Abstract
Heterogeneous macrophage lineages are present in the aortic and mitral valves of the heart during development and disease. These populations include resident macrophages of embryonic origins and recruited monocyte-derived macrophages prevalent in disease. Soon after birth, macrophages from haematopoietic lineages are recruited to the heart valves, and bone marrow transplantation studies in mice demonstrate that haematopoietic-derived macrophages continue to invest adult valves. During myxomatous heart valve disease, monocyte-derived macrophages are recruited to the heart valves and they contribute to valve degeneration in a mouse model of Marfan syndrome. Here, we review recent studies of macrophage lineages in heart valve development and disease with discussion of clinical significance and therapeutic applications.
Keywords: Heart valve, Myxomatous valve disease, Macrophage, Valve development
1. Introduction
In the past few years, macrophages have emerged as critical contributors to cardiovascular health and disease. In most organs, including the heart, resident macrophages are present beginning in embryonic development and persist into adulthood with immune surveillance and tissue homeostatic functions.1 In mouse models of heart failure, atherosclerosis, and valve disease, resident and recruited monocyte-derived macrophage populations expand, and inhibition of macrophage homing to injured tissue has emerged as a promising therapeutic strategy. In light of the successful Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) trial, there is enthusiasm for the potential of immune modulation cell-targeted therapy in cardiovascular disease.2 Here, we review recent discoveries of macrophage contributions to heart valve development and disease with discussion of potential therapeutic applications.
Heart valve disease is a common manifestation of cardiovascular disease with a frequency of up to 15% of people >65 years old affected.3–5 Myxomatous valve disease (MVD), usually involving the mitral valve, includes progressive extracellular matrix (ECM) abnormalities and thickening of valve leaflets that can lead to prolapse into the atria and regurgitation. Causes of MVD include congenital malformation, genetic mutations, ischaemic heart disease, and inflammatory endocarditis.5 Calcific aortic valve disease (CAVD) includes stenosis and mineralization of valve leaflets and is associated with aging, coronary artery disease, and other cardiovascular disease indicators.3,6 In both MVD and CAVD, ECM abnormalities and immune cell populations increase with the progression of disease leading to decreased cardiac function. The current standard of care is surgical valve replacement or repair, which can be contraindicated in aged individuals.7 Increased understanding of valve structural abnormalities and immune cell involvement should lead to urgently needed new therapeutic options.
Several recent studies have demonstrated the presence and importance of macrophage lineages in heart valve development, homeostasis, and disease. Infiltrating immune cells in adult valves were first identified by Visconti et al.8 who performed bone marrow-derived stem cell transplantation studies in mice, demonstrating that CD45+ donor cells are present in recipient valves. These cells were hypothesized to take on valve interstitial cell (VIC)-like functions within the context of the adult valves,9 but subsequent studies have shown that they maintain their immune cell phenotypes and are predominantly macrophages.10,11 More recent studies have shown that multiple immune cell types are present in adult and developing heart valves and they constitute approximately 8% of all cells in remodelling valves.11 At least 75% of these immune cells express macrophage markers of resident and recruited populations, in addition to smaller populations of dendritic cells and T cells. The specific roles and functions of these cells in heart development, homeostasis, and disease are largely unknown. However, they have been implicated in valve homeostasis, ECM remodelling, ageing, and disease progression in a variety of contexts.9–14
2. Macrophage lineages in cardiovascular development and disease
Studies in the late 19th century by Nobel Laureate Ilya Mechnikov first described macrophages as phagocytic cells that help to defend against pathogens and remove cell debris.15 More recently, it has become clear that macrophages populate all tissues, exhibiting organ-specific functions that affect not just host immunity, but also development, homeostasis, tissue repair, and disease.16 In addition, recent fate-mapping techniques have demonstrated that macrophages in many tissues, including the heart, emerge during embryonic development and can persist long-term through cell proliferation, rather than by monocyte recruitment in an uninjured setting.17 In contrast, bone marrow-derived macrophages arise from circulating monocytes and are recruited to target organs after stress or injury. Originally macrophages were characterized as proinflammatory M1 or reparative M2, but this dichotomy is oversimplified since the functions and origins of heterogeneous macrophage populations are variable and highly context dependent.18,19
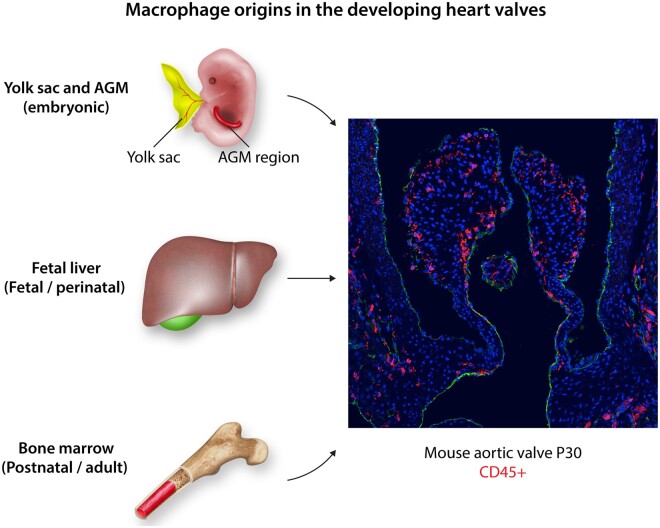
During mouse development, macrophages first appear during early gestation in the extraembryonic yolk sac during primitive haematopoiesis.20 Notably, at this stage, macrophages are the only immune cells made in the embryo and they express F4/80.21 Definitive haematopoiesis begins immediately thereafter with the appearance of haematopoietic stem cells emerging from the aorta-gonad-mesonephros (AGM) region that can give rise to all immune cell lineages.22 After embryonic day (E)10.5, these haematopoietic stem cells migrate to the primordial liver, which becomes the predominant site of haematopoiesis during embryogenesis.23 By E12–14, circulating monocyte-derived macrophages originally from the liver express CD11b and populate developing tissues, including the heart.24 In addition, within organs, macrophages can be locally produced through transformation of the haemogenic endothelium.14 Post-birth, however, haematopoiesis primarily occurs in the bone marrow and macrophage production is mediated through circulating blood monocytes (Figure 1).24 Expansion of macrophage lineages in tissue homeostasis and disease can occur through recruitment of monocyte-derived macrophages or proliferation of tissue-resident macrophages.
Figure 1.
Macrophage origins in developing heart valves. The sources of macrophages in the cardiovascular system during development include the yolk sac and AGM region in embryos, the foetal liver in foetuses and newborns, and the bone marrow during definitive haematopoiesis after birth.25 The right panel shows a mouse aortic valve at 1 month-of-age with CD45+ cells stained red that are predominantly heterogeneous macrophages.10
A normal uninjured adult heart has macrophages from two distinct origins that can be differentiated by the expression of the cell surface marker, CCR2.1 In this steady state condition, 6–8% of non-cardiomyocytes of the heart are resident macrophages, the majority of which lack CCR2 (termed CCR2−).26,27 These macrophages arise from the extraembryonic yolk sac and foetal liver, seed the heart during development, and are maintained throughout life.24,28,29 Soon after birth, the resident CCR2− macrophage population expands through proliferation and, beginning 2 weeks after birth, haematopoietic Cx3CR1-lineage CCR2− macrophages are recruited to the heart. Together, these populations constitute the tissue-resident CCR2− macrophages of the heart.24,28 During embryonic development, CCR2− macrophages are associated with the blood vessel progenitor populations and are required for normal coronary vascular development.28,30 Likewise, ablation of macrophages in the neonatal heart using clodronate liposomes prevents cardiac regeneration in response to injury, probably through loss of neovascularization.28,30,31 In addition, resident cardiac macrophages facilitate electrical conduction within the heart and have increased expression of genes associated with tissue repair.32,33 The recruited population of cardiac macrophages expresses CCR2 (termed CCR2+) and comprise only a small proportion of total cardiac macrophages in the uninjured state. These macrophages are short-lived and are monocyte-derived from bone marrow in a CCR2-dependent manner.1 While the functions of recruited CCR2+ macrophages in a healthy heart are not entirely defined, transcriptomic analysis reveals that they selectively express genes involved in the initiation of inflammation.33
Following cardiac injury such as myocardial infarction (MI), CCR2+ macrophages derived from circulating monocytes are recruited to the heart.28 This recruitment occurs in response to chemokine and cytokine expression induced after cardiac injury. Notably, increased expression of the ligand CCL2 (also called MCP-1, monocyte chemoattractant protein 1) by cardiac fibroblasts, endothelial cells, and other cell lineages in response to injury leads to homing of CCR2+ monocytes to the area of injury.34,35 CCR2+ monocyte and derivative macrophage recruitment is considered a maladaptive response associated with negative outcomes, including myocardial infarct expansion and left ventricular dysfunction.36 Thus, inhibition of CCR2+ macrophage recruitment in mice is protective against cardiovascular disease.35,37 Much less is known of human heart resident macrophage lineages and contributions to cardiac injury response, but recent detection of CCR2+ macrophages in human diseased hearts supports conserved mechanisms.33 Several studies have reported different approaches to targeting CCR2+ macrophage populations, including siRNA, antibody blockade, clodronate liposomes, and receptor targeting with small molecules, after MI in animal models with variable success.36–38 This remains an active area of research in efforts directed towards limiting cardiac injury and promoting recovery of cardiac function after myocardial infarction.
3. Macrophages in heart valve development
3.1 Overview of valve structure and function
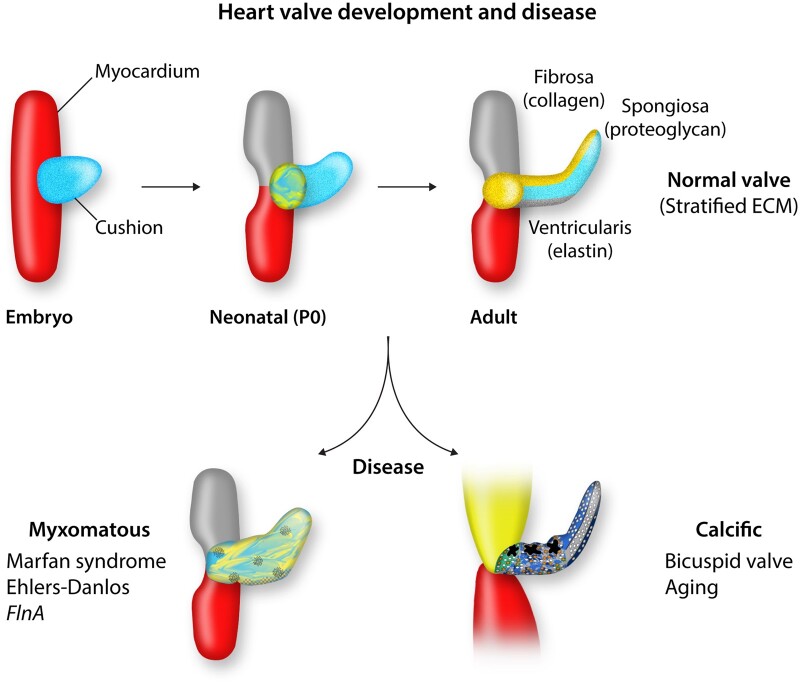
The semilunar and atrioventricular valves of the heart are primarily comprised of three functionally specialized ECM layers that are oriented relative to blood flow (Figure 2).39 The fibrosa, located away from directional blood flow, is predominantly fibrillar collagen, whereas the layer adjacent to blood flow contains elastin, and the intermediate layer is rich in chondroitin sulphate proteoglycans, notably versican.40 Altogether, this tri-laminar organization provides the rigidity and resiliency for the valves to endure differential pulsatile and oscillatory shear stresses throughout the cardiac cycle.41 During disease, ECM stratification often becomes disorganized with progressive thickening or mineralization which contributes to valve degeneration and dysfunction leading to compromised cardiac output.42
Figure 2.
Heart valve development and disease. During embryonic development, endocardial cushions are present in the outflow tract that elongate and stratify into valve leaflets after birth. Valve layers consist of fibrosa (yellow), spongiosa (blue), and atrialis (black). Bottom panels depict myxomatous alterations associated with connective tissue mutations (left) and calcification of aortic valves characteristic of aortic stenosis (right).
VICs are the main fibroblast-like cells that reside within the ECM layers and produce matrix during development and homeostasis. The valve leaflets are covered by valve endothelial cells (VECs) that are continuous with the endocardium and vascular endothelial cells of the great vessels in contact with blood flow. Additional cell types present in the developing and adult valves include monocyte-derived macrophages, dendritic cells, melanocytes, and small populations of T cells and mast cells.11 Recent work has revealed that 8–10% of heart valve cells are CD45+ and include macrophages, monocytes, and dendritic cells.10–12 These immune cells of the heart valves are heterogeneous in terms of expressed cell surface markers and developmental origins (Table 1). Very recent studies are just beginning to elucidate their functions and contributions to heart valve development and disease.14,44
Table 1.
Immune cells in developing and diseased heart valves
| Cell type | Markers | Immunological role | Notes |
|---|---|---|---|
| Valve development 14 | |||
| Macrophages | CD68+, Csf1r+, F4/80+ | ||
| Endocardially derived macrophages | Nfatc1-lineage+, CD68+, Csf1r+, CD206+ | Phagocytic and antigen presenting roles | Expression from E10.5 |
| Nfatc1-lineage+, F4/80+ | Expression after E13.5 | ||
| Ly6C low CD206+ | Phagocytic and pro-tissue reparatory | ||
| Non-endocardial-derived macrophages | Ly6C high CD206- | Pro-inflammatory | 8.6–11.2% of non-endocardially derived |
| Postnatal valve remodeling 10,11,14,43 | |||
| Leucocytes | CD45+ | Increased from P1 to P60 | |
| 1. Myeloid cells | |||
| Macrophages | F4/80+, CD11b+, CD64+, CD68+ | ||
| CD206+ macrophages | Ly6C low CD206+ | Phagocytic and pro-tissue reparatory | Increased after birth, predominant population at P7 |
| MHCII+ macrophages | Ly6C low MHCII low | Phagocytic | P1 to P28 |
| Ly6C low MHCII high | Antigen presenting | Increased with age | |
| Recruited macrophages | F4/80+ CCR2+ | Immune and defense response | Increased between P7 and P30 |
| Ly6C high CD206- | Decreased after birth | ||
| Dendritic cells | Klrb1, Xcr1 | Antigen processing and presenting | |
| Myeloid dendritic cells | CD11b+ CD11c+ F4/80- | Antigen presenting | |
| Non-myeloid dendritic cells | CD11b- CD11c+ | ||
| Mast cells | Kit, Cpa3, Tpsab1 | Regulation of macrophage activation | Decreased from P7 to P30 |
| 2. Lymphocytes | |||
| T cells | CD24a, Trbc1, CD3d, CD8a | Immune response | Decreased from P7 to P30 |
| Naïve T cells | Satb1, Phf3, Dntt | P7 | |
| Effector memory T cells | S100a10, Ppp43ca, Tnfrsf18 | Immune response to pathogens | P30 |
| MVD 13,44,76,78 | |||
| Leucocytes | CD45+ | Increased in MV of Axin2 KO, MFS, Filamin A KO mice and MI induced sheep models | |
| 1. Myeloid cells | |||
| Macrophages | F4/80+, CD11b+ | ||
| CD163+, CD68+, CD64+ | Increased in MFS pigs and human | ||
| Resident macrophages | CD206+ CCR2- | Increased in MV of MFS | |
| CD163+ CD14−; CD68+ CD14− | No change in MFS pigs and human | ||
| Recruited macrophages | MHCII+ CCR2+ | Pro-inflammatory | Increased in MV of MFS |
| CD163+ CD14+; CD68+ CD14+ | Pro-inflammatory | Increased in MFS pigs and human | |
| Neutrophils | Ly6G+ | Not detected in MV of MFS | |
| 2. Lymphocytes | |||
| T cells | CD3+ | Not detected in MV of MFS | |
3.2 Macrophages in heart valve development
The first evidence of heart valve formation in vertebrate development begins with the formation of endocardial cushions at E9.5 in mice (which corresponds to E31–E35 in humans) by endothelial-to-mesenchymal transformation (EndMT) of the endocardium in the atrioventricular canal and outflow tract (Figure 2).45 The mesenchymal and endothelial cells proliferate until E12.5–14.5 as the endocardial cushions expand and elongate to form the valve primordia.46,47 Macrophages expressing CD68 and CD206 are present in the endocardial cushions, as early E10.5.14 Genetic fate-mapping studies and flow cytometry demonstrate that a subset of these macrophages originate from specialized haemogenic endocardial cells that line the surface of valve primordia and appear during E9.5–E11.5.48 However, the majority of macrophages in the developing heart valves at E10.5–E15.5 originate elsewhere, likely from the yolk sac and/or foetal liver. By E15.5, distinct populations of valve macrophages with differential expression of CD206 and F4/80 are present in the developing heart valves.14
The specific functions of macrophages in developing heart valves are largely unknown. The endocardium-derived macrophages are more phagocytic than other macrophage populations and were speculated to be responsible for the clearance of apoptotic cells in embryonic valves during mid-late gestational stages.14,49,50 In fact, a recent RNAseq study of human embryonic aortic valve leaflets at different stages in development depicts a brief up-regulation in immune activity during mid-late gestation.51 Notably, genetic depletion of these endocardial-derived macrophages in mice resulted in increased numbers of apoptotic cells, suggestive of insufficient phagocytic clearance, in addition to thickened heart valves during adulthood.14 These findings highlight the importance of macrophages during cardiac valve development and suggest that they are important for the remodelling of valve leaflets with implications for long-term function and pathogenesis, notably with myxomatous ECM changes.
3.3 Macrophage lineages in postnatal heart valve remodelling and adult valve homeostasis.
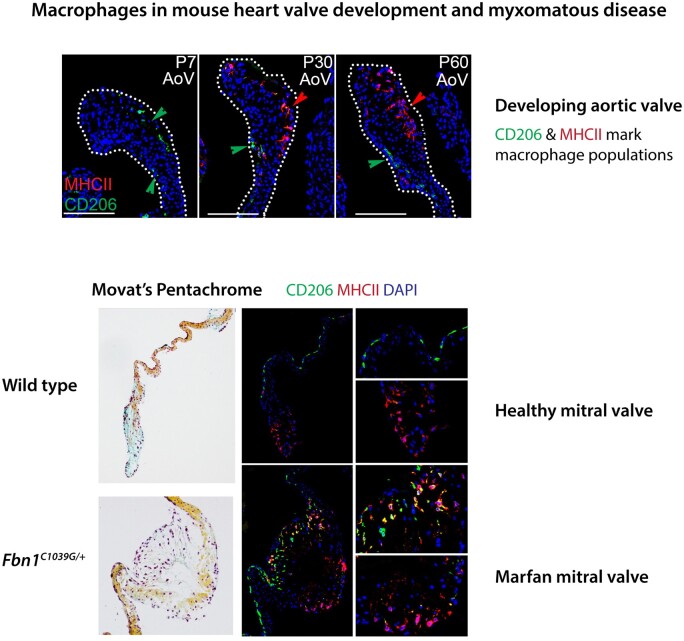
Heart valves undergo ECM remodelling into stratified layers after birth and do not fully mature until adulthood.40,49 Extensive characterization of the immune cell populations performed by our lab and others indicate that macrophages constitute the majority of the valvular immune cells during this period (Figure 3).10–12,14 These tissue-resident immune cells maintain expression of macrophage markers10 and do not convert to collagen or ECM-expressing VICs as originally proposed.9 As determined by fluorescence-activated cell sorting (FACS), macrophages present in neonatal heart valves express CD206+ consistent with embryonic origins and are the predominant immune cells in the valves until around P7.10 After P7, the numbers of MHCII+ macrophages begin to increase, similar to the transitions seen in the myocardium.10,24 Likewise, single-cell RNA sequencing (scRNAseq) analysis of normal valves at P7 and P30 shows that immune cell types in the developing valves not only include primarily macrophages, but also include mast cells, dendritic cells, and T cells.11 Approximately 8% of the total valve cells are immune cells at both P7 and P30. Two types of macrophages were detected and represent approximately 50% of immune cells at P7 and nearly 80% at P30. Notably, the per cent of CD206+ macrophages decreased from P7 to P30, consistent with results from FACS.10,11 Within the context of the valve, these macrophage populations exhibit distinct spatial location, with CD206+ macrophages predominantly under the endothelium at the region of coaptation, whereas MHCII+ macrophages are located at the distal tip of the aortic and mitral valve leaflets.10,11 This differential localization of macrophage subtypes is maintained into adulthood in mice.
Figure 3.
Macrophages in mouse heart development and MVD. (A–C) Localization of embryonic (CD206+) and postnatal (MHCII+) macrophage populations and localization in the developing mouse aortic valve change after birth. (Right panels) Increased numbers and altered localization of macrophages in myxomatous mitral valves of MFS mice (Fbn1C1039G/+) at 2 months-of-age.44
The increased numbers and localization of MHCII+ macrophages in the valve distal regions coincides with the time in which the primary site of haematopoiesis transitions from the foetal liver to the bone marrow, with a corresponding increase in CCR2+ monocyte-derived macrophages.52 This shift in macrophage origins is similar to that observed in the myocardium during the same time period when the developing bone marrow becomes the main source of monocytes.24 Genetic loss of CCR2 leads to reduced homing of monocyte-derived macrophages to the mature valves, with no major changes in valve leaflet stratification or morphology.44 However, ablation of phagocytic endocardially derived macrophages during development does lead to thickening and disorganization of aortic and mitral valves, implicating this subpopulation of macrophages in heart valve remodelling.14
In adult mice, bone marrow stem cell transplantation studies demonstrated continued investment of aortic valve leaflets with circulating CD45+ immune cells.8 Cell lineage analysis shows that the majority of these CD45+ cells express the CD11b monocyte marker and the numbers of these cells increase with age.12 Much less is known of macrophage populations in healthy human heart valves, but cells expressing CD64 or CD14 monocyte lineage markers in CD68+ macrophages are present in healthy human mitral valves, supporting a monocyte-derived population of tissue-resident macrophages in humans.10,44 Similarly, diverse populations of CD163+ and CD14+ monocyte lineage macrophages are present in adult porcine mitral valves.44 The functions of these tissue-resident macrophage populations in valve homeostasis have not been fully established, but they have been hypothesized to contribute to heart valve ECM remodelling, homeostasis, and repair.
4. Macrophage lineages in heart valve disease
4.1 Overview of myxomatous and calcific valve disease
Heart valve disease can develop before birth or be acquired later in life. Based on epidemiological studies, valve disease has a prevalence of 2.5% among 18–44-year-old individuals in the USA that increases to 13.5% in individuals over age 75, manifesting most commonly as aortic stenosis (AS) or mitral regurgitation (MR).4 Diseased valves generally conform to one of two broad pathological patterns: myxomatous degeneration or stenosis (Figure 2).42 Histologically, myxomatous degeneration, usually seen in the mitral valve, is characterized by diffuse accumulation of proteoglycan and fragmentation of collagen and elastin fibres, which can be caused by congenital malformation, cardiovascular dysfunction, or infectious endocarditis.5,53 These structural alterations result in a ‘floppy’ redundant valve that lacks the ability to seal properly and cause regurgitation. In contrast, aortic valve stenosis is characterized by collagen accumulation, loss of proteoglycan, and ultimately calcification.6 These fibrotic changes result in a sclerotic (‘stiff’) valve with a narrow valve orifice reducing cardiac output. Elucidating the molecular and cellular mechanisms underlying MVD and AS has been challenging because valves are not studied until symptomatic patients are examined by echocardiography, thereby representing advanced disease. Consequently, therapeutic options continue to be limited to surgical valve repair or replacement.5
4.2 Inflammatory mechanisms of CAVD
CAVD is prevalent in aged individuals and has risk factors similar to atherosclerosis and coronary artery disease.4,6 Molecular and cellular mechanisms of CAVD include induction of profibrotic and osteogenic regulatory pathways as well as proinflammatory signalling.54 Both innate and adaptive immune cells are present in calcified valves, and infiltration by T lymphocytes has been identified as a critical driver of CAVD.55,56 Macrophages also increase in CAVD, and increased numbers of CD11c+ myeloid cells, rather than CD206+ macrophages, have been associated with aortic valve calcification .57 In addition, CAVD is often associated with atherosclerosis and hypercholesteraemia that may affect the proinflammatory profile and pathologic macrophage populations.38 In contrast to MVD, T cells are increased in calcified valves and have been identified as critical inflammatory drivers of progressive CAVD.56 Thus, inflammatory mediators of CAVD progression are more dependent on adaptive immunity mechanisms, suggesting that anti-inflammatory therapeutic interventions will need to be targeted to specific immune cell-types dependent on the type of cardiovascular disease.
4.3 MVD origins and contributions of macrophages
MR is a major source of morbidity and mortality worldwide that can lead to arrhythmias, heart failure, and sudden death.58,59 Mitral valve regurgitation can be due to intrinsic structural deficiencies in the mitral valve leaflets (primary MR) or as a result of abnormal ventricular deformation and increased chamber size with advanced heart disease (secondary MR).5 Despite the two aetiologies, diseased valves appear histologically similar and commonly exhibit features of MVD, characterized by leaflet thickening, diffuse accumulation of proteoglycan, collagen fibre disruption, and elastin fragmentation. Proteolytic enzymes, such as matrix metalloproteinases [(MMP)-1, MMP-2, MMP-9, and MMP13] and cathepsin K, have also been detected during disease associated with pathologic remodelling.60–64 MVD can occur with congenital valve malformations or genetic aetiologies, including mutations in ECM genes, or alternatively can be acquired with autoimmune disease, inflammatory endocarditis, or secondarily with progressive heart failure after myocardial injury.65 Increased macrophage numbers in association with valve myxomatous ECM changes are common to MVD arising from various causes.
MVD with underlying genetic aetiologies often appears early in life and exhibits familial clustering.66,67 Syndromic MVD is commonly associated with genetic disorders of the connective tissue, including Marfan syndrome (MFS; Fibrillin-1),66 Ehlers-Danlos syndrome (Collagen types I, III, V, XI; Tenascin),68 Osteogenesis imperfecta (Collagen type I), and Larsen-like syndrome (Proteoglycan synthesis; B3GAT3).69 MVD is also present in Loeys–Dietz syndrome (Tgfβ receptors 1 and 2),70 Juvenile polyposis syndrome (Smad-4, BMP receptor 1a),71 and Aneurysm-osteoarthritis syndrome (Smad3).72 Together, these implicate matrix abnormalities and signalling as primary drivers of MVD. MVD can also appear in isolation (i.e. ‘non-syndromic’ MVD). The earliest confirmed non-syndromic mutation involved the X-linked FLNA (Filamin-A) gene, which causes valvular defects and progressive MVD in mice and humans.73–75 Mouse models of several of these mutations have been generated, and increased numbers of macrophages are present in diseased myxomatous valves,44,76 thus implicating macrophage infiltration as a potential secondary driver of MVD progression.
MVD in the absence of infective endocarditis has traditionally been regarded as a ‘non-inflammatory’ disease. In recent years, however, CD45+ haematopoietic cells have been detected in diseased human, sheep, and murine valves with myxomatous degeneration related to non-infective causes.13,53,76–78 Additional work characterizing these cells has demonstrated that they are predominantly macrophages, with smaller contributions from dendritic cells.10,11 A caveat for this interpretation is that CD45 expression can be induced by Tgfβ signalling in mitral VECs in MVD after myocardial infarction in sheep.78,79 However, increased numbers of CD206+ macrophages in regions of ECM remodelling and myeloid-lineage macrophages in association with increased immunogenic proteoglycans further supports roles for distinct populations of macrophages in myxomatous alterations in human valves.10,28,44 Despite the identification of these heterogeneous macrophage populations, little is known regarding their origins or functions in valve disease initiation or progression.
Using a combination of small/large animal models and clinical human valve specimens, we demonstrated that myxomatous valves across species exhibit an inflammatory micro-environment comprised of increased recruitment of pro-inflammatory macrophages and immunogenic ECM remodelling.44 The timing of macrophage infiltration is worth noting since these cells emerge in significant numbers after initial thickening is observed, but prior to MR. This might explain why there have historically been conflicting reports about the presence of immune cells in myxomatous valve specimens. More importantly, this suggests that macrophages play a role in the progression of MVD and that additional studies are needed to determine what initiates MVD. Nonetheless, the fact that versican and hyaluronan, ECM components known to induce sterile inflammation, are increased with no macrophage infiltration suggests that abnormal ECM remodelling in the valve contributes to the induction of an inflammatory response.80
Gene expression profiling of myxomatous valves from humans, dogs, and mice implicated inflammation and macrophage recruitment as an induced biological process with disease.13,44,81,82 In an Axin2-null model of MVD, expression of several chemokines and cytokines is induced, including C-C motif chemokine ligand 2 (CCL2), CCL3, and CCL7, prior to major myxomatous matrix changes.13 At the same time, increased numbers of macrophages are present in the valve interstitium. Tgfβ signalling also is increased in MVD from mouse, humans, and dogs and has been associated with ECM dysregulation and increased macrophage numbers in diseased valves.28,53,81,83,84 Thus, early stage MVD includes expression of inflammatory mediators and signalling pathways.
A mouse model of MFS generated with a targeted missense C1039G mutation in the Fibrillin1 locus (Fbn1C1039G/+) has been used to examine the origins and mechanisms of MVD. The initial valve thickening and malformations of mitral valves in these mice soon after birth are dependent on Tgfβ signalling.83 In addition, Wnt/β-catenin signalling is increased at an early stage of disease.13 Gene expression profiling of Fbn1C1039G/+ myxomatous vs. control valves implicated inflammation and immune cell recruitment as a prevalent process in MFS valve disease. Early in disease, CCL2 expression is induced in resident VICs in proximity to CCR2+ macrophages.44 In addition, immunogenic proteoglycan matrix expressing inter alpha trypsin inhibitor (IAI) is present in regions of the valves with increased numbers of CCR2+ macrophages.85 These macrophages do not express CD206, supporting a circulating monocyte origin. Similar expression of IAI and monocyte-derived macrophage markers were observed in human and porcine myxomatous mitral valves.10,44 Together, these data support a mechanism for myxomatous mitral valve disease whereby ECM alterations and induced expression of chemokines and cytokines by VICs leads to recruitment of CCR2+ macrophages at the early stages of disease.
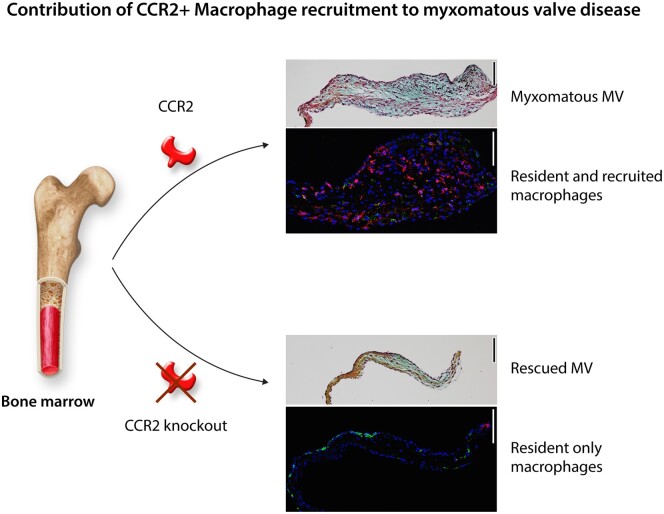
The specific contributions of infiltrating macrophages to the progression of MVD were examined in Fbn1C1039G/+ mice.44 Analysis of macrophage identities showed increased numbers of both CD206+ resident and CCR2+ recruited populations in valves at early stages of thickening, as well as ECM abnormalities including IAI induction. Notably, increased recruitment of T and B cells was not detected in myxomatous mitral valves. Bone marrow transplant studies confirmed the circulating monocyte origins of macrophages recruited into the valves. CCR2 knockout mice were bred with Fbn1C1039G/+ MFS mice to determine the contributions of the recruited macrophages to the progression of myxomatous disease. These studies confirmed that CCR2+ macrophages are present in diseased MFS valves and deletion of the CCR2 receptor prevents monocyte recruitment (Figure 4). Strikingly, valve leaflet thickening and ECM abnormalities including IAI induction were normalized with loss of CCR2. Thus, recruited CCR2+ macrophages contribute to valve thickening and myxomatous matrix changes as part of the pathogenesis of MVD in a mouse model of MFS.
Figure 4.
CCR2 depletion as a therapeutic approach for MVD. Increased recruited macrophages (CCR2+) are present in myxomatous mitral valves that express CCL2. Recruited macrophages are not present in CCR2-null mice and they have reduced myxomatous valve degeneration as adults.44
5. Clinical/translational perspectives: implications for treatment of valve disease
Effective therapy for heart valve disease remains a clinically unmet need due to a limited translation of molecular and cellular heart valve disease mechanisms discovered in animal models to human patient populations. The current standard of care in most cases is surgical repair or replacement of the diseased valve, although these surgeries are often not tolerated well, particularly in elderly patients.7,86 Moreover, surgical correction is often accompanied by long-term anti-coagulant therapy and re-operations due to limited durability and growth potential, especially for paediatric patients.87 As a result, development of new non-invasive therapies is imperative.
Over the past few decades, studies in animal models have implicated inflammation in the development of a wide variety of cardiovascular diseases, including atherosclerosis and myocardial infarction.88 As a result, these studies have uncovered promising new pathways and inflammatory mediators as therapeutic targets. Clinical translation of these studies to the bedside has been difficult, however, due to the complex and often overlapping roles that cellular and molecular mediators of inflammation play in tissue homeostasis and disease.89 For instance, steroids and methotrexate, while being immunosuppressive and subsequently tested on patients with myocardial infarction and rheumatoid arthritis, respectively, lack cellular specificity and interfere with protective functions of inflammation, such as wound healing and defense against infection.90 Alternative more targeted strategies directed towards specific cytokines, as shown in the landmark CANTOS clinical trial targeting a key pro-inflammatory chemokine, interleukin-1β, have provided significant therapeutic benefits by mitigating adverse cardiovascular outcomes.2 In light of this study, there has been a renewed interest in dissecting the cellular and molecular mediators of the immune system and their influence on a broad range of cardiovascular diseases.
Targeting myeloid cells has emerged as a potential therapeutic approach in cardiac fibrosis after injury and atherosclerotic vascular disease.38 Likewise, monocyte-derived CCR2+ macrophages were identified as a promising therapeutic target in MFS mice, since depletion of these recruited cells was protective against MVD progression.44 CCR2+ macrophages are uniquely pro-inflammatory and distinct from the non-inflammatory patrolling CCR2− macrophage subset, both of which are present in the injured heart and myxomatous valve.27,90,91 Moreover, a recent study demonstrated that localized injury to the myocardium leads to increased CCR2+ cells that are protective from additional injury and inhibit induction of a cardiac fibrotic response.92 Cell-specific targeting of CCR2+ macrophages is a promising therapeutic approach, as CCR2 inhibition would target detrimental macrophages while leaving protective macrophages alone, so that the latter can continue to maintain homeostasis, resolve inflammation, and/or promote healing. Appropriately, numerous investigations are ongoing in developing effective CCR2-specific inhibitors, several of which are currently in clinical trials for a wide range of diseases.93–95 Additional preclinical studies are still needed, however, to determine optimal dosage, administration, and long-term viability in treating cardiovascular disease.
Authors’ contributions
Writing and editing manuscript: A.J.K, N.X., and K.E.Y; figure drafts and table: N.X. and K.E.Y.
Acknowledgements
We thank Alexia Hulin for critical reading of the manuscript.
Conflict of interest: none declared.
Funding
Support for this work was provided by National Institutes of Health, National Heart, Lung and Blood Institute [R01 HL143881].
References
- 1.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ,, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC. The macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (part 4). J Am Coll Cardiol 2018;72:2213–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 3.Nasir K, Katz R, Takasu J, Shavelle DM, Detrano R, Lima JA, Blumenthal RS, O’Brien K, Budoff MJ. Ethnic differences between extra-coronary measures on cardiac computed tomography: multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2008;198:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iung B, Vahanian A. Epidemiology of valvular heart disease in the adult. Nat Rev Cardiol 2011;8:162–172. [DOI] [PubMed] [Google Scholar]
- 5.Levine RA, Hagege AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Merot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Puceat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH; Leducq Mitral Transatlantic Network. Mitral valve disease–morphology and mechanisms. Nat Rev Cardiol 2015;12:689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 8.Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res 2006;98:690–696. [DOI] [PubMed] [Google Scholar]
- 9.Hajdu Z, Romeo SJ, Fleming PA, Markwald RR, Visconti RP, Drake CJ. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol 2011;51:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulin A, Anstine LJ, Kim AJ, Potter SJ, DeFalco T, Lincoln J, Yutzey KE. Macrophage transitions in heart valve development and myxomatous valve disease. Arterioscler Thromb Vasc Biol 2018;38:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulin A, Hortells L, Gomez-Stallons MV, O’Donnell A, Chetal K, Adam M, Lancellotti P, Oury C, Potter SS,, Salomonis N, Yutzey KE. Maturation of heart valve cell populations during postnatal remodeling. Development 2019;146:pii:dev173047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anstine LJ, Horne TE, Horwitz EM, Lincoln J. Contribution of extra-cardiac cells in murine heart valves is age-dependent. J Am Heart Assoc 2017;6:pii:e007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hulin A, Moore V, James JM, Yutzey KE. Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease. Cardiovasc Res 2017;113:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigeta A, Huang V, Zuo J, Besada R, Nakashima Y, Lu Y, Ding Y, Pellegrini M, Kulkarni RP, Hsiai T, Deb A, Zhou B, Nakano H, Nakano A. Endocardially derived macrophages are essential for valvular remodeling. Dev Cell 2019;48:617–630.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavaillon JM. The historical milestones in the understanding of leukocyte biology initiated by Elie Metchnikoff. J Leukoc Biol 2011;90:413–424. [DOI] [PubMed] [Google Scholar]
- 16.Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity 2016;44:439–449. [DOI] [PubMed] [Google Scholar]
- 17.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 2013;14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams JW, Giannarelli C, Rahman A, Randolph GJ, Kovacic JC. Macrophage biology, classification, and phenotype in cardiovascular disease: JACC macrophage in CVD series (part 1). J Am Coll Cardiol 2018;72:2166–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res 2016;119:414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012;336:86–90. [DOI] [PubMed] [Google Scholar]
- 21.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014;41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 1996;86:897–906. [DOI] [PubMed] [Google Scholar]
- 23.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013;38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 2014;40:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development 2011;138:1017–1031. [DOI] [PubMed] [Google Scholar]
- 26.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res 2016;118:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 2014;115:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 2014;111:16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruithof BPT, Paardekooper L, Hiemstra YL, Goumans MJ, Palmen M, Delgado V, Klautz RJM, Ajmone Marsan N. Stress-induced remodelling of the mitral valve: a model for leaflet thickening and superimposed tissue formation in mitral valve disease. Cardiovasc Res 2020;116:931–943. [DOI] [PubMed] [Google Scholar]
- 30.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 2016;118:1498–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA,, Bassel-Duby R, Sadek HA, Olson EN. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124:1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wulfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 2017;169:510–522.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 2018;24:1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005;96:881–889. [DOI] [PubMed] [Google Scholar]
- 35.Bajpai G, Bredemeyer A,, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 2019;124:263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 2013;127:2038–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nahrendorf M. Myeloid cell contributions to cardiovascular health and disease. Nat Med 2018;24:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoen FJ. Mechanisms of function and disease of natural and replacement heart valves. Annu Rev Pathol 2012;7:161–183. [DOI] [PubMed] [Google Scholar]
- 40.Hinton RB Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 2006;98:1431–1438. [DOI] [PubMed] [Google Scholar]
- 41.Mahler GJ, Frendl CM, Cao Q, Butcher JT. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol Bioeng 2014;111:2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol 2011;73:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi JH, Do Y, Cheong C, Koh H, Boscardin SB, Oh YS,, Bozzacco L, Trumpfheller C, Park CG, Steinman RM. Identification of antigen-presenting dendritic cells in mouse aorta and cardiac valves. J Exp Med 2009;206:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim AJ, Xu N, Umeyama K, Hulin A, Ponny SR, Vagnozzi RJ, Green EA, Hanson P, McManus BM, Nagashima H, Yutzey KE. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation 2020;141:132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res 2009;105:408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res 2004;95:645–654. [DOI] [PubMed] [Google Scholar]
- 47.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn 2004;230:239–250. [DOI] [PubMed] [Google Scholar]
- 48.Nakano H, Liu X, Arshi A, Nakashima Y, van Handel B, Sasidharan R, Harmon AW, Shin JH, Schwartz RJ, Conway SJ,, Harvey RP, Pashmforoush M, Mikkola HK, Nakano A. Haemogenic endocardium contributes to transient definitive haematopoiesis. Nat Commun 2013;4:1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 2006;113:1344–1352. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Wu B, Farrar E, Lui W, Lu P, Zhang D, Alfieri CM, Mao K, Chu M, Yang D, Xu D, Rauchman M, Taylor V, Conway SJ, Yutzey KE, Butcher JT, Zhou B. Notch-Tnf signalling is required for development and homeostasis of arterial valves. Eur Heart J 2017;38:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gottlieb Sen D, Halu A, Razzaque A, Gorham JM, Hartnett J, Seidman JG, Aikawa E, Seidman CE. The transcriptional signature of growth in human fetal aortic valve development. Ann Thorac Surg 2018;106:1834–1840. [DOI] [PubMed] [Google Scholar]
- 52.Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, Rodewald HR, Rosenthal NA, Bajenoff M, Prinz M, Jung S, Sieweke MH. Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 2014;211:2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation 2012;126:S189–S–197.. [DOI] [PubMed] [Google Scholar]
- 54.Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, Hofmann-Bowman MA, Mortlock DP, Rogers MB, Sadeghi MM, Aikawa E. Calcific aortic valve disease: a consensus summary from the Alliance of Investigators on Calcific Aortic Valve Disease. Arterioscler Thromb Vasc Biol 2014;34:2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guauque-Olarte S, Droit A, Tremblay-Marchand J,, Gaudreault N, Kalavrouziotis D, Dagenais F, Seidman JG, Body SC, Pibarot P, Mathieu P, Bosse Y. RNA expression profile of calcified bicuspid, tricuspid, and normal human aortic valves by RNA sequencing. Physiol Genomics 2016;48:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raddatz MA, Madhur MS, Merryman WD. Adaptive immune cells in calcific aortic valve disease. Am J Physiol Heart Circ Physiol 2019;317:H141–H155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li G, Qiao W, Zhang W, Li F, Shi J, Dong N. The shift of macrophages toward M1 phenotype promotes aortic valvular calcification. J Thorac Cardiovasc Surg 2017;153:1318–1327.e1. [DOI] [PubMed] [Google Scholar]
- 58.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 59.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med 2005;352:875–883. [DOI] [PubMed] [Google Scholar]
- 60.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001;104:2525–2532. [DOI] [PubMed] [Google Scholar]
- 61.Gupta V, Barzilla JE, Mendez JS, Stephens EH, Lee EL, Collard CD, Laucirica R, Weigel PH, Grande-Allen KJ. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol 2009;18:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akhtar S, Meek KM, James V. Ultrastructure abnormalities in proteoglycans, collagen fibrils, and elastic fibers in normal and myxomatous mitral valve chordae tendineae. Cardiovasc Pathol 1999;8:191–201. [DOI] [PubMed] [Google Scholar]
- 63.Rabkin-Aikawa E, Farber M, Aikawa M, Schoen FJ. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis 2004;13:841–847. [PubMed] [Google Scholar]
- 64.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol 2003;42:271–277. [DOI] [PubMed] [Google Scholar]
- 65.Meier LA, Auger JL, Engelson BJ, Cowan HM, Breed ER, Gonzalez-Torres MI, Boyer JD, Verma M, Marath A, Binstadt BA. CD301b/MGL2(+) mononuclear phagocytes orchestrate autoimmune cardiac valve inflammation and fibrosis. Circulation 2018;137:2478–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Judge DP, Rouf R, Habashi J, Dietz HC. Mitral valve disease in Marfan syndrome and related disorders. J Cardiovasc Transl Res 2011;4:741–747. [DOI] [PubMed] [Google Scholar]
- 67.Le Tourneau T, Merot J, Rimbert A, Le Scouarnec S, Probst V, Le Marec H, Levine RA, Schott JJ. Genetics of syndromic and non-syndromic mitral valve prolapse. Heart 2018;104:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Atzinger CL, Meyer RA, Khoury PR, Gao Z, Tinkle BT. Cross-sectional and longitudinal assessment of aortic root dilation and valvular anomalies in hypermobile and classic Ehlers-Danlos syndrome. J Pediatr 2011;158:826–830.e1. [DOI] [PubMed] [Google Scholar]
- 69.Baasanjav S,, Al-Gazali L, Hashiguchi T, Mizumoto S, Fischer B, Horn D, Seelow D, Ali BR, Aziz SA, Langer R, Saleh AA, Becker C, Nurnberg G, Cantagrel V, Gleeson JG, Gomez D, Michel JB, Stricker S, Lindner TH, Nurnberg P, Sugahara K, Mundlos S, Hoffmann K. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet 2011;89:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–281. [DOI] [PubMed] [Google Scholar]
- 71.Andrabi S, Bekheirnia MR, Robbins-Furman P, Lewis RA, Prior TW, Potocki L. SMAD4 mutation segregating in a family with juvenile polyposis, aortopathy, and mitral valve dysfunction. Am J Med Genet A 2011;155A:1165–1169. [DOI] [PubMed] [Google Scholar]
- 72.van der Linde D, van de Laar IM, Bertoli-Avella AM, Oldenburg RA, Bekkers JA,, Mattace-Raso FU, van den Meiracker AH, Moelker A, van Kooten F, Frohn-Mulder IM, Timmermans J,, Moltzer E, Cobben JM, van Laer L, Loeys B, De Backer J, Coucke PJ, De Paepe A,, Hilhorst-Hofstee Y, Wessels MW, Roos-Hesselink JW. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol 2012;60:397–403. [DOI] [PubMed] [Google Scholar]
- 73.Sauls K, de Vlaming A, Harris BS, Williams K, Wessels A, Levine RA, Slaugenhaupt SA, Goodwin RL, Pavone LM, Merot J, Schott JJ, Le Tourneau T, Dix T, Jesinkey S, Feng Y, Walsh C, Zhou B, Baldwin S, Markwald RR, Norris RA. Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc Res 2012;96:109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lardeux A, Kyndt F, Lecointe S, Marec HL, Merot J, Schott JJ, Le Tourneau T, Probst V. Filamin-a-related myxomatous mitral valve dystrophy: genetic, echocardiographic and functional aspects. J Cardiovasc Transl Res 2011;4:748–756. [DOI] [PubMed] [Google Scholar]
- 75.Le Tourneau T, Le Scouarnec S, Cueff C, Bernstein D, Aalberts JJJ, Lecointe S, Merot J, Bernstein JA, Oomen T, Dina C, Karakachoff M, Desal H, Al Habash O, Delling FN, Capoulade R, Suurmeijer AJH, Milan D, Norris RA, Markwald R, Aikawa E, Slaugenhaupt SA, Jeunemaitre X, Hagege A, Roussel JC, Trochu JN, Levine RA, Kyndt F, Probst V, Le Marec H, Schott JJ. New insights into mitral valve dystrophy: a Filamin-A genotype-phenotype and outcome study. Eur Heart J 2018;39:1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sauls K, Toomer K, Williams K, Johnson AJ, Markwald RR, Hajdu Z, Norris RA. Increased infiltration of extra-cardiac cells in myxomatous valve disease. J Cardiovasc Dev Dis 2015;2:200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim AJ, Alfieri CM, Yutzey KE. Endothelial cell lineage analysis does not provide evidence for EMT in adult valve homeostasis and disease. Anat Rec (Hoboken )2019;302:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartko PE, Dal-Bianco JP, Guerrero JL, Beaudoin J,, Szymanski C, Kim DH, Seybolt MM, Handschumacher MD, Sullivan S, Garcia ML, Titus JS, Wylie-Sears J, Irvin WS, Messas E, Hagege AA, Carpentier A,, Aikawa E, Bischoff J,, Levine RA; Leducq Transatlantic Mitral Network. Effect of losartan on mitral valve changes after myocardial infarction. J Am Coll Cardiol 2017;70:1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bischoff J, Casanovas G, Wylie-Sears J, Kim DH, Bartko PE, Guerrero JL, Dal-Bianco JP, Beaudoin J, Garcia ML, Sullivan SM, Seybolt MM,, Morris BA, Keegan J, Irvin WS, Aikawa E, Levine RA. CD45 expression in mitral valve endothelial cells after myocardial infarction. Circ Res 2016;119:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol 2014;35:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lu CC, Liu MM, Culshaw G, Clinton M, Argyle DJ, Corcoran BM. Gene network and canonical pathway analysis in canine myxomatous mitral valve disease: a microarray study. Vet J 2015;204:23–31. [DOI] [PubMed] [Google Scholar]
- 82.Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, Miller JD. Nonbiased molecular screening identifies novel molecular regulators of fibrogenic and proliferative signaling in myxomatous mitral valve disease. Circ Cardiovasc Genet 2015;8:516–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 2004;114:1586–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne JO, Nusgens BV, Radermecker MA, Colige AC. Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res 2012;93:480–489. [DOI] [PubMed] [Google Scholar]
- 85.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lincoln J, Garg V. Etiology of valvular heart disease-genetic and developmental origins. Circ J 2014;78:1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc 2010;85:483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen H, Kreisel D, Goldstein DR. Processes of sterile inflammation. J Immunol 2013;191:2857–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noels H, Weber C, Koenen RR. Chemokines as therapeutic targets in cardiovascular disease. Arterioscler Thromb Vasc Biol 2019;39:583–592. [DOI] [PubMed] [Google Scholar]
- 90.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 2011;29:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010;121:2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vagnozzi RJ, Maillet M, Sargent MA,, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 2020;577:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Struthers M, Pasternak A. CCR2 antagonists. Curr Top Med Chem 2010;10:1278–1298. [DOI] [PubMed] [Google Scholar]
- 94.Zheng Y, Qin L, Zacarias NV, de Vries H, Han GW, Gustavsson M, Dabros M, Zhao C, Cherney RJ, Carter P, Stamos D, Abagyan R,, Cherezov V, Stevens RC, IJ AP, Heitman LH, Tebben A, Kufareva I, Handel TM. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature 2016;540:458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor BC, Lee CT, Amaro RE. Structural basis for ligand modulation of the CCR2 conformational landscape. Proc Natl Acad Sci USA 2019;116:8131–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]