Abstract
The reactivity of 5‐(alkynyl)dibenzothiophenium salts 1 is explored in the presence of different nucleophiles, dienes, and under photochemical conditions. Reaction with lithium acetylides affords diynes in moderate yields; while depending on the substitution pattern, the reaction with sulfinates delivers either the alkyne transfer products, alkynyl sulfones, or β‐(sulfonium) vinyl sulfones through addition to the C−C triple bond. Similar behavior is observed when tosylamines are used as nucleophiles. Salts of general formula 1 also react with dienes to render the corresponding Diels‐Alder cycloadducts. The vinyl sulfonium salts obtained by these routes further react with nucleophiles through a Michael addition, dibenzothiophene elimination sequence. Alternatively, they also engage in photoinduced radical cyclizations to produce substituted phenanthrenes. Attempts to use this specific addition/radical cyclization sequence for the construction of the 6a,7‐dehydroaporphine skeleton present in several families of alkaloids are also described.
Keywords: Addition reactions, Diynes, Electrophilic alkynylation, Sulfonium salts, Vinyl sulfones
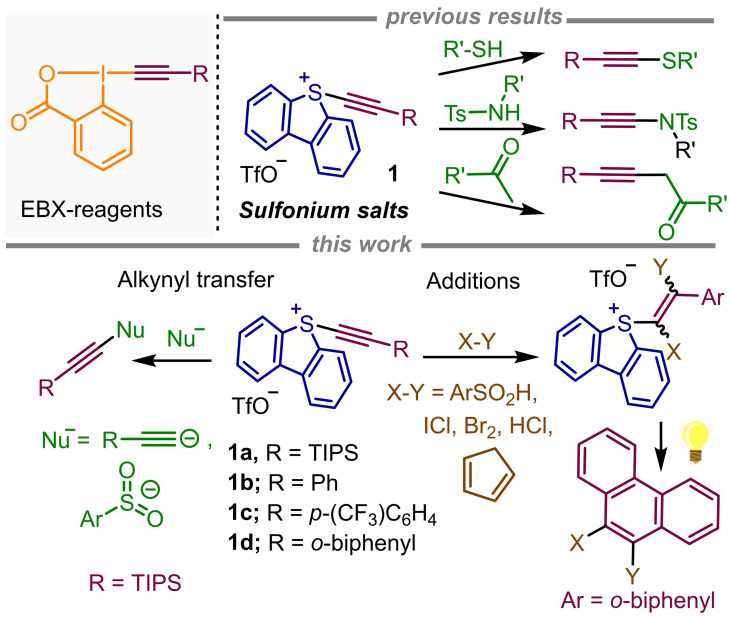
The synthetic potential of 5‐(alkynyl)benzothiophenium salts is further evaluated. In addition to their reactivity as electrophilic alkynylation reagents, they undergo regioselective additions to the C−C triple bond. The products thus obtained can be used for the synthesis of Z‐vinyl sulfones and 9,10‐substituted phenanthrenes.
Introduction
Since their first preparation in the mid‐1960s, the chemistry of alkynyl‐substituted iodonium(III) salts has been well established.[1] These species are known to serve as efficient alkynyl transfer reagents when reacting with nucleophiles, which make them convenient electrophilic acetylene synthons.[2, 3] Initial examples of that reactivity were published by Beringer,[4] and Ochiai;[5] their use in organic synthesis has been later popularized by Zhdankin,[6] Ochiai himself,[7] Stang[8] and Waser[9] using alkynylbenziodoxolones. Additionally, alkynyl‐substituted iodonium(III) salts are known precursors of vinyl iodonium salts either via Michael type nucleophilic additions,[10] or [4+2] cycloaddition reactions occurring at the C−C triple bond.[11] The use of these I(III) derivatives however, is not always free of inconveniences. Specifically, some of these I(III) compounds are thermally unstable and show strong exothermic decompositions on heating.[12]
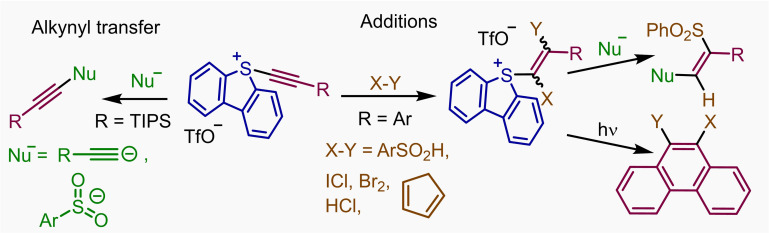
Having this shortcoming in mind, we recently developed S‐alkynyl(dibenzothiophenium) salts 1, which depict an improved safety profile and also efficiently serve as [R−C≡C]+ synthons for the metal‐free alkynylation of thiols, sulfonamides and activated methylene groups.[13] Herein, we further study the reactivity of this family of compounds in two directions. Initially, the transfer of the alkyne moiety to additional nucleophiles such as acetylides or sulfonates is evaluated; diynes, triynes and alkynylsulfones are obtained from these reactions. Secondly, the addition of acids and halogens to the C−C triple bond of 1 or the Diels‐Alder cycloaddition between the same alkyne moiety and dines are presented. Finally, some further reactivity of the S‐vinyl sulfonium salts thus obtained is described as well (Scheme 1); specifically, the photocatalyzed generation of vinyl radicals. Following this addition/radical generation/cyclization sequence, a series of phenanthrene derivatives have been obtained from 1 d. Our attempts to use this strategy for the preparation of the 6a,7‐dehydroaporphine skeleton are also reported; however, the yields obtained are rather poor.
Scheme 1.
From iodine to sulfur. General reactivity and synthetic applications of 5‐(alkynyl)dibenzothiophenium salts.
Results and Discussion
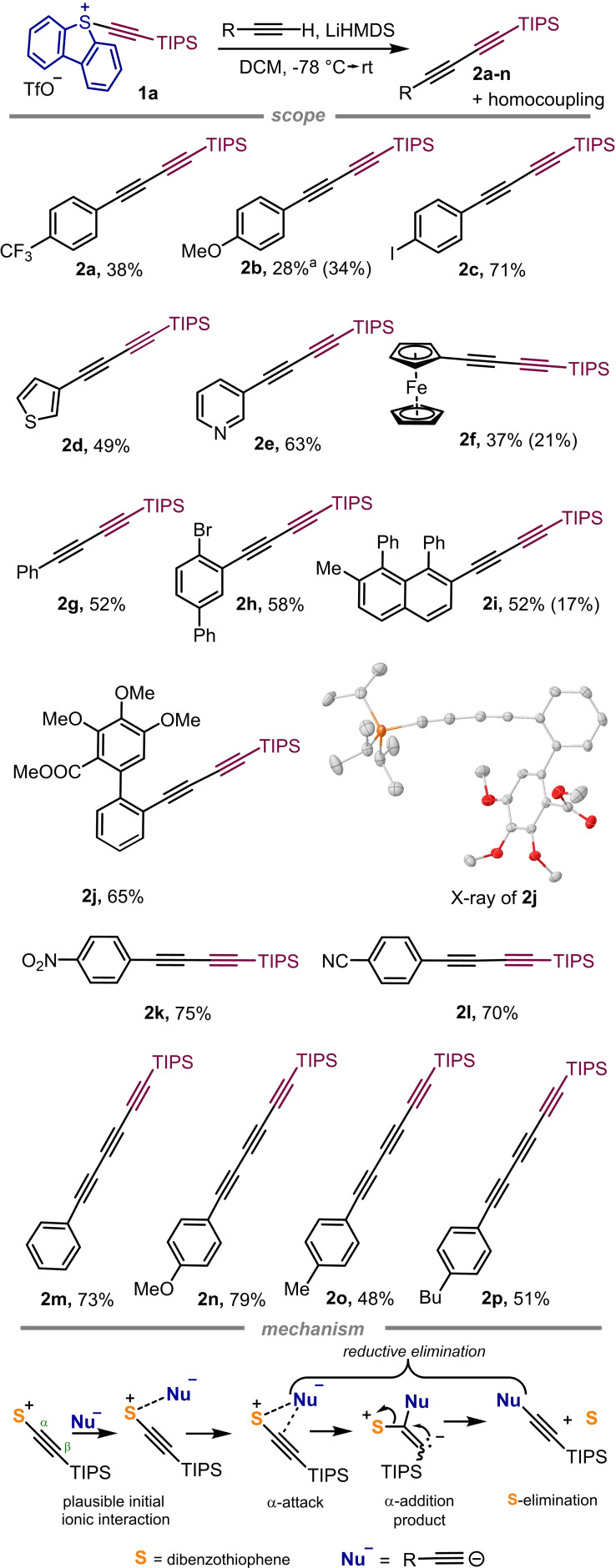
Reaction of 5‐(alkynyl) dibenzothiophenium salts with lithiated alkynes. Building upon the work of Stang, who was able to couple alkynyl iodonium tosylates with alkynylcopper(I) reagents to obtain asymmetric diacetylenes,[14] we submitted 1 a to the reaction with terminal alkynes in the presence of a stoichiometric amount of lithium hexamethyldisilazane (LiHMDS). Gratifyingly, deprotonated alkynes proved to react with 1 a yielding the corresponding diynes in moderate to good yields. In all cases, however, the formation of homocoupling products in different amounts was detected, being the proportion of the diynes in the mixture strongly dependent on the substrate used. Moreover, altering the ratio of either the sulfonium salt or the alkyne, or replacing LiHMDS with n‐butyllithium is detrimental, and causes the formation of increased amounts of homocoupling products. Suppressing the homocoupling however, is possible to a certain extension by addition of CuCN (1.0 equiv.) to the reaction mixture, which in situ generates dialkynylcuprates.[14] For example, the yield of diyne 2 b was improved from 28 % to 52 % using this modification of the protocol.
The scope of the transformation is shown in Figure 1. Unfortunately, we have not been able to establish a relation between the electronic nature of the substrates and the amount of homocoupling product formed. For example, both electron‐ poor p‐(CF3)‐phenylacetylene and electron‐rich p‐(OMe)‐phenylacetylene performed poorly (38 % and 28 % yield, respectively), while p‐(I)‐phenylacetylene delivered the desired diyne in a respectable 71 % yield. As expected, when the nucleophile used is already a 1,3‐diyne the corresponding TIPS‐capped triynes 2 m–p are delivered in moderate to good yields. The connectivity of 2 f and 2 j has been confirmed by X‐ray crystallography (see the Supporting Information).
Figure 1.
Substrate scope of the coupling of S‐(alkynyl) sulfonium salts with lithiated alkynes. All reagents were used in stoichiometric amounts and all reactions were quenched after 15 min. Yields are of the isolated asymmetric di‐ and triynes, in parenthesis the yields of the isolated homocoupling products.[a] The yield of 2 b was improved to 52 % when 1.0 equiv. of CuCN was added to the reaction mixture under otherwise identical conditions. X‐ray structure of 2 j, ellipsoids shown at 50 % probability and hydrogen atoms omitted for clarity.[15]
Our previous studies on the reaction of nucleophiles with isotope labelled 1 a makes us believe that the deprotonated alkyne most probably reacts via attack at the α‐position of the alkyne, following elimination of the dibenzothiophene unit and concomitant regeneration of the triple bond (Figure 1).[13b]
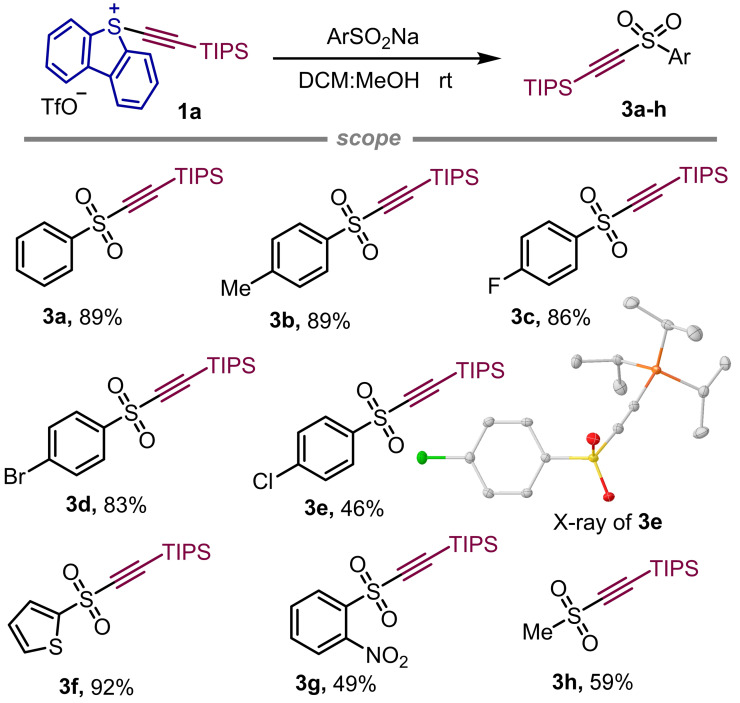
Reaction of S‐(alkynyl) dibenzothiophenium salts with sodium sulfonates and sulfinic acids. Exposure of 1 a to the sodium salt of (hetero)aryl or alkyl sulfinates in DCM : MeOH (1 : 1) cleanly resulted in the formation of alkynyl sulfones 3 a–h in moderate to excellent yields. With high probability, this reaction proceeds via attack of the sulfinate at the α‐position of the alkyne as well, following elimination of dibenzothiophene and regeneration of the triple bond (Figure 2). Considering the product outcome, sulfonium salt 1 a behaves as its I(III)‐analogues even if the operating reaction mechanism is not necessarily identical.[16]
Figure 2.
Substrate scope of the coupling of S‐(alkynyl) sulfonium salts with sodium sulfinates. Yields are of the isolated products. X‐ray structure of 3 e; ellipsoids shown at 50 % probability and hydrogen atoms are omitted for clarity.[15]
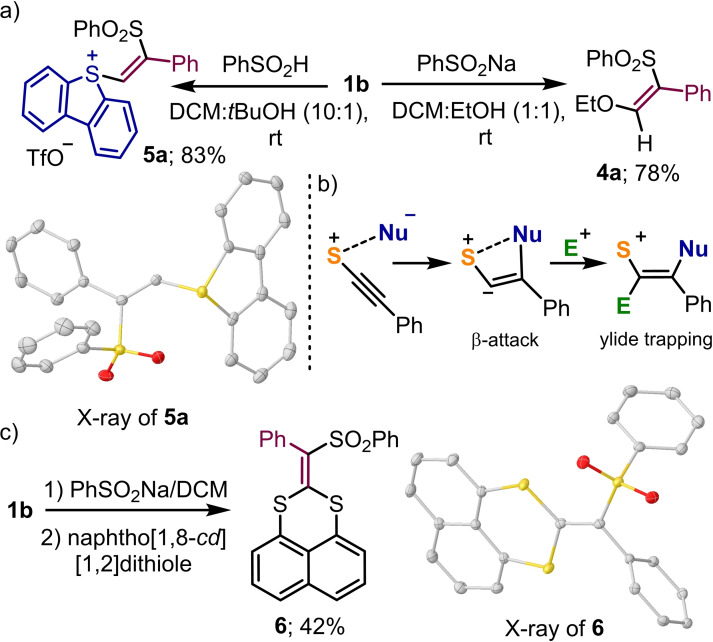
In marked contrast to the transformation just described, the reaction of sodium sulfinates with alkynyl sulfonium salt 1 b, which has a terminal Ph group at the alkyne moiety, does not deliver alkynyl sulfones but β‐ethoxy‐(Z)‐vinylsulfone 4 a (Scheme 2a). This different reactivity mode has been already described for selenonium and iodonium salts and reveals the preference of alkynyl sulfonium salts to suffer the attack of the nucleophile at the alkyne β‐position when steric factors do allow it.[17] Moreover, when 1 b was made react with benzenesulfinic acid in DCM : t‐BuOH, (10 : 1) the (Z)‐β‐sulfonylalkenylsulfonium salt 5 a is cleanly isolated. This shows that in the absence of an appropriate nucleophile able to attack the vinyl sulfonium intermediate, the reaction stops after the initial addition to the C−C triple bond. The absolute stereocontrol of the addition reaction towards the Z‐olefin might be considered a hint of the assisting role of the sulfonium moiety as directing group for the attack of the incoming nucleophile (Scheme 2b). Moreover, the intermediate ylide can be trapped with electrophiles other than a proton. For example, when the reaction of 1 b and sodium benzenesulfinate is carried out in the presence of excess of naphtho[1,8‐cd][1,2]dithiole without protic solvent, then 2‐methylenenaphtho[1,8‐de][1,3]dithiine 6 is obtained (Scheme 2c). The formation of this product can be explained via a sequence starting with the β‐attack of the sulfonate to 1 b, trapping of the ylidic intermediate by the disulfide, intramolecular Michael addition of the in situ generated sulfide moiety to the vinyl sulfonium fragment and final elimination of dibenzothiophene.
Scheme 2.
Synthesis of (Z)‐β‐sulfonylalkenylsulfonium salt 5 a and plausible mechanism for its formation. X‐ray structures of 5 a and 6; ellipsoids shown at 50 % probability, anion in 5 a and hydrogen atoms removed for clarity.[15]
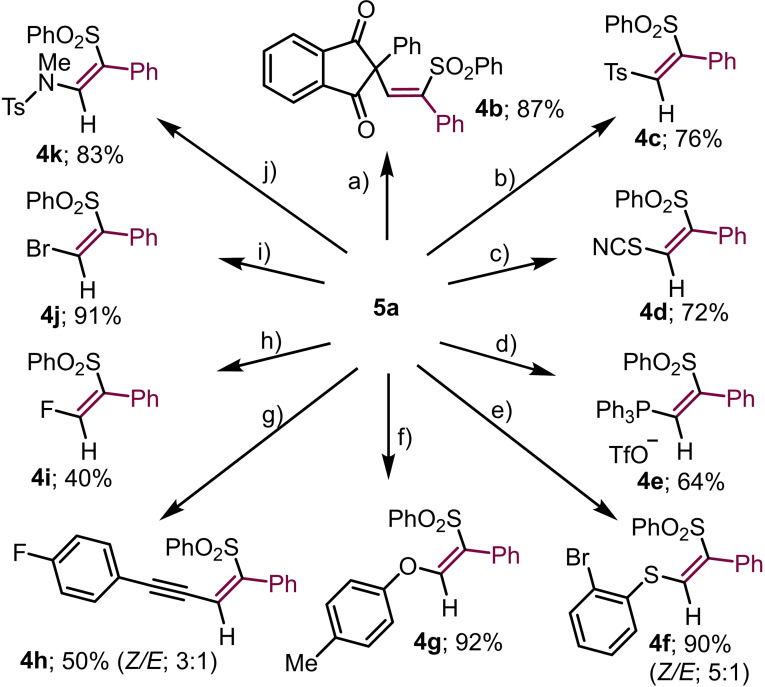
The reactivity of salt 5 a has been further examined; a set of ten different reactions involving nucleophiles of diverse nature that include C−, S−, N−, P−, O− and halogen‐based ones, are shown in Scheme 3. Given the excellent leaving group properties of the dibenzothiophene unit, all nucleophiles tested are able to smoothly react with 5 a and replace this moiety affording the desired β‐substituted vinyl sulfone with moderate to excellent yields. The substitution reaction takes place with retention of the Z‐configuration of the olefin in most of the cases, as could be confirmed either by NOE experiments or comparison with literature data.[17a] This is remarkable since the Z‐ products obtained are often the thermodynamically less stable ones. Olefin isomerization could only be detected for compounds 4 f and 4 h and even in these cases it occurred in moderate extent (Scheme 3). A direct attack of the nucleophile to the sulfonium moiety in 5 a, followed by reductive coupling between the nucleophile and the vinyl moiety provides a feasible explanation for this stereochemical outcome; however, a Michael‐type addition of the nucleophile to the α‐carbon of the vinyl moiety follow by elimination of dibenzothiophene cannot be completely excluded, in particular for those cases where some isomerization is detected.
Scheme 3.
Synthesis and reactivity (Z)‐β‐sulfonylalkenylsulfonium salt 5 a; a) 1,3‐diketone (1.0 equiv.), Cs2CO3 (1.0 equiv.), CH2Cl2, rt, 12 h; b) TsNa (1.0 equiv.), CH2Cl2 : t‐BuOH (1 : 1), rt, 12 h; c) NaSCN (1.1 equiv.), CH2Cl2/t‐BuOH (1 : 1), rt, 12 h; d) PPh3 (1.2 equiv.), CH2Cl2, rt, 12 h; e) o‐Br‐thiophenol (1.0 equiv.), Cs2CO3, CH2Cl2, rt, 12 h; f) p‐cresol (1.0 equiv.), Cs2CO3 (1.0 equiv.), CH2Cl2, rt, 12 h; g) LiHMDS (1.0 equiv), p‐F‐(C6H4)−C≡CH (1.0 equiv), CH2Cl2, rt, 12 h; h) KF (1.2 equiv.), CH2Cl2 : t‐BuOH (1 : 1), rt, 12 h; i) Bu4NBr (1.0 equiv.), CH2Cl2, rt, 12 h; j) MeNHTs (1.2 equiv.), Cs2CO3 (1.2 equiv.), CH2Cl2, rt, 12 h.
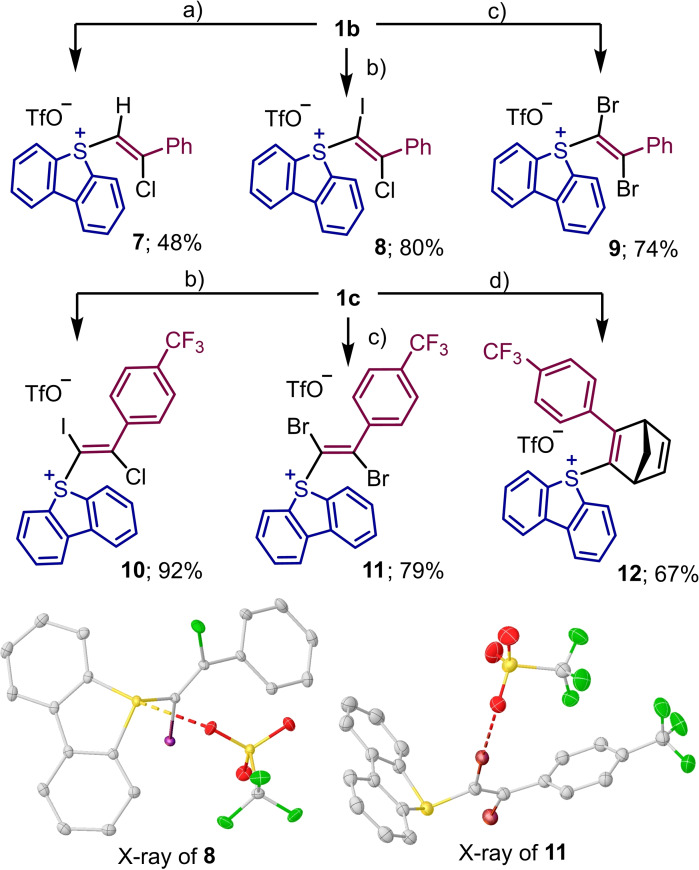
Reaction of 5‐(alkynyl) dibenzothiophenium salts with halogens and dienes. The straightforward addition of sulfonic acids to 1 b made us believe that β‐halovinyl sulfonium salts and α,β‐dihalovinyl sulfonium salts might also be accessed, respectively, via addition of HX or X2 (X=halogen) to the corresponding 5‐(alkynyl)dibenzothiophenium salts 1. These reactions actually work efficiently, and the desired mono‐ and dihalogenated products 7–11 have been obtained in good to excellent yields (Scheme 4). The atom connectivity of compounds 8 and 11 has been subsequently confirmed by X‐ray analysis.
Scheme 4.
Additions to the triple bond of 1 b–c; a) 2 M HCl in Et2O (1.5 equiv.), CH2Cl2, rt, 12 h; b) ICl (1.2 equiv.), CH2Cl2, rt, 12 h; c) Br2 1.0 (equiv.), CH2Cl2, rt, 12 h; d) cyclopentadiene (5.0 equiv.), CH3CN, 100 °C, μW, 2 h. X‐ray structures of 8 and 11; hydrogen atoms removed for clarity and ellipsoids shown at 50 % probability.[15]
The formation of 7, 8 and 10 is diastero‐ and regioselective and probably follows the mechanism already described in Scheme 2b. The synthetic potential of these products is obvious if one considers the known differences in reactivity of alkenyl chlorides, bromides, iodides, and sulfonium salts.[18] Interestingly, alkynyl sulfonium salts also undergo smoothly Diels‐Alder cycloaddition with cyclopentadiene to deliver the corresponding bicyclic cyclohexadiene 12 in good yield. This reactivity is again parallel to that already observed for alkynyl‐substituted hypervalent I(III) reagents.[19]
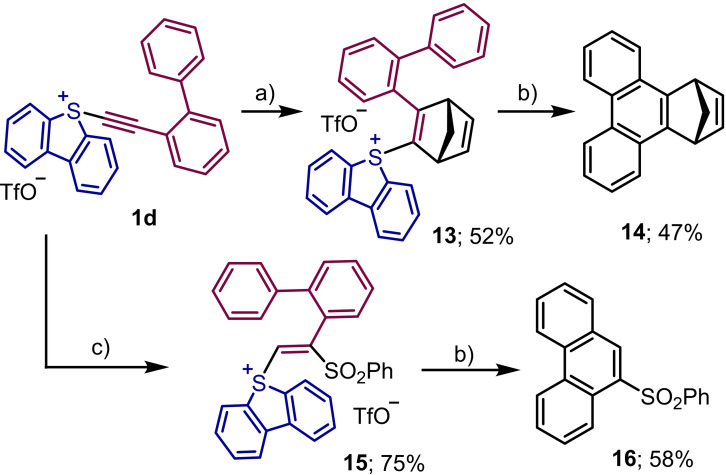
Additionally, having in mind the ability of sulfonium salts to generate organic radicals via mesolitic S−C bond cleavage after accepting one electron, we set up to study whether appropriately designed alkenyl‐substituted sulfonium salts could be used for the assembly of phenanthrene skeletons via radical induced cyclization.[20] Hence, we prepared 13 and 15 from 1 d following the methods herein reported, and submitted these products to photochemically induced one electron reduction using very similar reaction conditions to those recently developed by Procter and coworkers for the coupling between the exo‐aryl group of dibenzothiophenium salts and non‐functionalized (hetero)arenes[21]. To our delight, the proposed transformation took place smoothly and the expected phenanthrenes 14 and 16 were obtained, albeit with moderate yields (Scheme 5).[22]
Scheme 5.
Phenanthrene syntheses via photochemical methods; a) cyclopentadiene (5.0 equiv.), CH3CN, μW, 100 °C, 3 h; b) Cs2CO3 (1.1 equiv.), [Ru(bpy)3]Cl2 ⋅ 6 H2O (2 mol%), CH3CN, Blue LED (28 W), rt, 16 h.; c) PhSO2H (2.0 equiv.), CH2Cl2/t‐BuOH (8 : 1), rt, 12 h.
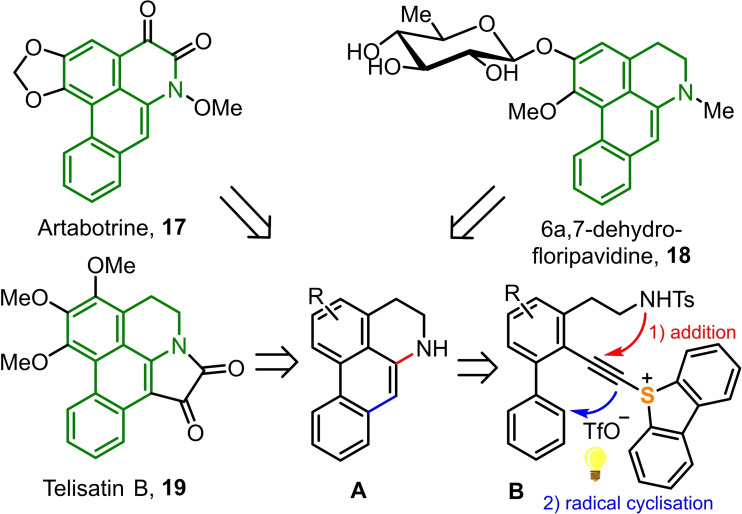
Synthesis of the 6 a ,7‐dihydroaporphine scaffold. Finally, we decided to explore whether the just described addition/cyclisation protocol would be useful for the synthesis of the 6a,7‐dihydroaporphine core A, a tetracyclic amine which constitutes the skeleton of dozens of alkaloids such as for example Artabotrine (17),[23] (−)‐6a,7‐dehydrofloripavidine (18),[24] or Telisatin B (19),[25] among others.[26] We envisaged that if access to sulfonium salts of general formula B could be provided, then, intramolecular hydroamination of the triple bond followed by cyclization of the photochemically generated vinyl radical should deliver the desired tetracycle (Scheme 6).
Scheme 6.
Synthetic strategy for the synthesis of the 6a,7‐dihydroaporphine scaffold.
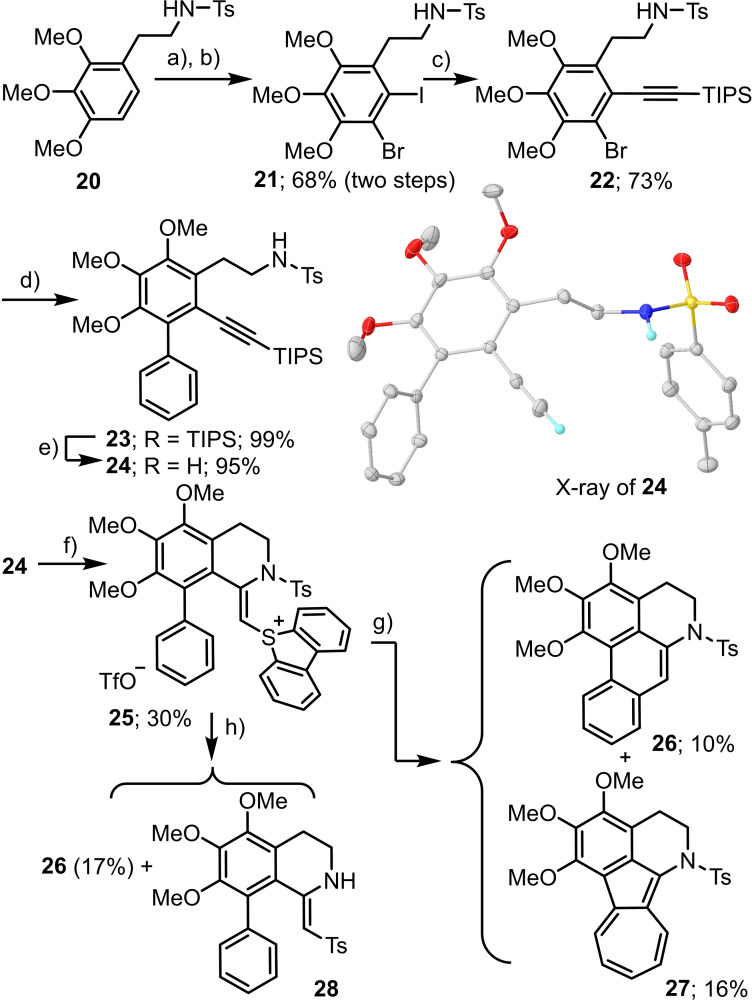
In order to evaluate the feasibility of the synthetic strategy just presented, known tosylamide 20 was chosen as appropriate starting material. Regioselective bromination and subsequent iodination of this compound following a protocol already described for similar substrates affords compound 21.[27] Sonogahira reaction with TIPS‐protected acetylene selectively delivered the corresponding alkyne 22, which was subsequently cross‐coupled with phenylboronic acid to produce biphenyl derivative 23. Unfortunately, all attempts to install the sulfonium moiety directly in 23 were unsuccessful probably due to the steric demand of the TIPS group. For this reason, 23 was treated with TBAF to afford the terminal acetylene 24 in excellent yield as a crystalline material. The atom connectivity of this product was established by X‐ray crystallography (Scheme 7). Treatment of 24 with activated dibenzothiophene S‐oxide finally afforded a sulfonium salt, which is not the expected alkynyl substituted one but already the result of the intramolecular addition of the sulfonamide moiety to the alkyne fragment 25 (Scheme 7). The stage was then set for the key photocyclization, which occurred under the optimized conditions already identified for the preparation of 14 and 16. Unfortunately, though the desired N‐tosyl dihydroaporphine 26 could be obtained from that experiment, the isolated yield is rather poor. In fact, the main product identified from the mixture is azulene 27; a structure that has been proposed to derive from the insertion of an in situ generated vinyl carbene into the proximal C−C bond of the adjacent phenyl rest.[28] Treatment of 25 with strong bases such as KH does not solve the selectivity problem. In fact, complex reaction mixtures are obtained from which 26 is again isolated in low yields. From a crystal formed from the crude reaction mixture, we could also identify 28 as side product by X‐ray diffraction; however, we were not able to isolate an analytically pure sample of that enamine.
Scheme 7.
Studies towards the synthesis of the 6a,7‐dihydroaporphine scaffold; a) NBS, 60 °C, 16 h; b) NIS, TFA, CH3CN, μW 110 °C, 16 h.; c) TIPS‐acetylene, Pd(PPh3)2Cl2, CuI, NEt3, THF, μW 90 °C, 60 h.; d) PhB(OH)2, Pd(PPh3)2Cl2, Na2CO3, DME/H2O, μW 120 °C, 26 h.; e) TBAF, THF, rt, 2 h.; f) dibenzothiophene‐S‐oxide, Tf2O, CH2Cl2, −50 °C→−20 °C, 16 h.; g) [Ru(bipy)3]2 ⋅ 6H2O (3 mol%), blue LEDs, Cs2CO3 (1.4 equiv.), CH3CN; h) KH (3.0 equiv.), KOt‐Bu (10 mol%). X‐ray of 24 drawn at 50 % probability level; only amide and ethynyl protons shown.[15]
Conclusion
We have further evaluated the reactivity of 5‐(alkynyl)dibenzothiophenium salts, which are easily prepared from the reaction of dibenzothiophene S‐oxide with TMS‐capped or even free alkynes. While TIPS‐substituted salts react with nucleophiles transferring the alkyne moiety; aryl substituted salts preferentially suffer addition reactions to the C−C triple bond to deliver 5‐vinyl sulfonium species. The further transformation of these initial adducts into Z‐configured vinyl sulfones and phenanthrenes is also described herein. Considering the multitude of products that might be gained following these protocols, we do believe that the chemistry of 5‐alkynyl sulfonium salts shows a vast synthetic potential, which is still under study in our laboratory.
Experimental Section
General experimental details: All reactions were carried out using pre‐dried glassware under nitrogen atmosphere unless otherwise stated. Dry and degassed solvents (THF, dichloromethane, toluene, n‐pentane, diethyl ether) were obtained with the MBraun Solvent Purification System (MB‐SPS‐800) or by distillation over appropriate drying agents and stored under nitrogen atmosphere. Flash chromatography was performed either on Merck 60 (40–63 μm) or Macherey‐Nagel 60 (40–63 μm) silica gel. Thin‐layer chromatography (TLC) analyses were performed using polygram SIL G/UV254 from Macherey‐Nagel and visualized by UV irradiation and/or phosphomolybdic acid, KMnO4 or p‐anisaldehyde dip. All commercially available products (Acros Organics, ABCR, Alfa Aesar, Sigma Aldrich, Fluorochem, TCI) were used as received. Compounds 1 a, 1 b and 1 c were synthesized according to known procedures.[13]
General procedure for the synthesis of compounds 2 a–2 p
The corresponding 1‐alkyne or 1,3 diyne (0.2 mmol, 1.0 equiv.) was dissolved in anhydrous DCM (2 mL, 0.1 M) under inert atmosphere and LiHMDS (0.2 mL of a 1 M solution in THF, 0.2 mmol, 1.0 equiv.) was added at −78 °C under stirring. The reaction mixture was allowed to warm up to 0 °C and stirred for additional 5 min. Successively, it was cooled back to −78 °C, and 1 a (103 mg, 0.2 mmol, 1.0 equiv.) was added in one portion. Then, the reaction mixture was allowed to warm up to r.t. and stirred for an additional 10 min. Finally, the reaction was quenched with water and extracted with EtOAc (3×5 mL). The desired products were obtained after purification by column chromatography using pentane as eluent. As representative example the spectroscopic characterization of 2 e is listed: 1 H NMR (300 MHz, CDCl3): δ=8.73 (s, 1H), 8.56 (d, J=4.2 Hz, 1H), 7.78 (d, J=7.9 Hz, 1H), 7.29–7.22 (m, 1H), 1.11 (s, 21H). 13 C NMR (125 MHz, CDCl3): δ=153.5, 149.3, 139.6, 123.1, 119.2, 89.9, 89.0, 78.0, 72.2, 18.7, 11.4 ppm. IR (neat): =2940, 2862, 2204, 2106, 1462, 1404, 996, 882, 659, 602 cm−1. HRMS (EI) m/z calcd for C18H25NSi+ [M]+: 283.1751; found: 283.1750. See the SI for the characterization of all other dienes and trienes.
General procedure for the synthesis of compounds 3 a–3 h
Sulfonium salt 1 a (103 mg, 0.2 mmol, 1.0 equiv.) and the corresponding sulfinate (0.24 mmol, 1.2 equiv.) were dissolved in DCM : MeOH 1 : 1 (5 mL), and the reaction mixture was stirred for 30 min at r.t. Upon completion of the reaction (monitoring via TLC), pentane (5 mL) was added, and the reaction mixture was transferred directly to a pre‐wetted (hexane) silica column and eluted with the same solvent to afford the desired products. As representative example the spectroscopic characterization of 3 g is listed: 1 H NMR (400 MHz, CDCl3): δ=8.33–8.26 (m, 1H), 7.99–7.92 (m, 1H), 7.87–7.79 (m, 2H), 1.20–1.06 (m, 21H) ppm. 13 C NMR (101 MHz, CDCl3): δ=148.3, 135.4, 135.2, 133.1, 131.0, 125.5, 102.6, 99.3, 18.5, 11.1 ppm. IR (ATR): =2948, 2867, 2360, 2340, 2124, 1542, 1336, 1159, 803, 575 cm−1. HRMS (ESI) m/z calcd for C17H26NO4SSi+ [M+H]+: 368.1346; found: 368.1342. See the SI for the characterization of all other alkynyl sulfones.
(Z)‐2‐Phenyl‐2‐[2‐phenyl‐2‐(phenylsulfonyl)vinyl]‐1H‐indene‐1,3(2H)‐dione 4 b: A mixture of 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (65.2 mg, 0.2 mmol, 1.0 equiv.) and 1,3‐indandione (29.2 mg, 0.2 mmol, 1.0 equiv.) in CH2Cl2 (2 mL) was stirred at room temperature for 12 h and then directly transferred to a pre‐wetted column (hexane). Pure 4 b (80.8 mg, 173.9 μmol, 87 %) was obtained as an orange oil by column chromatography (gradient hexanes→hexanes : EtOAc 4 : 1). 1 H NMR (400 MHz, CDCl3): δ=7.90 (s, 1H), 7.53 (t, J=7.2 Hz, 2H), 7.49–7.45 (m, 2H), 7.45–7.29 (m, 12H), 7.27–7.23 (m, 2H), 7.16 (d, J=7.2 Hz, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=192.8, 168.2, 150.7, 139.1, 138.4, 133.51, 133.45, 130.8, 130.6, 130.6, 129.6, 129.6 (2 overlapped 13C signals), 128.9, 128.8, 128.6, 128.5, 128.4, 128.3, 128.1, 127.5, 122.8, 119.4, 116.9 ppm. IR (neat): =3725, 3626, 1715, 1378, 1308, 1204, 1146, 1082, 689, 646 cm−1. HRMS (ESI) m/z calcd for C29H20NaO4S+ [M+Na]+: 487.0975; found: 487.0975.
(Z)‐1‐Methyl‐4‐{[2‐phenyl‐2‐(phenylsulfonyl)vinyl]sulfonyl} benzene 4 c: Compound 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.) and sodium p‐tolylsulfinate (35.6 mg, 0.2 mmol, 1.0 equiv.) were dissolved in CH2Cl2/t‐BuOH 1 : 1 (2 mL) and the reaction mixture was stirred at room temperature for 12 h. Pure 4 c (60.6 mg, 0.152 mmol, 76 %) was obtained as a colorless oil by column chromatography (gradient hexanes→hexanes : EtOAc 1 : 1). 1 H NMR (400 MHz, CDCl3): δ=7.98 (d, J=8.3 Hz, 2H), 7.75 (d, J=8.3 Hz, 2H), 7.58 (t, J=7.5 Hz, 1H), 7.47–7.32 (m, 5H), 7.29–7.24 (m, 2H), 7.22–7.18 (m, 2H), 6.87 (s, 1H), 2.47 (s, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=151.9, 145.3, 141.4, 138.6, 138.4, 134.4, 132.7, 130.4, 129.9, 129.8, 129.4, 129.1, 128.6, 128.5, 21.9 ppm. IR (neat): =3060, 3011, 1595, 1325, 1148, 1079, 907, 726, 670, 563 cm−1. HRMS (ESI) m/z calcd for C21H19O4S2 + [M+H]+: 399.0719; found: 399.0704.
(Z)‐[(1‐Phenyl‐2‐thiocyanatovinyl)sulfonyl]benzene 4 d: Salt 5 a (115.3 mg, 0.2 mmol, 1 equiv.) was added in one portion to a stirred solution of NaSCN (19.5 mg, 0.24 mmol, 1.2 equiv.) in a mixture of CH2Cl2 (1 mL) and t‐BuOH (1 mL). The reaction mixture was stirred at r.t. for 12 h. Column chromatography (gradient hexanes→hexanes : EtOAc 4 : 1) of the reaction mixture thus obtained afforded pure 4 d (43.4 mg, 0.144 mmol, 72 %) as a white solid. 1 H NMR (400 MHz, CDCl3): δ=7.65 (dd, J=8.4, 1.2 Hz, 2H), 7.60 (t, J=7.5 Hz, 1H), 7.47–7.42 (m, 2H), 7.37 (t, J=7.4 Hz, 1H), 7.29 (t, J=7.6 Hz, 2H), 7.22–7.18 (m, 2H), 6.99 (s, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=142.5, 138.1, 134.6, 131.7, 131.2, 130.2, 129.5, 129.4, 128.8, 128.1, 111.9 ppm. IR (neat): =3030, 1737, 1367, 1306, 1210, 1146, 1084, 753, 684, 651 cm−1. HRMS (ESI) m/z calcd for C15H11NNaO2S2 + [M+Na]+: 324.0123; found: 324.0122.
(Z)‐Triphenyl[2‐phenyl‐2‐(phenylsulfonyl)vinyl]phosphonium trifluoromethanesulfonate 4 e: A Schlenk flask equipped with a stirring bar was charged with 5 a (115.3 mg, 0.2 mmol, 1 equiv.) and Ph3P (63 mg, 0.24 mmol, 1.2 equiv.). Both reactants were dissolved in CH2Cl2 (2 mL) and the reaction mixture was stirred at room temperature for 12 h. After this the solvent was evaporated and diethyl ether (10 mL) was added to precipitate the salt, which was filtered off, washed once more with diethyl ether (10 mL) and dried in vacuo. Salt 4 e (83.8 mg, 0.128 mmol, 64 %) was obtained as a white solid. 1 H NMR (400 MHz, CD3CN): δ=8.54 (d, J=8.6 Hz, 2H), 8.31 (d, J=7.9 Hz, 2H), 7.98 (t, J=7.7 Hz, 2H), 7.95–7.88 (m, 5H), 7.87–7.82 (m, 2H), 7.77 (d, J=7.5 Hz, 3H), 7.64–7.59 (m, 3H), 7.46–7.38 (m, 2H), 7.31 (d, J=4.6 Hz, 4H), 6.85 (s, 1H) ppm. 31 P NMR (162 MHz, CD3CN): δ=16 ppm. 13 C NMR (101 MHz, CD3CN): δ=166.5, (d, 2 J P‐C=3.6 Hz), 157.3, 141.0, 136.8, 135.8, 135.7 (d, 4 J P‐C=3.2 Hz), 135.0 (d, 1 J P‐C=10.8 Hz), 134.6 (d, 4 J P‐C=10.7 Hz), 132.9, 132.4, 131.7, 131.3 (d, 1 J P‐C=13.3 Hz), 131.0 (d, 2 J P‐C=13.5 Hz), 130.8, 130.2, 130.2, 129.7 (d, 2 J P‐C=13.7 Hz), 129.6 (d, 2 J P‐C=14.0 Hz), 127.9, 125.3, 122.1 (q, 1 J C‐F=321 Hz), 122.0 (d, 1 J P‐C=93.7 Hz), 121.3 ppm. IR (ATR): =3730, 3628, 2266, 1648, 1255, 1148, 1029, 686, 636, 515 cm−1. HRMS (ESI) m/z calcd for C32H26O2PS+ [M−OTf]+: 505.1386; found: 505.1385.
(E/Z)‐(2‐Bromophenyl)[2‐phenyl‐2‐(phenylsulfonyl)vinyl] sulfane 4 f: A suspension of 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (65.2 mg, 0.2 mmol, 1.0 equiv.) and 2‐bromobenzenethiol (37.8 mg, 0.2 mmol, 1.0 equiv.) in CH2Cl2 (2 mL) was stirred at r.t. for 12 h and then directly transferred to a pre‐wetted chromatography column (hexanes). Pure 4 f (77.6 mg, 179.8 μmol, 90 %) was obtained as a white solid (mixture of Z/E isomers (5 : 1)) after column chromatography (gradient hexanes→hexanes : EtOAc 1 : 1). 1 H NMR (400 MHz, CDCl3): δ=7.85 (dd, J=8.4, 1.2 Hz, 2H), 7.67–7.61 (dt J=1.4, 8.0 Hz, 2H), 7.59–7.52 (m, 1H), 7.47–7.42 (m, 2H), 7.41–7.26 (m, 4H), 7.26–7.19 (m, 3H), 6.98 (s, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=144.7, 140.4, 136.3, 135.7, 134.4, 134.3, 134.0, 133.6, 130.6, 130.3, 130.0, 129.0, 128.8, 128.44, 128.39, 127.8 ppm. IR (ATR): =3728, 3626, 1737, 1557, 1447, 1306, 1146, 1084, 753, 664 cm−1. HRMS (ESI) m/z calcd for C20H16O2S2 + [M+H]+: 430.9770; found: 430.9769.
(Z)‐1‐Methyl‐4‐{[2‐phenyl‐2‐(phenylsulfonyl)vinyl]oxy} benzene 4 g: A suspension of 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (65.2 mg, 0.2 mmol, 1.0 equiv.) and p‐cresol (21.6 mg, 0.2 mmol, 1.0 equiv.) was stirred in CH2Cl2 (2 mL) at r.t. for 12 h. Then, the solvents were evaporated, and the residue was charged into a pre‐wetted chromatography column (hexanes). Pure 4 g (64.5 mg, 0.184 mmol, 92 %) was obtained as a white solid by column chromatography (gradient hexanes→hexanes : EtOAc 1 : 1). 1 H NMR (400 MHz, CDCl3): δ=7.98 (dd, J=8.4, 1.3 Hz, 2H), 7.61–7.56 (m, 1H), 7.52–7.46 (m, 2H), 7.43–7.36 (m, 5H), 7.11 (d, J=8.1 Hz, 2H), 6.90 (s, 1H), 6.83 (d, J=8.6 Hz, 2H), 2.31 (s, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=154.4, 150.3, 142.5, 134.9, 133.1, 131.3, 131.0, 130.4, 129.0, 128.8, 128.5, 128.1, 125.9, 117.2, 20.8 ppm. IR (ATR): =3080, 2923, 1632, 1600, 1504, 1224, 1141, 726, 686, 574 cm−1. HRMS (ESI) m/z calcd for C21H18NaO3S+ [M+Na]+: 373.0869; found: 373.0863.
(Z)‐[2‐Fluoro‐1‐(phenylsulfonyl)vinyl]benzene 4 i: 5 a (115.3 mg, 0.2 mmol, 1 equiv.) was added in one portion to a solution of KF (13.9 mg, 0.24 mmol, 1.2 equiv.) in CH2Cl2 (1 mL) and t‐BuOH (1 mL). The reaction mixture was stirred for 12 h at r. t. Then, the solvents were evaporated, and the residue was charged into a pre‐wetted chromatography column (hexanes). Pure product 4 i (21.0 mg, 80 μmol, 40 %) was obtained as a white solid by column chromatography (gradient hexanes→hexanes : EtOAc 4 : 1). 1 H NMR (400 MHz, CDCl3): δ=7.84 (d, J=7.5 Hz, 2H), 7.62 (t, J=7.5 Hz, 1H), 7.50 (t, J=7.8 Hz, 2H), 7.41 (d, J=7.3 Hz, 1H), 7.35 (t, J=7.4 Hz, 2H), 7.29–7.25 (m, 2H), 6.83 (d, J=78.5 Hz, 1H) ppm. 19 F NMR (376 MHz, CDCl3): δ=−101.96 (d, J=78.6 Hz) ppm. 13 C NMR (101 MHz, CDCl3): δ=152.8 (d, 1 J C‐F=292.7 Hz), 140.8, 133.9, 131.0 (d, 2 J C‐F=3.2 Hz), 130.2, 130.2, 129.9, 129.2, 128.7, 128.1 (d, 3 J C‐F=1.1 Hz) ppm. IR (neat): =3728, 3628, 1648, 1325, 1180, 1135, 1079, 732, 667, 553 cm−1. HRMS (ESI) m/z calcd for C14H12FO2S+ [M+H]+: 263.0537; found: 263.0538.
(Z)‐[2‐Bromo‐1‐(phenylsulfonyl)vinyl]benzene 4 j: Salt 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.) and NEt4Br (64.5 mg, 0.2 mmol, 1.0 equiv.) were dissolved in CH2Cl2 (2 mL). The reaction mixture was stirred at room temperature for 12 h and then directly transferred to a pre‐wetted column (hexanes). Analytically pure 4 j (58.8 mg, 181.9 μmol, 91 %) was obtained as a colorless oil by column chromatography (gradient hexanes→hexanes : EtOAc 8 : 2). 1 H NMR (400 MHz, CDCl3): δ=7.84–7.79 (m, 2H), 7.60 (ddt, J=7.9, 7.0, 1.2 Hz, 1H), 7.49–7.44 (m, 2H), 7.41–7.36 (m, 1H), 7.34–7.29 (m, 2H), 7.25–7.21 (m, 2H), 7.01 (s, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=147.7, 139.8, 134.2, 133.9, 130.0, 129.7, 129.0, 129.0 (2 overlapped 13C signals), 128.4, 117.4 ppm. IR (neat): =1576, 1445, 1319, 1146, 1082, 836, 718, 686, 533, 512 cm−1. HRMS (ESI) m/z calcd for C14H11BrNaO2S+ [M+Na]+: 344.9555; found: 344.9553.
(Z)‐N,4‐dimethyl‐N‐[2‐phenyl‐2‐(phenylsulfonyl)vinyl]benzene sulfonamide 4 k: A suspension of 5 a (115.3 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (78.2 mg, 0.24 mmol, 1.2 equiv.) and N,4‐dimethyl benzenesulfonamide (44.5 mg, 0.24 mmol, 1.2 equiv.) in CH2Cl2 (2 mL) was stirred at r.t. for 12 h. and then directly transferred to a pre‐wetted column (hexanes). Compound 4 k (71.0 mg 0.166 mmol, 83 %) was obtained as an off‐white solid after purification by column chromatography (gradient hexanes→hexanes : EtOAc 1 : 1). 1 H NMR (400 MHz, CDCl3): δ=8.38 (s, 1H), 7.74 (d, J=8.3 Hz, 2H), 7.56–7.47 (m, 3H), 7.40–7.33 (m, 4H), 7.28 (d, J=7.5 Hz, 1H), 7.16 (t, J=7.9 Hz, 2H), 6.93 (d, J=8.3 Hz, 2H), 2.47 (s, 3H), 2.46 (s, 3H) ppm. 13 C NMR (101 MHz, CD3CN): δ=145.3, 139.8, 136.7, 134.2, 133.0, 132.5, 130.4, 129.5, 129.3, 128.8, 128.3, 128.0, 127.5, 122.2, 34.8, 21.8 ppm. IR (ATR): =1630, 1357, 1287, 1170, 1141, 1082, 978, 943, 688, 545 cm−1. HRMS (ESI) m/z calcd for C22H22NNaO4S2 + [M+Na]+: 450.0804; found: 450.0794.
(Z)‐5‐[2‐Phenyl‐2‐(phenylsulfonyl)vinyl]‐5H‐dibenzo[b ,d] thiophen‐5‐ium trifluoromethanesulfonate 5 a: A Schlenk flask was equipped with a stirring bar and charged with 1 b (1.01 g, 2.32 mmol, 1.0 equiv.) and phenylsulfinic acid (330 mg, 2.32 mmol, 1.0 equiv.). The reactants were dissolved in a mixture of DCM (10 mL) and t‐BuOH (1 mL) and the reaction mixture was stirred at room temperature for 12 h. After this, the solvents were evaporated, and diethyl ether (2×40 mL) was added to wash the salt, which was finally dried in vacuo. Product 5 a was obtained as a beige solid (1.113 g, 1.93 mmol, 83 %); m.p.: 184–185 °C. 1 H NMR (400 MHz, CD3CN) δ=8.52 (d, J=8.2 Hz, 2H), 8.31 (d, J=7.8 Hz, 2H), 7.98 (t, J=7.7 Hz, 2H), 7.93 (d, J=8.5 Hz, 2H), 7.87–7.83 (m, 2H), 7.79 (t, J=7.5 Hz, 1H), 7.64–7.59 (m, 2H), 7.43 (t, J=8.7 Hz, 1H), 7.34–7.29 (m, 4H), 6.84 (s, 1H) ppm. 13 C NMR (101 MHz, CD3CN): δ=157.5, 141.1, 136.9, 136.3, 135.8, 132.9, 132.5, 131.7, 130.9, 130.6, 130.30, 130.28, 129.8, 129.6, 127.9, 125.4, 122.1 (q, 1 J C‐F=321 Hz) ppm. IR (ATR): =2360, 2337, 1447, 1309, 1253, 1224, 1148, 1028, 759, 635 cm−1. HRMS (ESI) m/z calcd for C26H19O2S2 + [M−OTf]+: 427.0821; found: 427.0821.
2‐[Phenyl(phenylsulfonyl)methylene]naphtho[1,8‐de][1,3] dithiine 6: 1 b (87.0 mg, 0.20 mmol, 1.0 equiv.), naphtho[1,8‐cd][1,2]dithiole (76.1 mg, 0.4 mmol, 2.0 equiv.), 15‐C‐5 (100.1 mg, 90 μL, 0.45 mmol, 2.25 equiv.) and sodium phenylsulfinate (49.2 mg, 0.3 mmol, 1.5 equiv.) were dissolved in DCM (3 mL) and the mixture stirred at r.t. under nitrogen overnight. Then, the solvents were evaporated and the residue charged into a pre‐wetted silica column (hexanes). Pure 6 (36.3 mg, 83.9 μmol, 42 %) was obtained as a white solid after column chromatography (gradient hexanes→hexanes : EtOAc 8 : 2). 1 H NMR (400 MHz, CDCl3): δ=7.83 (dd, J=8.4, 1.2 Hz, 2H), 7.63 (d, J=8.1 Hz, 1H), 7.61–7.55 (m, 2H), 7.50 (d, J=6.2 Hz, 1H), 7.48–7.35 (m, 6H), 7.30–7.26 (m, 1H), 7.10 (dd, J=11.3, 7.2 Hz, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=143.3, 140.7, 134.3, 133.5, 133.1, 131.8, 130.8, 129.6, 128.9, 128.8, 128.3, 128.2, 128.1, 127.5, 127.4, 126.7, 126.4, 124.1, 123.3, 122.7 ppm. IR (ATR): =1557, 1506, 1314, 1207, 1146, 1082, 684, 667, 651, 577 cm−1. HRMS (ESI) m/z calcd for C24H17O2S3 + [M+H]+: 433.0385; found: 433.0388.
(Z)‐5‐(2‐Chloro‐2‐phenylvinyl)‐5H‐dibenzo[b ,d]thiophen‐5‐ium trifluoromethanesulfonate 7: Salt 1 b (102 mg, 235 μmol, 1.0 equiv.) was dissolved in CH2Cl2 (2 mL), and HCl (0.18 mL, 2 M solution in Et2O; 360 μmol, 1.53 equiv.) was added. After stirring the reaction overnight the solvent was removed and diethyl ether (2×10 mL) was added to wash the resulting oil. Salt 7 (53.1 mg, 112.7 μmol, 48 %) was obtained as a white foam. 1 H NMR (400 MHz, CD3CN): δ=8.32 (d, J=8.6 Hz, 2H), 8.27 (d, J=8.1 Hz, 2H), 7.97 (t, J=8.2 Hz, 2H), 7.79 (t, J=7.2 Hz, 2H), 7.76–7.73 (m, 2H), 7.61–7.57 (m, 1H), 7.52–7.47 (m, 2H), 6.84 (s, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=156.4, 141.0, 135.6, 134.3, 134.0, 132.6, 130.7, 130.2, 129.3, 128.7, 125.4, 122.1 (q, 1 J C‐F=322 Hz), 113.7 ppm. IR (ATR): =1702, 1557, 1445, 1253, 1221, 1151, 1028, 924, 753, 635 cm−1. HRMS (ESI) m/z calcd for C20H14ClS+ [M−OTf]+: 321.0499; found: 321.0494.
(E)‐5‐(2‐Chloro‐1‐iodo‐2‐phenylvinyl)‐5H‐dibenzo[b ,d]thio‐phen‐5‐ium trifluoromethanesulfonate 8: Iodine monochloride (45.8 mg, 282 μmol, 1.2 equiv.) was added to a solution of 1 b (102 mg, 235 μmol, 1.0 equiv.) in CH2Cl2 (2 mL) and the reaction mixture stirred at r.t. overnight. Subsequently, the solvent was removed under reduced pressure and the residue washed with diethyl ether (3×10 mL). Removal of the solvent using a filtration cannula afforded 8 as a white solid, which was dried in vacuum (112.2 mg, 0.188 mmol, 80 %), m.p.: 255 °C (with decomposition). 1 H NMR (400 MHz, CD3CN): δ=8.32 (dd, J=8.1, 1.5 Hz, 2H), 8.28 (dd, J=8.1, 1.6 Hz, 2H), 8.07–8.02 (m, 2H), 7.87–7.81 (m, 2H), 7.62 (dd, J=8.0, 1.7 Hz, 2H), 7.59–7.51 (m, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=155.5, 141.8, 139.1, 136.5, 132.9, 132.9 (overlapped 13C signals), 130.9, 130.0, 129.8, 129.6, 125.6, 122.1 (q, 1 J C‐F=321 Hz), 84.0 ppm. IR (ATR): =3086, 2360, 2268, 1253, 1221, 1156, 1025, 758, 673, 638 cm−1. HRMS (ESI) m/z calcd for C20H13ClIS+ [M−OTf]+: 446.9466; found: 446.9466.
(E)‐5‐(1,2‐Dibromo‐2‐phenylvinyl)‐5H‐dibenzo[b ,d]thiophen‐5‐ium trifluoromethanesulfonate 9: Bromine (37.6 mg, 12 μL, 235 μmol, 1.0 equiv.) was added to a solution of 1 b (102 mg, 235 μmol, 1.0 equiv.) in CH2Cl2 (2 mL) and the reaction stirred at r.t. overnight. Removal of the solvent under reduced pressure afforded a solid that was washed with Et2O (3×10 mL) and dried in vacuum. Compound 9 (103.3 mg, 173.8 μmol, 74 %) was obtained as a yellow solid. 1 H NMR (400 MHz, CD3CN): δ=8.38 (d, J=8.1 Hz, 2H), 8.31 (d, J=8.6 Hz, 2H), 8.01 (t, J=7.7 Hz, 2H), 7.83 (t, J=8.4 Hz, 2H), 7.58 (dd, J=7.4, 2.3 Hz, 2H), 7.54–7.47 (m, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=144.4, 141.9, 138.7, 136.6, 132.8, 132.6, 129.9, 129.8, 129.5, 129.3, 125.6, 122.1 (q, 1 J C‐F=322 Hz), 108.2 ppm. IR (ATR): =1445, 1258, 1224, 1156, 1025, 758, 697, 670, 635, 518 cm−1. HRMS (ESI) m/z calcd for C20H13Br2S+ [M−OTf]+: 442.9099; found: 442.9100.
(E)‐5‐{2‐Chloro‐1‐iodo‐2‐[4‐(trifluoromethyl)phenyl]vinyl}‐5H‐dibenzo[b ,d]thiophen‐5‐ium trifluoromethanesulfonate 10: Iodine monochloride (268 mg, 1.65 mmol, 1.1 equiv.) was added in one portion to a stirred solution of 1 c (754 mg, 1.5 mmol, 1.0 equiv.) in CH2Cl2 (10 mL) and the reaction mixture stirred at r.t. overnight. Removal of the solvent under reduced pressure causes solidification of a residue, which was washed with hexane (3×100 mL). After drying in vacuum, salt 10 (917 mg, 1.38 mmol, 92 %) was obtained as a yellow solid. 1 H NMR [400 MHz, (CD3)2CO]: δ=8.58 (d, J=8.1 Hz, 2H), 8.54 (d, J=7.8 Hz, 2H), 8.12 (t, J=8.1 Hz, 2H), 7.93 (s, 6H) ppm. 19 F NMR [377 MHz, (CD3)2CO]: δ=−63.6, −78.8 ppm. 13 C NMR [101 MHz, (CD3)2CO]: δ=152.3, 143.2 (q, 4 J C‐F=1.3 Hz), 141.9, 136.4, 132.8, 131.5, 130.9, 130.5, 130.0, 126.9 (q, 3 J C‐F=3.6 Hz), 125.6, 124.6 (q, 1 J C‐F=272.6 Hz), 122.3 (q, 1 J C‐F=322.3 Hz), 87.6 ppm. IR (ATR): =3730, 3628, 1739, 1325, 1242, 1165, 1023, 670, 633, 512 cm−1. HRMS (ESI) m/z calcd for C21H12ClF3IS+ [M−OTf]+: 514.9340; found: 514.9344.
(E)‐5‐{1,2‐Dibromo‐2‐[4‐(trifluoromethyl)phenyl]vinyl}‐5H‐dibenzo[b ,d]thiophen‐5‐ium trifluoromethanesulfonate 11: Bromine (36.8 mg, 11.8 μL, 230 μmol, 1.0 equiv.) was added in one portion to a solution of salt 1 c (115.6 mg, 230 μmol, 1.0 equiv.) in CH2Cl2 (2 mL) and the reaction mixture thus obtained was stirred at room temperature for 1 h. Removal of the solvent under reduced pressure, afforded a solid, which was washed with Et2O (3×10 mL) and pentane (10 mL). Salt 11 (120.3 mg, 181.6 μmol, 79 %) was obtained as a white solid. 1 H NMR (400 MHz, CD3CN): δ=8.37 (d, J=7.7 Hz, 2H), 8.31 (dd, J=8.1, 0.9 Hz, 2H), 8.03 (td, J=7.7, 1.0 Hz, 2H), 7.86–7.80 (m, 4H), 7.76–7.71 (m, 2H) ppm. 19 F NMR (377 MHz, CD3CN): δ=−63.7, 79.3 ppm. 13 C NMR (101 MHz, CDCl3): δ=142.5, 142.1, 142.1, 136.8, 133.3, 133.0, 130.2, 130.0, 129.1, 127.1 (q, 3 J C‐F=3.8 Hz, CF3), 125.9, 125.7, 122.1 (q, 1 J C‐F=321 Hz), 110.5 ppm. IR (ATR): =3730, 1325, 1255, 1224, 1159, 1130, 1063, 1028, 761, 635 cm−1. HRMS (ESI) m/z calcd for C21H12Br2F3S+ [M−OTf]+: 512.8953; found: 512.8964.
5‐{3‐[4‐(Trifluoromethyl)phenyl]bicyclo[2.2.1]hepta‐2,5‐dien‐2‐yl}‐5H‐dibenzo[b ,d]thiophen‐5‐ium trifluoromethane‐sulfonate 12: Freshly distilled cyclopentadiene (168.2 μL, 132.2 mg, 2.0 mmol, 5 equiv.) was added to a solution of 1 c (200 mg, 0.40 mmol, 1.0 equiv.) in MeCN (2 mL), and the reaction mixture was heated to 100 °C for 2 h under microwave irradiation. Subsequently, the solvent was evaporated and the residue washed with n‐hexane (2×20 mL). The solid thus obtained was dried in vacuum affording 12 (152.4 mg, 0.268 mmol, 67 %, a mixture of rotamers) as a pale green solid. 1 H NMR (400 MHz, CD3CN): δ=8.38 (dd, J=8.2, 4.0 Hz, 2H), 8.30 (d, J=8.6 Hz, 1H), 8.03–7.94 (m, 6H), 7.87–7.82 (m, 1H), 7.71–7.60 (m, 2H), 6.97–6.92 (m, 1H), 6.24–6.20 (m, 1H), 4.25 (s, 1H), 2.77 (s, 1H), 2.22–2.15 (m, 1H), 1.99–1.96 (m, 1H) ppm. 19 F NMR (377 MHz, CD3CN): δ=−63.3, −79.3 ppm. 13 C NMR (101 MHz, CD3CN): δ=178.7, 143.3, 141.3, 141.2, 140.9, 136.6 (q, 4 J C‐F=1.3 Hz), 135.6, 135.5, 132.9 (q, 2 J C‐F=32.8 Hz), 132.68, 131.9, 131.3, 129.8, 129.44, 129.42, 129.42 (2 overlapped 13C signals), 129.0, 127.4, 127.1 (q, 3 J C‐F=3.8 Hz), 126.0, 125.6, 125.1 (q, 1 J C‐F=272.0 Hz), 122.1 (q, 1 J C‐F=320.2 Hz), 71.6, 60.0, 52.2 ppm (19 13C signals expected, 26 detected because of the hindered rotation of the DBT‐unit). IR (ATR): =3091, 2950, 1710, 1325, 1258, 1159, 1121, 1068, 1028, 635 cm−1. HRMS (ESI) m/z calcd for C26H18F3S+ [M−OTf]+: 419.1076; found: 419.1079.
5‐{3‐([1,1′‐Biphenyl]‐2‐yl)bicyclo[2.2.1]hepta‐2,5‐dien‐2‐yl}‐5H‐dibenzo[b,d]thiophen‐5‐ium trifluoromethanesulfonate 13: Freshly distilled cyclopentadiene (168.2 μL, 132.2 mg, 2.0 mmol, 5.0 equiv.) was added to a solution of 1 d (204 mg, 0.4 mmol, 1.0 equiv.) in MeCN (2 mL). The reaction mixture was heated at 100 °C for 3 h under microwave irradiation. Subsequently, the solvents were removed in vacuum and the solid thus obtained washed with Et2O (3×30 mL). Compound 13 (119.9 mg, 207.9 μmol, 52 %) was obtained as a brown solid. 1 H NMR (400 MHz, CD3CN): δ=δ 8.29 (t, J=9.4 Hz, 2H), 7.95–7.88 (m, 2H), 7.71 (d, J=8.7 Hz, 1H), 7.68–7.63 (m, 3H), 7.62–7.60 (m, 1H), 7.60–7.55 (m, 3H), 7.54–7.49 (m, 3H), 7.26 (d, J=8.0 Hz, 2H), 6.56–6.51 (m, 1H), 6.06–6.02 (m, 1H), 3.84–3.79 (m, 1H), 2.57–2.52 (m, 1H), 2.08–2.05 (m, 1H), 1.86–1.83 (m, 1H) ppm. 13 C NMR (101 MHz, CD3CN): δ=142.2, 141.7, 141.5, 141.1, 140.7, 135.4, 135.3, 132.5, 132.4, 132.1, 131.8, 131.7, 131.1, 130.0, 129.8, 129.6, 129.3, 129.2, 129.0, 128.8, 128.4, 128.0, 125.9, 125.5, 122.1 (q, 1 J C‐F=322 Hz), 72.0, 61.2, 51.1 ppm. IR (ATR): =3730, 3628, 3594, 1539, 1260, 1151, 1028, 755, 673, 651 cm−1. HRMS (ESI) m/z calcd for C31H23S+ [M−OTf]+: 427.1515; found: 427.1518.
1,4‐Dihydro‐1,4‐methanotriphenylene 14: A Schlenk flask equipped with a stirring bar was charged with 13 (115.3 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (71.7 mg, 0.22 mmol, 1.1 equiv.) and [Ru(bipy)3]Cl2×6H2O (3 mg, 4.0 μmol, 2.0 mol%), purged three times with nitrogen. After adding degassed MeCN (2 mL) the reaction mixture was exposed to blue LED light irradiation (28 W) at r.t. for 16 h. Subsequently, the solvents were evaporated and the residue charged into a pre‐wetted silica column (hexanes). Pure 14 (22.8 mg, 94 μmol, 47 %) was obtained after column chromatography (gradient hexanes→hexanes : CH2Cl2 4 : 1) as a white solid. 1 H NMR (400 MHz, CDCl3): δ=8.73 (d, J=8.1 Hz, 2H), 8.06 (d, J=7.6 Hz, 2H), 7.66–7.56 (m, 4H), 7.02 (s, 2H), 4.63 (s, 2H), 2.55 (d, J=6.6 Hz, 1H), 2.46 (d, J=6.6 Hz, 1H) ppm. 13 C NMR (101 MHz, CDCl3): δ=147.9, 143.8, 129.0, 128.7, 126.5, 125.3, 123.6, 123.5, 72.5, 49.2 ppm. IR (ATR): =3060, 2980, 2928, 2862, 1506, 1298, 753, 720, 670, 660 cm−1. HRMS (GCMS) m/z calcd for C19H14 + [M]+: 242.1090; found: 242.1090. Analytical data are identical to the previously reported ones.[29]
(Z)‐5‐{2‐([1,1′‐Biphenyl]‐2‐yl)‐2‐(phenylsulfonyl)vinyl}‐5H‐dibenzo‐[b,d]thiophen‐5‐ium trifluoromethanesulfonate 15: A Schlenk flask was equipped with a stirring bar and charged with 1 d (944.5 mg, 1.85 mmol, 1.0 equiv.) and phenylsulfinic acid (526 mg, 3.70 mmol, 2.0 equiv.). Subsequently, CH2Cl2 (16 mL) and t‐BuOH (2 mL) were added and the reaction mixture was stirred at r.t. overnight. After this, the solvents were evaporated to a minimal volume and Et2O (50 mL) was added, which causes precipitation of crude 15. The latter was filtered off, washed with Et2O (30 mL) and dried in vacuo to afford 15 (905.6 mg, 138.7 μmol, 75 %) as a pale yellow solid. 1 H NMR (400 MHz, CD3CN): δ=8.26 (d, J=7.9 Hz, 2H), 8.06 (d, J=8.1 Hz, 2H), 7.98–7.90 (m, 3H), 7.86 (d, J=7.4 Hz, 2H), 7.79–7.75 (m, 2H), 7.74–7.69 (m, 2H), 7.49 (t, J=7.6 Hz, 1H), 7.36 (t, J=7.7 Hz, 1H), 7.27–7.19 (m, 3H), 7.03 (t, J=7.8 Hz, 2H), 6.58 (d, J=9.5 Hz, 2H), 6.52 (s, 1H) ppm. 13 C NMR (101 MHz, CD3CN): δ=157.2, 143.8, 140.9, 139.8, 137.3, 136.2, 135.8, 132.9, 132.2, 131.9, 131.3, 130.9, 130.8, 130.6, 130.1, 129.9, 129.5, 129.2, 128.7, 128.6, 128.4, 125.4, 122.1 (q, 1 J C‐F=321 Hz) ppm. IR (ATR): =3735, 3626, 3064, 3014, 1445, 1255, 1148, 1025, 635 cm−1. HRMS (ESI) m/z calcd for C32H23O2S+ [M−OTf]+: 503.1134; found: 503.1137.
9‐(Phenylsulfonyl)phenanthrene 16: A Schlenk flask was equipped with a stirring bar and charged with 15 (130.5 mg, 0.2 mmol, 1.0 equiv.), Cs2CO3 (71.7 mg, 0.22 mmol, 1.1 equiv.) and [Ru(bipy)3]Cl2×6H2O (3 mg, 4.0 μmol, 2.0 mol%). Subsequently, CH3CN (2 mL) was added and the reaction mixture was exposed to blue LED light irradiation (28 W) at r.t. for 16 h. Subsequently, the solvents were evaporated and the residue charged into a pre‐wetted silica column (hexanes). Pure 16 (36.9 mg, 115.8 μmol, 58 %) was obtained by column chromatography (gradient hexanes→hexanes : EtOAc 4 : 1) as a pale‐yellow solid. 1 H NMR (400 MHz, CDCl3): δ=8.92 (s, 1H), 8.74–8.67 (m, 2H), 8.63 (d, J=9.0 Hz, 1H), 8.11 (d, J=8.5 Hz, 1H), 8.00 (d, J=7.3 Hz, 2H), 7.83 (t, J=8.4 Hz, 1H), 7.73 (t, J=7.1 Hz, 1H), 7.68 (t, J=7.0 Hz, 1H), 7.62–7.58 (m, 1H), 7.53 (t, J=7.3 Hz, 1H), 7.47 (t, J=7.4 Hz, 2H) ppm. 13 C NMR (101 MHz, CDCl3): δ=141.7, 134.3, 133.2, 133.1, 132.9, 131.4, 130.9, 130.2, 129.4, 129.2, 127.8, 127.7, 127.5, 127.5 (2 overlapped 13C signals), 126.1, 125.5, 123.4, 122.9 ppm. IR (ATR): =1447, 1322, 1151, 1082, 791, 753, 734, 686, 649, 566 cm−1. HRMS (ESI) m/z calcd for C20H14NaO2S+ [M+Na]+: 341.0607; found: 341.0608. Analytical data are identical to the previously reported ones.[30]
N‐(3‐Bromo‐2‐iodo‐4,5,6‐trimethoxyphenethyl)‐4‐methyl‐benzenesulfonamide 21: A solution of 20 (2.13 g, 5.83 mmol, 1.0 equiv.) and NBS (1.04 g, 5.84 mmol, 1.0 equiv.) in CCl4 (20 mL) was stirred at 50 °C for 16 h in the dark. After cooling to rt, the reaction was quenched with water (25 mL), and the aqueous phase was extracted with CH2Cl2 (3×30 mL). The combined organic phases were washed with sat. aq. Na2SO3 (30 mL) and brine (30 mL), dried over Na2SO4 and concentrated in vacuo. Purification by column chromatography (hexane/EtOAc, 7 : 3) gave N‐(5‐bromo‐2,3,4‐trimethoxyphenethyl)‐4‐methylbenzene‐sulfonamide (2.31 g, 5.20 mmol, 89 %) as an off‐white solid. 1 H NMR (300 MHz, CDCl3): δ=7.67 (d, J=8.1 Hz, 2H), 7.27 (d, J=8.3 Hz, 2H), 6.88 (s, 1H), 4.61 (t, J=5.9 Hz, 1H), 3.92–3.77 (m, 9H), 3.17 (q, J=6.5 Hz, 2H), 2.67 (t, J=6.8 Hz, 2H), 2.42 (s, 3H) ppm. 13 C NMR (75 MHz, CDCl3): δ=151.6, 150.4, 147.5, 143.5, 137.1, 129.8, 128.3, 127.9, 127.2, 111.6, 61.1, 61.1, 61.1, 43.7, 30.3, 21.7 ppm. IR (ATR): =2967, 2930, 2899, 2864, 2822, 1598, 1457, 1402, 1319, 1162, 1068, 1002, 807, 671, 554 cm−1. HRMS (ESI): m/z calcd for C18H23BrNO5S+ [M+H]+: 444.0475; found: 444.0477. To a solution of this compound (2.0 g, 4.50 mmol, 1.0 equiv.) in acetonitrile (19 mL) was added NIS (2.53 g, 11.25 mmol, 2.5 equiv.) and TFA (1.283 g, 0.86 mL, 11.25 mmol, 2.5 equiv.). The reaction mixture was stirred at 110 °C for 16 h under microwave irradiation. The reaction was quenched with sat. aq. Na2SO3 (50 mL) at rt, and the aqueous layer was extracted with DCM (3×30 mL). The combined organic phases were washed with brine (40 mL), dried over MgSO4 and concentrated under reduced pressure. The residue was purified by column chromatography (hexane/EtOAc, 9 : 1→3 : 1). Product 21 (1.94 g, 3.40 mmol, 76 %) was obtained as a brownish oil. 1 H NMR (300 MHz, CDCl3): δ=7.59 (d, J=8.0 Hz, 2H), 7.19 (d, J=8.0 Hz, 2H), 4.81 (t, J=5.4 Hz, 1H), 3.93–3.81 (m, 9H), 3.19 (q, J=6.4 Hz, 2H), 3.04 (t, J=6.8 Hz, 2H), 2.40 (s, 3H) ppm. 13 C NMR (75 MHz, CDCl3): δ=151.1, 150.7, 147.2, 143.4, 136.8, 132.5, 129.7, 127.0, 121.7, 101.6, 61.3, 61.0, 60.9, 42.3, 36.9, 21.7 ppm. IR (ATR): =2936, 2861, 1735, 1458, 1398, 1386, 1325, 1154, 1094, 1046, 999, 813, 662, 548 cm−1. HRMS (ESI): m/z calcd for C18H22BrINO5S+ [M+H]+: 569.9441; found: 569.9444.
N‐{3‐Bromo‐4,5,6‐trimethoxy‐2‐[(triisopropylsilyl) ethynyl]phenethyl}‐4‐methylbenzene‐sulfonamide 22: To a dry THF (11 mL) solution containing 21 (1.90 g, 3.33 mmol, 1.0 equiv.) and NEt3 (4.6 mL), Pd(PPh3)2Cl2 (233 mg, 331.9 μmol, 10 mol%), CuI (126 mg, 0.662 mmol, 0.2 equiv.) and TIPS‐ acetylene (3,65 g, 4.49 mL, 20.0 mmol, 6.01 equiv.) were added, and the mixture was stirred at 90 °C for 60 h under microwave irradiation. The suspension thus obtained was filtered through a pad of silica gel and eluted with CH2Cl2 (60 mL). The filtrate was concentrated in vacuo and the residue purified by column chromatography (hexane/EtOAc, 10 %→30 %). Sulfonamide 22 (1.52 g, 2.43 mmol, 73 %) was obtained as a colorless solid. 1 H NMR (300 MHz, CDCl3): δ=7.51 (d, J=8.1 Hz, 2H), 7.14 (d, J=8.0 Hz, 2H), 4.75 (t, J=5.2 Hz, 1H), 3.91 (s, 3H), 3.85 (s, 3H), 3.81 (s, 3H), 3.24 (q, J=6.1 Hz, 2H), 2.97 (t, J=6.5 Hz, 2H), 2.38 (s, 3H), 1.12 (s, 21H) ppm. 13 C NMR (75 MHz, CDCl3): δ=151.2, 150.5, 147.8, 143.2, 136.5, 131.1, 129.5, 126.9, 121.2, 117.0, 102.5, 100.3, 61.2, 61.1, 61.0, 43.1, 28.8, 21.7, 18.8, 11.4 ppm. IR (ATR): =2940, 2890, 2863, 2151, 1461, 1411, 1397, 1336, 1158, 1093, 1020, 812, 662, 549 cm−1. HRMS (ESI): m/z calcd for C29H43BrNO5SSi+ [M+H]+: 624.1809; found: 624.1802.
4‐Methyl‐N‐(2‐{4,5,6‐trimethoxy‐2‐[(triisopropylsilyl)ethynyl]‐[1,1′‐biphenyl]‐3‐yl}ethyl)‐benzenesulfonamide 23: To a solution of 22 (1.42 g, 2.27 mmol, 1.0 equiv.) and phenylboronic acid (555 mg, 4.55 mmol, 2.0 equiv.) in DME (9.5 mL), Na2CO3 (1.21 g, 11.4 mmol, 5.0 equiv.) and water (3.4 mL) were added. Finally, Pd(PPh3)2Cl2 (160 mg, 228 μmol, 10 mol%) was added to the suspension and the reaction mixture was stirred at 120 °C for 26 h. under microwave irradiation. After cooling to rt, the mixture was acidified with aq. HCl (1 m) to pH=1. The aqueous layer was extracted with EtOAc (3×40 mL), the combined organic phases were washed with brine (50 mL), dried over MgSO4 and concentrated under reduced pressure. Column chromatography (hexane/EtOAc, 10 : 1→3 : 1) yielded 23 (1.39 g, 2.24 mmol, 99 %) as a colorless oil. 1 H NMR (400 MHz, CDCl3): δ=7.60 (d, J=8.3 Hz, 2H), 7.38–7.24 (m, 5H), 7.20 (d, J=7.9 Hz, 2H), 4.88 (t, J=5.1 Hz, 1H), 3.93 (s, 3H), 3.87 (s, 3H), 3.54 (s, 3H), 3.25 (q, J=6.2 Hz, 2H), 3.02 (t, J=6.5 Hz, 1H), 2.38 (s, 3H), 0.88 (s, 21H) ppm. 13 C NMR (101 MHz, CDCl3): δ=151.1, 150.5, 147.2, 143.0, 137.0, 136.5, 135.8, 130.2, 129.9, 129.5, 127.9, 127.3, 127.2, 119.0, 103.4, 98.6, 61.2, 61.1, 61.0, 43.5, 28.4, 21.6, 18.6, 11.2 ppm. IR (ATR): =2939, 2889, 2863, 2146, 1460, 1416, 1405, 1333, 1158, 1090, 1048, 880, 813, 661, 549 cm−1. HRMS (ESI): m/z calcd for C35H48NO5SSi+ [M+H]+: 622.3017; found: 622.3013.
N‐(2‐(2‐ethynyl‐4,5,6‐trimethoxy‐[1,1′‐biphenyl]‐3‐yl)ethyl)‐4‐methylbenzenesulfonamide 24: Under an atmosphere of nitrogen, a Schlenk flask was charged with 23 (126 mg, 202.6 μmol, 1.0 equiv.), and dry THF (2 mL) was added. A solution of TBAF (1 M in THF, 0.26 mL, 0.26 mmol, 1.3 equiv.) was added at 0 °C and the reaction mixture was stirred at rt for 2 h. The reaction was quenched with sat. aq. NH4Cl (6 mL), and the aqueous phase was extracted with EtOAc (3×15 mL). The combined organic phases were washed with brine (20 mL), dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography on silica (hexane/EtOAc, 20 : 1→3 : 1) to deliver 24 (89 mg, 0.19 mmol, 94 %) as a colorless solid, m.p.: 154–155 °C. 1 H NMR (400 MHz, CDCl3): δ=7.66 (d, J=8.3 Hz, 2H), 7.45–7.29 (m, 5H), 7.23 (d, J=8.1 Hz, 2H), 4.83 (t, J=5.4 Hz, 1H), 3.93 (s, 3H), 3.87 (s, 3H), 3.53 (s, 3H), 3.25 (q, J=6.7 Hz, 2H), 3.03–2.97 (m, 3H), 2.39 (s, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=151.2, 150.5, 147.6, 143.1, 137.2, 136.2, 135.8, 130.4, 130.2, 129.6, 127.8, 127.6, 127.2, 117.5, 84.3, 80.5, 61.3, 61.0, 61.0, 43.4, 28.6, 21.7 ppm. IR (ATR): =2936, 2871, 2836, 1460, 1415, 1404, 1327, 1155, 1103, 1020, 814, 705, 661, 548 cm−1. HRMS (ESI): m/z calcd for C26H28NO5S+ [M+H]+: 466.1683; found: 466.1685.
Compound 25: In a Schlenk flask, dibenzothiophene‐S‐oxide (23.5 mg, 0.117 mmol, 1.0 equiv.) was dissolved in CH2Cl2 (1 mL). Tf2O (36.4 mg, 21.7 μL, 0.129 mmol, 1.10 equiv.) was added to the solution at −50 °C, and the red suspension obtained was stirred for 30 min at this temperature. Compound 24 (60.0 mg, 0.129 mmol, 1.1 equiv.) was added at −60 °C, and the reaction mixture was allowed to warm up to −20 °C overnight. The solvent was then removed in vacuo and the residue was washed with dry Et2O (2×2 mL) in an ultrasonic bath. Purification by column chromatography (CH2Cl2/acetone, 9 : 1→1 : 1) delivered 25 (38.0 mg, 47.6 μmol, 41 %) as a brown solid. 1 H NMR (400 MHz, CDCl3): δ=8.33 (d, J=8.1 Hz, 2H), 8.00 (d, J=7.9 Hz, 2H), 7.94 (d, J=8.4 Hz, 2H), 7.79 (t, J=7.6 Hz, 2H), 7.67 (t, J=7.7 Hz, 2H), 7.39 (d, J=8.2 Hz, 2H), 7.15–7.05 (m, 4H), 6.87 (t, J=6.8 Hz, 1H), 4.49 (s, 1H), 3.91 (s, 3H), 3.87 (t, J=6.5 Hz, 2H), 3.79 (s, 3H), 3.50 (s, 3H), 2.93 (t, J=6.5 Hz, 2H), 2.44 (s, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=151.4, 150.6, 150.2, 149.3, 146.1, 139.0, 135.4, 134.2, 133.3, 131.8, 130.5, 130.0, 129.9, 128.8, 128.6, 127.7, 127.6, 125.8, 123.6, 116.0, 61.3, 61.14, 61.11, 45.9, 21.9, 21.0 ppm (triflate‐carbon not observed). IR (ATR): =2939, 1597, 1448, 1402, 1332, 1257, 1232, 1156, 1028, 813, 751, 708, 635 cm−1. HRMS (ESI): m/z calcd for C38H34NO5S2 + [M−OTf]+: 648.1873; found: 648.1874.
1,2,3‐Trimethoxy‐6‐tosyl‐5,6‐dihydro‐4H‐dibenzo[de ,g] quinoline 26 and 4,5,6‐Trimethoxy‐1‐tosyl‐2,3‐dihydro‐1H‐azuleno[1,2,3‐ij]isoquinoline 27: A flame‐dried Schlenk flask was charged with 25 (34.9 mg, 43.7 μmol, 1.0 equiv.), Cs2CO3 (19.9 mg, 61.2 μmol, 1.4 equiv.) and [Ru(bpy)3]Cl2 ⋅ 6H2O (0.97 mg, 1.3 μmol, 3 mol%). CH3CN (1.0 mL) was added, and the solution was degassed with nitrogen for 5 min. The reaction mixture was irradiated with vigorous stirring at r.t. with blue LEDs (28 W) for 22 h. Subsequently, the solvent was removed in vacuo and the residue was purified by flash chromatography (hexane→hexane : EtOAc, 3 : 1) to give 26 (2.1 mg, 4.5 μmol, 10 %) as a green solid and 27 (3.2 mg, 6.9 μmol, 16 %) as a green‐blue solid.
26: 1H NMR (400 MHz, CDCl3): δ=9.53–9.47 (m, 1H), 8.00 (s, 1H), 7.90–7.84 (m, 1H), 7.64–7.54 (m, 2H), 7.45 (d, J=8.3 Hz, 2H), 7.04 (d, J=8.1 Hz, 2H), 4.07 (t, J=6.1 Hz, 2H), 4.02 (s, 3H), 3.96 (s, 3H), 3.79 (s, 3H), 2.81 (t, J=6.1 Hz, 2H), 2.28 (s, 3H) ppm. 13 C NMR (101 MHz, CDCl3): δ=151.4, 149.2, 146.1, 143.6, 137.9, 131.8, 131.0, 129.5, 128.9, 128.4, 127.2, 127.1, 126.8, 126.7, 122.2 (2 overlapped 13C signals), 119.9, 61.3, 60.8, 60.5, 44.5, 22.3, 21.5 ppm. IR (ATR): =2932, 2850, 1737, 1596, 1455, 1386, 1338, 1161, 1090, 1014, 721, 662, 567, 545 cm−1. HRMS (ESI): m/z calcd for C26H26NO5S+ [M+H]+: 464.1526; found: 464.1527.
27: 1H NMR (600 MHz, CDCl3): δ=8.71 (dd, J=8.6, 1.0 Hz, 1H), 8.37 (dt, J=11.1, 0.9 Hz, 1H), 7.36–7.33 (m, 2H), 7.18 (ddt, J=11.0, 8.4, 1.0 Hz, 1H), 7.02–7.00 (m, 2H), 6.99 (ddt, J=11.1, 8.5, 0.8 Hz, 1H), 6.88 (ddd, J=11.1, 8.4, 0.8 Hz, 1H), 4.15 (s, 3H), 4.00 (t, J=5.9 Hz, 2H), 3.93 (s, 3H), 3.77 (s, 3H), 2.43 (t, J=5.9 Hz, 2H), 2.28 (s, 3H). 13 C NMR (126 MHz, CDCl3): δ=151.4, 149.5, 143.8, 142.6, 138.4, 136.9, 135.8, 134.3, 132.8, 131.2, 130.1, 129.5, 127.5, 126.3, 124.0, 120.9, 115.0, 113.6, 61.7, 61.3, 60.7, 47.9, 21.6, 19.8. IR (ATR): =2935, 2840, 1604, 1391, 1350, 1290, 1159, 1139, 1044, 1006, 708, 660, 543 cm−1. HRMS (ESI): m/z calcd for C26H26NO5S+ [M+H]+: 464.1531; found: 464.1526.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft (INST 186/1237‐1 and INST 186/1324‐1) and the European Research Council (ERC CoG 771295) is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
K. Kafuta, C. J. Rugen, T. Heilmann, T. Liu, C. Golz, M. Alcarazo, Eur. J. Org. Chem. 2021, 2021, 4038.
In memory of Klaus Hafner.
Contributor Information
Dr. Kevin Kafuta, https://www.uni‐goettingen.de/en/569043.html
Prof. Dr. Manuel Alcarazo, Email: manuel.alcarazo@chemie.uni-goettingen.de.
References
- 1.Zhdankin V. V., Stang P. J., Tetrahedron 1998, 54, 10927–10966. [Google Scholar]
- 2.For a general monographies in I(III) chemistry see:
- 2a.Wirth T., Topics in Current Chemistry, Vol. 373, Springer International Publishing, Chamm, 2016; [Google Scholar]
- 2b.Hypervalent Iodine Chemistry, V. V. Zhdankin; John Wiley &Sons, Ltd, 2014.
- 3.For recent reviews see:
- 3a.Boelke A., Finkbeiner P., Nachtsheim B. J., Beilstein J. Org. Chem. 2018, 14, 1263–1280; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b.Yoshimura A., Zhdankin V. V., Chem. Rev. 2016,116, 3328–3435; [DOI] [PubMed] [Google Scholar]
- 3c.Brand J. P., Waser J., Chem. Soc. Rev. 2012, 41, 4165–4179. [DOI] [PubMed] [Google Scholar]
- 4.Beringer F. M., Galton S. A., J. Org. Chem. 1965, 30, 1930–1934. [Google Scholar]
- 5.Ochiai M., Masaki Y., Shiro M., J. Org. Chem. 1991, 56, 5511–5513. [Google Scholar]
- 6.Zhdankin V. V., Kuehl C. J., Krasutsky A. P., Bolz J. T., Simonsen A. J., J. Org. Chem. 1996, 61, 6547–6551. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a.Ochiai M., Kunishima M., Nagao Y., Fuji K., Shiro M., Fujita E., J. Am. Chem. Soc. 1986, 108, 8281–8283; [Google Scholar]
- 7b.Ochiai M., Ito T., Takaoka Y., Masaki Y., Kunishima M., Tani S., Nagao Y., J. Chem. Soc. Chem. Commun. 1990, 118–119. [Google Scholar]
- 8.Bachi M. D., Bar-Ner N., Crittell C. M., Stang P. J., Williamson B. L., J. Org. Chem. 1991, 56, 3912–3915. [Google Scholar]
- 9.
- 9a.Fernández-González D., Brand J. P., Waser J., Chem. Eur. J. 2010, 16, 9457–9461; [DOI] [PubMed] [Google Scholar]
- 9b.Fernández-González D., Brand J. P., Mondière R., Waser J., Adv. Synth. Catal. 2013, 355, 1631–1639; [Google Scholar]
- 9c.Frei R., Waser J., J. Am. Chem. Soc. 2013, 135, 9620–9623; [DOI] [PubMed] [Google Scholar]
- 9d.Frei R., Wodrich M. D., Hari D. P., Borin P. A., Chauvier C., Waser J., J. Am. Chem. Soc. 2014, 136, 16563–16573; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9e.Chen C. C., Waser J., Chem. Commun. 2014, 50, 12923–12926. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a.Beringer F. M., Galton S. A., J. Org. Chem. 1965, 30, 1930–1934; [Google Scholar]
- 10b.Ochiai M., Kitagawa Y., Toyonari M., Uemura K., Oshima K., Shiro M., J. Org. Chem. 1997, 62, 8001–8008; [DOI] [PubMed] [Google Scholar]
- 10c.Dixon L. I., Carroll M. A., Gregson T. J., Ellames G. J., Harrington R. W., Clegg W., Eur. J. Org. Chem. 2013, 12, 2334–2345. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a.Kotali E., Varvoglis A., Bozopoulos A., J. Chem. Soc. Perkin Trans. 1 1989, 827–832; [Google Scholar]
- 11b.Maas G., Regitz M., Moll U., Rahm R., Krebs F., Hector R., Stang P. J., Crittell C. M., Williamson B. L., Tetrahedron 1992, 48, 3527–3540; [Google Scholar]
- 11c.Stang P. J., Blume T., Zhdankin V. V., Synthesis 1993, 35–36; [Google Scholar]
- 11d.Murch P., Arif A. M., Stang P. J., J. Org. Chem. 1997, 62, 5959–5965. [Google Scholar]
- 12.See ref. 2b, Chapter 7, page 425: “The vast majority of organic λ3-iodanes lack thermal stability and some of them are explosive”.
- 13.
- 13a.Waldecker B., Kafuta K., Alcarazo M., Org. Synth. 2019, 96, 258–276; [Google Scholar]
- 13b.Waldecker B., Kraft F., Golz C., Alcarazo M., Angew. Chem. Int. Ed. 2018, 57, 12538–12542; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 12718–12722. [Google Scholar]
- 14.Kitamura T., Tanaka T., Taniguchi H., Stang P. J., J. Chem. Soc. Perkin Trans. 1 1991, 2892–2893. [Google Scholar]
- 15.Deposition Numbers 2068276 (2 f), 2068277 (2 j), 2068278 (3 e), 2068279 (5 a), 2068280 (6), 2068281 (8), 2068282 (10), 2068283 (11), 2068284 (11Br), 2068285 (24), and 2068286 (28) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 16.Tykwinski R. R., Williamson B. L., Fischer D. R., Stang P. J., Arif A. M., J. Org. Chem. 1993, 58, 5235–5237. [Google Scholar]
- 17.
- 17a.Watanabe S.-i., Yamamoto K., Itagaki Y., Iwamura T., Iwama T., Kataoka T., Tanabe G., Muraoka O., J. Chem. Soc. Perkin Trans. 1 2001, 239–247; [Google Scholar]
- 17b.Watanabe S.-i., Mori E., Nagai H., Iwamura T., Iwama T., Kataoka T., J. Org. Chem. 2000, 65, 8893–8898; [DOI] [PubMed] [Google Scholar]
- 17c.Watanabe S.-i., Mori E., Nagai H., Kataoka T., Synlett 2000, 49–52; [Google Scholar]
- 17d.Ochiai M., Kitagawa Y., Toyonari M., Uemura K., Oshima K., Shiro M., J. Org. Chem. 1997, 62, 8001–8008. [DOI] [PubMed] [Google Scholar]
- 18.See for example:
- 18a.Zeng X., Liu S., Yang Y., Yang Y., Hammond G. B., Xu B., Chem. 2020, 6, 1018–1031; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b.Chen J., Li J., Plutschack M. B., Berger F., Ritter T., Angew. Chem. Int. Ed. 2020, 59, 5616–5620; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5665–5669; [Google Scholar]
- 18c.Aukland M. H., Talbot F. J. T., Fernández-Salas J. A., Ball M., Pulis A. P., Procter D. J., Angew. Chem. Int. Ed. 2018, 57, 9785–9789; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 9933–9937; [Google Scholar]
- 18d.Desrat S., Remeur C., Gény C., Rivière G., Colas C., Dumontet V., Birlirakis N., Iorga B. I., Roussi F., Chem. Commun. 2014, 50, 8593–8596; [DOI] [PubMed] [Google Scholar]
- 18e.Chen D., Cao Y., Yuan Z., Cai H., Zheng R., Kong L., Zhu G., J. Org. Chem. 2011, 76, 4071–4074; [DOI] [PubMed] [Google Scholar]
- 18f.Poulsen T. B., Dickmeiss G., Overgaard J., Jørgensen K. A., Angew. Chem. Int. Ed. 2008, 47, 4687–4690; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 4765–4768; [Google Scholar]
- 18g.Negishi E., Alimardanov A., Xu C., Org. Lett. 2000, 2, 65–67. [DOI] [PubMed] [Google Scholar]
- 19.Shimitzu M., Takeda Y., Hiyama T., Chem. Lett. 2008, 37, 1304–1305. [Google Scholar]
- 20.For recent reviews on the topic see:
- 20a.Péter Á., Perry G. J. P., Procter D. J., Adv. Synth. Catal. 2020, 362, 2135–2142; [Google Scholar]
- 20b.Kozhushkov S., Alcarazo M., Eur. J. Inorg. Chem. 2020, 2486–2500; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20c.Kaiser D., Klose I., Oost R., Neuhaus J., Maulide N., Chem. Rev. 2019, 119, 8701–8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aukland M. H., Šiaučiulis M., West A., Perry G. J. P., Procter D. J., Nat. Catal. 2020, 3, 163–169. [Google Scholar]
- 22.For a similar cyclization see: Karreman S., Karnbrock S. B. H., Kolle S., Golz C., Alcarazo M., Org. Lett. 2021, 23, 1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijeratnea E. M. K., Gunatilaka A. A. L., Kingston D. G. I., Haltiwanger R. C., Eggleston D. S., Tetrahedron 1995, 51, 7877–7882. [Google Scholar]
- 24.Sari A., Gray A. I., Sariyar G., Nat. Prod. Res. 2004, 18, 265–268. [DOI] [PubMed] [Google Scholar]
- 25.Liu B., Jian L., Chen G., Song X., Han C., Wang J., Chem. Nat. Compd. 2014, 49, 1172–1174. [Google Scholar]
- 26.Aporphine Alkaloids. In: Natural Compounds. S. S. Azimova, M. S. Yunusov (Eds), Springer, New York, 2013.
- 27.Chien C.-W., Shi C., Lin C.-F., Ojima I., Tetrahedron 2011, 67, 6513–6523. [Google Scholar]
- 28.Feldman K. S., Cutarelli T. D., J. Am. Chem. Soc. 2002, 124, 11600–11601. [DOI] [PubMed] [Google Scholar]
- 29.Mansø M., Fernandez L., Wang Z., Moth-Poulsen K., Nielsen M. B., Molecules 2020, 25, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y. H., Lee H., Kim Y. J., Kim B. T., Heo J.-N., J. Org. Chem. 2008, 73, 495–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information