Abstract
Study Objectives
Recent findings indicate that noradrenergic and antimuscarinic processes are crucial for sleep-related reductions in pharyngeal muscle activity. However, there are few human studies. Accordingly, this study aimed to determine if a combined noradrenergic and antimuscarinic intervention increases pharyngeal dilator muscle activity and improves airway function in sleeping humans.
Methods
Genioglossus (GG) and tensor palatini electromyography (EMG), pharyngeal pressure, upper airway resistance, and breathing parameters were acquired in 10 healthy adults (5 female) during two overnight sleep studies after 4 mg of reboxetine (REB) plus 20 mg of hyoscine butylbromide (HBB) or placebo using a double-blind, placebo-controlled, randomized, cross-over design.
Results
Compared with placebo, peak and tonic GG EMG were lower (Mean ± SD: 83 ± 73 vs. 130 ± 75, p = 0.021 and 102 ± 102 vs. 147 ± 123 % wakefulness, p = 0.021, respectively) but the sleep-related reduction in tensor palatini was less (Median [25th, 75th centiles]: 53[45, 62] vs. 34[28, 38] % wakefulness, p = 0.008) with the drug combination during nonrapid eye movement (non-REM) sleep. These changes were accompanied by improved upper airway function including reduced pharyngeal pressure swings, airway resistance, respiratory load compensation, and increased breathing frequency during N2. REB and HBB significantly reduced rapid eye movement sleep compared with placebo (0.6 ± 1.1 vs. 14.5 ± 6.8 % total sleep time, p < 0.001).
Conclusions
Contrary to our hypothesis, GG muscle activity (% wakefulness) during non-REM sleep was lower with REB and HBB. However, sleep-related reductions in tensor palatini activity were less and upper airway function improved. These findings provide mechanistic insight into the role of noradrenergic and antimuscarinic processes on upper airway function in humans and have therapeutic potential for obstructive sleep apnea.
Clinical Trial Registration
Australian New Zealand Clinical Trials Registry, https://www.anzctr.org.au, trial ID: ACTRN12616000469415.
Keywords: obstructive sleep apnea, norepinephrine reuptake inhibitor, muscarinic antagonists, genioglossus, tensor palatini, upper airway physiology, sleep-disordered breathing
Statement of Significance.
Treatment options for obstructive sleep apnea (OSA) are often inadequate. There are currently no approved pharmacotherapies. At least 30% of OSA patients have minimal upper-airway dilator muscle activation during airway narrowing. These patients may benefit from a targeted pharmacological intervention to increase upper-airway muscle activity during sleep. This study suggests that a combined noradrenergic and antimuscarinic intervention improves multiple aspects of upper-airway function during non-REM sleep in healthy individuals. This drug combination also alters sleep architecture in a way that may be beneficial for individuals with REM sleep dominant OSA although potential undesirable effects on REM-dependent processes (e.g. memory and learning) require consideration. These novel mechanistic findings warrant further investigation in an OSA population to determine their potential therapeutic efficacy.
Introduction
OSA is characterized by repetitive narrowing and occlusion of the pharyngeal airway during sleep. These breathing disturbances lead to intermittent blood gas disturbances (hypoxemia and hypercapnia). Changes in chemical drive stimulate chemoreflexes to increase respiratory effort against the narrowed airway which often elicits arousal from sleep causing sleep fragmentation [1]. If left untreated, OSA increases the risk of multiple adverse consequences, including daytime sleepiness, depression [2], cardiovascular disease and stroke [3], neurocognitive impairment [4] and motor vehicle accidents, and workplace injuries [5]. Continuous positive airway pressure (CPAP) is currently the most efficacious therapy for severe OSA. It resolves the vast majority of breathing disturbances and can reduce symptoms of OSA including daytime sleepiness, neurocognitive impairment, and other quality of life traits [6]. However, CPAP is poorly tolerated, with ~50% of OSA patients being nonadherent to the therapy [7]. Given the burden of the disease and health and safety consequences of untreated OSA, new treatments are required.
The causes of OSA are multifactorial [8, 9]. However, the interaction between pharyngeal anatomy and sleep-related changes in upper airway muscle control are fundamental to OSA pathogenesis [9, 10]. Indeed, electrical stimulation of the hypoglossal nerve to increase the activity of the largest pharyngeal dilator muscle, genioglossus (GG), reduces OSA severity [11, 12]. However, there are currently no approved pharmacotherapies to increase upper airway muscle activity to treat OSA.
Nonetheless, promising animal studies have identified key mechanisms that contribute to GG muscle activity during wakefulness and sleep [13–15]. Withdrawal of noradrenergic drive has been identified as a major contributor to GG muscle activity during nonrapid eye movement (non-REM) sleep in rats [13]. Muscarinic receptor blockade restores GG muscle tone during rapid eye movement (REM)-like sleep in rats [14]. Recent translation of these findings to humans has shown that 200 mg of the tricyclic antidepressant desipramine which has strong noradrenergic and mild antimuscarinic effects, restored tonic GG muscle activity to near wakefulness levels in healthy humans [16]. Desipramine also reduced upper airway collapsibility (critical closing pressure of the upper airway [Pcrit]) and reduced OSA severity in patients with minimal muscle responsiveness [17].
Desipramine however, has only minor affinity for muscarinic receptors and less specific selectivity for norepinephrine reuptake sites compared with agents like reboxetine (REB) [18]. Thus, further investigation into whether a more selective combination of noradrenergic and antimuscarinic agents yields greater increases in upper airway dilator muscle activity during sleep is warranted. Therefore, the primary aim of this detailed physiological study was to determine whether a combination of REB (noradrenergic agent) and hyoscine butylbromide (HBB; antimuscarinic agent) prevents sleep-related reductions in GG muscle activity. Secondary aims were to determine whether REB and HBB increases tensor palatini muscle activity, and their effects on sleep and respiratory parameters. We hypothesized that compared with placebo, a combined dose of 4 mg REB and 20 mg HBB would increase GG muscle activity during non-REM and REM and restore muscle activity during sleep to near wakefulness levels.
Methods
Participants
Twelve healthy participants were recruited from NeuRA’s sleep and breathing laboratory healthy volunteers database or via poster advertisements placed on notice boards within the local medical and university precincts. Participants completed a health screen questionnaire over the phone before participation. Potential participants were excluded if they were known to suffer from OSA (apnea/hypopnea index [AHI] > 10 events h−1 sleep). Other key exclusion criteria included lactating or pregnant women, people with respiratory or cardiovascular disease, diabetes, neurological disorders, or anyone taking a medication known to affect sleep or breathing. These medications include: sleep or wakefulness promoters, psychoactive drugs (antidepressant, antiepileptic, antipsychotic, or anxiolytic), certain cardiovascular drugs (beta-blockers and alpha-2 agonists), antihistamines and pain medications including opioids. The study was approved by the South Eastern Sydney Local Health District Human Research Ethics Committee (15/234 HREC/15/POWH/449). The protocol was prospectively registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12616000469415).
Protocol
Participants were studied on two different nights, separated by a 1-week washout according to a double-blind, randomized, placebo-controlled, cross-over design (Figure 1). An independent party blinded to the study conditions performed the randomization. Initially, participants arrived at the laboratory at ~7:30 pm, provided informed written consent and completed the Epworth Sleepiness Scale (ESS) questionnaire. Key demographic and anthropometric variables were also obtained.
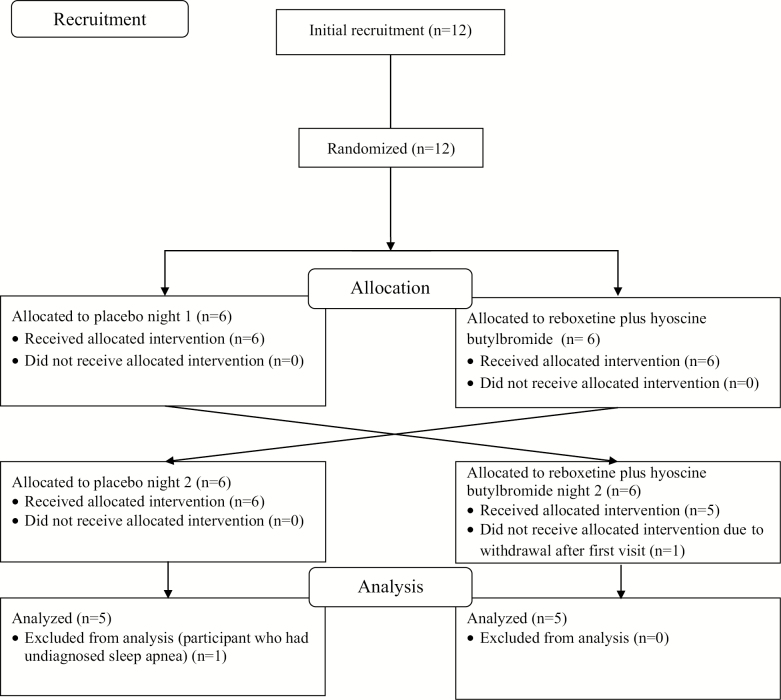
Figure 1.
CONSORT diagram highlighting the enrollment and participant flow including exclusions through the protocol and analysis steps for this double-blind, randomized, placebo-controlled, cross-over study. Refer to the text for further detail.
Following instrumentation (see below) and maneuvers such as swallows and tongue protrusions to quantify maximal pharyngeal muscle activity, at least 3 min of quiet breathing during wakefulness was recorded. Participants then received 4 mg of REB plus 20 mg of HBB or a placebo administered orally immediately before lights out. Both the active and placebo arms (2 sugar pills) were prepared in a single, water-soluble capsule of identical appearance to ensure participant and investigator blinding. During the following visit, participants received the alternate allocation. Participants were instructed to breathe through their nose and to sleep supine as much as possible. Ceiling mounted infrared cameras were used to confirm body position and periods of mouth breathing. If participants slept on their side for a prolonged duration, they were quietly instructed to return to sleeping on their back.
Participants were given an 8-h opportunity to sleep. Thirty minutes after awakening in the morning, participants completed the Karolinska Sleepiness Scale (KSS) to assess subjective sleepiness.
Equipment and measurements
Upper airway muscle activity
Electromyography (EMG) activity was measured using fine-wire intramuscular electrodes. After application of surface anesthesia (1% lignocaine), two Teflon coated fine-wire electrodes (2 mm of Teflon coating removed at tip of the wire) were inserted through a 25-gauge needle into the GG muscle orally 3–4 mm to either side of the frenulum at a depth of 1.5 cm to obtain a bipolar multiunit recording as described previously [19]. Similarly, to measure tensor palatini muscle activity, two Teflon coated fine-wire electrodes were inserted via 23-gauge needle into the palate at a 45° angle along the lateral surface of the pterygoid plate according to methodology described previously [19].
Respiratory parameters
Epiglottic pressure was determined using a polyurethane pressure-tipped catheter (Mikro-tip pressure transducer, model MPR-500; Millar). The catheter was advanced via the nostril and descended 1–2 cm caudal to the base of the tongue to monitor breathing effort (epiglottic pressure swings) and allow for calculation of upper airway resistance [19]. Nasal decongestant (oxymetazoline HCl) and local anesthetic (4% lignocaine) were applied 5 min before catheter insertion to minimize discomfort during placement.
Participants were fitted with a sealed nasal mask (Gel Mask; Philips Respironics, Murrysville, PA) attached to a pneumotachograph (model 3700A; Hans Rudolph, Inc., Kansas City, KS), carbon dioxide transducer (model 17630; VacuMed, Ventura, CA) and mask pressure transducer (Validyne, Northridge, CA) to measure airflow, mask pressure (Pmask), tidal volume, and end-tidal carbon dioxide. Pmask was referenced to atmosphere. Latex tape was placed over the participant’s mouths if mouth breathing occurred.
Polysomnography
Overnight in-laboratory sleep studies (polysomnography) were performed according to American Academy of Sleep Medicine (AASM) guidelines. This included electroencephalograms (EEG: F3, F4, C3, C4, O1, and O2 referenced to A1–A2) using surface gold cup electrodes (Grass Technologies) according to the International 10–20 system for electrode placement and left and right electrooculograms to allow for sleep staging and arousal detection. Participants were also fitted with finger pulse oximetry.
Data analysis
CED 1902 amplifiers were used to record upper airway EMG activity at a sampling rate of 1,000 Hz. EEG and EOG signals were recorded using a Grass amplifier system at a sampling rate of 250Hz (Model 15LT Bipolar, Natus Neurology, Warwick, RI). All remaining signals were sampled at 125 Hz. Data from all recording devices were acquired using a CED 1401 analog-to-digital converter and Spike2 software (Cambridge Electronic Design, Cambridge, UK) (see Figure 2 for raw example). Peak GG muscle activity was defined as the maximal value obtained from the rectified moving time average (MTA) signal (100 ms) during each inspiratory effort. Tonic GG activity was defined as the nadir value obtained from the rectified MTA signal (100 ms) during the expiratory phase. Unlike the GG, tensor palatini typically displays a constant level of activation during quiet breathing (tonic muscle). Thus, only the tonic value was reported for this muscle in these healthy individuals. Wakefulness EMG data were quantified as a percentage of maximal activation. Sleep EMG data are expressed as a percentage of the wakefulness level and also reported as a percentage of maximum activation (via swallow or tongue protrusion) and in microvolts. Sleep staging and arousal and respiratory event scoring were performed by a board registered sleep technician blinded to the study allocations. Sleep stages, respiratory events, and arousals (brief awakenings as detected in the EEG signals) were scored according to standard criteria [20]. Similar to previous OSA research [8], absence of OSA was defined as <10 events h−1 sleep.
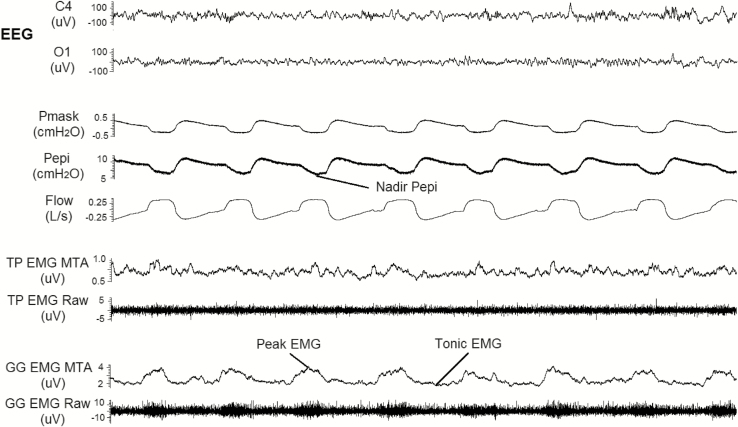
Figure 2.
An example of a 30-s epoch during one of the sleep studies on a placebo night (20 years old, female, apnea hypopnea index = 0.3 events h−1). Signals include: electroenecephalography (EEG recorded at O1 and C4), respiratory paramaters and pharyngeal muscle activity during non-REM sleep. Nadir epiglottic pressure (Pepi), peak, and tonic genioglossus (GG) muscle activity are highlighted. Pmask, mask pressure; TP, tensor palatini; GG, genioglossus; MTA, 100 ms moving time average of the rectified raw electromography (EMG) signal.
Similar to recently described methodology [21], a custom script was used to extract respiratory data, arousal timing and muscle activity on a breath-by-breath basis for the entire 8-h recording period. Breaths were manually checked and were included in the final analysis if they were recorded in the supine position and free of electrical interference (i.e. noise) and movement artifacts (i.e. swallows and coughs). Breaths were excluded during transient GG muscle activation that occurred after cortical arousal from sleep (for up to 10 s). Upper airway resistance was calculated on a breath by breath basis as the difference between mask and epiglottic pressures at a flow rate of 200 mL s−1.
Statistical analyses
Statistical analyses were performed using SPSS (IBM, version 24) and SigmaPlot (IBM version 12). Two-way repeated measures analysis of variance (ANOVA) were used to compare key PSG variables between conditions (drug combination and placebo) and sleep stages (N1, N2, N3, and REM). Two-tailed, paired Student’s t-tests or Wilcoxon signed rank tests were used for direct comparisons between placebo and REB plus HBB conditions as appropriate for all other variables. Data are expressed as mean ± standard deviation (SD) unless stated otherwise. Statistical significance was inferred at p < 0.05.
Results
Participants
Twelve participants were enrolled in the study. One participant experienced discomfort with the laboratory equipment and subsequently withdrew from the study after the first study night. Eleven participants successfully completed the two overnight studies. One participant was excluded from analysis due to the confounding effects of undiagnosed OSA on the key outcomes variables (baseline AHI on placebo was 34.7 vs. 18.5 events h−1 sleep on REB plus HBB). Participants did not take any medications for the duration of the study with the exception of one individual who was taking pantoprazole (20 mg) for gastro-esophageal reflux disease and loperamide hyperchloride (2 mg) for inflammatory bowel symptoms. Data from a total of 10 healthy participants were analyzed. Their anthropometric characteristics are outlined in Table 1.
Table 1.
Participant characteristics
| Sex | 5 male, 5 female |
| Age (years) | 31 ± 14 |
| Body mass index (kg m−2) | 24 ± 3 |
| Epworth sleepiness scale (0–24 point scale) | 7 ± 1 |
The apnea/hypopnea index was calculated from the placebo night. Values are mean ± SD.
Effect of REB and HBB on sleep architecture
There was a significant condition (drug combination vs. placebo) by sleep-stage distribution (N1, N2, N3, and REM % total sleep time) interaction effect (p = 0.004). REM sleep was significantly reduced with REB plus HBB compared to placebo (p < 0.001, Table 2). Of the 10 participants studied, REM sleep was abolished in seven with REB plus HBB. Conversely, the proportion of N2 sleep increased during the REB plus HBB night compared with placebo (p = 0.042, Table 2), while there was no systematic change in the proportion of N1 or slow wave sleep. REB plus HBB did not alter sleep efficiency (p = 0.334, Table 2). Respiratory events were rare in these healthy individuals and their frequency and effect on nadir oxygen saturation were not different between conditions (p = 0.830 and p = 0.408, respectively, Table 2). Similarly, the AHI during non-REM (p = 0.925) and REM sleep (p = 0.454) were not different between conditions. Morning alertness scores as measured by the KSS (p = 0.226) and the arousal index did not significantly differ between the two conditions (p = 0.338, Table 2).
Table 2.
Polysomnography parameters
| Placebo | REB + HBB | |
|---|---|---|
| Karolinska sleepiness scale | 5 ± 2 | 5 ± 2 |
| AHI (no. of events h−1 sleep) | 3 ± 4 | 3 ± 4 |
| Sleep efficiency (%) | 71 ± 15 | 75 ± 13 |
| N1 (% total sleep time) | 16 ± 8 | 21 ± 12 |
| N2 (% total sleep time) | 56 ± 10 | 65 ± 8* |
| Slow wave sleep (% total sleep time) | 13 ± 7 | 13 ± 9 |
| REM sleep (% total sleep time) | 15 ± 7 | 1 ± 1* |
| Arousal index (no. of arousals h−1 sleep) | 23 ± 10 | 26 ± 15 |
| Arousal duration (s) | 13 ± 3 | 14 ± 2 |
| Nadir SpO2 during sleep (%) | 92 ± 2 | 91 ± 3 |
Polysomnography parameters (n = 10) except for rapid eye movement (REM) sleep (n = 3). AHI, apnea/hypopnea index, SpO2, arterial oxygen saturation estimated via pulse oximetry, REB, reboxetine and HBB, hyoscine butylbromide. Values are mean ± SD.
*Significant difference compared with placebo.
Effect of REB plus HBB on upper airway muscle activity during sleep
Genioglossus
During the placebo night, a single motor unit predominated the GG EMG signal in one participant. Thus, multiunit GG EMG data during both conditions were available for analysis in n = 9 individuals during non-REM sleep.
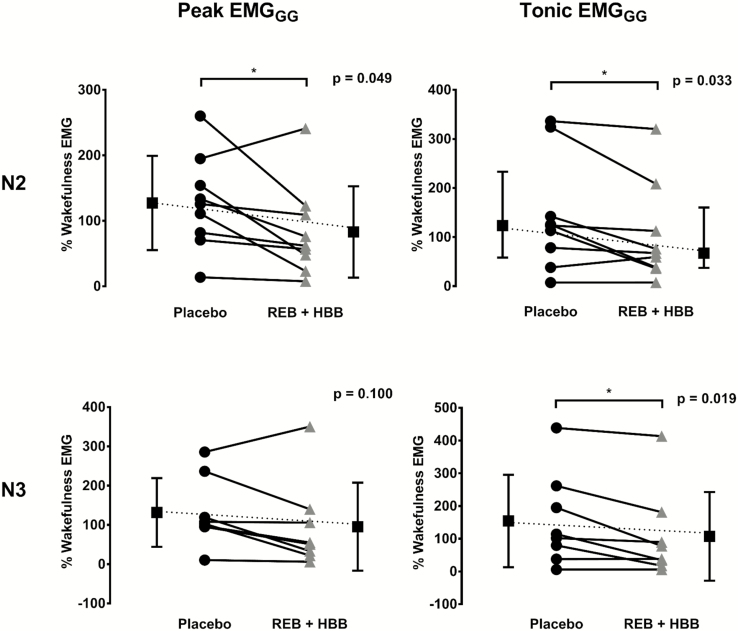
Before receiving the drug combination or placebo, peak and tonic GG muscle activity (% of maximum) awake were similar between study visits (4.3% ± 3.0% vs. 4.0% ± 2.0% max, p = 0.738 and 2.1% ± 1.6% vs. 1.7% ± 0.9% max, p = 0.401, respectively). GG EMG activity tended to increase during sleep from the wakefulness level during placebo whereas the opposite occurred on the REB plus HBB night (Figure 3). Indeed, following REB plus HBB, peak GG muscle activity as a percentage of the wakefulness EMG level was lower during N2 but not N3 sleep versus placebo (Figure 3). Tonic GG muscle activity as a percentage of the wakefulness EMG was lower in both N2 and N3 sleep following reboxetine plus HBB versus placebo (Figure 3). These findings were similar when expressed as percentage of maximal activity and in microvolts (e.g. peak GG EMG during reboxetine plus HBB in non-REM sleep compared with placebo was 3.0% ± 2.4% vs. 4.6% ± 2.9% max, p = 0.091 and 16.5 ± 17.5 vs. 22.9 ± 20.0 µV, p = 0.047, respectively).
Figure 3.
Peak and tonic genioglossus muscle activity expressed as a percentage of wakefulness genioglossus EMG during N2 and N3 sleep between conditions. REB, reboxetine; HBB, hyoscine butylbromide.
Tensor palatini
Tensor palatini EMG was successfully obtained in 8 of the 10 participants on both nights. In one participant, signal quality was poor and EMG activity could not be quantified. In another participant, the recording electrode was dislodged after a cough early during the wakefulness-recording period and was not replaced.
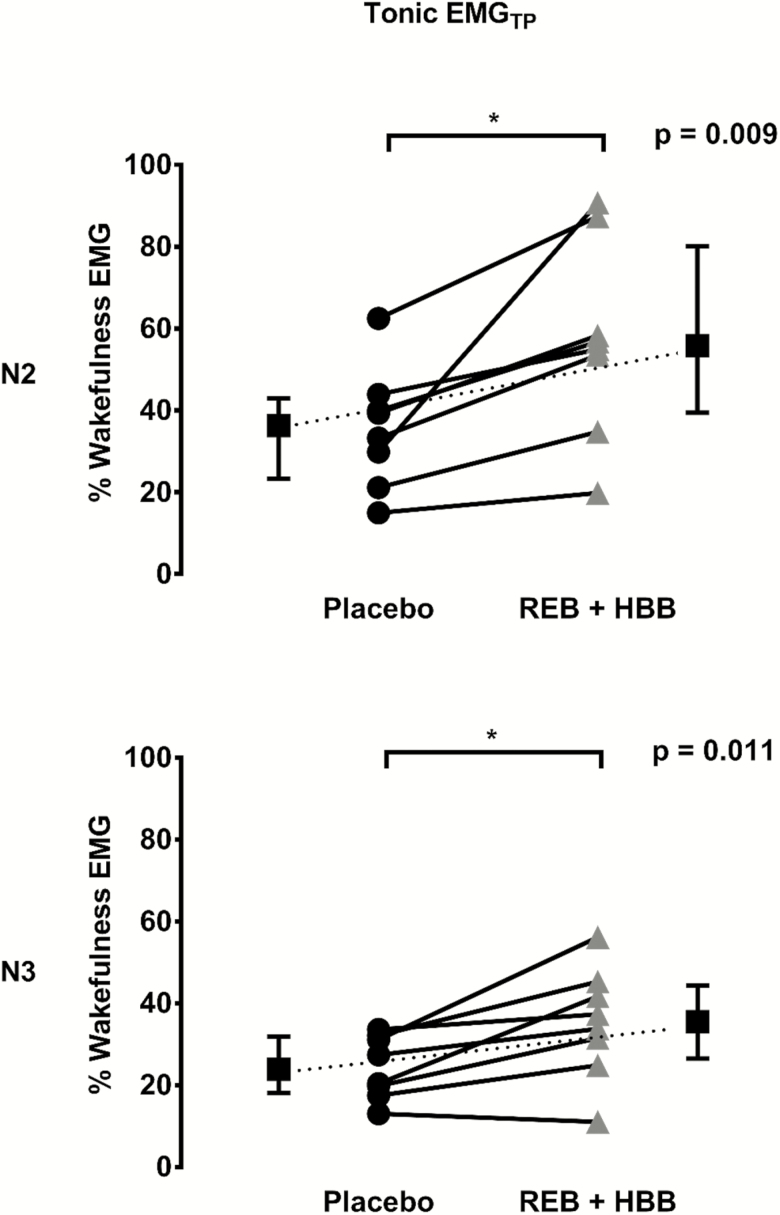
Before receiving the study intervention or placebo, tonic tensor palatini muscle activity (% of maximum) awake was similar between study visits (2.3 ± 1.3 vs. 2.1 ± 0.7% max, p = 0.665). Tensor palatini EMG decreased during sleep compared with the wakefulness level on both nights although to a greater extent during the placebo night (Figure 4). Indeed, tensor palatini EMG as a percentage of wakefulness EMG was higher in the REB plus HBB condition compared with placebo during N2 and N3 sleep (Figure 4). These findings were similar when expressed as a percentage of maximal activity. For example, tonic tensor palatini EMG during REB plus HBB in non-REM sleep compared with placebo was (1.2% ± 0.7% vs. 0.7% ± 0.3%; p = 0.057). When expressed as microvolts, tonic tensor palatini EMG was higher with REB plus HBB compared to placebo during non-REM sleep (1.7 ± 0.7 vs. 1.3 ± 0.8 µV; p = 0.028).
Figure 4.
Tensor palatini muscle activity as a percentage of wakefulness EMG during N2 and N3 sleep between conditions. REB, reboxetine; HBB, hyoscine butylbromide.
Effect of REB and HBB on respiratory parameters during sleep
Nadir epiglottic pressure swings were smaller and upper airway resistance was lower during N2 sleep on the REB plus HBB night compared with placebo (Table 3).
Table 3.
Changes in epiglottic pressure swings and upper airway resistance during non-REM sleep with reboxitine and hyoscine butylbromide
| N2 | N3 | |||
|---|---|---|---|---|
| Placebo | REB + HBB | Placebo | REB + HBB | |
| PEPI (cmH2O) | −3.4 ± 0.9 | −2.7 ± 1.0* | −4.2 ± 2.2 | −4.2 ± 1.7 |
| UARES (cmH2O L−1 s) | 5.9 ± 3.6 | 3.5 ± 2.5* | 5.6 ± 5.0 | 5.6 ± 3.2 |
| Number of breaths | 1548 ± 900 | 1970 ± 1175 | 511 ± 266 | 522 ± 338 |
PEPI, epiglottic pressure; UARES, upper airway resistance; number of breaths, average number of breaths analyzed per person in each condition. Data are mean ± SD.
*Significant difference between placebo and reboxetine (REB) plus hyoscine butylbromide (HBB) conditions within a sleep stage.
Breath frequency was elevated throughout N2 sleep during the REB plus HBB condition compared with placebo (Table 4). Elevated breath frequency during N2 was accompanied by shorter inspiratory and expiratory times. In N3, only inspiratory time was significantly shorter during the REB plus HBB condition compared to placebo.
Table 4.
Key respiratory parameters during wakefulness before the study intervention and during sleep after placebo or reboxitine and hyoscine butylbromide
| Placebo | REB + HBB | |
|---|---|---|
| Wake (before receiving the study intervention) | ||
| VI (L min−1) | 6.5 ± 1.6 | 5.8 ± 1.3 |
| VT (L) | 0.4 ± 0.2 | 0.4 ± 0.1 |
| FB (breaths min−1) | 16.0 ± 3.7 | 15.9 ± 2.5 |
| TI (s) | 1.8 ± 0.6 | 1.7 ± 0.4 |
| TE (s) | 2.4 ± 0.8 | 2.9 ± 0.4 |
| PET CO2 (mmHg) | 39.1 ± 3.8 | 40.9 ± 2.5 |
| PIF (L s−1) | 0.4 ± 0.1 | 0.3 ± 0.1 |
| N2 | ||
| Placebo | REB + HBB | |
| VI (L min−1) | 4.9 ± 0.8 | 5.7 ± 1.7 |
| VT (L) | 0.3 ± 0.1 | 0.3 ± 0.1 |
| FB (breaths min−1) | 14.4 ± 1.7 | 16.8 ± 2.5* |
| TI (s) | 1.8 ± 0.2 | 1.6 ± 0.3* |
| TE (s) | 2.5 ± 0.4 | 2.2 ± 0.4* |
| PET CO2 (mmHg) | 43.0 ± 4.2 | 43.7 ± 2.8 |
| PIF (L s−1) | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Slow wave sleep | ||
| VI (L min−1) | 5.0 ± 1.1 | 5.4 ± 1.5 |
| VT (L) | 0.3 ± 0.1 | 0.3 ± 0.1 |
| FB (breaths min−1) | 14.7 ± 2.2 | 15.9 ± 2.4 |
| TI (s) | 1.8 ± 0.3 | 1.7 ± 0.3* |
| TE (s) | 2.4 ± 0.5 | 2.2 ± 0.4 |
| PET CO2 (mmHg) | 43.9 ± 4.6 | 44.9 ± 2.9 |
| PIF (L s−1) | 0.3 ± 0.1 | 0.3 ± 0.1 |
REB, reboxetine; HBB, hyoscine butylbromide; VI, inspired minute ventilation; VT, tidal volume; FB, breathing frequency; average TI, inspiratory time; TE, expiratory time; PET CO2, end-tidal CO2; PIF, peak inspiratory flow. Data are mean ± SD.
*Significant difference compared with placebo.
Discussion
Contrary to our hypothesis, the main finding of this study was that a combined 4 mg dose of REB plus 20 mg of HBB does not increase GG muscle activity compared with placebo during sleep in healthy individuals without OSA. Rather, both peak and tonic GG muscle activity (as a percentage of wakefulness) were lower compared with placebo. However, the sleep-related reduction in tensor palatini muscle activity was less versus placebo and upper airway function improved with REB plus HBB. Indeed, REB plus HBB reduced upper airway resistance during N2 sleep. Furthermore, N2 increased and REM sleep was abolished in 7 out of the 10 participants with REB plus HBB. This highlights the importance of noradrenergic activation and muscarinic receptor blockade on sleep architecture. These novel findings provide mechanistic insight into the role of noradrenergic and antimuscarinic processes on upper airway function in humans, and they have implications for OSA as discussed below.
Effect of REB and HBB on sleep architecture
Consistent with our findings of reduced REM sleep, REB was found to increase REM latency in rats in a dose dependent manner [22, 23]. Similarly, 2 mg of REB markedly reduced REM sleep in a study involving 13 healthy men [24]. In addition, consistent with our findings, hyoscine has also been shown to reduce REM sleep, increase REM latency, and increase N1 and N2 sleep [25, 26]. However, there are few published studies that have specifically examined the effects of HBB on sleep in humans. In one study, 10 mg of HBB increased REM onset latency by ~80 min in a group of 10 healthy volunteers [27]. Finally, while noradrenergic activation of α1 and β receptors can have potent arousal and wake-promoting effects [28], in accordance with previous human studies [16, 27], sleep efficiency remained similar to placebo in our study of a combined noradrenergic and antimuscarinic intervention.
Sleep state-related changes in upper airway muscle activity and function
GG muscle activity is mediated by at least three key sources of neural drive [10, 29]. Firstly, central respiratory pattern generator neurons located within the medulla provide drive to the upper airway muscles including the GG. This source produces periodic discharge patterns with typically higher levels of drive during inspiration [29]. Secondly, mechanoreceptors located within the larynx and pharyngeal airway respond reflexively to changes in airway pressure to alter GG muscle activity [10, 19]. Lastly, sleep-sensitive neuromodulators or “wakefulness stimuli” including serotonergic and noradrenergic systems provide excitatory input to hypoglossal motoneurons, to increase GG activity [30, 31]. Both central pattern generator neurons and mechanoreceptors reflex feedback are believed to contribute to the phasic pattern of GG muscle activation (more during inspiration, less during expiration, Figure 2). Conversely, withdrawal of wakefulness stimuli is believed to be an important modulator of tonic drive (drive unrelated to respiratory phase). Changes in tonic drive to the upper airway dilators can modulate upper airway resistance and collapsibility [32].
In people with and without OSA, GG and tensor palatini muscle activity decrease rapidly at sleep onset [10, 33–35]. These findings have provided strong support for the concept that OSA is caused by an interaction between pharyngeal anatomy and sleep-related reductions in muscle contractility. Indeed, respiratory events are common during the sleep transition period. However, consistent with earlier work [35, 36], the findings from the placebo arm of the current study indicate increased rather than decreased GG muscle activity during established sleep compared with wakefulness. These prior findings are consistent with the current observations of increased upper airway resistance and epiglottic pressure swings during the placebo arm and knowledge that the GG is exquisitely sensitive to changes in airway pressure [19, 37].Thus, mechanisms beyond sleep-related reductions in GG muscle activity likely importantly contribute to OSA pathogenesis for some people.
On the other hand, while hypoglossal and trigeminal motor neurons can share common drive [38, 39], tensor palatini (and likely other airway dilators) are less sensitive to relatively small changes in airway pressure [19, 37, 40, 41]. These differences in sensitivity to airway pressure and the extent of sleep-related reductions in non-GG dilator muscle activity are likely key drivers of increased upper airway resistance and negative pharyngeal pressure swings during sleep. Indeed, under the same conditions tensor palatini remained ~65% lower compared with the wakefulness level during non-REM sleep. Our data is supported by findings that nadir epiglottic pressure correlates strongly with peak GG EMG but not tensor palatini EMG, suggesting negative pressure is not a primary modulator of tensor palatini muscle activity [37]. Therefore, the decrease in tensor palatini EMG might be explained by a withdrawal of wakefulness stimuli as opposed to negative pressure changes.
Effect of REB and HBB on upper airway muscles and respiratory parameters during sleep
Lower peak GG muscle activity with REB plus HBB during non-REM sleep versus placebo was unexpected and is in contrast to recent noradrenergic animal [13] and human studies with desipramine [16, 17]. There are several possibilities that may explain this finding. Consistent with an earlier study that showed the importance of tensor palatini in mediating upper airway resistance [42], tensor palatini muscle activity was higher with reboxetine plus HBB compared with placebo and upper airway resistance and negative pharyngeal pressure swings were reduced. Thus, the upper airway was more stable and there were less local stimuli for the GG to respond, potentially contributing to lower levels of activation. Consistent with decreased upper airway resistance, respiratory load compensation (inspiratory time) was less with the drug combination during N2 sleep. Differences in the pharmacological properties and dosage of the agents used to alter noradrenergic and antimuscarinic activity in these different studies may have also contributed such that the current combination may have greater affinity for trigeminal versus hypoglossal motor neurons. Indeed, although desipramine and reboxetine both impact the noradrenergic system, desipramine is a tricylic antidepressant whereas reboxetine is a serotonin and norepinephrine reuptake inhibitor with different neuropharmacological properties [43, 44]. In addition, dose equivalency studies for depression treatment suggest that 200 mg of desipramine, as used in recent upper airway physiology studies [16, 17], is roughly equivalent to 11 mg of reboxetine [45]. Alternatively, similar to desipramine [46, 47], reboxetine plus HBB may reduce nasal resistance which would contribute to less pronounced epiglottic pressure swings and improved airway stability. There may have also been drug-mediated changes to other upper airway muscles not measured in the current study that contributed to the observed improvement in upper airway function.
Finally, breathing frequency was ~15% higher during N2 with REB plus HBB versus placebo. In addition, consistent with a direct stimulatory effect on breathing, the typical sleep-related reduction in minute ventilation did not occur during the REB plus HBB condition. Thus, respiratory stimulant effects of the drug combination may have also contributed to improved upper airway function in the current study. Further work is required to clarify these potential mechanisms.
Methodological considerations
The current study has several novel features and strengths including a double-blind randomized design, gold standard methodology to quantify EMG activity and upper airway mechanics and use of healthy individuals to avoid the confounders of OSA (i.e. repetitive upper airway collapse and blood gas changes which alter EMG activity). However, there were limitations including a relatively small sample size and single night studies. Despite this, robust differences in pharyngeal muscle activity and function with REB plus HBB were detected. Nonetheless, future studies that include a larger number of participants following chronic use of these agents in people with OSA to extend generalizability and to determine the clinical relevance of these findings for targeted pharmacotherapy are required.
This study also used a combined pharmacologic intervention rather than studying each agent separately. Thus, we do not know the relative contribution of each agent alone to the key outcomes of the current study. Plasma concentration was not quantified in this study to determine the bioavailability and action of these drugs. However, pharmacokinetic studies indicate that the bioavailability of REB is 94% and achieves a maximum plasma concentration 2 h after administration [18]. The maximum plasma concentration of HBB is reached ~15–90 min after administration. However, the bioavailability of HBB is quite low [48]. Thus, REB may have been the major contributor mediating the current findings although this remains to be tested.
Finally, one of the key criteria for REM sleep is reduced EMG activity. Thus, it is possible that the marked reduction in REM sleep with REB plus HBB detected in the current study may be attributed to, at least in part, increased EMG activity rather than REM suppression per se. However, careful post hoc evaluation of scored polysomnographic records was performed to identify other potential markers of REM sleep in the absence of reduced EMG activity (e.g. sharp conjugate eye movements, presence of saw tooth waves in the EEG). This secondary analysis did not change the marked REM suppression finding with REB plus HBB.
Summary and potential clinical implications for OSA
Four milligrams of REB plus 20 mg of HBB is associated with reduced GG but increased tensor palatini muscle activity compared with placebo during non-REM sleep. The net result is improved upper airway function reflected by reduced pharyngeal pressure swings and upper-airway resistance. Indeed, in the participant with OSA who was inadvertently recruited, the AHI almost halved with the drug combination. These findings provide important mechanistic insight into the role of noradrenergic and antimuscarinic processes on upper airway stability during sleep and may have important implications for pharmacological targets for OSA.
Given that OSA results in partially reversible depression with CPAP therapy in some patients [2], while further studies in OSA are required, there may be an advantage of using an antidepressant that also reduces OSA. REB plus HBB also has a major influence on sleep architecture via REM suppression. Abolition of REM sleep may have conflicting implications for OSA and REM sleep dependent processes such as memory consolidation and cognitive function. However, while some studies suggest that suppression or deprivation of REM sleep impairs procedural memory and cognitive function [49, 50], others indicate no effect on memory or cognitive flexibility [51] or improvement in certain aspects of skill memory [24]. Similar to other antidepressants [16, 17], acute abolition of REM sleep with the agents used in the current study may be a useful experimental approach to further examine these unresolved questions. Similarly, by definition, suppression of REM sleep will reduce/eliminate REM OSA. Recent studies have shown multiple adverse consequences of REM specific OSA which is very common [52, 53]. Thus, REB plus HBB could be useful to study the implications of removal of REM OSA. While longer term studies are required to determine if the current acute REM suppression effects are maintained over time and their potential clinical benefit, the current findings raise several important questions and opportunities for future research.
Funding
This work was funded by NeuroSleep, a National Health and Medical Research Council of Australia (NHMRC) Centre for Research Excellence Grant (1060992). D.J.E. is supported by a NHMRC Senior Research Fellowship (1116942). R.G. is funded by an NHMRC Senior Principal Research Fellowship (1106974).
Authors’ contributions
D.J.E. and J.C. were responsible for study conception and experimental design. D.J.E., J.C., and R.L. contributed to data collection, analysis, and interpretation. D.J.E., J.C., and R.L. wrote the draft version of the manuscript. All authors contributed to the final version and provided valuable intellectual input.
Conflict of interest statement. D.J.E holds a Cooperative Research Centre (CRC) Grant which is a collaboration between the Australian Government, Academia and Industry (Industry partner: Oventus Medical), serves as a consultant for Bayer and has a research grant from Apnimed. R.G. is a Program Leader of a CRC Grant (Philips Healthcare major industry partner) and has provided medical advisory board services to Merck and Teva. A.W. works as a consultant for Varnum, Cambridge Sound Management, Nox, Bayer, Philips, and Galvani, and has received grants from Philips and Varnum. He also has a financial interest in Apnimed Corp., a company developing pharmacologic therapies for sleep apnea. R.L. and J.C.C. do not have any competing interests to disclose.
Acknowledgments
The authors would like to thank Professors Simon Gandevia and Robert Herbert for coordinating the randomization process for the study. They would also like to thank Mr Benjamin Tong for scoring and staging of the sleep studies and Dr Rodrigo Martins for assisting with data collection and participant set-up.
Work Performed: Neuroscience Research Australia (NeuRA), Sydney, NSW, Australia.
References
- 1.Eckert DJ, et al. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985). 2014;116(3):302–313. [DOI] [PubMed] [Google Scholar]
- 2.Edwards C, et al. Depressive symptoms before and after treatment of obstructive sleep apnea in men and women. J Clin Sleep Med. 2015;11(9):1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golbin JM, et al. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008;5(2):200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan AS, et al. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanna A. Obstructive sleep apnoea, motor vehicle accidents, and work performance. Chron Respir Dis. 2013;10(1):29–33. [DOI] [PubMed] [Google Scholar]
- 6.Avlonitou E, et al. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16(2):563–569. [DOI] [PubMed] [Google Scholar]
- 7.Weaver TE, et al. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert DJ, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carberry JC, et al. Personalized management approach for OSA. Chest. 2018;153(3):744–755. [DOI] [PubMed] [Google Scholar]
- 10.Fogel RB, et al. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol. 2005;564(Pt 2):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastwood PR, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strollo PJ Jr, et al. ; STAR Trial Group. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. [DOI] [PubMed] [Google Scholar]
- 13.Chan E, et al. Endogenous excitatory drive modulating respiratory muscle activity across sleep-wake states. Am J Respir Crit Care Med. 2006;174(11):1264–1273. [DOI] [PubMed] [Google Scholar]
- 14.Grace KP, et al. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187(3):311–319. [DOI] [PubMed] [Google Scholar]
- 15.Horner RL, et al. A resource of potential drug targets and strategic decision-making for obstructive sleep apnoea pharmacotherapy. Respirology. 2017;22(5):861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taranto-Montemurro L, et al. Desipramine increases genioglossus activity and reduces upper airway collapsibility during non-REM sleep in healthy subjects. Am J Respir Crit Care Med. 2016;194(7):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taranto-Montemurro L, et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation. Eur Respir J. 2016;48(5):1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hajós M, et al. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev. 2004;10(1):23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carberry JC, et al. Mechanisms contributing to the response of upper-airway muscles to changes in airway pressure. J Appl Physiol (1985). 2015;118(10):1221–1228. [DOI] [PubMed] [Google Scholar]
- 20.Berry RB, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen CD, et al. An automated and reliable method for breath detection during variable mask pressures in awake and sleeping humans. PLoS One. 2017;12(6):e0179030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez C, et al. Depression and poor sleep: the effect of monoaminergic antidepressants in a pre-clinical model in rats. Pharmacol Biochem Behav. 2007;86(3):468–476. [DOI] [PubMed] [Google Scholar]
- 23.Wong EH, et al. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol Psychiatry. 2000;47(9):818–829. [DOI] [PubMed] [Google Scholar]
- 24.Rasch B, et al. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12(4):396–397. [DOI] [PubMed] [Google Scholar]
- 25.Gillin JC, et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991;30(2):157–169. [DOI] [PubMed] [Google Scholar]
- 26.Sagales T, et al. Differential effects of scopolamine and chlorpromazine on REM and NREM sleep in normal male subjects. Clin Pharmacol Ther. 1969;10(4):522–529. [DOI] [PubMed] [Google Scholar]
- 27.Rauniar GP, et al. Comparative effects of hyoscine butylbromide and atropine sulphate on sleep architecture in healthy human volunteers. Indian J Physiol Pharmacol. 1998;42(3):395–400. [PubMed] [Google Scholar]
- 28.Berridge CW, et al. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogel RB, et al. Within-breath control of genioglossal muscle activation in humans: effect of sleep-wake state. J Physiol. 2003;550(Pt 3):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelev A, et al. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532(Pt 2):467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenik VB, et al. REM sleep-like atonia of hypoglossal (XII) motoneurons is caused by loss of noradrenergic and serotonergic inputs. Am J Respir Crit Care Med. 2005;172(10):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierce R, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30(2):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas CL, et al. Discharge patterns of human tensor palatini motor units during sleep onset. Sleep. 2012;35(5):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson V, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31(4):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worsnop C, et al. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol (1985). 1998;85(3):908–920. [DOI] [PubMed] [Google Scholar]
- 36.Basner RC, et al. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol. 1991;83(2):189–200. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra A, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162(3 Pt 1):1058–1062. [DOI] [PubMed] [Google Scholar]
- 38.Jordan AS, et al. Common drive in hypoglossal and trigeminal motor neurons. Am J Respir Crit Care Med. 2011;183(9):1280. [DOI] [PubMed] [Google Scholar]
- 39.Trinder J, et al. Motor unit activity in upper airway muscles genioglossus and tensor palatini. Respir Physiol Neurobiol. 2013;188(3):362–369. [DOI] [PubMed] [Google Scholar]
- 40.Dotan Y, et al. Asynchrony of lingual muscle recruitment during sleep in obstructive sleep apnea. J Appl Physiol (1985). 2015;118(12):1516–1524. [DOI] [PubMed] [Google Scholar]
- 41.Oliven R, et al. Alteration in upper airway dilator muscle coactivation during sleep: comparison of patients with obstructive sleep apnea and healthy subjects. J Appl Physiol (1985). 2018;124(2):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tangel DJ, et al. Influence of sleep on tensor palatini EMG and upper airway resistance in normal men. J Appl Physiol (1985). 1991;70(6):2574–2581. [DOI] [PubMed] [Google Scholar]
- 43.Grandoso L, et al. Comparative study of the effects of desipramine and reboxetine on locus coeruleus neurons in rat brain slices. Neuropharmacology. 2004;46(6): 815–823. [DOI] [PubMed] [Google Scholar]
- 44.Cottingham C, et al. Noradrenergic antidepressant responses to desipramine in vivo are reciprocally regulated by arrestin3 and spinophilin. Neuropharmacology. 2012;62(7):2354–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayasaka Y, et al. Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord. 2015;180:179–184. [DOI] [PubMed] [Google Scholar]
- 46.Jaffuel D, et al. Is the muscle the only potential target of desipramine in obstructive sleep apnea syndrome? Am J Respir Crit Care Med. 2017;195(12):1677–1678. [DOI] [PubMed] [Google Scholar]
- 47.Taranto-Montemurro L, et al. Reply: is the muscle the only potential target of desipramine in obstructive sleep apnea syndrome? Am J Respir Crit Care Med. 2017;195(12):1678–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tytgat GN. Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs. 2007;67(9):1343–1357. [DOI] [PubMed] [Google Scholar]
- 49.Karni A, et al. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–682. [DOI] [PubMed] [Google Scholar]
- 50.Smith CT, et al. Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem. 2004;11(6):714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Göder R, et al. Sleep and cognition at baseline and the effects of REM sleep diminution after 1 week of antidepressive treatment in patients with depression. J Sleep Res. 2011;20(4):544–551. [DOI] [PubMed] [Google Scholar]
- 52.Aurora RN, et al. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med. 2018;197(5):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conwell W, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16(2):519–526. [DOI] [PubMed] [Google Scholar]