Abstract
Objectives
People living with HIV (PLWH) have a high risk of kidney injury. Measurement of serum creatinine, along with proteinuria, is not sensitive to detect early kidney injury. Here, we investigated novel urinary biomarkers of early renal injury in PLWH.
Methods
We performed a cross‐sectional study of 166 antiretroviral‐naïve PLWH and 99 HIV‐negative persons who all had an estimated glomerular filtration rate > 90 mL/min/1.73 m2. We compared the levels of seven urinary biomarkers between the two groups using the propensity score matching (PSM) approach and explored the risk factors associated with elevated urinary biomarkers in PLWH.
Results
Eighty‐three pairs were successfully matched based on PSM. Compared with the HIV‐negative group, the HIV‐positive group had higher ratios of N‐acetyl‐β‐D‐glucosaminidase (NAG) to urine creatinine (UCr), alpha1‐microglobulin (α1‐M) to UCr, kidney injury marker‐1 (KIM‐1) to UCr, neutrophil gelatinase‐associated lipocalin to UCr, and epidermal growth factor to UCr, whereas the Tamm–Horsfall protein to UCr ratio and the abnormal albumin to UCr ratio were not significantly different. Positive correlations were observed between HIV RNA level and NAG: UCr (rs = 0.32; P < 0.001) and α1‐M:UCr (rs = 0.24; P = 0.002) ratios, and negative correlations were observed between CD4 cell count and NAG:UCr (rs = –0.34; P < 0.001), KIM‐1:UCr (rs = –0.16; P = 0.042) and α1‐M:UCr (rs = –0.36; P < 0.001) ratios. In multivariate linear regression analyses, older age, lower total cholesterol and higher HIV RNA were independently associated with higher NAG:UCr; older age, lower total cholesterol and lower CD4 cell count were independently associated with higher α1‐M:UCr.
Conclusions
In comparioson with HIV‐negative participants, PLWH were more likely to have tubular injury. Early antiretroviral treatment might mitigate the development of kidney injury.
Keywords: HIV infection, kidney injury, propensity score matching, urinary biomarkers
Introduction
With the advent of combination antiretroviral therapy (ART), the prognosis of individuals with HIV infection has substantially improved. As life expectancy has improved, non‐AIDS‐related comorbidities have become increasingly important causes of morbidity and mortality in people living with HIV (PLWH) [1]. Kidney disease has emerged as a common comorbidity in PLWH, and HIV‐infected persons have a higher incidence of end‐stage renal disease than those without HIV infection [2, 3, 4]. Therefore, it is critical to identify early kidney damage in order to reduce the risks of overt renal injury. Unfortunately, traditional markers of kidney disease, such as estimated glomerular filtration rate (eGFR) and proteinuria, have low sensitivity and specificity for the early diagnosis of impaired kidney function [5].
The development of proteomics has resulted in the discovery of novel urinary biomarkers for detecting early kidney damage [6]. In the Women’s Interagency HIV Study (WIHS), HIV‐infected women had more extensive tubulointerstitial and glomerular injury than uninfected women [7], and urinary interleukin‐18 (IL‐18), kidney injury molecule‐1 (KIM‐1) and albuminuria showed independent and complementary associations with longitudinal kidney function decline [8]. However, most studies were conducted in HIV‐infected patients without comparisons with the general population, or in unselected HIV‐infected patients regardless of ART [5, 7]. The subject selection bias and the multiple potential confounders that existed make it hard to draw conclusions regarding causality between HIV infection and early kidney injury [7]. The propensity score matching (PSM) approach is widely used to reduce bias in the estimation of treatment effects, exposures and interventions in observational studies, which can address covariate imbalance and enhance research efficiency. Herein, we undertook a cross‐sectional study of seven novel urinary markers in antiretroviral‐naïve HIV‐infected individuals and HIV‐uninfected individuals who all had eGFR > 90 mL/min/1.73 m2, to investigate the impact of HIV infection on early renal injury through PSM analysis and to identify clinical factors associated with higher biomarker levels among HIV‐infected participants.
Methods
Study population and design
HIV‐positive patients were enrolled in the study between August 2018 and June 2019 from the out‐patient service of the Infection and Immunity Department, Shanghai Public Health Clinical Center, and HIV‐negative participants were enrolled from the Physical Examination Center, Shanghai Public Health Clinical Center during the same period. The inclusion criteria were as follows: (1) for the HIV‐infected group, a confirmed diagnosis of HIV‐1 infection using the western blot method by the Centers for Disease Control and Prevention and no initiation of ART; (2) for the HIV‐negative group, a negative HIV‐1 antibody test; (3) eGFR ≥ 90 mL/min/1.73 m2; (4) Chinese citizens > 18 years old. The exclusion criteria for both groups were: (1) hypertension (blood pressure ≥ 140/90 mmHg, self‐reported hypertension or self‐reported medication), diabetes mellitus (fasting glucose ≥ 7.0 mmol/L, self‐reported diabetes, or self‐reported medication), chronic hepatitis B [defined as hepatitis B virus (HBV) surface antigen (HBsAg) positive or detectable HBV DNA] or hepatitis C [defined as detectable hepatitis C virus (HCV) RNA or HCV antibody positive], or a history of autoimmune diseases (including systemic lupus erythematosus, vasculitis and Sjogren syndrome), gout or hyperuricaemia; (2) current opportunistic infections, including tuberculosis, cryptococcosis and Pneumocystis pneumonia; (3) a definite history of malignant tumour; (4) a history of nephritis; (5) any protein‐positive or microsopic haematuria (three or more red blood cells per high‐power microscopic field visible) in routine urine analysis; (6) for female subjects, pregnancy or being in the lactation period; (7) participation in other clinical studies. After inclusions and exclusions, a total of 166 HIV‐positive patients and 99 HIV‐negative controls were included in the analysis. Using PSM analysis with confounding variables, 83 subjects with HIV infection were matched with 83 subjects without HIV infection.
This study was approved by the independent ethics committees of Shanghai Public Health Clinical Center, Fudan University. Informed consent was obtained from all study participants, and all clinical investigations were conducted according to the principles expressed in the 1995 Declaration of Helsinki.
Measurement of urinary biomarkers
All participants underwent a routine urine test and random urine specimens were collected. Random urine specimens were immediately refrigerated at 4°C and centrifuged at 1000 g for 10 min to remove cellular debris. The supernatant was aliquoted into 1.5‐cc vials and stored at –80°C. Previous studies have shown that the novel urinary biomarkers can be used to determine the specific site of injury within the nephron as follows: (1) glomerular/endothelial injury: albumin‐to‐creatinine ratio (ACR); (2) proximal tubular dysfunction: alpha 1‐microglobulin (α1‐M), N‐acetyl‐β‐D‐glucosaminidase (NAG) and KIM‐1; (3) loop of Henle protection: Tamm–Horsfall Protein (THP) [9]; (4) distal tubular injury: neutrophil gelatinase‐associated lipocalin (NGAL) [10]; (5) tubulointerstitial injury and fibrosis: epidermal growth factor (EGF). Except two protection markers (THP and EGF), urinary levels of the other five markers (α1‐M, NAG, KIM‐1, NGAL and ACR) are considered to correlate positively with kidney damage [6, 11]. Urinary NAG, NGAL, α1‐M, KIM‐1 and THP were measured using commercially available enzyme‐linked immunosorbent assay (ELISA) kits (Cusabio Biotech Co., Wuhan, China). Urinary EGF was measured using a commercially available ELISA (Raybiotech, Norcross, GA, USA). Urinary albumin was measured using a commercial assay (BN ProSpec System; Siemens, Marburg, Germany) and urine Cr (UCr) was measured using the Abbott enzymatic Cr assay (Architect C1600; Abbott, Milan, Italy). Coefficients of variation were < 6% for NAG, < 8% for NGAL, α1‐M, KIM‐1 and THP, and < 10% for EGF. The levels of urinary markers were normalized to the urine Cr concentration, and the cut‐off value for ACR was 30 mg/g [12].
Clinical and laboratory evaluations
The other variables examined were as follows: age, sex, body mass index (BMI), serum creatinine (SCr), eGFR (estimated using the simplified modification of diet in renal disease equation for SCr), uric acid (UA), serum albumin, total cholesterol (TC) and triglycerides (TG). On the same day as collection of the urine sample, a physical examination, computed tomography of the chest and a sputum acid‐fast bacilli smear were performed, and serum cryptococcal antigen, current CD4 cell count, plasma HIV RNA, HBsAg, HBV DNA, HCV antibody and HCV RNA were measured.
Statistical analysis
Continuous variables are presented as median with interquartile range (IQR), and categorical variables are presented as counts and percentages. The significance of differences in clinical characteristics and urinary biomarkers between the HIV‐infected group and the HIV‐uninfected group was determined using the Mann–Whitney U test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Given the differences in the characteristics between eligible participants in the two groups, PSM was used to match subjects with similar clinical characteristics. The variables used in the propensity score included sex, age, BMI, UA concentration and eGFR. Matching was performed with the use of a 1:1 matching protocol using the nearest‐neighbour matching algorithm without replacement, with a caliper width equal to 0.2 of the standard deviation of the logit of the propensity score. The Spearman correlation test was performed to assess the correlation between clinical parameters and urinary biomarkers. Linear regression analyses were performed to determine factors associated with levels of urinary biomarkers, and when a significance level of P < 0.20 was reached in univariate analysis, the factor was assessed in multivariate analysis. Statistical analyses were performed and graphs created using spss version 23.0 (SPSS Inc., Chicago, IL) and r (V.3.6.0). A two‐sided P value of < 0.05 was considered statistically significant.
Results
Urinary biomarkers to UCr ratios of participants before and after matching
A total of 166 HIV‐positive patients with a median age of 30 [interquartile range (IQR) 25–36] years and 99 HIV‐negative controls with a median age of 29 (IQR 25–33.75) years were included in the study (Table 1). HIV‐positive patients had comparable age and BMI but not male‐to‐female ratio to the HIV‐negative controls. Compared with the HIV‐negative group, the HIV‐positive group showed higher median levels of SCr (71.69 vs. 68.32 μmol/L, respectively; P = 0.001) and UA (390.57 vs. 343.01 μmol/L, respectively; P < 0.001) and a lower median level of eGFR (116.57 vs. 120.79 mL/min/1.73 m2, respectively; P = 0.005). NAG:UCr, NGAL:UCr, α1‐M:UCr, KIM‐1:UCr and EGF:UCr and frequencies of abnormality in ACR differed significantly between the two groups (all P < 0.05), whereas THP:UCr did not differ significantly between the two groups (Fig. 1).
Table 1.
Characteristics of the HIV‐positive and HIV‐negative groups before and after propensity score matching analysis
| Variable | Before matching | P | After matching | P | ||
|---|---|---|---|---|---|---|
| HIV‐positive (n = 166) | HIV‐negative (n = 99) | HIV‐positive (n = 83) | HIV‐negative (n = 83) | |||
| Age (years) | 30 (25–36) | 29 (25–33.75) | 0.287 | 28 (25–33.75) | 29 (25–35.75) | 0.441 |
| Male gender | 156 (93.98) | 78 (78.79) | <0.001 | 76 (91.57) | 73 (87.95) | 0.442 |
| BMI (kg/m2) | 21.97 (20.28–24.61) | 21.97 (20.19–23.76) | 0.538 | 21.72 (19.88–24.03) | 21.93 (20.19–23.86) | 0.748 |
| SCr (μmol/L) | 71.69 (65.01–79.20) | 68.32 (58.31–75.93) | 0.001 | 70.10 (63.89–79.43) | 70.08 (62.21–76.69) | 0.424 |
| eGFR (mL/min/1.73 m2) | 116.57 (103.89–129.30) | 120.79 (114.43–137.37) | 0.005 | 122.11 (107.63–133.39) | 117.55 (109.55–133.14) | 0.988 |
| Serum albumin (g/L) | 46.50 (44.33–48.46) | 46.03 (44.56–48.01) | 0.480 | 46.61 (44.53–48.69) | 46.14 (44.61–48.22) | 0.537 |
| Uric acid (μmol/L) | 390.57 (337.24–449.72) | 343.01 (284.23–393.97) | <0.001 | 369.10 (304.51–417.43) | 354.38 (306.65–401.07) | 0.258 |
| TC (mmol/L) | 4.18 (3.67–4.61) | 4.12 (3.67–4.72) | 0.967 | 4.1900 (3.6150–4.6250) | 4.13 (3.65–4.72) | 0.954 |
| TG (mmol/L) | 1.31 (0.94–1.97) | 0.89 (0.67–1.13) | <0.001 | 1.29 (1.00 – 1.97) | 0.89 (0.69–1.17) | <0.001 |
| Current CD4 count (cells/μL) | 277 (180–393) | – | – | 292 (202.50–401.50) | – | – |
| HIV RNA (log10 copies/mL) | 4.38 (3.88–4.83) | – | – | 4.34 (3.73–4.77) | – | – |
| NAG:UCr (log10 U/g) | 1.45 (1.20–1.78) | 1.07 (0.80–1.29) | <0.001 | 1.39 (1.17–1.63) | 1.07 (0.80–1.29) | <0.001 |
| NGAL:UCr (log10 ng/g) | 3.93 (3.56–4.23) | 3.33 (2.90–3.75) | <0.001 | 3.92 (3.55–4.29) | 3.30 (2.90–3.80) | <0.001 |
| α1‐M:UCr (log10 ng/g) | 3.62 (3.41–3.83) | 3.17 (2.79–3.40) | <0.001 | 3.57 (3.36–3.79) | 3.19 (2.79–3.42) | <0.001 |
| KIM‐1:UCr (log10 ng/g) | 2.66 (2.40–2.87) | 2.45 (2.13–2.86) | 0.008 | 2.64 (2.44–2.92) | 2.42 (2.12–2.87) | 0.011 |
| EGF:UCr (log10 μg/g) | 3.89 (3.73–4.04) | 3.81 (3.60–3.97) | 0.005 | 3.94 (3.81–4.08) | 3.80 (3.59–3.98) | <0.001 |
| THP:UCr (log10 μg/g) | 3.05 (2.82–3.26) | 3.07 (2.73–3.24) | 0.542 | 3.05 (2.79–3.27) | 3.07 (2.70–3.25) | 0.721 |
| Abnormal ACR | 8 (4.82) | 0 (0) | 0.027 | 4 (4.82) | 0 (0) | 0.120 |
Continuous data are presented as median (interquartile range), and categorical variables are presented as n (%). P‐values are from χ2 tests for categorical variables and Mann–Whitney U tests for continuous variables.
ACR, albumin‐to‐creatinine ratio; BMI, body mass index; UCr, Urine creatinine; eGFR, estimate glomerular filtration rate; SCr, serum creatinine; TC, total cholesterol; TG, triglycerides; UA, uric acid; NAG, N‐acetyl‐β‐D‐glucosaminidase; NGAL, neutrophil gelatinase‐associated lipocalin; α1‐M, alpha 1‐microglobulin; KIM‐1, kidney injury molecule‐1; THP, Tamm–Horsfall protein; EGF, epidermal growth factor.
Fig. 1.
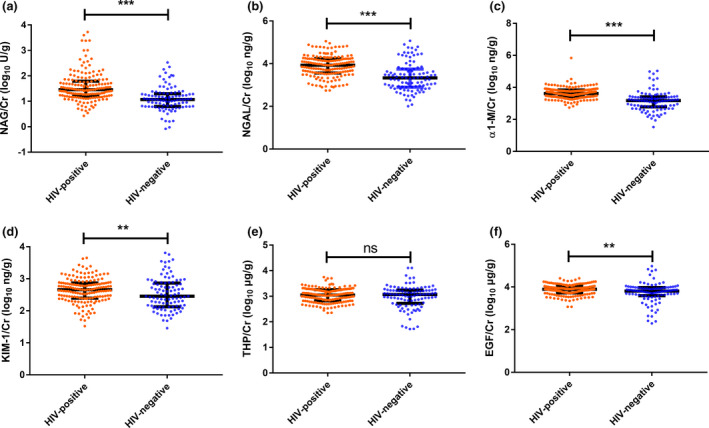
The distribution of urinary biomarkers after normalizing by urine creatinine (UCr) concentration between people living with HIV (PLWH) and HIV‐negative controls before propensity score matching. (a) N‐acetyl‐β‐D‐glucosaminidase (NAG):UCr; (b) N‐acetyl‐β‐D‐glucosaminidase (NGAL):UCr; (c) alpha 1‐microglobulin (α1‐M):UCr; (d) kidney injury molecule‐1 (KIM‐1):UCr; (e) Tamm–Horsfall protein (THP):UCr; (f) epidermal growth factor (EGF):UCr. (ns, not significant; *0.01 < P ≤ 0.05; **0.001 < P ≤ 0.01; *** P < 0.001)
For the significance of differences in eGFR, SCr, sex and UA between the two groups, we used PSM analysis to achieve a balance. The variables used in the propensity score included sex, age, BMI, UA and eGFR. In the propensity‐matched participants, the final covariates, namely, age, sex, BMI, eGFR, SCr and UA, showed no significant difference between the two groups (all P > 0.05). Urinary marker ratios of NAG:UCr, NGAL:UCr, α1‐M:UCr, KIM‐1:UCr and EGF:UCr were higher in the HIV‐infected group than in the HIV‐negative group (all P < 0.05). However, the urine ratio of THP:UCr was not significantly different between the two groups (P = 0.721), and the frequency of abnormality in ACR was also not significantly different between the two groups (P = 0.120).
Correlations of urinary biomarkers with clinical parameters
To investigate factors associated with urinary biomarker levels, we generated a correlation matrix (Fig. 2). In HIV‐positive patients, NAG:UCr showed significant correlations with NGAL:UCr (rs = 0.18; P = 0.023), α1‐M:UCr (rs = 0.67; P < 0.001), KIM‐1:UCr (rs = 0.27; P < 0.001) and THP:UCr (rs = 0.23; P = 0.003). Positive correlations were observed between HIV RNA level and NAG:UCr (rs = 0.32; P < 0.001) and α1‐M:UCr (rs = 0.238; P = 0.002), and negative correlations were observed between CD4 count and NAG:UCr (rs = –0.34; P < 0.001), α1‐M:UCr (rs = –0.36; P < 0.001), KIM‐1:UCr (rs = –0.16; P = 0.042), age (rs = –0.25; P = 0.001) and HIV RNA (rs = –0.28; P < 0.001). Age showed positive correlations with NAG:UCr (rs = 0.328; P < 0.001), α1‐M:UCr (rs = 0.364; P < 0.001), KIM‐1:UCr (rs = 0.330; P < 0.001) and ACR abnormality (rs = 0.163; P = 0.035) and a negative correlation with EGF:UCr (rs = –0.383; P < 0.001). In addition, NAG:UCr, NGAL:UCr, α1‐M:UCr, KIM‐1:UCr, EGF:UCr and THP:UCr were positively correlated with each other in the HIV‐negative group (all P < 0.001; Fig. 2b).
Fig. 2.
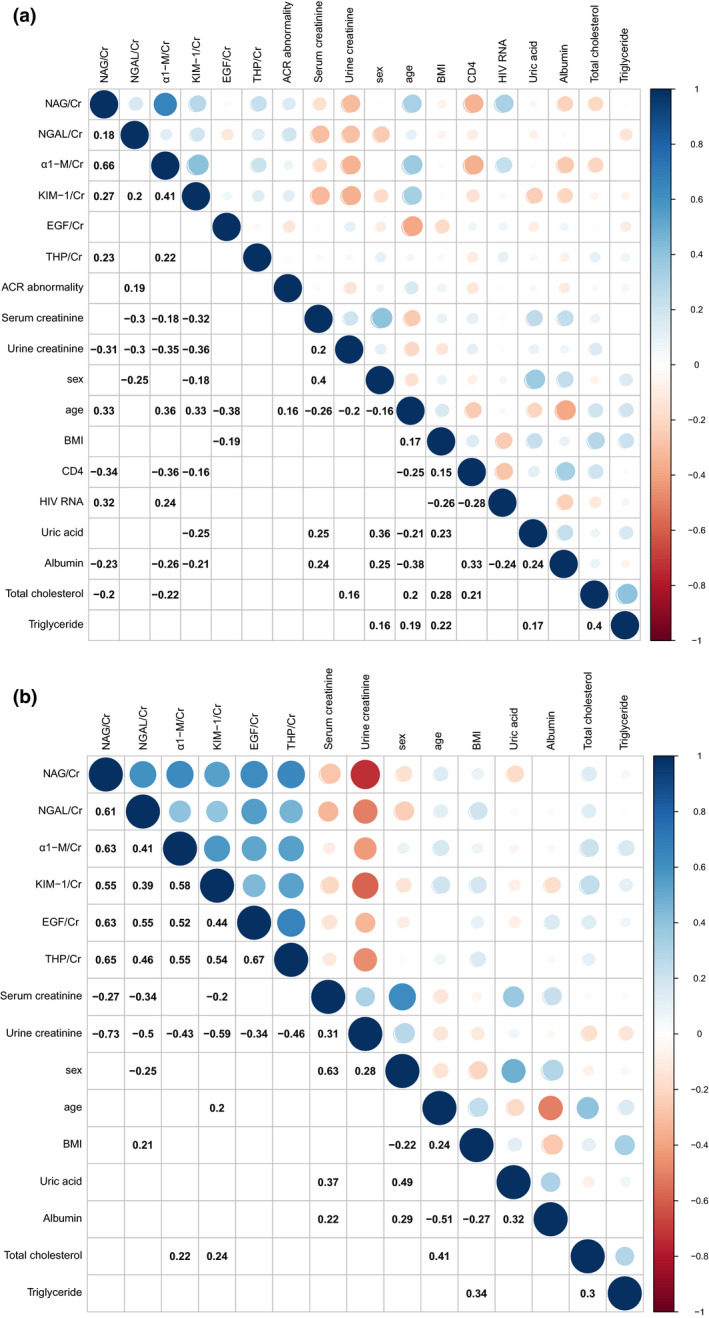
Spearman correlation between urinary biomarkers and clinical parameters in the HIV‐positive group (a) and the HIV‐negative group (b) before propensity score matching. The circle colour indicates the value of Spearman correlation coefficients. Blue indicates a positive correlation and red indicates a negative correlation. White grid without blue or red circle indicates no significant correlation (P > 0.05). Cr indicates urine creatinine; NAG, N‐acetyl‐β‐D‐glucosaminidase; NGAL, neutrophil gelatinase‐associated lipocalin; α1‐M, alpha 1‐microglobulin; KIM‐1, kidney injury molecule‐1; THP, Tamm–Horsfall protein; EGF, epidermal growth factor; BMI, body mass index.
Factors associated with ratios of urinary biomarkers to UCr
We then explored factors associated with kidney injury biomarkers in 166 HIV‐positive patients. In the univariable linear regression model, age, BMI, TC, TG, albumin, SCr, UA, CD4 count and HIV RNA were included as independent variables. Figure 3 shows factors associated with log‐transformed ratios of urinary biomarkers to UCr in the univariate linear regression analysis. In the multiple linear regression, older age (β = 0.288 per decade; P < 0.001), lower TC level (β = −0.211; P = 0.006) and higher HIV RNA level (β = 0.176; P = 0.023) were all independently associated with higher NAG:UCr (Fig. 4). Multiple linear regression showed that older age (β = 0.253 per decade; P = 0.002), lower TC level (β = −0.169; P = 0.023) and lower CD4 count (β = −0.175; P = 0.031) were independently associated with higher α1‐M:UCr (Fig. 4). Lower SCr remained as an independent factor associated with higher NGAL:UCr. No factors were significantly associated with urinary KIM‐1:UCr and THP : UCr. Of note, older age (β = −0.384 per decade; P < 0.001) was associated with lower EGF:UCr. However, no factors were independently associated with KIM‐1:UCr and THP:UCr.
Fig. 3.
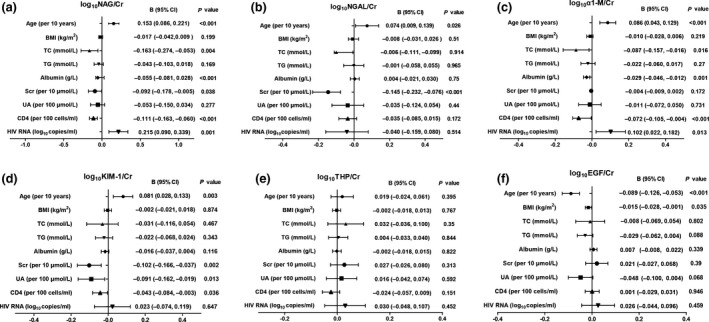
Factors associated with a higher ratio of urinary biomarker to creatinine (UCr) ratios in univariate linear regression analysis. (a) N‐acetyl‐β‐D‐glucosaminidase (NAG):UCr; (b) neutrophil gelatinase‐associated lipocalin (NGAL):UCr; (c) alpha 1‐microglobulin (α1‐M):UCr; (d) kidney injury molecule‐1 (KIM‐1):UCr; (e) Tamm–Horsfall protein (THP):UCr; (f) epidermal growth factor (EGF):UCr. BMI, body mass index; TC, total cholesterol; TG, triglycerides; SCr, serum creatinine; UA, uric acid; CI, confidence interval.
Fig. 4.
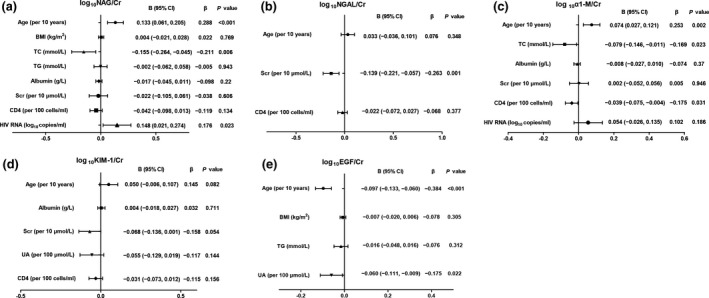
Factors associated with a higher ratio of urinary biomarker to creatinine (UCr) in multivariate linear regression analysis. (a) N‐acetyl‐β‐D‐glucosaminidase (NAG):UCr; (b) neutrophil gelatinase‐associated lipocalin (NGAL):UCr; (c) alpha 1‐microglobulin (α1‐M):UCr; (d) kidney injury molecule‐1 (KIM‐1):UCr; (e) epidermal growth factor (EGF):UCr. BMI, body mass index; TC, total cholesterol; TG, triglycerides; SCr, serum creatinine; UA, uric acid; CI, confidence interval.
Discussion
In this study, we used PSM to reduce the influence of data bias and confounding variables and to enable more robust comparisons to be made between the HIV‐infected individuals and the general population, and found that HIV‐positive participants had significantly higher urinary NAG:UCr, NGAL:UCr, α1‐M:UCr, KIM‐1:UCr and EGF:UCr compared with the HIV‐negative participants. We also identified factors associated with the levels of urinary biomarkers.
To our knowledge, this is the first study to compare the levels of urinary biomarkers of early kidney injury in antiretroviral‐naïve HIV‐infected patients and HIV‐uninfected participants in China. None of the participants in either of the groups enrolled in our study had a history of renal disease, tumours, autoimmune disease, gout, current hypertension, diabetes or hepatitis B/C, which eliminated the effects of other diseases that cause kidney injuries. Moreover, most previous research has focused on PLWH using certain antiretroviral medications that have been demonstrated to have nephrotoxic effects [11, 13]. In a sense, our results show the relationship between early kidney injury and HIV infection using a critical study design based on PSM approach. Although all participants enrolled in our study had normal kidney function, various types of subclinical kidney damage indicated by novel markers might occur in PLWH. These urinary biomarkers could be used in clinical practice to assess kidney function in order to intervene, if possible, to prevent loss of renal function.
After PSM, the proximal tubular markers NAG:UCr, KIM‐1:UCr and α1‐M:UCr and the distal tubular marker NAGL:UCr were significantly higher in the HIV‐positive group. However, no significant differences in urinary THP:UCr and the proportions of abnormal ACR were found. These results suggest that HIV infection was mainly associated with injury of the proximal and distal tubules, rather than the glomerulus. The frequencies of abnormality in ACR, a glomerular injury marker, differed significantly between the two groups before PSM. However, the abnormality of ACR showed no significant difference between the two groups after PSM, which may have been a consequence of the PSM analysis eliminating the differences in eGFR, UA and male:female ratio between the PLWH and the HIV‐negative controls. These findings are consistent with previous studies, in which the urinary excretion of tubular proteins increased even before diagnosis of proteinuria and/or a decrease in glomerular function, indicating that renal tubular injury is an early event in patients with HIV infection [14, 15].
The Multiple AIDS Cohort Study (MACS) found that HIV infection was associated with higher urinary interleukin (IL)‐18 and KIM‐1, indicating that HIV infection may promote proximal tubular injury [2]. In the WIHS, it was also found that HIV‐infected women had more extensive kidney injury, with higher urinary concentrations of IL‐18, KIM‐1, NGAL and ACR, compared with HIV‐uninfected participants [7]. Unlike the WIHS and MACS, our study was conducted in treatment‐naïve patients with preserved glomerular filtration rate, excluded comorbidities such as hypertension, diabetes and tumours, and used PSM to reduce potential confounding.
It was interesting that the protective marker EGF:UCr was elevated with HIV infection. EGF, which is a tubule‐specific protein derived from the ascending portion of Henle’s loop and the distal convoluted tubule and is considered critical for cell differentiation and regenerative tubular reserve, may slow progression of chron ic kidney disease (CKD) through alleviating renal interstitial fibrosis and tubular atrophy [16, 17]. Urinary EGF has been shown to be inversely correlated with interstitial fibrosis, diabetic nephropathy, immunoglobulin A (IgA) nephropathy, adult polycystic kidney disease and paediatric CKD [18]. However, in the presence of proinflammatory stimuli, EGF further exacerbated injury [19]. In our study, the eligible participants had preserved glomerular filtration rates and no renal disease history. We hypothesized that the elevations of EGF:UCr in PLWH were a negative feedback to modulate the tubular response to injury, in which the role of urinary EGF in HIV‐associated early kidney injury has not yet been clearly elucidated. In contrast, we also observed a negative correlation between age and urinary EGF:UCr, which suggests that an aging kidney tubule in PLWH might have limited potential for regeneration and recovery from damage.
In our study, risk factors associated with each type of tubular injury were also assessed. We found that proximal tubular injury markers, particularly NAG:UCr and α1‐M:UCr, had strong associations with HIV‐related factors such as CD4 count and HIV RNA level. Shlipak et al. also found that, although HIV infection was not significantly associated with urinary KIM‐1 and NGAL levels, HIV‐related characteristics including lower CD4 count and higher HIV viral load were independently associated with higher urinary IL‐18 concentrations as a biomarker of tubulointerstitial injury [7]. These results are similar to those of previous studies, which suggested that urinary biomarkers of subclinical tubular damage were strongly associated with HIV infection and uncontrolled viraemia after adjustment for demographic and conventional risk factors [20]. Direct HIV infection of renal epithelial cells has been verified through both in situ hybridization and in situ polymerase chain reaction (PCR) [21, 22]. Hence, renal tubular cells are considered a critical reservoir of HIV‐1. In 2009, Mikulak et al. demonstrated that HIV‐1 could infect epithelial cells and that this was mediated by the DEC‐205 receptor, a member of the C‐type lectin‐like receptor family expressed on human renal proximal tubular epithelial cells [23]. This finding might indicate a mechanism of early tubular injury associated with HIV infection.
The study has several limitations. Firstly, this was a single‐centre study and had a relatively small sample size, which certainly weakens the generality of our conclusions. Secondly, this was a cross‐sectional study, and so it is hard to draw conclusions regarding the causative relationship between urinary biomarker levels and HIV infection, although we used the PSM approach. In addition, although we observed elevated levels of urinary biomarkers in HIV‐infected patients, there was no direct evidence of early kidney injury obtained by renal biopsies. Longitudinal studies should be conducted to confirm whether these biomarkers influence the prognosis of PLWH.
In summary, PLWH with normal kidney function had significant elevations in urinary biomarkers for proximal tubular injury and distal tubular injury, but not glomerular injury, compared with HIV‐negative persons. HIV infection was associated with higher NAG:UCr and α1‐M:UCr, which are markers for proximal tubule injury. Initiation of effective antiretroviral therapy and reductions in the usage of nephrotoxic drugs may reduce the kidney disease burden in this population.
Author contributions
RF Zhang, XQ Le and J Chen designed the study. JR Wang, JJ Sun, L Liu, YZ Shen, TK Qi, ZY Wang, Y Tang, W Song and DP Liu collected the data. JN Xun supervised the experiment. XQ Le, X Zhang, JJ Sun and DP Liu analysed the data. XQ Le, DP Liu and ZY Gong interpreted the data. XQ Le, DP Liu, ZY Gong and C Steinhart wrote and revised the manuscript. All authors reviewed, revised and approved the final version of the manuscript.
Acknowledgements
We thank Caiyun Zhu and Jian Zhou at the Health Management Centre of Shanghai Public Health Clinical Center for providing us with clinical materials and specimens for the HIV negative group.
Conflicts of interest: The authors declared that they have no competing interests.
Financial disclosure: This work was supported by the National Science and Technology Major Project of China (2017ZX10202101), the Shanghai Commission of Science and Technology (20MC1920100), the Shanghai Municipal Key Clinical Specialty (shslczdzk01102), and the Academy Level Project of Shanghai Public Health Clinical Center (KY‐GW‐2019‐13).
References
- 1.Schouten J, Wit FW, Stolte IGet al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59: 1787–1797. [DOI] [PubMed] [Google Scholar]
- 2.Jotwani V, Scherzer R, Estrella MMet al. Association of HIV infection with biomarkers of kidney injury and fibrosis in the multicenter AIDS cohort study. Antivir Ther 2017; 22: 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas GM, Lau B, Atta MGet al. Chronic kidney disease incidence, and progression to end‐stage renal disease, in HIV‐infected individuals: a tale of two races. J Infect Dis 2008; 197: 1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanepoel CR, Atta MG, D'Agati VDet al. Kidney disease in the setting of HIV infection: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 2018; 93: 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muiru AN, Shlipak MG, Scherzer Ret al. Kidney disease risk factors associate with urine biomarkers concentrations in HIV‐positive persons; a cross‐sectional study. BMC Nephrol 2019; 20: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu I, Parikh CR. Screening for kidney diseases: older measures versus novel biomarkers. Clin J Am Soc Nephrol 2008; 3: 1895–1901. [DOI] [PubMed] [Google Scholar]
- 7.Jotwani V, Scherzer R, Abraham Aet al. Does HIV infection promote early kidney injury in women? Antivir Ther 2014; 19: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlipak MG, Scherzer R, Abraham Aet al. Urinary markers of kidney injury and kidney function decline in HIV‐infected women. J Acquir Immune Defic Syndr 1999; 2012: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 2017; 13: 525–544. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra R, Siew ED. Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 2017; 12: 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan L, Asriel B, Eaton EF, Wyatt CM. Potential kidney toxicity from the antiviral drug tenofovir: new indications, new formulations, and a new prodrug. Curr Opin Nephrol Hypertens 2018; 27: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens PE, Levey AS, Lamb EJ. The kidney disease improving global outcomes (KDIGO) guideline update for chronic kidney disease: evolution not revolution. Clin Chem 2013; 59: 462–465. [DOI] [PubMed] [Google Scholar]
- 13.Jotwani V, Scherzer R, Estrella MMet al. HIV infection, tenofovir, and urine alpha1‐microglobulin: a cross‐sectional analysis in the multicenter AIDS cohort study. Am J Kidney Dis 2016; 68: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiseha T, Gebreweld A. Urinary markers of tubular Injury in HIV‐infected patients. Biochem Res Int 2016; 2016: 1501785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando M, Yanagisawa N, Ajisawa A, Tsuchiya K, Nitta K. Kidney tubular damage in the absence of glomerular defects in HIV‐infected patients on highly active antiretroviral therapy. Nephrol Dial Transplant 2011; 26: 3224–3229. [DOI] [PubMed] [Google Scholar]
- 16.Nowak N. Protective factors as biomarkers and targets for prevention and treatment of diabetic nephropathy: from current human evidence to future possibilities. J Diabetes Investig 2020; 11: 1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju W, Nair V, Smith Set al. Tissue transcriptome‐driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015; 7: 316ra193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang WR, Parikh CR. Biomarkers of acute and chronic kidney disease. Annu Rev Physiol 2019; 81: 309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lechner J, Malloth NA, Jennings Pet al. Opposing roles of EGF in IFN‐alpha‐induced epithelial barrier destabilization and tissue repair. Am J Physiol Cell Physiol 2007; 293: C1843–C1850. [DOI] [PubMed] [Google Scholar]
- 20.Sheets KM, Atta MG, Fine DMet al. Longitudinal assessment of proximal tubular dysfunction in HIV seropositive and seronegative persons: correlates and implications. J Acquir Immune Defic Syndr 1999; 2017: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasi M, Balakumaran B, Chen Pet al. Renal epithelial cells produce and spread HIV‐1 via T‐cell contact. AIDS 2014; 28: 2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen AH, Sun NC, Shapshak P, Imagawa DT. Demonstration of human immunodeficiency virus in renal epithelium in HIV‐associated nephropathy. Mod Pathol 1989; 2: 125–128. [PubMed] [Google Scholar]
- 23.Mikulak J, Teichberg S, Faust T, Schmidtmayerova H, Singhal PC. HIV‐1 harboring renal tubular epithelial cell interaction with T cells results in T cell trans‐infection. Virology 2009; 385: 105–114. [DOI] [PubMed] [Google Scholar]


