Abstract
Pyrethroid insecticides are the only pesticides approved for the treatment of head lice (pediculosis capitis) infestations in Japan. However, in Okinawa Prefecture, 96% of head lice are resistant to pyrethroids. Here, we conducted a clinical trial to assess the safety and efficacy of a dimethicone preparation against head lice infestations in Okinawa Prefecture. Dimethicone‐containing lotion was applied over the entire scalp three times over a 7‐day period. Lice bodies (nymphs/adults) and eggs (nits) were counted before (day 0) and after treatment (day 8); a subset of eggs was collected to estimate viability based on hatch rate. Efficacy was evaluated based on improvement (reduction) in head lice counts post‐treatment with respect to baseline. Safety was evaluated based on subjects’ scalp condition and adverse event incidence. Utility was a composite end‐point combining efficacy and safety. In total, 35 subjects were enrolled. Efficacy and safety were evaluated in 23 and 35 subjects, respectively. No side‐effects of note were reported during the treatment period. The dimethicone lotion resulted in a utility rating of “marginally useful” or higher in over 80% of the study population, signifying the formulation to be both safe and effective. The dimethicone lotion was also a potent ovicide; 99.4% of eggs collected after treatment failed to hatch when incubated. Eradication of head lice remained successful for at least 4 weeks after the final topical dimethicone application in 25 of the 28 subjects reached by telephone survey. Lice bodies and eggs were genotyped to analyze the prevalence of three knockdown resistance (kdr)‐type mutations within the voltage‐sensitive sodium channel known to confer pyrethroid resistance. One or more kdr mutations were confirmed in 30 of the 32 subjects from whom specimens were collected (93.8%). Dimethicone was confirmed to be both safe and effective in treating pyrethroid‐resistant head lice.
Keywords: dimethicone, head lice, knockdown resistance mutation, pyrethroid, pyrethroid resistant
1. INTRODUCTION
As of December 2020, the only pesticide approved in Japan for head lice (pediculosis capitis) or other sucking lice infestations is phenothrin 0.4%, an over‐the‐counter (OTC) pyrethroid formulation. Pyrethroid preparations (e.g. Sumithrin Shampoo®, Earth Shiramitori Shampoo®) are extremely safe, and have been widely used for over 35 years; however, the existence of pyrethroid‐resistant head lice has been reported for over 20 years, primarily in Europe and the USA.1, 2 One 2010 investigation discovered pyrethroid resistance in 96% of head lice in Okinawa Prefecture, much higher than the nationwide prevalence of 8.5%.3, 4 The development of safe and highly effective louse‐killing agents (pediculicides) is desperately needed in households, schools, and clinical settings alike, as Okinawans increasingly encounter difficult‐to‐treat head lice that cannot be eradicated by pyrethroid formulations, and resistant pediculosis seem to have risen in recent years, even outside Okinawa Prefecture.5
Non‐pyrethroid formulations used outside of Japan include ivermectin 0.5% lotion, malathion 0.5% lotion, benzyl alcohol 5% lotion, spinosad 0.9% suspension, and abametapir 0.74% lotion.6 Another is dimethicone (a.k.a. dimethylpolysiloxane), an OTC product that is considered effective against pyrethroid‐resistant head lice.7
In 2016, our team demonstrated the safety and efficacy of an ivermectin 0.5% lotion, available OTC in the USA, in a clinical study motivated by the need for non‐pyrethroid treatment options for pediculosis.8 This report details the results of a new clinical trial conducted to establish the safety and efficacy of a lotion formulation of dimethicone in treating pyrethroid‐resistant head lice infestations.
2. METHODS
2.1. Patients
The major inclusion criteria were: age of 6 months or more, and confirmation of one or more live head lice (including adults and nymphs) or 20 or more eggs (nits) in 3 min of visual inspection.
The exclusion criteria were: scalp disease, injury, or wound(s) not attributable to pediculosis; conditions requiring antibiotic or hormone drugs; history of severe irritation or allergic symptoms in response to topical agents or cosmetics; severe heart/kidney/liver dysfunction or respiratory, cardiovascular, hepatic, renal, or other concomitant disease; confirmed or potential pregnancy; currently breast‐feeding; or age of less than 6 months. Candidates were deemed ineligible if they: were unable to discontinue any medication that could potentially affect the evaluation of the study drug; had their hair dyed, bleached, permed, or straightened within 2 weeks of enrollment; had participated in another study within 3 months of enrollment; or were judged unsuitable for any other reason by the physician in charge. In addition, enrolled subjects were excluded from the efficacy evaluation if only nits (no lice bodies) were found by visual inspection, but none of the collected nits hatched.
Eligible candidates aged older than 6 months but younger than 16 years were enrolled after obtaining voluntary, written informed consent from a proxy (i.e., parent or other guardian), who first received an explanation of the study aims and details. Eligible candidates of 16–20 years of age were enrolled after obtaining voluntary, written informed consent from the candidate and a proxy (i.e., parent or other guardian), who first received an explanation of the study aims and details. Eligible candidates older than 20 years of age were enrolled after obtaining their voluntary, written informed consent following an explanation of the study aims and details.
2.2. Medicine
The novel pediculicide ST2702 is a lotion formulation consisting of 4% dimethicone (the active ingredient), which forms a non‐sticky base that dimethicone is highly soluble in. This preparation (“dimethicone” below) was provided by Earth Corporation. A patch test of ST2702 was conducted on 40 healthy subjects; there were no adverse reactions reported, confirming the safety of the product. A phase I study in 10 healthy subjects did not reveal adverse events or side‐effects.
2.3. Study design
Only dimethicone was tested in this open‐label study (UMIN registration no. 000043440). This clinical study was conducted as contract research within the Department of Dermatology of the University of the Ryukyus, following its approval by the university’s Clinical Trial Review Committee in 2017. Subjects’ human rights, safety, and welfare were protected through the provision of full explanations of the trial’s aims, method, risks, and privacy protection‐related matters in compliance with ethical principles in the spirit of the Declaration of Helsinki.
Figure 1 shows a scheme of the study regimen. On day 0, each subject (or a family member) applied a single dose of dimethicone to dried hair at home, spreading it evenly over the entire scalp to fully coat the hair down to the hairline. Subjects were instructed to use approximately 25 mL if their hair was short and approximately 50 mL if long as a guideline. After setting for 5 min, the product was washed out using normal hair shampoo. Dimethicone was applied again on days 3 and 7, for a total of three times in 1 week. On day 8, subjects visited the university hospital or a local clinic to be examined for the safety and efficacy assessments. To assess egg viability, 10 nits each were collected on days 0 and 8. Physical removal of lice and nits by combing/brushing as well as pesticidal treatment using agents besides dimethicone were prohibited until re‐assessment on day 8. Four weeks after the final treatment, each subject (or a family member) was contacted by the research team by telephone to check whether their head lice had been successfully eradicated.
FIGURE 1.
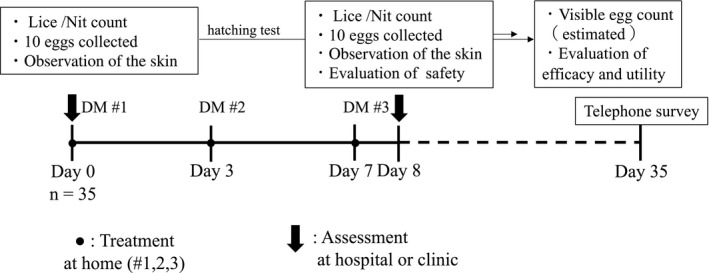
Schematic of the regimen. Dimethicone (DM) was applied to subjects’ hair at three timepoints. Lice bodies and nits were separately counted, and (up to) 10 eggs were sampled for the calculation of hatch rate, on both day 0 (baseline) and day 8 (post‐treatment). The long‐term success of dimethicone in curing head lice infestations was evaluated 4 weeks after the final treatment using a telephone survey
2.4. Evaluation of efficacy
First, the degree of infestation on day 0 before the first dimethicone treatment (“baseline”) and on day 8 (“post‐treatment”) was determined by a dermatologist, who visually inspected the subject’s entire scalp for 3 min and recorded the numbers of head lice and nits observed.
Lice count, namely the total number (n) of nymph plus adult bodies, was rated as follows: “none”, 0; “mild”, 1–4; “moderate”, 5–9; or “severe”, 10 or more. Nit count, namely the total number (n) of unhatched nits (viable plus non‐viable nits; non‐empty eggs below) and hatched shells (casings), was rated as follows: “none”, 0; “mild”, 1–49; “moderate”, 50–99; or “severe”, 100 or more. Egg viability (%) was assessed as a hatch rate, namely the proportion of non‐empty eggs sampled from a patient that ultimately hatched after incubation. On each day, after counting nits over the entire scalp, 10 eggs were collected with the associated strand of hair using scissors, transferred to a Petri dish, and maintained in an incubator at 30°C and 65% humidity. On day 0, a maximum of 10 eggs were collected in total; the hatch rate was calculated by dividing the number of eggs that hatched (viable eggs) by the number of non‐empty eggs within this sample. On day 8, in excess of 10 eggs were collected (if that many were present) and 10 non‐empty eggs were chosen from among them; the hatch rate was calculated by dividing the number of viable eggs by this subset of (up to) 10. Finally, estimated viable egg count (n) was calculated on each day as the product of the corresponding nit count and egg viability.
In addition, the presence of scratch marks was recorded based on the following criteria: 0, “none”; 1, “mild” (only minor rash noted); 2, “moderate” (rash with severe inflammation covering <10% of entire scalp); or 3, “severe” (rash with severe inflammation covering ≥10% of entire scalp).
2.5. Evaluation of safety
Safety was evaluated in terms of patients’ skin reactions before and after dimethicone treatment based on visual inspection of hair and scalp by a dermatologist. The following criteria were used: 0, “no reaction”; 1, “slight erythema”; 2, “obvious erythema”; 3, “erythema and either edema or papules”; 4, “erythema and edema/papules and small blisters”; and 5, “large blisters”.
2.6. End‐points
Our efficacy end‐point was post‐treatment improvement in lice and estimated viable egg counts, as classified by the rating scale introduced above, according to the following grading system:
Significant: either lice count or estimated viable egg count improved (i.e., decreased) by two or more steps with respect to baseline and reached zero and the other measure did not worsen.
Moderate: either lice count or estimated viable egg count improved by two or more steps with respect to baseline and the other measure did not worsen.
Mild: either lice count or estimated viable egg count improved by one step with respect to baseline and the other measure did not worsen.
Unchanged: both lice count and estimated viable egg count were the same post‐treatment as at baseline.
Deterioration: either lice count or estimated viable egg count worsened (i.e., increased) with respect to baseline or the scratch‐mark score worsened by two or more steps compared to baseline.
Our safety end‐point was the post‐treatment change in skin reaction severity, as classified by the rating scale introduced above, according to the following grading system:
Safe: skin reaction score not worse than baseline and no side‐effects observed.
Largely safe: skin reaction score one step worse than baseline, but no side‐effects observed.
Questionable safety: skin reaction score two or more steps worse than baseline and side‐effects without a definitive causal relationship to dimethicone observed.
Unsafe: skin reaction score two or more steps worse than baseline and side‐effects with obvious causal relationship to dimethicone observed.
Utility was assessed as a composite end‐point combining efficacy and safety:
Very useful: efficacy = “significant” and safety = “safe”.
Useful: efficacy = “moderate” or higher and safety = “largely safe” or higher.
Marginally useful: efficacy = “mild” and safety = “largely safe” or higher.
Not useful: efficacy = “unchanged” and safety = “largely safe” or higher.
Undesirable: efficacy = “deterioration” or safety = “questionable safety” or lower.
Dimethicone was judged to be useful if rated as “marginally useful” or higher in 80% or more of the sample of patients examined to determine utility. One month after the final treatment, subjects were contacted by telephone and asked whether their pediculosis had been fully eradicated. Patients were considered lice free if the respondent reported neither live lice nor any increase in nits.
2.7. Analysis of the frequency of knockdown resistance (kdr) mutations in the voltage‐sensitive sodium channel (VSSC)
In order to verify the presence of the point mutations at M815I, T917I, and L920F in the body or egg of a human head lice specimen, polymerase chain reaction (PCR) and direct DNA sequencing was done according to the primer design by Kasai et al.1 The human head lice sample, placed in a 1.5‐mL PCR reaction tube, was homogenized in a mixture of extraction solution (20 μL) and tissue‐preparation solution (5 μL) (REDExtract‐N‐Amp™ Tissue PCR Kit; Sigma) for the extraction of DNA. Then, the solution was heated at 95°C for 3 min and was neutralized. Initial fragment amplification was carried out using primers F52‐PhSC and R55‐PhSC for M815I analysis and F56‐PhSC and R53‐PhSC for T917I and L920F analysis, using KOD‐Plus Neo (Toyobo). The amplified fragments of the expected size were purified using ExoSAP‐IT (USB) at a temperature of 37°C for 30 min and then 80°C for 15 min. DNA sequencing was carried out using primers R55‐PhSC for M815I and F56‐PhSC for T917I and L920F analysis, respectively (Table S1) with a BigDye Terminator version 1.1 Cycle Sequencing Kit (Applied Biosystems Japan). Direct DNA sequencing was performed on the 3730 DNA Analyzer (Applied Biosystems Japan). The targeted amino acid replacement was analyzed using the software MEGA 6.0 (http://www.megasoftware.net/) and ATGC for Windows version 9.0.0 (Genetyx).
3. RESULTS
3.1. Patients
In total, 35 subjects were enrolled in the clinical trial between May and September 2017 (M/F, 2/33; age range, 1.5–42 years), of which 26 (74.3%) were children aged 9 years or younger (Table 1, Table S1 and S2). These 35 patients included four sibling pairs (n = 8) and one parent–child pair (n = 2).
TABLE 1.
Patient characteristics
| Characteristic | |
|---|---|
| Age, mean (range), years, n = 35 | 9.5 (1.5–42) |
| Sex, male : female, n = 35 | 2:33 |
| Phenothrin history, yes/no, n = 12 | 10/2 |
| Knockdown resistance (kdr) mutation, +/−, n = 32 | 30/2 |
Efficacy was evaluated in 23 subjects, as 12 who met the exclusion criteria were excluded from this analysis. Specifically, 10 were excluded because none of the nits collected on day 0 (i.e., before dimethicone application) hatched; the other two (cases 13 and 34) were excluded for self‐reported usage of combs during the treatment period. Safety was evaluated in all 35 subjects.
3.2. Efficacy
Head lice count, and estimated viable egg counts at baseline (day 0) and post‐treatment (day 8) obtained for the 23 subjects were ultimately included in the efficacy evaluation (Table 2). Estimated viable egg count did not worsen post‐treatment in any of these subjects: it was calculated as zero in 20 subjects, and unchanged in only one individual (Table S1).
TABLE 2.
Lice and nit counts, hatch rate, and estimated viable egg count, n = 23
| Measure | Day 0 | Day 8 |
|---|---|---|
| Lice count, mean (range) | 1.9 (0‐4) | 0.0 (0) |
| Nit count, mean (range) | 36.7 (4–120) | 22.4 (0–110) |
| Hatch rate, mean (range)%, n = 22 | 58.6% (12.5–100.0) | 1.2% (0–25.0) |
| Estimated viable egg count, mean (range), n = 21 | 23.1 (0–48.7) | 0.1 (0–1.25) |
Dimethicone resulted in improvement that was “significant” in 17.3% of subjects (4/23), “moderate” in 0% (0/23), and “mild” in 78.3% (18/23); while improvement was “unchanged” in 4.3% of subjects (1/23) (Table 3). One or more lice bodies were observed in 11 of 23 subjects at baseline, all of whom had become lice‐free by day 8 (Table S1).
TABLE 3.
Efficacy, safety, utility, and long‐term outcomes
| Outcome | n (%) |
|---|---|
| Efficacy, n = 23 | |
| Significant | 4 (17.3) |
| Moderate | 0 (0.0) |
| Mild | 18 (78.3) |
| Unchanged | 1 (4.3) |
| Deterioration | 0 (0.0) |
| Safety, n = 35 | |
| Safe | 35 (100) |
| Largely safe | 0 (0) |
| Questionable safety | 0 (0) |
| Unsafe | 0 (0) |
| Utility, n = 23 | |
| Very useful | 4 (17.3) |
| Useful | 0 (0.0) |
| Marginally useful | 18 (78.3) |
| Not useful | 1 (4.3) |
| Undesirable | 0 (0.0) |
| Long‐term success (1 month post‐treatment), n = 28 | |
| No lice | 25 (89.2) |
| With lice | 3 (10.7) |
3.3. Safety
Dimethicone was evaluated to be “safe” overall because none of the 35 subjects exhibited any erythema or other skin reactions or adverse events after dimethicone application to the scalp, neither did any receive a worse scratch‐mark score post‐treatment than at baseline (Table 3, Tables S1 and S2).
3.4. Utility
Utility was assessed separately for each subject based on his/her efficacy and safety ratings. Dimethicone was found to be “very useful” for four subjects (17.3%), “marginally useful” for 18 subjects (78.3%), and “not useful” for one subject (4.3%). Dimethicone was evaluated to have utility in the treatment of pediculosis capitis, because it was rated as “marginally useful” or higher for 95.7% of the utility population, surpassing the criterion of 80% or more (Table 3).
3.5. Long‐term success
Subjects were contacted for a telephone survey 1 month after the final treatment to assess persistent pediculosis and symptoms. The survey was completed by 28 of the 35 subjects (or a member of his/her family). In total, 89.2% (25/28) of the subjects reported no itchiness, and denied finding any head lice or nits; the remaining three reported persistence of infestation and an increase in nits (Table 3).
3.6. Genotyping of head lice
No lice or nits could be collected from three of the 35 subjects (cases 18, 34, and 35). In total, 96 samples from the remaining 32 subjects (2–6 samples/subject) were sequenced to identify kdr mutations in the VSSC gene. The amino acid sequences of 87 of the 96 samples were successfully obtained. In total, 81.6% (71/87) of the samples carried a kdr mutant allele at a minimum of one of the three loci analyzed. Pyrethroid resistance, defined as the presence of at least one mutant allele in at least one sample (from the same subject), was observed in 93.8% (30/32) of patients (Table 1). Samples obtained from the same subject did not always have matching genotypes (Table S3).
4. DISCUSSION
Dimethicone is used in products such as shampoos and conditioners for its properties as a hair coating agent, as well as in oral medications for gastrointestinal bloating. Dimethicone is believed to act by coating the surface of lice, killing adults and nymphs by restricting their movement and blocking the spiracles and airways, and preventing eggs from hatching by blocking pores and sealing the operculum, the structure from which first‐instar nymphs emerge. As of 2020, dimethicone preparations are sold in 32 countries, primarily within the European Union, and there have been no reports of severe health harm resulting from their use. They are widely used as safe medications that are effective against even phenothrin‐resistant head lice.7
We judged dimethicone to have utility in the treatment of pediculosis capitis because it was rated as “marginally useful” or higher for 95.7% of the utility population (22/23), surpassing the criterion of 80% or more. Only patient was it graded “not useful” (case 22), because one of the five eggs collected at the end of the treatment period (day 8) ended up hatching. Despite this low efficacy rating, dimethicone had a curative effect in this case, as her egg viability rate dropped from 100.0% at baseline to 25.0% after using the preparation.
It is difficult to determine with the naked eye whether a nit attached to a hair shaft is a live, unhatched egg, or an empty, already‐hatched casing. Knowing that removing large numbers of nits at baseline would influence the subsequent evaluation, we decided to calculate hatch rate on day 0 by collecting a maximum of 10 nits, and dividing the number of eggs that hatched by the number of those (up to) 10 identified as non‐empty eggs. Dimethicone exhibited pediculicidal activity against nits as well, evidenced by the low viability of all eggs collected on day 8 versus day 0: 0.6% (1/174) versus 37.9% (67/177).
A majority of subjects reached for the follow‐up telephone survey 1 month after dimethicone treatment were cured of infestation (89.2%, 25/28): the girl who received a utility rating of “not useful” (case 22) was among them. For one of the three exceptions (case 13), we suspect that dimethicone failed to eradicate the girl’s lice because of an increase in nits even after day 8. Lice seemed to be temporarily eradicated in the other two (cases 9 and 15), but the fact that their eggs increased in the 1 month after treatment suggests that dimethicone failed to exterminate the lice, or alternatively, that the children were re‐infested after successful extermination.
Our results provide evidence that dimethicone preparations are effective against even difficult‐to‐treat cases of phenothrin‐resistant pediculosis capitis. For example, dimethicone eradicated head lice in cases 11 and 12, two sisters who had struggled with the condition for the preceding 3 years despite repeated use of phenothrin products and combs. Likewise, it eradicated infestation in case 6 by a month after the end of the trial, on whose scalp eight lice (bodies) were counted at baseline in just 3 min of visual inspection. Although this patient’s lice count was rated as “moderate” according to the study protocol (i.e., 5 ≤ n ≤ 9), it seems reasonable to regard her true condition as severe.
Dimethicone was rated just “marginally useful” in a majority of the utility population (18/23). One plausible reason why it was not rated more useful is that our counts may not have reflected the infestations’ true severity, given the difficulty of determining accurate counts of lice bodies by unassisted visual inspection.
The regimen of a 2016 clinical trial for head lice infestation conducted by a team including the authors provided for patients to be treated with phenothrin initially, but switched to an ivermectin lotion if phenothrin was judged to be ineffective.8 In that study, efficacy was evaluated based on the numbers of head lice (bodies) and nits collected from a patient’s hair and scalp by 20 comb strokes at four timepoints in total. The efficacy of phenothrin was inconclusive since the evaluation method consisted of just 20 comb strokes. Strokes are restricted in comb‐ or brush‐based evaluation techniques because the action of combing itself removes lice and nits, confounding straightforward assessment of the efficacy of a study drug.
We addressed this shortcoming in the current trial by deciding to count lice bodies and nits by visual inspection instead of using a comb. This decision allowed us to exclude the confounding and therapeutic effects of combing, although it required skill to count the number of lice accurately in this study.
Our trial successfully confirmed dimethicone’s ovicidal efficacy, both in a larger population as well as in terms of egg viability rate after application compared with before. We also were able to gain insight into its long‐term efficacy through the use of a follow‐up telephone survey 4 weeks after the final treatment.
Pediculicidal resistance was estimated to occur in 96% of head lice in Okinawa Prefecture in a 2010 Japanese national survey of kdr‐type mutations in the head louse VSSC.4 We were able to genotype samples originating from 32 of 35 subjects in the present trial; head lice in 30 of them were confirmed to carry mutant kdr alleles, meaning 93.8% of patients were infested with pyrethroid‐resistant head lice.
Knockdown resistance mutations exhibited diverse patterns: while many lice carried homozygous mutants at all three loci, some were homozygous mutants at one locus but wild‐type at the other two; others had homozygous mutations at two loci but wild‐type at one; and still others had a different pattern at each locus (i.e., homozygous‐, heterozygous‐mutant, and wild‐type). Intriguingly, head lice specimens from the same patients did not always have matching genotypes. The diversity in the present trial notwithstanding, nearly all head lice in Okinawa Prefecture genotyped in the 2010 nationwide survey were homozygous mutants.3, 4 This suggests that interbreeding is increasing the proportion of pyrethroid resistance‐conferring genes, since pyrethroid‐susceptible lice still exist in Okinawa Prefecture, albeit in small numbers.
We were able to inquire about the history of phenothrin shampoo usage before trial enrollment with 20 of the 35 subjects. Five of them denied pyrethroid usage; the remaining 15 had used it but claimed it had failed to eradicate their head lice. At least one kdr mutation in louse VSSC was identified in the samples of 14 of the 15 subjects with a history of past pyrethroid usage (the lone exception, case 34, was unable to be genotyped) as well as four of the five subjects without such a history. Our observations reconfirm the fact that pyrethroid‐resistant head lice are epidemic in Okinawa irrespective of carriers’ past usage of pyrethroid products, given the high prevalence of resistance‐conferring genes in the prefecture.
In summary, the dimethicone lotion tested in this trial was demonstrated to be both safe and effective in treating pyrethroid‐resistant head lice.
CONFLICT OF INTEREST
This work was supported financially by Earth Corporation, who played no active role in the design of the study, interpretation of the results, or the writing of the manuscript.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
Drug ST2702 was developed by Earth Corporation, and was approved by the Ministry of Health, Labour, and Welfare (MHLW) as a quasi‐drug on April 6, 2021.
Abbreviations: kdr, knockdown resistance; OTC, over‐the‐counter; VSSC, voltage‐sensitive sodium channel; PCR, polymerase chain reaction.
REFERENCES
- 1.Chosidow O, Chastang C, Brue C, Bouvet E, Izri M, Monteny N, et al. Controlled study of malathion and d‐phenothrin lotions for Pediculus humanus var capitis‐infested schoolchildren. Lancet. 1994;344:1724–7. [DOI] [PubMed] [Google Scholar]
- 2.Hodgdon HE, Yoon KS, Previte DJ, Kim HJ, Aboelghar GE, Lee SH, et al. Determination of knockdown resistance allele frequencies in global human head louse populations using the serial invasive signal amplification reaction. Pest Manag Sci. 2010;66:1031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasai S, Ishii N, Natsuaki M, Fukutomi H, Komagata O, Kobayashi M, et al. Prevalence of kdr‐like mutations associated with pyrethroid resistance in human head louse populations in Japan. J Med Entomol. 2009;46:77–82. [DOI] [PubMed] [Google Scholar]
- 4.Tomita T, Kasai S, Komagata O, Kobayashi M. Development of pyrethroid drug resistance in head lice development and effective control measures. Jpn J Dermatol. 2011;121:2898–9. [Google Scholar]
- 5.Minakawa S, Matsuzaki Y, Yamaguchi S, Takahashi K, Kayaba H, Sawamura D. Pediculus humanus capitis: pyrethroid resistance and utility of scanning electron microscopy. J Dermatol. 2019;46:e418–e419. [DOI] [PubMed] [Google Scholar]
- 6.Coates SJ, Thomas C, Chosidow O, Engelman D, Chang AY. Ectoparasites Pediculosis and tungiasis. J Am Acad Dermatol. 2020;82:551–69. [DOI] [PubMed] [Google Scholar]
- 7.Ihde ES, Boscamp JR, Loh JM, Rosen L. Safety and efficacy of a 100% Dimethicone pediculocide in school‐age children. BMC Pediatr. 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komoda M, Yamaguchi S, Takahashi K, Yanase K, Umezawa M, Miyajima A, et al. Efficacy and safety of a combination regimen of phenothrin and ivermectin lotion in patients with head lice in Okinawa, Japan. J Dermatol. 2020;47:720–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


