Abstract
Serological tests for SARS-CoV-2 are a critical component of disease control strategies. SARS-CoV-2 serology tests used in clinical diagnostic should not accurately evaluate total levels the antibodies but also closely correlate with neutralizing antibodies titers.
However, only limited data is available reporting correlation of neutralization antibody assays with commercial high-throughput serological assays widely used in clinical laboratories.
We performed evaluation of the GenScript cPass neutralizing antibody detection assay, to assess its value for routine clinical use to measure neutralizing titers in patients who recovered from coronavirus disease 2019 (COVID-19) or have been vaccinated. We tested its clinical performance against the commonly used Ortho Vitros IgG assay.
Our combined data shows that GenScript cPass neutralizing antibody assay has satisfactory analytical and clinical performance and good correlation with Ortho Vitros IgG, supporting its use as a tool for accurate SARS-COV-2 immune surveillance of recovered or vaccinated individuals.
Keywords: SARS-CoV-2 serology, SARS CoV-2 antibody
1. Introduction
To understand immunity after natural infection or vaccination, a functional analysis of antibody response needs to be evaluated including the presence of high-affinity neutralizing antibodies [1]. Therefore, serology that provides accurate measurements of anti-SARS-Cov-2 antibodies becomes an essential tool in tracking Covid-19 immunity and clinical trials for vaccine and treatment development.
Most available assays that determine anti-SARS-CoV2 neutralizing antibodies rely on utilization of virus or pseudo-virus in the tissue culture settings and are based on infectivity read-out/measurement of cell’s infectivity. Those assays are performed in enhanced biosafety level facilities, are not standardized and labor-intensive, low throughput, and therefore are not suitable for clinical use.
GenScript cPass SARS-CoV-2 Neutralization Antibody Detection Assay is the first commercial assay granted FDA_EUA that semi-quantitatively measures levels of neutralizing antibodies [2]. The majority of neutralizing antibodies produced during SARS-CoV2 infection or post-vaccination are directed against the receptor binding domain (RBD) of the spike protein of SARS-CoV2 virus, and inhibit the interaction between RBD and angiotensin-converting enzyme 2 (ACE2) expressed on the surface of the host’s endothelial cells [3]. The cPass assay measures levels of antibodies that inhibit interaction between two recombinant proteins: RBD-HPR and ACE2. It is based on ELISA, therefore it is independent of the use of the virus/pseudo-virus and cell cultures, it allows for high-throughput, automation and shorter turnaround time [4].
Here, we present an evaluation of analytical performance of cPass SARS-CoV-2 Neutralization Antibody Detection Assay and the assay’s clinical performance against Ortho Vitros IgG assay for the assessment of post-immunity in infected or vaccinated individuals.
2. Materials and methods
Specimens for our validation study were obtained under a protocol (H47459) approved by the Baylor College of Medicine Institutional Review Board. Positive patients were previously diagnosed with COVID-19 by RT-PCR or transcription mediated amplification methods at our institution. Post-vaccine (at least 3 weeks post second dose of Pfizer-BioNTech or Moderna vaccines) specimens were collected by venipuncture into K2EDTA tubes or serum separator tubes and processed upon receipt by the laboratory, with plasma or serum stored for up to 5 days at 4 °C until analysis. A total of 131 specimens were analyzed and a total of 18 scavenged convalescent-phased plasma samples were used for the concordance study (samples from donor program were available for the neutralization assay, eligible individuals were confirmed to be PCR positive for SARS-CoV-2, were symptom free for at least 14 days prior to plasma donation, and met all standard blood donation criteria according to FDA requirements).
Analytical specificity and sensitivity of the cPass Neutralization assay were assessed as concordance with the positive or negative RT-PCR status of the specimen using 25 confirmed positive and 10 negative samples at 1:20 dilution. The collection and description of the deidentified patient cohorts for both the positive and pre-pandemic samples are previously described [5], [6].
Intra- and inter-assay precision studies were performed in accordance with CLSI EP5-A2 guidelines on negative and positive specimens. Intra-run precision was assessed by measurement of 12 replicates within one run, and inter-assay precision was assessed by measurement of negative and positive samples once a day for a time period of at least 20 days (n = 10). Assay precision was expressed as a coefficient of variation (%CV) of % inhibition for positive specimens.
Interference testing was performed by spiking negative or positive samples with of hemoglobin, conjugated bilirubin, and triglyceride-rich lipid (Sun Diagnostics, New Gloucester, ME). For negative samples % difference in measured OD was calculated, for positive samples % inhibition was calculated.
Analytical specificity was assessed by testing 18 different sera positive for common respiratory viruses’ samples which were tested at 1:20 dilution.
Linearity was assessed by preparing serial dilutions of commercial standards with known levels of neutralizing antibodies (2.5–150 µg/ml), measured and expected values were plotted.
2.1. GenScript cPass™ SARS-CoV-2 neutralization antibody detection assay
The assay was performed according to the manufacturer’s protocol. In brief, samples and supplied controls were diluted 1:10 with dilution buffer and mixed with RBD-HRP. After a 30 min incubation at 37 °C, 100 μl of samples, sample dilutions or controls were added to a 96 well plate pre-coated with recombinant ACE2 protein. Plate was incubated for 15 min at 37 °C, sample mixture removed and cell wells were washed with provided wash buffer. After the addition of substrate, reaction was stopped and plates read at 450 nm immediately afterwards. Data was interpreted as percentage reduction (%reduction) based on OD450 intensity. Manufacture recommended cut-off of ≥ 30% signal reduction was used to indicate the presence of anti-SARS-CoV-2 neutralizing antibodies.
2.2. Ortho VITROS SARS-CoV-2 IgG assay
VITROS Anti-SARS-CoV-2 IgG was performed following the manufacturer's instructions on the Vitros 5600 automated chemistry analyzer (Ortho Clinical Diagnostics). The Vitros CoV2 IgG assay is a qualitative, chemiluminescence immunoassay designed to detect IgG antibodies to the S1 subunit of the SARS‐CoV‐2 spike glycoprotein. Results of the test are reported as either reactive (S/Co ≥ 1.0) or nonreactive (S/Co < 1.0). Current FDA guidelines recommends high titer convalescent plasma, corresponding to an S/Co of ≥9.5 [2] on Ortho VITROS Anti‐SARS‐CoV‐2 IgG.
2.3. Statistical analysis
All statistical analysis was performed using GraphPad Prism.
3. Results
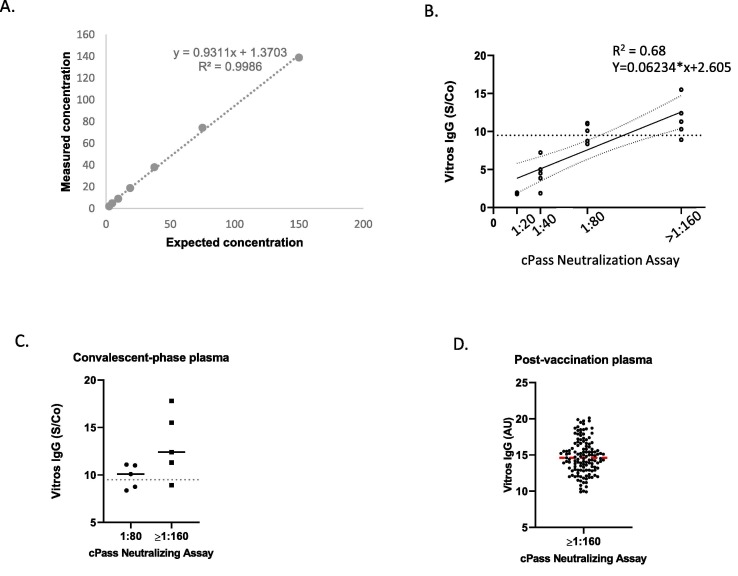
We investigated inter- and intra assay variance of the cPass Assay by testing a representative subset of samples. We observed a very low 8.3% CV for positive sample with a mean % inhibition >90%. Inter-assay studies yielded %CVs of % inhibition of 3.5% for a positive specimen (Table 1 A). We analyzed linearity of cPass Assay (Fig. 1 A) by preparing and testing several dilutions of known standards covering a wide range of values. cPass Assay did not deviate from linearity in the entire range of tested values, suggesting good analytical accuracy. Linearity was excellent (R2 = 0.99; y-intercept = 1.4) in the measurement range.
Table 1.
(A) Intra- and inter-assay precision study results. (B) Concordance between qualitative Vitros CoV2 IgG and cPass Neutralizing Assay for convalescent-phased plasma (CPP) and post-vaccine specimens.
| A. |
||
|---|---|---|
| Sample | Intra assay | Inter assay |
| Positive (%CV %inhibition) | 8.3% | 3.5% |
| B. |
||
|---|---|---|
| CPP positive* at 1:80 titer | Post-vaccine specimens positive* at 1:80 titer | |
| % Positive Agreement | 91 | 100 |
| % Negative Agreement | 71 |
| C. |
||
|---|---|---|
| Sample | Interferant | % Difference to control OD |
| negative sample | Hemolysate | 5% |
| Conjugated bilirubin | 6.6% | |
| Triglyceride-rich lipid | 4.4% | |
| % Difference in %inhibition | ||
| positive sample | Hemolysate | 1.46% |
| Conjugated bilirubin | 0.64% | |
| Triglyceride-rich lipid | 0.48% | |
*≥30% inhibition.
**Only 2 post-vaccine specimens had values <9.5 S/Co on Vitros IgG; both had ≥30% inhibition at 1:80 titer.
Fig. 1.
(A) Linearity assessment of anti-SARS-CoV-2S-RBDIgG assays. (B) Linear correlation of the Vitros anti-SARS-CoV-2 IgG assay (signal to cut-off) and end point titers with ≥30% inhibition cPass Assay. Dashed line (y axis) represents S/CO ≥ 9.5 values; 95% CI marked. (C) Correlation between Vitros IgG results and positive endpoint neutralizing titer vs Vitros IgG for convalescent-phase plasma and (D) post-vaccine specimens.
We tested analytical performance of cPass assay in our laboratory. Sensitivity of the assay was 96% with 24/25 of confirmed Covid-19 specimens’ yielding ≥ 30% inhibition at 1:20 titer. Specificity was found to be 100%, all tested known Covid-19 negative samples (n = 10) yield ≤ 30% inhibition at 1:20 titer.
FDA recommends titers of convalescent plasma suitable for treatment of 1:160 or higher, however a titer of 1:80 may be considered acceptable if an alternative matched unit is not available [7]. We analyzed correlation between cPass neutralizing antibody assay and Vitros CoV2 IgG. Total of 131 post-vaccine and 18 convalescent-phased plasma specimens were used for the concordance study. Samples were considered positive when Vitros IgG S/Co ≥ 9.5 and ≥30% inhibition at end-point titer of 1:80 measured by cPass assay. For the post-vaccine specimens, we observed perfect positive agreement of 100% between the assays, 2 specimens had values <9.5 on Vitros and ≥30% inhibition at end-point titer of 1:80. For the convalescent-phase plasma specimens, we found positive agreement and negative agreement at 1:80 titer were 91% and 71% respectively (Table 1B).
To further investigate the correlation of Vitros IgG Assay S/Co values with %inhibition in the cPass, a linear regression analysis was performed using all sera for which a positive endpoint titer was available (n = 148). We found a good correlation (R2 = 0.63, y-intercept 1.07) between Vitros IgG Assay and cPass endpoint positive titer (Fig. 1B).
Next, we analyzed correlation between S/Co values from Vitros IgG Assay and positive end-point titers on cPass Assay. Again, for convalescent-phased specimens 1:80 and 1:160 positive endpoint titers were tested, for post-vaccine specimens 1:160 positive end-point titer was analyzed. As expected, lower Vitros IgG assay values corresponded to lower positive end-point titer on cPass Assay. Mean values of S/Co of 10 for 1:80 and S/Co of 12 for 1:160 titer for convalescent-phased specimens were obtained, post-vaccine specimen yielded a S/Co value of 14.7 at high titer of 1:160 (Fig. 1 C, D).
Interference studies were performed to examine the effect of common interferents on the cPass Assay. Our results showed no significant changes in sample OD (for negative samples) or %inhibition (for positive samples) from neat when the samples were spiked with hemoglobin, conjugated bilirubin or triglyceride-rich lipid. Analysis of specimen positive for common respiratory viruses but negative for SARS-CoV-2 by RT-PCR showed no cross-reactivity with cPass SARS-CoV-2 Neutralization Antibody at 1:20 titer (data not shown).
4. Discussion
The relationship between SARS-CoV-2 antibodies and neutralizing activity remains an essential and open issue. SARS-CoV-2 neutralizing antibodies titers are currently gaining importance for supporting vaccine development, and to aid convalescent plasma therapy. Commonly used clinical serology tests do not assess antibody function as neutralizing [4]. We performed clinical evaluation of the GenScript cPass assay, to assess its value for routine clinical diagnostics to measure neutralizing capability in SARS-CoV-2 elicited antibody responses.
We observed a high specificity of 100% and an overall clinical sensitivity of 96%. The assay shows robust analytical performance with low intra and inter assay variation. Results showed that this assay presents excellent analytical performances, both for precision and linearity.
Comparison of different serology assays is complicated by differences in each method’s principle (measuring antigen binding vs %inhibition of RBD-ACE2 interaction). At high 1:160 titers, our data showed good negative test agreement, moderate overall agreement and low positive agreement between cPass assay and Vitros IgG at S/Co ≥ 9.5 for convalescent-phased samples. This result might arise from difference methods’ principle (measuring antigen binding vs %inhibition of RBD-ACE2 interaction) and low number of specimens tested. Poor concordance between commercial high-throughput serology assays for SARS-CoV-2 and titers of neutralizing antibodies has been reported [8], suggesting that serological results for SARS-CoV-2 antibodies should be interpreted with caution.
In conclusion, the cPass Assay can be used as an additional assay to estimate the neutralizing antibody status of COVID-19 infected or vaccinated individuals and convalescent-phased specimens evaluated as potential treatment. The value of the cPass Assay is related to its short turn-around time, possibility of automation and high throughput as well as standardization across laboratories.
References
- 1.Wajnberg A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science:abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA_EUA, https://www.fda.gov/media/141477/download.
- 3.Tan C.W., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 4.Meyer B., et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg. Microbes Infect. 2020;9(1):2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.E. Garnett, et al., Clinical validation and performance evaluation of the automated Vitros Total Anti-SARS-CoV-2 Antibodies assay for screening of serostatus in COVID-19. medRxiv, 2020: p. 2020.06.09.20126474 DOI: 10.1101/2020.06.09.20126474. [DOI] [PMC free article] [PubMed]
- 6.Jung J., et al. Clinical performance of a semi-quantitative assay for SARS-CoV2 IgG and SARS-CoV2 IgM antibodies. Clin. Chim. Acta. 2020;510:790–795. doi: 10.1016/j.cca.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.C.T. Lily Li, Convalescent Plasma: What the Data Shows for this Promising Potential COVID-19 Treatment 2021.
- 8.Tang M.S., et al. Association between SARS-CoV-2 neutralizing antibodies and commercial serological assays. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa211. [DOI] [PMC free article] [PubMed] [Google Scholar]