Abstract
Morphine is an opioid analgesic indicated in the treatment of acute and chronic moderate to severe pain. From a pharmacodynamic standpoint, morphine exerts its effects by agonizing mu‐opioid receptors predominantly, resulting in analgesia and sedation. Pharmacokinetically, morphine is primarily metabolized in the liver via glucuronidation by the enzyme uridine diphosphate glucuronosyltransferase family 2 member B7 and encounters the transporter proteins organic cation transporter isoform 1 and P‐glycoprotein (adenosine triphosphate–binding cassette subfamily B member 1) as it is being distributed throughout the body. The genes coding for the proteins impacting either the pharmacokinetics or pharmacodynamics of morphine may bear genetic variations, also known as polymorphisms, which may alter the function of the proteins in such a manner that an individual may have disparate treatment outcomes. The purpose of this review is to highlight some of the genes coding for proteins that impact morphine pharmacokinetics and pharmacodynamics and present some treatment considerations.
Keywords: ABCB1, OCT1, OPRM1, P‐glycoprotein, pharmacogenomics, UGT2B7
Morphine is a nonsynthetic opioid agonist with a US Food and Drug Administration indication for moderate to severe acute and chronic pain.1, 2 Morphine exerts its action via activation of opioid receptors, specifically delta‐, mu‐, and kappa‐opioid receptors, resulting in analgesia, anxiolysis, euphoria, sedation, respiratory depression, and gastrointestinal smooth muscle contraction.3, 4 Of the 3 aforementioned opioid receptors, morphine predominantly interacts with mu‐opioid receptors (MORs), which are distributed in several locations throughout the central nervous system.3, 4, 5
Morphine serves as the prototypical opiate with regard to analgesic and side effects for other opiate derivatives.6 It is absorbed in the upper intestine and the mucosa of the rectum, while it is eliminated renally and via enterohepatic circulation, primarily as morphine‐3‐glucuronide (M3G).3, 4 Morphine is available as branded and generic products in multiple dosage forms and can be administered orally, parenterally, epidurally, and rectally.1 Morphine dosing is individualized on the basis of factors such as the patient's degree of opioid tolerance, general condition, and medical status, as well as current medications and the type and severity of pain.2 Due to the abuse potential and risk of developing an addiction disorder, morphine is a Schedule II narcotic under the Controlled Substances Act of 1970.8 Common adverse effects attributable to morphine therapy include respiratory depression, somnolence, constipation, and nausea.1
Overview of Pharmacogenomics and Morphine
Although clinical practice guidelines have been developed to standardize the treatment of pain,9 ≈10% to 30% of patients are not able to reach their pain management goals due to either inadequate analgesia or untoward side effects.10 There are many potential factors that can contribute to variable pain management outcomes, such as age, sex, or differences in pain perception and interindividual differences attributable to genetic variations, also known as polymorphisms. According to Angst et al,11 genetic effects are thought to be responsible for ≈12% to 60% of response variability in opioid treatment. Genetic polymorphisms may impact morphine pharmacokinetics (PK), which refers to the absorption, distribution, metabolism, and elimination of a drug from the body; or pharmacodynamics (PD), which refers to the effect that the drug has on the body. An example of a genetic polymorphism impacting the PK of morphine would be a mutation in a gene coding for an enzyme that metabolizes morphine that results in a reduction in the rate at which morphine is metabolized. As such, one would expect serum morphine levels to be higher in a person with this mutation when compared to a person without such a mutation, and this may increase the risk of untoward effects, such as respiratory depression.2 An example of a genetic polymorphism impacting the PD of morphine would be a mutation in the gene coding for the drug receptor target for morphine that results in reduced binding affinity. A person carrying this mutation may experience inadequate pain relief compared to a person carrying the normal receptor gene and may precipitate the need for a higher morphine dose or additional analgesics. Pharmacogenomics is the study of the impact that genetic polymorphisms have on drug response, and the use of clinical pharmacogenomics testing can help to elucidate genetic polymorphisms that play a role in the PK and PD for morphine.7
Genetic Polymorphisms Impacting Morphine PK and PD
Morphine encounters several proteins in the body, including uridine diphosphate glucuronosyltransferase family 2 member B7 (UGT2B7), organic cation transporter isoform 1 (OCT1), opioid receptor mu 1 (OPRM1), and P‐glycoprotein (P‐gp). We will discuss some of the more frequently studied genetic polymorphisms impacting these proteins and their associated functional characteristics.
Uridine Diphosphate Glucuronosyltransferase Family 2 Member B7
UGT2B7 belongs to the uridine diphosphate glucuronosyltransferase group of phase II metabolic enzymes that are involved in the glucuronidation of compounds such as steroid hormones, bile acids, retinoids, and fatty acids.7, 12 UGT2B7 is predominantly expressed in the endoplasmic reticulum of hepatocytes and is also found in the gastrointestinal tract, kidney, pancreas, and brain.12 Figure 1 shows that UGT2B7 converts morphine to M3G (90%) and morphine‐6‐glucuronide (M6G) (10%). Although M3G is the major metabolite, it does not confer any analgesic activity and cannot cross the blood‐brain barrier (BBB).2, 3 However, M3G is bioactive and is associated with neuroexcitatory effects, including allodynia, myoclonus, and seizures. M6G is an active metabolite and a potent analgesic that can cross the BBB and enhance the effects of morphine.10
Figure 1.
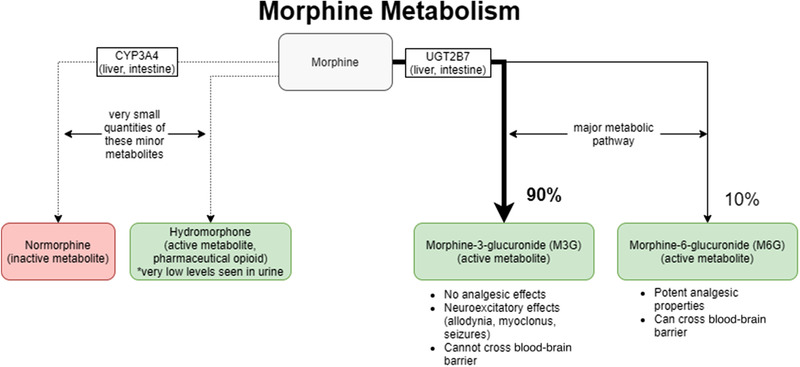
Morphine metabolism. Morphine is predominantly metabolized via glucuronidation by the phase II enzyme UGT2B7 to the major metabolites morphine‐3‐glucuronide (M3G) and morphine‐6‐glucuronide (M6G). Green indicates an active metabolite, and red indicates an inactive metabolite. CYP3A4, cytochrome P450 3A4.
There has been conflicting evidence in the literature regarding the functional characteristics of some UGT2B7 gene polymorphisms. Some of the most frequently studied UGT2B7 gene polymorphisms are UGT2B7 802T>C (rs7439366) and UGT2B7 –900G>A (rs7438135). The UGT2B7 802T>C polymorphism has been characterized in some studies as impacting interindividual variability in treatment outcomes.13, 14 For example, one study concluded that carriers of the UGT2B7 802T allele experienced a higher peak and extended analgesia compared to subjects homozygous for the UGT2B7 802C allele, indicating that the 802T allele may result in reduced glucuronidation activity.15 However, other studies do not demonstrate an association between the 802T allele and treatment outcome variability.12, 16, 17 Similar to the UGT2B7 –900G>A variant, there have been contradictory findings in the literature, as one study concluded that the 900A allele conferred greater activity over the wild‐type 900G allele,18 while another study did not find any significant impact on morphine PK due to this variant.19 In addition to UGT2B7 polymorphisms impacting PK, some studies have evaluated associations between UGT2B7 polymorphisms and disease risk20 or morphine adverse effects.21 Further research on UGT2B7 polymorphisms is needed to gain a better understanding of how to predict the functional capabilities of this essential morphine metabolic enzyme.
Organic Cation Transporter Isoform 1
OCT1 is an influx transporter encoded by the solute carrier family 22 member 1 (SLC22A1) gene.22 OCT1 is located in several tissues, but is abundantly expressed in the liver with much lower levels in other tissues, including intestine and skeletal muscle.22 Morphine is metabolized by UGT2B7, which is predominantly located in the liver, and morphine is a high‐affinity substrate for OCT1, which is also predominantly located in the liver. Therefore, SLC22A1 gene polymorphisms that reduce the transporting capabilities of OCT1 would also theoretically reduce the metabolism of morphine, resulting in higher serum levels of morphine compared to wild‐type SLC22A1. Many of the most frequently studied SLC22A1 gene polymorphisms confer a loss of function to the OCT1 transporter, including OCT1*2, OCT1*3, OCT1*4, OCT1*5, and OCT1*6.23 According to one study, carriers of the loss‐of‐function OCT1 polymorphisms had significantly higher plasma concentrations compared to the noncarriers.23 Furthermore, compared to the carriers of 2 active OCT1 alleles, the area under the concentration‐time curve of morphine were 1.7‐fold higher in the carriers of no active OCT1 alleles and 1.5‐fold higher in the carriers of 1 active OCT1 allele.23 Among children, morphine clearance was significantly lower in homozygote carriers of loss‐of‐function OCT1 variants.24
Opioid Receptor Mu 1
The OPRM1 gene encodes the MOR, which belongs to the G‐protein coupled receptor superfamily of transmembrane receptors.25 MOR is expressed throughout the brain,25 including the ventral tegmental area, nucleus accumbens, and the prefrontal cortex.25 MOR is the primary site of action for endogenous and exogenous opioid‐mediated pharmacologic responses26 and mediates the analgesia, tolerance, and reward effects of opioids.27 Additionally, due to MOR expression in the brain stem areas associated with respiration and on enteric neurons, morphine can also induce respiratory depression and inhibition of intestinal motility and intestinal secretions.28
Exploring polymorphisms in the OPRM1 gene would lend insight into how pharmacogenomics can impact morphine PD. One of the most frequently studied polymorphisms is the OPRM1 118 A>G variant (rs1799971), which has been shown to alter MOR signaling and/or expression in the human brain.27 The OPRM1 118A>G polymorphism is located in the +118 position in exon 1, which codes for the Asn40Asp substitution.29 There have been discordant findings in regard to the functional significance of the OPRM1 118A>G polymorphism in the scientific literature.
Some studies have shown that OPRM1 118G/G homozygotes required a higher dose of morphine compared to patients with the wild‐type OPRM1 118A/A homozygotes. Campa et al26 examined the impact of OPRM1 polymorphisms on morphine pain relief in a cohort of 145 Italian patients and revealed that OPRM1 118A/A homozygotes were associated with a significantly higher decrease in pain compared to G/G homozygotes, and A/G heterozygotes showed no significant difference compared to G/G homozygotes. Furthermore, the study showed that patients sharing at least one OPRM1 118G allele were the poorest morphine responders and that OPRM1 118 A/A homozygotes were good responders to morphine.26 In another study of 207 patients using morphine for pain, seventy‐eight 118A/A homozygotes, seventeen 118A/G heterozygotes, and four 118G/G heterozygotes reported adequate pain control.30 The study concluded that 118GG homozygotes needed more morphine than 118A/G heterozygotes and 118A/A homozygotes.30 Finally, a study of 147 Taiwanese patients receiving morphine after a surgical procedure determined that 118G/G homozygotes consumed more morphine than 118A/A homozygotes during the first 48 hours postoperatively.31 Although the aforementioned studies along with other studies currently present in the literature have demonstrated statistically significant findings in regards to dosing differences based on OPRM1 gene polymorphisms, there are also studies that did not demonstrate differences in morphine dosing based on OPRM1 gene polymorphisms.32, 33 However, considering OPRM1 polymorphisms in regards to morphine treatment may be promising, as the existing data demonstrate that OPRM1 is moderately actionable.34 Further studies are needed with a larger sample size to reach a consensus regarding the functional characteristics of OPRM1 gene polymorphisms and their impact on morphine PD.
Adenosine Triphosphate–Binding Cassette Subfamily B Member 1
P‐gp is a member of the superfamily of adenosine triphosphate (ATP)‐binding cassette transporters and is an ATP‐dependent drug efflux pump for xenobiotic compounds with broad substrate specificity.35 P‐gp is encoded by the ATP‐binding cassette subfamily B member 1 (ABCB1) gene and is endogenously expressed at the BBB19 and in the plasma membranes of cells in the small intestine, liver, and kidneys.36 As such, P‐gp can impact the PK of morphine, particularly regarding drug absorption from the intestines and first‐pass metabolism by the liver. P‐gp is a polymorphic protein with ≈1279 single‐nucleotide polymorphisms (SNPs) in the ABCB1 gene region, of which 62 SNPs have been identified in the coding region of ABCB1 as of April 30, 2009.37 The 3 most frequently studied ABCB1 SNPs in the coding region are rs1045642, rs1128503, and rs2032582. These 3 polymorphisms exhibit higher frequencies in White and Asian populations and lower frequencies in African populations.38
The rs1045642 variant corresponds to the ABCB1 C3435T polymorphism. In one study, it was shown that rs1045642 strongly and independently affects morphine responsiveness and that patients homozygous for the ABCB1 3435T allele and the OPRM1 118A allele were the best responders to morphine.26 However, another study showed that patients managed on morphine for pain associated with undergoing a cesarean section that were homozygous for the ABCB1 3435T allele trended toward a higher risk of developing persistent postoperative pain compared to the CT and CC genotypes.39
The rs1128503 variant corresponds to the ABCB1 C1236T polymorphism. A meta‐analysis exploring the impact of the rs1128503 variant on chemotherapy determined that patients with the CT and TT genotypes had a better response to chemotherapy than patients with wild‐type CC homozygotes.38 The results from the meta‐analysis may be potentially applicable to the impact of the rs1128503 variant on morphine PK. Furthermore, one study concluded that ABCB1 1236TT genotype had a significantly lower frequency of fatigue compared to the ABCB1 1236 CC homozygotes.40
The rs2032582 variant corresponds to the ABCB1 G2677T/A polymorphism. One study showed that the rs2032582 variant had a strong association with central side effects on morphine therapy.41 Another study showed that the ABCB1 2677TT homozygotes had a significantly lower frequency of fatigue while on morphine therapy compared to the wild‐type ABCB1 2677 GG homozygotes.40 Furthermore, there was a higher frequency of vomiting (grades 1‐3) in carriers of at least 1 ABCB1 2677G allele than in other patients.40
Despite the significant amount of literature available in regard to the 3 aforementioned SNPs, there has not been a consensus in regards to their phenotypic presentations.10, 37, 42
Other Genes of Interest That May Impact Morphine Pharmacogenetics
In addition to UGT2B7, OCT1, OPRM1, and ABCB1, there are other genes of interest cited in the literature that may impact morphine pharmacogenetics. The genes encoding for catechol‐O‐methyltransferase (COMT) and beta‐arrestin 2 (ARRB2) may be helpful in understanding morphine pharmacogenetics.
The ARRB2 protein is a MOR‐interacting protein that is involved with MOR signal transduction and regulation.43 ARRB2 is expressed at high levels in the central nervous system and is thought to be involved with agonist‐mediated desensitization of G‐protein coupled receptors and cause specific dampening of cellular responses to stimuli,44 such as the opioid agonist activity of morphine on the MOR. There are 2 studies in the literature that explored the impact of ARRB2 on morphine PK, but there were no significant findings in either study.43, 45
COMT is an enzyme that catalyzes the breakdown of the catechol group on molecules, such as dopamine, norepinephrine, and epinephrine and has shown an association with pain sensitivity and analgesic response.46The Val158Met substitution is the most studied polymorphism and is associated with a 3‐ to 4‐fold decrease in catalytic activity.46, 47 Therefore, the Val/Val genotype is associated with higher COMT activity than the Val/Met and Met/Met genotypes. Some studies have shown that patients expressing the Met/Met variant required less morphine to achieve analgesia,46, 47, 48 while another study showed that the Val/Met, but not the Met/Met genotype, was associated with reduced consumption of opioids for analgesia.49
Conclusion
The genes UGT2B7, OCT1, OPRM1, and ABCB1 show significant promise in predicting treatment responses for morphine therapy. The minimally effective analgesic concentration required for morphine analgesia varies from 6.3 to 53.6 ng/mL, suggesting a nearly 10‐fold difference in morphine sensitivity among individuals.50, 51 Determining morphine dosing via empirical methods can lead to delayed analgesia in some patients and potential overdose in others.52 Pharmacogenomics can help explain differences in morphine sensitivity that may have otherwise been misconstrued as medication nonadherence, unexplained adverse effects, or drug‐seeking behavior. For example, as previously evidenced in Campa et al, patients who were simultaneously ABCB1 T/T and OPRM1 A/A homozygotes were the best responders to morphine therapy and patients with at least one OPRM1 G allele were among the worst responders.26 The patients with at least 1 OPRM1 G allele may report to their clinicians that they are experiencing persistent pain and may request an increase in morphine dose or additional pain medicine. Without knowing their pharmacogenomic profile, the clinicians may mistakenly conclude that these patients are exhibiting drug‐seeking behavior, as they were dosed similarly to the other patients in the cohort.
There are multiple barriers that challenge the clinical use of these genes in pharmacogenomic testing algorithms, and in a larger sense, the clinical implementation of pharmacogenomics testing with surrounding morphine therapy. First, in the case of all 4 of these genes, there is not a unanimous consensus regarding the phenotypic characterization for most of the alleles. This challenges the translation of real‐world evidence for these genes into clinical decision support tools for health care practitioners seeking to use pharmacogenomics at the bedside. Second, there are other genes of interest that require further interrogation to fully understand morphine pharmacogenomics, and there needs to be a greater understanding of the interplay between genetic polymorphisms at different genes. For example, as P‐gp is located on the liver and UGT2B7 is located inside the liver, there may be a unique impact on morphine therapy if a patient presents with an overactive P‐gp mutation and a reduced‐function UGT2B7 allele. Finally, there needs to be further interrogation of genetic targets of interest across different populations to understand differences in genotypic distribution and to identify novel polymorphisms. For example, a Brazilian cohort exhibited differences in the allelic frequency of ABCB1 3435T, where American Indians had an allelic frequency of 51.4% and people of African descent had an allelic frequency of 32.8%.53 Furthermore, the CYP2C19*3 allele was not present in this cohort,53 as CYP2C19*3 is more commonly found among Oceanian (14.64%) and East Asian (7.25%) populations.54 Addressing this barrier will help improve the representation of ethnically diverse populations in pharmacogenomic testing algorithms and improve their generalizability. Further research is needed to generate more real‐world evidence supporting the use of pharmacogenomics in morphine therapy and develop clinical decision‐making tools to translate research findings into tangible improvements in treatment outcomes.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This project was supported (in part) by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The authors thank the Howard University Research Centers in Minority Institution, National Workforce Development Program, and the Howard University College of Pharmacy Center of Excellence for their continued support in our research endeavors.
References
- 1.Morphine sulfate entire monograph ‐ Epocrates Online. Online.epocrates.com. https://online.epocrates.com/drugs/11010/morphine‐sulfate/Monograph. Published 2018. Accessed November 9, 2018.
- 2.Fda.gov. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202515s000lbl.pdf. Accessed March 16, 2021.
- 3.PubChem. Morphine. Nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/morphine. Accessed November 11, 2018.
- 4.Morphine. http://www.drugbank.ca/drugs/DB00295. Accessed November 9, 2018.
- 5.Lewis RJ. Hawley's Condensed Chemical Dictionary. 15th ed.Wiley‐Interscience; 2007. [Google Scholar]
- 6.Morgan B, Aroke EN, Dungan J. The role of pharmacogenomics in anesthesia pharmacology. Annu Rev Nurs Res. 2017;35(1):241‐256. [DOI] [PubMed] [Google Scholar]
- 7.Ting S, Schug S. The pharmacogenomics of pain management: prospects for personalized medicine. J Pain Res. 2016;9:49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Department of Justice, Drug Enforcement Administration. Drugs of Abuse: A DEA Resource Guide/2020 Edition. https://www.dea.gov/sites/default/files/2020‐04/Drugs%20of%20Abuse%202020‐Web%20Version‐508%20compliant‐4‐24‐20_0.pdf. Published 2020. Accessed March 15, 2021.
- 9.About CDC's opioid prescribing guideline. https://www.cdc.gov/drugoverdose/prescribing/guideline.html. Published February 17, 2021. Accessed March 15, 2021.
- 10.Bastami S, Gupta A, Zackrisson A‐L, Ahlner J, Osman A, Uppugunduri S. Influence of UGT2B7, OPRM1 and ABCB1 gene polymorphisms on postoperative morphine consumption. Basic Clin Pharmacol Toxicol. 2014;115(5):423‐431. [DOI] [PubMed] [Google Scholar]
- 11.Angst MS, Phillips NG, Drover DR, et al. Pain sensitivity and opioid analgesia: a pharmacogenomic twin study. Pain. 2012;153(7):1397‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holthe M, Rakvág TN, Klepstad P, et al. Erratum: Sequence variations in the UDP‐glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J. 2003;3(4):248‐248. [DOI] [PubMed] [Google Scholar]
- 13.Blevins‐Primeau AS, Sun D, Chen G, et al. Functional significance of UDP‐glucuronosyltransferase variants in the metabolism of active tamoxifen metabolites. Cancer Res. 2009;69(5):1892‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Peng P, Mei Q, et al. The impact of UGT2B7 C802T and CYP3A4*1G polymorphisms on pain relief in cancer patients receiving oxycontin. Support Care Cancer. 2018;26(8):2763‐2767. [DOI] [PubMed] [Google Scholar]
- 15.Meissner KM, Meyer zu Schwabedissen HM, Göpfert CG, et al. UDP glucuronosyltransferase 2B7 single nucleotide polymorphism (rs7439366) influences heat pain response in human volunteers after i.v. morphine infusion. Crit Care. 2011;15(S1):P363. [Google Scholar]
- 16.Peterkin VC, Bauman JN, Goosen TC, et al. Limited influence of UGT1A1*28 and no effect of UGT2B7*2 polymorphisms on UGT1A1 or UGT2B7 activities and protein expression in human liver microsomes. Br J Clin Pharmacol. 2007;64(4):458‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coffman BL, Rios GR, King CD, Tephly TR. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab Dispos. 1997;25(1):1‐4. [PubMed] [Google Scholar]
- 18.Matic M, Norman E, Rane A, et al. Effect of UGT2B7 ‐900G>A (‐842G>A; rs7438135) on morphine glucuronidation in preterm newborns: results from a pilot cohort. Pharmacogenomics. 2014;15(12):1589‐1597. [DOI] [PubMed] [Google Scholar]
- 19.De Gregori S, De Gregori M, Ranzani GN, Allegri M, Minella C, Regazzi M. Morphine metabolism, transport and brain disposition. Metab Brain Dis. 2012;27(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B‐X, Qiao B, Lam AK‐Y, Zhao X‐L, Zhang W‐Z, Liu H. Association between UDP‐glucuronosyltransferase 2B7 tagSNPs and breast cancer risk in Chinese females. Clin Exp Pharmacol Physiol. 2018;45(5):437‐443. [DOI] [PubMed] [Google Scholar]
- 21.Kung C‐C, Chen S‐S, Yang H‐J, Lai C‐J, Chen L‐K. Pharmacogenetic study of pruritus induced by epidural morphine for post cesarean section analgesia. Taiwan J Obstet Gynecol. 2018;57(1):89‐94. [DOI] [PubMed] [Google Scholar]
- 22.Shu Y, Brown C, Castro RA, et al. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83(2):273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzvetkov MV, dos Santos Pereira JN, Meineke I, Saadatmand AR, Stingl JC, Brockmöller J. Morphine is a substrate of the organic cation transporter OCT1 and polymorphisms in OCT1 gene affect morphine pharmacokinetics after codeine administration. Biochem Pharmacol. 2013;86(5):666‐678. [DOI] [PubMed] [Google Scholar]
- 24.Goswami S, Gong L, Giacomini K, Altman RB, Klein TE. PharmGKB summary: very important pharmacogene information for SLC22A1. Pharmacogenet Genomics. 2014;24(6):324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chartoff, E. H., Connery, H. S. It's MORe exciting than mu: crosstalk between mu opioid receptors and glutamatergic transmission in the mesolimbic dopamine system. Front Pharmacol. 2014;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campa D, Gioia A, Tomei A, Poli P, Barale R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin Pharmacol Ther. 2008;83(4):559‐566. [DOI] [PubMed] [Google Scholar]
- 27.Chatti I, Woillard J‐B, Mili A, et al. Genetic analysis of Mu and kappa opioid receptor and COMT enzyme in cancer pain Tunisian patients under opioid treatment. Iran J Public Health. 2017;46(12):1704‐1711. [PMC free article] [PubMed] [Google Scholar]
- 28.Siuda ER, 3rd Carr R, Rominger DH, Violin JD. Biased mu‐opioid receptor ligands: a promising new generation of pain therapeutics. Curr Opin Pharmacol. 2017;32:77‐84. [DOI] [PubMed] [Google Scholar]
- 29.Ray LA, Hutchison KE. A polymorphism of the mu‐opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28(12):1789‐1795. [DOI] [PubMed] [Google Scholar]
- 30.Klepstad P, Rakvåg TT, Kaasa S, et al. The 118 A >G polymorphism in the human mu‐opioid receptor gene may increase morphine requirements in patients with pain caused by malignant disease. Acta Anaesthesiol Scand. 2004;48(10):1232‐1239. [DOI] [PubMed] [Google Scholar]
- 31.Chou W‐Y, Yang L‐C, Lu H‐F, et al. Association of mu‐opioid receptor gene polymorphism (A118G) with variations in morphine consumption for analgesia after total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50(7):787‐792. [DOI] [PubMed] [Google Scholar]
- 32.Coulbault L, Beaussier M, Verstuyft C, et al. Environmental and genetic factors associated with morphine response in the postoperative period. Clin Pharmacol Ther. 2006;79(4):316‐324. [DOI] [PubMed] [Google Scholar]
- 33.Klepstad P, Fladvad T, Skorpen F, et al. Influence from genetic variability on opioid use for cancer pain: a European genetic association study of 2294 cancer pain patients. Pain. 2011;152(5):1139‐1145. [DOI] [PubMed] [Google Scholar]
- 34.Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37(9):1105‐1121. [DOI] [PubMed] [Google Scholar]
- 35.Nih.gov. https://ghr.nlm.nih.gov/gene/ABCB1. Accessed March 15, 2021.
- 36.Lund M, Petersen TS, Dalhoff KP. Clinical implications of P‐glycoprotein modulation in drug‐drug interactions. Drugs. 2017;77(8):859‐883. [DOI] [PubMed] [Google Scholar]
- 37.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P‐glycoprotein). Pharmacogenet Genomics. 2011;21(3):152‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Chen Q, Zuo D, Wang H, Hua Y, Cai Z. ABCB1 (rs1128503) polymorphism and response to chemotherapy in patients with malignant tumors‐evidences from a meta‐analysis. Int J Clin Exp Med. 2015;8(1):265‐272. [PMC free article] [PubMed] [Google Scholar]
- 39.Sia AT, Sng BL, Lim EC, Law H, Tan EC. The influence of ATP‐binding cassette sub‐family B member ‐1 (ABCB1) genetic polymorphisms on acute and chronic pain after intrathecal morphine for caesarean section: a prospective cohort study. Int J Obstet Anesth. 2010;19(3):254‐260. [DOI] [PubMed] [Google Scholar]
- 40.Fujita K‐I, Ando Y, Yamamoto W, et al. Association of UGT2B7 and ABCB1 genotypes with morphine‐induced adverse drug reactions in Japanese patients with cancer. Cancer Chemother Pharmacol. 2010;65(2):251‐258. [DOI] [PubMed] [Google Scholar]
- 41.Ross JR, Riley J, Taegetmeyer AB, et al. Genetic variation and response to morphine in cancer patients: catechol‐O‐methyltransferase and multidrug resistance‐1 gene polymorphisms are associated with central side effects. Cancer. 2008;112(6):1390‐1403. [DOI] [PubMed] [Google Scholar]
- 42.Lin JH, Yamazaki M. Role of P‐glycoprotein in pharmacokinetics: clinical implications: Clinical implications. Clin Pharmacokinet. 2003;42(1):59‐98. [DOI] [PubMed] [Google Scholar]
- 43.Ambrose‐Lanci LM, Vaswani M, Clarke T‐K, et al. Association study of the β‐arrestin 2 gene (ARRB2) with opioid and cocaine dependence in a European‐American population. Psychiatr Genet. 2012;22(3):141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ARRB2 arrestin beta 2 [Homo sapiens (human)] ‐ Gene ‐ NCBI. Nih.gov. https://www.ncbi.nlm.nih.gov/gene/409. Accessed March 15, 2021.
- 45.Jimenez N, Anderson GD, Shen DD, et al. Is ethnicity associated with morphine's side effects in children? Morphine pharmacokinetics, analgesic response, and side effects in children having tonsillectomy: Ethnicity morphine response/side effects in children. Paediatr Anaesth. 2012;22(7):669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Gregori M, Garbin G, De Gregori S, et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur J Clin Pharmacol. 2013;69(9):1651‐1658. [DOI] [PubMed] [Google Scholar]
- 47.Rakvåg TT, Ross JR, Sato H, Skorpen F, Kaasa S, Klepstad P. Genetic variation in the catechol‐O‐methyltransferase (COMT) gene and morphine requirements in cancer patients with pain. Mol Pain. 2008;4:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lucenteforte E, Vannacci A, Crescioli G, et al. Opioid response in paediatric cancer patients and the Val158Met polymorphism of the human catechol‐O‐methyltransferase (COMT) gene: an Italian study on 87 cancer children and a systematic review. BMC Cancer. 2019;19(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu B, Zhang X, Xu G, et al. Association between COMT polymorphism Val158Met and opioid consumption in patients with postoperative pain: a meta‐analysis. Neurosignals. 2018;26(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 50.FH M. Essence of anesthetic control before pain management of PCA. J Jpn Soc Clin Anesth. 2010;30:669‐675. [Google Scholar]
- 51.Pico L, Hernot S, Negre I, Fletcher D. Preoperative titration of morphine improves immediate postoperative analgesia after total hip arthroplasty. Can J Anesth. 2000;47(4):309‐314 [DOI] [PubMed] [Google Scholar]
- 52.Yoshida K, Nishizawa D, Ide S, Ichinohe T, Fukuda K‐I, Ikeda K. A pharmacogenetics approach to pain management. Neuropsychopharmacol Rep. 2018;38(1):2‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos PCJL, Soares RAG, Santos DBG, et al. CYP2C19 and ABCB1 gene polymorphisms are differently distributed according to ethnicity in the Brazilian general population. BMC Med Genet. 2011;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CPIC® guideline for clopidogrel and CYP2C19. https://cpicpgx.org/guidelines/guideline‐for‐clopidogrel‐and‐cyp2c19/. Accessed March 28, 2021.


