Abstract
Introduction
To evaluate rotational fibrin‐based thromboelastometry (ROTEM® FIBTEM) with amplitude of clot firmness at 5 min (A5) as an early point‐of‐care parameter for predicting progression to severe postpartum hemorrhage, and compare its predictive value with that of fibrinogen.
Material and methods
Prospective cohort study in the Netherlands including women with 800–1500 ml of blood loss within 24 h following birth. Blood loss was quantitatively measured by weighing blood‐soaked items and using a fluid collector bag in the operating room. Both FIBTEM A5 values and fibrinogen concentrations (Clauss method) were measured between 800 and 1500 ml of blood loss. Predictive accuracy of both biomarkers for the progression to severe postpartum hemorrhage was measured by area under the receiver operating curves (AUC). Severe postpartum hemorrhage was defined as a composite endpoint of (1) total blood loss >2000 ml, (2) transfusion of ≥4 packed cells, and/or (3) need for an invasive intervention to cease bleeding.
Results
Of the 391 women included, 72 (18%) developed severe postpartum hemorrhage. Median (IQR) volume of blood loss at blood sampling was 1100 ml (1000–1300) with a median (interquartile range [IQR]) fibrinogen concentration of 3.9 g/L (3.4–4.6) and FIBTEM A5 value of 17 mm (13–20). The AUC for progression to severe postpartum hemorrhage was 0.53 (95% confidence interval [CI] 0.46–0.61) for FIBTEM A5 and 0.58 (95% CI 0.50–0.65) for fibrinogen. Positive predictive values for progression to severe postpartum hemorrhage for FIBTEM A5 ≤12 mm was 22.5% (95% CI 14–33) and 50% (95% CI 25–75) for fibrinogen ≤2 g/L.
Conclusions
The predictive value of FIBTEM A5 compared to fibrinogen concentrations measured between 800 and 1500 ml of blood loss following childbirth was poor to discriminate between women with and without progression towards severe postpartum hemorrhage.
Keywords: blood transfusion, fibrinogen, FIBTEM A5, maternal mortality, point‐of‐care testing, postpartum hemorrhage, rotational thromboelastometry, severe acute maternal morbidity
Abbreviations
- AUC
area under the receiver operating curve
- A5
amplitude of clot firmness at 5 minutes
- CI
confidence interval
- FIBTEM
fibrin‐based thromboelastometry
- IQR
interquartile range
- ROTEM®
rotational thromboelastometry
- TeMpOH‐2
Towards better prognostic and diagnostic strategies for Major Obstetric Hemorrhage study
- RCOG
Royal College of Obstetricians and Gynaecologists.
Key message.
The clinical value of ROTEM® FIBTEM A5 for predicting the severity of bleeding when routinely measured during the onset of postpartum hemorrhage is limited.
1. INTRODUCTION
Postpartum hemorrhage is a significant contributor to maternal morbidity in high‐income countries and the leading cause of maternal mortality worldwide.1, 2, 3, 4 Hemorrhage following childbirth most commonly has a primary obstetric cause, and although postpartum hemorrhage should be treated prior to development of coagulopathy, it can be aggravated by hemostatic impairment.5 Research into the maternal coagulation profile during postpartum hemorrhage has indicated that a low fibrinogen concentration (≤2 g/L) is associated with severity of bleeding.6, 7, 8, 9 However, detection of a fibrinogen deficiency during hemorrhage is often delayed due to the long turnaround times of conventional coagulation tests (eg Clauss fibrinogen assay), with results generally available only after at least 60 min.10 For this reason, conventional coagulation tests are not suitable for predicting progression of bleeding and to guide hemostatic interventions (eg administration of fibrinogen concentrate) during the acute phase of postpartum hemorrhage.11
Viscoelastometric point‐of‐care testing using rotational thromboelastometry (ROTEM®, Tem International GmbH, Munich, Germany) provides an alternative approach to detect early changes in coagulation parameters by analyzing clot formation, firmness, and lysis.12 The ROTEM® fibrin‐based assay (FIBTEM) determines the role of the extrinsic coagulation pathway to clot firmness independent of platelets.12 The fibrinogen concentration is the major contributing factor in the FIBTEM assay.12 Results from the FIBTEM amplitude of clot firmness at 5 min (A5) can be obtained 10–15 min after blood sampling, and correlates well with plasma‐derived fibrinogen concentrations during pregnancy and postpartum hemorrhage.13, 14 A FIBTEM A5 value of ≤12 mm has been suggested as the most accurate cut‐off point to select women with a fibrinogen concentration of ≤2 g/L during postpartum hemorrhage (A. Gillissen, unpublished data). In a previous study, FIBTEM A5 was found to be a rapidly available biomarker for predicting progression of postpartum hemorrhage.15 Nevertheless, it remains unclear whether implementation of FIBTEM A5 measurements as part of standard care during the onset of postpartum hemorrhage has the potential to diagnose low fibrinogen concentrations accurately and whether it is able to discriminate between women with and without progression to severe postpartum hemorrhage. We hypothesized that an early FIBTEM A5 measurement is able to predict progression to severe postpartum hemorrhage for the following reasons. First, a deterioration in FIBTEM A5 measurements during the early stages of postpartum hemorrhage might indicate that blood loss is greater than appreciated. Secondly, a deterioration in FIBTEM A5 measurements might indicate an impairment in hemostasis, suggesting that these women have a higher than average propensity to bleed in relation to the primary obstetric cause. Early identification of hemostatic impairment allows for earlier use of targeted hemostatic interventions. If so, FIBTEM A5 could be a promising point‐of‐care parameter to identify women who could possible benefit from targeted fibrinogen replacement therapy during the earliest stages of postpartum hemorrhage, possibly preventing progression to severe bleeding.
National guidelines on the prevention and management of postpartum hemorrhage from the UK (Royal College of Obstetricians and Gynaecologists [RCOG]) and the Netherlands (Dutch Society of Obstetrics and Gynaecology [NVOG]) recommend routine coagulation testing in the case of ongoing bleeding exceeding 1000 ml of blood loss following birth.16, 17 However, in anticipation of postpartum hemorrhage, in some women intravenous access with simultaneous collection of blood for coagulation screening may already have been established before reaching the threshold of 1000 ml blood loss. Therefore, the aim of this study was to evaluate the value of FIBTEM A5 as a predictor for progression to severe postpartum hemorrhage when collected between 800 and 1500 ml blood loss after birth, and to compare its predictive value with that of the conventional Clauss fibrinogen assay.
2. MATERIAL AND METHODS
This study was part of the Towards better prognostic and diagnostic strategies for Major Obstetric Hemorrhage (TeMpOH‐2) study. The TeMpOH‐2 study was a multicenter prospective cohort study of pregnant women in the Netherlands carried out between February 2015 and April 2018. Pregnant women at least 18 years of age with a gestational age of at least 24 weeks were recruited at the outpatient clinics and maternity wards of three participating hospitals: Leiden University Medical Center (tertiary hospital), Erasmus Medical Center Rotterdam (tertiary hospital) and Isala Clinics in Zwolle (secondary hospital). Included women were monitored for the occurrence of postpartum hemorrhage (in the Netherlands defined as ≥1000 ml blood loss within 24 h after birth) and followed until discharge from hospital. In all hospitals, consecutive measurements of blood loss at time of blood sampling and total volume of blood loss when bleeding ceased was measured by weighing gauzes or other soaked materials and using a collector bag and suction system in the operating room. The clinician in charge was instructed to take at least one blood sample between 800 and 1500 ml of blood loss. These samples were taken for full blood count, fibrinogen measurement (according to the Clauss method)18 and FIBTEM A5 measurement performed on a ROTEM® Delta device (Tem International GmbH). All measurements on the ROTEM® Delta device were conducted with a single‐use reagent and in accordance with the recommendations of the manufacturer. In one hospital, the ROTEM® Delta device was positioned in a utility room equipped with laboratory devices at the maternity ward and measurements were carried out by well‐trained research nurses and clinical midwives. In the other two participating hospitals, the ROTEM® Delta devices were located in the laboratory and samples were handled by laboratory staff. Blood samples were collected before administration of blood components and fibrinogen concentrate. If multiple blood samples were taken between 800 and 1500 ml of blood loss, the first sample above 1000 ml was selected for analysis as recommended by the Dutch and English national guidelines on management of postpartum hemorrhage, which specifically recommends routine coagulation testing in the case of bleeding exceeding 1000 ml of blood loss following birth.16, 17 Women who used anticoagulants or had a known coagulation disorder were excluded from analysis. FIBTEM A5 results were not made available to the clinicians and women were treated according to the Dutch national guidelines for the management of postpartum hemorrhage.17
Trained research nurses obtained information on maternal and obstetric characteristics from medical files that were available at the maternity ward and operating theater. The following parameters were recorded: maternal age at time of birth, body mass index at beginning of pregnancy, gestational age at time of birth, parity, ethnicity (white or non‐white), multiple pregnancy, presence of preeclampsia or HELLP syndrome, mode of birth (vaginal birth or cesarean section), cause of hemorrhage (uterine atony, uterine rupture, placental pathology [including placenta previa, retained placenta or placental remnants, abnormally invasive placenta, and placental abruption], laceration of birth canal, and surgical bleeding), administration of uterotonic agents (including oxytocin, sulprostone, ergometrine, misoprostol and carbetocin), administration of non‐uterotonic agents (including tranexamic acid, fibrinogen concentrate, and recombinant factor VIIa), administration of clear fluids (crystalloids and colloids) and blood products (including packed cells, fresh frozen plasma and platelets) and invasive interventions to control bleeding (including intrauterine balloon tamponade, uterine artery ligation, uterine compression sutures, uterine artery embolization and peripartum hysterectomy).
Severe postpartum hemorrhage was defined as a composite outcome of a total blood loss exceeding 2000 ml, a transfusion of ≥4 packed red blood cells and/or need for any invasive intervention, defined as intrauterine balloon tamponade, uterine artery ligation, uterine compression sutures, uterine artery embolization or peripartum hysterectomy. These outcomes are considered severe postpartum hemorrhage‐related core outcome sets based on two international Delphi consensus studies.19, 20
Continuous data are presented as medians with interquartile ranges (IQR) and categorical data are summarized as frequencies with percentages (%). The clinical value of FIBTEM A5 and fibrinogen to predict progression to severe postpartum hemorrhage was investigated by area under the receiver operating characteristics curves (AUC) with 95% confidence intervals (CI) and positive and negative predictive values. The correlation between FIBTEM A5 values and fibrinogen concentrations was assessed by the Spearman’s rank correlation coefficient (rs). Considering that the Dutch and English national guidelines specifically recommend coagulation testing when bleeding exceeds 1000 ml, the clinical value of FIBTEM A5 and fibrinogen to predict progression to severe postpartum hemorrhage was also examined in a subgroup including only blood samples taken between 1000 and 1500 ml of blood loss. A complete case analysis was performed and missing data were not imputed. Statistical analyses were performed using STATA Statistical Software: Release 14 (StataCorp LP, College Station, TX, USA).
2.1. Ethical approval
The TeMpOH‐2 study was approved by the ethical committee of the Leiden University Medical Center (P13.246; 24 February 2014) and by the institutional review board of each participating hospital. Written informed consent was obtained antenatally from women included in the study. However, the ethical committee provided the possibility to ask women for verbal informed consent during the onset of postpartum hemorrhage in case they had not yet been included during pregnancy. In these cases, written informed consent was obtained when bleeding had ceased. The TeMpOH‐2 study is registered at ClinicalTrials.gov (NCT02149472; 29 May 2014).
3. RESULTS
During the 3‐year inclusion period of the TeMpOH‐2 study there were 17 203 women of at least 18 years of age, with a gestational age of 24 weeks onwards who gave birth in one of the three hospitals. Of these women, 1605 (9.3%) had lost at least 1000 ml of blood, of whom 391 fulfilled the inclusion criteria, consented to participate, and had a valid corresponding FIBTEM A5 and fibrinogen measurement between 800 and 1500 ml of blood loss after childbirth (Figure 1). Of the 391 women included, 72 women (18%) had severe postpartum hemorrhage.
FIGURE 1.
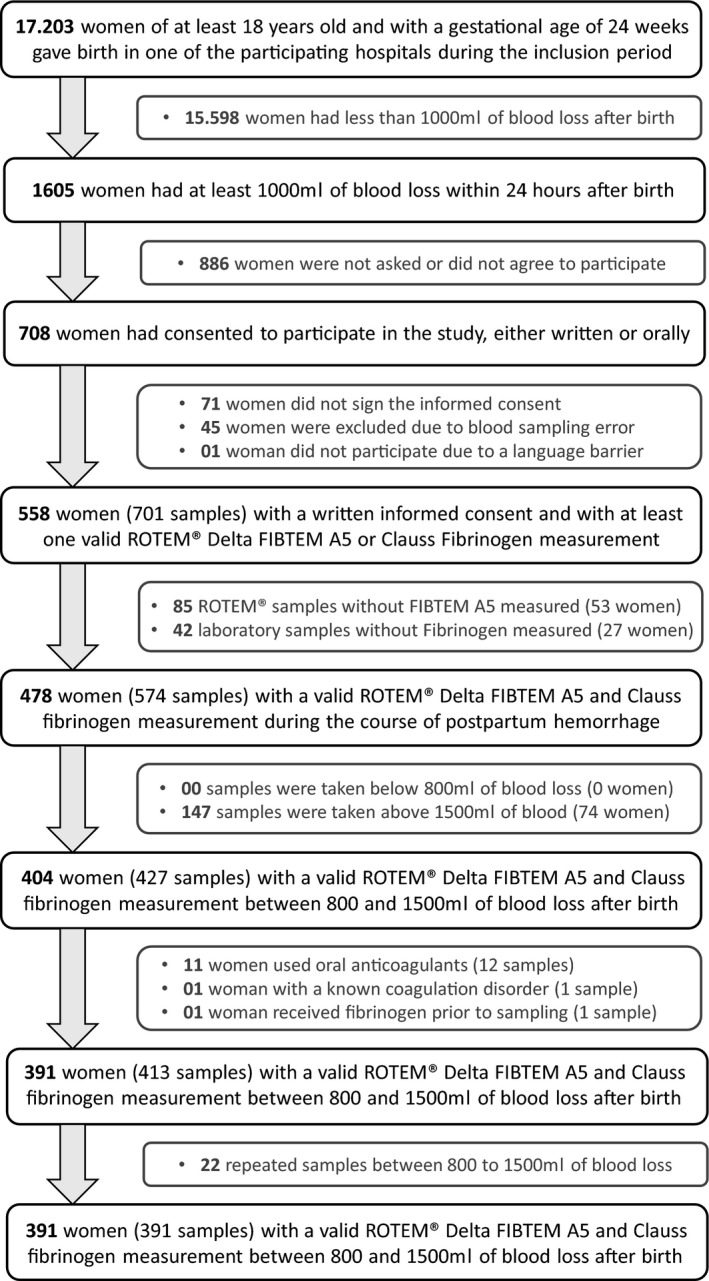
Flowchart of study enrollment
3.1. Baseline characteristics
Characteristics at study entry are presented in Table 1. The main cause of bleeding was either uterine atony (n = 145/391, 37%) or retained placenta or placental remnant (n = 148/391, 38%). Median (IQR) blood loss at time of blood sampling was 1100 ml (1000–1300) with a median (IQR) fibrinogen concentration of 3.9 g/L (3.4–4.6) and a median (IQR) FIBTEM A5 value of 17 mm (13–20). The lowest median (IQR) fibrinogen concentration and FIBTEM A5 value at study entry were associated with placental abruption, respectively, 2.5 g/L (1.7–3.7) and 13 mm (8–16) (see Table S1 for FIBTEM A5 values and fibrinogen concentrations for other postpartum hemorrhage causes). Sixteen (4%) women had a fibrinogen concentration ≤2 g/L and 80 (20%) women had a FIBTEM A5 value ≤12 mm. Colloids were infused in 28% (n = 108, with a median [IQR] volume of 500 ml [0–500]) and crystalloids in 62% (n = 241, with a median [IQR] volume of 500 ml [500–1000]) of the women before blood sampling.
TABLE 1.
Baseline characteristics for women with postpartum hemorrhage and a valid corresponding fibrin‐based thromboelastometry amplitude of clot firmness at 5 min (FIBTEM A5) and fibrinogen measurement between 800 and 1500 ml of blood loss following birth
|
Total n = 391 |
Severe postpartum hemorrhage | ||
|---|---|---|---|
|
No n = 319 |
Yes n = 72 |
||
| Maternal age, years* | 32 (28–35) | 32 (28–35) | 32 (28–35) |
| Gestational age, weeks* | 39 (38–40) | 39 (38–40) | 39 (37–40) |
| Body mass index, kg/m2 * | 24 (22–28) | 24 (22–28) | 25 (22–29) |
| Caucasian, n (%) | 336 (86%) | 276 (87%) | 60 (83%) |
| Nulliparity, n (%) | 201 (51%) | 166 (52%) | 35 (49%) |
| Multiple pregnancy, n (%) | 34 (9%) | 23 (7%) | 11 (15%) |
| Preeclampsia, n (%) | 32 (8%) | 24 (8%) | 8 (11%) |
| Mode of birth, n (%) | |||
| Vaginal birth | 304 (78%) | 246 (77%) | 58 (81%) |
| Cesarean section | 87 (22%) | 73 (23%) | 14 (19%) |
| Cause of hemorrhage, n (%) | |||
| Uterine atony | 145 (37%) | 120 (38%) | 25 (35%) |
| Retained placenta or remnants of placental tissue | 148 (38%) | 112 (35%) | 36 (50%) |
| Placenta previa | 5 (1%) | 4 (1%) | 1 (1.5%) |
| Placental abruption | 3 (1%) | 3 (1%) | 0 (0%) |
| Abnormally invasive placenta | 5 (1%) | 2 (1%) | 3 (4%) |
| Laceration of the birth canal | 42 (11%) | 36 (11%) | 6 (8%) |
| Iatrogenic surgical bleeding | 39 (10%) | 38 (12%) | 1 (1.5%) |
| Uterine rupture | 4 (1%) | 4 (1%) | 0 (0%) |
| Blood loss at study entry, ml* | 1100 (1000–1300) | 1100 (1000–1300) | 1100 (1000–1300) |
| Hb level at study entry, g/dl* | 10.3 (9.0–11.4) | 10.5 (9.4–11.4) | 9.5 (8.1–11.1) |
| Fibrinogen at study entry, g/L* | 3.9 (3.4–4.6) | 4.0 (3.4–4.7) | 3.9 (2.8–4.4) |
| FIBTEM A5 at study entry, mm* | 17 (13–20) | 17 (13–20) | 16 (13–21) |
Reported as median with (IQR).
3.2. Management of hemorrhage
All women (n = 391) received a uterotonic agent, of whom 79 (20%) had a second uterotonic agent, 8 (2%) a third uterotonic agent and 2 (0.5%) women a fourth uterotonic agent during hemorrhage. Tranexamic acid was administered in 60 (15%) women and fibrinogen concentrate in 12 (3%) women; none of the women received recombinant factor VII. There were 21 invasive interventions in 19 (5%) women, including 13 intrauterine balloons, 4 uterine compression sutures, 2 uterine artery embolization and 2 peripartum hysterectomies. One woman had intrauterine balloon tamponade in combination with uterine artery embolization and one woman had intrauterine balloon tamponade followed by peripartum hysterectomy. Seventy‐one women (18%) received transfused packed red blood cells, 8 (2%) fresh frozen plasma, and 9 (2%) platelet concentrates during bleeding.
3.3. Outcomes
Median (IQR) total blood loss was 1400 ml (1100–2000); 66 (17%) women had a total blood loss >2000 ml and 13 (3%) women had four or more packed red blood cells transfused. In 72 women (18%) the composite endpoint was defined as severe postpartum hemorrhage (18%). The distribution of outcomes according to increasing fibrinogen concentrations and FIBTEM A5 values at study entry are presented in Table 2 (and visually displayed in Figure S1). No maternal deaths occurred in this study.
TABLE 2.
Distribution of outcomes categorized to fibrinogen concentrations and fibrin‐based thromboelastometry amplitude of clot firmness at 5 min (FIBTEM A5) values measured between 800 and 1500 ml of blood loss following birth
| Total (n = 391) | Fibrinogen, g/L | FIBTEM A5, mm | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
≤2 (n = 16) |
2.1–3.0 (n = 49) |
3.1–4.0 (n = 138) |
>4 (n = 178) |
≤12 (n = 80) |
13–15 (n = 74) |
16–22 (n = 191) |
≥23 (n = 46) |
||
| Total volume of blood loss, L* | 1.4 (1.1–2.0) | 2 (1.8–2.6) | 1.5 (1.2–2) | 1.5 (1.2–1.9) | 1.3 (1.1–1.7) | 1.6 (1.3–2.0) | 1.5 (1.2–1.9) | 1.3 (1.1–1.7) | 1.5 (1.2–2.0) |
| Severe postpartum hemorrhage, n (%) | 72 (18%) | 8 (50%) | 11 (23%) | 23 (17%) | 28 (16%) | 18 (23%) | 16 (22%) | 30 (16%) | 8 (17%) |
| Women with blood loss >2000 ml, n (%) | 66 (17%) | 7 (44%) | 8 (16%) | 21 (15%) | 28 (16%) | 14 (18%) | 14 (19%) | 30 (16%) | 8 (17%) |
| Women receiving ≥4 packed cells, n (%) | 13 (3%) | 3 (19%) | 2 (4%) | 3 (2%) | 5 (3%) | 4 (5%) | 1 (1%) | 6 (3%) | 2 (4%) |
| Women with invasive intervention, n (%) | 19 (5%) | 2 (13%) | 6 (12%) | 3 (2%) | 8 (5%) | 5 (6%) | 5 (7%) | 6 (3%) | 3 (7%) |
Reported as median with (IQR).
3.4. Predictive value
Fibrinogen concentrations and FIBTEM A5 values were moderately correlated (r s = 0.53). The AUC for progression to severe postpartum hemorrhage was 0.58 (95% CI 0.50–0.65) for fibrinogen and 0.53 (95% CI 0.46–0.61) for FIBTEM A5 when measured between 800 and 1500 ml of blood loss following childbirth (Figure 2). Fibrinogen had an AUC for progression to total blood loss >2000 ml of 0.55 (95% CI 0.47–0.62), AUC for progression to ≥4 packed red blood cells of 0.63 (95% CI 0.44–0.81) and AUC for progression to any invasive intervention of 0.59 (95% CI 0.42–0.75). FIBTEM A5 had an AUC for progression to total blood loss >2000 ml of 0.50 (95% CI 0.42–0.58), AUC for progression to ≥4 packed red blood cells of 0.51 (95% CI 0.33–0.70) and AUC for progression to any invasive intervention of 0.53 (95% CI 0.38–0.68) (Figures [Link], [Link], [Link], [Link]). Positive predictive value for progression to severe postpartum hemorrhage for a fibrinogen concentration ≤2 g/L was 50% (95% CI 25–75) and 22.5% (95% CI 14–33) for a FIBTEM A5 value ≤12 mm when measured between 800 and 1500 ml of blood loss. Negative predictive values for a fibrinogen concentration >2 g/L and FIBTEM A5 value >12 mm were 83% (95% CI 79–87) and 83% (95% CI 78–87), respectively.
FIGURE 2.
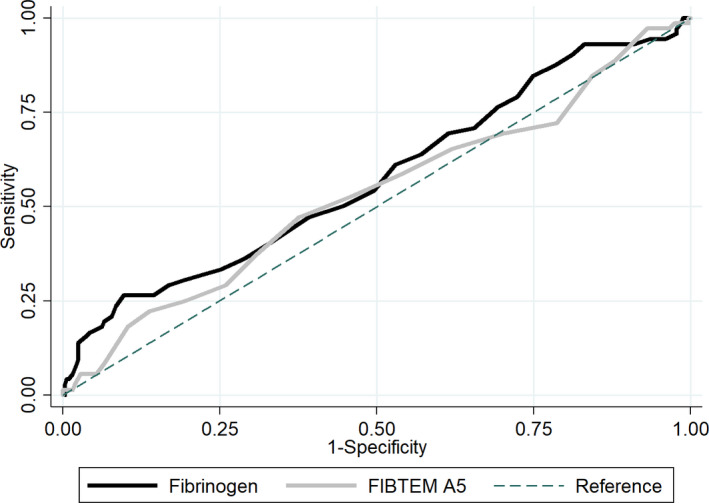
ROC curves for fibrinogen (black) and fibrin‐based thromboelastometry amplitude of clot firmness at 5 min (FIBTEM A5) (gray) for progression to severe postpartum hemorrhage when measured between 800 and 1500 ml of blood loss following birth
3.5. Subgroup analysis
There were 306 women with postpartum hemorrhage and a valid corresponding FIBTEM A5 and fibrinogen measurement between 1000 and 1500 ml of blood loss following childbirth. Median (IQR) total blood loss was 1500 ml (1200–2000) and 61 women (20%) developed severe postpartum hemorrhage. AUC for progression to severe postpartum hemorrhage was 0.57 (95% CI 0.49–0.65) for fibrinogen and 0.55 (95% CI 0.47–0.64) for FIBTEM A5 (Figure S5); these AUC values were indistinguishable from AUCs for fibrinogen and FIBTEM A5 when measured between 800 and 1500 ml of blood loss.
4. DISCUSSION
This multicenter, prospective cohort study found that the point‐of‐care test FIBTEM A5 correlates only moderately with the Clauss fibrinogen assay and lacks the ability to single out women who develop severe postpartum hemorrhage when routinely measured between 800 and 1500 ml. Women with a fibrinogen concentration ≤2 g/L had a 50% risk of developing severe postpartum hemorrhage vs 23% of women with a FIBTEM A5 value ≤12 mm.
The main strength of this study was that we prospectively collected fibrinogen and FIBTEM A5 measurements in a large cohort, within a specific range of blood loss during the onset of hemorrhage. Using postpartum hemorrhage‐related core outcome sets, our results may be used in systematic reviews and/or meta‐analyses regarding viscoelastometric point‐of‐care testing.19, 20 Our median (IQR) FIBTEM A5 value of 17 mm (13–20) measured between 800 and 1500 ml of blood loss appeared lower than the median (IQR) FIBTEM A5 value of 21 mm (18–23) measured in healthy laboring women.21 This is not surprising given that fibrinogen, for which FIBTEM A5 is a surrogate measure, falls early in the course of postpartum hemorrhage.6 Generalizability is hampered by challenges in having women provide consent and executing trial procedures in acute situations where women have rapid blood loss, a previously encountered problem.22, 23 An examination of potential cases showed that women who lost large volumes of blood in short time frames were more frequently not included. Trial procedures were hampered in more serious bleeding, leading to 74 women with blood samples solely taken >1500 ml and 80 with only one valid FIBTEM A5 or Clauss fibrinogen measurement. This could have resulted in underestimation of fibrinogen concentrations ≤2 g/L or FIBTEM A5 values ≤12 mm, and fewer women with severe postpartum hemorrhage. However, the proportion of women who had a fibrinogen concentration ≤2 g/L (4%) was similar to that in the UK and Denmark.15, 22 Given that we collected a limited number of variables, we were unable to present all known risk factors associated with severe postpartum hemorrhage as baseline characteristics. In addition, the numbers of women with specific characteristics predisposing to coagulopathy or severe postpartum hemorrhage were small, restricting possible subgroup analyses to determine whether FIBTEM A5 might be more predictive in some, for example, women with a placental abruption (n = 3). Managing clinicians were intended to be blinded for the FIBTEM A5 results. However, it appeared that clinicians from one of the participating hospitals had been able to see the FIBTEM A5 results. The finding that of the 19 women with a FIBTEM A5 value ≤12 mm in this hospital, none received fibrinogen concentrate suggests that little was done with this information. No noticeable differences were found between hospitals regarding patient characteristics, FIBTEM A5 values, fibrinogen concentrations, incidence or management of postpartum hemorrhage.
Clinical utility of FIBTEM A5 as a predictor for progression of postpartum hemorrhage has only been described once.15 In contrast to our results, that study from the UK found that FIBTEM A5 could be used as a predictive biomarker for progression to transfusion of ≥4 packed cells (AUC 0.78, 95% CI 0.69–0.88), need for invasive intervention (AUC 0.69, 95% CI 0.52–0.86) and total blood loss >2500 ml (AUC 0.75, 95% CI 0.66–0.85).15 However, this study also included samples taken above 1500 ml and women were not enrolled when bleeding stopped simultaneously or soon after reaching the entry criteria.15 The fact that we did include such women may explain the lower proportion of women in our study with ≥4 packed cells (3% vs 9%) or any invasive intervention (5% vs 11%).15 However, these findings could also indicate differences in management or transfusion policies. Nevertheless, the proportion of women with fibrinogen concentrations ≤2 g/L appeared to be the same in the UK (4%, n = 12/341).15 These combined findings suggest that FIBTEM A5 is not a good predictor for progression of bleeding when routinely taken during the onset of postpartum hemorrhage at a blood loss ≤1500 ml.
The low number of women with a fibrinogen concentration ≤2 g/L reflects the hypercoagulable state of pregnancy.24, 25, 26 Although low fibrinogen concentrations are associated with severity of bleeding,6, 7, 27 the likelihood of a fibrinogen concentration ≤2 g/L with a blood loss of ≤1500 ml is low.8, 9, 28 Our study population was relatively low risk; only a small proportion had a fibrinogen concentration ≤2 g/L or a FIBTEM A5 value ≤12 mm. Hemostatic impairment is more likely to occur in the presence of more severe ongoing bleeding or risk factors for coagulopathy. Furthermore, in our study many women stopped bleeding soon after 1000 ml. Therefore, the role of a single routine FIBTEM A5 measurement during onset of hemorrhage ≤1500 ml appears limited, and may explain the poor predictive value of FIBTEM A5 for progression to severe postpartum hemorrhage. However, multiple consecutive measurements during ongoing bleeding might still detect a downfall in FIBTEM A5 values, indicating fibrinogen deficiency, and could identify women who may benefit from targeted fibrinogen replacement therapy. Additionally, FIBTEM A5 might be useful in specific causes of ongoing bleeding more likely to involve coagulopathy, such as trauma‐related hemorrhage.
Whether correction of fibrinogen based on FIBTEM A5 improves maternal outcome is still being investigated. The OBS2 trial showed that infusion of fibrinogen concentrate in women with a FIBTEM A5 value ≤15 mm and a bleeding exceeding 1500 ml did not improve outcome.23 Subgroup analyses showed that FIBTEM A5 values >12 mm or fibrinogen concentrations >2 g/L are sufficient to maintain hemostasis.23 Another study from the UK compared a fixed‐ratio transfusion protocol with targeted fibrinogen replacement therapy on the basis of FIBTEM A5 results and obstetric hemorrhage >1500 ml.29 A significant reduction in total use of allogeneic blood products was found when fibrinogen concentrate was infused based on FIBTEM A5 values <7 mm, or values <12 mm with ongoing bleeding.29
This is important considering that guidelines on postpartum bleeding recommend the use of fixed‐ratio protocols. The observational counterpart of the OBS2 found no significant hemostatic impairment when fresh frozen plasma was withheld in FIBTEM A5 values >15 mm.30 Therefore, FIBTEM A5 seems to have potential to avoid unnecessary transfusion of blood products. However, our study found a moderate correlation between FIBTEM A5 values and fibrinogen concentrations. The same moderate correlation (r s = 0.59) was found between both test results in the study from the UK that evaluated FIBTEM A5 as a predictive biomarker.15 Only one small observational study found a strong correlation (r s = 0.86) between FIBTEM A5 values and fibrinogen concentrations, but volume of blood loss at time of blood sampling was not described,14 an important omission considering that an increase in correlation between FIBTEM A5 values and fibrinogen concentrations in higher volumes of blood loss was previously found (A Gillissen, unpublished data). This raises the question of whether FIBTEM A5 is accurate enough in guiding or withholding hemostatic interventions during the whole course of postpartum hemorrhage. This requires further study and calls for more accurate point‐of‐care tests to quantify plasma fibrinogen concentrations.31
Our results show that the clinical utility of FIBTEM in postpartum bleedings below 1500 ml is limited and should encourage researchers and clinical caregivers to reserve the use of FIBTEM A5 to more severe bleedings, especially considering the high costs associated with ROTEM® tests. FIBTEM A5 might still be useful in a selected population of women at high risk of coagulopathy or with ongoing bleeding reaching a certain, yet undefined but presumably >1500 ml, volume of blood loss. Further research in these situations is needed as to whether FIBTEM A5 is able to detect development of fibrinogen deficiency to identify women at risk of progression to severe postpartum hemorrhage who may benefit from targeted fibrinogen replacement. As stated by the RCOG and National Institute of Health and Care Excellence, the role of viscoelastometric point‐of‐care testing during postpartum hemorrhage requires evaluation before FIBTEM A5 can be included in guidelines.16, 32
5. CONCLUSION
Our study results suggest that FIBTEM A5 is not a good predictor for progression of bleeding when routinely measured as part of standard clinical care during the onset of postpartum hemorrhage. Nevertheless, FIBTEM A5 might still be a promising point‐of‐care test with clinical advantages in women with ongoing hemorrhage exceeding 1500 ml of blood loss, especially considering the potential to provide rapid results at which blood products can be withheld. However, given the moderate correlation between FIBTEM A5 values and fibrinogen concentrations, and the costs that come with running and interpreting ROTEM® tests, more clinical and cost‐effectiveness research is needed before FIBTEM A5 can be included in guidelines on prevention and management of postpartum hemorrhage.
CONFLICT OF INTERESTS
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHOR CONTRIBUTIONS
The conceptualization of the study design was performed by PR, AG and JvdB. Data analyses were performed by PR and CCD. All authors contributed equally to the interpretation of the data. PR wrote the first manuscript, which was revised by all authors.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1
ACKNOWLEDGMENTS
The authors thank research technician D. Priem‐Visser, research nurses C. Kolster‐Bijdevaate, M. S. Bourgonje‐Verhart, C. E. Bleeker‐Taborh, E. Roos‐van Milligen, R. J. M. Berkhout, E. Sucu, E. C. Willems of Brilman‐Tuinhof de Mode, M. Stigter‐Dekker, N. C. W. van Rijn and J. van Rhee, medical students M. van de Sande, R. H. Wouters and L. S. Smits, and clinical midwives of the Leiden University Medical Center, Erasmus Medical Center Rotterdam and Isala Zwolle for their contributions to the TeMpOH‐2 study.
Ramler PI, Gillissen A, Henriquez DDCA, et al. Clinical value of early viscoelastometric point‐of‐care testing during postpartum hemorrhage for the prediction of severity of bleeding: A multicenter prospective cohort study in the Netherlands. Acta Obstet Gynecol Scand. 2021;100:1656–1664. 10.1111/aogs.14172
Funding information
The TeMpOH‐2 study was supported by an internal grant from Sanquin Research (PPOC 13‐029). Tem International GmbH (Munich, Germany) provided the Leiden University Medical Center with a ROTEM® device on the basis of a loan agreement without additional charge. The other two hospitals already owned ROTEM® devices that were made available for this study. Reagents used in the ROTEM® devices were paid by the study without discount.
REFERENCES
- 1.Kassebaum NJ, Bertozzi‐Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brace V, Penney G, Hall M. Quantifying severe maternal morbidity: a Scottish population study. BJOG. 2004;111:481‐484. [DOI] [PubMed] [Google Scholar]
- 3.Zwart JJ, Richters JM, Ory F, de Vries JI, Bloemenkamp KW, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population‐based study of 371,000 pregnancies. BJOG. 2008;115:842‐850. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199(133):e1‐8. [DOI] [PubMed] [Google Scholar]
- 5.Allard S, Green L, Hunt BJ. How we manage the haematological aspects of major obstetric hemorrhage. Br J Haematol. 2014;164:177‐188. [DOI] [PubMed] [Google Scholar]
- 6.Charbit B, Mandelbrot L, Samain E, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266‐273. [DOI] [PubMed] [Google Scholar]
- 7.Cortet M, Deneux‐Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum hemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108:984‐989. [DOI] [PubMed] [Google Scholar]
- 8.de Lloyd L, Bovington R, Kaye A, et al. Standard hemostatic tests following major obstetric hemorrhage. Int J Obstet Anesth. 2011;20:135‐141. [DOI] [PubMed] [Google Scholar]
- 9.Gillissen A, van den Akker T, Caram‐Deelder C, et al. Coagulation parameters during the course of severe postpartum hemorrhage: a nationwide retrospective cohort study. Blood Adv. 2018;2:2433‐2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toulon P, Ozier Y, Ankri A, Fleron MH, Leroux G, Samama CM. Point‐of‐care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost. 2009;101:394‐401. [PubMed] [Google Scholar]
- 11.Solomon C, Collis RE, Collins PW. Hemostatic monitoring during postpartum hemorrhage and implications for management. Br J Anaesth. 2012;109:851‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiting P, Al M, Westwood M, et al. Viscoelastic point‐of‐care testing to assist with the diagnosis, management and monitoring of hemostasis: a systematic review and cost‐effectiveness analysis. Health Technol Assess. 2015;19(1–228):v‐vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huissoud C, Carrabin N, Benchaib M, et al. Coagulation assessment by rotation thrombelastometry in normal pregnancy. Thromb Haemost. 2009;101:755‐761. [PubMed] [Google Scholar]
- 14.Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum hemorrhage by thrombelastometry. BJOG. 2009;116:1097‐1102. [DOI] [PubMed] [Google Scholar]
- 15.Collins PW, Lilley G, Bruynseels D, et al. Fibrin‐based clot formation as an early and rapid biomarker for progression of postpartum hemorrhage: a prospective study. Blood. 2014;124:1727‐1736. [DOI] [PubMed] [Google Scholar]
- 16.Mavrides E, Allard S, Chandraharan E, et al.; on behalf of the Royal College of Obstetricians and Gynaecologists . Prevention and Management of Postpartum Hemorrhage: Green‐top Guideline No. 52. BJOG. 2017;124:e106‐e49. [DOI] [PubMed] [Google Scholar]
- 17.Nederlandse Vereniging voor Obstetrie en Gynaecologie . Hemorrhagia postpartum (HPP). Arnhem; 2013. https://www.nvog.nl/wp‐content/uploads/2018/02/Hemorrhagia‐postpartum‐HPP‐3.0‐14‐11‐2013.pdf
- 18.Stang LJ, Mitchell LG. Fibrinogen. Methods Mol Biol. 2013;992:181‐192. [DOI] [PubMed] [Google Scholar]
- 19.Schaap T, Bloemenkamp K, Deneux‐Tharaux C, et al. Defining definitions: a Delphi study to develop a core outcome set for conditions of severe maternal morbidity. BJOG. 2019;126:394‐401. [DOI] [PubMed] [Google Scholar]
- 20.Meher S, Cuthbert A, Kirkham JJ, et al. Core outcome sets for prevention and treatment of postpartum hemorrhage: an international Delphi consensus study. BJOG. 2019;126:83‐93. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Eley VA, Wyssusek KH, et al. Baseline parameters for rotational thromboelastometry in healthy labouring women: a prospective observational study. BJOG. 2020;127:820‐827. [DOI] [PubMed] [Google Scholar]
- 22.Wikkelso AJ, Edwards HM, Afshari A, et al. Pre‐emptive treatment with fibrinogen concentrate for postpartum hemorrhage: randomized controlled trial. Br J Anaesth. 2015;114:623‐633. [DOI] [PubMed] [Google Scholar]
- 23.Collins PW, Cannings‐John R, Bruynseels D, et al. Viscoelastometric‐guided early fibrinogen concentrate replacement during postpartum hemorrhage: OBS2, a double‐blind randomized controlled trial. Br J Anaesth. 2017;119:411‐421. [DOI] [PubMed] [Google Scholar]
- 24.Cerneca F, Ricci G, Simeone R, Malisano M, Alberico S, Guaschino S. Coagulation and fibrinolysis changes in normal pregnancy. Increased levels of procoagulants and reduced levels of inhibitors during pregnancy induce a hypercoagulable state, combined with a reactive fibrinolysis. Eur J Obstet Gynecol Reprod Biol. 1997;73:31‐36. [DOI] [PubMed] [Google Scholar]
- 25.Brenner B. Hemostatic changes in pregnancy. Thromb Res. 2004;114:409‐414. [DOI] [PubMed] [Google Scholar]
- 26.Szecsi PB, Jorgensen M, Klajnbard A, Andersen MR, Colov NP, Stender S. Hemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103:718‐727. [DOI] [PubMed] [Google Scholar]
- 27.Gayat E, Resche‐Rigon M, Morel O, et al. Predictive factors of advanced interventional procedures in a multicenter severe postpartum hemorrhage study. Intensive Care Med. 2011;37:1816‐1825. [DOI] [PubMed] [Google Scholar]
- 28.Green L, Knight M, Seeney F, et al. The haematological features and transfusion management of women who required massive transfusion for major obstetric hemorrhage in the UK: a population based study. Br J Haematol. 2016;172:616‐624. [DOI] [PubMed] [Google Scholar]
- 29.Mallaiah S, Barclay P, Harrod I, Chevannes C, Bhalla A. Introduction of an algorithm for ROTEM‐guided fibrinogen concentrate administration in major obstetric hemorrhage. Anaesthesia. 2015;70:166‐175. [DOI] [PubMed] [Google Scholar]
- 30.Collins PW, Cannings‐John R, Bruynseels D, et al. Viscoelastometry guided fresh frozen plasma infusion for postpartum hemorrhage: OBS2, an observational study. Br J Anaesth. 2017;119:422‐434. [DOI] [PubMed] [Google Scholar]
- 31.Imai K, Kotani T, Nakano T, Ushida T, Kikkawa F. Clinical utility and limitations of FibCare® for the rapid measurement of fibrinogen concentrations: the first clinical experience. Taiwan J Obstet Gynecol. 2018;57:899‐900. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Health and Care Excellence . Detecting, managing and monitoring hemostasis: viscoelastometric point‐of‐care testing (ROTEM, TEG and Sonoclot systems). www.nice.org.uk/guidance/dg13. Diagnostics guidance Publisched: 20 August 2014.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Table S1


