Abstract
Objective
Refractory migraine (Ref‐M) represents a conundrum that headache experts have to face with. We aim to investigate whether a peculiar profile may characterize patients with Ref‐M according to 2020 European Headache Federation criteria. Furthermore, to substantiate a dysfunctional dopaminergic pathway involvement in these patients, we explored the effectiveness of olanzapine.
Materials & Methods
Eighty‐four patients (fitting previous Ref‐M criteria of the 2014) were treated with erenumab for six months. Differences between clinical and demographic features of responder (Ref‐M according to 2014 criteria) and not‐responder (Ref‐M according to 2020 criteria) patients to CGRP‐mAbs were investigated and their predictive values assessed. In fifteen patients with Ref‐M not responders to CGRP‐mAbs, olanzapine was administered (5 mg/die) for 3 months and frequency and pain intensity of migraine attacks were estimated.
Results
Patients with Ref‐M not responsive to CGRP‐mAbs (29/84) when compared with Ref‐M responsive to CGRP‐mAbs showed higher baseline frequency of migraine attacks, medication overuse and pain catastrophizing scale (PCS) scores. Logistic regression analyses showed that frequency of attacks, medication overuse and PCS score represent independent negative predictors of CGRP‐mAbs response. A ≥50% reduction of headache days/month was observed after olanzapine treatment in 67% of patients with Ref‐M not responsive to CGRP‐mAbs.
Conclusions
We outline that higher frequency of migraine attacks, medication overuse and pain catastrophizing characterize patients with Ref‐M not responsive to CGRP‐mAbs. In this frame, olanzapine effectiveness on frequency and pain intensity of migraine attacks supports the hypothesis that migraine refractoriness may be subtended by a prominent involvement of the dopaminergic pathway.
Keywords: antipsychotic drugs, chronic migraine, monoclonal antibodies, olanzapine, refractory
1. INTRODUCTION
Despite the advent of novel and specific therapeutic strategies, refractory migraine (Ref‐M) still represents a conundrum that headache experts have to face with. Indeed, it has been estimated that from 5 to 30% of migraine patients could be considered as refractory.1 This variability depends on the criteria employed to define Ref‐M, which is a dynamic concept in constant redefinition according to pharmacological advances.2, 3, 4, 5, 6, 7 The European Headache Federation (EHF) consensus of 2014 defined patients with Ref‐M those with failure and/or contraindications to at least 3 migraine preventative drugs among beta‐blockers, calcium channel blockers, anticonvulsants, antidepressants, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and onabotulinumtoxinA.4 Moreover, previous compelling demographic and clinical evidence suggested a peculiar Ref‐M patient's profile, characterized by a long disease history, symptomatic drug overuse, comorbid sleep disorders and overall psychiatric symptoms.2, 8 More recently, the Ref‐M criteria have been updated considering failure and/or contraindications for all the available migraine preventative drugs including those acting on the calcitonin‐gene related peptide (CGRP) pathway and debilitating headache for at least 8 days per month for no less than 6 consecutive months.7 Drug failure may include lack of efficacy or lack of tolerability.
Nevertheless, the pathophysiological mechanisms underlying Ref‐M are still matter of debate, although the prominence of specific neurotransmitter pathways as well as the imbalance between them has been suggested.9 According to this point of view, patients with Ref‐M, being subtended by the prominence of pathways on which the existing drugs seem not to act, may probably need different therapeutic approaches.
Interestingly, the dopaminergic pathway, poorly targeted by current preventive migraine medications, seems to play a critical role in more resistant migraine endophenotypes such as those associated with depressive or anxious symptoms and sleep disorders as well as medication overuse,10 known to be negative predictor of response to migraine preventive therapies.
The proof of this concept comes from previous pivotal studies showing the efficacy of selective anti‐dopaminergic drugs such as olanzapine in patients suffering from drug‐resistant chronic migraine.11, 12 However, these observations were conducted before the advent of onabotulinumtoxinA and CGRP‐monoclonal antibodies (CGRP‐mAbs).
Starting from these assumptions, according to the last Ref‐M consensus,7 we investigate whether: i) demographic data, parameters of disease severity and scores related to comorbidities (sleep disorders, depressive and anxiety symptoms, pain catastrophizing) may differ between chronic migraine patients CGRP‐mAbs‐responders (‘old‐refractoriness’) and chronic migraine patients CGRP‐mAbs not responders (‘new‐refractoriness’); ii) demographic data, parameters of disease severity and scores related to comorbidities (sleep disorders, depressive and anxiety symptoms) could represent independent predictors of CGRP‐mAbs’ lack of efficacy in chronic migraine patients.
We hypothesize that specific clinical and demographic features can identify a ‘profile’ predicting the lack of response to CGRP‐mAbs.
We speculate an underlying dysfunctional dopaminergic pathway in these patients, and, to this aim, we investigated the efficacy of daily administration of olanzapine, an anti‐dopaminergic drug, in a percentage of patients with Ref‐M not responsive to CGRP‐mAbs.
2. METHODS
This was a real‐word experience on eighty‐three patients with chronic migraine (according to the International Headache Society criteria) recruited from the migraine population being referred to the Headache Centre of the Department of Neurology at the University of Campania ‘Luigi Vanvitelli’ between February 2019 and July 2019 and followed up for nine months.13 Chronic migraine patients aged between 18 and 65 years received and failed at least four oral preventive medication classes (beta‐blockers, calcium channel blockers, anticonvulsants, antidepressants, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers) and onabotulinumtoxinA due to lack of efficacy, intolerable side effects or contraindications. Efficacy was defined as meaningful reduction (≥30% of reduction in headache days/month) in the frequency of headaches after the administration of drugs for at least three months as recommended by the European Headache Federation treatment guidelines.14, 15 The choice of 30% of monthly migraine days reduction as threshold to define the response to preventive treatments in our sample of refractory chronic migraine patients has been made in accordance with the guidelines for controlled trials of prophylactic treatment of chronic migraine in adults,16 afterwards considered as an appropriate efficacy cut‐off in many pharmacological studies focused on chronic migraine phenotype. Tolerability failure was defined as documented discontinuation due to adverse events at any time.
All patients received monthly one subcutaneous (s.c.) dose of erenumab until the sixth administration (for more information see Russo et al. 2020).17
The baseline (defined as the monthly mean of the 3 months preceding erenumab treatment) and the following headache attack frequencies were evaluated by reviewing patients’ standardized paper headache diaries.
After the sixth administration, patients with a clinically meaningful improvement (≥30% reduction of headache days per month) keep on the monthly erenumab administration, whereas patients with no clinically meaningful improvement (<30% reduction of headache days per month) discontinued erenumab administration and started therapy with olanzapine up to 5 mg per day.27
Before the first administration of erenumab (T0), all patients underwent an extensive interview aimed to assess clinical parameters of disease severity such as headache days per month, pain intensity (assessed by numerical rating scale [NRS]), acute pain medication intake, disease duration and migraine‐related disability (by MIDAS) and impact by Headache Impact Test (HIT‐6). Furthermore, patients underwent questionnaires aimed at exploring a) comorbid depression and anxiety by the Beck Depression Inventory‐II (BDI‐II), Hamilton Depression Rating Scale (HDRS), Hamilton Anxiety Rating Scale (HARS); b) quality of sleep by the Medical Outcomes Study (MOS) Sleep Scale c) quality of life by the migraine‐specific quality of life questionnaire (MSQ). Finally, Pain Catastrophizing Scale (PCS), Allodynia Symptom Checklist‐12 (ASC‐12) and Migraine attacks‐Subjective Cognitive impairments scale (MIG‐SCOG)18 were administered. Contrariwise, at the end of the sixth month (T1) of erenumab administration and after three months of olanzapine treatment (T2) all patients underwent a further interview aimed to assess clinical parameters of disease severity such as headache days per month, pain intensity (assessed by numerical rating scale [NRS]) of migraine attacks and acute pain medication intake.
Migraine patients with frank psychiatric comorbidities (psychosis, bipolar disorders or severe anxious or depressive symptoms) were excluded from the study. A part of the present patients’ population (54 patients) has been included in a previous published real‐world experience to assess multidimensional aspects of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments.17 The protocol was reviewed and approved by the Ethical Committee of the University of Campania ‘Luigi Vanvitelli’. Each patient gave a free, informed consent for participation in the study and the analysis and publication of the protocol data.
2.1. Statistical analysis
Categorical data were reported as number and percentage, while continuous data were reported as mean ±standard deviation (SD) and scale scores were reported as median and interquartile range (IQR). We used the chi‐square test to compare categorical variables and ANOVA to compare continuous variables, while we used the Mann‐Whitney U test to compare medians. Statistical significance was set at p < .05 and the Bonferroni correction has been applied to correct for multiple comparison. All analyses were performed using STATA version 14 (StataCorp, College Station, TX, USA).
In a second analytic step, we calculated simple binary logistic regression analyses, completed by a multiple binary logistic regression analysis (Forced Entry Method), in order to identify which parameters of disease severity (age, attack frequency, pain intensity, disease duration, medication overuse, MIDAS, HIT‐6, ASC‐12, HARS, HDRS and PCS scores) and scores related to comorbidities (depressive and anxiety symptoms, sleep disorders) represent independent predictors of response to monthly erenumab treatment.
Due to the observational design of the study, we did not plan a sample size calculation.
3. RESULTS
3.1. Demographic and clinical characteristics of patients with Ref‐M (before CGRP‐mAbs treatment)
The whole population consisted of 84 patients. The population of patients with Ref‐M not responsive to CGRP‐mAbs consisted of 29 patients (34% of patients treated with monthly erenumab administration for at least 6 months). Most patients were female (63 pts: 75%), with a mean age of 46.9 ± 11.9 years (range 18–75). The average time since the onset of migraine was 30 (±13.9) years. Abortive medications overuse was observed in 64% of patients and they had previously failed to benefit from at least a detoxification or acute medication withdrawal strategy. All patients had at least 4 oral preventive treatment failures, and all of them had also received at least three onabotulinumtoxinA administrations showing no benefits on the headache frequency and intensity (6.3 ± 0.8). Moreover, 64 patients (77%) showed mild depression symptoms (according to the HDRS or the self‐administrated BDI‐II), and 61 patients (73%) reported moderate anxiety comorbidity (according to the HARS). Finally, 64 patients (77%) reported CA and 66 patients (79%) experienced cognitive symptoms during migraine attacks. Demographic and baseline headache characteristics of patients included in the study are reported in Table 1.
TABLE 1.
Descriptive statistics of baseline characteristics of 84 patients
| Female n (%) | 63 (75%) |
| Age (Mean ± SD) | 46.91 ± 11.98 |
| Headache history (Mean ± SD) | 30.00 ± 13.96 |
| Medication overuse n (%) | 54 (64%) |
| Previous preventive (Mean ± SD) | 6.3 ± 0.81 |
| Baseline frequency of attacks (Mean ± SD) | 20.04 ± 6.79 |
| Headache pain intensity (Mean NRS ± SD) | 8.68 ± 1.08 |
| MIDAS (Median ± IQR ranges) | 88 ± 80.5 |
| HIT‐6 (Median ± IQR ranges) | 66 ± 7 |
| MSQ (Median ± IQR ranges) | 63.5 ± 26.5 |
| Pain catastrophizing scale (Median ± IQR ranges) | 36 ± 14.5 |
| Helplessness (Median ± IQR ranges) | 15.5 ± 7.25 |
| Rumination (Median ± IQR ranges) | 16 ± 6 |
| Magnification (Median ± IQR ranges) | 3 ± 3 |
| Beck depression inventory‐II (Median ± IQR ranges) | 17 ± 13 |
| Hamilton depression scale (Median ± IQR ranges) | 15.5 ± 11 |
| Hamilton anxiety scale (Median ± IQR ranges) | 17 ± 14.25 |
| MOS sleep scale (Median ± IQR ranges) | 24 ± 7 |
| ASC‐12 (Median ± IQR ranges) | 6 ± 9 |
| Mig‐Scog (Median ± IQR ranges) | 11 ± 7 |
Abbreviations: ASC‐12, allodynia symptoms checklist‐12; HIT‐6, headache impact test‐6; IQR, interquartile range; MIDAS, migraine disability assessment score questionnaire; MIG‐SCOG, migraine attacks subjective cognitive impairments scale; MOS, sleep scale: medical outcomes study sleep scale; MSQ, migraine‐specific quality of life questionnaire; NRS, numerical rating scale; SD, standard deviation.
3.2. Demographic and clinical characteristics of patients with Ref‐M responsive to CGRP‐mAbs vs patients with Ref‐M not responsive to CGRP‐mAbs
Patients with Ref‐M not responsive to CGRP‐mAbs (29/84; 35%) when compared with patients with Ref‐M responsive to CGRP‐mAbs (55/84; 65%) showed significantly higher baseline frequency of migraine attacks (23.5 ± 7.2 vs 18.18 ± 5.82, p = .0002), migraine‐related disability evaluated by MIDAS (134.1 ± 83.3 vs 87.9 ± 66.0, p = .004), medication overuse (83% vs 55%, p = .009) and pain catastrophizing scores (41 ± 10 vs 34 ± 13, p = .006) (corrected for multiple comparison). Contrariwise, no between‐group differences were found in other parameters of disease severity as well as scores related to comorbidities (depressive and anxiety symptoms and quality of sleep) (Table 2). Finally, simple and multiple binary logistic regression analyses showed that attacks frequency, medication overuse and PCS score represent independent negative predictors of response to CGRP‐mAbs (Table 3).
TABLE 2.
Descriptive statistics of baseline characteristics of patients responders and non‐responders to monthly Erenumab treatment
| Responders (55) | Non‐Responders (29) | p‐value | |
|---|---|---|---|
| Female n (%) | 41 (75%) | 22 (76%) | .89 |
| Age (Mean ± SD) | 44.85 ± 11.59 | 50.83 ± 11.92 | .01 |
| Headache history (Mean ± SD) | 28.42 ± 13.85 | 32.93 ± 13.91 | .08 |
| Previous preventive n (SD) | 6.2 ± 0.76 | 6.4 ± 0.79 | .76 |
| Medication overuse n (%) | 30 (55%) | 24 (83%) | .009 |
| Frequency of attacks (Mean ± SD) | 18.18 ± 5.82 | 23.48 ± 7.21 | .0002 |
| Headache pain intensity (Mean NRS ± SD) | 8.75 ± 0.91 | 8.55 ± 1.35 | .78 |
| MIDAS (Median ± IQR ranges) | 87.86 ± 66.00 | 134.14 ± 83.27 | .004 |
| HIT‐6 (Median ± IQR ranges) | 66 ± 5.5 | 66 ± 9 | .08 |
| MSQ (Median ± IQR ranges) | 53 ± 24 | 69 ± 26 | .29 |
| Pain catastrophizing scale (Median ± IQR) | 34 ± 13 | 41 ± 10 | .006 |
| Helplessness (Median ± IQR) | 15 ± 7 | 19 ± 8 | .01 |
| Rumination (Median ± IQR) | 16 ± 6.5 | 18 ± 7 | .13 |
| Magnification (Median ± IQR) | 3 ± 3 | 4 ± 3 | .01 |
| Beck depression inventory‐II (Median ± IQR) | 16 ± 11.25 | 20 ± 21 | .29 |
| Hamilton depression scale (Median ± IQR) | 15 ± 9.5 | 16 ± 16 | .73 |
| Hamilton anxiety scale (Median ± IQR) | 17 ± 11 | 18 ± 18 | .89 |
| MOS sleep scale (Median ± IQR) | 24 ± 8 | 24 ± 8 | .61 |
| ASC‐12 (Median ± IQR) | 6.5 ± 7.75 | 6 ± 11 | .84 |
| Mig‐Scog (Median ± IQR) | 11 ± 6.5 | 11 ± 8 | .34 |
Abbreviations: ASC‐12, allodynia symptoms checklist‐12; HIT‐6, headache impact test‐6; IQR, interquartile range; MIDAS, migraine disability assessment score questionnaire; MIG‐SCOG: migraine attacks‐subjective cognitive impairments scale; MOS, sleep scale: medical outcomes study sleep sclae; MSQ, migraine‐specific quality of life questionnaire; NRS, numerical rating scale; SD, standard deviation.
Bold indicates statistically significant values.
TABLE 3.
Simple and multiple binary logistic regression analyses assessing which parameters is able to predict the response to erenumab treatment [95% bias‐corrected and accelerated bootstrap (BCa) confidence intervals (CIs) on1000 samples]
| Variable | p‐value | SE | 95% CIs for Odds Ratio | ||
|---|---|---|---|---|---|
| Lower | Odds | Upper | |||
| Simple Regression | |||||
| Disease history (years) | .155 | 0.02 | 0.94 | 0.97 | 1.01 |
| Age (years) | .034 | 0.02 | 0.92 | 0.95 | 1.00 |
| Pain intensity (NRS) | .432 | 0.25 | 0.78 | 1.18 | 1.80 |
| Frequency of attacks (days/month) | .001 | 0.03 | 0.81 | 0.87 | 0.95 |
| Medication overuse headache | .014 | 0.14 | 0.08 | 0.25 | 0.75 |
| Pain catastrophizing scale | .013 | 0.03 | 0.88 | 0.93 | 0.98 |
| ASC−12 | .924 | 0.04 | 0.91 | 0.99 | 1.08 |
| Beck depression inventory ‐II | .260 | 0.02 | 0.93 | 0.97 | 1.02 |
| MIDAS | .014 | 0.01 | 0.98 | 0.99 | 1.00 |
| HIT−6 | .201 | 0.04 | 0.86 | 0.94 | 1.03 |
| MOS sleep scale | .364 | 0.04 | 0.89 | 0.96 | 1.04 |
| Multiple regressiona | |||||
| Medication overuse headache | .050 | 0.18 | 0.08 | 0.29 | 1.00 |
| Frequency of attacks (days/month) | .009 | 0.03 | 0.82 | 0.89 | 0.97 |
| Pain catastrophizing scale | .037 | 0.02 | 0.88 | 0.93 | 0.99 |
p‐value <0.001.
R2 =0.20 (Nagelkerke).
Abbreviations: SE, Standard Error; NRS, numerical rating scale; NRS, numerical rating scale; ASC‐12, allodynia symptoms checklist‐12; MIDAS, migraine disability assessment score questionnaire; HIT‐6, headache impact test‐6; MSQ, migraine‐specific quality of life questionnaire; MOS sleep scale, medical outcomes study sleep scale.
Model χ2 (2) =20.56.
3.3. Olanzapine efficacy, safety and tolerability in patients with Ref‐M not responsive to CGRP‐mAbs
After a three‐month olanzapine treatment (T2), 67% (n = 10) of patients were considered ‘responders’ to olanzapine (eg ≥30% reduction of headache days/month), whereas the remaining 33% (5 pts) were considered ‘non‐responders’ (eg <30% reduction of headache days/month). Interestingly, all the responders to olanzapine (67%) showed ≥50% reduction of headache days/month, switching from chronic to episodic migraine (from 24.5 ± 7.7 at baseline to 14.3 ± 8.2 after three months of olanzapine treatment, p < .01). Finally, a significant improvement has been observed in headache pain intensity (from 8.80 ± 1.1 at baseline to 7.3 ± 1.7 after three months of olanzapine treatment, p < .001) (Figure 1).
FIGURE 1.
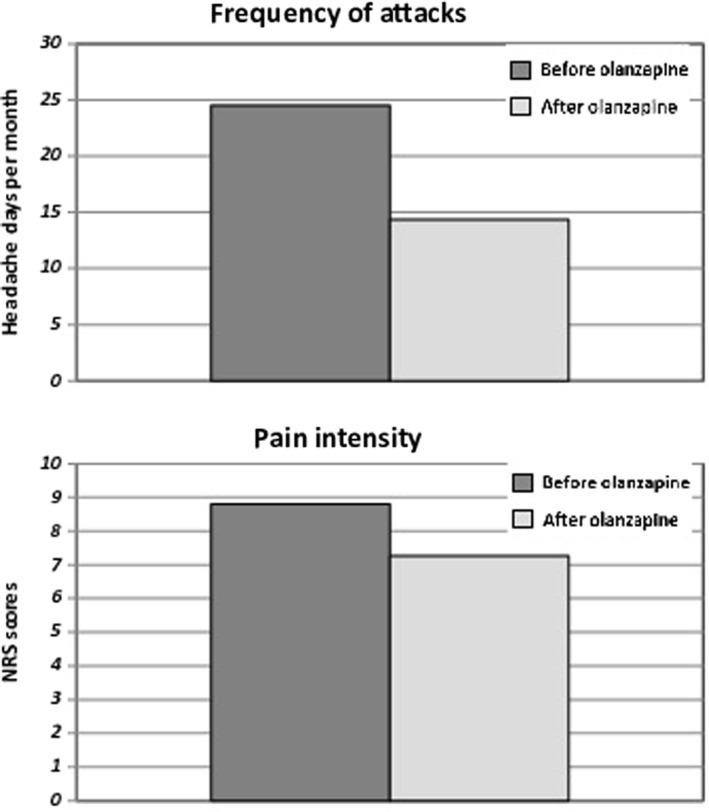
Comparison of frequency of monthly headache attacks and pain intensity during headache attacks in patients with Ref‐M before and after 3‐month olanzapine treatment
Nine patients (60%) reported side effects related to olanzapine treatment. Specifically, 7 patients reported weight gain, 4 patients reported excessive somnolence, while 3 patients reported orthostatic hypotension (Table 4).
TABLE 4.
Descriptive statistics of characteristics of the 15 patients treated with Olanzapine
| Female n (%) | 12 (80%) | |
| Age (Mean ± SD) | 48.66 ± 11.18 | |
| Headache history (Mean ± SD) | 30.80 ± 13.98 | |
| Baseline frequency of attacks (Mean ± SD) | 24.53 ± 7.75 | |
| Frequency of attacks after three months (Mean ± SD) | 14.33 ± 8.25 | p = .002 |
| Headache pain intensity (Mean NRS ± SD) | 8.80 ± 1.14 | |
| Headache Pain intensity after three months (Mean NRS ± SD) | 7.27 ± 1.75 | p = .003 |
| Patients with 2‐hours pain free to triptans n (%) | 13 (87%) |
Abbreviations: SD, standard deviation; NRS, numerical rating scale.
4. DISCUSSION
In the present study, we outline the demographic and clinical profile of patients with chronic Ref‐M not responsive to CGRP‐mAbs (according to EHF 2020 criteria) compared with those responsive to CGRP‐mAbs (‘refractoriness’ according to EHF 2014 criteria) in a cohort of patients with chronic migraine with previous failure of at least 5 prophylactic drug therapies (including onabotulinumtoxinA).
Moreover, according to the hypothesis of a prevalent involvement of the dopaminergic pathway in the refractoriness phenomenon, we substantiate the efficacy of daily intake of olanzapine in a percentage of patients with Ref‐M not responsive to CGRP‐mAbs.
Since the first attempt of Reisman in 1952 to define the concept of Ref‐M, several criteria have been proposed over time.2, 3, 4, 5, 6, 7 Indeed, the progressively advent of new therapeutic strategies has required a continuous updating of migraine refractoriness criteria, gradually reducing the number of patients falling into this definition. On the other hand, there is always a subgroup of chronic patients fitting Ref‐M criteria. Identifying demographic and clinical features of these patients allowed delineating a peculiar profile supporting the stimulating hypothesis that the Ref‐M endo‐phenotype could be subtended by a different—or more complex—pathophysiological mechanism.2, 10 In line with this concept, previous observations (according to the previous definition of refractoriness) have demonstrated that patients with Ref‐M were characterized by a long disease history and the coexistence of depressive or anxious symptoms (not such as to allow a categorization in a well‐defined psychiatric disorder) as well as sleep disturbances.19, 20, 21
Our findings further support the peculiar clinical profile of patients with Ref‐M as a group, according to European Headache Federation consensus of 2014 (and then independently from the response to CGRP‐mAbs) characterized by a long disease history (30.0 ± 13.9) and depressive or anxious symptoms (77% and 73% respectively) and medication overuse (64%). Moreover, we observed in these patients a high prevalence of aberrant pain coping (eg pain catastrophizing), sleep disturbances, cutaneous allodynia and cognitive symptoms in the course of migraine attacks (Table 1 for further information).
However, the main finding of the present study is that patients with Ref‐M according to the recent European Headache Federation consensus, 2020 (eg not responsive to CGRP‐mAbs) exhibited a higher frequency of migraine attacks per month, medications overuse and a worse ability to cope with actual or anticipated pain (eg pain catastrophizing). Contrariwise, no differences were found in other parameters of disease severity as well as in scores related to comorbidities (depressive and anxiety symptoms and quality of sleep).
Similarly, Ornello et al., have recently demonstrated the higher frequency of migraine attacks and greater symptomatic drug overuse in patients with Ref‐M not responsive to CGRP‐mAbs.22 Although medication overuse is known as a prognostic factor in terms of both migraine chronification and poor response to preventive treatments, the mechanisms by which the overuse of symptomatic drugs leads to increased frequency of attacks or refractoriness is still matter of debate. Alteration of cortical neuronal excitability, central sensitization, changes in the dopaminergic pathways as well as in the endocannabinoid system have been suggested.23 Furthermore, neuroimaging studies have consistently demonstrated changes in the orbitofrontal cortex and the mesocorticolimbic dopamine circuitry in patients with medication overuse headache (MOH).24, 25, 26 In line with our findings, it has been recently showed that erenumab efficacy in migraine prevention is not influenced by patients’ psychological traits such as depressive and anxious symptoms, impulsiveness, alexithymia and emotion regulation.27 On the other hand, it has been demonstrated that migraine patients, beyond depressive and anxiety symptoms, employ maladaptive pain coping strategies encompassing the so‐called ‘pain catastrophizing’, characterized by a negative orientation towards actual or anticipated pain experience.28, 29 Patients who ‘catastrophize’ have trouble in inhibiting thoughts about pain (rumination), exaggerate and worry about the negative consequences of pain (magnification) and believe that there is nothing they can do to alleviate the pain (helplessness). Pain catastrophizing leads to an increased pain experience, complaining and behaviour evolving into preoccupation and compulsive seeking of pain relief, with consequent decreased quality of life.30
Among patients with migraine, ‘catastrophizing’ habits are associated with more frequent migraine attacks and chronic migraine, poorer treatment response, increased medical consultations, impaired functioning and reduced health‐related quality of life.31, 32, 33 In this context, the higher PCS scores observed in patients with Ref‐M non‐responsive to CGRP‐mAbs as well as the PCS capability to predict those patients who will not respond to CGRP‐mAbs administration, identify the maladaptive coping strategy as a critical risk factor for refractoriness despite the use of innovative and high effective therapies. Pathophysiological substrates of refractoriness are quite unknown in migraine and although genetic heterogeneity, structural and functional brain changes have been widely evoked, focusing on neurotransmitters differences may shed some lights on the matter.34
Migraine is a complex multiphasic disorder involving in its pathophysiology multiple neurotransmitters such as CGRP but also dopamine, serotonin, glutamate, orexin, nitric oxide and disparate others with different prevalence in the several phases of migraine cycle.35 Therefore, it can be argued that the prominence of specific neurotransmitter pathways as well as the imbalance between them may differ between patients leading to different phenotypes.
Since the seminal works of Sicuteri in the seventies of the last century, several studies have strongly supported the role of an increased dopaminergic activity as a key pathophysiological mechanism underlying at least some phase of migraine cycle as well as some subgroups of migraine patients.36, 37, 38, 39, 40, 41 Converging clinical evidence support the involvement of dopamine as an endogenous protagonist during migraine prodromal phase witnessed by the so‐called ‘dopaminergic symptoms’ such as yawning, drowsiness, irritability and hyperactivity, probably subtended by the increased frequency of the D2 receptor allele (DRD2 NcoI C allele) leading to a greater expression of dopaminergic receptors in these patients.
Consistently, patients with chronic migraine, experiencing a sort of ‘never‐ending migraine attack’, are characterized by higher dopamine plasma levels compared with patients suffering from chronic tension type headache and healthy subjects.42
Very recently, an innovative comprehensive model involving, on one hand, dopamine neurotransmitters and the nucleus accumbens and—on the other hand—the chronification of pain, medication overuse and pain catastrophizing has been suggested via abnormal reward mechanisms.40 By means of a reward homeostat (the so‐called ‘hedonostat’), dopamine concentrations, which are perturbed by chronic pain as well as emotional stimuli, are monitored by the nucleus accumbens by inputs from cortical and subcortical areas known to be part of the so‐called neurolimbic pain network.43 Deviations from such a set point are buffered by the opponent hedonostat effectors that readjust dopamine synthesis, its release and signalling to the pre‐perturbation levels. However, robust and sustained increases in dopaminergic trafficking induced by repetitive migraine attacks as well as maladaptive emotional stimuli (eg pain catastrophizing) override homeostatic feedback control inducing allostatic/maladaptive neuroadaptations. In other terms, the massive dopaminergic tone surges in reward, motivation and learning centres leading to migraine chronification and abnormal coping strategies such as pain catastrophizing and urgency to seek and consume analgesic drugs (eg medication overuse). Similarly, this could manifest in spontaneous pain or in exaggerated responses to painful (hyperalgesia) and normally non‐painful (allodynia) stimuli, in combination with negative affective and cognitive states and a persistent desire to eliminate pain via behavioural or pharmacologic measures.44
Interestingly, dopamine abnormalities play their role also in the occurrence of depressive and anxious symptoms as well as in the genesis of the sleep disorders known to characterize patients with chronic migraine as well as patients with Ref‐M.45, 46
Altogether, the data are in line with recent observations demonstrating that greater the involvement of the dopaminergic system the more disabling the migraine phenotype is (eg long‐lasting attacks, high frequency of osmophobia, allodynia and unilateral cranial autonomic symptoms.47
In this scenario, it would be of interest to substantiate whether atypical antipsychotic drugs are able to reverse massive dopamine concentrations in patients suffering from Ref‐M. This approach is further reinforced by pharmacological insights such as the efficacy as migraine rescue of the D2 dopamine receptor antagonists such as prochlorperazine, metoclopramide and domperidone. Similarly,48 the well‐demonstrated flunarizine efficacy as migraine preventive treatment (despite flunarizine is not available in the United States or many other countries) seems to be due to its dopamine antagonist properties more than to its activity as calcium channel blocker.49
Silberstein and colleagues investigated the efficacy of olanzapine as preventive migraine therapy in a cohort of refractory chronic migraine patients.11 The authors observed a statistically significant decrease in headache days per month (from 27.5 ± 4.9 to 21.1 ± 10.7) and headache pain intensity (from 8.7 ± 1.6 to 2.2 ± 2.1) after three months of olanzapine treatment. However, these observations date back to 2002 in a clinical scenario in which onabotulinumtoxinA and CGRP‐monoclonal antibodies (CGRP‐mAbs) were not available yet. Contrariwise, we focused on patients with Ref‐M not responsive to both onabotulinumtoxinA and CGRP‐mAbs showing a statistically significant reduction in both migraine attacks frequency and mean pain intensity of headache episodes. More specifically, a ≥50% reduction in headache days per month with the consequent conversion from chronic to episodic migraine has been observed in 67% of patients treated with olanzapine (from 24.5 ± 7.7 headache/month at baseline to 14.3 ± 8.2 headache/month after three months of olanzapine treatment, p < .01). We speculate that the efficacy of olanzapine in patients Ref‐M may be due to its ability to act not only on pain perception, but also on emotional modulation and internal representation of pain experience and, finally, on the ability to cope with it.12 More specifically, a drug characterized by such a broad receptor profile may be able to more comprehensively re‐modulate the neurolimbic changes that underlie Ref‐M.
We do not know if the increased involvement of dopaminergic circuits would be an innate characteristic of a group of migraine patients leading to a specific—severe—phenotype, or if it is the result of the migraine burden as well as of its comorbidities or coexistent conditions. Surely, the identification of these patients could represent a further step towards the precision and patient‐oriented medicine.
On the other hand, we would underline that the aim of the present study is not to propose olanzapine as a migraine preventive therapy (although it could be considered in some selected patients) but to substantiate, by means of a pharmacological indirect proof, the possibility that different migraine endophenotypes may be underpinned by equally different pathophysiological mechanisms and neurotransmitter pathways such as the dopaminergic one. Moreover, although on a limited patients’ sample, olanzapine has shown the typical profile of the previous not specific preventive anti‐migraine drugs such as the high prevalence of side effects, which were overcome by CGRP‐mAbs.
We are aware that this study is not except from some limitations. First, patients did not undergo an extensive psychiatric evaluation able to identify subtle psychiatric or personality disorders that could represent the trigger for migraine attacks and, on the other hand, the background of a good response to antipsychotic treatment. However, the good response to triptans exhibited by Ref‐M patients receiving daily olanzapine treatment, tend to support that migraine attacks were subtended by a migraineous pathophysiology.50 Nevertheless, the lack of an extensive psychiatric evaluation, even representing a study limitation, brings a generalizability closer to the common clinical practice in which there is not always the possibility to carry out an extensive psychodiagnostics interview that allows to identify subtler disturbances in migraine patients. The low sample size certainly represents another limitation of the study that nevertheless is proposed as an experiment to inspire future, ad hoc designed studies. Finally, about the percentage of patients with Ref‐M treated with olanzapine, it cannot be excluded that its efficacy in reducing headache frequency per month may be due, at least partially, to other non‐dopaminergic pharmacological mechanisms. Indeed, olanzapine is an atypical antipsychotic drug characterized by a broad receptor profile: it acts as a D2 dopamine receptor antagonist but also as 5‐HT2A and 5‐HT2C serotonin receptors antagonist and alpha1adrenolytic as well as antihistamine antagonist (the latter not relevant for migraine treatment).11 Therefore, it could be suggested that its anti‐migraine effect is due to serotonin antagonism shared with methysergide as well as to alfa2‐adrenoreceptors agonism subtending the antinociceptive effect of clozapine. However, with the exclusion of CGRP‐mAbs, this is a speculative limitation affecting all ‘repositioning’ migraine treatments.
5. CONCLUSIONS
In conclusion, we demonstrated a peculiar clinical profile of Ref‐M patients characterized by higher frequency of migraine attacks per month, medications overuse and pain catastrophizing able to identify these migraine patients in clinical practice. It is likely that refractoriness phenomenon may be subtended by a prominence of pathways that the existing drugs are not able to modulate, and, to date, the dopaminergic pathway seems to be the less modulated by using the current migraine preventive armamentarium. We speculate that future specific therapies able to modulate also dopaminergic pathways could represent the gold standards of migraine treatments, further reducing the percentage of patients falling into the category of Ref‐M.
CONFLICT OF INTEREST
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MS has received speaker honoraria from Novartis and Lilly. AT has received speaker honoraria from Novartis, Schwarz Pharma/UCB, Lundbeck, Abbvie and Glaxo. GT has received speaker honoraria from Sanofi‐Aventis, Merck Serono, Bayer Schering Pharma, Novartis, Biogen‐Dompe´ AG, Teva and Lilly; has received funding for travel from Bayer Schering Pharma, Biogen‐Dompe´ AG, Merck Serono, Novartis, and Sanofi Aventis; and serves as an associate editor of Neurological Sciences. AR has received speaker honoraria from Allergan, Lilly, Novartis and Teva and serves as an associate editor of Frontiers in Neurology (Headache Medicine and Facial Pain session). The other authors have nothing to declare.
AUTHOR CONTRIBUTIONS
MS involved in data analysis, literature review, results interpretation, manuscript drafting and revision. AT involved in data analysis, results interpretation and manuscript revision. FSDC involved in literature review, results interpretation and manuscript revision. GB involved in results interpretation and manuscript revision. GT involved in results interpretation and manuscript revision. AR involved in data analysis, results interpretation, manuscript drafting and revision.
ACKNOWLEDGEMENT
None declared
Funding information
This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are available from the corresponding author (dottor.russo@gmail.com).
REFERENCES
- 1.Schulman EA, Brahin EJ. Refractory headache: historical perspective, need, and purposes for an operational definition. Headache. 2008;48(6):770‐777. [DOI] [PubMed] [Google Scholar]
- 2.D'Antona L, Matharu M. Identifying and managing refractory migraine: barriers and opportunities? J Headache Pain. 2019;23(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goadsby PJ, Schoenen J, Ferrari MD, et al. Towards a definition of intractable headache for use in clinical practice and trials. Cephalalgia. 2006;26:1168‐1170. [DOI] [PubMed] [Google Scholar]
- 4.Martelletti P, Katsarava Z, Lampl C, et al. Refractory chronic migraine: a consensus statement on clinical definition from the European headache federation. J Headache Pain. 2014;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman EA, Lake AE, Goadsby PJ, et al. Defining refractory migraine and refractory chronic migraine: proposed criteria from the refractory headache special interest section of the American headache society. Headache. 2008;48:778‐782. [DOI] [PubMed] [Google Scholar]
- 6.Silberstein SD, Dodick DW, Pearlman S. Defining the pharmacologically intractable headache for clinical trials and clinical practice. Headache. 2010;50:1499‐1406. [DOI] [PubMed] [Google Scholar]
- 7.Sacco S, Braschinsky M, Ducros A, et al. European headache federation consensus on the definition of resistant and refractory migraine: Developed with the endorsement of the European Migraine & Headache Alliance (EMHA). J Headache Pain. 2020;21(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres‐Ferrús M, Ursitti F, Alpuente A, et al. From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. 2020;21:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agostoni EC, Barbanti P, Calabresi P, et al. Current and emerging evidence‐based treatment options in chronic migraine: a narrative review. J Headache Pain. 2019;30;20(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akdag Uzun Z, Kurt S, Karaer UH. The relationship with restless legs syndrome, fibromyalgia, and depressive symptoms in migraine patients. Neurol Sci. 2018;39(8):1409‐1414. [DOI] [PubMed] [Google Scholar]
- 11.Silberstein SD, Peres MF, Hopkins MM, et al. Olanzapine in the treatment of refractory migraine and chronic daily headache. Headache. 2002;42(6):515‐518. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez XF, Sundararajan T, Covington EC. A systematic review of atypical antipsychotics in chronic pain management: olanzapine demonstrates potential in central sensitization, fibromyalgia, and headache/migraine. Clin J Pain. 2018;34(6):585‐591. [DOI] [PubMed] [Google Scholar]
- 13.Headache classification committee of the international headache society (IHS) . The International Classification of Headache Disorders. 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 14.Steiner TJ, Martelletti P. Aids for management of common headache disorders in primary care. J Headache Pain. 2007;8(Suppl 1):S2. [PubMed] [Google Scholar]
- 15.Silberstein S, Tfelt‐Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484‐495. [DOI] [PubMed] [Google Scholar]
- 16.Silberstein S, Tfelt‐Hansen P, Dodick DW, et al. Task Force of the International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28(5):484‐495. [DOI] [PubMed] [Google Scholar]
- 17.Russo A, Silvestro M, Scotto di Clemente F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real‐world experience. J Headache Pain. 2020;21(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russo A, Silvestro M, Garramone F, et al. A subjective cognitive impairments scale for migraine attacks: validation of the Italian version of the MIG‐SCOG. Neurol Sci. 2020;41(5):1139‐1143. [DOI] [PubMed] [Google Scholar]
- 19.Baskin SM, Lipchik GL, Smitherman TA. Mood and anxiety disorders in chronic headache. Headache. 2006;46(Suppl 3):S76‐S87. [DOI] [PubMed] [Google Scholar]
- 20.Saper JR. “are you talking to me?” confronting behavioral disturbances in patients with headache. Headache. 2006;46(Suppl 3):S151‐S156. [DOI] [PubMed] [Google Scholar]
- 21.Freedom T, Evans RW. Headache and sleep. Headache. 2013;53(8):1358‐2136. [DOI] [PubMed] [Google Scholar]
- 22.Ornello R, Casalena A, Frattale I, et al. Real‐life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bigal ME, Lipton RB. Overuse of acute migraine medications and migraine chronification. Curr Pain Headache Rep. 2009;13(4):301‐307. [DOI] [PubMed] [Google Scholar]
- 24.Schiano di Cola F, Caratozzolo S, Liberini P, et al. Response predictors in chronic migraine: medication overuse and depressive symptoms negatively impact onabotulinumtoxin‐a treatment. Front Neurol. 2019;10(10):678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riederer F, Marti M, Luechinger R, et al. Grey matter changes associated with medication‐overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatry. 2012;13:517‐525. [DOI] [PubMed] [Google Scholar]
- 26.Riederer F, Schaer M, Gantenbein AR, et al. Cortical alterations in medication‐overuse headache. Headache. 2017;57:255‐265. [DOI] [PubMed] [Google Scholar]
- 27.Altamura C, Costa C, Fofi L, et al. Migraineurs’ psychological traits do not influence response to erenumab. Neurol Sci. 2020;41(Suppl 2):467‐468. [DOI] [PubMed] [Google Scholar]
- 28.Bond DS, Buse DC, Lipton RB, et al. Clinical Pain Catastrophizing in Women With Migraine and Obesity. Headache. 2015;55(7):923‐933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santangelo G, Russo A, Trojano L, et al. Cognitive dysfunctions and psychological symptoms in migraine without aura: a cross‐sectional study. J Headache Pain. 2016;17(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89(1):11‐36. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52‐64. [DOI] [PubMed] [Google Scholar]
- 32.Peters ML, Vlaeyen JW, Weber WE. The joint contribution of physical pathology, pain‐related fear and catastrophizing to chronic back pain disability. Pain. 2005;113:45‐50. [DOI] [PubMed] [Google Scholar]
- 33.Severeijns R, Vlaeyen JW, van den Hout MA, et al. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165‐172. [DOI] [PubMed] [Google Scholar]
- 34.Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noseda R, Borsook D, Burstein R. Neuropeptides and neurotransmitters that modulate thalamo‐cortical pathways relevant to migraine headache. Headache. 2017;57:97‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbanti P, Fabbrini G. Migraine and the extrapiramidal system. Cephalalgia. 2002;22:2‐11. [DOI] [PubMed] [Google Scholar]
- 37.Bussone G, Boiardi A, La Mantia L, et al. Clinical usefulness of a dopaminergic agonist in headache diagnosis. Int J Clin Pharmacol Res. 1986;6:23‐26. [PubMed] [Google Scholar]
- 38.Bès A, Dupui P, Güell A, et al. Pharmacological exploration of dopamine hypersensitivity in migraine patients. Int J Clin Pharmacol Res. 1986;6:189‐192. [PubMed] [Google Scholar]
- 39.Barbanti P, Bronzetti E, Ricci A, et al. Increased density of dopamine D5 receptor in peripheral blood lymphocytes of migraineurs: A marker for migraine? Neurosci Lett. 1996;207:1‐4. [DOI] [PubMed] [Google Scholar]
- 40.Barbanti P, Fabbrini G, Ricci A, et al. Migraine patients show an increased density of dopamine D3 and D4 receptors on lymphocytes. Cephalalgia. 2000;20:15‐19. [DOI] [PubMed] [Google Scholar]
- 41.DaSilva AF, Nascimento TD, Jassar H, et al. Dopamine D2/D3 imbalance during migraine attack and allodynia in vivo. Neurology. 2017;88:1634‐1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenen J. Is chronic migraine a never‐ending migraine attack? Pain. 2011;152:239‐240. [DOI] [PubMed] [Google Scholar]
- 43.Maizels M, Aurora S, Heinricher M. Beyond neurovascular: migraine as a dysfunctional neurolimbic pain network. Headache. 2012;52(10):1553‐1565. [DOI] [PubMed] [Google Scholar]
- 44.Russo A, Silvestro M, Trojsi F, et al. Cognitive networks disarrangement in patients with migraine predicts cutaneous allodynia. Headache. 2020;60(7):1228‐1243. [DOI] [PubMed] [Google Scholar]
- 45.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20:1036‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vgontzas A, Pavlović JM. Sleep disorders and migraine: review of literature and potential pathophysiology mechanisms. Headache. 2018;58(7):1030‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barbanti P, Aurilia C, Egeo G, et al. Dopaminergic symptoms in migraine: A cross‐sectional study on 1148 consecutive headache center‐based patients. Cephalalgia. 2020;40(11):1168‐1176. [DOI] [PubMed] [Google Scholar]
- 48.Marmura MJ. Use of dopamine antagonists in treatment of migraine. Curr Treat Options Neurol. 2012;14(1):27‐35. [DOI] [PubMed] [Google Scholar]
- 49.Wöber C, Brücke T, Wöber‐Bingöl C, et al. Dopamine D2 receptor blockade and antimigraine action of flunarizine. Cephalalgia. 1994;14(3):235‐240. [DOI] [PubMed] [Google Scholar]
- 50.Bigal ME, Ferrari M, Silberstein SD, et al. Migraine in the triptan era: lessons from epidemiology, pathophysiology, and clinical science. Headache. 2009;49(Suppl 1):S21‐33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are available from the corresponding author (dottor.russo@gmail.com).


