Abbreviations
- BMI
body mass index
- C1‐INH‐HAE
hereditary angio‐oedema due to C1‐inhibitor deficiency/dysfunction
- CI
confidence interval
- FPS‐R
Faces Pain Scale—Revised tool
- HAE
hereditary angio‐oedema
- HCP
healthcare professional
- SD
standard deviation
- TEAE
treatment‐emergent adverse event
- TOSR
time to onset of symptom relief
- TTMS
time to minimum symptoms
CONFLICT OF INTEREST
HF served as a member of advisory boards for BioCryst, CSL Behring, Octapharma, and Takeda; received grants/honoraria from BioCryst, CSL Behring, Octapharma, Takeda, and Sobi; and served as a clinical trial investigator for BioCryst, CSL Behring, and Takeda. AR received research funding/travel grants/honoraria from BioCryst, Cephalon, CSL Behring, Pharming, Takeda, and Teva and served as an advisor/speaker for CSL Behring and Takeda. TC served as a member of advisory boards for BioCryst, CSL Behring, Novartis, Octapharma, Pharming, and Takeda; served as a member of speaker bureaus for CSL Behring, Merck, Novartis, and Takeda; received grants/honoraria from BioCryst, CSL Behring, Novartis, and Takeda; received funding to attend conferences/educational events from CSL Behring, Novartis, and Takeda; served as a clinical trial/registry investigator for BioCryst, CSL Behring, Novartis, Pharming, and Takeda; and served as a researcher from the IdiPAZ program for promoting research activities. MCOL received travel grants/honoraria from Octapharma and Takeda. AK received travel grants from Pharming and Takeda and received honoraria from CSL Behring and Takeda. MV was a full‐time employee of Takeda at the time of the study and is currently working for Alexion Pharmaceuticals. JH is a full‐time employee of Takeda and holds stock/stock options in Takeda. WA served as a member of advisory boards/speaker bureaus for BioCryst, CSL Behring, Pharming, and Takeda; received research grants from CSL Behring and Takeda; received funding to attend conferences/educational events and donations to departmental fund from Takeda; and is a clinical trial investigator for BioCryst and Takeda.
AUTHOR CONTRIBUTION
Henriette Farkas: Conceptualization (equal); Data curation (equal); Methodology (equal); Writing‐review & editing (equal). Avner Reshef: Data curation (equal); Writing‐review & editing (equal). Teresa Caballero: Data curation (equal); Writing‐review & editing (equal). María Claudia Ortega: Data curation (equal); Writing‐review & editing (equal). Aharon Kessel: Data curation (equal); Writing‐review & editing (equal). Moshe Vardi: Conceptualization (equal); Methodology (equal); Supervision Writing‐review & editing (equal). James Hao: Formal analysis Writing‐review & editing (equal). Werner Aberer: Data curation (equal); Writing‐review & editing (equal).
To the Editor,
Hereditary angio‐oedema (HAE) is rare disease characterized by recurrent, unpredictable, and debilitating attacks of subcutaneous/submucosal tissue swelling.1, 2 The reported median age of onset of HAE due to C1 inhibitor deficiency/dysfunction (type 1/2; C1‐INH‐HAE) is 11‐12 years.1, 3 Treatment options for pediatric patients are limited, owing to low childhood diagnosis rates and low representation in investigative clinical trials.4
We present a multicenter, open‐label, single‐arm, phase 3 study (NCT01386658) investigating the use of icatibant, a bradykinin B2 receptor antagonist, to treat HAE attacks in pediatric patients with a confirmed diagnosis of C1‐INH‐HAE.5 In Part 1, patients (aged 2 to <18 years with confirmed diagnosis of C1‐INH‐HAE) received an icatibant injection in the presence or absence of an attack. Icatibant showed acceptable safety and tolerability, and the treatment response to the first icatibant injection (n = 22) was consistent with that observed in adults, with median time to onset of symptom relief (TOSR) of 1.0 hour. The European Medicines Agency subsequently approved icatibant in 2017 for use in pediatric patients aged 2‐17 years.6
Here, we report results from Part 2 of the study, investigating continued icatibant use across additional attacks in adolescents. The study design and Part 1 results were previously reported.5 In Part 2, adolescent patients (aged 11 to <18 years at enrollment; pubertal/post‐pubertal [assessed by Tanner scale]) continued to receive one icatibant injection per attack for up to two additional attacks. See Supplemental Methods S1.1 to S1.5 for detailed methodology.
Patients received subcutaneous icatibant at 0.4 mg/kg up to 30 mg (approved adult dose) ≤12 hours after symptom onset. Repeat treatment of a previously treated attack was not allowed. Treatment was administered by patients/caregivers after appropriate training or by a healthcare professional. The primary end‐point was TOSR by composite symptom score, defined as the duration from treatment to the earliest time with ≥20% improvement in composite score and no worsening in any component score. Additional end‐points were time to initial symptom relief, time to minimum symptoms (TTMS), and TOSR and TTMS for self‐assessed pain (Faces Pain Scale—Revised tool [FPS‐R]). Safety and tolerability were also evaluated.
Summary statistics were provided for continuous variables. Median times to events (95% confidence interval [CI]) were estimated using Kaplan‐Meier methodology. A post hoc analysis of the effect of time to treatment on TOSR was performed for attacks treated at home, using Pearson and Spearman methodologies for correlation coefficients.
In Part 2, nine pubertal/post‐pubertal patients received one icatibant administration to treat 18 separate attacks (Table 1; Figure S1). All patients completed assessments at Day 8 and all but one at Day 90; none discontinued prematurely. Ten attacks were treated with self‐administered icatibant, six with caregiver‐administered icatibant, and two with healthcare professional–administered icatibant (in one patient, both mild laryngeal attacks).
TABLE 1.
Demographics and HAE history for adolescent patients who experienced additional icatibant administrationsa to treat attacks in Part 2 of the study (safety population)
| Characteristic | N = 9 |
|---|---|
| Age at second icatibant administration, y, mean (SD) | 17.4 (2.45) |
| Range | 11.6‐19.4 |
| Age at third icatibant administration, y, mean (SD) | 17.9 (1.90) |
| Range | 13.5‐19.4 |
| Female, n (%) | 5 (55.6) |
| White, n (%) | 8 (88.9) |
| BMI at second icatibant administration, kg/m2, mean (SD) | 23.3 (2.26) |
| BMI at third icatibant administration, kg/m2, mean (SD) | 23.7 (2.50) |
| Type of HAE attack prior to icatibant administration, n (%) | First | Second | Third |
|---|---|---|---|
| Cutaneous | 4 (44.4) | 4 (44.4) | 3 (33.3) |
| Abdominal | 3 (33.3) | 0 (0.00) | 2 (22.2) |
| Cutaneous and abdominal | 1 (11.1) | 4 (44.4) | 3 (33.3) |
| Laryngeal | 1 (11.1) | 1 (11.1) | 1 (11.1) |
Abbreviations: BMI, body mass index; HAE, hereditary angio‐oedema; SD, standard deviation.
In Part 1, three patients received icatibant in the absence of an HAE attack; their second icatibant administration in Part 2 was therefore treatment of their first on‐study attack.
All nine patients were included in the primary end‐point analysis after second icatibant administration (for new attacks in Part 2), and eight patients after third administration (for subsequent attacks; one patient did not have baseline measurement for a cutaneous/abdominal attack); all experienced symptom relief after treatment. Median TOSR (95% CI) was 1.0 (1.0‐2.3) and 1.1 (1.0‐3.0) hours after second and third administration, respectively (Figure 1A,B). The two laryngeal attacks had TOSRs of 4.0 and 1.0 hours.
FIGURE 1.
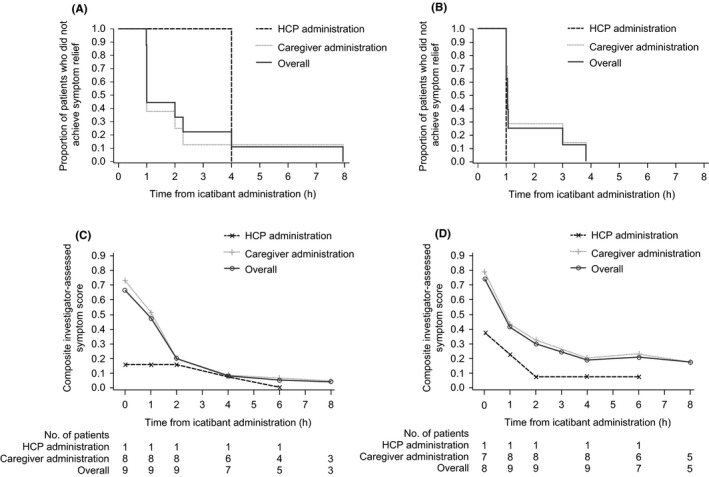
(A) Kaplan‐Meier curves for TOSR after the second icatibant administration. (B) Kaplan‐Meier curves for TOSR after the third icatibant administration. (C) Mean composite symptom score over time after the second icatibant administration. (D) Mean composite symptom score over time after the third icatibant administration. The composite symptom score was calculated as an average of 8 cutaneous/abdominal or 13 laryngeal assessment components. Caregiver administration includes administration by home healthcare provider, patient, parent, legal guardian, or self. HCP, healthcare professional; TOSR, time to onset of symptom relief
Most attacks had a short time to initial symptom relief: ~80% and ~90% started to resolve by 1 and 2 hours, respectively, after second administration, and ~55% and ~90% by 1 and 2 hours, respectively, after third administration. Median TTMS (95% CI) was 1.2 (1.0‐2.0) and 2.2 hours (1.0‐not estimable) after second and third administration, respectively (n = 7 for both). Two patients were censored for the third administration because minimum symptoms were not achieved within the 8 hour observation period. Mean composite symptom score improved over time (Figure 1C, 1D, and Table S1).
Median (95% CI) TOSR for FPS‐R (eight patients with data) was 1.1 (1.0‐2.1) and 1.0 (1.0‐1.2) hours after second and third administration, respectively. Median TTMS (95% CI) for FPS‐R was 2.5 (1.0‐4.0) and 14.9 (3.8‐24.1) hours, respectively; one patient was censored for analysis of the third administration (minimum symptoms not achieved within 8 hours). Table S2 for FPS‐R scores over time. Only one patient used rescue medication (C1 inhibitor), 19 hours after second icatibant administration for a cutaneous attack.
In the home setting, eight patients received self/caregiver‐administered icatibant to treat 16 non‐laryngeal attacks. Icatibant was administered within a mean (standard deviation [SD]) time of 2.7 (3.04) hours and a median (inter‐quartile range) time of 1.6 (0.5‐3.9) hours from symptom onset. Individual TOSR for attacks treated ≤1 hour was all ~1 hour, whereas TOSR for attacks treated >1 hour varied (1‐8 hours) (Figure S2). By the Pearson model, time to treatment significantly correlated with TOSR by composite symptom score (15 attacks; r = 0.826; P < .01) and FPS‐R (14 attacks; r = 0.650; P = .01). By the Spearman model, time to treatment trended toward correlation with TOSR by composite symptom score (r = 0.439; P = .10) but not FPS‐R (r = 0.223; P = .44). A 1‐hour threshold was used to define early vs late treatment in the Icatibant Outcome Survey.7 Here, there was a non‐significant trend (P = .09) toward decreased TOSR by composite symptom score for treatment <1 hour vs ≥1 hour (mean [SD], 1.0 [0.04] vs 2.8 [2.33] hours; seven and eight attacks, respectively).
Treatment‐emergent adverse events (TEAEs) were reported by four and five patients after the second and third administration, respectively (Table 2). No TEAEs were considered related to icatibant, and all were mild/moderate except for two severe TEAEs after third administration (folliculitis and ear pain). No serious TEAEs or discontinuations due to TEAEs were reported. Most injection site reactions were mild/moderate and most resolved ≤6 hours. Severe injection site reactions were reported in three patients and one patient after the second and third administration, respectively, which resolved ≤8 hours (Table S3). There were no clinically significant changes in laboratory values, vital signs, or reproductive hormone levels (Table S4), and no anti‐drug antibodies were detected.
TABLE 2.
Summary of TEAEsa after second and third icatibant administration, by System Organ Class and Preferred Term
| TEAE | Second icatibant administration N = 9 | Third icatibant administration N = 9 | ||
|---|---|---|---|---|
| Patients, n (%) | Events, n | Patients, n (%) | Events, n | |
| Any TEAE | 4 (44.4) | 5 | 5 (55.6) | 7 |
| Infections and infestations | 3 (33.3) | 3 | 1 (11.1) | 1 |
| Influenza | 1 (11.1) | 1 | – | – |
| Nasopharyngitis | 1 (11.1) | 1 | – | – |
| Bacterial vaginitis | 1 (11.1) | 1 | – | – |
| Folliculitis | – | – | 1 (11.1) | 1 |
| Gastrointestinal disorders | 0 | 0 | 2 (22.2) | 2 |
| Toothache | – | – | 1 (11.1) | 1 |
| Vomiting | – | – | 1 (11.1) | 1 |
| General disorders and administration site conditions | 0 | 0 | 2 (22.2) | 2 |
| Pyrexia | – | – | 2 (22.2) | 2 |
| Ear and labyrinth disorders | 0 | 0 | 1 (11.1) | 1 |
| Ear pain | – | – | 1 (11.1) | 1 |
| Eye disorders | 1 (11.1) | 1 | 0 | 0 |
| Allergic conjunctivitis | 1 (11.1) | 1 | – | – |
| Respiratory, thoracic, and mediastinal disorders | 0 | 0 | 1 (11.1) | 1 |
| Oropharyngeal pain | – | – | 1 (11.1) | 1 |
| Skin and subcutaneous disorders | 1 (11.1) | 1 | 0 | 0 |
| Papule | 1 (11.1) | 1 | – | – |
Abbreviations: TEAE, treatment‐emergent adverse event.
None of the reported TEAEs were considered by investigators to be related to icatibant treatment.
Because HAE attacks recur throughout a patient's lifetime, it is important that repeated on‐demand treatments maintain effectiveness, with no safety or tolerance issues. Limitations of the present analyses include the non‐controlled, non‐randomized design and the small population due to the rarity of C1‐INH‐HAE. During Part 2 of this study, median TOSRs and safety profiles after second and third icatibant administration were consistent with those after first icatibant use. Findings were also similar to those reported in adults with multiple attacks in the open‐label extension phases of randomized controlled trials, although comparisons are limited by differences in study designs and populations.8, 9, 10
This study confirms that icatibant is suitable for self‐ or caregiver administration in adolescents after appropriate training, which is a guideline recommendation for patients of all ages to facilitate prompt treatment of attacks.1, 4, 6 Icatibant provides consistent and effective symptom relief and is well tolerated in the on‐demand treatment of recurrent HAE attacks in adolescents with C1‐INH‐HAE, similarly to adult patients.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank all patients, investigators, and their study staff. Under the direction of the authors, Alpa Parmar, PhD, CMPP, employee of Excel Medical Affairs, provided writing assistance for this publication. Editorial assistance in formatting, proofreading, copyediting, and fact‐checking also was provided by Excel Medical Affairs. The interpretation of the data was made by the authors independently.
Funding information
This study was supported by Shire Human Genetic Therapies, Inc, a Takeda company. Shire Human Genetic Therapies, Inc, a Takeda company, provided funding to Excel Medical Affairs for support in writing and editing this publication.
REFERENCES
- 1.Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema–the 2017 revision and update. Allergy. 2018;73(8):1575‐1596. [DOI] [PubMed] [Google Scholar]
- 2.Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136‐1148. [DOI] [PubMed] [Google Scholar]
- 3.Christiansen SC, Davis DK, Castaldo AJ, Zuraw BL. Pediatric hereditary angioedema: onset, diagnostic delay, and disease severity. Clin Pediatr (Phila). 2016;55(10):935‐942. [DOI] [PubMed] [Google Scholar]
- 4.Farkas H, Martinez‐Saguer I, Bork K, et al. HAWK. International consensus on the diagnosis and management of pediatric patients with hereditary angioedema with C1 inhibitor deficiency. Allergy. 2017;72(2):300‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkas H, Reshef A, Aberer W, et al. Treatment effect and safety of icatibant in pediatric patients with hereditary angioedema. J Allergy Clin Immunol Pract. 2017;5(6):1671‐1678. [DOI] [PubMed] [Google Scholar]
- 6.European Medicines Agency . Firazyr 30 mg solution for injection in pre‐filled syringe. https://www.ema.europa.eu/en/documents/product‐information/firazyr‐epar‐product‐information_en.pdf. Accessed July 20, 2020.
- 7.Maurer M, Aberer W, Bouillet L, et al. Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS One. 2013;8(2):e53773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malbrán A, Riedl M, Ritchie B, et al. Repeat treatment of acute hereditary angioedema attacks with open‐label icatibant in the FAST‐1 trial. Clin Exp Immunol. 2014;177(2):544‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baş M, Greve J, Hoffmann TK, et al. Repeat treatment with icatibant for multiple hereditary angioedema attacks: FAST‐2 open‐label study. Allergy. 2013;68(11):1452‐1459. [DOI] [PubMed] [Google Scholar]
- 10.Lumry WR, Farkas H, Moldovan D, et al. Icatibant for multiple hereditary angioedema attacks across the controlled and open‐label extension phases of FAST‐3. Int Arch Allergy Immunol. 2015;168(1):44‐55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


