Abstract
Objectives
Effective coverage of non‐communicable disease (NCD) care in sub‐Saharan Africa remains low, with the majority of services still largely restricted to central referral centres. Between 2015 and 2017, the Rwandan Ministry of Health implemented a strategy to decentralise outpatient care for severe chronic NCDs, including type 1 diabetes, heart failure and severe hypertension, to rural first‐level hospitals. This study describes the facility‐level implementation outcomes of this strategy.
Methods
In 2014, the Ministry of Health trained two nurses in each of the country’s 42 first‐level hospitals to implement and deliver nurse‐led, integrated, outpatient NCD clinics, which focused on severe NCDs. Post‐intervention evaluation occurred via repeated cross‐sectional surveys, informal interviews and routinely collected clinical data over two rounds of visits in 2015 and 2017. Implementation outcomes included fidelity, feasibility and penetration.
Results
By 2017, all NCD clinics were staffed by at least one NCD‐trained nurse. Among the approximately 27 000 nationally enrolled patients, hypertension was the most common diagnosis (70%), followed by type 2 diabetes (19%), chronic respiratory disease (5%), type 1 diabetes (4%) and heart failure (2%). With the exception of warfarin and beta‐blockers, national essential medicines were available at more than 70% of facilities. Clinicians adhered to clinical protocols at approximately 70% agreement with evaluators.
Conclusion
The government of Rwanda was able to scale a nurse‐led outpatient NCD programme to all first‐level hospitals with good fidelity, feasibility and penetration as to expand access to care for severe NCDs.
Keywords: non‐communicable disease, type 1 diabetes, rheumatic heart disease, first‐level hospital, national scale‐up, rural, Rwanda, Africa
Introduction
In lower‐income sub‐Saharan African (SSA) countries, effective coverage of longitudinal services for chronic non‐communicable diseases (NCDs) has been rare [1]. NCDs are characterised by diversity in their prevalence and severity. In response to the call to Universal Health Coverage (UHC), efforts have been made in a number of countries to decentralise care for common NCDs such as mild‐to‐moderate hypertension, intermittent and mild persistent asthma, and non‐insulin‐dependent type 2 diabetes to district (first‐level) hospitals and health centres using the WHO Package of Essential NCD Interventions (WHO PEN) and other integrated strategies [2]. In contrast, there have been few models of decentralised and integrated care for less common and more severe chronic NCDs, such as type 1 diabetes, insulin‐dependent type 2 diabetes, advanced rheumatic heart disease, cardiomyopathies, severe hypertension and moderate to severe persistent asthma [3]. Severe NCDs are those that result in large losses of healthy life for affected individuals due to early and high levels of disability and mortality in the absence of treatment [4, 5]. To date, no published studies have evaluated a nationally scaled outpatient NCD programme inclusive of integrating severe NCDs at first‐level hospitals.
Rwanda’s health infrastructure and workforce were decimated during the 1994 Genocide against the Tutsi. Since then, there has been progressive decentralisation and integration of outpatient services for a spectrum of chronic diseases, including HIV and tuberculosis, neuropsychiatric disorders and other chronic NCDs [3]. This process started with decentralisation of services from tertiary referral centres serving between 5 and 10 million people, to district hospitals serving around 255 000 people, and subsequently to primary health centres serving around 20 000 people. Services were grouped into competency‐related clusters and focused initially on more severe conditions. Nurse‐led task sharing was essential to this strategy because of a relative shortage of physicians [6]. During the late 1990s, specialised neuropsychiatric nurses and psychologists were trained and deployed to district hospitals (DH) to address psychosis and other severe neuropsychiatric disorders [7]. By the late 2000s, other cadres of nurses had been trained to deliver care for tuberculosis and HIV at these secondary‐care facilities as well [6]. However, services such as insulin management, anticoagulation and echocardiography for severe, chronic NCDs such as type 1 diabetes and advanced rheumatic heart disease remained exclusively centralised.
To address the challenge of increasing access to services for both severe and common NCDs, the Rwanda Ministry of Health (RMOH) collaborated with the non‐governmental organisation Partners In Health (PIH)/Inshuti Mu Buzima (IMB). Between 2006 and 2008, the RMOH and PIH/IMB worked in three rural DHs to establish outpatient care teams for chronic NCDs [3]. Detailed, early assessments of programme implementation and outcomes from these facilities have been published elsewhere [3, 8]. Briefly, this nurse‐led model integrated the diagnosis, treatment and follow‐up of type 1 and 2 diabetes mellitus, heart failure, severe hypertension, severe chronic respiratory disease (CRD) and other advanced conditions into a single entity with shared clinic space, staff, equipment, supply chain management, monitoring and evaluation systems and mentored training. Each advanced NCD clinic was led by two NCD‐trained nurses, supervised by a generalist physician and supported by a social worker, and a data officer. Simplified diagnostic and management protocols and forms for entry into an electronic medical record system were developed. Medical specialists initially trained nurses at one hospital clinic, who then trained those at the other two hospitals over a three‐month period. Advanced skills included simplified echocardiographic diagnosis, diuretic management, interpretation of HbA1c testing and insulin titration. These advanced NCD clinic nurses also trained and mentored nurses at primary care facilities, enabling progressive decentralisation and integration of chronic care for common NCDs such as non‐insulin‐dependent type 2 diabetes and non‐severe hypertension and asthma alongside chronic care for common mental disorders, HIV and tuberculosis at health centres in their supported districts [3, 9]. Hypertension cases were considered severe if they had a systolic blood pressure >180 mmHg or a diastolic blood pressure >110 mmHg. Asthma cases were considered severe if they qualified as ‘severe persistent’, which includes respiratory symptoms throughout the day, nighttime awakenings more than one time per week, and extreme limitation in daily activities.
Following a national NCD workshop at Rwinkwavu DH in 2010, the RMOH set a ’80 × 40 × 20’ target for 2013, which sought to reduce premature mortality from all NCDs and injuries by 80% in individuals younger than 40 years by the year 2020 [10]. In 2014, as one of the steps needed to achieve this goal, the RMOH began to decentralise chronic care for severe NCDs across all of Rwanda’s 42 DHs. The RMOH used the three PIH/IMB‐supported advanced NCD clinics as training facilities and sources of supervision.
Evaluations at the initial PIH/IMB‐supported RMOH DHs demonstrated good implementation feasibility and fidelity with promising clinical outcomes [9, 11, 12, 13, 14, 15, 16]. Here, we describe the fidelity, penetration and feasibility of scaling this NCD clinic approach for severe NCDs across all 42 DHs in Rwanda. We report on the implementation process, results of 2015 and 2017 post‐intervention cross‐sectional surveys of NCD clinic staffing, equipment and medication availability, a 2017 cross‐sectional survey of clinical decision making at these hospitals, and enrolment data from HMIS. We also discuss lessons learned to date from this scale‐up effort towards universal health coverage (UHC).
Methods
Setting
Rwanda is a small (26 338 km2), low‐income, predominantly rural country in East Africa with the highest population density (445/km2) in mainland Africa with a rising population from <6 million in 1994 to about 12.5 million in 2020. The country is geographically and administratively divided into five provinces constituted by 30 districts. Each district includes at least one DH, with the larger districts having at least two hospitals. The average catchment area for each DH is 255 000 people. Each district includes multiple sectors which serve as the catchment area for health centres. DHs are staffed primarily with nurses and general practitioners. Few DHs have medical specialists, such as internists and paediatricians. In 2016, there were 10 795 nurses and 1392 physicians in the country [17].
Scale‐up of first‐level hospital clinics for severe NCDs
All DHs identified two nurses as designated NCD providers to attend a two‐month national baseline training on NCD prevention, diagnosis, treatment and follow‐up. The decision to train nurses as opposed to physicians was based on their known competencies in HIV care, higher retention at DHs and greater numbers. A general practitioner was identified at each hospital to provide supervision and support, especially for complex cases. This initial training began in September 2014 for a subset of the DHs with a gradual increase in training of at least two nurses from all DHs by the end of 2015. NCD nurses then completed a one‐month practical training at a fully operational NCD clinic in one of the three model districts. Key learning points of the training were clinical care protocols, clinical workflow, pharmacy and supply chain, staffing availability and district leadership support. The specific skills obtained focus on the mastery of multi‐specialty diagnostic and treatment protocols, counselling and mentorship. Upon completion of didactic and practical trainings, NCD nurses were expected to return to their respective DHs and work together with their leadership to establish integrated NCD clinics with necessary space, trained staff, relevant equipment and medicines, and administrative support. The RMOH also implemented ministerial orders so that the public procurement and supply chain system could ensure that appropriate NCD essential medicines, laboratory tests and equipment would be available at the DHs.
Study design
A retrospective review of repeated cross‐sectional surveys from site visit reports and clinical observation checklists in 2015 and 2017 (n = 29 DHs, and n = 42 DHs, respectively) was conducted to assess implementation outcomes. We also reviewed national registries and other visit report information, including informal interviews with hospitals leadership and clinical staff.
Post‐intervention cross‐sectional surveys
In 2015, six to twelve months after an initial training of 29 DHs, a first round of cross‐sectional surveys of the respective district NCD clinics was performed. A team of three individuals, including one RMOH NCD staff, one RMOH NCD clinician and one PIH/IMB NCD staff, visited each NCD clinic of the 29 DHs. Each DH visit was two days long and had the following objectives: (i) observe and mentor NCD nurses on clinic organisation (appointment tracking, patient flow, referral systems, equipment and medication availability, and management of medical files); (ii) observe operations of different departments of the hospitals (administration, pharmacy, laboratory and finances); (iii) complete an observation checklist regarding facility‐level characteristics; and (iv) discuss with the DH leadership regarding visit observations, accomplishments, challenges and recommendations for next steps. The visiting team also obtained data from informal interviews with key hospital staff of the relevant departments. In the case of medication and laboratory cross‐sectional survey, the availability of the specific medicine or laboratory test was visually verified by the visiting team.
In 2017, another round of observation visits was conducted in all DHs; by this point, an additional 13 DHs had opened NCD clinics, bringing the national total to 42. This observation round included additional clinical cross‐sectional surveys, mentorship and supervision. The visiting team consisted of physicians (internist, paediatrician or general practitioner), RMOH staff and nurse specialists all trained in mentorship. The three‐part mentorship activity included one‐on‐one and group mentoring, as well as a didactic presentation. Once again, the visiting team collected data regarding feasibility of the programme design through observation checklists with some additional medicines included in the 2017 cross‐sectional survey. A clinical fidelity survey focused on adherence to guidelines on NCD management as defined by: (i) the percentage of patient encounters with the correctly prescribed medication regimen, and (ii) the percentage of patient encounters with appropriately prescribed laboratory follow‐up. Sample selection involved randomly selecting and reviewing 10 medical files per disease condition per facility by the visiting physician, who then completed a checklist. Specifically, the visiting physician identified whether the clinical decision for laboratory testing and medication regimen followed the national clinical protocol.
Retrospective review of national patient registration data
To assess penetration, national patient volumes were collected via the electronic RMOH Health Management Information System (HMIS). From 2015 to present, health facilities reported patient volumes each month from their respective NCD clinic registries. Initially, type 1 diabetes was the only disease group with categorised patient volume data beginning in 2015. Patients who are deceased, lost to follow‐up or transferred out of care are not included in the reported patient volumes.
Analysis
Our analysis applies an implementation research framework described by Proctor et al, with a focus on feasibility, fidelity and penetration [18]. Data from first and second round evaluations were reviewed to assess feasibility based on the following: (i) medication availability for diabetes, cardiovascular disease and CRD; (ii) equipment availability, including electrocardiography (ECG), ultrasound with cardiac probes, electrolyte and creatinine testing, and haemoglobin A1c (HbA1c). Due to the complexity of ensuring consistent access to commodities and maintaining delicate equipment, access to these inputs are a rate limiting step of many efforts to decentralised care. For this reason, we use these as metrics of feasibility. We assessed fidelity based on the per cent agreement between NCD clinic nurses and visiting mentors surrounding clinical decision making. Finally, we assessed penetration based on: (i) availability of an operational NCD Clinic, with DH NCD Clinics being defined as ‘operational’ if an NCD‐trained nurse performed clinical duties on a routine, weekly basis in a dedicated clinical space; (ii) proportion of the nurses staffing the NCD clinics who completed formal NCD training; (iii) patient enrolment via data from HMIS by disease and over time.
Descriptive statistical methods in Stata 13 were used to analyse absolute numbers and proportions.
Ethics
The authors obtained ethics approval for this study from the Rwanda National Ethics Committee.
Patient and Public Involvement
This study was led by the Ministry of Health and is an evaluation of its intervention at public district hospitals. This intervention has been refined through consideration of patient and provider feedback at public facilities.
Results
Feasibility
Table 1 shows the first‐round cross‐sectional survey data from 2015, and the follow‐up data from 2017 for laboratory testing and equipment availability. Both creatinine and electrolyte testing were widely available in both 2015 and 2017. HbA1c testing availability increased from 10% of DHs to 100% of hospitals during this period. Prothrombin (PT)/INR testing availability was not available at any DH in 2015, but increased to 40% of hospitals by 2017. ECG and cardiac ultrasound availability increased from <15% of hospitals to more than 35%.
Table 1.
Availability of laboratory testing, and equipment at district‐level NCD clinics from 2015 to 2017
|
2015 (n = 29) |
2017 (n = 42) |
|
|---|---|---|
| Laboratory testing | ||
| District hospitals performing creatinine and electrolyte testing | 29 (100%) | 42 (100%) |
| District hospitals performing HbA1c testing | 18 (62%) | 42 (100%) |
| District hospitals performing PT/INR testing | N/A | 17 (40%) |
| Equipment | ||
| District hospitals with ECG equipment | 3 (10%) | 15 (36%) |
| District hospitals with ultrasound equipment and cardiac probes | 4 (14%) | 16 (38%) |
Across the DH pharmacies, the reported first‐line essential NCD medications were present in at least 75% of all DHs in 2015 (see Figure 1). In 2017, the availability of most medications increased with the exceptions of glibenclamide, atenolol, spironolactone and beclomethasone inhaler. Several new medications were added in 2017, including cardiovascular medications, such as aspirin and warfarin. In 2017, warfarin was notably present in only 44% of DHs.
Figure 1.
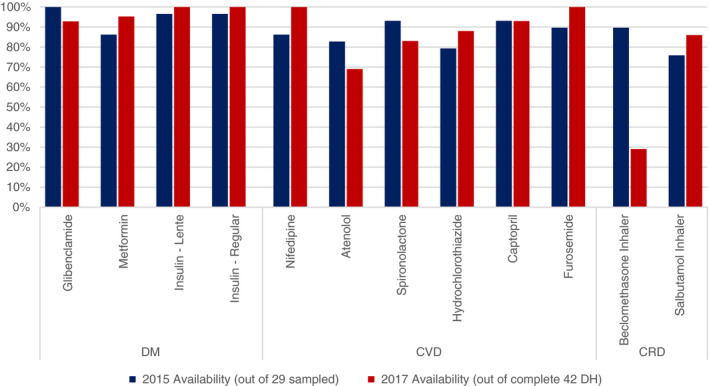
Feasibility of NCD medication availability from 2015 (out of 29 DH sampled) to 2017 (out of 42 DH).
Fidelity
The medical chart review evaluators found a high level of fidelity to protocols. After reviewing 420 patient encounters for each disease, the clinical agreement between evaluators and nurses in 2017 was approximately 70% for laboratory testing and treatment regimens across all diseases except for chronic respiratory disease and heart failure (Figure 2). Chronic respiratory disease treatment showed a per cent agreement >80%, while heart failure laboratory testing was at 64%.
Figure 2.
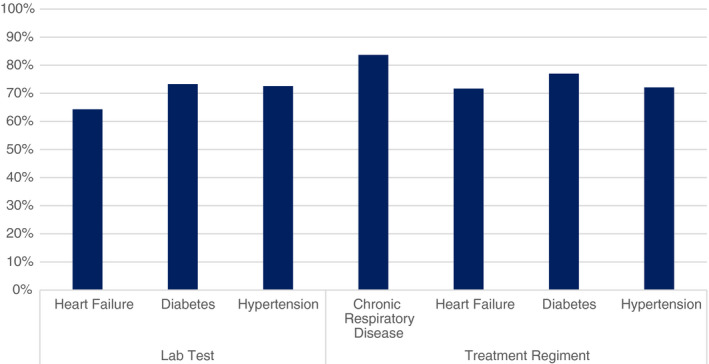
Quality of NCD care at all DHs in 2017.
Penetration
As of 2015, 29 of Rwanda’s 42 DHs (69%) had initiated NCD clinic operations. By 2017, all 42 DHs (100%) were found to have operational NCD clinics, which included staffing of at least one formally trained NCD nurse. Furthermore, 28 (67%) of the 42 district hospitals had two formally trained NCD nurses.
Approximately 27 000 patients were enrolled at NCD clinics in first‐level hospitals by 2019. The distribution of enrolled NCD patients at all first‐level hospitals included hypertension (70%), followed by type 2 diabetes (19%), chronic respiratory disease (5%), type 1 diabetes (4%) and heart failure (2%) (Figure 3). The specification of severe cases by insulin‐dependence or stage of disease was not available.
Figure 3.
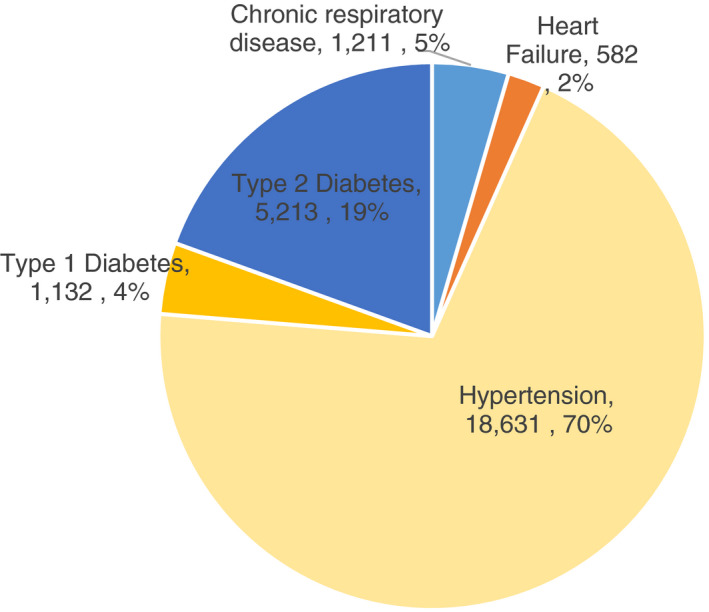
NCD distribution across all DH NCD clinics in Rwanda, HMIS, 2019.
As an example of the enrolment trend of severe NCDs at DHs, Figure 4 shows that the volume of patients with type 1 diabetes enrolled steadily increased from 2015 to 2019. The annual increase was approximately 175 patients resulting in an almost tripling of patient volume.
Figure 4.
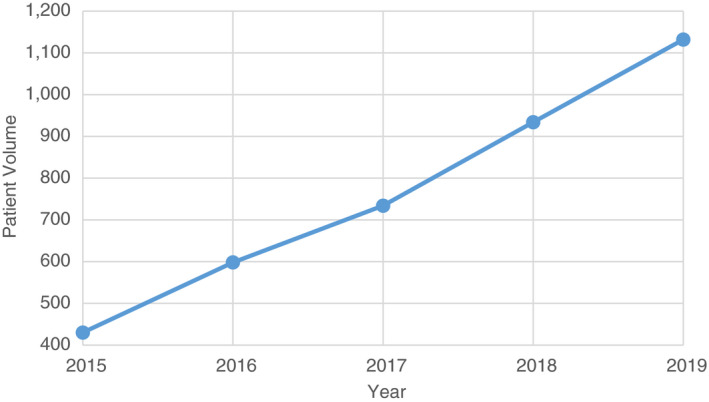
National enrolment of Type 1 Diabetes patients at DHs, HMIS, 2015–2019.
Discussion
This study demonstrates Rwanda’s ability to rapidly scale access to integrated chronic care services for severe NCDs at all first‐level hospitals. Specifically, the national programme demonstrated good adoption and feasibility at facilities as well as good penetration and fidelity in a number of key areas, including human resources, diagnostic and monitoring tools, and medications availability. This initial effort towards national decentralisation of NCD care highlights the potential for UHC through carefully planned and implemented training programmes, whereby initial pilot sites develop into national training sites. Furthermore, this national scale‐up experience illustrates the key role that first‐level hospitals can play to provide greater access to care for a number of severe, complex diseases that were previously only addressed at referral hospitals.
Challenges encountered with this national decentralisation effort included staff turnover. Between 2015 and 2017, 14 (17%) of the 84 trained nurses ceased working in NCD clinics. Possible reasons for this level of turnover include factors that affect all staffing at rural DH’s, such as limited educational opportunities for children [18]. It is also possible that work in the NCD clinic is perceived as being more demanding than other positions that are compensated similarly. Further research is needed to better understand the motivations and challenges faced by this new cadre. In the meantime, efforts to address turnover include maintaining a steady pipeline of trainees and careful selection of staff for training. Refresher training, mentorship and supervision are also essential for knowledge retention and motivation among new NCD nurses. Mentorship and supervision can come in the form of local physicians or visiting specialist clinicians from referral hospitals providing direct real‐time education, remote support for technical questions by phone, or individual teaching sessions. Peer‐to‐peer onsite training is also valuable. Another route to improve retention is greater formal recognition and professionalisation of specialised NCD practice through education degrees and/or credentialing, which may lead to greater financial incentives [19].
An additional challenge encountered in implementation was low availability of laboratory testing and ultrasound equipment. Over the course of the programme, additional portable ultrasound machines and electrocardiograms (ECGs) were purchased for 38% and 36% of hospitals, respectively. Similarly, point‐of‐care INR testing was not routine, but by 2017, 40% of hospitals had purchased point‐of‐care INR testing equipment. These gaps in equipment availability point to an opportunity for re‐prioritisation by governments for such essential equipment as well as greater regional and/or global efforts towards pooled procurement for these types of initial capital investments [20].
The majority (mean of 83%) of NCD essential medicines were available at district pharmacies in 2017, indicating that NCD drugs were well‐integrated in the national supply chain. This supply chain was established through careful planning, coordination and communication from the central medical store down to the peripheral levels of care. As the breadth of medications available in rural facilities was expanded, the supply chain was simultaneously strengthened to compensate for this increasing demand. These findings are substantially higher than that of other low‐income countries in Africa, including Ethiopia, Malawi, and Democratic Republic of the Congo. For example, the Service Provision Assessment surveys showed other countries in the region with availability of insulin to be available in less than 60% of first‐level hospitals. Ethiopia was more comparable to Rwanda with 79% availability [21]. Two exceptions to this relatively high availability of medications in Rwanda are beclomethasone and warfarin. Anecdotally, the low availability of beclomethasone (30% by 2017) was likely due to concerns by DH directors regarding the high cost of this medication. Warfarin was available in slightly less than half of all DHs by 2017, due to many DHs having not yet identified patients requiring warfarin. As a result, warfarin and INR testing were not dispensed to those hospitals.
Study limitations
Limitations of this study include the lack of baseline data of the availability of medications, equipment and other resources prior to the national scale‐up initiative. Furthermore, the particular day of facility visits may not be representative of the entire study period. Review of national registries and clinical charts are dependent on accurate record keeping which may have been inconsistent. The patient enrolment data over time were limited to type 1 diabetes and would benefit from additional data across all disease groups.
Future directions
More work is needed to assess national programmatic and patient‐level costs and treatment outcomes for integrated outpatient NCD services at first‐level hospitals in Rwanda. The Rwanda MOH has designed disease‐specific clinical forms for use in the hospital‐level NCD clinic and is nationally scaling an NCD electronic medical record platform that will enable routine data capture for individual patients. Additionally, the Rwanda Human Resource for Health programme has increased the availability of well‐trained internists and paediatricians in the country [22]. Quarterly mentorship visits by these physician specialists are planned and are expected to contribute both to continuous quality improvement and better cross‐sectional survey of clinical performance. Cardiologists and endocrinologists in the country are also working to develop continuing education and supervision strategies for specific services such as simplified echocardiography, anticoagulation and insulin titration [11, 12, 13, 14]. The programme has previously been found to cost approximately $50 000 in initial start‐up expenses in a single DH, and around $70 000 annually in operating expenses [8]. This translates to an approximate annual cost per patient of $109 incurred by the district hospital. Costs of care to the patient are supported by Rwanda’s national community‐based health insurance programme, Mutuelle de Santé, which provides coverage for 90% of all health costs to the patient at each visit. Future research will investigate actual direct and indirect costs to patients participating in the programme nationally.
Recently, WHO in the African region has begun to develop a Package of Essential NCD Interventions – Plus (PEN‐Plus) strategy to decentralise chronic care for severe NCDs such as type 1 diabetes, heart failure, advanced rheumatic heart disease and sickle cell disease based on the experience of Rwanda and other countries [23]. This PEN‐Plus strategy is meant to build on and accelerate implementation of WHO PEN chronic care services for more common NCDs at primary health centres [24].
Rwanda is working to decentralise and integrate care for common NCDs such as uncomplicated hypertension at the primary care level along with care for other chronic infectious conditions (HIV and TB) and neuropsychiatric conditions (depression, anxiety and epilepsy) [15, 16]. These efforts should allow primary hospital outpatient services to focus on more complex, less common diseases. Additionally, PEN‐Plus clinicians can serve as mentors for NCD clinicians at primary health centres.
Conclusion
To the best of our knowledge, the RMOH has implemented the first systematic national strategy to decentralise integrated care for severe chronic NCDs in a low‐ or lower middle income sub‐Saharan African country. The approach Rwanda has taken is nurse‐led and has been executed rapidly once initial training sites were established. This strategy has the potential to accelerate access to diagnosis and treatment for both severe and common NCDs across the continent as part of a movement towards UHC.
Acknowledgements
We acknowledge the critical talent and service provided by the NCD nurses across all district hospitals in Rwanda. We also acknowledge the role of valuable partners of the RMOH, including PIH/IMB.
Sustainable Development Goal: Good Health and Well‐being
Data availability statement
Additional data are available by emailing the corresponding author.
References
- 1.World Health Organization . Tracking Universal Health Coverage. World Health Organization: Geneva, 2015. Accessed July 2, 2020. [Google Scholar]
- 2.WHO . Package of essential noncommunicable disease interventions for primary health care in low‐resource settings. Geneva Wold Heal. Organ., p. 66, 2010. Accessed July 2, 2020.
- 3.Bukhman G, Kidder A, eds. The Partners in Health Guide to Chronic Care Integration for Endemic Non‐Communicable Diseases. Rwanda Edition. Cardiac, Renal, Diabetes, Pulmonary, and Palliative Care. Boston: Partners In Health; 2011: 1–350. (Available from: http://ncdsynergies.org/planning_tool/pih‐guide‐to‐chronic‐care‐integration [2 July 2020].
- 4.Johansson KA, Coates MM, Økland J‐Met al. Health by disease categories. In: Cookson R, Griffin S, Norheim OCulyer A (eds). Distributional Cost‐Effectiveness Analysis: A Handbook of Equity‐Informative Health Economic Evaluation. Oxford University Press: Oxford, 2020. Accessed July 2, 2020. [Google Scholar]
- 5.Kjell Arne Johansson J‐M Ø, Skaftun EK, Bukhman G, Norheim OF, Coates MM, Haaland ØA. Measuring Baseline Health with Individual Health‐Adjusted Life Expectancy (iHALE); 2019. (Available from: https://www.medrxiv.org/content/). 10.1101/19003814. [2 July 2020]. [DOI]
- 6.Shumbusho F, van Griensven J , Lowrance Det al. Task shifting for scale‐up of HIV care: evaluation of nurse‐centered antiretroviral treatment at rural health centers in Rwanda. PLoS Medicine 2009: 6: e1000163. Accessed July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ait Mohand A, Kayiteshonga Y. The set‐up of a national mental health program in Rwanda. Basel, Switzerland: 9th European Congress on Tropical Medicine and International Health, 2015: 1–31. Accessed July 2, 2020. [Google Scholar]
- 8.Eberly LA, Rusangwa C, Ng'ang'a Let al. Cost of integrated chronic care for severe non‐communicable diseases at district hospitals in rural Rwanda. BMJ Glob Health 2019: 4: e001449. Accessed July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndayisaba A, Harerimana E, Borg Ret al. A clinical mentorship and quality improvement program to support health center nurses manage type 2 diabetes in rural Rwanda. J. Diabetes Res 2017: 2017: 1–10. Accessed July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Binagwaho A, Muhimpundu MA, Bukhman G. 80 under 40 by 2020: an equity agenda for NCDs and injuries. Lancet 2014: 383: 3–4. Accessed July 2, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Tapela N, Habineza H, Anoke Set al. Diabetes in Rural Rwanda: high retention and positive outcomes after 24 months of follow‐up in the setting of chronic care integration. Int J Diabetes Clin Res 2016: 3: 1–6. Accessed July 2, 2020. [Google Scholar]
- 12.Rusingiza EK, El‐Khatib Z, Hedt‐Gauthier Bet al. Outcomes for patients with rheumatic heart disease after cardiac surgery followed at rural district hospitals in Rwanda. Heart 2018: 104: 1707–1713. Accessed July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan GF, Bukhman AK, Miller ACet al. A simplified echocardiographic strategy for heart failure diagnosis and management within an integrated noncommunicable disease clinic at district hospital level for Sub‐Saharan Africa. JACC Heart Fail 2013: 1: 230–236. Accessed July 2, 2020. [DOI] [PubMed] [Google Scholar]
- 14.Eberly LA, Rusingiza E, Park PHet al. 10‐Year heart failure outcomes from nurse‐driven clinics in rural Sub‐Saharan Africa. J Am Coll Cardiol 2019: 73: 977–980. Accessed July 2, 2020. [DOI] [PubMed] [Google Scholar]
- 15.Mantini N, Ngoga G, Park Pet al. Reducing Severe Hypertension Through Nurse‐Led Integrated Noncommunicable Disease Care Delivery at Decentralized Facilities in Rural Rwanda. American Heart Association Scientific Sessions: Chicago, IL, 2018. Accessed July 2, 2020. [Google Scholar]
- 16.Ngoga G, Park PH, Borg Ret al. Outcomes of decentralizing hypertension care from district hospitals to health centers in Rwanda, 2013–2014. Public Health Action 2019: 9: 142–147. Accessed July 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health of Rwanda . Annual Health Statistics Booklet: Key Statistics in the Health Sector for the year 2016. Kigali, 2016. Accessed July 2, 2020.
- 18.Proctor EK, Brownson RC. Measurement issues in dissemination and implementation research. In Dissemination and Implementation Research in Health: Translating Science to Practice. 2012. 10.1093/acprof:oso/9780199751877.003.0013. [July 2, 2020]. [DOI]
- 19.Wurie HR, Samai M, Witter S. in rural Sierra Leone: findings from life histories; 2016. (Available from: https://www.ncbi.nlm.nih.gov/pubmed/26833070). [2 July 2020]. [DOI] [PMC free article] [PubMed]
- 20.“Procurement Tools.” Procurement Tools ‐ Sourcing & Management of Health Products ‐ The Global Fund to Fight AIDS, Tuberculosis and Malaria. (Available from: www.theglobalfund.org/en/sourcing‐management/procurement‐tools/#pooled‐procurement). [2 July 2020].
- 21.The DHS Program ‐ Survey Search. The DHS Program. (Available from: https://dhsprogram.com/What‐We‐Do/survey‐search.cfm?pgtype=main&SrvyTp=type) [31 May 2019].
- 22.Binagwaho A, Kyamanywa P, Farmer PEet al. Rwanda's human resources for health program: a new partnership. N Engl J Med 2013: 369: 2054–2059. Accessed July 2, 2020. [DOI] [PubMed] [Google Scholar]
- 23.WHO PEN and Integrated Outpatient Care for Severe, Chronic NCDs at First Referral Hospitals in the African Region (PEN‐Plus). Report on Regional Consultation. 29 July – 1 August 2019. Brazzaville, Congo: World Health Organization, Regional Office for Africa; 2020. (Available from: https://www.afro.who.int/publications/who‐pen‐and‐integrated‐outpatient‐care‐severe‐chronic‐ncds‐first‐referral‐hospitals). [2 July 2020]. [Google Scholar]
- 24.Implementation Tools . Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low‐resource Settings. Geneva: World Health Organization; 2013: 1–210.. Accessed July 2, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data are available by emailing the corresponding author.


