Abstract
Objective
To assess the efficacy of using a bone substitute material (BSM) in the fixture–socket gap in patients undergoing tooth extraction and immediate implant placement.
Materials and methods
MEDLINE, EMBASE, and CENTRAL databases were searched for randomized controlled trials (RCTs). RCTs were screened for eligibility, and data were extracted by two authors independently. Risk of bias (ROB) was assessed using Cochrane's ROB tool 2.0. Primary outcomes were implant failure, overall complications, and soft‐tissue esthetics. Secondary outcomes were vertical buccal bone resorption, vertical interproximal bone resorption, horizontal buccal bone resorption, and mid‐buccal mucosal recession. Meta‐analysis was performed using random‐effects model with generic inverse variance weighing. GRADE was used to grade the certainty of the evidence.
Results
After screening 19 544 potentially eligible references, 20 RCTs were included in this review, with a total of 848 patients (916 sites). Most included RCTs were deemed of some concerns (53%) or at low (38%) risk of bias, except for overall complications (high ROB). Implant failure did not differ significantly RR = 0.92 (confidence intervals [CI] 0.34 to 2.46) between using a BSM compared with not using a BSM (NoBSM). BSM use resulted in less horizontal buccal bone resorption (MD = −0.52 mm [95% CI −0.74 to −0.30]), a higher esthetic score (MD = 1.49 [95% CI 0.46 to 2.53]), but also more complications (RR = 3.50 [95% CI 1.11 to 11.1] compared with NoBSM. Too few trials compared types of BSMs against each other to allow for pooled analyses. The certainty of the evidence was considered moderate for all outcomes except implant failure (low), overall complications (very low), and vertical interproximal bone resorption (very low).
Conclusion
BSM use during immediate implant placement reduces horizontal buccal bone resorption and improves the periimplant soft‐tissue esthetics. Although BSM use increases the risk of predominantly minor complications.
Keywords: bone regeneration, bone substitutes, dental implant, tooth extraction, tooth socket
What is known:
A recent review reported no significant added value in using a BSM in the fixture‐socket gap; while another, reported a favorable mitigating effect of BSM use on horizontal bone resorption. However, these reviews included various study designs, displayed heterogeneous outcomes definitions and results.
What this study adds:
This is the first review to assess the evidence presented in relevant randomized clinical trials. It demonstrates that BSM use reduces the amount of horizontal buccal bone resorption and improves peri‐implant soft‐tissue esthetics, but also results in a higher risk of minor complications.
1. INTRODUCTION
Tooth extraction marks the initiation of the socket healing process; which consists of three main stages, namely inflammatory, proliferative, and bone (re)modeling. Consequently, the bundle bone resorbs and the alveolar ridge tends to return to its pre‐eruption form, thus resulting in reduction of the alveolar ridge dimensions.1, 2, 3 After atraumatic tooth extraction, immediate implant placement (IIP) is a common procedure with an approximate success rate of 95%. Moreover, IIP reduces treatment duration, and omits the need for another implant placement surgery when compared with delayed implant placement.4, 5, 6
The difference in the circumference between the extracted tooth and the implant creates a gap; the “fixture‐socket gap.” Several studies suggested that the use of a bone‐substitute material (BSM) in the fixture–socket gap preserves socket volume, minimizes socket remodeling, and supports de‐novo bone formation .2, 4, 7 This might improve the peri‐implant bone regeneration process.
Various types of BSMs can be used during IIP, such as autograft, allograft, xenograft, or alloplast. Each of these BSMs has merits and drawbacks based on their properties. The type of BSM used can affect the dimensional change of the socket, percentage of regenerated vital bone, and the amount of connective tissue present in the site.8, 9, 10, 11
Trials assessing the efficacy of using a BSM in the fixture–socket gap during IIP are often limited in sample size, tend to suffer from heterogeneous reporting, and frequently have conflicting results.2, 12, 13, 14, 15 An overview, critical appraisal, and pooled analysis of studies looking at the efficacy of using BSMs for IIP is still lacking.
This systematic review aims to assess the efficacy of using a BSM in the fixture‐socket gap compared with not using any grafting material (NoBSM) in patients undergoing tooth extraction and IIP. In addition, we aimed to evaluate whether efficacy was different between the different types of BSMs that were used.
2. MATERIAL AND METHODS
2.1. Reporting
The methodology of this systematic review followed the Cochrane handbook for systematic reviews of interventions.16 Reporting was in accordance with the PRISMA checklist. The protocol of this systematic review was registered in the PROSPERO database (CRD42020164451).17, 18
2.2. Eligibility criteria
RCTs assessing the efficacy of BSMs in adult participants (≥18 years old) undergoing tooth extraction and IIP with a minimum follow‐up of 4 months were eligible for inclusion. Trials that only used the patient's blood extracts, or tooth graft to fill the fixture–socket gap were excluded. Additionally, trials that used soft‐tissue augmentation as the primary intervention were excluded.
2.3. Information sources and search strategy
Searches were conducted up to September 15, 2020 in MEDLINE (via PubMed), EMBASE and the Cochrane's Central Register of Controlled Trials (CENTRAL), and Google Scholar. The search strategy for MEDLINE is presented in (Table S1). Additionally, all online issues of the following specific journals were screened by two authors (JZ and NY): Journal of Clinical Periodontology, Journal of Periodontology, Journal of Dental Research, Journal of Clinical Implant Dentistry and Related Research, the International Journal of Oral and Maxillofacial Implants, the International Journal of Periodontics and Restorative Dentistry, and the European Journal of Oral Implantology. One author (JZ) performed snowballing by searching the reference lists of systematic reviews on similar topics and searching for relevant trials protocols on the Pan‐African (https://pactr.samrc.ac.za) and national Institutes of Health's (https://clinicaltrials.gov) clinical‐trials registries.
All references were imported in Endnote X9 software,19 in which records were screened and duplicates were removed. Two authors (JZ and NY) first screened titles and abstracts of articles independently and subsequently assessed full‐texts of the articles for inclusion in the review. Conflicts were resolved through discussion, and in case of disagreement, a third author (AE) was the referee for the final verdict. Data extraction was performed independently by two authors (JZ and NY) and discrepancies were resolved through discussion. The authors used a customized table that was first piloted then amended as necessary. When efficacy outcomes were not available in the full text or supporting information files, JN and NY attempted to contact the corresponding author to acquire the necessary missing information.
2.4. Outcomes
Implant failure was defined as clinically detectable implant mobility.20 Any complications reported by the authors of the original article were included under the overall complications as efficacy outcome. Peri‐implant soft‐tissue esthetics was assessed clinically through the pink esthetic score (PES),21 The PES ranges from 0 to 14 points, with the minimum clinically acceptable value ≥8 points.22, 23 Vertical bone resorption outcome was stratified into vertical buccal and vertical interproximal bone resorption. Horizontal bone resorption outcome was stratified into horizontal buccal and overall horizontal bone resorption. All bone resorption outcomes were measured either clinically or radiographically. Mid‐buccal mucosal recession was measured clinically, and defined as any apical migration of the mid‐buccal implant mucosa from baseline. When multiple follow‐up durations were reported, the longest was used in meta‐analysis. When an RCT used multiple BSM arms, we combined groups that were similar (e.g., multiple types of BSMs).16
2.5. Risk of bias
Two authors (JZ and AE) independently assessed risk of bias (ROB) using the Cochrane risk of bias tool version 2.0 for RCTs.24 All conflicts were resolved through discussion. The Robvis web application was used to produce and visualize relevant plots.25 Authors JN and AE conducted the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) independently via the GRADEpro GDT web application and resolved conflicts through discussion.26, 27
2.6. Data analysis
Meta‐analysis was performed for all outcomes for which quantitative analysis was feasible, using review manager (Revman) 5.4 software.28 Summary estimates and 95% confidence intervals (CI) were generated using the generic inverse variance method in a random‐effects model. Risk ratios (RR) and mean differences (MD) were used for dichotomous (implant failure, overall complications) and continuous outcomes (soft‐tissue esthetics, vertical and horizontal bone resorption and mucosal recession), respectively. Heterogeneity was evaluated using the P‐value of the X 2 test, I 2, Tau2, and visual inspection of overlap between confidence intervals.26, 29 When substantial (I 2 = 50%–90%) heterogeneity was present, pooled estimates were not calculated. If measures of dispersion (e.g., standard deviation) were not reported, values were imputed using mean values obtained from other trials reporting the measure of dispersion for that outcome.16 Descriptive summary of individual trials was provided when summary estimates could not be calculated.
Subgroup analysis was performed to identify potential effect modification when at least two trials were present per subgroup.16 Subgroups that were analyzed included the duration of the follow‐up (short‐term [≤1 year] vs long‐term [>1‐year]), use of a barrier (membrane vs no membrane), surgical approach (flap/no flap), and healing protocol (submerged/non‐submerged). Trials reporting change from baseline and trials reporting only follow‐up measurements were pooled together for the respective outcome.16 Sensitivity analysis was performed for trials with imputed values, and for trials at high risk of bias.
3. RESULTS
3.1. Study selection
The electronic search (Figure 1) retrieved 19 544 references after removal of duplicates. After title and abstract screening, 85 references remained for full‐text evaluation, of which 20 were deemed eligible for inclusion (Table S2). Meta‐analysis was possible for 17 trials30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 while the other three33, 47, 48 were discussed narratively.
FIGURE 1.
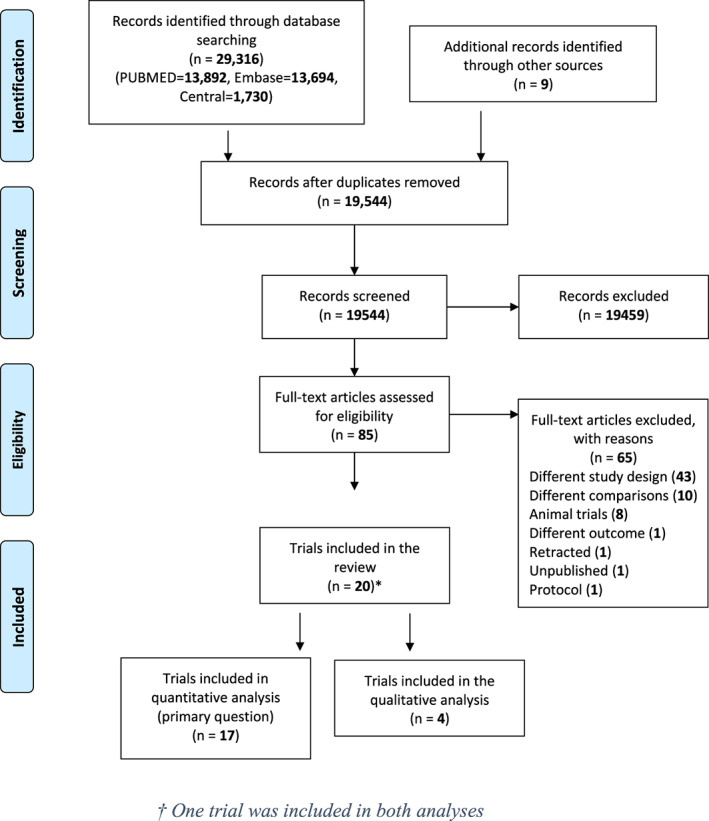
Literature search flowchart. *One trial was included in both analyses
3.2. Characteristics of included studies
Characteristics of the included trials are presented in (Table 1 and Table S3). The 17 trials comparing BSM to NoBSM included 743 randomized participants (804 implants). Longest reported follow‐up ranged from 4 months from placement to 48 months from prosthetic loading, and the predominant site of implant placement was the anterior maxilla.
TABLE 1.
Characteristics of the included trials comparing BSM to no filling material (NoBSM)
| Author (year) | Country (setting) | M:F ratio | Mean age in years ± SD or (range) | Longest follow‐up in months (reference) | Sample size (patients randomized) | Number of implants | Maxilla or Mandible (anterior or posterior) | Intervention | Co‐intervention | Control | Outcomes used in the meta‐analysis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | ||||||||
| Bittner et al.30 | USA (educational) | 9:23 | 52.3 ± 4 | 12 (placement) | 16 | 16 | 16 | 16 | maxilla (anterior) | Xenograft (Bio‐oss collagen) | None | None | Mid‐buccal mucosal recession | ||
| Chen et al.31 | Australia (private practice) | 15:12 | 14:21 | 41.15 ± 1.2 | 42.36 ± 0.72 | 24 (loading) | 27a | 35a | 27a | 35a | maxilla (anterior) | Autograft | Resorbable membrane (Resolut) | Resorbable membrane (Resolut) | Implant failure |
| Non‐resorbable membrane (Gore‐tex) | |||||||||||||||
| Connective‐tissue graft | Connective‐tissue graft | Complications | |||||||||||||
| Chen et al.32 | Australia (private practice) | 7:13 | 3:7 | 46.8 ± 10.8 | 43.2 ± 10.8 | 36 (loading) | 20a | 10 | 20a | 10 | maxilla (anterior) | Xenograft (Bio‐oss) | Collagen membrane (BioGuide) | None | Complications |
| Vertical buccal bone resorption | |||||||||||||||
| Horizontal buccal bone resorption | |||||||||||||||
| Cornelini et al.33 | Italy (educational) | 9:11 | 45 years (21–61) | 6 (placement) | 10 | 10 | 10 | 10 | Both (anterior) | Xenograft (Bio‐oss) | Collagen membrane (BioGuide) | Collagen membrane (BioGuide) | Vertical interproximal bone resorption | ||
| Daif34 | Egypt (educational) | 10:18 | 34 (22–48) | 6 (loading) | 14 | 14 | 14 | 14 | mandible (anterior) | Alloplast (ß‐TCP) | None | None | N/A | ||
| Angelis35 | Italy (private practice) | 21:19 | 21:19 | 46.4 (20–77) | 47.7 (24–75) | 12 (loading) | 40 | 40 | 40 | 40 | Both (Both) | Xenograft (Endobon) | Collagen membrane (OsseoGuard) | Collagen membrane (OsseoGuard) | Soft‐tissue esthetics |
| Implant failure | |||||||||||||||
| Complications | |||||||||||||||
| Vertical interproximal bone resorption | |||||||||||||||
| Gher et al.36 | USA (educational) | 15:5 | 14:6 | 58 (26–80) | 53.95 (27–81) | 6 (placement) | 20 | 20 | 22 | 21 | Both (Both) | Allograft (DFDBA [Calcitek]) | Non‐resorbable membrane (Gore‐tex) | Non‐resorbable membrane (Gore‐tex) | Vertical buccal bone resorption |
| Girlanda et al.37 | Brazil (educational) | 4:18 | (21–58) | 6 (placement) | 11 | 11 | 11 | 11 | Maxilla (anterior) | Xenograft (Bio‐oss collagen) | None | None | Mid‐buccal mucosal recession | ||
| Grassi et al.38 | Italy (educational) | 6:9 | 6:8 | 47.3 ± 8.7 | 42.6 ± 10.7 | 6 (placement) | 15 | 14 | 15 | 14 | Maxilla (anterior) | Xenograft (Bio‐gen) | None | None | Vertical buccal bone resorption |
| Horizontal buccal bone resorption | |||||||||||||||
| Jacobs et al.39 | USA (educational) | 8:11 | 6:8 | 53 ± 20 | 65 ± 14 | 10 (placement) | 19 | 14 | 19 | 14 | Maxilla (anterior) | Xenograft (Bio‐oss) | Collagen dressing (Collagen plug) | None | Soft‐tissue esthetics |
| Vertical buccal bone resorption | |||||||||||||||
| Horizontal buccal bone resorption | |||||||||||||||
| Mid‐buccal mucosal recession | |||||||||||||||
| Mastrangelo et al.40 | Italy (private practice) | 31:20 | 32:19 | 44 ± 6.7 | 36 (loading) | 51 | 51 | 64 | 51 | Maxilla (anterior) | Xenograft (Bio‐oss) | Collagen membrane (Ostebiol) | None | Soft‐tissue esthetics | |
| Implant failure | |||||||||||||||
| Vertical interproximal bone resorption | |||||||||||||||
| Mohamed et al.41 | Egypt (educational) | 2:8 | 2:3 | 33 ± 5 | 35 ± 11 | 6 (placement) | 10a | 5 | 14a | 7 | maxilla (anterior) | Allograft (Puros) | None | None | Vertical buccal bone resorption |
| Alloplast (Bio‐resorp) | |||||||||||||||
| Paknejad et al.42 | Iran (educational) | 3:17 | 38.8 (37–57) | 4–6 (placement) | 20 | 14 | 13 | maxilla (anterior) | Xenograft (Compact bone) | None | None | Vertical buccal bone resorption | |||
| Prosper et al.43 | Italy (educational) | 39:44 | 46.2 ± 14.3 | 48 (placement) | 83 | 56 | 55 | Both (posterior) | Alloplast (Biosite) | None | Resorbable membrane (Osseoquest) | Implant failure | |||
| Sanz et al.44 | Italy | 22:21 | 19:24 | ? | 4 (placement) | 45b | 46b | 43 | 43 | Maxilla (anterior) | Xenograft (Bio‐oss collagen) | None | None | Implant failure | |
| Spain | Vertical buccal bone resorption | ||||||||||||||
| UK | |||||||||||||||
| (educational) | Horizontal buccal bone resorption | ||||||||||||||
| Spinato et al.45 | Italy (private practice) | 11:30 | 42.5 (22–70) | 32 (placement) | 41 | 22 | 23 | Maxilla (anterior) | Xenograft | None | None | Complications | |||
| Allograft | Vertical interproximal bone resorption | ||||||||||||||
| Autograft | |||||||||||||||
| Combination | mid‐buccal mucosal recession | ||||||||||||||
| Yuenyongorarn et al.46 | USA (educational) | 4:6 | 2:8 | 65.4 (41–83) | 12 (placement) | 10 | 10 | 10 | 10 | Maxilla (anterior) | Xenograft (Bio‐oss) | None | None | Implant failure | |
| Vertical interproximal bone resorption | |||||||||||||||
| mid‐buccal mucosal recession | |||||||||||||||
Combined groups.
One patient had a failure and four lost to the follow up.
? = not mentioned.
Eleven trials used a xenograft,30, 32, 33, 35, 37, 38, 39, 40, 42, 44, 46 two used an alloplast,34, 43 one used an autograft,31 one an allograft,36 and one used a combination these.45 For one trial,41 we combined both the alloplast and allograft BSM groups into one intervention group. As an adjunct to the BSM, six trials31, 32, 33, 35, 36, 40 used a barrier membrane, one a collagen plug,39 and another a connective‐tissue graft.31 Five trials31, 33, 35, 36, 43 used a barrier membrane in the control group.
Four trials41, 47, 48, 49 with a total of 105 participants (112 implants) compared different types of BSMs with each other. Two trials compared allograft to alloplast,41, 48 one trial49 autograft to allograft, and one alloplast to autograft.47 The trials did not use barrier membranes, though one trial49 did use platelet‐rich fibrin (PRF) to seal the gap between the implant and the socket. The longest follow‐up ranged from 6 to 44 months from placement.
3.3. Risk of bias assessment
For the outcome implant failure (Figure S1), two trials were at low risk of bias,35, 40 while the other four31, 43, 44, 46 were of some concerns. For overall complications as outcome, one trial35 was at low risk of bias, with the remaining three trials at a high risk of bias (Figure appx1). This was mainly due to deviation from the intended protocol,32 or only reporting complications narratively rather than quantitatively.34, 45 Two out of the three trials that evaluated soft‐tissue esthetics (Figure appx3) were at low risk,35, 40 while the third39 had some concerns.
For the outcome vertical buccal bone resorption (Figure appx6), five trials were judged to have some concerns,32, 36, 39, 41, 42 and two as low ROB.38, 44 Two trials35, 40 were judged to have a low ROB with regard to vertical interproximal resorption (Figure appx8), with the other three33, 45, 46 at some concerns. For horizontal buccal bone resorption, two trials38, 44 were at low ROB, and two trials32, 39 had some concerns (Figure appx10). One trial37 reported the overall horizontal bone resorption, which was at low ROB. Two30, 37 out of five trials assessing mid‐buccal mucosal recession (Figure appx12) outcome were at low ROB while three39, 45, 46 were of some concerns.
Of the trials comparing different types of BSMs, three trials47, 48, 49 were at a high ROB for vertical interproximal bone resorption. One trial41 was of some concerns for vertical buccal bone resorption. One trial49 evaluated implant failure and crestal bone resorption. This trial was judged at high ROB and of some concerns, for these outcomes, respectively.
3.4. Synthesis of results
3.4.1. Implant failure
Fifteen trials assessed implant failure,30, 31, 32, 33, 34, 35, 36, 38, 40, 41, 42, 43, 44, 45, 46 but no trial reported time to implant failure. Only six of these trials31, 35, 40, 43, 44, 46 reported implant failure as a dichotomous (yes/no) outcome, with at least one event observed. The pooled RR for implant failure was 0.92 (95% CI 0.34 to 2.46) (Figure 2) with no detected heterogeneity (I 2 = 0%). No significant difference for the sub‐groups based on duration of follow‐up, surgical approach, or use of a barrier31, 35, 40 (Figures appx14–appx16) was observed. Only one trial49 involved in the second question contributed a single failure event in the allograft group with a statistically non‐significant result (RR = 0.33 [95% CI 0.01 to 7.81]).
FIGURE 2.
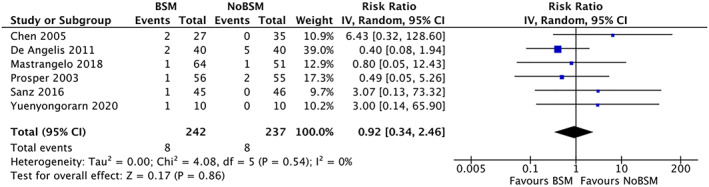
Forest‐plot of “implant failure” using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.2. Overall complications
Twelve trials30, 31, 32, 33, 34, 35, 38, 41, 42, 43, 44, 45 reported complications, but only four reported one or more events.31, 32, 35, 45 The pooled RR was 3.50 (95% CI 1.11 to 11.08) with no detected heterogeneity (I 2 = 0), indicating that use of a BSM in patients undergoing IIP increased the risk of complications (Figure 3). The 12 complications in the BSM group, and the three complications in the NoBSM group, were predominantly minor (93.4%). Complications in the BSM group included three events of abscess formation, and two events of pain at prosthetic loading. Other complications consisted of peri‐implant infection, persistent inflammation of the buccal mucosa, peri‐implant mucositis, small lesions in the peri‐implant mucosa, post‐operative pain, cover screw loosening, and loosening of the provisional abutment. Complications observed in the NoBSM group consisted of de‐cementation of the final prosthesis, post‐operative pain, and cover screw loosening. Sensitivity analysis for trials with low ROB for overall complications resulted in only one trial.35 Though the point estimate of the pooled analysis and this trial were similar, it was not statistically significant (RR = 3.00 [0.64 to 13.98]) (Figure appx22).
FIGURE 3.

Forest‐plot of “overall complications” using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.3. Soft‐tissue esthetics
Three trials35, 39, 40 reported soft‐tissue esthetics using the PES. The mean PES was 1.49 (95% CI 0.46 to 2.53) points higher in the BSM group compared with not using a BSM (Figure 4). Only xenografts were used in the trials and there was moderate heterogeneity (I 2 = 57%). For trials reporting long‐term follow‐up (≥1 year), the mean difference was 1.82 (95% CI 1.09 to 2.55) with no detected heterogeneity.
FIGURE 4.
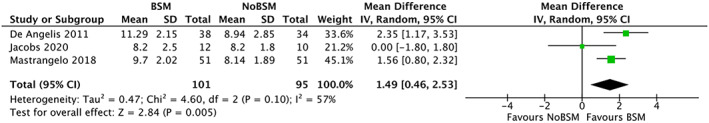
Forest‐plot of “esthetics” (pink esthetic score 0‐14) using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.4. Vertical buccal bone resorption
Thirteen trials reported vertical bone resorption, of which seven32, 36, 38, 39, 41, 42, 44 reported vertical buccal resorption. Six trials32, 36, 38, 41, 42, 44 reported the change from baseline and one trial39 reported only the follow‐up measurements. The pooled mean difference in vertical buccal bone resorption was −0.11 mm (95% CI −0.33 to 0.10) using a BSM compared with control (NoBSM) (Figure 5), with limited heterogeneity (I 2 = 11%). Sub‐group analysis of trials that used membranes either in the BSM group only32 or in both groups36 reported a significantly lower mean resorption of −1.5 mm (95% CI −2.66 to −0.34) in the BSM group (Figure 6). One trial41 compared vertical buccal resorption for different types of BSMs, though did not find a statistically significant difference.
FIGURE 5.
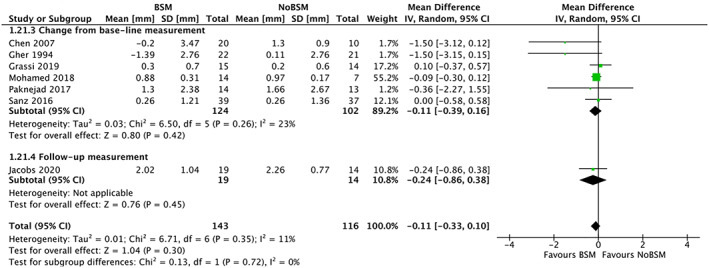
Forest‐plot of “vertical buccal bone resorption” (in mm) using a bone‐substitute material (BSM) vs no filling material (NoBSM)
FIGURE 6.

Forest‐plot of “vertical buccal bone resorption” (in mm) by membrane‐use when using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.5. Vertical inter‐proximal bone resorption
Of the 13 trials looking at vertical bone resorption five33, 35, 40, 45, 46 reported interproximal resorption (Figure 7). Four of these reported change from baseline,33, 40, 45, 46 while the fifth only reported the follow‐up measurement.35 Two trials33, 46 did not report standard deviations for vertical inter‐proximal bone resorption, hence these values were imputed accordingly. One trial43 measured the vertical interproximal bone resorption, but did not provide the mean or standard deviation and was excluded from the analysis. Vertical inter‐proximal bone resorption was lower in the BSM group than the NoBSM group, though not statistically significant (−0.05 mm [95% CI −0.16 to 0.06]). Combining trials reporting change from baseline and trials reporting only follow‐up measurements, resulted in substantial heterogeneity (I 2 = 94.2) and as such no pooled estimate was calculated. Three trials47, 48, 49 compared different types of BSMs, and all reported statistically non‐significant differences regarding vertical interproximal bone resorption.
FIGURE 7.
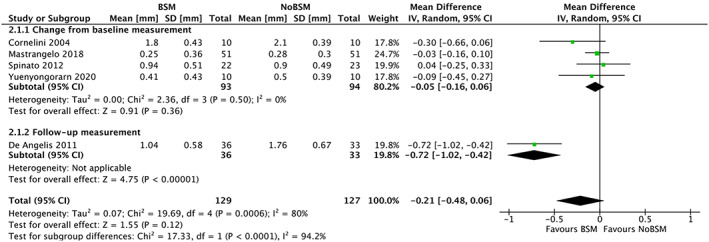
Forest‐plot of “vertical interproximal bone resorption” (in mm) using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.6. Horizontal buccal bone resorption
Five trials reported horizontal bone resorption, of which four32, 38, 39, 44 reported horizontal buccal resorption. Xenograft was the only type of BSM used in these trials. One trial31 reported only the amount of vertical and horizontal defect reduction and was excluded from the analysis. Three trials32, 38, 44 reported change from baseline, with one39 reporting only the follow‐up measurements. Significantly less horizontal buccal bone resorption was observed in the BSM group −0.52 mm (95% CI −0.74 to −0.30), with no heterogeneity (I 2 = 0%) between trials (Figure 8). Both subgroup analyses (Figures appx20 and appx21) based on surgical approach32, 38, 39, 44 and healing protocol,32, 38, 39, 44 did not show a significant difference regarding horizontal buccal bone resorption.
FIGURE 8.
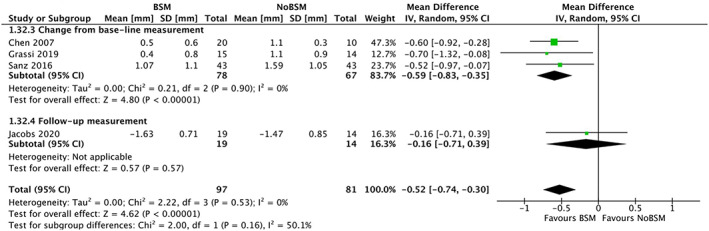
Forest‐plot of “horizontal buccal bone resorption” (in mm) using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Only one trial37 reported the bucco‐palatal horizontal dimensions through the ridge width at 1 mm apical to the crest. A lower mean horizontal buccal bone resorption of −0.22 mm (95% CI −0.25 to −0.19) was observed in the BSM group compared with the NoBSM group. One trial49 compared the mean horizontal buccal bone resorption of allograft (0.72 mm ± 1.46) to autograft (−0.08 mm ± 1.95). This trial reported significantly (P = 0.026) less resorption when using an allograft.
3.4.7. Mid‐buccal mucosal recession
Five trials30, 37, 39, 45, 46 reported the amount of mid‐buccal mucosal recession as a continuous variable. One trial32 reported recession as present or absent with no threshold mentioned, and thus was excluded from the analysis. Pooled analysis of the five other trials showed a non‐significant increase in mid‐buccal mucosal recession of 0.02 mm (95% CI −0.23 to 0.28) in the BSM group, with no detectable heterogeneity (I 2 = 0%; Figure 9). There was no difference between the three trials30, 45, 46 reporting change from baseline (0.02 mm [95% CI −0.25 to 0.30]), and the other two trials37, 39 reporting only follow‐up measurements (0.02 mm [95% CI −0.68 to 0.71]).
FIGURE 9.
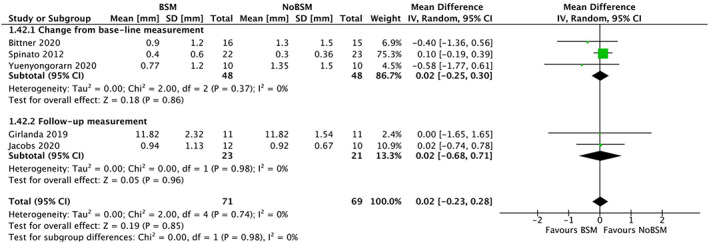
Forest‐plot of “mid‐buccal mucosal recession” (in mm) using a bone‐substitute material (BSM) vs no filling material (NoBSM)
3.4.8. Grade
Grading of evidence and reasons for downgrading are presented in Table S4. A moderate certainty of evidence was judged for soft‐tissue esthetics, vertical buccal bone resorption, horizontal buccal bone resorption, and mid‐buccal mucosal recession outcomes. Implant failure was judged to be at low certainty of evidence judged by the wide confidence intervals and small sample size. Overall complications and vertical interproximal bone resorption were judged to be at very low certainty of evidence. The reasons for this downgrading were wide confidence intervals, small sample size, few complications events, and substantial heterogeneity (I 2 = 94%) for the vertical interproximal bone resorption. Moreover, the majority of the involved trials in these two outcomes were of some concerns or at high ROB.
4. DISCUSSION
In this systematic review, we have evaluated the evidence on the efficacy of using a BSM in the fixture‐socket gap in patients undergoing tooth extraction and IIP. In view of the wide confidence intervals, BSM may increase or decrease the risk of implant failure (low certainty). Overall complications were more frequent (very low certainty) though these were predominantly minor. Esthetic scores were significantly higher (moderate certainty) in patients undergoing IIP with BSM.
The results should be interpreted with the considerations that a significant number of trials had at least some concerns for ROB for the relevant outcomes. As a consequence, most recommendations were based on a moderate certainty of evidence except for implant failure (low), complications (very low) and vertical inter‐proximal bone resorption (very low). The certainty of the evidence was downgraded because of imprecision, ROB, and inconsistency (Table S4).
Finding no significant differences for implant failure could be explained by the very high success rates of IIP6, 50 in both review arms, and low absolute risk of developing (serious) complications which might cause implant failure. No trials reported the time to implant failure as an outcome. Although there does not appear to be a clear reason, one possible explanation could be unfamiliarity in the dentistry field with more complex statistical methods, such as survival analysis. Not using survival analysis methods, and therefore, not taking selective censoring into account may result in biased estimates of implant failure.16
We found more complications when using a BSM compared with not using one.10 However, the complications were predominantly minor, mainly peri‐implant mucositis, post‐operative pain, prosthetic complications, and only one case of peri‐implantitis. The higher risk of complications may be due to the use of regenerative materials and the associated technique‐sensitive approach.51 Poor reporting combined with the use of ambiguous definition of what constitute as complications could explain the limited number of complication events observed in included RCTs. The effect of BSM use on soft‐tissue esthetics could be explained by the incorporation of the BSM particles in the soft‐tissue, and the less horizontal resorption of the supporting bone. This might result in thicker and more supported soft tissues with improved long‐term soft‐tissue esthetics.10, 12, 52, 53, 54, 55
Our review did not demonstrate a mitigating effect of a BSM on vertical buccal bone resorption. The loss of the bundle bone during extraction, in addition to the amount of cancellous component at the alveolar crest of thin labial plates, seems to outweigh the potential positive effect of BSM on the amount of vertical buccal bone resorption.56 Moreover, the trials' inclusion criteria that favor IIP such as the presence of an intact post‐extraction labial plate, minimized the expected resorption in both arms.4, 45 Another explanation could be the limited diagnostic performance of the Cone‐beam computed tomography (CBCT) in measuring the thin peri‐implant bone.57, 58, 59 Furthermore, the slow resorption rates of the radio‐opaque graft materials, could decrease the validity of radiographic and clinical measurements of the vertical buccal bone resorption in the BSM group.60
An effect in favor or against the use of a BSM could not be demonstrated for interproximal bone resorption. This finding could be explained by the dependency of the interproximal bone height mainly on the attachment level on the neighboring natural teeth.4, 61 Also, the benefit of using a BSM on the mid‐buccal mucosal recession could not be demonstrated nor refuted. The intactness of the labial plate and the non‐submerged healing protocol in the five trials30, 37, 39, 45, 46 reporting mid‐buccal mucosal recession could help understanding this finding.62, 63
To our knowledge, there is no clear histological explanation for the significantly less horizontal buccal resorption when using a BSM.64 All of the trials involved in this outcome used a xenograft, which has a slow resorption rate.65 Therefore, the hard‐tissue structure in the fixture‐socket gap could be vital bone, non‐resorbed BSM particles in a connective‐tissue matrix, or a combination of both.65 A radiographic, histologic, or clinical distinction should be made to identify the nature of the peri‐implant hard‐tissue structure, and its implications. After tooth extraction, the horizontal resorption is the most pronounced, compared with the vertical resorption.66, 67 Mitigating horizontal bone resorption in sites with thin buccal plate (≤2 mm) would provide the patients with many benefits; especially in the esthetic zone.66, 67, 68 These benefits include better and stable peri‐implant soft‐tissue esthetics, in addition to improved implant function, and hygiene.54, 69, 70 Thin biotype and inadequate oral hygiene are risk factors for developing peri‐implantitis and subsequent reduction in the quality of life.71, 72
This review systematically assessed the efficacy and strength of evidence of BSMs on a wide range of clinical outcomes assessed in RCTs published in both generic and dentistry‐specific databases. Both quantitative and qualitative assessments of various clinically relevant outcomes were performed. It also complied to the guidelines provided by international literature regarding reporting, risk of bias assessment, and evaluation of the strength of evidence.
There are some limitations to fully appreciate the findings in this review. No trials reported the time to implant failure; consequently, implant failure was analyzed as a dichotomous outcome. Only a limited number of trials reporting implant failure as the primary outcome were found. These trials had a small sample size, and as a result, the precision of our pooled estimate is limited. The inconsistent methods of reporting and heterogeneity in the measurements and definitions used for the various outcomes posed a challenge, resulting in some concessions (e.g., combining outcome categories). Sub‐groups based on type of BSM used, labial plate thickness, or fixture‐socket dimensions could not be performed because only one trial was present for some of these subgroups. Moreover, due to the paucity of trials we could not compare the efficacy of different types of BSMs to each other regarding implant failure, overall complications, and soft‐tissue esthetics.
Five systematic reviews on the topic of this article have been published. Each of these reviews included both observational and experimental study designs. Two reviews13, 15 could not provide a conclusion in favor or against the use of a BSM due to insufficient evidence. Another review12 did not provide a quantitative analysis because of high heterogeneity of the included studies. However, this review concluded that the use of guided bone regeneration techniques preserves the peri‐implant hard and soft tissues. One review73 studied the broad outcome “crestal bone loss”; and reported a non‐significant difference with substantial heterogeneity (I 2 = 59.6%). Our findings are in accordance with a systematic review by Lee and colleagues14 who reported a MD of 0.01 (95% CI −0.05 to 0.08), and a weighted mean difference (WMD) of 0.84 mm (95% CI 0.53 to 1.14) for implant failure, and the change in horizontal bone dimensions, respectively. However, in this review the definition of the outcome assessing changes in ridge width was not clear. Moreover, this review included only seven trials, one of which been retracted due to the absence of the ethical approval to conduct the trial.74
5. CONCLUSIONS
Compared with no BSM, a beneficial or harmful effect of BSM on implant failure could not be demonstrated nor refuted (low level of certainty).
BSM use results in an increased risk of minor complications (very low level of certainty), reduces the amount of horizontal buccal resorption (moderate level of certainty), and improves long‐term peri‐implant soft‐tissue esthetics (moderate level of certainty) compared with no BSM. Therefore, BSM use is recommended in the esthetic zone and sites with thin buccal plate, after discussing the benefits and drawbacks of using a BSM with the patient.
A difference in the amount of vertical buccal or interproximal bone resorption (moderate level of certainty), or the amount of mid‐buccal mucosal recession (moderate level of certainty) between BSM use and no BSM use could not be demonstrated nor refuted.
6. IMPLICATIONS FOR FUTURE RESEARCH
RCTs of sufficient sample size and consistent reporting of the outcomes.
RCTs assessing the added value of using a membrane with a BSM in IIP.
RCTs comparing the efficacy of various BSMs to xenografts in IIP.
Studies assessing the validity and reliability of the instruments used to measure peri‐implant bone dimensions and mucosal recession.
ABBREVIATIONS
- BSM
bone‐substitute material
- BSMs
bone‐substitute materials
- NoBSM
no bone‐substitute material
- IIP
immediate implant placement
- DIP
delayed implant placement
- RCT
randomized‐controlled trial
- RCTs
randomized‐controlled trials
- ROB
risk of bias
- PES
pink esthetic score
- RR
relative risk
- MD
mean difference
- CI
confidence intervals
- PRF
platelet‐rich fibrin
- CBCT
cone‐beam computed tomography
- PRISMA
preferred reporting items for systematic reviews and meta‐analyses
- GRADE
grading of recommendations, assessment, development and evaluations
- USA
United States
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
John Zaki: Conceived the idea; authored the manuscript, screened the titles, selected the trials and extracted the data; assessed the risk of bias, and graded the evidence; designed the analysis plan. Rob J.P.M Scholten: Conceived the idea; designed the analysis plan; authored the manuscript; revised the manuscript. Nermin Yussif: Conceived the idea; screened the titles, selected the trials and extracted the data. Ahmed El‐Khadem: Assessed the risk of bias, and graded the evidence. Kevin Jenniskens: Authored the manuscript; revised the manuscript.
Supporting information
Table S1 Search strategy for MEDLINE (PUBMED)
Table S2. Excluded publications with reasons
Table S3. Characteristics of the included trials comparing different bone‐substitute materials with each other
Table S4. Summary of findings table
Figure S1. Risk of bias summary for “implant failure”
Appendix S1 Supporting Information.
Figure appx1. Risk of bias summary of “complications” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx2. The weighted summary of “complications” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx3. Risk of bias summary of “soft‐tissue esthetics” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx4. The weighted summary of “soft‐tissue esthetics” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx5. The weighted summary of “implant failure” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx6. Risk of bias summary of “vertical crestal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx7. The weighted summary of “vertical crestal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx8. The risk of bias summary of “vertical interproximal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx9. The weighted summary of “vertical interproximal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx10. The risk of bias summary of “horizontal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx11. The weighted summary of “horizontal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx12. The risk of bias summary of “mid‐buccal mucosal recession” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx13. The weighted summary of “mid‐buccal mucosal recession” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx14. The forest plot of “implant failure” by duration of follow‐up when using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx15. The forest plot of “implant failure” by surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx16. The forest plot of “implant failure” by membrane‐use using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx17. The forest plot of “vertical buccal bone resorption” by the surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx18. The forest plot of “vertical buccal bone resorption” by membrane‐use using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx19. The forest plot of “vertical buccal bone resorption” by healing protocol using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx20. The forest plot of “horizontal buccal bone resorption” by surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM).
Figure appx21. The forest plot of “horizontal buccal bone resorption” by healing protocol using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx22. The forest‐plot representing the sensitivity analysis of “overall complications” based on trials at low risk of bias using a bone‐substitute material (BSM) vs no filling material (NoBSM)
ACKNOWLEDGMENT
In memory of Ola Kamel, you made it happen; thank you!
The review received no funding.
Zaki J, Yusuf N, El‐Khadem A, Scholten RJPM, Jenniskens K. Efficacy of bone‐substitute materials use in immediate dental implant placement: A systematic review and meta‐analysis. Clin Implant Dent Relat Res. 2021;23(4):506–519. 10.1111/cid.13014
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article and from the corresponding author upon reasonable request.
REFERENCES
- 1.Araujo MG, Silva CO, Misawa M, Sukekava F. Alveolar socket healing: what can we learn? Periodontol 2000. 2015;68(1):122‐134. [DOI] [PubMed] [Google Scholar]
- 2.Araujo MG, Silva CO, Souza AB, Sukekava F. Socket healing with and without immediate implant placement. Periodontol 2000. 2019;79(1):168‐177. [DOI] [PubMed] [Google Scholar]
- 3.Botticelli D, Berglundh T, Lindhe J. Hard‐tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820‐828. [DOI] [PubMed] [Google Scholar]
- 4.Chappuis V, Araujo MG, Buser D. Clinical relevance of dimensional bone and soft tissue alterations post‐extraction in esthetic sites. Periodontol 2000. 2017;73(1):73‐83. [DOI] [PubMed] [Google Scholar]
- 5.Cosyn J, De Lat L, Seyssens L, Doornewaard R, Deschepper E, Vervaeke S. The effectiveness of immediate implant placement for single tooth replacement compared to delayed implant placement: a systematic review and meta‐analysis. J Clin Periodontol. 2019;46:224‐241. [DOI] [PubMed] [Google Scholar]
- 6.Mello CC, Lemos CAA, Verri FR, Dos Santos DM, Goiato MC, Pellizzer EP. Immediate implant placement into fresh extraction sockets versus delayed implants into healed sockets: a systematic review and meta‐analysis. Int J Oral Maxillofac Surg. 2017;46(9):1162‐1177. [DOI] [PubMed] [Google Scholar]
- 7.Atieh MA, Alsabeeha NH, Payne AG, Duncan W, Faggion CM, Esposito M. Interventions for replacing missing teeth: alveolar ridge preservation techniques for dental implant site development. Cochrane Database Syst Rev. 2015;5:CD010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016;98B:6‐9. [DOI] [PubMed] [Google Scholar]
- 9.Jambhekar S, Kernen F, Bidra AS. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: a systematic review of randomized controlled clinical trials. J Prosthet Dent. 2015;113(5):371‐382. [DOI] [PubMed] [Google Scholar]
- 10.MacBeth N, Trullenque‐Eriksson A, Donos N, Mardas N. Hard and soft tissue changes following alveolar ridge preservation: a systematic review. Clin Oral Implants Res. 2017;28(8):982‐1004. [DOI] [PubMed] [Google Scholar]
- 11.Papageorgiou SN, Papageorgiou PN, Deschner J, Gotz W. Comparative effectiveness of natural and synthetic bone grafts in oral and maxillofacial surgery prior to insertion of dental implants: systematic review and network meta‐analysis of parallel and cluster randomized controlled trials. J Dent. 2016;48:1‐8. [DOI] [PubMed] [Google Scholar]
- 12.AlKudmani H, Al Jasser R, Andreana S. Is bone graft or guided bone regeneration needed when placing immediate dental implants? A systematic review. Implant Dent. 2017;26(6):936‐944. [DOI] [PubMed] [Google Scholar]
- 13.Clementini M, Tiravia L, De Risi V, Vittorini Orgeas G, Mannocci A, de Sanctis M. Dimensional changes after immediate implant placement with or without simultaneous regenerative procedures: a systematic review and meta‐analysis. J Clin Periodontol. 2015;42(7):666‐677. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Park D, Koo KT, Seol YJ, Lee YM. Validity of a regenerative procedure for a minor bone defect with immediate implant placement: a systematic review and meta‐analysis. Acta Odontol Scand. 2019;77(2):99‐106. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed HHB, Serag Eldien AM, Zahran A. Augmentation versus no augmentation for immediate postextraction implants. Int J Dent. 2018;2018:5209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Vol 2019. 2nd ed.Chichester, UK: John Wiley & Sons; 2019. [Google Scholar]
- 17.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:2046‐4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.University of York . 'PROSPERO' The International Prospective Register of Systematic Reviews. https://www.crd.york.ac.uk/prospero/ Accessed date March 15, 2021.
- 19.The EndNote Team . EndNote, EndNote X9 ed. Philadelphia, PA: Clarivate Analytics; 2013. [Google Scholar]
- 20.Smith DE, Zarb GA. Criteria for success of osseointegrated endosseous implants. J Prosthet Dent. 1989;62(5):567‐572. [DOI] [PubMed] [Google Scholar]
- 21.Furhauser R, Florescu D, Benesch T, Haas R, Mailath G, Watzek G. Evaluation of soft tissue around single‐tooth implant crowns: the pink esthetic score. Clin Oral Implants Res. 2005;16(6):639‐644. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Chiang C, Zhang Y. Esthetic evaluation of natural teeth in anterior maxilla using the pink and white esthetic scores. Clin Implant Dent Relat Res. 2018;20(5):770‐777. [DOI] [PubMed] [Google Scholar]
- 23.Cosyn J, Eghbali A, De Bruyn H, Dierens M, De Rouck T. Single implant treatment in healing versus healed sites of the anterior maxilla: an aesthetic evaluation. Clin Implant Dent Relat Res. 2012;14(4):517‐526. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness LA, Higgins J. Risk‐of‐bias VISualization (robvis): an R package and shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods. 2020;12(1):55‐61. 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 26.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations.: The GRADE Working Group; 2013.
- 27.Evidence Prime I . GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. Hamilton, ON: McMaster University; 2015. [Google Scholar]
- 28.Cochrane RevMan . Review Manager (RevMan), 5.4 Ed. London: The Cochrane Collaboration; 2020. [Google Scholar]
- 29.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bittner N, Fau‐Planzos L, Planzos L, et al. Evaluation of horizontal and vertical buccal ridge dimensional changes after immediate implant placement and immediate temporization with and without bone augmentation procedures: short‐term, 1‐year results. A randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2020;40(1):83‐93. [DOI] [PubMed] [Google Scholar]
- 31.Chen ST, Darby IB, Adams GG, Reynolds EC. A prospective clinical study of bone augmentation techniques at immediate implants. Clin Oral Implants Res. 2005;16(2):176‐184. [DOI] [PubMed] [Google Scholar]
- 32.Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non‐submerged immediate implants: clinical outcomes and esthetic results. Clin Oral Implants Res. 2007;18(5):552‐562. [DOI] [PubMed] [Google Scholar]
- 33.Cornelini R, Cangini F, Martuscelli G, Wennstrom J. Deproteinized bovine bone and biodegradable barrier membranes to support healing following immediate placement of transmucosal implants: a short‐term controlled clinical trial. Int J Periodontics Restorative Dent. 2004;24(6):555‐563. [PubMed] [Google Scholar]
- 34.Daif ET. Effect of a multiporous beta‐tricalicum phosphate on bone density around dental implants inserted into fresh extraction sockets. J Oral Implantol. 2013;39(3):339‐344. [DOI] [PubMed] [Google Scholar]
- 35.Angelis ND, Felice P, Pellegrino G, Camurati A, Gambino P, Esposito M. Guided bone regeneration with and without a bone substitute at single post‐extractive implants: 1‐year post‐loading results from a pragmatic multicentre randomised controlled trial. Eur J Oral Implantol. 2011;4(4):313‐325. [PubMed] [Google Scholar]
- 36.Gher ME, Quintero G, Assad D, Monaco E, Richardson AC. Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol. 1994;65(9):881‐891. [DOI] [PubMed] [Google Scholar]
- 37.Girlanda FF, Feng HS, Correa MG, et al. Deproteinized bovine bone derived with collagen improves soft and bone tissue outcomes in flapless immediate implant approach and immediate provisionalization: a randomized clinical trial. Clin Oral Investig. 2019;23(10):3885‐3893. [DOI] [PubMed] [Google Scholar]
- 38.Grassi FR, Grassi R, Rapone B, Alemanno G, Balena A, Kalemaj Z. Dimensional changes of buccal bone plate in immediate implants inserted through open flap, open flap and bone grafting and flapless techniques: a cone‐beam computed tomography randomized controlled clinical trial. Clin Oral Implants Res. 2019;30(12):1155‐1164. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs BP, Zadeh HH, De Kok I, Cooper L. A randomized controlled trial evaluating grafting the facial gap at immediately placed implants. Int J Periodontics Restorative Dent. 2020;40(3):383‐392. [DOI] [PubMed] [Google Scholar]
- 40.Mastrangelo F, Gastaldi G, Vinci R, et al. Immediate postextractive implants with and without bone graft: 3‐year follow‐up results from a multicenter controlled randomized trial. Implant Dent. 2018;27(6):638‐645. [DOI] [PubMed] [Google Scholar]
- 41.Huda M, Sherirn HM, Amr Z, Ahmed R. Clinical evaluation of the stability of the immediately loaded dental implants with and without bone grafting materials in the maxillary premolar extraction sockets. EDJ. 2018;64(3):1145‐1154. [Google Scholar]
- 42.Paknejad M, Akbari S, Aslroosta H, Panjnoush M, Hajheidary S. Effect of flapless immediate implantation and filling the Buccal gap with Xenograft material on the buccal bone level: a randomized clinical trial. J Dent (Tehran). 2017;14(6):344‐351. [PMC free article] [PubMed] [Google Scholar]
- 43.Prosper P, Gherlone EF, Redaelli S, Quaranta M. Four‐year follow‐up of larger‐diameter implants placed in fresh extraction sockets using a resorbable membrane or a resorbable alloplastic material. Int J Oral Maxillofac Implants. 2003;18(6):856‐864. [PubMed] [Google Scholar]
- 44.Sanz M, Lindhe J, Alcaraz J, Sanz‐Sanchez I, Cecchinato D. The effect of placing a bone replacement graft in the gap at immediately placed implants: a randomized clinical trial. Clin Oral Implants Res. 2017;28(8):902‐910. [DOI] [PubMed] [Google Scholar]
- 45.Spinato S, Agnini A, Chiesi M, Agnini AM, Wang HL. Comparison between graft and no‐graft in an immediate placed and immediate nonfunctional loaded implant. Implant Dent. 2012;21(2):97‐103. [DOI] [PubMed] [Google Scholar]
- 46.Yuenyongorarn P, Kan JYK, Rungcharassaeng K, et al. Facial gingival changes with and without socket gap grafting following single maxillary anterior immediate tooth replacement: 1‐year results. J Oral Implantol. 2020;46(5):496‐505. [DOI] [PubMed] [Google Scholar]
- 47.Adam SAN, Elarab AE, Rahman ARA, Rahim DFA, Nouby S. Evaluation of implant stability and marginal bone loss in immediate implant using “nano bone” versus “autogenous bone” for the treatment of patients with unrestorable single tooth: a randomized controlled trial. J Osseointegr. 2019;12(1):8‐17. [Google Scholar]
- 48.Viswambaran M, Arora V, Tripathi RC, Dhiman RK. Clinical evaluation of immediate implants using different types of bone augmentation materials. Med J Armed Forces India. 2014;70(2):154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noelken R, Pausch T, Wagner W, Al‐Nawas B. Peri‐implant defect grafting with autogenous bone or bone graft material in immediate implant placement in molar extraction sites‐1‐ to 3‐year results of a prospective randomized study. Clin Oral Implants Res. 2020;31(11):1138‐1148. [DOI] [PubMed] [Google Scholar]
- 50.Lin G, Ye S, Liu F, He F. A retrospective study of 30,959 implants: risk factors associated with early and late implant loss. J Clin Periodontol. 2018;45(6):733‐743. [DOI] [PubMed] [Google Scholar]
- 51.Lim G, Lin GH, Monje A, Chan HL, Wang HL. Wound healing complications following guided bone regeneration for ridge augmentation: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2018;33(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 52.Avila‐Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta‐analysis. J Clin Periodontol. 2019;46:195‐223. [DOI] [PubMed] [Google Scholar]
- 53.Chu SJ, Salama MA, Salama H, et al. The dual‐zone therapeutic concept of managing immediate implant placement and provisional restoration in anterior extraction sockets. Compend Contin Educ Dent. 2012;33(7):524‐532. [PubMed] [Google Scholar]
- 54.Chen ST, Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla: a systematic review. Int J Oral Maxillofac Implants. 2014;29:186‐215. [DOI] [PubMed] [Google Scholar]
- 55.Farronato D, Pasini PM, Orsina AA, Manfredini M, Azzi L, Farronato M. Correlation between buccal bone thickness at implant placement in healed sites and buccal soft tissue maturation pattern: a prospective three‐year study. Materials (Basel). 2020;13(3):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 57.Bohner LOL, Mukai E, Oderich E, et al. Comparative analysis of imaging techniques for diagnostic accuracy of peri‐implant bone defects: a meta‐analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(4):432‐440. [DOI] [PubMed] [Google Scholar]
- 58.Naveau A, Shinmyouzu K, Moore C, Avivi‐Arber L, Jokerst J, Koka S. Etiology and measurement of peri‐implant crestal bone loss (CBL). J Clin Med. 2019;8(2):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ritter L, Elger MC, Rothamel D, et al. Accuracy of peri‐implant bone evaluation using cone beam CT, digital intra‐oral radiographs and histology. Dentomaxillofac Radiol. 2014;43(6):20130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elnayef B, Porta C, Suarez‐Lopez Del Amo F, Mordini L, Gargallo‐Albiol J, Hernandez‐Alfaro F. The fate of lateral ridge augmentation: a systematic review and meta‐analysis. Int J Oral Maxillofac Implants. 2018;33(3):622‐635. [DOI] [PubMed] [Google Scholar]
- 61.Jung RE, Heitz‐Mayfield L, Schwarz F, Groups of the 2nd Osteology Foundation Consensus M . Evidence‐based knowledge on the aesthetics and maintenance of peri‐implant soft tissues: osteology foundation consensus report part 3‐aesthetics of peri‐implant soft tissues. Clin Oral Implants Res. 2018;29:14‐17. [DOI] [PubMed] [Google Scholar]
- 62.Pohl V, Furhauser L, Haas R, Pohl S. Gingival recession behavior with immediate implant placement in the anterior maxilla with buccal dehiscence without additional augmentation‐a pilot study. Clin Oral Investig. 2020;24(4):1455‐1464. [DOI] [PubMed] [Google Scholar]
- 63.Benic GI, Mokti M, Chen CJ, Weber HP, Hammerle CH, Gallucci GO. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: a clinical and cone beam computed tomography study. Clin Oral Implants Res. 2012;23(5):560‐566. [DOI] [PubMed] [Google Scholar]
- 64.Willenbacher M, Al‐Nawas B, Berres M, Kammerer PW, Schiegnitz E. The effects of alveolar ridge preservation: a meta‐analysis. Clin Implant Dent Relat Res. 2016;18(6):1248‐1268. [DOI] [PubMed] [Google Scholar]
- 65.Zhao H, Hu J, Zhao L. Histological analysis of socket preservation using DBBM. A systematic review and meta‐analysis. J Stomatol Oral Maxillofac Surg. 2020;121(6):729‐735. [DOI] [PubMed] [Google Scholar]
- 66.Couso‐Queiruga E, Stuhr S, Tattan M, Chambrone L, Avila‐Ortiz G. Post‐extraction dimensional changes: a systematic review & meta‐analysis. J Clin Periodontol. 2020;48(1):126‐144. [DOI] [PubMed] [Google Scholar]
- 67.Seyssens L, De Lat L, Cosyn J. Immediate implant placement with or without connective tissue graft: a systematic review and meta‐analysis. J Clin Periodontol. 2020;48(2):284‐301. [DOI] [PubMed] [Google Scholar]
- 68.Tsigarida A, Toscano J, de Brito BB, et al. Buccal bone thickness of maxillary anterior teeth: a systematic review and meta‐analysis. J Clin Periodontol. 2020;47(11):1326‐1343. [DOI] [PubMed] [Google Scholar]
- 69.0.02w?>Nikiforidou M, Tsalikis L, Angelopoulos C, Menexes G, Vouros I, Konstantinides A. Classification of periodontal biotypes with the use of CBCT. A cross‐sectional study. Clin Oral Investig. 2016;20(8):2061‐2071. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Zhou T, Zhou N, Man Y. The thickness of labial bone affects the esthetics of immediate implant placement and provisionalization in the esthetic zone: a prospective cohort study. Clin Implant Dent Relat Res. 2019;21(3):482‐491. [DOI] [PubMed] [Google Scholar]
- 71.Insua A, Monje A, Wang HL, Inglehart M. Patient‐centered perspectives and understanding of peri‐implantitis. J Periodontol. 2017;88(11):1153‐1162. [DOI] [PubMed] [Google Scholar]
- 72.Schwarz F, Derks J, Monje A, Wang HL. Peri‐implantitis. J Periodontol. 2018;89:S267‐S290. [DOI] [PubMed] [Google Scholar]
- 73.Kinaia BM, Kazerani S, Korkis S, Masabni OM, Shah M, Neely AL. Effect of guided bone regeneration on immediately placed implants: meta‐analyses with at least 12 months follow up after functional loading. J Periodontol. 2019;(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 74.Bottini LP, Ricci L, Piattelli A, Perrotti V, Iezzi G. RETRACTED: bucco‐lingual crestal bone changes around implants immediately placed in fresh extraction sockets in association or not with porcine bone: a non‐blinded randomized controlled trial in humans. J Periodontol. 2012;88(12):1374. 10.1902/jop.2012.120396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategy for MEDLINE (PUBMED)
Table S2. Excluded publications with reasons
Table S3. Characteristics of the included trials comparing different bone‐substitute materials with each other
Table S4. Summary of findings table
Figure S1. Risk of bias summary for “implant failure”
Appendix S1 Supporting Information.
Figure appx1. Risk of bias summary of “complications” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx2. The weighted summary of “complications” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx3. Risk of bias summary of “soft‐tissue esthetics” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx4. The weighted summary of “soft‐tissue esthetics” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx5. The weighted summary of “implant failure” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx6. Risk of bias summary of “vertical crestal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx7. The weighted summary of “vertical crestal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx8. The risk of bias summary of “vertical interproximal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx9. The weighted summary of “vertical interproximal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx10. The risk of bias summary of “horizontal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx11. The weighted summary of “horizontal buccal bone resorption” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx12. The risk of bias summary of “mid‐buccal mucosal recession” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx13. The weighted summary of “mid‐buccal mucosal recession” when using a bone‐substitute material vs no filling material (BSM vs NoBSM)
Figure appx14. The forest plot of “implant failure” by duration of follow‐up when using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx15. The forest plot of “implant failure” by surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx16. The forest plot of “implant failure” by membrane‐use using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx17. The forest plot of “vertical buccal bone resorption” by the surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx18. The forest plot of “vertical buccal bone resorption” by membrane‐use using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx19. The forest plot of “vertical buccal bone resorption” by healing protocol using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx20. The forest plot of “horizontal buccal bone resorption” by surgical approach using a bone‐substitute material (BSM) vs no filling material (NoBSM).
Figure appx21. The forest plot of “horizontal buccal bone resorption” by healing protocol using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Figure appx22. The forest‐plot representing the sensitivity analysis of “overall complications” based on trials at low risk of bias using a bone‐substitute material (BSM) vs no filling material (NoBSM)
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article and from the corresponding author upon reasonable request.


