Summary
Background
Human skin, which is constantly exposed to solar ultraviolet radiation (UVR), has a unique ability to respond by increasing its pigmentation in a protective process driven by melanogenesis in human epidermal melanocytes (HEMs). However, the molecular mechanisms used by HEMs to detect and respond to UVR remain unclear.
Objectives
To investigate the function and potential mechanism of opsin 5 (OPN5), a photoreceptor responsive to UVR wavelengths, in melanogenesis in HEMs.
Methods
Melanin content in HEMs was determined using the NaOH method, and activity of tyrosinase (TYR) (a key enzyme in melanin synthesis) was determined by the l‐DOPA method. OPN5 expression in UVR‐treated vs. untreated HEMs and explant tissues was detected by reverse‐transcription quantitative polymerase chain reaction (RT‐qPCR), Western blotting and immunofluorescence. Short interfering RNA‐mediated OPN5 knockdown and a lentivirus OPN5 overexpression model were used to examine their respective effects on TYR, tyrosinase‐related protein 1 (TRP1), TRP2 and microphthalmia‐associated transcription factor (MITF) expression, under UVR. Changes in expression of TYR, TRP1 and TRP2 caused by changes in OPN5 expression level were detected by RT‐qPCR and Western blot. Furthermore, changes in signalling pathway proteins were assayed.
Results
We found that OPN5 is the key sensor in HEMs responsible for UVR‐induced melanogenesis. OPN5‐induced melanogenesis required Ca2+‐dependent G protein‐coupled receptor‐ and protein kinase C signal transduction, thus contributing to the UVR‐induced MITF response to mediate downstream cellular effects, and providing evidence of OPN5 function in mammalian phototransduction. Remarkably, OPN5 activation was necessary for UVR‐induced increase in cellular melanin and has an inherent function in melanocyte melanogenesis.
Conclusions
Our results provide insight into the molecular mechanisms of UVR sensing and phototransduction in melanocytes, and may reveal molecular targets for preventing pigmentation or pigment diseases.
Short abstract
What is already known about this topic?
Ultraviolet radiation (UVR) induces a protective response to DNA damage mediated by melanin synthesis in human epidermal melanocytes (HEMs).
Tyrosinase (TYR), with tyrosinase‐related proteins (TRP1, TRP2), are the key enzymes for melanin synthesis.
Microphthalmia‐associated transcription factor regulates key genes for melanocyte development and differentiation, and can stimulate melanogenesis by activating transcription of TYR and other pigmentation genes, including TRP1.
Opsin 5 (OPN5) is known to function as a photoreceptor responsive to wavelengths in the near UV spectrum.
What does this study add?
UVR induces melanogenesis in HEMs via OPN5.
OPN5 regulates expression of TYR, TRP1 and TRP2 through the calcium‐dependent G protein‐coupled and protein kinase C signalling pathways.
OPN5 has an inherent role in HEMs in mediating melanogenesis.
What is the translational message?
OPN5 was discovered as a key sensor for UVR‐induced melanogenesis in human skin melanocytes.
It could be a target for early treatment of pigmentation or pigment diseases, to provide a more personalized and economically feasible method.
Linked Comment: L.V.M. de Assis and A.M. de Lauro Castrucci. Br J Dermatol 2021; 185:249–250.
Plain language summary available online
Skin provides a protective barrier from the external environment.1, 2 Solar ultraviolet radiation (UVR) is known as the main factor for stimulating pigmentation of the skin.3, 4 UVR‐induced pigmentation is a protective response mediated by melanogenesis in human epidermal melanocytes (HEMs) to shield DNA from UVR‐induced damage.2, 5 Solar UVR at the Earth’s surface is comprised of ∼95% UVA (320–400 nm) and ∼5% UVB (280–320 nm),6 and human skin elicits different responses to the two types of UVR.
When the skin is exposed to UVB radiation, keratinocytes synthesize various biochemical factors, such as α‐melanocyte stimulating hormone, endothelin‐1, stem cell factor and prostaglandin E2.7 These keratinocyte‐derived factors are transported to melanocytes in a paracrine manner, and through a series of signal transduction events they induce microphthalmia‐associated transcription factor (MITF) activation, which leads to transcriptional activation of tyrosinase‐related proteins (TRPs) in HEMs, and to melanogenesis.7
UVA causes primarily oxidative damage and leads to immediate pigment darkening within minutes8 via an unknown mechanism. Some recent studies have found that the UVA activation pathway in HEMs is mediated by TRP channels and leads to rapid Ca2+ release from internal stores and Ca2+‐dependent early melanogenesis.2 However, little is known about the mechanism of UVR (UVA) sensing and phototransduction in HEMs.
Opsins (OPNs) belong to the photosensitive G protein‐coupled receptor (GPCR) superfamily, which mediate phototransduction through GPCR signalling pathways.9, 10, 11, 12 Opsins are light sensitive due to a bound chromophore, retinal, which, in either the cis or all‐trans form, initiates UV‐activated signal transduction.2 Opsins act as photoreceptors in animals and participate in light adaptation to the external environment. Recently, expression of photoreceptors (opsins) in skin has been reported in several organisms, including humans and mice.13, 14, 15, 16, 17, 18, 19 The functions of these opsins are still being determined. Neuropsin (OPN5) is another opsin family member. The gene was previously found in mouse and human genomes, with mRNAs detected in mammalian neural tissue, and the protein found in skin and eyes of mice and humans.17, 20, 21, 22, 23, 24, 25, 26, 27 Studies have shown that OPN5 can respond to the wavelength of violet light (λmax = 380 nm).21, 28, 29 Some researchers observed that OPN5 is involved in regulating the seasonal breeding behaviour of birds and the activity cycle of mice;22, 30 it also mediates the entrainment of circadian light in the retina.26 Another study showed that the OPN5–dopamine pathway mediates light‐dependent development of blood vessels in mouse eyes.21 In addition, a study has shown that OPN5 is expressed in the hypothalamus and mediates phototransduction of violet light to inhibit thermogenesis of hypothalamic neurons.24 Although current research indicates that OPN5 may participate in mammalian physiological responses as a photoreceptor,24 the role of OPN5 in the HEM reaction to UVR leading to melanogenesis is still unclear.
Here, we demonstrate that OPN5 is the key sensor in HEMs responsible for UVR‐induced melanogenesis. OPN5‐mediated melanin production is activated by protein kinase C (PKC) through the G protein–calcium signalling pathway. After phosphorylation of PKC, MITF is further activated, and finally, expression of tyrosinase (TYR), TRP1 and TRP2 is upregulated. In addition, OPN5 plays an inherent role in the synthesis of melanocyte pigments. Our research provides insights into the molecular mechanisms of UVR in human skin melanocytes and may reveal molecular targets for preventing pigmentation or pigment diseases.
Materials and methods
Cell and explant culture
HEMs from children’s foreskin, acquired from Affiliated Hospital of Guizhou Medical University, Guiyang, China, were cultured in Medium 254 (M254500; Cascade Biologics®, Thermo Fisher Scientific, Waltham, MA, USA) and Human Melanocyte Growth Supplement (S0165; Cascade Biologics), and propagated for ≤ 7 population doublings. PIG1 (an immortalized human melanocyte cell line) was purchased from ATCC (ATCC® CRL‐2208™; ATCC, Manassas, VA, USA) and maintained in Gibco Dulbecco’s Modified Eagle Medium (DMEM) (11965092; Thermo Fisher) supplemented with 10% fetal bovine serum (FBS) (FBSSA500‐S; AusGeneX, Molendinar, QLD, Australia). Foreskin explants were cultured in DMEM supplemented with 10% FBS. Cells and explants were cultured at 37 °C in a humidified incubator with 5% CO2.
Pharmacological preparation and storage
All‐trans retinal [R2500; Sigma‐Aldrich (Shanghai) Trading Co. Ltd., Shanghai, China] stocks (12 mmol L–1) were prepared in 100% ethanol under dim red light and stored in the dark in brown glass vials at –20 °C until use.
ML329 (HY‐101464; MedChemExpress, Princeton, NJ, USA) stocks (12 mmol L–1) were prepared in 100% dimethyl sulfoxide and stored in the dark in brown glass vials at –20 °C or –80 °C until use.
Cell viability tests: optimization of ultraviolet radiation and all‐trans retinal dose
Cell viability was determined using the Cell Counting Kit‐8 (CCK‐8; Dojindo, Kumamoto, Japan). HEMs were incubated overnight in 96‐well culture plates at a concentration of 1·2 × 104 cells per well. When 70–80% confluence was reached, cells were irradiated with UVR. Following 48 h of incubation, 10 µL CCK‐8 solution was added and incubated at 37 °C for an additional 2 h. Absorbance at 450 nm was then measured, and cell viability was calculated as the percentage of the absolute optical density of each sample relative to that of the control value. To optimize UVR dose and all‐trans retinal dose, we first determined the effects of different doses of UVR (1·5, 2·5, 3·5, 4·5 or 5·5 J cm–2) and of all‐trans retinal (0·1, 0·25, 0·5, 1, 2, 4 or 6 µmol mL–1) on the viability of HEMs, as described (Figure S1; see Supporting Information). The 1·5‐J cm–2 UVR dose and 0·5 µmol mL–1 all‐trans retinal were used in subsequent experiments.
Ultraviolet radiation assays
UVR irradiation was carried out using a UV light therapy unit (Sigma High‐tech Co., Ltd, Shanghai, China). We used a dichroic mirror (280–400 nm) combined with 280‐nm longpass and 400‐nm shortpass filters for total UVR. The UV dose was measured using a UV radiometer (Sigma High‐tech). The UVR radiation dose (1·5 J cm–2, 30 W cm–2 for 1–1·5 min) was applied at a controlled temperature (37 ± 0·5 °C), and cells were harvested after 24 or 48 h for use in experiments. After each stimulus, explants or cells were returned to the original incubator at 37 ± 0·5 °C. Control cells were also subjected to similar conditions but were not exposed to UVR.
Melanin identification and quantification
Fontana‐Masson staining
HEMs were seeded into 12‐well plates at a density of 1·2 × 104 cells. After 24 h in the plates, the cells were exposed to 1·5 J cm–2 UVR for 48 h, then fixed with 4% paraformaldehyde (30525‐89‐4; Sigma‐Aldrich, St Louis, MO, USA) for 10 min. Tissue processing and preparation of paraffin‐embedded tissue blocks were performed by fixing the tissue in 10% neutral‐buffered formalin solution for 24 h at 4 °C. Then, Fontana‐Masson staining was performed on HEMs and foreskin explants (Figure S2a–d; see Supporting Information), according to the manufacturer’s instructions (G2031; Beijing Solarbio Science & Technology Co., Ltd, Beijing, China). After the end of the procedure, the cells were immediately analysed by an inverted microscope (Nikon, Tokyo, Japan). Quantification analysis was performed by ImageJ (https://imagej.nih.gov/ij/, 1997‐2018). At least three fields for each condition or timepoint were quantified.
NaOH method
HEMs were seeded into a six‐well plate at a density of 1·2 × 104 cells and cultured for 24 h. HEMs were irradiated with UVR, then 24 or 48 h after irradiation, 400 µL of cell culture medium were collected and transferred to a 1·5‐mL test tube containing 400 µL of 1 mol L–1 NaOH to determine extracellular melanin. The remaining medium was discarded and cells were harvested after digestion with 0·25% trypsin/0·01% ethylenediaminetetraacetic acid (EDTA) solution. We added 200 µL of the cell suspension to 200 µL trypan blue (Solarbio, China) solution, and live and non‐live cells were counted in a haemocytometer. The residual volume of the cell suspension (800 µL) was centrifuged (1000 g, 5 min), the supernatant was discarded, and 400 µL of 1 mol L–1 NaOH was added to the pellet. Melanin‐containing tubes were heated at 75 °C for 2·5–3 h, centrifuged (1000 g, 10 min), and the supernatant collected. We added five copies (100 µL) of each sample to the wells of a 96‐well flat‐bottomed plate, and total absorbance of melanin at 405 nm was measured with a microplate reader (BioTek Agilent, Winooski, VT, USA). This value was interpolated from the standard curve of synthetic melanin (Sigma‐Aldrich). Total melanin content is shown (Figure S2e) as the sum of the intracellular and extracellular melanin content normalized by the number of cells in each well. Statistical analyses were performed with the raw data, which were graphed as the percentage relative to the mean of each HEM control group.
Assay of tyrosinase activity
TYR activity was measured 24 h and 48 h after UVR irradiation by the l‐DOPA method as follows. HEMs were seeded into a six‐well plate at a density of 1·2 × 104 cells and cultured for 24 h. Twenty‐four hours or 48 h after UVR radiation stimulation, the medium was discarded, and HEMs washed twice with phosphate‐buffered saline (PBS) and harvested with 0·25% trypsin/0·01% EDTA, then centrifuged (1000 g, 5 min). The pellet was washed with PBS, centrifuged (1000 g, 5 min), and the pellet incubated with 1% Triton X‐100 at −80 °C for 1 h. After this period, samples were thawed at room temperature and centrifuged (10000 g, 10 min). The supernatant was transferred to a 1·5‐mL tube, and protein concentration determined by the BCA assay according to manufacturer’s instructions (Beijing Solarbio Science & Technology Co., Ltd, Beijing, China). A volume containing 50 µg of protein and 50 µL of 10 mmol L–1 l‐DOPA was added to a 96‐well flat bottom plate, completing with 1% Triton X‐100 solution up to 100 µL. After incubation at 37 °C for 1 h, total absorbance of each sample in duplicate was measured at 490 nm (Figure S2f).
Immunofluorescence
Cellular immunofluorescence
Cells were inoculated onto coverslips at a density of 1·2 × 104 cells per well. After the cells were irradiated with UVR, they were washed three times with PBS. The cells were fixed with 95% ethanol at room temperature for 10 min and then dried at room temperature. Cells were blocked with 10% FBS in PBS for 30 min In a 5% CO2 incubator at 37 °C. Primary antibodies were incubated overnight at 4 °C. Incubation with fluorescence‐labelled secondary antibody was for 45 min at room temperature. Primary and secondary labelled antibodies used were as follows: anti‐TYR mouse monoclonal antibody (ab738; Abcam, Cambridge, UK) conjugated to Alexa Fluor 488‐labelled goat antimouse IgG (A0428; Beyotime Biotechnology, Haimen, Jiangsu, China); anti‐MITF mouse monoclonal antibody (ab12039; Abcam) conjugated to Cy3‐labelled goat antimouse IgG (A0521; Beyotime); and anti‐OPN5 rabbit polyclonal antibody (bs‐12024R; Bioss, Beijing, China) conjugated to Alexa Fluor 488‐labelled goat antirabbit IgG (A0423, Beyotime). Nuclear staining was performed with 4′,6‐diamidino‐2‐phenylindole. Cells were mounted and visualized under a confocal microscope (Carl Zeiss AG, Oberkochen, Germany). (See Figure S2g, h.)
Immunofluorescence staining of tissue sections
Samples were processed after the explants had been irradiated with UVR for 48 h. Samples were immediately placed in 4% paraformaldehyde at 4 °C overnight, washed with PBS and then paraffin embedded. Sections were cut at 5–8 µm, dried at 37 °C, deparaffinized in xylene, hydrated in a graded series of ethanol, and subjected to microwave EDTA antigen retrieval. Samples were blocked with 5% bovine serum albumin and 0·5% Tween‐20 in PBS. Slides were incubated with primary antibodies overnight at 4 °C. Incubation with fluorescence‐labelled secondary antibody was for 50 min at room temperature. Primary and secondary labelled antibodies used were as follows: anti‐TYR mouse monoclonal antibody (ab738; Abcam) conjugated to Cy3‐labelled goat antimouse IgG (A0521; Beyotime); anti‐MITF mouse monoclonal antibody (ab12039; Abcam) conjugated to Cy3‐labelled goat antimouse (A0521; Beyotime); anti‐MelanA mouse monoclonal antibody (ab140503; Abcam) conjugated to Cy3‐labelled goat antimouse (A0521, Beyotime); and anti‐OPN5 rabbit polyclonal antibody (bs‐12024R; Bioss) conjugated to Alexa Fluor 488‐labelled goat antirabbit (A0428; Beyotime). (See Figure S2i.)
Knockdown of opsin 5 gene expression with short interfering RNA
Four pooled short interfering (si)RNA oligos targeting OPN5 or negative control siRNAs were purchased from TransheepBio (Shanghai, China). Knockdown of OPN5 in HEMs was performed using siRNA technology according to the manufacturer’s protocol. The silencing efficiency of four different siRNA sequences (below) were analysed 48 h post transfection via reverse‐transcription quantitative polymerase chain reaction (RT‐qPCR) compared with that of negative control siRNA not targeting any known gene. The siRNA sequence with the strongest silencing effect on OPN5 was used for the follow‐up study (Figure S3a, b; see Supporting Information). Third‐passage HEMs were seeded into six‐well plates at a concentration of 1 × 104 cells per well. When 30–40% confluence was reached, the cells were transfected using Lipofectamine 2000 (TransheepBio) with a final siRNA concentration of 60 nmol L–1. After siRNA transfection, the cells were cultured for 48 h for further detection. The OPN5 siRNA sequences were as follows: 5′‐CCCGCUGAAAUAAUGACUATT‐3′, 5′‐GCUGAGACCCGCUGAAAUATT‐3′, 5′‐GGAAAUGGAUAUGUCCUUUTT‐3′, 5′‐CCCGCUGAAAUAAUGACUATT‐3′, and 5′‐UUCUCCGAACGUGUCA CGUTT‐3′ as the sequence of the control.
Transfection of PIG1 cells with a lentiviral system to overexpress opsin 5
PIG1 cells were transfected with lentivirus vector containing either the full‐length DNA sequence of human OPN5 (LV‐EFS>h OPN5‐CMV>eGFP/T2A/Puro) or a control sequence (LV‐CMV > EGFP/T2A/Puro) (both purchased from Cyagen Biosciences Inc., Guangzhou, China). To overexpress OPN5, PIG1 cells were transfected with control or OPN5 high‐expression vectors for 48 h. The fluorescence expression of OPN5 transfection was observed using a fluorescence microscope. Then, the expression level of OPN5 in the transfected PIG1 cells was determined by RT‐qPCR and Western blot. (See Figure S3c–f.)
Quantitative real‐time reverse‐transcription polymerase chain reaction analysis
Total RNA was isolated with the RNAprep Pure Cell Kit [DP430, TIANGEN Biotech (Beijing) Co. Ltd, Beijing, China]. Next, total RNA (2·5 µg) was reverse transcribed to obtain cDNA with the Fastking gDNA Dispelling RT SuperMix reverse transcriptase kit (KR170801: TIANGEN). Quantitative real‐time SYBR Green RT‐qPCR technology (TIANGEN) was used to determine expression levels of the selected target genes. Thermocycling conditions were 95 °C for 3 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 15 s. When the temperature rose from 65 °C to 95 °C, the signal was detected every 0·5 °C, and the melting curve was drawn. Primer sequences [Generay Biotech (Shanghai) Co., Ltd, Shanghai, China] used are as follows: OPN5 forward, 5′‐CTAGACGAAAGAAGAAGCTGAGACC‐3′, OPN5 reverse, 5′‐GCGGTGACAAAAGCAAGAGA‐3′; GAPDH forward, 5′‐GACATCCGCAAAGACCTG‐3′, GAPDH reverse, 5′‐GGAAGGTGGACAGCGAG‐3′; TRP1 forward, 5′‐AGGATAGCCTCCGGCATT‐3′; TRP1 reverse, 5′‐TTCCACCTCCACAAGACT‐3′; TRP2 forward, 5′‐AACTGCGAGCGGAAGAAACC‐3′, TRP2 reverse, 5′‐CGTAGTCGGGGTGTACTCTCT‐3′; MITF forward, 5′‐CGACGGGAGAAAGGGTGT‐3', MITF reverse, 5′‐CCTCGGAACTGGGACTGA‐3′; TYR forward, 5′‐TGCACAGAGACGACTCTTG‐3′, TYR reverse, 5′‐GAGCTGATGGTATGCTTTGCTAA‐3′.
Western blot analysis
RIPA buffer (R0010; Beijing Solarbio) was used to prepare cell lysates. After the protein was quantified and denatured, the lysate (40 µg) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane. The membranes were incubated with the following primary antibodies: anti‐OPN5 (GTX100173; GeneTex, Irvine, CA, USA), anti‐TYR (GTX16389, GeneTex), anti‐TRP1 (ab235446; Abcam), anti‐TRP2 (ab221144; Abcam), anti‐MITF (ab12039; Abcam), anti‐phospho‐MITF (ab59201; Abcam), anti‐PKC (AF6197; Affinity Biosciences, Cincinatti, OH, USA), anti‐phospho‐PKC (AF3197; Affinity), anti‐phospho‐CAMKII (MD1677‐100; MDL Medical Discovery Leader Biotech Co., Ltd, Beijing, China; mdlbiotech.com), anti‐CAMKII (MD2007‐100; MDL), anti‐actin (MD409‐020), anti‐p38 MAPK (MD2025‐100; MDL), anti‐phospho‐p38 MAPK (MD2084‐100; MDL), anti‐ERK1/2 (MD1853‐100), anti‐phospho‐ERK1/2 (MD1412‐100; MDL), anti‐JNK (MD1929‐20; MDL) and anti‐phospho‐JNK (MD1483‐20; MDL) for specific protein detection, and then incubated with horseradish peroxidase‐conjugated secondary antibodies. Specific bands were visualized using a chemiluminescent reaction (electrogenerated chemiluminescence) (E003‐50; 7Seabiotech, Shanghai, China; 7seapharmatech.com).
Calcium imaging and calcium flux analysis
Cells treated with 0·5 μmol L–1 all‐trans retinal were stimulated with UVR and incubated in Ca2+‐free extracellular buffer. Cells were incubated in the dark for 20 min in extracellular solution with 3 μmol L–1 Fluo‐3 AM (CAS 121714‐22‐5; AAT Bioquest, Inc., Sunnyvale, CA, USA) and transferred to the imaging chamber. Cells incubated in all‐trans retinal, but not irradiated, were analysed in parallel in each experiment and used for normalization. In addition, cells were incubated in the dark for 20 min in extracellular solution with 3 μmol L–1 Fluo‐3 AM. Cells were centrifuged at 1000 g for 5 min at room temperature, washed again with PBS, and then resuspended in 0·5 mL PBS buffer solution. Then the intracellular calcium ion concentration was measured by flow cytometry (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All experiments were performed independently at least three times. All values are expressed as mean ± SEM. Statistical significance was determined by one‐way anova followed by Tukey’s test. Differences were considered significant at P < 0·05. All analyses were carried out in GraphPad Prism Version 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Opsin 5 senses ultraviolet radiation in human epidermal melanocytes
Because recent studies have shown that in HEMs, UVR induces a retinal‐ and Ca2+‐dependent increase in cellular melanin concentration within hours of exposure,2, 8 and the mechanism involved appears to be different from that of UVB‐induced pigmentation, we studied the effect of UVR stimulation on HEMs, to determine the underlying mechanism.
Firstly, we studied the effects of different doses of UVR or all‐trans retinal on the viability of HEMs. At a dose of 1·5 J cm–2 UVR, all cells remained viable 48 h later (Figure S1): this dose was used in all subsequent experiments.
Exposure of HEMs to UVR resulted in a measurable increase in melanin and the increase in melanin depended on the presence of all‐trans retinal (Figure S2a–e).2, 8 We also tested tyrosinase activity using the l‐DOPA method (Figure S2f), as well as testing the expression of TYR and MITF by immunofluorescence (Figure S2g–i). The TYR and MITF immunofluorescence intensity of the UVR treatment group was higher than that of the control group. Simultaneously, we further tested the expression of TYR, TRP1, TRP2 and MITF at the protein and gene levels via Western blotting and RT‐qPCR. Compared with the control group, expression of TYR, TRP1, TRP2, MITF protein and genes in the UVR irradiation group increased significantly (Figure S2j–n). These results suggest that UVR induces melanogenesis in HEMs.
Next, we investigated whether HEMs can express direct sensors of UVR. OPN5 has an absorption spectrum in the shorter wavelengths of visible light.20, 21, 28, 29 Mouse and human OPN5 is a known UV‐sensitive photoreceptor linked to a Gi‐type G protein, with maximum absorption peaks in the UV (380‐nm) and visible light (471‐nm) regions.26 A recent study showed that in cocultured human melanocytes and keratinocytes, the expression level of OPN5 increased in a dose‐dependent manner after UVA irradiation.31 Based on these findings, we investigated whether OPN5 responds to UVR in HEMs.
Firstly, we tested the expression of OPN5 in HEMs via RT‐qPCR and Western blotting (Figure 1a, b) and by immunofluorescence (Figure 1c). Following UVR irradiation, expression levels of the OPN5 gene in HEMs appeared to increase significantly (Figure 1d), while the protein level increased slightly, compared with controls (Figure 1e). In the explant model, we used immunofluorescence staining to label melanocytes with anti‐MelanA antibody, and found that the OPN5 immunofluorescence intensity of the UVR irradiation group was higher than that of the control group (Figure 1f). These results show that, in our study, OPN5 in HEMs responds to UVR irradiation.
Figure 1.
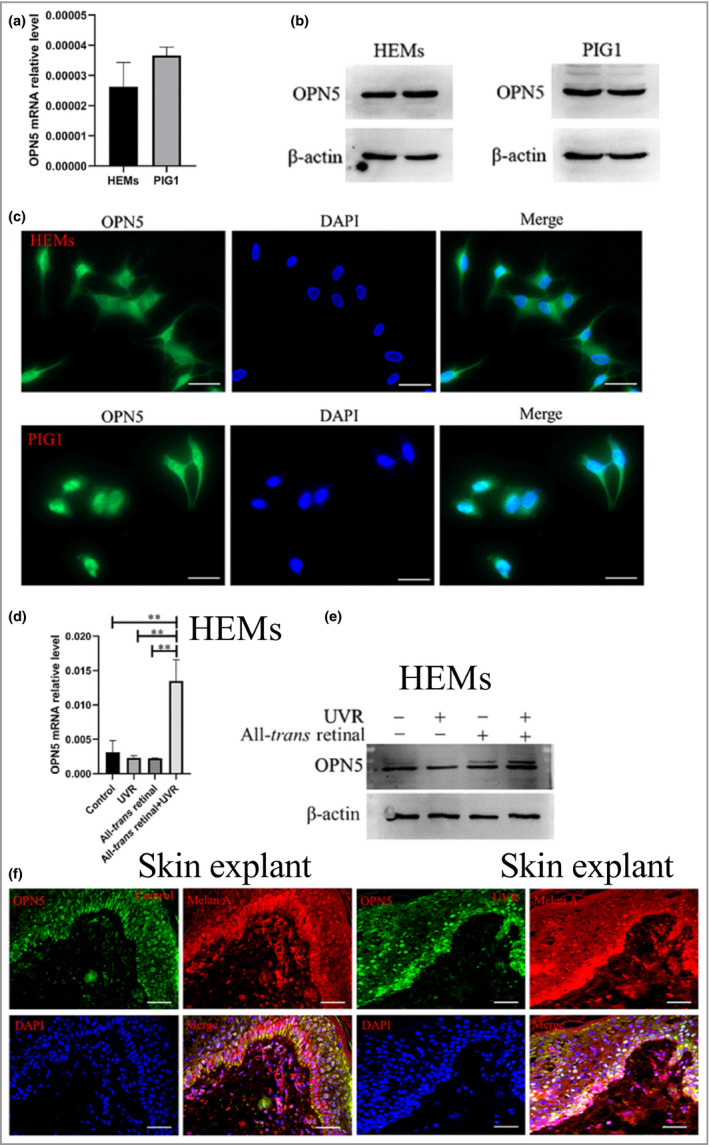
Opsin 5 (OPN5) senses ultraviolet radiation (UVR) in human epidermal melanocytes (HEMs). (a) OPN5 mRNA levels were normalized to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) levels. (b) Western blot analysis of OPN5 protein expression in HEMs and transfected human melanocyte line PIG1. (c) Representative images show OPN5 protein expression in HEMs (top) and PIG1 cells (bottom). The left panel represents localization of opsins (Alexa Fluor 488, green) at the plasma membrane, the middle panel represents the nucleus stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (blue), and the right panel represents an overlay of the middle panel and the phase contrast image. Scale bars = 20 µm. (d, e) HEMs were irradiated with UVR, and expression levels of OPN5 gene and protein were determined by quantitative real‐time reverse‐transcription polymerase chain reaction and Western blot analysis. All data are shown as mean ± SEM of three independent experiments. Beta‐actin was used as loading control. Statistical significance was determined by one‐way anova. **P < 0·01. (f) OPN5 expression (Alexa Fluor 488, green) colocalized with melanocyte marker MelanA (Cy3, red) in skin explant with immunofluorescence staining, without UVR (left) or with UVR (right). Scale bars = 50 µm.
Does opsin 5 mediate melanogenesis in human epidermal melanocytes?
Effects of opsin 5 knockdown on tyrosinase, tyrosinase‐related protein and microphthalmia‐associated transcription factor expression
To investigate whether OPN5 can mediate melanogenesis in HEMs, we knocked down OPN5 in HEMs with si‐OPN5. The efficiency of siRNA was tested by RT‐qPCR and Western blotting (Figure S3a, b). Melanin was measured using the NaOH method and, interestingly, we found that the amount of melanogenesis in the si‐OPN5 group was significantly lower than that in the control group (Figure 2a). RT‐qPCR was used to analyse changes of TYR and MITF gene expression in HEMs, and Western blotting was used to analyse changes of TYR, TRP1, TRP2 and MITF protein expression. Expression levels in the si‐OPN5 group were seen to be significantly lower than those in the control group (Figure 2b–d). These results indicate that OPN5 has a role in melanogenesis in HEMs.
Figure 2.
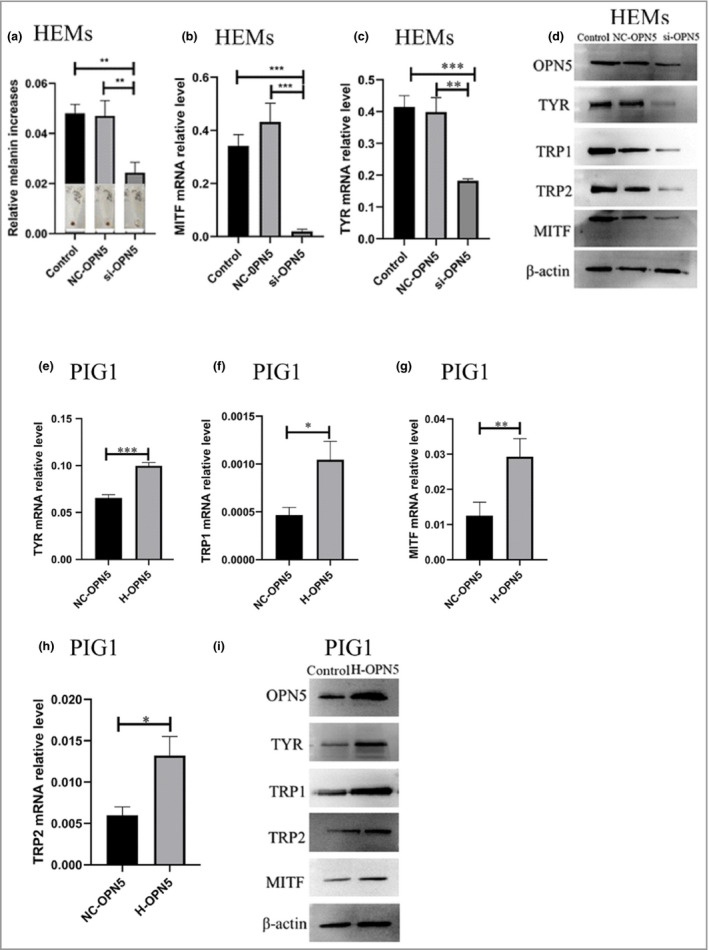
Opsin 5 (OPN5) mediates melanogenesis. (a) Human epidermal melanocytes (HEMs) were transfected with small interfering (si)RNA directed against OPN5 (si‐OPN5), or a negative control (NC‐OPN‐RNA). After short interfering (si)RNA inhibited OPN5, melanin changes in HEMs were measured by the NaOH method. (b, c) After siRNA inhibited OPN5, quantitative real‐time reverse‐transcription quantitative polymerase chain reaction (RT‐qPCR) was used to analyse changes of tyrosinase (TYR) and microphthalmia‐associated transcription factor (MITF) gene expression in HEMs. (d) After siRNA inhibited OPN5, Western blotting was used to analyse the changes of TYR, tyrosinase‐related protein 1 (TRP1), TRP2 and MITF protein expression in HEMs. Beta‐actin was used as a loading control. (e–i) After OPN5 was overexpressed (as high‐expression H‐OPN5‐RNAi) in PIG1 cells, RT‐qPCR and Western blotting were used to analyse gene and protein expression levels of TYR, TRP1, TRP2 and MITF. Beta‐actin was used as loading control. All data are shown as mean ± SEM of three independent experiments. Statistical significance was determined by one‐way anova followed by Tukey’s test. *P < 0·05, **P < 0·01, ***P < 0·001.
Effects of opsin 5 overexpression on tyrosinase, tyrosinase‐related protein and microphthalmia‐associated transcription factor expression change
To further confirm the function of OPN5 in melanocytes, PIG1 cells were transfected with a lentivirus vector designed to overexpress OPN5, and the effects on OPN5 gene and protein expression were observed by immunofluorescence, RT‐qPCR and Western blotting (Figure S3c–e). Gene and protein expression changes of TYR, TRP1, TRP2 and MITF were analysed by RT‐qPCR and Western blot, respectively. Overexpression of OPN5 resulted in upregulated expression of TYR, TRP1, TRP2 and MITF (Figure 2e–i). These findings further indicate that OPN5 is involved in melanogenesis in melanocytes.
Ultraviolet radiation upregulates tyrosinase, tyrosine‐related proteins 1 and 2, and microphthalmia‐associated transcription factor, via opsin 5
To study whether OPN5 is a UVR‐induced melanogenesis sensor. HEMs were irradiated with UVR after si‐OPN5 mediated OPN5 gene knockdown. Expression of TYR, TRP1, TRP2 and MITF proteins in the experimental group did not change significantly (Figure 3a). To further confirm that OPN5 may act as a UVR sensor, OPN5‐overexpressing PIG1 cells were irradiated with UVR. Expression levels of TYR, TRP1, TRP2 and MITF proteins in the UVR irradiation group were observed to be significantly higher than those in the control group (Figure 3b), suggesting that UVR upregulates expression of TYR, TRP1, TRP2 and MITF via OPN5 in melanocytes.
Figure 3.
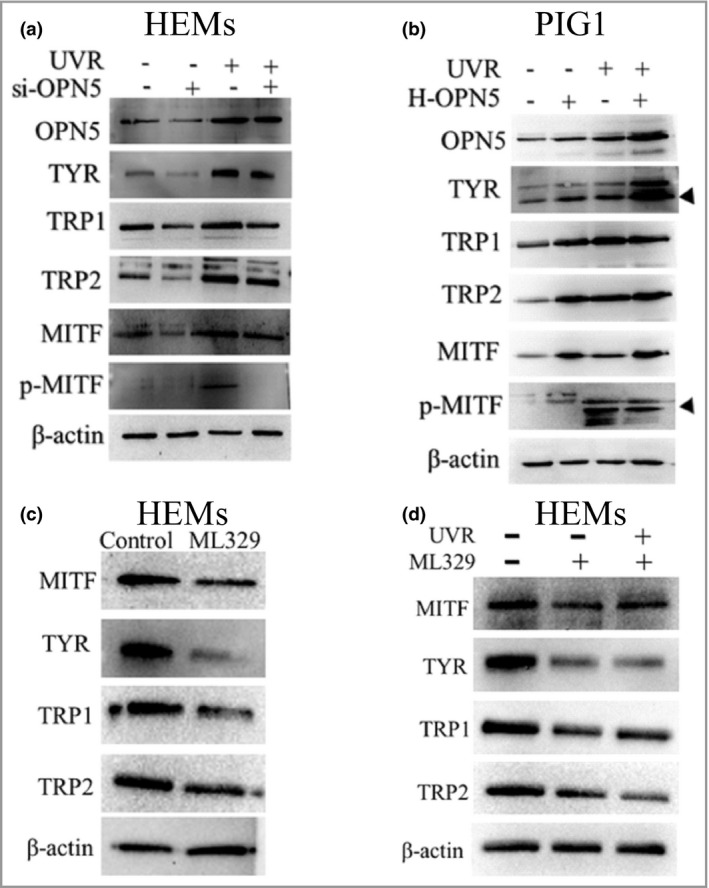
Ultraviolet radiation (UVR) upregulates expression of tyrosinase (TYR), tyrosinase‐related protein 1 (TRP1) and TRP2 through opsin 5 (OPN5). (a) Cells were transfected with short interfering (si)RNA against OPN5 irradiated with or without UVR and lysed after 48 h. Lysates were analysed by Western blotting using the indicated antibodies. (b) Cells were transfected with a lentivirus vector overexpressing OPN5 (H‐OPN5) irradiated with or without UVR and lysed after 48 h. Lysates were analysed by Western blotting using the indicated antibodies. (c, d) After inhibiting expression of microphthalmia‐associated transcription factor (MITF) with ML329, cells were irradiated without or with UVR, and lysed 48 h later to observe the expression of TYR, TRP1 and TRP2 protein levels. p‐, phosphorylated. Beta‐actin was used as a loading control.
Next, we tested whether UVR upregulates expression of TYR, TRP1 and TRP2 via MITF. Previous research reports that MITF regulates transcription of TYR, TRP1 and TRP2.32 ML329, an inhibitor of MITF, can reduce the expression of multiple MITF target genes.33 We blocked MITF, which is essential for the TYR pathway, with ML329. Our results showed that inhibition of MITF in HEMs significantly reduced expression of TYR, TRP1 and TRP2 (Figure 3c). Moreover, after treating HEMs with ML329 and then irradiating with UVR, we found that expression of TYR, TRP1 and TRP2 did not change (Figure 3d). These results indicate that OPN5 mediates melanogenesis in HEMs through the MITF signalling pathway.
Opsin 5 regulates the expression of tyrosinase, tyrosine‐related protein 1 and tyrosine‐related protein 2 via the calcium‐dependent G protein‐coupled signalling pathway
Previous work has shown that quail OPN5 or rat OPN5 are involved in the Ca2+ signalling cascade.34 A more recent study found that OPN5 in a HEK293 human embryonic kidney cell line and OPN5 in mouse neuroblastoma (Neuro2a) cell lines mediate light‐induced Ca2+ responses.35 Furthermore, a large number of studies have shown that the Ca2+ reaction is involved in melanogenesis in melanocytes.2, 8, 36 Based on these findings, we hypothesized that UVR induces the Ca2+ response to regulate melanogenesis through OPN5.
To confirm this hypothesis, we measured the intracellular Ca2+ level in melanocytes with a Fluo‐3/AM probe after UVR. The intracellular Ca2+ in the UVR irradiation group was significantly higher than that in the control group (Figure 4a, b), and UVR stimulated phosphorylation of Ca2+/calmodulin‐dependent protein kinase II (CAMKII), a key enzyme in the calcium pathway.12, 19 Then, we measured the UVR‐induced response to si‐OPN5 in melanocytes and found that while UVR increased phosphorylated (p‐)CAMKII levels, this is abolished when OPN5 is silenced (Figure 4c). Next, we measured the UVR‐induced CAMKII expression level in the overexpression OPN5 model, and found that the protein level of p‐CAMKII increased significantly (Figure 4d). These results further indicate that the UVR‐induced Ca2+ response is regulated via OPN5.
Figure 4.
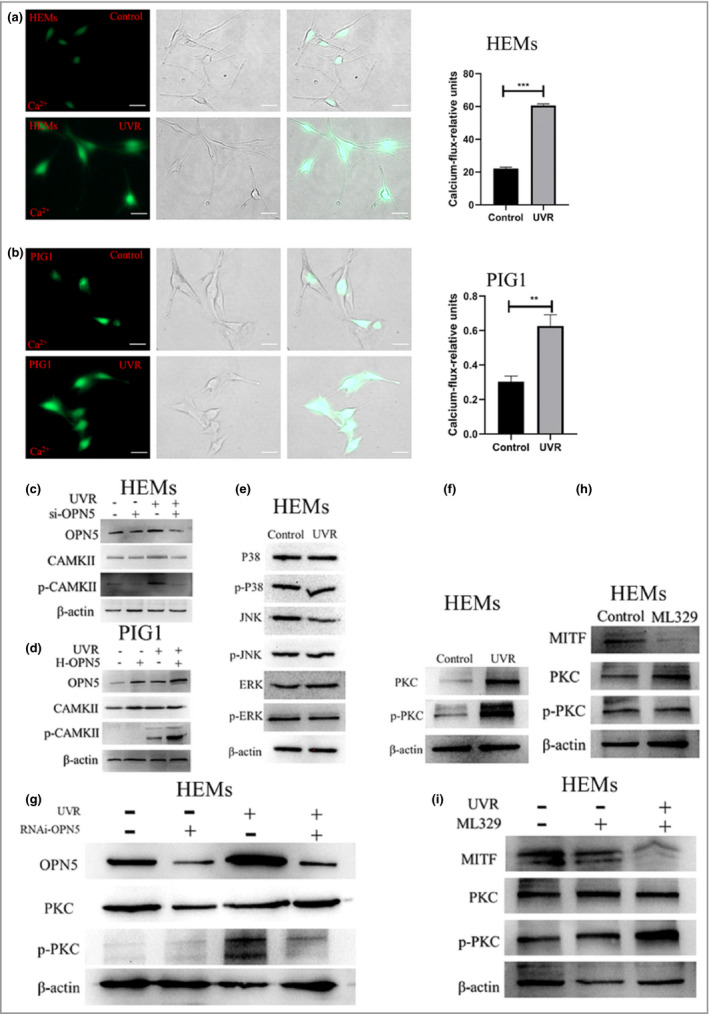
Ultraviolet radiation (UVR) activates the Ca2+/protein kinase C (PKC)/microphthalmia‐associated transcription factor (MITF) signalling pathway through opsin 5 (OPN5) and upregulates expression of tyrosinase (TYR), tyrosinase‐related protein 1 (TRP1) and TRP2. (a, b) Images of representative human epidermal melanocytes (HEMs) and PIG1 human melanocyte cells loaded with the Ca2+ indicator Fluo‐3 and irradiated with UVR. Scale bar = 20 µm. HEMs and PIG1 calcium fluxes were quantified by flow cytometry. Data are shown as mean ± SEM of three independent experiments. Statistical significance was determined by one‐way anova followed by Tukey’s test. **P < 0·01 ***P < 0·001. (c, d) Cells were transfected with (c) short interfering (si)RNA against OPN5, or (d) lentivirus overexpressing OPN5 (H‐OPN5), then irradiated with or without UVR and lysed after 48 h. Lysates were analysed by Western blotting using the indicated antibodies. (e, f) Cells were irradiated with and without UVR, and mitogen‐activated protein kinase (MAPK) and PKC protein and phosphorylated (p‐) protein levels were determined by Western blot analysis. (g) Cells were transfected with short interfering RNA (RNAi) against OPN5 irradiated with UVR and lysed after 48 h. Lysates were analysed by Western blotting using the indicated antibodies. (h, i) After inhibiting the expression of MITF with ML329, cells were irradiated with or without UVR, and lysed 48 h later to observe PKC protein expression. Beta‐actin was used as a loading control. CAMKII, calmodulin‐dependent protein kinase II.
Skin melanogenesis may be mediated by several melanogenesis signalling pathways.37 Some studies have shown that the increase in Ca2+ can activate the mitogen‐activated protein kinase (MAPK) signalling pathway or the PKC signalling pathway.35, 38 That is, phosphorylation of p38 MAPK39 or PKC40, 41 increases the expression of MITF and TYR, which leads to melanogenesis. In this study, we did not observe a UVR‐induced phosphorylation of MAPK (Figure 4e). However, interestingly, we found that UVR irradiation induced phosphorylation of PKC (Figure 4f). We further demonstrated that using siRNA‐mediated downregulation of OPN5 in HEMs blocked UVR‐induced phosphorylation of PKC (Figure 4g). These results indicate that OPN5 mediates melanogenesis through the PKC signalling pathway in melanocytes.
Previous studies have suggested that MITF might be a transcription factor of PKC‐β.42 Other studies have shown that MITF is a downstream phosphorylation target of PKC activity.43, 44 To confirm the upstream and downstream relationship between PKC and MITF, we used ML329 to inhibit MITF and then assayed PKC expression. The results showed that after inhibiting MITF, expression levels of PKC did not change (Figure 4h). We further demonstrated that after downregulation of MITF with ML329 in melanocytes, the UVR‐induced phosphorylation of PKC was not blocked (Figure 4i). These results further indicate that PKC is located upstream of MITF and is involved in melanogenesis.
We conclude that UVR activates OPN5 and leads to increased intracellular calcium. In response to calcium, CAMKII is phosphorylated, further inducing phosphorylation of PKC, and then MITF finally upregulates expression of TYR, TRP1 and TRP2 in human melanocytes (Figure 5).
Figure 5.
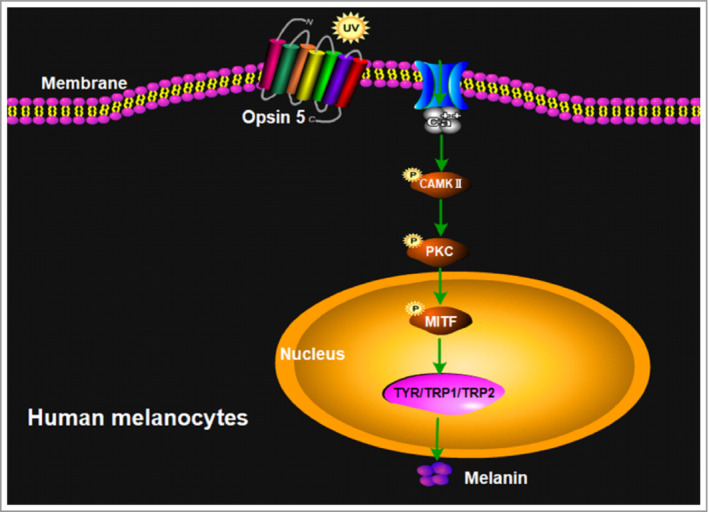
Summary model of the key findings in this study. Opsin 5 upregulates expression of microphthalmia‐associated transcription factor (MITF), tyrosinase (TYR), tyrosinase‐related protein 1 (TRP1) and TRP2 in melanocytes after ultraviolet (UV) radiation exposure through the calcium‐dependent G protein‐coupled and protein kinase C (PKC) signalling pathway. CAMKII, calmodulin‐dependent protein kinase II.
Discussion
The ability of humans or animals to detect and respond to light stimuli is critical to survival.2 The eye uses opsin (a photoreceptor) to convert light into electrical signals to mediate visual and nonvisual processes.9 HEMs are capable of rapidly detecting UVR by first increasing intracellular Ca2+ and later producing more melanin.2, 8, 36 We now demonstrate that OPN5 is critical for UVR signalling in melanocytes and provide evidence that OPN5 activates Ca2+ channels in a unique extraocular phototransduction pathway. Activation of this pathway leads to melanogenesis, thus allowing for rapid UVR detection and response in melanocytes.
Opsins are a class of GPCRs that convert the energy of a photon into a cellular signalling response.45, 46, 47 Recent studies have found that opsins (OPN1, OPN2, OPN3, OPN4, OPN5) are expressed in skin,14, 15, 16, 17, 18, 19, 24, 27 but their functions are not yet fully understood. Opsins may have different absorption spectra in different species. Both mouse and human OPN5 act as UV‐sensitive photoreceptors linked to Gi‐type G proteins, with absorption maxima in the 380‐nm (UV) and 471‐nm (visible light) regions.26 Some studies indicate that OPN5 may respond to UVA irradiation in cocultures of human melanocytes and keratinocytes.31 Another study suggested that after UVA irradiation of human melanocytes, the expression level of OPN5 increased most significantly at 48 h.48 In our study, OPN5 recognized and responded to UVR in melanocytes (Figure 1d–f). OPN5 is phylogenetically closely related to peropsin and retinal photoisomerases that have all‐trans retinal chromophores.20 Recent studies have shown that the addition of all‐trans retinal to cells expressing OPN5 protein can reconstruct the pigment.29 The pigment reconstituted with all‐trans retinal has an absorption maximum in the visible region. This seems to be consistent with previous reports2, 8 that the remodelled pigment can mediate melanogenesis in HEMs (Figure S2).
Previous studies have shown that OPN3 may be the sensor responsible for inducing hyperpigmentation through blue light in HEMs.19 Interestingly, some studies indicated that OPN3 does not mediate calcium‐dependent phototransduction of UVR, blue light or green light in HEMs and that OPN3 is a negative regulator of melanin levels in HEMs.49 Recent reports have confirmed that OPN3 is a key molecule responsible for the survival of HEMs.27 These findings suggest that the functions of opsins may be diverse, and indeed recent studies have shown such diversity of function, such as participating in development of blood vessels in mouse eyes,21 regulating circadian rhythms in mouse skin,24 and mediating thermogenesis of hypothalamic neurons.25 In our study, OPN5 acted as a UVR sensor in HEMs to mediate melanogenesis. When the OPN5 gene is silenced, UVR no longer upregulates expression of TYR, TRP1 and TRP2 (Figure 3a).
We then studied the signalling pathway leading to this OPN5‐mediated phenomenon. Previous studies have reported that GPCRs drive calcium mobilization in intracellular storage in melanocytes and participate in the downstream signalling cascade.2, 8, 36, 50 Several recent studies have shown that OPN5 mediates the light‐induced Ca2+ signalling cascade.30, 34, 35 To assess the contribution of intracellular Ca2+ to this signalling pathway, we measured the changes in Ca2+ in HEMs induced by UVR (Figure 4a, b), and the results are consistent with previous reports.2, 8 Moreover, UVR induced upregulation of p‐CAMKII (Figure 4c), a key enzyme in the calcium pathway.8, 19 To test whether Ca2+ mobilization starts downstream of OPN5 activation, we knocked down OPN5 in HEMs by si‐OPN5 and found that UVR increased p‐CAMKII levels, which is abolished when OPN5 is silenced (Figure 4c). These results indicate that the UVR‐induced Ca2+ response is mediated through OPN5. Furthermore, some studies have shown that the increase in Ca2+ activates the MAPK or PKC signalling pathways to participate in melanogenesis.19, 38 In our research, we confirmed that the PKC signalling pathway, rather than the MAPK signalling pathway, is involved in melanogenesis (Figure 4e–g). Moreover, previous studies have suggested that MITF may be a transcription factor of PKC‐β,42 while some have reported that MITF is a downstream phosphorylation target of PKC activity.42, 43 To confirm the upstream and downstream relationship between PKC and MITF, we used ML329 to inhibit MITF, and then after UVR irradiation, we found that the phosphorylation level of p‐PKC increased (Figure 4i). These results indicate that UVR upregulates expression of TYR, TRP1 and TRP2 through the OPN5–calcium‐dependent/PKC signalling pathways (Figure 5).
In summary, our research focuses on the effects of the inherent and sensor functions of OPN5 on melanogenesis in HEMs. OPN5 depends on the calcium pathway and PKC signalling pathway to upregulate the expression of TYR, TRP1 and TRP2. Therefore, it plays an important role in the melanogenesis of skin epidermal melanocytes. As OPN5 has been shown to be a key sensor for UVR‐induced melanogenesis in human skin melanocytes, it could be a target for early treatment of pigmentation or pigment diseases, to provide a more personalized and economically feasible method.
Author Contribution
Lan Yinghua: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Software (equal); Writing‐original draft (equal). Zeng wen: Data curation (equal); Formal analysis (equal); Methodology (equal); Software (equal). Dong Xian: Methodology (supporting). Hongguang Lu: Conceptualization (lead); Project administration (lead); Writing‐review & editing (lead).
Supporting information
Figure S1 Different doses of ultraviolet radiation and all‐trans retinal affect the viability of human epidermal melanocytes.
Figure S2 Ultraviolet radiation stimulates melanogenesis.
Figure S3 Knockdown of the opsin 5 gene (OPN5) in human epidermal melanocytes with OPN5 short interfering RNA, and overexpression assay of OPN5 in a PIG1 melanocyte cell line.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Acknowledgments
We thank Professor Yu Wang for his valuable comments on this article.
Funding sources Supported by the National Natural Science Foundation of China (no. 81673069, no. 81972920).
Conflicts of interest The authors declare they have no conflicts of interest.
Y.L. and W.Z. contributed equally to this work.
Plain language summary available online
References
- 1.Ross AA, Müller KM, Weese JS, Neufeld JD. Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc Natl Acad Sci USA 2018; 115:E5786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA 2013; 110:2383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature 2007; 445:843–50. [DOI] [PubMed] [Google Scholar]
- 4.Miller AJ, Tsao H. New insights into pigmentary pathways and skin cancer. Br J Dermatol 2010; 162:22–8. [DOI] [PubMed] [Google Scholar]
- 5.Brenner M, Coelho SG, Beer JZet al. Long‐lasting molecular changes in human skin after repetitive in situ UV irradiation. J Invest Dermatol 2009; 129:1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi W, Miyamura Y, Wolber Ret al. Regulation of human skin pigmentation in situ by repetitive UV exposure: molecular characterization of responses to UVA and/or UVB. J Invest Dermatol 2010; 130:1685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui R, Widlund HR, Feige Eet al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell 2007; 128:853–64. [DOI] [PubMed] [Google Scholar]
- 8.Wicks NL, Chan JW, Najera JAet al. UVA phototransduction drives early melanin synthesis in human melanocytes. Curr Biol 2011; 21:1906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell 2009; 139:246–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Gao X, Goswami Det al. Molecular assembly of rhodopsin with G protein‐coupled receptor kinases. Cell Res 2017; 27:728–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas RJ. Mammalian inner retinal photoreception. Curr Biol 2013; 23:R125–33. [DOI] [PubMed] [Google Scholar]
- 12.Lan Y, Wang Y, Lu H. Opsin 3 is a key regulator of ultraviolet A‐induced photoageing in human dermal fibroblast cells. Br J Dermatol 2020; 182:1228–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Assis LV, Moraes MN, da Silveira C‐Met al. The effect of white light on normal and malignant murine melanocytes: a link between opsins, clock genes, and melanogenesis. Biochim Biophys Acta 2016; 1863:1119–33. [DOI] [PubMed] [Google Scholar]
- 14.de Assis LVM, Mendes D, Silva MMet al. Melanopsin mediates UVA‐dependent modulation of proliferation, pigmentation, apoptosis, and molecular clock in normal and malignant melanocytes. Biochim Biophys Acta Mol Cell Res 2020; 1867:118789. [DOI] [PubMed] [Google Scholar]
- 15.de Assis LVM, Moraes MN, Magalhães‐Marques KK, Castrucci AML. Melanopsin and rhodopsin mediate UVA‐induced immediate pigment darkening: unravelling the photosensitive system of the skin. Eur J Cell Biol 2018; 97:150–62. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi M, Ikeyama K, Denda Set al. Expressions of rod and cone photoreceptor‐like proteins in human epidermis. Exp Dermatol 2009; 18:567–70. [DOI] [PubMed] [Google Scholar]
- 17.Haltaufderhyde K, Ozdeslik RN, Wicks NLet al. Opsin expression in human epidermal skin. Photochem Photobiol 2015; 91:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HJ, Son ED, Jung JYet al. Violet light down‐regulates the expression of specific differentiation markers through Rhodopsin in normal human epidermal keratinocytes. PLOS ONE 2013; 8:e73678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regazzetti C, Sormani L, Debayle Det al. Melanocytes sense blue light and regulate pigmentation through opsin‐3. J Invest Dermatol 2018; 138:171–8. [DOI] [PubMed] [Google Scholar]
- 20.Tarttelin EE, Bellingham J, Hankins MWet al. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett 2003; 554:410–16. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen MT, Vemaraju S, Nayak Get al. An opsin 5–dopamine pathway mediates light‐dependent vascular development in the eye. Nat Cell Biol 2019; 21:420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ota W, Nakane Y, Hattar S, Yoshimura T. Impaired circadian photoentrainment in Opn5‐null mice. iScience 2018; 6:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buhr ED, Yue WW, Ren Xet al. Neuropsin (OPN5)‐mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci USA 2015; 112:13093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhr ED, Vemaraju S, Diaz Net al. Neuropsin (OPN5) mediates local light‐dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol 2019; 29:3478–87.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang KX, D’Souza S, Upton BAet al. Violet‐light suppression of thermogenesis by opsin 5 hypothalamic neurons. Nature 2020; 585:420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojima D, Mori S, Torii Met al. UV‐sensitive photoreceptor protein OPN5 in humans and mice. PLOS ONE 2011; 6:e26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Lan Y, Lu H. Opsin3 downregulation induces apoptosis of human epidermal melanocytes via mitochondrial pathway. Photochem Photobiol 2020; 96:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamashita T, Ono K, Ohuchi Het al. Evolution of mammalian Opn5 as a specialized UV‐absorbing pigment by a single amino acid mutation. J Biol Chem 2014; 289:3991–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita T, Ohuchi H, Tomonari Set al. Opn5 is a UV‐sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci USA 2010; 107:22084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakane Y, Ikegami K, Ono Het al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci USA 2010; 107:15264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu QM, Yi WJ, Su MYet al. Induction of retinal‐dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif 2017; 50:e12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 2006; 12:406–14. [DOI] [PubMed] [Google Scholar]
- 33.Faloon PW, Bennion M, Weiner WSet al. A small molecule inhibitor of the MITF molecular pathway. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US), 2010. –. Available at: https://www.ncbi.nlm.nih.gov/books/NBK47352/ (last accessed 21 January 2021). [PubMed] [Google Scholar]
- 34.Nieto PS, Valdez DJ, Acosta‐Rodríguez VA, Guido ME. Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC‐5. Presence of OPN5 in the rat retina. PLOS ONE 2011; 6:e26417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiyama T, Suzuki H, Takahashi T. Light‐induced rapid Ca2+ response and MAPK phosphorylation in the cells heterologously expressing human OPN5. Sci Rep 2014; 4:5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellono NW, Oancea E. UV light phototransduction depolarizes human melanocytes. Channels (Austin) 2013; 7:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang YS, Oh SW, Park SHet al. Melanogenic effects of maclurin are mediated through the activation of cAMP/PKA/CREB and p38 MAPK/CREB signaling pathways. Oxid Med Cell Longev 2019; 2019:9827519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, Yudin Y, Rohacs T. Diacylglycerol kinases regulate TRPV1 channel activity. J Biol Chem 2020; 295:8174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn JH, Jin SH, Kang HY. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch Dermatol Res 2008; 300:325–9. [DOI] [PubMed] [Google Scholar]
- 40.Villareal MO, Han J, Yamada Pet al. Hirseins inhibit melanogenesis by regulating the gene expressions of Mitf and melanogenesis enzymes. Exp Dermatol 2010; 19:450–7. [DOI] [PubMed] [Google Scholar]
- 41.Park HY, Perez JM, Laursen Ret al. Protein kinase C‐beta activates tyrosinase by phosphorylating serine residues in its cytoplasmic domain. J Biol Chem 1999; 274:16470–8. [DOI] [PubMed] [Google Scholar]
- 42.Park HY, Wu C, Yonemoto Let al. MITF mediates cAMP‐induced protein kinase C‐beta expression in human melanocytes. Biochem J 2006; 395:571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakajima H, Wakabayashi Y, Wakamatsu K, Imokawa G. An extract of Melia toosendan attenuates endothelin‐1‐stimulated pigmentation in human epidermal equivalents through the interruption of PKC activity within melanocytes. Arch Dermatol Res 2011; 303:263–76. [DOI] [PubMed] [Google Scholar]
- 44.Sato‐Jin K, Nishimura EK, Akasaka Eet al. Epistatic connections between microphthalmia‐associated transcription factor and endothelin signaling in Waardenburg syndrome and other pigmentary disorders. FASEB J 2008; 22:1155–68. [DOI] [PubMed] [Google Scholar]
- 45.Lamb TD, Collin SP, Pugh EN Jr. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nat Rev Neurosci 2007; 8:960–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nayak G, Zhang KX, Vemaraju Set al. Adaptive thermogenesis in mice is enhanced by opsin 3‐dependent adipocyte light sensing. Cell Rep 2020; 30:672–86.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musilova Z, Cortesi F, Matschiner Met al. Vision using multiple distinct rod opsins in deep‐sea fishes. Science 2019; 364:588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng W, Lu H. Opsin as a photoreceptor after UVA irradiation to regulate MITF signal pathway and influence melanogenesis in the cultured human melanocyte. J Invest Dermatol 2019; 139 (Suppl.):S127. [Google Scholar]
- 49.Ozdeslik RN, Olinski LE, Trieu MMet al. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci USA 2019; 116:11508–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellono NW, Najera JA, Oancea E. UV light activates a Gαq/11‐coupled phototransduction pathway in human melanocytes. J Gen Physiol 2014; 143:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Different doses of ultraviolet radiation and all‐trans retinal affect the viability of human epidermal melanocytes.
Figure S2 Ultraviolet radiation stimulates melanogenesis.
Figure S3 Knockdown of the opsin 5 gene (OPN5) in human epidermal melanocytes with OPN5 short interfering RNA, and overexpression assay of OPN5 in a PIG1 melanocyte cell line.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


