Abstract
Aims
To investigate whether effects on chronic kidney disease risk factors could explain the apparent reduction in kidney outcomes (composite of macroalbuminuria, doubling of serum creatinine, renal replacement therapy, or renal death), primarily driven by changes in albuminuria, after treatment with the glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) liraglutide and semaglutide in patients with type 2 diabetes in the LEADER and SUSTAIN 6 trials.
Materials and Methods
We evaluated the mediation effect of glycated haemoglobin (HbA1c), systolic blood pressure (BP), and body weight on the kidney effects of GLP‐1RAs. Diastolic BP, haemoglobin, heart rate, low‐density lipoprotein and total cholesterol, and white blood cell count were also investigated. The mediation effect was estimated by the novel Vansteelandt statistical method. Subgroups with estimated glomerular filtration rate (eGFR) <60 and ≥60 mL/min/1.73 m2 were examined in LEADER.
Results
We observed that HbA1c mediated 25% (95% confidence interval [CI] −7.1; 67.3) and 26% (95% CI noncalculable), and systolic BP 9% (95% CI 2.8; 22.7) and 22% (95% CI noncalculable) of kidney effects of GLP‐1RAs in LEADER and SUSTAIN 6, respectively. Small or no mediation was observed for the other parameters; for example, body weight mediated 9% (95% CI −7.9; 35.5) in the former and did not mediate effects in the latter study. Mediation by HbA1c was greater in patients with eGFR ≥60 mL/min/1.73 m2 (57%) versus those with eGFR <60 mL/min/1.73 m2 (no mediation).
Conclusions
Our results suggest that HbA1c and systolic BP may moderately mediate kidney benefits of liraglutide and semaglutide, with all other variables having a small to no effect. Potential kidney benefits may be driven by other mediators or potentially by direct mechanisms.
Keywords: chronic kidney disease, glucagon‐like peptide‐1 receptor agonist, kidney mediation, liraglutide, semaglutide, type 2 diabetes
1. INTRODUCTION
Chronic kidney disease (CKD) is a frequent complication of type 2 diabetes associated with significantly increased mortality, morbidity,1 and healthcare costs.2 The global prevalence of CKD is growing, and is currently estimated at 9.1%.3 Over 40% of the global CKD prevalence is attributable to diabetes, making the latter the most important contributing factor.4 Treatment choices to reduce the risk of kidney function decline have been limited, as have the options to control glycaemia in CKD.5
Results from three cardiovascular (CV) outcome trials with glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), namely, “Liraglutide Effect and Action in Diabetes: Evaluation of CV outcome Results” (LEADER), “Trial to Evaluate CV and Other Long‐Term Outcomes with Semaglutide in Subjects with Type 2 Diabetes” (SUSTAIN 6), and “Researching CV Events with a Weekly Incretin in Diabetes” (REWIND), have indicated that patients receiving liraglutide, semaglutide or dulaglutide, respectively, were at a significantly lower risk of a major adverse CV event (MACE) and had a significantly lower occurrence of a composite kidney disease outcome, compared with patients receiving placebo.6, 7, 8, 9 The risk reductions of the kidney composite outcome observed in LEADER, SUSTAIN 6 and REWIND were 22% (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.67; 0.92), 36% (HR 0.64, 95% CI 0.46; 0.88), and 15% (HR 0.85, 95% CI 0.77; 0.93) in patients receiving liraglutide, semaglutide or dulaglutide, respectively, versus placebo, and these effects were mainly driven by a reduced risk of macroalbuminuria.6, 7, 8, 9 After exclusion of the macroalbuminuria component, the HR was 0.88 (95% CI 0.68; 1.13) with liraglutide and 1.05 (95% CI 0.57; 1.93) with semaglutide, compared with placebo.6, 7
Native glucagon‐like peptide‐1 has multiple metabolic effects, including stimulation of insulin secretion and biosynthesis, reduction of gastric emptying and food intake, and interaction with the renin‐angiotensin pathway.10 These and other mechanisms lead to reductions in known CKD risk factors such as glycated haemoglobin (HbA1c), systolic blood pressure (BP), and body weight.10 Some of these effects are attenuated in the setting of type 2 diabetes; however, it is possible to restore or magnify these effects by supplying GLP‐1RAs in supraphysiological doses.11 In the LEADER and SUSTAIN 6 trials, several risk factors for CKD, including HbA1c, systolic BP and body weight, were improved with liraglutide and semaglutide. In the present post hoc analysis, we investigated whether the effects of liraglutide and semaglutide on these and other identified risk factors may mediate their benefits on the kidneys.
2. MATERIALS AND METHODS
2.1. Trial design
The LEADER (NCT01179048) and SUSTAIN 6 (NCT01720446) trial designs and methods have been previously published in detail.6, 7 In brief, both trials were multicentre, double‐blind, placebo‐controlled trials. Patients with type 2 diabetes, aged ≥50 years with established CV disease, or ≥60 years with ≥1 CV risk factor (N = 9340 in LEADER and N = 3297 in SUSTAIN 6), were randomly assigned to a GLP‐1RA or placebo, both added to standard of care. In LEADER, liraglutide up to 1.8 mg subcutaneously (s.c.) once daily or matching placebo was given in a 1:1 ratio. In SUSTAIN 6, semaglutide 0.5 mg or 1.0 mg, or matching placebo, was given s.c. once weekly in a 1:1:1:1 ratio, pooled as semaglutide versus placebo for this analysis. The median duration of follow‐up was 3.8 years in LEADER and 2.1 years in SUSTAIN 6. In both trials, patients requiring continuous renal replacement therapy were excluded.6, 7
The present post hoc analysis was performed by trial in the full population and by CKD subgroup: estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 (normal kidney function or CKD stage 1‐2) or <60 mL/min/1.73 m2 (CKD stages 3‐5). This subgroup analysis was not performed for SUSTAIN 6 because of the relatively low number of patients in the latter subgroup.
2.2. Outcomes
We examined the prespecified secondary composite kidney outcome from both trials, composed of (a) new‐onset persistent macroalbuminuria, (b) persistent doubling of serum creatinine and reaching eGFR of ≤45 mL/min/1.73 m2, (c) need for continuous renal replacement therapy, or (d) death from kidney disease.6, 7, 8
2.3. Mediation analysis
The mediaton analysis was carried out using the statistical method of Vansteelandt et al.12 The aim of the analysis was to assess whether the well‐known effects (eg, on glycaemic control or weight loss) of a GLP‐1RA (liraglutide or semaglutide) could explain the beneficial effect on time to first kidney event. Such potential indirect (mediated) effects may be interpreted as the difference in time to first kidney event between a GLP‐1RA and placebo that may be explained by change in one or more observed variables (mediators). The effect remaining after controlling for the observed mediators reflects effects that are direct or that occur via unobserved mediators.
The Vansteelandt method is based on a dynamic model that allows for the incorporation of new mediator values as they become available during the trial; however, they are incorporated in the model as separate values (eg, as observed values from each trial visit). The method also allows for adjustment for confounders (such as concomitant medication at baseline or during trial). The present analysis was not adjusted for confounders. A more detailed explanation of the method has been published separately.12
The assessment of the mediated effect involved estimating the counterfactual probability for a patient in the GLP‐1RA treatment group to be event‐free at a given time if their mediator values changed to the levels that would have been seen had the patient been assigned to the placebo treatment group.
To estimate the indirect (mediated) effect, the probability of a patient in the GLP‐1RA group to be event‐free assuming their actual mediator values was compared with the probability for the same patient assuming placebo values. The total effect was estimated as the difference in the probabilities of being event‐free for patients in the GLP‐1RA and placebo groups using their actual mediator values.
The indirect‐to‐total‐effect ratio was used to calculate the percentage of mediation for each variable (0%, no mediation; 100%, complete mediation), while not allowing for adding effects of different mediators together. This calculation was performed at 36 months in LEADER and 24 months in SUSTAIN 6, corresponding to the time points of the last visit at which laboratory variables were tested for all patients in each trial. The number of patients under observation for kidney events decreased sharply after these time points because of trial close‐out at 24 months in SUSTAIN 6, and varied from 42 to 60 months in LEADER. A 95% CI for the percentage mediation was calculated using a bootstrap resampling procedure using 1000 samples. Due to a lower number of events in SUSTAIN 6, 95% CIs could only be calculated for mediators in the LEADER trial.
2.4. Identifying potential mediators
The potential mediators selected and analysed in this study included variables that (a) were likely to be changed by treatment with liraglutide and semaglutide, and (b) could potentially be linked to development and progression of CKD. Z‐scores from a mixed model were used to indicate the potential of the selected variables to be important mediators. Positive z‐scores indicate that the variable could be a potential mediator of the effect of liraglutide and semaglutide on the composite renal endpoint. It has previously been shown in LEADER and SUSTAIN 6 that HbA1c, systolic BP, and body weight were lowered by GLP‐1RA treatment.6, 7 Variables that had a potential link to kidney outcomes based on published literature that were available in the trial data (ie, diastolic BP, haemoglobin, heart rate, low‐density lipoprotein [LDL] cholesterol, total cholesterol, and white blood cell count) were analysed13, 14, 15, 16, 17 (Table 1). White blood cell count was selected as a marker for anti‐inflammatory effects of liraglutide and semaglutide, in the absence of other biomarkers,18 and because it has been shown to correlate with body mass index and plasma insulin levels.19, 20 Values of all variables included in the mediation analysis were measured at trial visits before or at the same visit as a kidney event was determined. Lastly, given the high prevalence of concomitant use of renin‐angiotensin‐aldosterone system (RAAS)‐blocking drugs (79.7%‐81.4% across both trials), a sensitivity analysis with RAAS‐blocking agents as a confounder was also carried out.
TABLE 1.
Potential mediators of the kidney benefit with liraglutide and semaglutide from LEADER and SUSTAIN 6, respectively
| LEADER | SUSTAIN 6 | |||
|---|---|---|---|---|
| Variable | Liraglutide vs. placebo, ETD (95% CI)a , b | Liraglutide vs. placebo: z‐score | Semaglutide vs. placebo, ETD (95% CI)a | Semaglutide vs. placebo: z‐score |
| Body weight, kg | −2.26 (−2.54; −1.99) | −16.18 | −3.55 (−3.95; −3.15) | −17.27 |
| Diastolic BP, mmHg | 0.59 (0.19; 0.99) | 2.88 | 0.21 (−0.39; 0.82) | 0.69 |
| Haemoglobin, mmol/L | 0.05 (0.03; 0.08) | 3.68 | 0.07 (0.02; 0.12) | 2.90 |
| HbA1c, % | −0.40 (−0.45; −0.34) | −13.49 | −0.86 (−0.95; −0.76) | −17.79 |
| Heart rate, beats/min | 2.98 (2.54; 3.42) | 13.28 | 2.22 (1.57; 2.87) | 6.68 |
| LDL cholesterol, mmol/L | −0.04 (−0.08; −0.01) | −2.28 | −0.06 (−0.11; −0.01) | −2.24 |
| Systolic BP, mmHg | −1.20 (−1.92; −0.48) | −3.28 | −1.89 (−2.91; −0.86) | −3.62 |
| Total cholesterol, mmol/L | −0.04 (−0.09; 0.00) | −1.92 | −0.08 (−0.15; −0.02) | −2.56 |
| White blood cell count, 109/L | −0.03 (−0.10; 0.05) | −0.62 | −0.09 (−0.20; 0.03) | −1.47 |
Note: Negative ETD indicates a larger reduction with liraglutide and semaglutide vs placebo. Positive ETD indicates a larger increase with liraglutide and semaglutide vs placebo.
Abbreviations: BP, blood pressure; CI, confidence interval; ETD, estimated treatment difference; HbA1c, glycated haemoglobin; LDL, low‐density lipoprotein.
Change from baseline to 24 months.
Change from baseline to 36 months.
2.5. Ethics
Both trials were approved by local institutional review boards or ethics committees at each centre. All patients provided written informed consent. The trials were conducted in accordance with the Declaration of Helsinki.21
2.6. Role of the funding source
The sponsor of this study had a role in study design, data analysis, and data interpretation. Three of the authors of this report are employees of the sponsor and, as such, were involved in the preparation, review, and approval of the manuscript. The corresponding author had final responsibility for the decision to submit for publication.
3. RESULTS
3.1. Baseline characteristics
In the LEADER trial, 9340 patients were randomized to liraglutide (N = 4668) or placebo (N = 4672) and in the SUSTAIN 6 trial, 3297 patients were randomized to semaglutide (N = 1648) or placebo (N = 1649). The median follow‐up was 3.8 years in LEADER and 2.1 years in SUSTAIN 6. Baseline characteristics were similar between trials and between treatment arms of both trials; these are summarized in Supplementary Table S1.
3.2. Potential mediators of the kidney‐protective effect of liraglutide and semaglutide
In LEADER and SUSTAIN 6, the composite kidney outcome was observed in 605 of 9340 and in 162 of 3297 participants, respectively. With liraglutide and semaglutide 0.5 and 1.0 mg compared to placebo, HbA1c was lowered by 0.4, 0.7 and 1.0 percentage points, respectively, systolic BP was lowered by 1.2, 1.3 and 2.6 mmHg, respectively, and body weight was lowered by 2.3, 2.9 and 4.3 kg, respectively (Table 1).
The probability of a composite kidney outcome occurring in patients in the liraglutide and semaglutide arm versus the placebo arm, together with the counterfactual probability of a kidney event if patients in the GLP‐1RA arm experienced the same mediator levels as those in the placebo arm, is shown in Figure 1. HbA1c mediated 25% (95% CI −7.1; 67.3; Figure 1A) and 26% (95% CI noncalculable; Figure 1B) of the benefit observed with GLP‐1RA treatment on the kidney composite in LEADER and SUSTAIN 6, respectively. Systolic BP mediated 9% in LEADER (95% CI 2.8; 22.7; Figure 1C) and 22% (95% CI noncalculable) of the kidney benefit in SUSTAIN 6 (Figure 1D). Body weight mediated 9% in LEADER (95% CI −7.9; 35.5; Figure 1E), but did not mediate kidney benefit in SUSTAIN 6 (Figure 1F).
FIGURE 1.
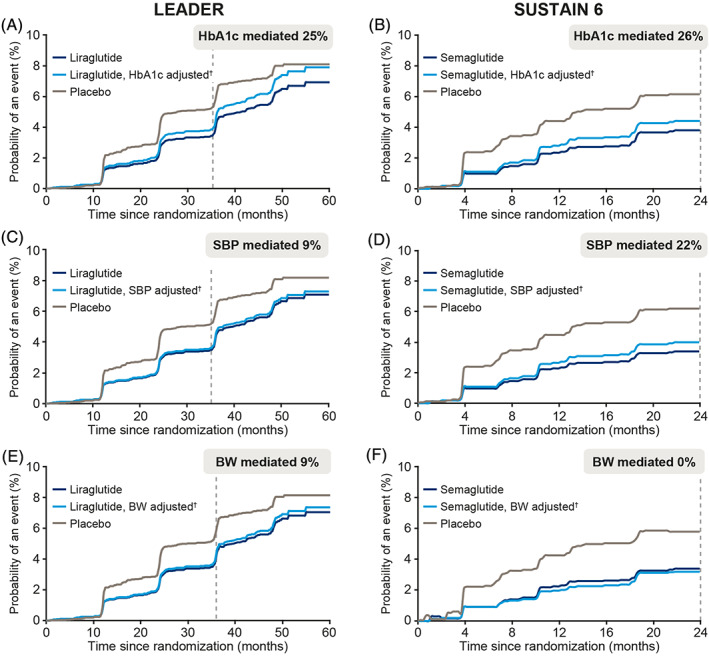
Levels of mediation by (A, B) glycated haemoglobin (HbA1c), (C, D) systolic blood pressure (SBP) and (E, F) body weight (BW) on the effect of liraglutide and semaglutide on time to first kidney outcome in the LEADER trials (at 36 months) and SUSTAIN 6 (at 24 months), respectively. Levels of mediation are demonstrated by examining the difference between the light and dark blue lines. †Adjusted to the counterfactual probability of an event at a given time if patients in the liraglutide and semaglutide arm changed to mediator levels they would have experienced in the placebo arm. Kidney event was defined as a composite of (i) new‐onset persistent macroalbuminuria, (ii) persistent doubling of serum creatinine and reaching estimated glomerular filtration rate (eGFR) of ≤45 mL/min/1.73 m2, (iii) need for continuous renal replacement therapy, or (iv) death from kidney disease
After accounting for the concomitant use of RAAS‐blocking drugs as a confounder, the level of mediation remained the same for HbA1c and systolic BP in LEADER (25% and 9%, respectively) and body weight in SUSTAIN 6 (no mediation). The percentage of mediation remained similar for body weight in LEADER (10% vs. 9%) and systolic BP in SUSTAIN 6 (26% vs. 22%). The only change in the sensitivity analysis was observed for HbA1c in SUSTAIN 6 (53% vs. 26%).
The additional candidate mediators investigated in the analysis (diastolic BP, haemoglobin, heart rate, LDL and total cholesterol, and white blood cell count) had small, if any, mediation effect (0%‐4% in LEADER and 0%‐11% in SUSTAIN 6; Supplementary Tables S2 and S3). Although of unknown clinical significance, diastolic BP was statistically significantly increased with liraglutide, and heart rate was significantly increased with both liraglutide and semaglutide versus placebo.6, 7
3.3. Potential mediators of the kidney‐protective effect of liraglutide in eGFR subgroups
In LEADER, the level of mediation differed by eGFR subgroup (Figure 2). A greater level of mediation by HbA1c was observed in patients with eGFR ≥60 mL/min/1.73 m2 (57%; 95% CI noncalculable; n = 7358), compared with patients with eGFR <60 mL/min/1.73 m2 (no mediation; n = 1982). Systolic BP mediated a numerically higher portion of the kidney benefit of liraglutide in patients with eGFR <60 mL/min/1.73 m2 (15%), compared with the subgroup with eGFR ≥60 mL/min/1.73 m2 (8%). A mediation effect of 29% by body weight was observed in patients with eGFR ≥60 mL/min/1.73 m2, whereas there was no mediation observed in the subgroup with eGFR <60 mL/min/1.73 m2. Data were too sparse to evaluate mediation according to eGFR subgroups in SUSTAIN 6 (n = 843 patients with eGFR <60 mL/min/1.73 m2 at baseline with 88 composite kidney outcomes).
FIGURE 2.
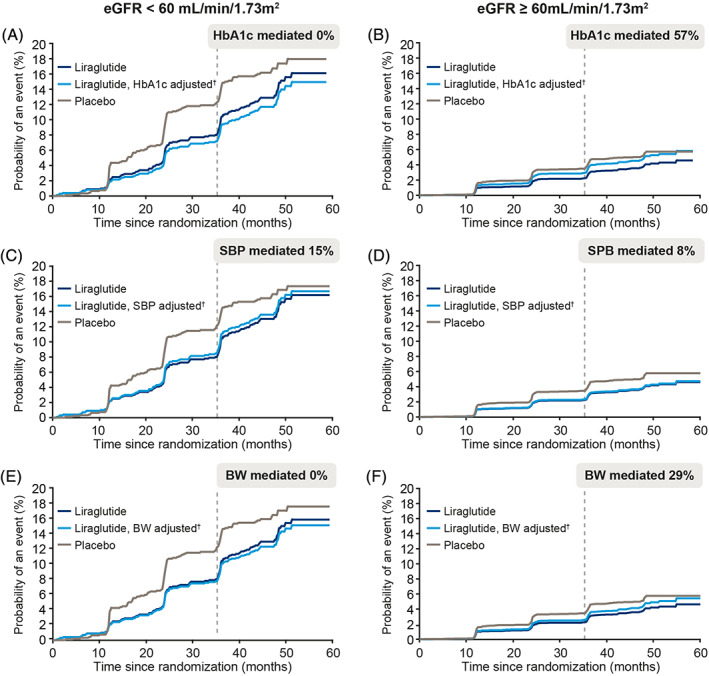
Levels of mediation by (A, B) glycated haemoglobin (HbA1c), (C, D) systolic blood pressure (SBP) and (E, F) body weight (BW) on the effect of liraglutide on time to first kidney outcome in two patient subgroups by estimated glomerular filtration rate (eGFR) from the LEADER trial. Levels of mediation are demonstrated by examining the difference between the light and dark blue lines. †Adjusted to the counterfactual probability of an event at a given time if patients in the liraglutide arm changed to mediator levels they would have experienced in the placebo arm. Kidney event was defined as a composite of (i) new‐onset persistent macroalbuminuria, (ii) persistent doubling of serum creatinine and reaching eGFR of ≤45 mL/min/1.73 m2, (iii) need for continuous renal replacement therapy, or (iv) death from kidney disease
4. DISCUSSION
The results of this post hoc analysis suggest that only a modest portion of the beneficial effect of liraglutide and semaglutide treatment on kidney outcomes observed in LEADER and SUSTAIN 6 is mediated by changes in HbA1c, systolic BP, and body weight. The effect attributable to HbA1c and body weight was consistent between the trials (25%‐26% and 0%‐9%, respectively). The level of mediation by systolic BP was numerically lower in LEADER (9%) than that in SUSTAIN 6 (22%). Other factors, including diastolic BP, haemoglobin, heart rate, cholesterol, and white blood cell count appeared to play a small, if any, role.
The validity of this analysis from LEADER and SUSTAIN 6 is supported by further observations. First, in the REWIND trial, HbA1c mediated 26% and systolic BP mediated 15% of the total effect of dulaglutide on kidney outcomes.9 These similar results were achieved with a different statistical model (Vansteelandt method in this study and Cox proportional hazards in REWIND). Levels of mediation by body weight and data according to CKD stage at baseline were not reported in REWIND.9 Second, the AWARD‐7 trial compared dulaglutide with insulin glargine in patients with diabetic kidney disease and a mean eGFR of 38 mL/min/1.73 m2 at baseline.22 While HbA1c levels were lowered substantially and to a similar extent by both treatments, ensuring glycaemic equipoise, the eGFR decrease was significantly smaller with dulaglutide than with insulin glargine.22 This indicates possible direct effects of the GLP‐1RA on the eGFR decrease, rather than mediation by glycaemia.
A recent cardiovascular mediation analysis of LEADER, in which the same analytical method was used as in the present analysis, identified HbA1c as a strong mediator of MACE.23 The observed level of mediation for the MACE outcome by HbA1c was 82%, and thus appreciably higher than the 25% observed in the present kidney mediation analysis, while the mediation of the effect on MACE by systolic BP (13%) and body weight (14%) was similar to our results on kidney mediation.23
In the LEADER subgroup analysis, a more pronounced mediation effect was observed for HbA1c and body weight in patients with normal kidney function or CKD stage 1 to 2 (eGFR ≥60 mL/min/1.73 m2), compared with patients with CKD stages 3 to 5 (eGFR <60 mL/min/1.73 m2). None of the observed kidney‐protective effects of liraglutide were found to be mediated by HbA1c or body weight in patients with CKD stages 3 to 5. Our observation that HbA1c and body weight do not mediate the kidney‐protective effect of liraglutide in patients with CKD stages 3 to 5 suggests that other mechanisms, possibly direct and not measured in the trials, may be particularly important in this patient subgroup.
It is intriguing that in previous subgroup analyses studying the kidney effects of liraglutide and semaglutide by kidney function, a greater benefit on eGFR loss was reported in patients with pre‐existing CKD stages 3 to 5 compared with patients with better kidney function.8, 24 A LEADER subgroup analysis by baseline eGFR showed that the eGFR decrease was smaller in the liraglutide group than in the placebo group in the subgroup with baseline eGFR 30 to 59 mL/min/1.73 m2 (P < 0.001), whereas it did not differ significantly between treatment arms in those with eGFR >60 mL/min/1.73 m2 or <30 mL/min/1.73 m2.8 In another combined subgroup analysis of LEADER and SUSTAIN 6, the annual loss of eGFR in patients treated with liraglutide and semaglutide was markedly slower in patients with pre‐existing CKD.24 For example, in LEADER, the beneficial effect of slower eGFR decline was significantly more pronounced in patients with a baseline eGFR <60 mL/min/1.73 m2 (annual estimated treatment difference [ETD] 0.67 mL/min/1.73 m2 slower eGFR reduction with liraglutide vs. placebo) than in patients with baseline eGFR ≥60 mL/min/1.73 m2 (annual ETD 0.15 mL/min/1.73 m2 slower eGFR reduction with liraglutide vs. placebo; subgroup interaction P = 0.008).24 Data from retrospective observational studies in clinical practice have supported the protective effect of liraglutide on the kidneys,25, 26 particularly in those with eGFR <45 mL/min/1.73 m2.26 Similarly in SUSTAIN 6, the beneficial effect of slower eGFR reduction was more pronounced in patients with a baseline eGFR <60 mL/min/1.73 m2 (annual ETD 1.62 mL/min/1.73 m2 slower eGFR reduction with semaglutide vs. placebo) than in patients with baseline eGFR ≥60 mL/min/1.73 m2 (annual ETD 0.63 mL/min/1.73 m2 slower eGFR reduction with semaglutide vs. placebo; subgroup interaction P = 0.06).24
Interestingly, a meta‐analysis of four large trials comparing standard versus intensive control of HbA1c on microvascular outcomes in patients with type 2 diabetes suggested that an improvement in HbA1c was associated with an approximately 20% reduced risk for unfavourable kidney outcomes.27 Those results are in line with our mediation estimate and they were also primarily driven by macroalbuminuria and, to a lesser extent, by kidney failure.27
It was surprising that the percentage of mediation by body weight was higher for liraglutide (9%) compared with semaglutide (0%), given that semaglutide had a larger treatment effect on body weight. However, the precision of the estimates should be considered. Additionally, in both trials, the observed treatment effects on body weight were statistically and clinically significant, albeit numerically higher for semaglutide. While we cannot rule out a potential relationship between the amount of body weight loss and the extent of mediation, our results indicate that body weight was not an important mediator of the kidney effect in LEADER nor in SUSTAIN 6.
The percentages of mediation observed for each candidate cannot be added together to give the overall mediation effect. Other mediators or direct mechanisms could potentially explain the observed benefit of GLP‐1RAs on kidney outcomes, including direct anti‐inflammatory effects, reduced levels of reactive oxygen species and protective effect against oxidative stress,28 and haemodynamic or natriuretic effects,29 as well as improved endothelial function (particularly in the kidneys)30 and suppression of angiotensin II production.31 However, as no mediators available for analysis in the present study could be used to assess the effect of these potential mechanisms, these potential mechanisms await testing in future clinical studies.
Another class that has shown promise in reducing progression of CKD is the sodium‐glucose cotransporter protein‐2 inhibitors.32, 33, 34 In a recent mediation analysis of the beneficial effects of canagliflozin treatment on kidney outcomes (CANVAS Program), nine out of 18 studied candidate mediators have shown some level of mediating effect: gamma glutamyltransferase (4.1%); urine pH (7.5%); systolic BP (8.9%); serum albumin (19.5%); urinary albumin‐creatinine ratio (UACR; 23.9%); serum urate (35.4%); haemoglobin (41.3%); haematocrit (51.1%); and erythrocytes (56.7%).17 Thus, the strongest mediators were related to blood volume and/or haematopoiesis, and not to glycaemic control.17 Compared with our analysis, the level of mediation by systolic BP was similar between CANVAS and LEADER/SUSTAIN 6 (9% and 9%‐22%, respectively); however, haemoglobin appeared to mediate a larger portion of the total effect of canagliflozin compared with liraglutide and semaglutide (41% vs. 4%‐11%, respectively). However, it should be noted that the definition of kidney outcome in CANVAS (a composite of 40% eGFR decline, end‐stage kidney disease, or death from kidney disease) differed from that in LEADER and SUSTAIN 6.6, 7, 17
We consider a strength of our study to be the use of the novel statistical approach to mediation analysis by Vansteelandt et al,12 in place of the conventional time‐dependent Cox proportional hazards model evaluated using two separate models. The Vansteelandt method is believed to have an improved ability to detect a mediated effect compared with Cox regression, which can be hampered by the need to fulfil the proportional hazard assumption and by not effectively adjusting for endogenous variables. An additional benefit of our methodology is that, as the Cox model is based on a single summary measure of the mediator, it may result in an underestimation of the mediated proportion (ie, it may underestimate biomarker effects). Such underestimation can occur, for example, when the association with the last recorded level of the mediator and the outcome is confounded by previous levels of the mediator.12 Thus, the Cox method may not capture the full complexity of the mediator and may not allow confounding to be controlled adequately.35 Additionally, the Cox method does not readily lend itself to a counterfactual interpretation (a “what if” approach), as used in the modern theory of mediation analysis. Nevertheless, results from the Cox model in REWIND appeared to support the data of the Vansteelandt approach in the present analysis.
A limitation of mediation analyses is that they do not allow differentiating between effects of individual components of each mediator. For example, it cannot be concluded whether it is the early response, the average level over time within patients, interpatient variability, or a combination of several elements that contribute to the mediated effect. Limitations of our study include the different median follow‐up durations in LEADER and SUSTAIN 6, and the associated low number of kidney events in SUSTAIN 6. Owing to this limitation, CIs could not be calculated for data from the SUSTAIN 6 trial. Furthermore, CIs of the mediated effects in LEADER were large, implying a high level of uncertainty. However, adjusting for the concomitant use of RAAS blockers as a confounder indicated that the results of our mediation analysis are robust, with the exception of mediation by HbA1c in SUSTAIN 6. For the latter, the estimation procedure became unstable, probably owing to the sparsity of data from SUSTAIN 6. There may be other potential mediators that were not assessed in the present analysis, as the field of important biomarkers evolves continuously and this analysis was limited to candidate mediators included in the trial designs of LEADER and SUSTAIN 6. For example, we have used white blood cell count as the only available proxy for anti‐inflammatory effects of liraglutide and semaglutide, as C‐reactive protein, a potentially more precise biomarker of inflammation, was not available. Additionally, it is important to note the inherent uncertainty in the total treatment effect of liraglutide and semaglutide, making it impossible to estimate the percentage of mediation with precision. Specifically for kidney outcomes, the benefit observed with liraglutide and semaglutide was driven primarily by the macroalbuminuria component (a surrogate outcome). This precluded us from testing UACR as a potential mediator, as well as analysing the components of the composite kidney outcome. Finally, post hoc analyses such as this cannot differentiate markers from causal factors. Thus, the results presented here should be interpreted with caution and should be used primarily for hypothesis‐generating purposes.
The LEADER and SUSTAIN 6 trials were not powered to prove benefits of liraglutide and semaglutide on kidney outcomes. Additionally, most patients participating in these two trials were at a relatively low kidney risk at baseline. To assess the effect of semaglutide in patients with CKD and type 2 diabetes, a kidney outcome trial (FLOW; NCT03819153) has recently been initiated. The FLOW trial will include more than 3000 adult patients with eGFR between 25 and 75 mL/min/1.73 m2, treated with a RAAS‐blocking agent. The completion of FLOW is estimated for 2024.
In summary, the present mediation analysis suggests that lowering HbA1c, body weight and systolic BP accounts for a minor part of the beneficial effect on the kidneys observed with liraglutide and semaglutide. Additionally, the effect of these mediators seems to vary depending on CKD stage. Further studies are needed to elucidate the potential direct mechanisms of GLP‐1RA treatment on reducing the progression of CKD in patients with type 2 diabetes.
CONFLICT OF INTEREST
J.F.E.M. has received speaker honoraria from Astra, Boehringer Ingelheim, Eli Lilly & Co, Medice, Novo Nordisk, Roche, and Vifor Pharma, research support from the European Union, Canadian Institutes of Health Research, Astra, Boehringer Ingelheim, Novo Nordisk, Roche and Sandoz, and consultation fees from Astra, Bayer, Novo Nordisk and Vifor Pharma. J.B.B.'s contracted consulting fees and travel support for contracted activities are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Eli Lilly, Fortress Biotech, MannKind, NovaTarg, Novo Nordisk, Sanofi, Senseonics, vTv Therapeutics and Zafgen, as well as grant support from NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics. J.B.B. is also a consultant to Cirius Therapeutics Inc, CSL Behring, Fortress Biotech, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, Stability Health and Zealand Pharma, holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio and Stability Health, and is supported by grants from the National Institutes of Health in part for this effort (UL1TR002489, P30DK124723). T.I. is an employee of Novo Nordisk and a significant stockholder. L.A.L. has received honoraria for advisory board participation from, and has provided continuing medical education on behalf of, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Servier, and has received research grants from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Lexicon, Novo Nordisk and Sanofi. R.E.P. has received research grants from Gilead Sciences, Lexicon Pharmaceuticals, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Sanofi‐Aventis US LLC, and Takeda, has acted as a speaker for AstraZeneca, Novo Nordisk and Takeda, and as a consultant for AstraZeneca, Boehringer Ingelheim, Eisai, Inc., GlaxoSmithKline, Janssen Scientific Affairs LLC, Ligand Pharmaceuticals Inc., Lilly, Merck, Novo Nordisk, Pfizer and Takeda. All payments are made directly to his employer (AdventHealth). S.R. is a Novo Nordisk employee and stockholder. T.V. has served on scientific advisory panels, been part of speaker's bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi and Sun Pharmaceuticals. B.W. is a Novo Nordisk employee and stockholder. V.P. has participated in steering committees, advisory boards, and/or received honoraria and consultation fees from Amgen, Janssen, GlaxoSmithKline, Astellas, Boehringer Ingelheim, Baxter, Mitsubishi Tanabe, Mundipharma, Retrophin, Merck, Abbvie, Novo Nordisk, AstraZeneca, Gilead, Durect, Servier, Tricida, Novartis, Eli Lilly, Relypsa, Pharmalink, Bayer and Bristol‐Myers Squibb.
AUTHOR CONTRIBUTIONS
All authors contributed significantly to the design and conduct of the study, and acquisition of clinical data. Søren Rasmussen performed the statistical analyses. All authors reviewed and interpreted the data, and were involved in drafting and critically revising the manuscript. The corresponding author is the guarantor, had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors approved the final version of the manuscript and take full responsibility for the content.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
LEADER, SUSTAIN 6 and the present analysis were funded by Novo Nordisk A/S. The authors thank all trial personnel and participants, and Sonia Vyskocilova, PhD, and Izabel James, MBBS, of Watermeadow Medical, an Ashfield company (funded by Novo Nordisk A/S), for medical writing and editorial assistance. Parts of the data included here have been published as an abstract and presented as an oral presentation at the American Society of Nephrology Kidney Week in 2019 (J Am Soc Nephrol 2019; 30:SA OR082), and at the European Association for the Study of Diabetes virtual meeting on September 23, 2020 (abstract 134).
Mann JFE, Buse JB, Idorn T, et al. Potential kidney protection with liraglutide and semaglutide: Exploratory mediation analysis. Diabetes Obes Metab. 2021;23(9):2058–2066. 10.1111/dom.14443
DATA AVAILABILITY STATEMENT
De identified individual participant data, study protocol, and redacted Clinical Study Report will be available according to Novo Nordisk data sharing commitments. The data will be made available permanently after research completion, and approval of product and product use in both the EU and US. Data will be shared with bona fide researchers submitting a research proposal requesting access to data and for use as approved by the Independent Review Board according to the IRB Charter (see novonordisk trials.com). Access request proposal form and access criteria can be found at novonordisk trials.com. The data will be made available on a specialised SAS data platform.
REFERENCES
- 1.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System . Annual data report. Chapter 7: healthcare expenditures for persons with CKD. 2018. https://www.usrds.org/2018/view/v1_07.aspx (Accessed September 2020).
- 3.GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990‐2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y, Bowe B, Mokdad AH, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567‐581. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a kidney disease: improving global outcomes (KDIGO) consensus conference. Kidney Int. 2020;97:1117‐1129. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo‐controlled trial. Lancet. 2019;394:131‐138. [DOI] [PubMed] [Google Scholar]
- 10.Bjerre Knudsen L, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol. 2019;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6‐week course of glucagon‐like peptide 1 on glycaemic control, insulin sensitivity, and beta‐cell function in type 2 diabetes: a parallel‐group study. Lancet. 2002;359:824‐830. [DOI] [PubMed] [Google Scholar]
- 12.Vansteelandt S, Linder M, Vandenberghe S, Steen J, Madsen J. Mediation analysis of time‐to‐event endpoints accounting for repeatedly measured mediators subject to time‐varying confounding. Stat Med. 2019;38:4828‐4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163:1555‐1565. [DOI] [PubMed] [Google Scholar]
- 14.Hillis GS, Hata J, Woodward M, et al. Resting heart rate and the risk of microvascular complications in patients with type 2 diabetes mellitus. J Am Heart Assoc. 2012;1:e002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radcliffe NJ, Seah JM, Clarke M, MacIsaac RJ, Jerums G, Ekinci EI. Clinical predictive factors in diabetic kidney disease progression. J Diabetes Investig. 2017;8:6‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Y, Park S, Lee S, et al. Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes: a nationwide population‐based study. PLoS One. 2020;15:e0231328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Neal B, Perkovic V, et al. Mediators of the effects of canagliflozin on kidney protection in patients with type 2 diabetes. Kidney Int. 2020;98:769‐777. [DOI] [PubMed] [Google Scholar]
- 18.Gabay C, Kushner I. Acute‐phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448‐454. [DOI] [PubMed] [Google Scholar]
- 19.Pratley RE, Wilson C, Bogardus C. Relation of the white blood cell count to obesity and insulin resistance: effect of race and gender. Obes Res. 1995;3:563‐571. [DOI] [PubMed] [Google Scholar]
- 20.Kuo TY, Wu CZ, Lu CH, et al. Relationships between white blood cell count and insulin resistance, glucose effectiveness, and first‐ and second‐phase insulin secretion in young adults. Medicine. 2020;99:e22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Medical Association . World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191‐2194. [DOI] [PubMed] [Google Scholar]
- 22.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605‐617. [DOI] [PubMed] [Google Scholar]
- 23.Buse JB, Bain SC, Mann JFE, et al. Cardiovascular risk reduction with Liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care. 2020;43(7):1546‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkovic V, Bain S, Bakris G, et al. eGFR loss with glucagon‐like peptide‐1 (GLP‐1) analogue treatment: data from SUSTAIN 6 and LEADER. Nephrol Dial Transplant. 2019;34:FP482. [Google Scholar]
- 25.Pasternak B, Wintzell V, Eliasson B, et al. Use of glucagon‐like peptide 1 receptor agonists and risk of serious renal events: Scandinavian cohort study. Diabetes Care. 2020;43:1326‐1335. [DOI] [PubMed] [Google Scholar]
- 26.Osonoi T, Saito M, Osonoi Y, Douguchi S, Ofuchi K, Katoh M. Liraglutide improves estimated glomerular filtration rate slopes in patients with chronic kidney disease and type 2 diabetes: a 7‐year retrospective analysis. Diabetes Technol Ther. 2020;22(11):828‐834. [DOI] [PubMed] [Google Scholar]
- 27.Zoungas S, Arima H, Gerstein HC, et al. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta‐analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431‐437. [DOI] [PubMed] [Google Scholar]
- 28.Oh YS, Jun HS. Effects of glucagon‐like peptide‐1 on oxidative stress and Nrf2 signaling. Int J Mol Sci. 2017;19(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutzwiller JP, Tschopp S, Bock A, et al. Glucagon‐like peptide 1 induces natriuresis in healthy subjects and in insulin‐resistant obese men. J Clin Endocrinol Metab. 2004;89:3055‐3061. [DOI] [PubMed] [Google Scholar]
- 30.Tsimihodimos V, Filippatos TD, Elisaf MS. SGLT2 inhibitors and the kidney: effects and mechanisms. Diabetes Metab Syndr. 2018;12:1117‐1123. [DOI] [PubMed] [Google Scholar]
- 31.Skov J, Dejgaard A, Frokiaer J, et al. Glucagon‐like peptide‐1 (GLP‐1): effect on kidney hemodynamics and renin‐angiotensin‐aldosterone system in healthy men. J Clin Endocrinol Metab. 2013;98:E664‐E671. [DOI] [PubMed] [Google Scholar]
- 32.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295‐2306. [DOI] [PubMed] [Google Scholar]
- 33.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 34.Davidson JA. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med. 2019;131:251‐260. [DOI] [PubMed] [Google Scholar]
- 35.Lapointe‐Shaw L, Bouck Z, Howell NA, et al. Mediation analysis with a time‐to‐event outcome: a review of use and reporting in healthcare research. BMC Med Res Methodol. 2018;18:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
De identified individual participant data, study protocol, and redacted Clinical Study Report will be available according to Novo Nordisk data sharing commitments. The data will be made available permanently after research completion, and approval of product and product use in both the EU and US. Data will be shared with bona fide researchers submitting a research proposal requesting access to data and for use as approved by the Independent Review Board according to the IRB Charter (see novonordisk trials.com). Access request proposal form and access criteria can be found at novonordisk trials.com. The data will be made available on a specialised SAS data platform.


