Abstract
A post hoc analysis of the Diabeloop WP7 multicentre, randomized controlled trial was performed to investigate the efficacy of the Diabeloop Generation‐1 (DBLG1) closed‐loop system in controlling the hypoglycaemia induced by physical activity (PA) in real‐life conditions. Glycaemic outcomes were compared between days with and without PA in 56 patients with type 1 diabetes (T1D) using DBLG1 for 12 weeks. After the patient announces a PA, DBLG1 reduces insulin delivery and, if necessary, calculates the amount of preventive carbohydrates (CHO). Daily time spent in the interstitial glucose range less than 70 mg/dL was not significantly different between days with and without PA (2.0% ± 1.5% vs. 2.2% ± 1.1%), regardless of the intensity or duration of the PA. Preventive CHO intake recommended by the system was significantly higher in days with PA (41.1 ± 35.5 vs. 21.8 ± 28.5 g/day; P < .0001), and insulin delivery was significantly lower (31.5 ± 10.5 vs. 34.0 ± 10.5 U/day; P < .0001). The time spent in hyperglycaemia and the glycaemic variation coefficient increased significantly on days with PA. In real‐life conditions, the use of DBLG1 avoids PA‐induced hypoglycaemia. Insulin adjustments and preventive CHO recommendation may explain this therapeutic benefit.
Keywords: artificial pancreas, closed‐loop system, continuous glucose monitoring, glycaemic control, hypoglycaemia, insulin pump therapy, physical activity, physical intervention, randomized controlled trial, type 1 diabetes
1. INTRODUCTION
The ideal goal of insulin therapy for type 1 diabetes (T1D) is to normalize blood glucose levels, without increasing exposure to hypoglycaemia. A meta‐analysis of 27 randomized controlled trials (RCTs) by Weisman et al.1 showed that closed‐loop (CL) artificial pancreas systems allow staying within the glycaemic target for a significantly longer time compared with conventional pump therapy (continuous subcutaneous insulin infusion with blinded continuous glucose monitoring [CGM] or unblinded sensor‐augmented pump [SAP] therapy). However, CL systems often do not protect against sudden drops in blood glucose caused by moderate intensity physical exercise (PE).2, 3, 4
In 12 patients under glycaemic control with a Medtronic‐derived CL system, Patel et al.5 showed that a simple method to prevent hypoglycaemia was to eat a snack just before and in the middle of the PE period (see also de Bock et al.6). Turksoy et al.7 reported that consuming an appropriate amount of carbohydrates (CHO) combined with predictive hypoglycaemia alarms can prevent most hypoglycaemic events.
The Diabeloop Generation‐1 (DBLG1) CL system is a hybrid, single‐hormone CL device, which has a self‐learning algorithm that regulates insulin delivery as a function of CGM, CHO intake and PA lasting more than 15 minutes.8, 9 We have previously shown that DBLG1 completely suppressed exercise‐induced hypoglycaemia in 14 free‐living patients consuming unrestricted diets for 3 days.9
Regular physical activity (PA) is beneficial for patients with T1D because it improves well‐being and fitness, reduces cardiovascular risk and helps with reaching target lipid profile and glycaemic goals.10, 11 A previous 12‐week randomized study in 63 free‐living patients eating an unrestricted diet (Diabeloop WP7 trial) showed that DBLG1 improves glucose control compared with SAP systems.8 Unlike SAP systems, DBLG1 records PA data in its internal memory. Therefore, the Diabeloop WP7 database was accessed to compare hypoglycaemia on days with and without PA.
2. METHODS
2.1. Study design
Post hoc analysis of the Diabeloop WP7 study, a 12‐week, multicentre, open‐label, randomized, controlled, crossover trial of DBLG1 in 68 patients with T1D under real‐life conditions8 (ClinicalTrials.gov NCT02987556), was performed. The trial involved 12 French centres, from March 2017 until August 2018. A full description of the study is presented in Benhamou et al.8 Briefly, participants were adult patients with T1D and an HbA1c of 10% or less, who were treated with wearable insulin pump therapy. Patients were randomly assigned (1:1) to receive DBLG1‐driven insulin therapy or sensor‐assisted pump therapy over 12 weeks, followed by an 8‐week washout period and the subsequent crossover intervention. Only the CL crossover period was analysed.
Similar to other studies in real‐life conditions, participants were free to decide the characteristics of their PA. They were only asked to announce the duration and intensity (mild, moderate, intense) of each PA session, 60 minutes before starting it.8 We have previously shown12 that the patient's self‐assessment of PA intensity is reliable. Thus, patients who perform PA at 50% of VO2 max self‐rate it as moderate and those performing PA at 75% of VO2 max self‐rate it as intense. When the patient declares a PA at least 2 hours before its onset, and if the glycaemic forecast is lower than 160 mg/dL (at the beginning of PA), a preventive CHO intake is requested depending on (a) the intensity of the PA and (b) the deviation from the glycaemic target.
2.2. The Diabeloop system
A Cellnovo (or Kaleido) insulin patch pump is managed by the Diabeloop application installed on an android smartphone, and is connected to a Dexcom G5 Mobile CGM system using Bluetooth Low Energy technology (for technical details, see8, 9, 13, 14).
DBLG1 comprises the announcement of PA and the software calculation of a preventive CHO intake. The latter is calibrated according to the duration and intensity of the PA to be performed.8, 9 The CGM provides one glycaemia value every 5 minutes to the system. The self‐learning algorithm rapidly optimizes insulin delivery during hypoglycaemic or hyperglycaemic challenges (in approximately 7 hours; it takes approximately 7 days to perform long‐term optimization).
2.3. Outcome measures
The Diabeloop database was accessed to extract the following data (recorded during the DBLG1 periods): (a) days with or without recorded PA, (b) PA duration and intensity, (c) time between the announcement and the start of the PA (notifications made at the beginning or after the session were considered “without announcement”), (d) daily time (24 hours) and overnight time spent in the interstitial glucose range less than 70 mg/dL (time below range [TBR]), (e) preventive CHO intake recommended by DBLG1, (f) preventive CHO intake estimated and recorded by the patient, (g) insulin delivery, (h) mean CGM and variation coefficient. Cut‐points for each glycaemic range were those used in the Diabeloop WP7 RCT8 and defined in the consensus report of outcome measures for artificial pancreas clinical trials.15
2.3.1. Primary outcome measure
The primary outcome was the difference in TBR between days with and without PA.
2.3.2. Secondary outcome measures
Secondary outcomes included the following comparisons between days with and without PA: TBR as a function of the intensity, duration and delay of PA announcement, preventive CHO, insulin delivery, mean glycaemia estimated from interstitial glucose and variation coefficient, time spent in the normoglycaemic range (TIR 70‐180 mg/dL) and in the hyperglycaemic range of more than 180 mg/dL (time above range [TAR]).
2.4. Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical comparisons of the primary outcome (TBR between days with and without PA) were made using two methods: (a) a mixed model for repeated measures, with corrections for baseline HbA1c and centre, and (b) an ordinal logistic model with multinomial distribution (1% TBR classes, cumulative logit link function), with corrections for baseline HbA1c and Centre. For preventive CHOs (system‐recommended and patient‐declared), statistical comparisons were made with the Wilcoxon test (days with vs. days without PA) or with the Kruskal‐Wallis test (PA intensity, duration and delay of announcement). Other statistical comparisons were made with the above mixed model.
3. RESULTS
3.1. Participants
Sixty‐three patients completed both CL 12‐week treatment periods and were included in the study.8 Their mean age was 48.2 ± 13.4 years, 62% were female and they had a medium‐high education level. Mean diabetes duration was 28 ± 13.6 years; mean HbA1c level was 7.6% ± 0.9% (59.4 ± 9.8 mmol/mol).
3.2. Registered PA events
The Diabeloop database registered 1256 PA events. Seven patients did not record any PA, and were excluded from the analysis. The remaining 56 participants recorded 19.9 ± 24.1 PA events/patient. There were 938 days with at least one PA event announced and 4260 days with no announced PA. Patients announced in advance 71.4% of their PA sessions. One PA session was announced the day before (21.6 hours) and the remaining sessions were announced with an average upstream time of 79.4 ± 82.8 minutes (min‐max 1‐596 minutes; median 59 minutes) (Table S1 and Figure S1). The duration of PA was 82.3 ± 58.3 minutes (min‐max 8–480 minutes; median 60 minutes) (Table S2 and Figure S2), with intensity, “mild” (40%), “moderate” (41%) or “intense” (19%), respectively.
3.3. Primary outcome
According to the mixed model for repeated measures, TBR was not significantly different between days with or without PA announced to the software (2.0% ± 1.5% vs. 2.2% ± 1.1%, respectively; P = .282) (Figure 1, top panel). One patient spent 1 day with TBR = 0% and most patients were in the TBR range of >0% to 4% range. Statistical analysis with the ordinal logistic model (with 1% TBR classes) confirmed that there was no more TBR with PA than without PA (Figure S3). In fact, the TBR was significantly lower with PA than without PA (P = .0065).
FIGURE 1.
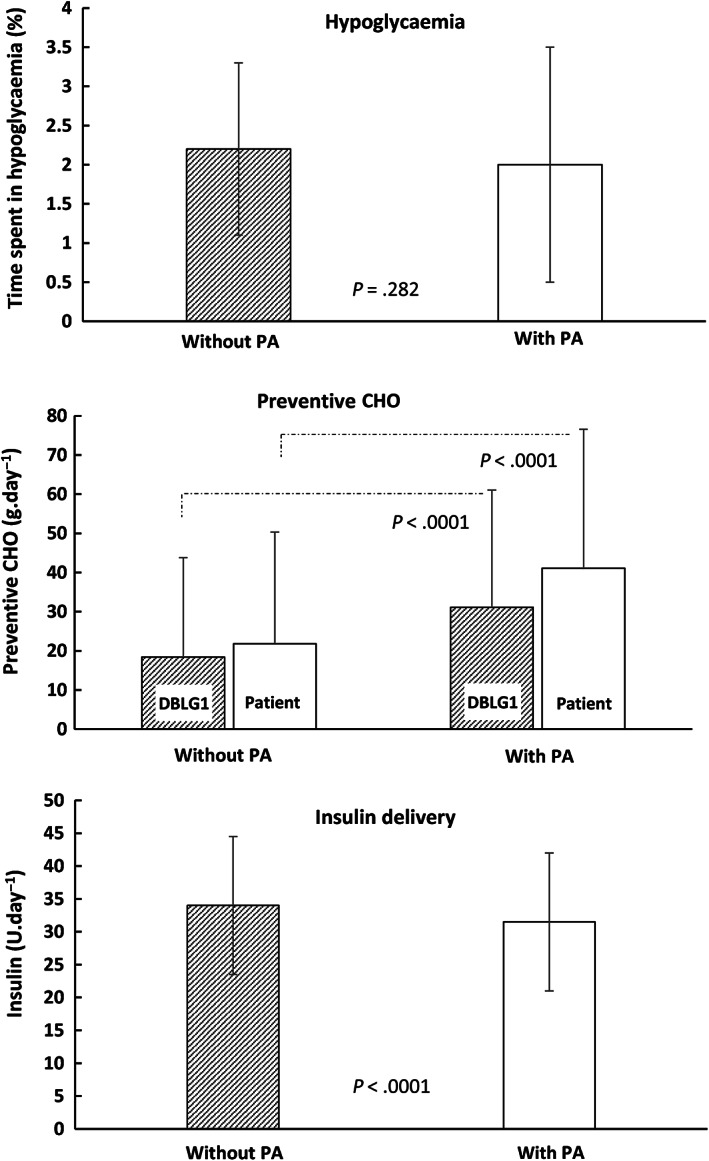
Time spent in hypoglycaemia during days with or without physical activity, and associated changes in carbohydrate (CHO) intake and insulin delivery. (Top) Time spent in hypoglycaemia (interstitial glucose <70 mg/dL). (Middle) Preventive CHO intake. “DBLG1” indicates preventive CHO intake recommended by the system. “Patient” indicates preventive CHO intake recorded by the patient. (Bottom) Insulin delivery
3.4. Secondary outcomes
TBR was not significantly influenced by: (a) PA intensity (TBR = 1.6% ± 1.8% [N = 36] for mild, 2.0% ± 1.4% for moderate [N = 50] and 2.2% ± 1.7% [N = 34] for intense; P = .209; N indicates the number of patients) or (b) PA duration (TBR = 1.9% [N = 13] for <30 minutes, 2.1% [N = 46] for 30‐60 minutes, 1.9% [N = 44] for 60–90 minutes, 2.0% [N = 52] for >90 minutes; P = .953). The time elapsed between the announcement and the onset of PA did not significantly influence the TBR (1.7% ± 0.3%, 2.3% ± 0.3%, 2.2% ± 0.3% and 2.3% ± 0.3% for announcement at 60 or more minutes before PA, 30–60 minutes before PA, <30 minutes before PA and at the start of PA, respectively; P = .285). Overnight TBR was not significantly different after days with or without PA (1.2% ± 0.2% vs. 1.6% ± 0.2%, respectively; P = .507).
Preventive CHO intake was significantly higher with PA than without PA (Figure 1, middle panel). This was the case for the preventive CHO recommended by DBLG1 (31.1 ± 29.9 vs. 18.4 ± 25.4 g/day, P < .0001), as well as for the preventive CHO recorded by the patient (41.1 ± 35.5 vs. 21.8 ± 28.5 g/day, P < .0001) (Figure 1, middle panel). Recorded CHO intake increased with increasing PA duration (e.g. 49.4 ± 38.3 vs. 37.8 ± 30.3 g/day for >90‐ and <30‐minute periods, respectively; P < .001). The mean delay between the intake and the PA onset was 36.4 ± 57.0 minutes (min‐max 1‐1295 minutes; median 59 minutes). The same patient who announced a PA session the day before (21.6 hours) announced the intake at the same time.
Total insulin delivery was significantly lower on days with PA (31.5 ± 10.5 U/day) compared with days without PA (34.0 ± 10.5 U/day; P < .0001) (Figure 1, bottom panel). Similar results were obtained with basal insulin (10.5 ± 0.5 U/day vs. 11.1 ± 0.5 U/day on days with and without PA, respectively; P < .001), and for bolus insulin (21.0 ± 1.0 U/day vs. 23.0 ± 1.0 U/day; P < .001). The HbA1c covariate had a positive and significant effect on basal, bolus and total insulin doses (2.7 ± 5.9, 4.9 ± 8.7 and 7.5 ± 13.4, respectively; P < .001 for each variable; Centre had no effect).
Figure S4 shows the median sensor IQRs of CGM levels: mean value was slightly, but significantly higher with PA (158.6 ± 12.0 mg/dL) than without PA (156.3 ± 12.0 mg/dL; P = .044). The average CGM coefficient of variation was higher with PA (32.0% ± 3.7%) than without PA (30.9% ± 3.7%; P = .019).
An increase in the daily time spent in TAR was observed with PA (28.7% ± 9.3%) versus without PA (26.8% ± 8.6%, P = .017). A very small, but significant decrease was found in the daily time spent in TIR with PA (69.1% ± 8.2%) compared with TIR without PA (70.9% ± 8.2%, P = .017).
4. DISCUSSION
No more hypoglycaemia was found on days with PA compared with days without PA when using DBLG1 for 12 weeks, regardless of the duration or intensity of the PA. This finding was accompanied by an increase in preventive CHO intake and a reduction in insulin delivery. A small but significant increase in hyperglycaemic time was found in days with PA.
Various approaches have been used to prevent or manage hypoglycaemia, and help people with T1D to engage in safe and regular PA.2, 3, 4 These include education concerning the timing and type of PA.2, 4 The PEAK group11 issued a consensus statement on glucose targets, insulin dose adjustments and nutritional issues. The American Diabetes Association recommends that adults with T1D engage in at least 150 minutes of moderate to vigorous aerobic activity per week.16 This objective was partially achieved in our study, where patients underwent PA sessions (mostly mild to moderate) of an average duration of 136.5 minutes per week.
Current guidelines recommend limiting PA‐induced hypoglycaemia by reducing or suppressing insulin delivery. In patients using insulin pumps, manually reducing the subcutaneous insulin infusion within 60‐90 minutes before starting PA lowers “insulin on board” and reduces the occurrence of hypoglycaemia.11, 17, 18 This seems difficult to achieve in real‐life conditions19 because it implies anticipating PA sessions at least 1 hour before the start of exercise (only a small benefit was observed with insulin administration adjustments made 5 minutes before20). Moreover, marked reductions in blood glucose are not prevented simply by reducing insulin delivery.5 In such a case, a CHO supplement5, 6, 7 or an unrestricted diet9 prevents exercise‐induced hypoglycaemia. The control of PA‐induced hypoglycaemia by DBLG1 was associated with a calibrated increase in preventive CHO intake (mean value = 20 g) and a small but significant reduction of insulin delivery (−2.5 units).
The participants in our study consumed a preventive CHO of around 30% more than that recommended by DBLG1, and this may have contributed to the increase in hyperglycaemic time on days with PA versus days without PA (28 minutes). Activation of counter‐regulatory mechanisms during intense anaerobic or aerobic PA may also have played a role in the observed hyperglycaemia.21, 22
The present post hoc analysis of the Diabeloop WP7 trial clearly confirms and extends previous studies5, 6, 7, 9 that have shown that PA‐induced hypoglycaemia can be avoided by adding CHO supplements or an unrestricted diet (Table 1). Dual‐hormone systems (insulin and glucagon) can prevent PA‐hypoglycaemia,3 but increase the cost and complexity of insulin pump devices.
TABLE 1.
Prevention of physical activity (PA)‐induced hypoglycaemia with closed‐loop (CL) systems combined with dietary interventions
| System | Reference | N | Time frame | Dietary intervention | Result |
|---|---|---|---|---|---|
| Medtronic PID‐IFB | 5 | 12 | 105 min | CHO supplement (15‐30 g) | No decrease in glucose levels |
| Medtronic 670G | 6 | 8 | 4 d | CHO supplementa | No exercise‐induced hypoglycaemia |
| Multivariable HEA | 7 | 6 | 3 d | CHO supplement (14 ± 7.8 g) | Most hypoglycaemic events were avoided |
| DBLG1 | 9 | 13 | 3 d | Unrestricted (CHO = 12‐17 g)b | No exercise‐induced hypoglycaemia |
| DBLG1 | This study | 63 | 12 wk | Unrestricted (CHO ~30 g) | No more hypoglycaemia on days with PA |
Abbreviations: CHO, carbohydrates; DBLG1, Diabeloop first‐generation CL system; HEA, hypoglycaemia early alarm; N, number of patients; PID‐IFB, proportional‐integral‐derivative with insulin feedback.
Calculated for each participant.
Value obtained from the Diabeloop SP6 database (not reported in Hanaire et al.7).
4.1. Study limitations
The study limitations were similar to those of other real‐life studies. In particular, participants were free to decide the characteristics of their PA, and only the duration and intensity (mild, moderate, intense) of each PA session were documented in the Diabeloop database. Participants did not sufficiently observe the CHO intake recommended by DBLG1. Preventive CHO may favour weight gain. We were unable to compare the performance of DBLG1 with that of standard care, because only DBLG1 recorded upcoming PA announcements (duration and intensity of PA). The post hoc nature of this analysis precluded the assessment of statistical power. Physiological factors that may contribute to glycaemic control during PA were not studied (e.g. counter‐regulations involving the autonomic nervous system and/or glucagon).23 Besides TIR (CGM 70‐180 mg/dL), TBR (CGM <70 mg/dL) and TAR (CGM >180 mg/dL), other glycaemic excursions were not analysed. The algorithm of the CL system does not distinguish PA performed in the postprandial versus the postabsorptive state.
In conclusion, when using DBLG1 in real‐life conditions, there is no more hypoglycaemia on days with PA compared with days without PA. This absence of supplementary hypoglycaemia was independent of the PA intensity and duration or the delay in notification. Small but significant increases in hyperglycaemic time and glycaemic variability were found on days with PA. Future studies should take this concern into account, in order to improve the algorithms of CL systems comprising the announcement of PA.
CONFLICT OF INTEREST
SF declares congress invitations from Sanofi, Eli Lilly, MSD, Novo Nordisk, Roche, Abbott and Boehringer; she has received speaker honoraria from Lilly and Novo Nordisk, and served on advisory board panels for Novo Nordisk, Diabeloop, Roche, Sanofi, Janssen and Lifescan. She also owns shares in Diabeloop SA. PYB has received speaker honoraria from Abbott, Roche, Eli Lilly, Novo Nordisk and Sanofi, and has served on advisory board panels for Abbott, Diabeloop, Roche, Medtronic, Dexcom, Insulet, Lifescan, Eli Lilly, Novo Nordisk and Sanofi. SB has received personal compensation for board participation and speaking fees from Abbott, Eli Lilly, Novo Nordisk, Medtronic and Sanofi Aventis. LC has received personal compensation for board participation and speaking fees from Eli Lilly, Lifescan, Novo Nordisk, Roche Diagnostics, Medtronic and Sanofi Aventis. BD has received personal compensation for board participation and speaking fees from Eli Lilly, Novo Nordisk, Sanofi Aventis, MSD and Astra Zeneca. MD is employed by Univ. Grenoble Alpes, CEA LETI, F‐38000 Grenoble. BG participated as an advisory panel/board member of Sanofi, Eli Lilly, NovoNordisk, Novartis, GSK, MSD, Boehringer Ingelheim, AstraZeneca, Abbott, Medtronic and Roche Diagnostics; he also participated as a clinical investigator for Sanofi, Eli Lilly, NovoNordisk, GSK, BMS, AstraZeneca, Medtronic, Abbott, Roche Diagnostics, MSD, Novartis, Janssen and Boehringer Ingelheim, and received research support from Medtronic, Vitalaire, Sanofi, Eli Lilly and Novo Nordisk. HH has received personal compensation for board participation and speaking fees from Abbott, Dexcom, Eli Lilly, Lifescan, Novo Nordisk, Roche Diagnostics, Medtronic, Sanofi Aventis and BD. EH is an employee of Diabeloop S.A. NJ received personal compensation for board participation and speaking fees from Eli Lilly, Novo Nordisk, Sanofi Aventis and Roche. CA has received congress invitations from Eli Lilly and AstraZeneca, and has received speaker honoraria from Diabeloop. ER is a consultant/advisor for Abbott, Air Liquide SI, Bastide Médical, Beckton‐Dickinson, Cellnovo, Dexcom, Eli‐Lilly, Hillo, Insulet, Johnson & Johnson (Animas, LifeScan), Medirio, Medtronic, Novo‐Nordisk, Roche Diagnostics and Sanofi‐Aventis, and has received research grant/material support from Abbott, Dexcom, Insulet, Roche Diagnostics and Tandem Diabetes Care. YR has received personal compensation for board participation and speaking fees from Novo Nordisk, Sanofi, Eli Lilly, Medtronic, Takeda, Abbott and Roche. PS has received speaking fees from Sanofi, Abbott and Lilly, and for participation on the boards of Novo‐Nordisk and Sanofi. C. Thivolet received personal compensation for board participation and speaking fees from Medtronic, Insulet, Sanofi, Lilly, Novo‐Nordisk and Abbott. CS, C. Thomas and PH have no conflicts of interest to declare. GC is CMO of Diabeloop SA, owns shares in Diabeloop SA, is employed by CERITD and received personal compensation for board participation, research funding and speaking fees from Astra‐Zeneca, Boehringer, Eli Lilly, Johnson & Johnson, MSD, Novo‐Nordisk, Sanofi‐Aventis and Voluntis.
AUTHOR CONTRIBUTIONS
SF, PYB, SB, LC, BD, BG, HH, NJ, CA, ER, YR, PS, ChT and GC designed the study. SF and GC wrote the protocol and liaised with regulatory authorities. SF, PYB, SB, LC, BD, BG, HH, NJ, CA, ER, YR, PS, ChT and GC were involved with patient enrolment and follow‐up. SF, PYB, SB, LC, BG, HH, NJ, CA, ER, YR, PS, CS, ChT, ClT, PH and GC interpreted data. MD engineered the algorithm. SF wrote the manuscript. All the authors revised the manuscript. SF and PH revised and edited the final version of the manuscript, which was accepted by all authors.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14442.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
The authors acknowledge Ricardo P. Garay (Craven, Villemoisson‐sur‐Orge, France) for editorial support. Diabeloop S.A. (Paris, France) provided the CL artificial pancreas. This study was funded by the French Innovation Fund and Diabeloop S.A. CERITD designed and conducted the study. Parts of the results were presented at the ATTD 2020 meeting (February 2020, Madrid), and at the virtual congress of the Francophone Diabetes Society in September 2020.
Franc S, Benhamou P‐Y, Borot S, et al. No more hypoglycaemia on days with physical activity and unrestricted diet when using a closed‐loop system for 12 weeks: A post hoc secondary analysis of the multicentre, randomized controlled Diabeloop WP7 trial. Diabetes Obes Metab. 2021;23(9):2170–2176. 10.1111/dom.14442
Funding information Diabeloop SA; French Innovation Fund
DATA AVAILABILITY STATEMENT
Data will be available upon request to SF.
REFERENCES
- 1.Weisman A, Bai JW, Cardinez M, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta‐analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501‐512. [DOI] [PubMed] [Google Scholar]
- 2.Cockcroft EJ, Narendran P, Andrews RC. Exercise‐induced hypoglycaemia in type 1 diabetes. Exp Physiol. 2020;105:590‐599. [DOI] [PubMed] [Google Scholar]
- 3.Tagougui S, Taleb N, Molvau J, et al. Artificial pancreas systems and physical activity in patients with type 1 diabetes: challenges, adopted approaches, and future perspectives. J Diabetes Sci Technol. 2019;13:1077‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaharieva DP, Messer LH, Paldus B, et al. Glucose control during physical activity and exercise using closed loop technology in adults and adolescents with type 1 diabetes. Can J Diabetes. 2020;44:740‐749. [DOI] [PubMed] [Google Scholar]
- 5.Patel NS, Van Name MA, Cengiz E, et al. Mitigating reductions in glucose during exercise on closed‐loop insulin delivery: the ex‐snacks study. Diabetes Technol Ther. 2016;18:794‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bock M, Dart J, Roy A, et al. Exploration of the performance of a hybrid closed loop insulin delivery algorithm that includes insulin delivery limits designed to protect against hypoglycemia. J Diabetes Sci Technol. 2017;11:68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turksoy K, Samadi S, Feng J, et al. Meal detection in patients with type 1 diabetes: a new module for the multivariable adaptive artificial pancreas control system. IEEE J Biomed Health Inform. 2016;20:47‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benhamou PY, Franc S, Reznik Y, et al. Closed‐loop insulin delivery in adults with type 1 diabetes in real‐life conditions: a 12‐week multicentre, open‐label randomised controlled crossover trial. Lancet Digit Health. 2019;1:e17‐e25. [DOI] [PubMed] [Google Scholar]
- 9.Hanaire H, Franc S, Borot S, et al. Efficacy of the Diabeloop closed‐loop system to improve glycaemic control in patients with type 1 diabetes exposed to gastronomic dinners or to sustained physical exercise. Diabetes Obes Metab. 2020;22:324‐334. [DOI] [PubMed] [Google Scholar]
- 10.Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross‐sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38:1536‐1543. [DOI] [PubMed] [Google Scholar]
- 11.Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5:377‐390. [DOI] [PubMed] [Google Scholar]
- 12.Franc S, Daoudi A, Pochat A, et al. Insulin‐based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: the DIABRASPORT randomized study. Diabetes Obes Metab. 2015;17:1150‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quemerais MA, Doron M, Dutrech F, et al. Preliminary evaluation of a new semi‐closed‐loop insulin therapy system over the prandial period in adult patients with type 1 diabetes: the WP6.0 Diabeloop study. J Diabetes Sci Technol. 2014;8:1177‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benhamou PY, Huneker E, Franc S, et al. Customization of home closed‐loop insulin delivery in adult patients with type 1 diabetes, assisted with structured remote monitoring: the pilot WP7 Diabeloop study. Acta Diabetol. 2018;55:549‐556. [DOI] [PubMed] [Google Scholar]
- 15.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39:1175‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes A. 5. Facilitating behavior change and well‐being to improve health outcomes: standards of medical Care in Diabetes‐2020. Diabetes Care. 2020;43:S48‐S65. [DOI] [PubMed] [Google Scholar]
- 17.Roy‐Fleming A, Taleb N, Messier V, et al. Timing of insulin basal rate reduction to reduce hypoglycemia during late post‐prandial exercise in adults with type 1 diabetes using insulin pump therapy: a randomized crossover trial. Diabetes Metab. 2019;45:294‐300. [DOI] [PubMed] [Google Scholar]
- 18.Zaharieva DP, McGaugh S, Pooni R, et al. Improved open‐loop glucose control with basal insulin reduction 90 minutes before aerobic exercise in patients with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Care. 2019;42:824‐831. [DOI] [PubMed] [Google Scholar]
- 19.Elliott L, Fidler C, Ditchfield A, et al. Hypoglycemia event rates: a comparison between real‐world data and randomized controlled trial populations in insulin‐treated Diabetes. Diabetes Ther. 2016;7:45‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rickels MR, DuBose SN, Toschi E, et al. Mini‐dose glucagon as a novel approach to prevent exercise‐induced hypoglycemia in type 1 diabetes. Diabetes Care. 2018;41:1909‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marliss EB, Vranic M. Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes. 2002;51(Suppl 1):S271‐S283. [DOI] [PubMed] [Google Scholar]
- 22.Zaharieva DP, Riddell MC. Prevention of exercise‐associated dysglycemia: a case study‐based approach. Diabetes Spectr. 2015;28:55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MH, Vogrin S, Paldus B, et al. Glucose and counterregulatory responses to exercise in adults with type 1 diabetes and impaired awareness of hypoglycemia using closed‐loop insulin delivery: a randomized crossover study. Diabetes Care. 2020;43:480‐483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
Data will be available upon request to SF.


