items from the CAPS‐IV were not removed for the analyses; removal may have provided a more accurate assessment of the relation between changes in CAPS‐IV and PSQI scores.
Table 2.
Blinded Outcome Measures at Primary Endpoint
| Control (n = 16) | Active MDMA (n = 46)a | ||||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | p | Hedges’ g | 95% CI |
| CAPS‐IV total score | |||||||
| Baseline | 86.13 | 10.39 | 89.57 | 17.94 | |||
| Primary endpoint | 73.75 | 21.16 | 54.76 | 30.95 | |||
| Change | −12.38 | 16.38 | −33.98 | 26.46 | .003 | 0.88 | [−5.18, 6.93] |
| PSQI total scoreb | |||||||
| Baseline | 12.00 | 3.83 | 12.91 | 4.17 | |||
| Primary endpoint | 12.56 | 3.85 | 9.15 | 4.39 | |||
| Change | 0.56 | 3.05 | −3.53 | 5.03 | .003 | 0.88 | [−0.27, 2.03] |
| PSQI subscale scores | |||||||
| Sleep Quality | |||||||
| Baseline | 1.75 | 0.78 | 1.98 | 0.88 | |||
| Primary endpoint | 1.94 | 0.93 | 1.35 | 0.90 | |||
| Change | 0.19 | 0.91 | −0.62 | 0.98 | .006 | 0.82 | [0.58, 1.06] |
| Sleep Latency | |||||||
| Baseline | 1.87 | 0.89 | 2.07 | 1.06 | |||
| Primary endpoint | 2.25 | 0.86 | 1.61 | 1.20 | |||
| Change | 0.38 | 0.72 | −0.42 | 1.01 | .005 | 0.84 | [0.60, 1.07] |
| Duration of Sleep | |||||||
| Baseline | 1.63 | 1.15 | 1.41 | 1.26 | |||
| Primary endpoint | 1.56 | 1.26 | 0.87 | 1.17 | |||
| Change | −0.06 | 0.85 | −0.49 | 1.39 | .256 | .31 | [−0.01, 0.63] |
| Sleep Efficiency | |||||||
| Baseline | 1.25 | 1.29 | 1.61 | 1.24 | |||
| Primary endpoint | 1.06 | 1.24 | 0.87 | 1.09 | |||
| Change | −0.19 | 1.05 | −0.69 | 1.38 | .191 | 0.38 | [0.05, 0.70] |
| Sleep Disturbance | |||||||
| Baseline | 2.06 | 0.57 | 2.04 | 0.60 | |||
| Primary endpoint | 2.13 | 0.50 | 1.80 | 0.78 | |||
| Change | 0.06 | 0.57 | −0.22 | 0.74 | .124 | 0.42 | [0.25, 0.60] |
| Use of Sleep Medication | |||||||
| Baseline | 1.63 | 1.31 | 1.72 | 1.38 | |||
| Primary endpoint | 1.50 | 1.37 | 1.41 | 1.34 | |||
| Change | −0.13 | 1.26 | −0.24 | 1.48 | .775 | 0.07 | [−0.29, 0.43] |
| Daytime Dysfunction | |||||||
| Baseline | 1.81 | 0.75 | 2.07 | 0.65 | |||
| Primary endpoint | 2.06 | 0.77 | 1.24 | 0.67 | |||
| Change | 0.25 | 0.93 | −0.82 | 0.89 | < .001 | 1.21 | [0.98, 1.43] |
Note. MDMA = 3,4‐methylenedioxymethamphetamine; CAPS‐IV = Clinician‐Administered PTSD Scale for DSM‐IV; PSQI = Pittsburgh Sleep Quality Index. aActive MDMA at primary endpoint for PSQI (n = 45) and CAPS (n = 46). bFrom baseline to endpoint, total PSQI scores dropped by 3 points or more for two participants in the control group (12.5%) and 25 participants in the active MDMA group (53.2%); scores did not drop by 3 points or more for 14 participants in the control group (87.5%) and 20 in the active MDMA group (42.6%), p = .003.
Figure 2.
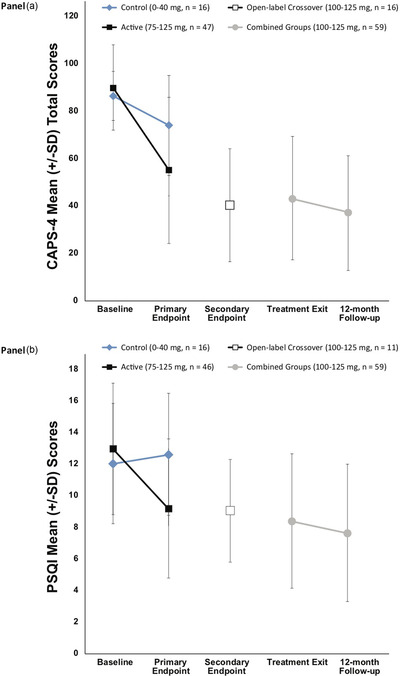
Change over time (A) Pittsburgh Sleep Quality Index (PSQI) and (B) Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV) Total Scores
Note. The primary endpoint occurred 1 month after the second blinded 3,4‐methylenedioxymethamphetamine (MDMA)/placebo session. The blind was broken after the primary endpoint. The active dose groups (100–125 mg) had one additional open‐label MDMA session and completed an assessment 2 months after the third session (i.e., TE). The comparator group (0–40 mg) and the 75–mg group crossed over to receive three open‐label (100–125 mg) sessions, with an assessment 1 month after the second open‐label MDMA session (i.e., secondary endpoint) and again 2 months after the third open‐label MDMA session (i.e., TE). The 12‐month follow‐up visit occurred after the final open‐label MDMA session. Groups were pooled for TE and 12‐month follow‐up endpoints, as all participants had received active doses of MDMA in either the blinded or open‐label crossover segments. PTSD = posttraumatic stress disorder.
Regarding PSQI subscale scores, significant differences between groups were detected at the primary endpoint for three of seven subscales (Table 2). Improvements in Sleep Quality subscale scores (e.g., “During the past month, how would you rate your sleep quality overall?”) were significantly greater in the active group compared to the control group, with a mean score drop of −0.62 (SD = 0.98) among individuals in the active group, reflecting improved sleep quality, compared to a mean increase of 0.19 (SD = 0.91) in the control group, reflecting worsened sleep quality, p = .006. Individuals in the active‐dose group also saw more improvement in sleep latency (i.e., requiring less time to fall asleep), with a mean score change of −0.42 (SD = 1.01) among active participants compared to a mean increase of 0.38 (SD = 0.72) among control participants, p = .005. Finally, participants in the active‐dose group reported less daytime dysfunction (i.e., difficulty staying awake and maintaining adequate enthusiasm to carry out the day‐to‐day activity) at the primary endpoint, reporting a mean score change of −0.82 (SD = 0.89) compared to a mean increase of 0.25 (SD = 0.93) among control participants (i.e., a worsening of daytime dysfunction), p < .001.
On the night of Experimental Session 1, no participants in the control group received a sleep aid (see Supplementary Table S1). For the active‐dose group (n = 46), on the night of Experimental Session 1, three participants (6.5%) received zolpidem, two received melatonin (4.3%), and one received zopiclone (2.2%); on the night of Experimental Session 2, four received zolpidem (8.7%), and one received melatonin (2.2%).
Open‐Label Endpoints: Treatment Exit and Long‐Term Follow‐Up
Because all participants received active doses of MDMA at some point during the study in either blinded or open‐label sessions, the control and active‐dose groups were combined for analyses of baseline, TE, and 12‐month follow‐up outcome analyses (Table 3). In the MMRM analysis, CAPS‐IV total scores significantly decreased from baseline to TE, p < .001, and baseline to 12‐month follow‐up, p < .001, with scores continuing to decline at 12‐month follow‐up compared to TE, p = .011. We found similar results for the PSQI, with scores significantly decreasing from baseline to TE, p < .001, and baseline to 12‐month follow‐up, p < .001. Scores continued to decline at 12‐month follow‐up compared to TE, p = .030. Age was the only significant covariate, p = .017, with participants under 30 years of age demonstrating a higher degree of sleep improvement compared to those older than 30. Changes in CAPS‐IV and PSQI scores from baseline to TE (n = 58; see Figure 3) were significantly correlated, r = .371, p = .004.
Table 3.
Within‐Subject Outcome Measures at Treatment Exit (TE) and 12‐Month Follow‐up
| Variable | LS M | SE | p | n | % |
|---|---|---|---|---|---|
| CAPS‐IV total score | |||||
| Baseline | 88.50 | 2.08 | 27 | 44.3 | |
| Treatment exit | 42.97 | 3.39 | 34 | 55.7 | |
| Change from baseline to TEa | −45.53 | 3.52 | < .001 | ||
| 12‐month follow‐up | 36.43 | 3.11 | |||
| Change from baseline to 12‐month follow‐up | −52.06 | 3.48 | < .001 | ||
| Change from TE to 12‐month follow‐up | −6.53 | 2.50 | .011 | ||
| PSQI total score | |||||
| Baseline | 12.65 | 0.52 | |||
| Treatment exit | 8.37 | 0.55 | |||
| Change from baseline to TEa | −4.28 | 0.60 | < .001 | ||
| 12‐month follow‐up | 7.34 | 0.57 | |||
| Change from baseline to 12‐month follow‐up | −5.31 | 0.68 | < .001 | ||
| Change from TE to 12‐month follow‐up | −1.03 | 0.46 | .029 | ||
|
PSQI total score ≥ 3‐point drop (baseline to 12‐month follow‐up) |
|||||
| Yes | — | ||||
| No | — |
Note. N = 62. LS = Least Squares; CAPS‐IV = Clinician‐Administered PTSD Scale for DSM‐IV; PSQI = Pittsburgh Sleep Quality Index.
Figure 3.
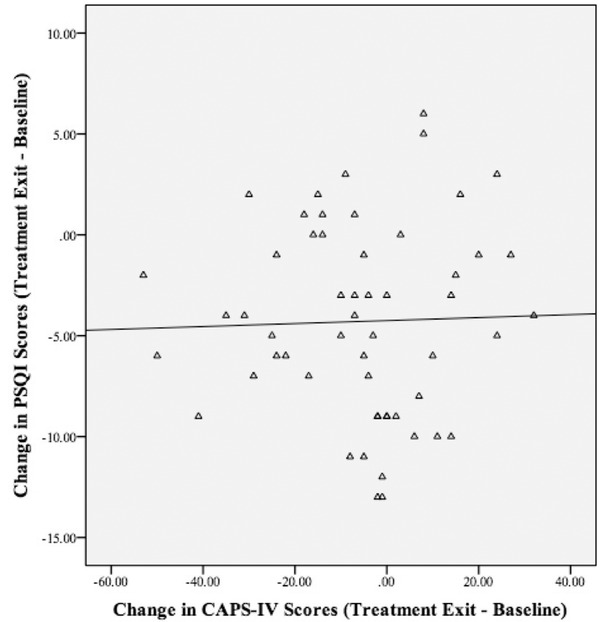
Correlation Plot of Change in Pittsburgh Sleep Quality Index (PSQI) and Clinician‐Administered PTSD Scale for DSM‐IV (CAPS‐IV) Total Scores Baseline to Treatment Exit
Note. The change in CAPS‐IV and PSQI scores from Baseline to TE (TE; n = 58) was significantly correlated, r = .371, p = .004.
To explore whether a clinically significant drop of 3 points or more on the PSQI at the primary endpoint impacted CAPS‐IV scores, we performed a subgroup analysis. A drop of 3 points or more on the PSQI at primary endpoint was associated with lower CAPS‐IV scores at TE (group difference: least squares (LS) M = −14.41, SD = 7.06), p = .046, and 12‐month follow‐up (group difference: LS M = −23.06, SD = 6.00), p < .001. At 12‐month follow‐up, 21 participants had a PSQI total score of 5 or lower compared to only two participants with a total PSQI score of 5 or lower at baseline.
Discussion
The present results provide evidence for the beneficial effects of MDMA‐assisted psychotherapy in treating sleep disturbance in individuals with PTSD. At the primary endpoint, participants in the active‐dose MDMA group reported significantly improved scores on measures of both sleep and PTSD symptoms compared to control participants, with approximately half (53.2%) of the active‐dose participants showing a clinically meaningful (i.e., 3‐point or more) reduction in PSQI scores, suggesting that MDMA‐assisted psychotherapy promotes improvements in both sleep and PTSD symptoms. In addition, sleep quality and PTSD symptoms improved significantly between TE and 12‐month follow‐up, indicating an enduring, clinically meaningful benefit of MDMA‐assisted psychotherapy on both domains. At 12‐month follow‐up, 21 out of 63 participants no longer met the criteria for sleep disturbance, as evidenced by a PSQI total score of 5 or lower.
We observed a significant between‐group difference in scores on the PSQI Sleep Latency and Daytime Dysfunction subscales at the primary endpoint, such that individuals in the active dose group reported requiring less time to fall asleep and less daytime dysfunction. These findings are in accordance with findings from studies of objective measures of sleep showing that in PTSD, both sleep and wakefulness are characterized by heightened arousal. Sleep‐related characteristics of PTSD include reduced slow‐wave sleep and increased Stage 1 sleep and rapid eye movement density. Reduced attention, increased distractibility, and reduced information processing speed may well reflect heightened arousal during wakefulness, suggesting that decreases in arousal may account for both decreased sleep latency and decreased daytime dysfunction (Koffel et al., 2016).
Although sleep‐specific items from the CAPS‐IV were retained rather than removed, a subgroup analysis showed that a 3‐point or more drop in PSQI scores at the primary endpoint was associated with lower CAPS‐IV scores at TE and 12‐month follow‐up such that participants who had a clinically meaningful improvement in sleep at primary endpoint also demonstrated more PTSD symptom improvement at TE and 12‐month follow‐up. Furthermore, the change in sleep quality was positively correlated with the change in PTSD symptom severity for the overall sample at posttreatment.
In contrast to reports of improved sleep lasting weeks or longer after treatment, MDMA can temporarily hamper sleep in healthy controls and individuals with PTSD, with reports of worsening insomnia lasting for approximately 3 days (Liechti et al., 2001; Mithoefer et al., 2018, 2019; Ot'alora, et al., 2018; Vizeli & Liechti, 2017). Mithoefer et al. (2019) reported safety outcomes from six Phase 2 trials, including participants represented in the present analyses, showing that on the night of experimental sessions, 38% of participants in the control group experienced insomnia compared with 29% of those in the MDMA group. The following day, the occurrence of insomnia increased for the MDMA group (47%) but stayed nearly the same for the control group (36%). Improved sleep assessed 1–2 months later demonstrates that these acute effects are transient.
There were several limitations of the present study. First, the sample size was relatively small and consisted of mostly White/Caucasian (85.7%) participants. Second, the main outcome measures of PTSD and sleep quality were based on self‐report assessments. Although the results from some studies that have compared subjective and objective sleep estimates have demonstrated that individuals with disturbed sleep tend to underestimate their total sleep time and overestimate their sleep onset latency (Kobayashi et al., 2012), these findings are inconclusive and warrant further investigation. Future studies can investigate the associations between subjective and objective measures of sleep after MDMA‐assisted psychotherapy to see if they are related and, if applicable, how they differ.
Given that sleep disturbance is not a unitary construct and different aspects of sleep disturbance are likely related in different ways to the risk, pathophysiology, maintenance of, and recovery from PTSD, future researchers should strive to elucidate the extent to which MDMA may differentially impact sleep symptoms, including well‐established PTSD‐associated sleep symptoms, such as nightmares, distressed awakenings, soaking sweats during sleep, and short sleep duration, and more recently identified PTSD‐associated sleep symptoms, such as acting‐out dreams and symptoms of obstructive sleep apnea (OSA). Researchers can utilize PTSD‐specific assessments of sleep symptoms, such as the PTSD addendum to the PSQI, and additionally assess OSA symptoms as well as pain and temperature dysregulation, which are known to interfere with sleep and may require separate treatment.
Although it is unclear whether MDMA‐assisted psychotherapy impacts the underlying hyperarousal symptoms of PTSD in a way that current evidence‐based treatments for PTSD do not, there are two important considerations to keep in mind when considering the present findings. First, some apparent residual symptoms may have been due to other sleep disorders that require separate treatment, such as OSA. Moreover, it is possible that MDMA trials are enrolling a more treatment‐resistant population.
The current report included the largest sample to date of data regarding sleep from participants with PTSD enrolled in clinical trials of MDMA‐assisted psychotherapy. At the posttreatment assessment, individuals in the MDMA group experienced a higher degree of improvements in sleep as well as steeper reductions in PTSD symptom severity compared to the control group. At 12‐month follow‐up, these improvements endured, suggesting that MDMA‐assisted psychotherapy may be particularly suited for promoting sleep regulation in patients with PTSD.
Open Practices Statement
All of the studies described were registered at ClinicalTrials.gov (http://www.clinicaltrials.gov; NCT01958593, NCT01211405, NCT01689740, and NCT01793610). The analyses were not formally preregistered; the sponsor and researchers intended to pool data from the pilot studies. Neither the data nor the materials have been made available on a permanent third‐party archive. Access to data and materials is limited to researchers who complete a data use proposal; information on data use proposals can be obtained through http://www.maps.org/datause.
Open Research Badges
This article has earned a Preregistered Research Designs badge for having a preregistered research design, available at https://www.clinicaltrials.gov/ct2/show/NCT01958593?term=NCT01958593&draw=2&rank=1, https://www.clinicaltrials.gov/ct2/show/NCT01211405?term=NCT01211405&draw=2&rank=1, https://www.clinicaltrials.gov/ct2/show/NCT01689740?term=NCT01689740&draw=2&rank=1, https://www.clinicaltrials.gov/ct2/show/NCT01793610?term=NCT01793610&draw=2&rank=1.
Supporting information
Supplemental Table. Number of Participants who Received a Sleep Aid on the Night of an Experimental Session during the Blinded Segment
Author Note Linnae Ponte received salary support from the Multidisciplinary Association for Psychedelic Studies (MAPS) when she was previously employed full‐time by MAPS. Lisa Jerome received salary support for full‐time employment with MAPS Public Benefit Corporation (PBC). Scott Hamilton received salary support from MAPS PBC as a biostatistician. Michael Mithoefer received research funds from MAPS Public Benefit Corporation as a clinical investigator and clinical trial medical monitor and for training and supervision of research psychotherapists. Berra Yazar‐Klosinski received salary support for full‐time employment with MAPS. Allison Feduccia received salary support for full‐time employment with MAPS PBC. Eric Vermetten received salary support from MAPS PBC support as an investigator.
References
- American Psychiatric Association . (2013). The diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Backhaus, J., Junghanns, K., Broocks, A., Riemann, D., & Hohagen, F. (2002). Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research, 53(3), 737–740. 10.1016/s0022-3999(02)00330-6. [pii] [DOI] [PubMed] [Google Scholar]
- Bershad, A. K., Miller, M. A., Baggott, M. J., & de Wit, H. (2016). The effects of MDMA on socio‐emotional processing: Does MDMA differ from other stimulants? Journal of Psychopharmacology, 30(12), 1248–1258. 10.1080/17470919.2016.1143026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, D. D., Weathers, F. W., Nagy, L. M., Kaloupek, D. G., Gusman, F. D., Charney, D. S., & Keane, T. M. (1995). The development of a Clinician‐Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. 10.1007/BF02105408 [DOI] [PubMed] [Google Scholar]
- Buysse, D. J., Germain, A., Moul, D. E., Franzen, P. L., Brar, L. K., Fletcher, M. E., Begley, A., Houck, P. R., Mazumdar, S., Reynolds, 3rd, C. F., & Monk, T. H. (2011). Efficacy of brief behavioral treatment for chronic insomnia in older adults. Archives of Internal Medicine, 171(10), 887–895. 10.1001/archinternmed.2010.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse, D. J., Reynolds, 3rd, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4. [pii] [DOI] [PubMed] [Google Scholar]
- Clum, G. A., Nishith, P., & Resick, P .A. (2001). Trauma‐related sleep disturbance and self‐reported physical health symptoms in treatment‐seeking female rape victims. Journal of Nervous and Mental Disease, 189(9), 618–622. https://doi.10.1097/00005053‐200109000‐00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeViva, J. C. (2014). Treatment utilization among OEF/OIF veterans referred for psychotherapy for PTSD. Psychol Serv, 11(2), 179–184. 10.1037/a0035077 [DOI] [PubMed] [Google Scholar]
- El‐Solh, A. A., O'Brien, N., Akinnusi, M., Patel, S., Vanguru, L., & Wijewardena, C. (2019). Predictors of cognitive behavioral therapy outcomes for insomnia in veterans with post‐traumatic stress disorder. Sleep Breath, 23(2), 635–643. 10.1007/s11325-019-01840-4 [DOI] [PubMed] [Google Scholar]
- Feduccia, A. A., Holland, J., & Mithoefer, M. C. (2018). Progress and promise for the MDMA drug development program. Psychopharmacology, 235(2), 561–571. 10.1007/s00213-017-4779-2 [DOI] [PubMed] [Google Scholar]
- Germain, A. (2013). Sleep disturbances as the hallmark of PTSD: Where are we now? American Journal of Psychiatry, 170(4), 372–382. 10.1176/appi.ajp.2012.12040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain, A., Buysse, D. J., Shear, M. K., Fayyad, R., & Austin, C. (2004). Clinical correlates of poor sleep quality in posttraumatic stress disorder. Journal of Traumatic Stress, 17(6), 477–484. 10.1007/s10960-004-5796-6 [DOI] [PubMed] [Google Scholar]
- Germain, A., McKeon, A. B., & Campbell, R. L. (2017). Sleep in PTSD: Conceptual model and novel directions in brain‐based research and interventions. Current Opinion in Psychology, 14, 84–89. 10.1016/j.copsyc.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Germain, A., Moul, D. E., Franzen, P. L., Miewald, J. M., Reynolds, 3rd, C. F., Monk, T. H., & Buysse, D. J. (2006). Effects of a brief behavioral treatment for late‐life insomnia: Preliminary findings. Journal of Clinical Sleep Medicine, 2(4), 403–406. [PubMed] [Google Scholar]
- Greer, G. R., & Tolbert, R. (1998). A method of conducting therapeutic sessions with MDMA. Journal of Psychoactive Drugs, 30(4), 371–379. [DOI] [PubMed] [Google Scholar]
- Harvey, A. G., Jones, C., & Schmidt, D. A. (2003). Sleep and posttraumatic stress disorder: A review. Clinical Psychology Review, 23(3), 377–407. 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Jetly, R., Heber, A., Fraser, G., & Boisvert, D. (2015). The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD‐associated nightmares: A preliminary randomized, double‐blind, placebo‐controlled cross‐over design study. Psychoneuroendocrinology, 51, 585–588. 10.1016/j.psyneuen.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Kamilar‐Britt, P., & Bedi, G. (2015). The prosocial effects of 3,4‐methylenedioxymethamphetamine (MDMA): Controlled studies in humans and laboratory animals. Neuroscience & Biobehavioral Reviews, 57, 433–446. 10.1016/j.neubiorev.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick, M. G., Baggott, M. J., Mendelson, J. E., Galloway, G. P., Liechti, M. E., Hysek, C. M., & de Wit, H. (2014). MDMA effects consistent across laboratories. Psychopharmacology (Berl), 231(19), 3899–3905. 10.1007/s00213-014-3528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, I., Huntley, E., Lavela, J., & Mellman, T. A. (2012). Subjectively and objectively measured sleep with and without posttraumatic stress disorder and trauma exposure. Sleep, 35(7), 957–965. 10.5665/sleep.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel, E., Khawaja, I. S., & Germain, A. (2016). Sleep disturbances in posttraumatic stress disorder: Updated review and implications for treatment. Psychiatr Annals, 46(3), 173–176. 10.3928/00485713-20160125-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow, B., Artar, A., Warner, T.D., Melendrez, D., Johnston, L., Hollifield, M., Germain, A., & Koss, M. (2000). Sleep disorder, depression, and suicidality in female sexual assault survivors. Crisis, 21(4), 163–170. 10.1027//0227-5910.21.4.163 [DOI] [PubMed] [Google Scholar]
- Krakow, B., Germain, A., Warner, T.D., Schrader, R., Koss, M., Hollifield, M., Germain, A., & Koss, M. (2001). The relationship of sleep quality and posttraumatic stress to potential sleep disorders in sexual assault survivors with nightmares, insomnia, and PTSD. Journal of Traumatic Stress, 14(4), 647–665. 10.1023/A:1013029819358 [DOI] [PubMed] [Google Scholar]
- Krakow, B., Haynes, P. L., Warner, T. D., Santana, E., Melendrez, D., Johnston, L., Hollifield, M., Sisley, B. M., Koss, M., & Shafer, L. (2004). Nightmares, insomnia, and sleep‐disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. Journal of Traumatic Stress, 17(3), 257–268. 10.1023/B:JOTS.0000029269.29098.67 [DOI] [PubMed] [Google Scholar]
- Krakow, B., Hollifield, M., Johnston, L., Koss, M., Schrader, R., Warner, T. D., Tandberg, D., Lauriello, J., McBride, L., Cutchen, L., Cheng, D., Emmons, S., Germain, A., Melendrez, D., Sandoval, D., & Prince, H. (2001). Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: A randomized controlled trial. JAMA, 286(5), 537–545. 10.1001/jama.286.5.537 [DOI] [PubMed] [Google Scholar]
- Krakow, B., Melendrez, D., Pedersen, B., Johnston, L., Hollifield, M., Germain, A., Koss, M., Warner, T. D., & Schrader, R. (2001). Complex insomnia: Insomnia and sleep‐disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biologiacal Psychiatry, 49(11), 948–953. 10.1016/s0006-3223(00)01087-8 [DOI] [PubMed] [Google Scholar]
- Krediet, E., Janssen, D. G., Heerdink, E. R., Egberts, T. C., & Vermetten, E. (2020). Experiences with medical cannabis in the treatment of veterans with PTSD: Results from a focus group discussion. European Neuropsychopharmacology, 36, 244–254. 10.1016/j.euroneuro.2020.04.009 [DOI] [PubMed] [Google Scholar]
- Liechti, M .E., Gamma, A., & Vollenweider, F. X. (2001). Gender differences in the subjective effects of MDMA. Psychopharmacology, 154(2), 161–168. 10.1007/s002130000648 [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., David, D., Kulick‐Bell, R., Hebding, J., & Nolan, B. (1995). Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. American Journal of Psychiatry, 152(11), 1659–1663. 10.1176/ajp.152.11.1659 [DOI] [PubMed] [Google Scholar]
- Miller, K. E., Brownlow, J. A., & Gehrman, P. R. (2019). Sleep in PTSD: Treatment approaches and outcomes. Current Opinion in Psychology , 34, 12–17. 10.1016/j.copsyc.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer, M. C. (2017). A manual for MDMA‐assisted psychotherapy in the treatment of posttraumatic stress disorder (Version 8.1). https://s3‐us‐west‐1.amazonaws.com/mapscontent/research‐archive/mdma/TreatmentManual_MDMAAssistedPsychotherapyVersion+8.1_22+Aug2017.pdf [DOI] [PMC free article] [PubMed]
- Mithoefer, M. C., Feduccia, A. A., Jerome, L., Mithoefer, A., Wagner, M., Walsh, Z., Hamilton, S., Yazar‐Klosinski, B., Emerson, A., & Doblin, R. (2019). MDMA‐assisted psychotherapy for treatment of PTSD: Study design and rationale for Phase 3 trials based on pooled analysis of six Phase 2 randomized controlled trials. Psychopharmacology, 236(9), 2735–2745. 10.1007/s00213-019-05249-5 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, M., Wymer, J., Holland, J., Hamilton, S., Yazar‐Klosinski, B., Emerson, A., & Doblin, R. (2018). 3,4‐methylenedioxymethamphetamine (MDMA)‐assisted psychotherapy for post‐traumatic stress disorder in military veterans, firefighters, and police officers: A randomised, double‐blind, dose–response, Phase 2 clinical trial. Lancet Psychiatry, 5(6), 486–497. 10.1016/s2215-0366(18)30135-4 [DOI] [PubMed] [Google Scholar]
- Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., & Doblin, R. (2011). The safety and efficacy of {+/‐}3,4‐methylenedioxymethamphetamine‐assisted psychotherapy in subjects with chronic, treatment‐resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology, 25(4), 439–452. 10.1177/0269881110378371. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., Martin, S. F., Yazar‐Klosinski, B., Michel, Y., Brewerton, T. D., & Dobin, R. (2013). Durability of improvement in post‐traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4‐methylenedioxymethamphetamine‐assisted psychotherapy: A prospective long‐term follow‐up study. Journal of Psychopharmacology, 27(1), 28–39. 10.1177/0269881112456611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishith, P., Resick, P. A., & Mueser, K. T. (2001). Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. Journal of Traumatic Stress, 14(3), 469–479. 10.1023/A:1011152405048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen, P., Traber, R., Widmer, V., & Schnyder, U. (2013). A randomized, controlled pilot study of MDMA (+/‐ 3,4‐Methylenedioxymethamphetamine)‐assisted psychotherapy for treatment of resistant, chronic post‐traumatic stress disorder (PTSD). Journal of Psychopharmacology, 27(1), 40–52. 10.1177/0269881112464827 [DOI] [PubMed] [Google Scholar]
- Ot'alora, G. M., Grigsby, J., Poulter, B., Van Derveer, J. W., Giron, S. G., Jerome, L., Feduccia, A. A., Hamilton, S., Yazar‐Klosinski, B., Emerson, A., Mithoefer, M. C., & Doblin, R. (2018). 3,4‐Methylenedioxymethamphetamine‐assisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized Phase 2 controlled trial. Journal of Psychopharmacology, 32(12), 1295–1307. 10.1177/0269881118806297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen, S., Hamre, H. S., Lang, N., & Bjorvatn, B. (2020). Doxazosin for the treatment of nightmare disorder: A diary‐based case study. SAGE Open Medical Case Reports, 8, 2050313×20936079. 10.1177/2050313.20936079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, A., Kanady, J. C., & Neylan, T. C. (2020). Sleep disturbance in PTSD and other anxiety‐related disorders: An updated review of clinical features, physiological characteristics, and psychological and neurobiological mechanisms. Neuropsychopharmacology, 45(1), 55–73. 10.1038/s41386-019-0486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin, M. E., Brady, K. T., Dansky, B. S., & Kilpatrick, D. G. (1995). Understanding comorbidity between PTSD and substance use disorders: Two preliminary investigations. Addictive Behaviors, 20(5), 643–655. https://www.ncbi.nlm.nih.gov/pubmed/8712061 [DOI] [PubMed] [Google Scholar]
- Seal, K. H., Maguen, S., Cohen, B., Gima, K. S., Metzler, T. J., Ren, L., Bertenthal, D., & Marmar, C. R. (2010). VA mental health services utilization in Iraq and Afghanistan veterans in the first year of receiving new mental health diagnoses. J Trauma Stress, 23(1), 5–16. 10.1002/jts.20493 [DOI] [PubMed] [Google Scholar]
- Smith, C., & Koola, M. M. (2016). Evidence for using doxazosin in the treatment of posttraumatic stress disorder. Psychiatric Annals, 46(9), 553–555. 10.3928/00485713-20160728-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker, V. I., & Montgomery, P. (2008). Disturbed sleep in post‐traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 169–184. 10.1016/j.smrv.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Ulmer, C. S., Edinger, J. D., & Calhoun, P. S. (2011). A multi‐component cognitive‐behavioral intervention for sleep disturbance in veterans with PTSD: A pilot study. Journal of Clinical Sleep Medicine, 7(1), 57–68. https://www.ncbi.nlm.nih.gov/pubmed/21344046 [PMC free article] [PubMed] [Google Scholar]
- van Liempt, S. (2012). Sleep disturbances and PTSD: A perpetual circle? European Journal of Psychotraumatology, 3(1), 19142. 10.3402/ejpt.v3i0.19142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt, S., Arends, J., Cluitmans, P. J., Westenberg, H. G., Kahn, R. S., & Vermetten, E. (2013). Sympathetic activity and hypothalamic‐pituitary‐adrenal axis activity during sleep in post‐traumatic stress disorder: A study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology, 38(1), 155–165. 10.1016/j.psyneuen.2012.05.015 [DOI] [PubMed] [Google Scholar]
- van Liempt, S., Vermetten, E., Lentjes, E., Arends, J., & Westenberg, H. (2011). Decreased nocturnal growth hormone secretion and sleep fragmentation in combat‐related posttraumatic stress disorder: Potential predictors of impaired memory consolidation. Psychoneuroendocrinology, 36(9), 1361–1369. 10.1016/j.psyneuen.2011.03.009 [DOI] [PubMed] [Google Scholar]
- Vermetten, E., Germain, A., & Neylan, T. C. (2018). Sleep and combat‐related post‐traumatic stress disorder. Springer. 10.1007/978-1-4939-7148-0. [DOI] [Google Scholar]
- Vizeli, P., & Liechti, M. E. (2017). Safety pharmacology of acute MDMA administration in healthy subjects. Journal of Psychopharmacology, 31(5), 576–588. 10.1177/0269881117691569 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W. (2004). Clinician‐Administered PTSD Scale (CAPS): Technical manual. Western Psychological Services. [Google Scholar]
- Weathers, F. W., Keane, T. M., & Davidson, J. R. (2001). Clinician‐administered PTSD scale: a review of the first ten years of research. Depress Anxiety, 13(3), 132–156. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11387733 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Ren, R., Sanford, L. D., Yang, L., Ni, Y., Zhou, J., Zhang, J., Wing, Y. ‐K., Shi, J., Lu, L. & Tang, X. (2020). The effects of prazosin on sleep disturbances in post‐traumatic stress disorder: A systematic review and meta‐analysis. Sleep Medicine, 67, 225–231. 10.1016/j.sleep.2019.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Number of Participants who Received a Sleep Aid on the Night of an Experimental Session during the Blinded Segment


