Abstract
Health-care workers have an increased incidence of allergic disease compared with the general public and are exposed to a variety of high-level disinfectants. Although exposure to these agents has been associated with allergic disease, findings between epidemiology and animal studies often conflict respecting immunological mechanisms. Therefore, we hypothesized that previous exposure to a representative IgE-mediated sensitizer (ortho-phthalaldehyde [OPA]) alters immune responses to a representative T-cell-mediated sensitizer (didecyldimethlyammonium chloride [DDAC]). Here, BALB/c mice were topically exposed to OPA (0.5%) for 3 days, rested, then topically exposed to DDAC (0.0625%, 0.125%, and 0.25%) for 14 days. Coexposure resulted in phenotypic changes in draining lymph node (dLN) cells, including a decreased frequency of CD8+ T cells and increased frequency and number of B cells compared with DDAC-only treated mice. The coexposed mice also had enhanced Th2 responses, including significant alterations in: dLN Il4 (increased), B-cell activation (increased), CD8+ T-cell activation (decreased), and local and systemic IgE production (increased). These changes were not observed if mice were exposed to DDAC prior to OPA. Exposure to OPA alone shows Th2 skewing, indicated by increased activation of skin type 2 innate lymphoid cells, increased frequency and activation of draining lymph node B cells, and increased levels of type 2 cytokines. These findings suggest that the OPA-induced immune environment may alter the response to DDAC, resulting in increased IgE-mediated immune responses. This data may partially explain the discordance between epidemiological and laboratory studies regarding disinfectants and provide insight into the potential immunological implications of mixed chemical exposures.
Keywords: OPA, DDAC, chemical-allergy, IgE, coexposure
In order to increase the safety of health-care facilities, chemical antimicrobials are increasingly being utilized. Although this has reduced the incidence of health-care-associated infections (Alfa et al., 2015; Quinn et al., 2015), high-level disinfectant use may result in negative health effects. Epidemiological data indicate that health-care workers have an elevated risk for development of sensitization and allergic asthma from exposure to chemicals compared with nonhealth-care workers (Warshaw et al., 2008). Additionally, a recent National Health Interview Survey indicated that workers in the health care and social assistance industry had the highest prevalence of asthma (8.8%) among all categories of working adults (Mazurek and Syamlal, 2018). Although the mechanisms underlying this increase in allergic disease are complex and multi-factorial, one common attribute is the exposure to low molecular weight (LMW) organic chemicals, particularly concerning occupational asthma (Jarvis et al., 2005).
The mechanisms underlying the development of chemical-associated allergic diseases are not yet fully understood, and involve many novel mediators (Shane et al., 2019a). In general, following the Gell and Coombs classification scheme (Coombs and Gell, 1963), chemical allergy is characterized as either an Immunoglobulin E (IgE)- or T-cell-mediated response. These responses are defined by different mechanisms of action, where IgE-mediated responses require the generation of IgE and Th2 effector responses (eliciting allergy via the activation of mast cells), and T-cell-mediated responses require the activation of Th1 and CD8+ T cells (eliciting cell-mediated responses through secretion cytokines). LMW chemicals have been shown to elicit responses via either of these mechanisms, with T-cell-mediated responses predominating (Kimber and Dearman, 2005).
Quaternary ammonium compounds (QACs) are among the most common allergens in the health-care profession and are associated with IgE- and T-cell-mediated allergic responses (Bernstein et al., 1994; Gonzalez et al., 2014; Purohit et al., 2000; Shaffer and Belsito, 2000; Suneja and Belsito, 2008). Didecyldimethylammonium chloride (DDAC) is a broad-spectrum QAC that exhibits antimicrobial activity against a number of pathogens (Argy et al., 1999; Skaliy et al., 1980; Walsh et al., 2003) and is increasingly being used in a wide variety of applications (Ohnuma et al., 2011), including as a disinfectant in health-care settings. Previously, our laboratory has shown that dermal DDAC exposure results in a hypersensitivity response dominated by T-cell-mediated mechanisms, resulting in a substantial increase in the activation of CD8+ T cells (Anderson et al., 2016).
Ortho-phthalaldehyde (OPA) is an aromatic dialdehyde used as a high-level disinfectant in health-care settings for the sterilization of heat-sensitive equipment (Akamatsu et al., 2005; Walsh et al., 1999). OPA is often used as a replacement for glutaraldehyde, another well-recognized chemical sensitizer associated with the development of occupational asthma (Gannon et al., 1995). Despite being used as a “safe alternative” for glutaraldehyde, a number of case reports have identified OPA as a causative agent of allergic disease both in health-care workers (Robitaille and Boulet, 2015), and in patients who are repeatedly exposed to OPA-treated equipment (Atiyeh et al., 2015). Previously, our laboratory has identified OPA as a sensitizer (EC3 value of 0.051% in local lymph node [LN] assay), which induced elevations in specific and total IgE along with elevations in the Th2 cytokine IL-4 in mouse models (Anderson et al., 2010).
DDAC and OPA are both widely used in health-care settings, albeit for largely different purposes. DDAC is predominantly used as a surface disinfectant used ubiquitously throughout health-care settings, whereas OPA is used for the sterilization of reusable heat-sensitive equipment. Due to their different applications, the chance for repeated coexposures to both chemicals is high, especially for staff and nurses working in specialized units, such as endoscopy, where exposure to OPA is high due to sterilization procedures and health effects due to OPA exposure have been reported (Franchi and Franco, 2005; Fujita et al., 2006). One limitation of toxicological studies is that often, the agent being assessed is tested in exclusion. This means that immunologically “naïve” cellular populations/animals are exposed to a single agent at a time. The field of infectious diseases has long recognized that a skewed immunological response caused by one pathogen can influence the immune response to secondary pathogens (Mwinzi et al., 2001; Riner et al., 2016). However, very few studies have addressed how coexposure to chemicals influences immune responses and health outcomes. Although evidence suggests that both DDAC and OPA are capable of eliciting sensitization via different immunological mechanisms, to our knowledge the impact of exposure to both chemicals has not been assessed, despite the high potential for coexposure in occupational settings. Based on our previous murine studies, DDAC was selected as the representative T-cell-mediated sensitizer and OPA as the IgE-mediated sensitizer. Here, we assess whether prior exposure to the IgE-mediated sensitizer OPA alters the overall immune response to the T-cell-mediated sensitizer DDAC in a murine model.
MATERIALS AND METHODS
Animals
Female BALB/c mice were used for the murine models. The BALB/c mouse strain has a Th2 bias and is commonly used to evaluate IgE-mediated sensitization (Klink and Meade, 2003; Woolhiser et al., 2000). The mice were purchased from Taconic at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each shipment of animals was randomly assigned to a treatment group, weighed and individually identified (via tail marking) using a permanent marker or tattoo. A preliminary analysis of variance on body weights was performed to ensure a homogeneous distribution of animals across treatment groups. The animals were housed at a maximum of 5/cage in ventilated plastic shoebox cages with hardwood chip bedding. NIH-31 modified 6% irradiated rodent diet (Harlan Teklad) and tap water were provided from water bottles, ad libitum. The animal facility temperature was maintained at 68°F–72°F and the relative humidity between 36% and 57%. A light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the AAALAC International accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Chemicals and exposures
OPA (CAS No. 643-79-8), DDAC (CAS No. 7173-51-5), and acetone (CAS No. 67-64-1) were purchased from Sigma-Aldrich (Milwaukee, Wisconsin). Concentrations and durations of dosing were chosen based on data from previous studies (Anderson et al., 2010; Shane et al., 2019b), OPA, 0.5% for 3 days, was the lowest concentration that produced significant elevations in serum IgE. Mice were exposed on the dorsal surface of each ear (50 µl/mouse; 25 µl/ear) daily for the number of days/chemical as indicated in the experimental scheme (Figure 1A). The coexposure dosing resulted in a total of 6 treatment groups; (1) A + A (Acetone + Acetone), (2) A + DDAC (Acetone + 0.25% DDAC), (3) OPA + A (0.5% OPA + Acetone), (4) OPA + Low (L)-DDAC (0.5% OPA + 0.0625% DDAC), (5) OPA + Mid (M)-DDAC (0.5% OPA + 0.125% DDAC), and (6) OPA + High (H)-DDAC (0.5% OPA + 0.25% DDAC). Animals were euthanized by either sodium pentobarbital injection, or CO2 asphyxiation 24 h following the last exposure.
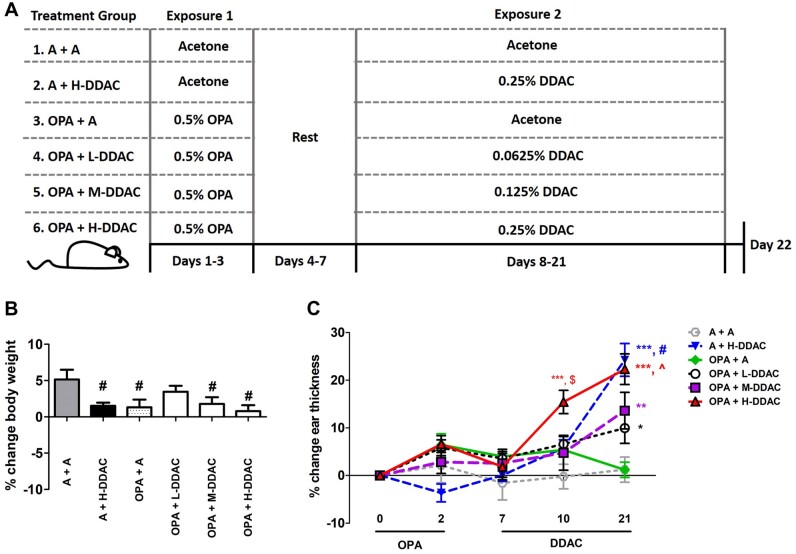
Figure 1.
Evaluation of toxicity and irritation following a dual exposure scenario. A, A diagram of the experimental scheme used throughout the article. B, % Change in body weight was calculated from the beginning of the study (day 1) versus the end of the study (day 22). C, % Change in ear thickness was measured throughout the study as a change from day 0 (before dosing began) through the end of the study. N = 5 mice per group; data are representative of 2 independent replicate studies. For body weights, statistical significance was determined using a 1-way AVOVA, with a post hoc Dunnett’s multiple comparisons test versus “A + A” control (gray bar). #p < .05. For the ear thickness data, statistical significance was determined using a 2-way ANOVA with Bonferroni posttest. *s indicate significant differences from A + A control (*p < .05, **p < .01, and ***p < .001), $ indicates significant difference in ortho-phthalaldehyde (OPA) + H-didecyldimethlyammonium chloride (DDAC) over all other data sets (p < .05–.001), ^indicates significant difference in OPA + H-DDAC over all other data sets, except A + H-DDAC (p < .05–.001), # indicates significant difference in A + H-DDAC over all other data sets; except OPA+H-DDAC (p < .05–.001).
Body weights and irritation
Mice were weighed at day 0 of the study and on day 22 to determine % change in body weight. For irritancy evaluation, the thickness of the right and left ear pinnae of each mouse was measured using a modified engineer micrometer (Mitutoyo Co.) before the first administration of OPA (day 0) and were measured throughout the study to determine % change in ear thickness. Two measurements were taken at each time point for each ear and all measurements were averaged for % change calculations.
Total IgE analysis
Blood samples were collected via cardiac puncture following euthanasia and sera was then separated by centrifugation and frozen at −20°C for subsequent analysis. To quantify serum IgE levels a standard colorimetric sandwich ELISA was performed according to manufacturer directions (Mouse IgE Ready-Set-Go! eBioscience). To determine local production of IgE the IgE+B220+ (IgE+ B-cells) population was analyzed by flow cytometry as previously described using IgE-FITC (R35-72; BD Biosciences; Shane et al., 2017).
Single-cell preparation and flow cytometry analysis
DLN cell suspensions (2 nodes/animal) were prepared by mechanical disruption of tissues between frosted microscope slides in phosphate-buffered saline and live cells were counted on a Cellometer using Acridine Orange/Propidium Iodide (AO/PI, Nexcelom). Ear cell suspensions were prepared by splitting ear into ventral and dorsal halves, followed by an enzymatic digestion for 90 min at 37°C with 0.25 mg/ml Liberase-TL Research grade (Roche) in RPMI with 100 µg/ml DNase I (Sigma-Aldrich). Digestion was stopped by the addition of 3 ml of RPMI + 10% fetal bovine serum (FBS), the ears + media were transferred to gentleMACS C Tubes (Miltenyi Biotec), then cells were mechanically disrupted on a gentleMACS Dissocciator (Miltenyi Biotec). Following disruption, cells were passed through a 70-μm cell strainer to make a single-cell suspension, washed with RPMI 10% FBS, then live cells were counted on a Cellometer using AO/PI (Nexcelom) in order to quantify cells. Single-cell suspensions were re-suspended in staining buffer containing antimouse CD16/32 antibody (BD Biosciences) then incubated with a cocktail of fluorochrome-conjugated antibodies specific for cell surface antigens, for the LN phenotyping: (antimouse: CD4-BV605 [GK1.5], CD8-PE-eF594 [53-6.7], B220-V500 [RA3-6B2], MHC-II-AF700 [M5/114.15.2], CD11b-PerCPCy5.5 [M1/70], CD11c-eF450 [N418], IgE-FITC [R35-72], CD44-eF780 [IM7], and CD86-APC [GL1]). For type 2 innate lymphoid cell (ILC2) staining: PerCP-Cy5.5-conjugated α-CD19 (eBio1D3), α-CD3e (145-2C11,) α-Ly6-G (RB6-8C5), α-CD11b (M1/70), α-Ter119 (Ter119), α-CD11c (N418), FITC-conjugated α-CD2 (RM2-5), α-CD90.2-APC-eF780 (53-2.1), α-CD25-PE-eF610 (PC61.5), α-CD127-PE (A7R34), α-KLRG1-APC (2F1; all eBioscience), α-inducible T-cell costimulator (ICOS)-PE-Cy7 (C398.4A; Biolegend), and α-CD45-BV605 (30-F11; BD Bioscience). Cells were then washed, fixed in Cytofix buffer (BD Biosciences), resuspended in staining buffer, events were collected on an LSR II flow cytometer (BD Biosciences), and analyzed using Flowjo software v10. Gating of populations is indicated in the legends for Figures 2, 3, and 5. For Table 1, CD4 T cells were identified as CD4+CD8−B220− lymphocytes, CD8 T cells were identified as CD8+CD4−B220− lymphocytes, B cells were identified as B220+CD4−CD8− lymphocytes and LN dendritic cells (DCs) were identified as CD11c+CD11b+MHCII+ cells. Gating for ILC2 cells was performed as described in (Shane et al., 2019b).
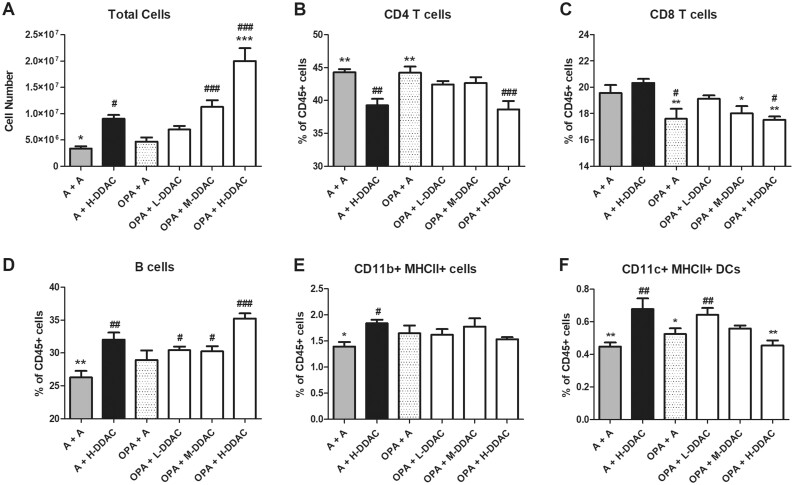
Figure 2.
Phenotypic analysis of draining lymph node (dLN) cell populations. A, Total number of dLN cells. Frequencies of cells were determined using analysis of flow cytometry data, cells were first gated on single cells, then CD45+ cells and are shown as a frequency of total CD45+ cells; B, CD4+ T cells (CD3+ CD4+); C, CD8+ T cells (CD3+ CD8+); D, B cells (B220+); E, MHCII+ CD11b+ cells; F, MHCII+ CD11c+ cells. N = 5 mice per group; data are representative of 2 independent replicate studies. Statistical significance was determined using a 1-way AVOVA, with a post hoc Dunnett’s multiple comparisons test versus “A + H-didecyldimethlyammonium chloride” control (black bar, significance indicated by *s), and versus “A + A” control (gray bar, significance indicated by #s). */#p < .05, **/##p < .01, and ***/###p < .001.
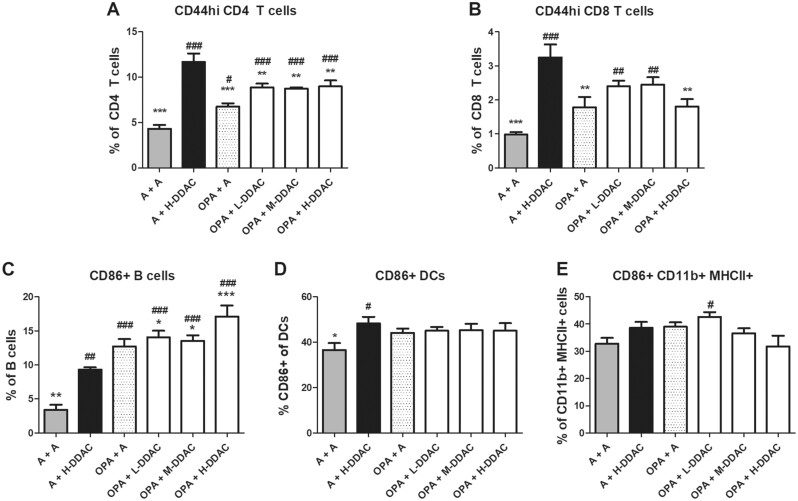
Figure 3.
Activation status of cell populations in draining lymph node (dLN) following exposure to ortho-phthalaldehyde, didecyldimethlyammonium chloride (DDAC), or both. Activation status of cells in the dLN was assessed by flow cytometry by determining the % of CD44+ cells of total CD4+ T cells (A), or of total CD8+ T cells (B), or the % of CD86 + cells on total B cells (C), dendritic cells (D) or CD11b+MHCII+ cells. N = 5 mice per group; data are representative of 2 independent replicate studies. Statistical significance was determined using a 1-way AVOVA, with a post hoc Dunnett’s multiple comparisons test versus “A + DDAC” control (black bar, significance indicated by *s) and versus “A + A” control (gray bar, significance indicated by #s). */#p < .05, **/##p < .01, and ***/###p < .001.
Figure 5.
Ortho-phthalaldehyde (OPA) skews the local microenvironment towards Th2 dominant responses. Immunological parameters were assayed in mice following 3 days of acetone vehicle control (white bars) or OPA (black bars) exposure and 4 days of rest. A, Total cells isolated from the site of chemical application (ear skin) and flow cytometric analysis of total type 2 innate lymphoid cell cells and their activation status as determined by median fluorescent intensity of inducible T-cell costimulator expression. B, Protein levels in total ear lysate as quantified by Luminex assay. C, Total number of immune cells isolated from the skin draining lymph node (dLN). D, Average proportion of B cells (black), CD4+ T cells (dark gray) and CD8+ T cells (light gray), making up the lymphocyte compartment of the skin dLNs. E, Frequencies of activated B cells (CD86+), and CD4/CD8 T cells (CD44) in the skin dLNs. N = 5 mice per group. Statistical significance was determined using a 2-tailed unpaired t test, where *p < .05, **p < .01, and ***p < .001.
Table 1.
Total Numbers of Cells in the DLNs
| A + A | A + H-DDAC | OPA + A | OPA + L-DDAC | OPA + M-DDAC | OPA + H-DDAC | |
|---|---|---|---|---|---|---|
| Total cells (×106) | 3.37 ± 0.44* | 9.02 ± 0.74 | 4.68 ± 0.81 | 6.97 ± 0.71 | 11.30 ± 1.25 | 20.00 ± 2.45*** |
| CD4 T cells (×106) | 1.48 ± 0.18 | 3.55 ± 0.34 | 2.08 ± 0.39 | 2.96 ± 0.31 | 4.85 ± 0.60 | 7.74 ± 1.02*** |
| CD8 T cells (×106) | 0.65 ± 0.07* | 1.84 ± 0.16 | 0.81 ± 0.13* | 1.34 ± 0.14 | 2.06 ± 0.29 | 3.52 ± 0.46*** |
| B cells (×106) | 0.89 ± 0.14** | 2.87 ± 0.17 | 1.35 ± 0.22* | 2.11 ± 0.20 | 3.39 ± 0.31 | 7.02 ± 0.76*** |
| CD11c + MHCII+ (×104) | 1.53 ± 0.25** | 6.02 ± 0.53 | 2.45 ± 0.50* | 4.36 ± 0.26 | 6.26 ± .062 | 9.29 ± 1.59* |
| CD11b+MHCII+ (×105) | 0.47 ± 0.08*** | 1.65 ± 0.11 | 0.75 ± 0.13** | 1.11 ± 0.10 | 1.93 ± 0.12 | 3.05 ± 0.36*** |
A, acetone; L, low (0.0625% DDAC); M, mid (0.125% DDAC); H, high (0.25% DDAC); OPA, (0.5% OPA). N = 5 mice/group, representative of 2 independent experiments.
(*)indicates significant difference compared with DDAC control (A + H-DDAC) *p < .05, **p < .01, and ***p < .001; light gray indicates significant decreases, whereas dark gray indicates significant increases compared with DDAC control.
Ear protein analysis
Ears (one per mouse) were mechanically disrupted on a TissueLyser II (Qiagen) in 0.75 ml T-PER protein extraction reagent (Pierce) and soluble proteins were quantified by BCA protein assay (Pierce). Cytokines were measured using the Th1/Th2/Th9/Th17/Th22/Treg Cytokine 17-Plex Mouse ProcartaPlex Panel (ThermoFisher) according to manufacturer’s instructions and data was acquired using a Luminex 200 system (Millipore).
Ex Vivo LN Cell Stimulations
Lymph node cells (5 × 105) were seeded into 96-well u-bottom plates with soluble α-CD3 (BD Pharmingen) (2 µg/ml) and α-CD28 (BD Pharmingen; 2 µg/ml) in RPMI media containing L-glutamine and HEPES (Cellgro) with 10% fetal bovine serum (Hyclone), and Gentamicin solution (Sigma-Aldrich; cRPMI). Cells were incubated for 24 h in a humidified incubator (37°C/5% CO2). Cytokines in the supernatants were measured using the Th1/Th2/Th9/Th17/Th22/Treg Cytokine 17-Plex Mouse ProcartaPlex Panel (ThermoFisher) according to manufacturer’s instructions and data was acquired using a Luminex 200 system (Millipore).
Gene expression analysis
Ears were mechanically disrupted on a TissueLyser II in Buffer RLT (Qiagen). Total RNA was extracted using Qiagen’s RNeasy mini spin column kits with DNase treatment on a QIAcube workstation. RNA concentrations and purity were analyzed on a NanoDrop spectrophotomer (Thermo Fisher Scientific). The cDNA (1–2 μg) was prepared on an Eppendorf Mastercycler using Applied Biosystems’ High Capacity Reverse Transcription kit. The cDNA was used as template for real-time PCR reactions containing TaqMan PCR Master Mix with gene-specific primers (Applied Biosystems) on a 7500 real-time PCR System. Relative fold gene expression changes for Il4 (2−ΔΔCT) were determined compared with the DDAC control and normalized for expression of housekeeping gene Actb.
Statistical analyses
For analysis, data were first tested for homogeneity using Bartlett’s χ2 test. If homogeneous, a 1-way ANOVA was conducted for single timepoint studies. If the ANOVA showed significance at p < .05, Dunnett’s multiple range t test was used to compare treatment groups with the DDAC control, unless otherwise noted in the figure legends. For longitudinal studies (ear irritation) a 2-way ANOVA was conducted, with a Bonferroni posttest for comparison between groups. For the studies where only exposure to OPA was analyzed (Figure 5), statistical significance was determined using a 2-tailed unpaired Student t test. Statistical analysis was performed using Prism v.5.0 (GraphPad Software). Statistical significance is designated by *p < .05, **p < .01, and ***p < .001.
RESULTS
Effect on Overt Toxicity and Irritation
Animals were treated following the experimental scheme outlined in Figure 1A. Animals showed no signs of overt toxicity (ie, >20% body weight loss or physical signs of distress) throughout the study, and no animals lost weight by the end of the 22 days of exposure (Figure 1B). However, mice that were exposed to acetone alone (A +A) did gain significantly more weight throughout the study compared with all other treatment groups with the exception of OPA + L-DDAC. Irritation was assessed throughout the study by measuring changes in ear thickness. Following exposure to H-DDAC, the mice that were pretreated with OPA initially showed an increased level of irritation compared with the group that was exposed to H-DDAC alone (day 10). However, by the end of the study, both the A + H-DDAC and OPA + H-DDAC groups had equal levels of irritation, with lower levels of irritation observed for the OPA + L-DDAC and OPA + M-DDAC treatment groups (Figure 1C and Supplementary Table 1).
DLN Immune Responses
To evaluate the effect that coexposure played on the development of adaptive immune responses, we assessed cellular populations and phenotypes in the draining LNs (dLNs). Mice that were treated with OPA alone (OPA + A) did not have an increased number of dLN cells by the end of the study (day 22), compared with the acetone control (A + A). Treatment with DDAC alone (A+ H-DDAC) resulted in increased number of lymphocytes, but the mice that were previously exposed to OPA (OPA + H-DDAC) had on average more than twice the number of dLN cells as the H-DDAC control group (20.0 × 106 ± 2.47 vs 9.02 × 106 ± 0.74; Figure 2A). In addition to changes in the overall number of cells, the composition of the dLN cells changed depending on exposure. Overall, compared with the A + A group, treatment with DDAC alone (A + H-DDAC) decreased frequencies of CD4+ T cells, while decreased frequencies of CD8+ T cells were observed in mice treated with OPA alone (OPA + A; Figs. 2B and 2C). Although coexposure did not affect the frequency of CD4+ T cells (Figure 2B), mice exposed to OPA prior to DDAC had a significantly reduced frequency of CD8+ T cells compared with those treated with DDAC alone (Figure 2C), at a frequency similar to those exposed to OPA alone (OPA + A). Although not reaching statistical significance, pretreatment with OPA also increased the frequency of B cells above the frequency seen with DDAC alone (35.24% ± 0.77% vs 31% ± 1.06%, respectively; Figure 2D).
For all groups, approximately half of the CD11c+MHCII+ DCs expressed CD11b and showed similar trends in both frequency and number as did total DCs (CD11c+MHCII+; data not shown). The majority of CD11b+MHCII+ cells (Figure 2E) did not express CD11c, indicating that these cells were likely LN resident/migratory macrophages or inflammatory monocytes. Although the A+ H-DDAC group was elevated compared with the acetone control mice (A + A), the frequency of CD11b+MHCII+ cells did not vary between experimental treatment groups (Figure 2E). Mice treated with H-DDAC alone (A + H-DDAC), had significantly more DCs in the dLN than A + A control mice, mice treated with OPA (OPA + A), and the coexposed mice (Figure 2F). Numerically, the OPA + H-DDAC group had statistically significant increases in all cell types analyzed compared with the A + H-DDAC group, whereas the OPA + A had significant decreases in number of CD8+ T cells, B cells, DCs, and CD11b+MHCII cells (Table 1), although this was likely due to a difference in timing since last stimulus.
To further evaluate the immunological status in the dLNs we evaluated the activation status of the different subsets of immune cells by assessing the levels of CD44 and CD86 expression on T cells, and B cells and DC subsets, respectively (Figure 3 and Table 2). When compared with the acetone control (A + A), all treatment scenarios resulted in increased frequencies of activated CD4+ T cells; mice that we pre-exposed to OPA showed significant reductions in CD4 T cell activation as compared with the A + H-DDAC control (Figure 3A). Treatment with DDAC alone (A + H-DDAC) resulted in the highest activation of CD8+ T cells (Figure 3B), with a significant reduction in CD8+ T cell activation in the H-DDAC group when pre-exposed to OPA (OPA + H-DDAC) compared with the DDAC control (A + H-DDAC). In contrast, B-cell activation was increased in the mice pre-exposed to OPA, with the OPA + H-DDAC group having the largest percentage of activated B cells (Figure 3C). While treatment with H-DDAC alone significantly increased activation of DCs above the acetone control, no differences in activation status of DCs or CD11b+MHCII+ cells were observed with coexposure (Figs. 3D and 3E). Numerically, the OPA + A group showed decreased numbers of activated CD4+/CD8+ T cells and DCs, compared with the H-DDAC group, while the OPA+H-DDAC group showed increased numbers of activated CD4+ T cells, B cells, CD11b+ cells, and IgE-producing B cells (Table 2).
Table 2.
Activated Cells in the Draining LNs (Numbers)
| A + A | A + H-DDAC | OPA + A | OPA + L-DDAC | OPA + M-DDAC | OPA + H-DDAC | |
|---|---|---|---|---|---|---|
| CD44+ CD4 T cells (×105) | 0.65 ± 0.12*** | 4.13 ± 0.43 | 1.43 ± 0.33** | 2.59 ± 0.23 | 4.25 ± 0.55 | 6.99 ± 1.09** |
| CD44+ CD8 T cells (×104) | 0.65 ± 0.10*** | 6.06 ± 0.94 | 1.45 ± 0.36** | 3.15 ± 0.29 | 4.96 ± 0.71 | 6.64 ± 1.40 |
| CD86 + B cells (×105) | 0.32 ± 0.12 | 2.69 ± 0.22 | 1.79 ± 0.38 | 2.93 ± 0.28 | 4.60 ± 0.54 | 12.49 ± 2.56*** |
| IgE+ B cells (×105) | 0.70 ± 0.34 | 3.12 ± 0.70 | 1.58 ± 0.51 | 1.86 ± 0.35 | 4.42 ± 0.64 | 18.00 ± 2.94*** |
| CD86+ CD11c+ (×104) | 0.56 ± 0.12** | 2.95 ± 0.40 | 1.09 ± 0.25* | 1.95 ± 0.09 | 2.87 ± 0.36 | 4.34 ± 0.95 |
| CD86+ CD11b+ (×104) | 1.55 ± 0.30** | 6.39 ± 0.53 | 2.91 ± 0.51 | 4.69 ± 0.39 | 7.11 ± 0.63 | 9.96 ± 2.01* |
A, acetone; L, low (0.0625% DDAC); M, mid (0.125% DDAC); H, high (0.25% DDAC), OPA, (0.5% OPA). N = 5 mice/group, representative of 2 independent experiments.
(*) indicates significant difference compared with DDAC control (A + DDAC), *p < .05, **p < .01, and ***p < .001; light gray indicates significant decreases, whereas dark gray indicates significant increases compared with DDAC control.
Coexposure Results in Elevated Th2 Immune Responses (IL-4 and IgE)
As the cell populations elevated and activated suggested that prior exposure to OPA modulated the subsequent immune responses to DDAC, we next aimed to evaluate direct mediators of Th2 immune responses. To that end, we evaluated IL-4 and IgE in the dLNs. Gene expression analysis of Il4 revealed that exposure to the OPA control group (OPA + A) resulted in increased expression of Il4 compared with the DDAC control group (A + H-DDAC), and that the coexposed groups expressed similar levels of Il4 mRNA as the OPA group (Figure 4A). We next assessed IL-4 protein levels in dLN cells after ex vivo T-cell stimulation (Figure 4B). Although all groups produced more IL-4 than the acetone control (A + A), only the H-DDAC group that was previously exposed to OPA (OPA + H-DDAC) was able to produce significant levels of IL-4 above the DDAC control (A + H-DDAC). We next evaluated local (Figure 4C) and systemic (Figure 4D) total IgE levels and found, similar to the IL-4 production capabilities, the group that was exposed to OPA followed by H-DDAC, produced significantly more IgE than the group exposed to DDAC alone (A + H-DDAC). Importantly, when the exposure scenario is reversed (DDAC treatment prior to OPA exposure), the coexposed group no longer showed elevations in total IgE (local or systemic) or B cell activation (Supplementary Figure 1).
Figure 4.
Ortho-phthalaldehyde + didecyldimethlyammonium chloride (DDAC) exposure increases IgE-mediated immune responses. A, Gene expression analysis of Il4 in draining lymph nodes (dLNs), evaluated compared with DDAC control (A+ H-DDAC). B, IL-4 protein levels were measured via Luminex assay in cell culture supernatants of stimulated dLN cells isolated from the indicated groups. C, Frequency of IgE+ B cells was measured using flow cytometry. D, Total levels of IgE were analyzed in the serum at the end of the study. N = 5 mice per group; data are representative of 2 independent replicate studies. Statistical significance was determined using a 1-way AVOVA, with a post hoc Dunnett’s multiple comparisons test versus “A+ H-DDAC” control (black bar, significance indicated by *s) and versus “A + A” control (gray bar, significance indicated by #s). */#p < .05, **/##p < .01, and ***/###p < .001.
Local Environment Likely Alters Subsequent Immune Responses
To tease out the mechanism behind the altered immune responses observed in the coexposed mice and provide a snapshot of the local immune responses immediately prior to DDAC exposure, we assessed the immune responses in mice exposed to OPA for 3 days then rested for 4 days. Total numbers of immune cells and ILC2 activation status were assessed at the site of chemical application (Figure 5A). Following OPA exposure, the total number of immune (CD45+) cells in the ears increased. While total numbers of ILC2s did not increase, ILC2s increased their level of ICOS expression, indicative of activation. Significant increases in the cytokines GM-CSF, IL-4, IFN-γ, and TNF-α were detected in protein lysates collected from the ears (Figure 5B).
In the ear-dLNs, OPA exposure induced significant increases in cell number (Figure 5C). OPA exposure also affected the composition of the lymphocyte compartment, significantly increasing the frequency of B cells, at the expense of the CD4+ and CD8+ T-cell frequencies (Figure 5D). In addition, OPA exposure resulted in the activation of B cells, CD4+ T cells, and CD8+ T cells (Figure 5E). Although all subsets of lymphocytes numerically increased due to OPA exposure, the total number of B cells and the number of activated (CD86+) B cells had the largest fold increases (Table 3). Stimulated T cells isolated from OPA-treated mice produced significant levels of a variety of T-cell-derived cytokines, with the highest fold increases (>6-fold) being for GM-CSF, IL-4, IL-13, IL-10, and IL-9 (Supplementary Table 2).
Table 3.
Total Cell Numbers and Numbers of Activated Cells Following 3 days of OPA Exposure and 4 Days of Rest
| Acetone (VC) | OPA | Fold Increase (OPA/VC) | |
|---|---|---|---|
| Total Cells | |||
| B cells (×106) | 0.72 ± 0.13 | 6.72 ± 1.16*** | 9.33 |
| CD4 T cells (×106) | 1.63 ± 0.23 | 8.25 ± 1.39** | 5.06 |
| CD8 T cells (×106) | 0.73 ± 0.09 | 3.29 ± 0.53** | 4.51 |
| Activated Cells | |||
| CD86+ B cells (×105) | 0.42 ± 0.09 | 16.86 ± 3.48** | 40.14 |
| CD44+ CD4 T cells (×105) | 1.15 ± 0.22 | 14.64 ± 2.98** | 12.73 |
| CD44+ CD8 T cells (×105) | 0.08 ± 0.02 | 1.05 ± 0.23** | 13.125 |
N = 5 mice/group, statistical significance was determined using a 2-tailed unpaired t test.
(*) indicates significant increase compared with VC,
p < .01 and
p < .001.
DISCUSSION
In both occupational and consumer environments exposure to more than one immune-altering chemical is a common occurrence. Repetitive and concurrent exposures may ultimately impact disease development, symptoms, and progression. For these reasons, the results from laboratory animal studies are not always 100% predictors of disease symptoms observed in humans. Heavy use of QACs, including DDAC, for instance, is often associated with the development of asthma or respiratory disease in hospital workers (Gonzalez et al., 2014). As DDAC is a low-volatile chemical (Vincent et al., 2007) there is a decreased risk that individuals will be exposed via inhalation exposure. Although it is possible that fine mist solutions might be inhaled, the most predominant route of exposure will be through contact with the skin. However, in the laboratory setting, using standard immunotoxicological assays, dermal DDAC exposure primarily fit the classification as a T-cell-mediated sensitizer (Anderson et al., 2016). Although there certainly are cases of skin sensitization developing after exposure to DDAC (Dejobert et al., 1997; Mowitz and Ponten, 2015; Ruiz Oropeza et al., 2011), the increased chances of developing asthma like symptoms are not suggested from our previous study. One factor leading to the discrepancies observed between laboratory animal studies and human observations might be due to differences in exposure durations (Shane et al., 2017); yet, it is also likely that the prior immune status of the individual will affect the developing immunologic response to a new stimulus. However, the summation of these immunological effects is difficult to quantify.
In this study, we used an exposure scenario with 2 relevant sensitizing chemicals (Figure 1A) with immunologically distinct profiles to determine if one exposure would influence another. Using this novel study design, we assessed a number of immunological factors including immunophenotyping, cytokine, and total IgE analysis to determine if, as a whole, the IgE-mediated sensitizer OPA would influence immune responses to the T-cell-mediated sensitizer DDAC. Although mixed hypersensitivity responses have been reported for DDAC (Geier et al., 2013; Houtappel et al., 2008; Vandenplas et al., 2013) and OPA (Fujita et al., 2006), for the purpose of this study, based on our previous studies conducted in mice, DDAC was selected as the representative T-cell-mediated sensitizer and OPA as the IgE-mediated sensitizer.
Although there were no obvious signs of overt toxicity observed (significant weight loss, obvious signs of physical stress, or altered behavior), acetone control mice gained the most weight (along with the OPA+ L-DDAC) suggesting that there might be minor overall health effects following coexposure to OPA and/or DDAC. However, these effects were not exacerbated by exposure to both antimicrobials, and overall seem negligible, as no mice lost weight (Figure 1B). Additionally, mice that were previously exposed to OPA showed increased irritation following exposure to H-DDAC at an earlier timepoint (by day 10) versus those exposed to H-DDAC alone, which peaked at the end of the study. However, by the end of the study the OPA + H-DDAC group and the A + H-DDAC group had equal levels of irritation (Figure 1C). The earlier irritation response might be due to the “OPA primed” environment, but the equalized response by the end of the study shows that increased levels of irritation do not likely play a significant role in the changes in immunological responses observed in our studies.
The evaluation of cellular profiles in the LNs draining the site of chemical application can give valuable insight regarding the development of adaptive immune responses. As types of hypersensitivity responses are largely defined by the adaptive immune responses which they elicit, the profiling of these cell populations can define the class of chemical sensitizer (types I vs IV). As previously mentioned, for the purposes of these studies we have defined OPA as a representative IgE-mediated chemical sensitizer based on the increased number and activation of B cells, CD4+ T cells, Il4, and IgE in a murine model (Anderson et al., 2010). The local DDAC immune response is characterized by increases in frequency, number, and activation of T cells. Although CD4+ T cells are activated following exposure, the responses are more dramatic for CD8+ T cells, indicating a skewing towards a Th1 dominant/T-cell-mediated immune response (Anderson et al., 2016). In animals that have been exposed to OPA prior to DDAC exposure, decreased frequencies of CD8+ T cells were observed (Figure 2C) in addition to decreases in the activation of CD4+ and CD8+ T cells, compared with mice exposed to DDAC alone (Figs. 3A and 3B). In addition to the dampening of the overall Th1-type responses, the increased activation of B cells (Figure 3C) indicates a skewing towards more of a Th2/IgE-mediated immune response in the coexposed mice. This Th2 skewing is further supported by increased levels of IL-4 (at both the gene and protein levels), as well as increased levels of B-cells expressing IgE in the dLN, and total levels of IgE assessed in the serum (Figure 4). Although immune function was not directly evaluated, the complementary data obtained from cellular phenotyping, gene expression and protein analysis of specific cytokines, and IgE analysis work together to support the hypothesis that exposure to OPA prior to DDAC exposure augments Th2 responses to DDAC.
Importantly, the same effects were not observed when the exposures were reversed (when mice were treated with DDAC for 2 weeks, followed by OPA exposure) (Supplementary Figure 1). In fact, exposure to DDAC prior to OPA exposure resulted in increased CD8+ T-cell activation and decreased B-cell activation, with no change in Il4 or total IgE levels, compared with those mice treated with OPA alone. Overall, the impact of DDAC skewing OPA immune responses were not as dramatic as the reverse scenario and might be indicative of OPA overwhelming the immune response. However, this shows that the observed changes in cellular phenotype and increases in IL-4 and IgE were not simply due to the additive effects of exposure to 2 sensitizing chemicals, but that the IgE-mediated skewing is dependent upon the order in which these exposures occurred (OPA exposure preceding DDAC exposure).
As the aforementioned data indicated that OPA might prime the immunological environment in such a way that the responses to DDAC are altered, we investigated the local immunological environment at the time of DDAC exposure. As the site of exposure can have impacts on the developing immune response (Shane et al., 2019a), we assayed responses both in the skin (the site of chemical application) and the dLNs. ILC2s are found at barrier sites such as the skin and are known to produce Th2 type cytokines (Artis and Spits, 2015). Additionally, ILC2s have been shown to drive allergic responses in several different models (Kim et al., 2013; Martinez-Gonzalez et al., 2015; Noval Rivas et al., 2016). Following OPA exposure skin ILC2s did not increase in number, but did become activated, as indicated by the upregulation of ICOS (Figure 5). ICOS has been shown to be upregulated on activated ILC2s (Roediger et al., 2013), and correlates with increased ILC2 survival and functional ability to produce type 2 cytokines (Maazi et al., 2015). We have previously shown that DDAC exposure also results in the activation of ILC2s (Shane et al., 2019b), so it is possible that the further activation of these cells may contribute to the enhanced Th2 skewing environment.
OPA exposure alone increased cytokines both typically associated with Th1 (IFN-γ and TNF-α) and Th2 responses (GM-CSF and IL-4), but the greatest increase compared with acetone control was observed with IL-4 (9.3-fold; Figure 5). Likewise, while all lymphocytes were activated following OPA exposure, B cells were disproportionally expanded, and activated, as highlighted in Table 3. This demonstrates that at the time which immune reactions against exposure to DDAC were initiated, the immune environment was skewed towards the development of Th2 type immune responses, both at the site of exposure (the skin) and in the skin-dLNs. Similarly, a study by Johnson and colleagues showed that OPA is a respiratory sensitizer in mice and induces strong Th2 responses (increases in IgE+ B cells, IL-4, IL-5, and IL-13 in the dLNs) at occupationally relevant exposure concentrations (Johnson et al., 2011). We believe that this data supports the conclusion that the OPA-induced immunological environment shapes the subsequent immune responses, in this case, driving the immune response to DDAC towards an IgE-mediated hypersensitivity reaction.
Together, the data from the coexposure and OPA-only experiments strongly support the overall hypothesis; the IgE-mediated sensitizer OPA alters the overall immune response to the T-cell-mediated sensitizer DDAC by creating a Th2 skewed immune environment. It is possible, that in health-care settings, where multiple chemicals are commonly used, that this skewed immunological environment is present in workers. These studies may help explain, in part, why work-related asthma is a symptom of DDAC exposure in health-care workers, although not identified as an IgE-mediated sensitizer in animal studies. At a minimum, the studies presented within this article show the capability of one chemical to alter the immune response to another. As humans are exposed to a multitude of immunological stimuli throughout their lives, understanding how prior immune status alters responses to a specific agent is important in understanding the overall health impact of the agent being evaluated. We believe this is an important issue in toxicological and immunotoxicological studies, and one that warrants consideration in study design, and further investigation.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
This work was supported by internal funds from the Health Effects Laboratory Division of the National Institute for Occupational Safety and Health.
DECLARATION OF CONFLICTING INTERESTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Supplementary Material
Contributor Information
Hillary L Shane, Allergy and Clinical Immunology Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Morgantown, West Virginia 26505.
Ewa Lukomska, Allergy and Clinical Immunology Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Morgantown, West Virginia 26505.
Lisa Weatherly, Allergy and Clinical Immunology Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Morgantown, West Virginia 26505.
Rachel Baur, Allergy and Clinical Immunology Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Morgantown, West Virginia 26505.
Stacey E Anderson, Allergy and Clinical Immunology Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, Morgantown, West Virginia 26505.
REFERENCES
- Akamatsu T., Minemoto M., Uyeda M. (2005). Evaluation of the antimicrobial activity and materials compatibility of orthophthalaldehyde as a high-level disinfectant. J. Int. Med. Res. 33, 178–187. [DOI] [PubMed] [Google Scholar]
- Alfa M. J., Lo E., Olson N., MacRae M., Buelow-Smith L. (2015). Use of a daily disinfectant cleaner instead of a daily cleaner reduced hospital-acquired infection rates. Am. J. Infect. Control 43, 141–146. [DOI] [PubMed] [Google Scholar]
- Anderson S. E., Shane H., Long C., Lukomska E., Meade B. J., Marshall N. B. (2016). Evaluation of the irritancy and hypersensitivity potential following topical application of didecyldimethylammonium chloride. J. Immunotoxicol. 13, 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E., Umbright C., Sellamuthu R., Fluharty K., Kashon M., Franko J., Jackson L. G., Johnson V. J., Joseph P. (2010). Irritancy and allergic responses induced by topical application of ortho-phthalaldehyde. Toxicol. Sci. 115, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argy G., Bricout F., d’Hermies F., Cheymol A. (1999). Study of prophylaxis by didecyl dimethyl ammonium chloride against herpes simplex virus infection in nude mice. C. R. Acad. Sci. 322, 863–870. [DOI] [PubMed] [Google Scholar]
- Artis D., Spits H. (2015). The biology of innate lymphoid cells. Nature 517, 293–301. [DOI] [PubMed] [Google Scholar]
- Atiyeh K., Chitkara A., Achlatis S., Branski R. C., Amin M. R. (2015). Allergic reaction to ortho-phthalaldehyde following flexible laryngoscopy. 125, 2349–2352. [DOI] [PubMed] [Google Scholar]
- Bernstein J. A., Stauder T., Bernstein D. I., Bernstein I. L. (1994). A combined respiratory and cutaneous hypersensitivity syndrome induced by work exposure to quaternary amines. J. Allergy Clin. Immunol. 94, 257–259. [DOI] [PubMed] [Google Scholar]
- Coombs R. R. A., and Gell, P. G. H. (1963). The classification of allergic reactions underlying disease. In Clinical Aspects of Immunology (Gell P. G. H., Coombs R. R. A. Eds.). Blackwell, Oxford. [Google Scholar]
- Dejobert Y., Martin P., Piette F., Thomas P., Bergoend H. (1997). Contact dermatitis from didecyldimethylammonium chloride and bis-(aminopropyl)-lauryl amine in a detergent-disinfectant used in hospital. Contact Dermatitis 37, 95–96. [DOI] [PubMed] [Google Scholar]
- Franchi A., Franco G. (2005). Evidence-based decision making in an endoscopy nurse with respiratory symptoms exposed to the new ortho-phthalaldehyde (OPA) disinfectant. Occup. Med. (Lond.) 55, 575–578. [DOI] [PubMed] [Google Scholar]
- Fujita H., Ogawa M., Endo Y. (2006). A case of occupational bronchial asthma and contact dermatitis caused by ortho-phthalaldehyde exposure in a medical worker. J. Occup. Health 48, 413–416. [DOI] [PubMed] [Google Scholar]
- Gannon P. F., Bright P., Campbell M., O’Hickey S. P., Burge P. S. (1995). Occupational asthma due to glutaraldehyde and formaldehyde in endoscopy and X ray departments. Thorax 50, 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier J., Lessmann H., Krautheim A., Fuchs T. (2013). Airborne allergic contact dermatitis caused by didecyldimethylammonium chloride in a geriatric nurse. Contact Dermatitis 68, 123–125. [DOI] [PubMed] [Google Scholar]
- Gonzalez M., Jegu J., Kopferschmitt M. C., Donnay C., Hedelin G., Matzinger F., Velten M., Guilloux L., Cantineau A., de Blay F. (2014). Asthma among workers in healthcare settings: Role of disinfection with quaternary ammonium compounds. Clin. Exp. Allergy 44, 393–406. [DOI] [PubMed] [Google Scholar]
- Houtappel M., Bruijnzeel-Koomen C. A., Rockmann H. (2008). Immediate-type allergy by occupational exposure to didecyl dimethyl ammonium chloride. Contact Dermatitis 59, 116–117. [DOI] [PubMed] [Google Scholar]
- Jarvis J., Seed M. J., Elton R., Sawyer L., Agius R. (2005). Relationship between chemical structure and the occupational asthma hazard of low molecular weight organic compounds. Occup. Environ. Med. 62, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. J., Reynolds J. S., Wang W., Fluharty K., Yucesoy B. (2011). Inhalation of ortho-phthalaldehyde vapor causes respiratory sensitization in mice. J. Allergy (Cairo) 2011, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. S., Siracusa M. C., Saenz S. A., Noti M., Monticelli L. A., Sonnenberg G. F., Hepworth M. R., Van Voorhees A. S., Comeau M. R., Artis D. (2013). TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 5, 170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. (2005). What makes a chemical a respiratory sensitizer? Curr. Opin. Allergy Clin. Immunol. 5, 119–124. [DOI] [PubMed] [Google Scholar]
- Klink K. J., Meade B. J. (2003). Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicol. Sci. 75, 89–98. [DOI] [PubMed] [Google Scholar]
- Maazi H., Patel N., Sankaranarayanan I., Suzuki Y., Rigas D., Soroosh P., Freeman G. J., Sharpe A. H., Akbari O. (2015). ICOS: ICOS-ligand interaction is required for type 2 innate lymphoid cell function, homeostasis, and induction of airway hyperreactivity. Immunity 42, 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez I., Steer C. A., Takei F. (2015). Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol. 36, 189–195. [DOI] [PubMed] [Google Scholar]
- Mazurek J. M., Syamlal G. (2018). Prevalence of asthma, asthma attacks, and emergency department visits for asthma among working adults - National Health Interview Survey, 2011-2016. MMWR Morb. Mortal. Wkly. Rep. 67, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowitz M., Ponten A. (2015). Foot dermatitis caused by didecyldimethylammonium chloride in a shoe refresher spray. Contact Dermatitis 73, 374–376. [DOI] [PubMed] [Google Scholar]
- Mwinzi P. N., Karanja D. M., Colley D. G., Orago A. S., Secor W. E. (2001). Cellular immune responses of schistosomiasis patients are altered by human immunodeficiency virus type 1 coinfection. J. Infect. Dis. 184, 488–496. [DOI] [PubMed] [Google Scholar]
- Noval Rivas M., Burton O. T., Oettgen H. C., Chatila T. (2016). IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 138, 801–811.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma A., Yoshida T., Horiuchi H., Fukumori J., Tomita M., Kojima S., Takahashi N., Fukuyama T., Hayashi K., Yamaguchi S., et al. (2011). Altered pulmonary defense system in lung injury induced by didecyldimethylammonium chloride in mice. Inhal. Toxicol. 23, 476–485. [DOI] [PubMed] [Google Scholar]
- Purohit A., Kopferschmitt-Kubler M. C., Moreau C., Popin E., Blaumeiser M., Pauli G. (2000). Quaternary ammonium compounds and occupational asthma. Int. Arch. Occup. Environ. Health 73, 423–427. [DOI] [PubMed] [Google Scholar]
- Quinn M. M., Henneberger P. K., Braun B., Delclos G. L., Fagan K., Huang V., Knaack J. L. S., Kusek L., Lee S.-J., Le Moual N., et al. (2015). Cleaning and disinfecting environmental surfaces in health care: Toward an integrated framework for infection and occupational illness prevention. Am. J. Infect. Control 43, 424–434. [DOI] [PubMed] [Google Scholar]
- Riner D. K., Ndombi E. M., Carter J. M., Omondi A., Kittur N., Kavere E., Korir H. K., Flaherty B., Karanja D., Colley D. G. (2016). Schistosoma mansoni infection can jeopardize the duration of protective levels of antibody responses to immunizations against hepatitis B and tetanus toxoid. PLoS Negl. Trop. Dis. 10, e0005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille C., Boulet L.-P. (2015). Occupational asthma after exposure to ortho-phthalaldehyde (OPA). Occup. Environ. Med. 72, 381–381. [DOI] [PubMed] [Google Scholar]
- Roediger B., Kyle R., Yip K. H., Sumaria N., Guy T. V., Kim B. S., Mitchell A. J., Tay S. S., Jain R., Forbes-Blom E., et al. (2013). Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Oropeza A., Fischer Friis U., Duus Johansen J. (2011). Occupational contact urticaria caused by didecyl dimethyl ammonium chloride. Contact Dermatitis 64, 297–298. [DOI] [PubMed] [Google Scholar]
- Shaffer M. P., Belsito D. V. (2000). Allergic contact dermatitis from glutaraldehyde in health-care workers. Contact Dermatitis 43, 150–156. [DOI] [PubMed] [Google Scholar]
- Shane H. L., Long C. M., Anderson S. E. (2019. a). Novel cutaneous mediators of chemical allergy. J. Immunotoxicol. 16, 13–27. [DOI] [PubMed] [Google Scholar]
- Shane H. L., Lukomska E., Kashon M. L., Anderson S. E. (2019. b). Topical application of the quaternary ammonium compound didecyldimethylammonium chloride activates type 2 innate lymphoid cells and initiates a mixed-type allergic response. Toxicol. Sci. 168, 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane H. L., Lukomska E., Stefaniak A. B., Anderson S. E. (2017). Divergent hypersensitivity responses following topical application of the quaternary ammonium compound, didecyldimethylammonium bromide. J. Immunotoxicol. 14, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaliy P., Thompson T. A., Gorman G. W., Morris G. K., McEachern H. V., Mackel D. C. (1980). Laboratory studies of disinfectants against Legionella pneumophila. Appl. Environ. Microbiol.gy 40, 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja T., Belsito D. V. (2008). Occupational dermatoses in health care workers evaluated for suspected allergic contact dermatitis. Contact Dermatitis 58, 285–290. [DOI] [PubMed] [Google Scholar]
- Vandenplas O., D’Alpaos V., Evrard G., Jamart J., Thimpont J., Huaux F., Renauld J. C. (2013). Asthma related to cleaning agents: A clinical insight. BMJ Open 3, e003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent G., Kopferschmitt-Kubler M. C., Mirabel P., Pauli G., Millet M. (2007). Sampling and analysis of quaternary ammonium compounds (QACs) traces in indoor atmosphere. Environ. Monit. Assess. 133, 25–30. [DOI] [PubMed] [Google Scholar]
- Walsh S. E., Maillard J. Y., Russell A. D. (1999). Ortho-phthalaldehyde: A possible alternative to glutaraldehyde for high level disinfection. J. Appl. Microbiol. 86, 1039–1046. [DOI] [PubMed] [Google Scholar]
- Walsh S. E., Maillard J. Y., Russell A. D., Catrenich C. E., Charbonneau D. L., Bartolo R. G. (2003). Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 94, 240–247. [DOI] [PubMed] [Google Scholar]
- Warshaw E. M., Schram S. E., Maibach H. I., Belsito D. V., Marks J. G. Jr, Fowler J. F. Jr, Rietschel R. L., Taylor J. S., Mathias C. G., DeLeo V. A., et al. (2008). Occupation-related contact dermatitis in North American health care workers referred for patch testing: Cross-sectional data 1998 to 2004 . Dermatitis 19, 261–274. [PubMed] [Google Scholar]
- Woolhiser M. R., Munson A. E., Meade B. J. (2000). Comparison of mouse strains using the local lymph node assay. Toxicology 146, 221–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.