Abstract
Owing to its heterogeneity and rarity, management of disseminated marginal zone B‐cell lymphoma (MZL) remains largely understudied. We present prospective data on choice of systemic treatment and survival of patients with MZL treated in German routine practice. Of 175 patients with MZL who had been documented in the prospective clinical cohort study Tumour Registry Lymphatic Neoplasms (NCT00889798) collecting data on systemic treatment, 58 were classified as extranodal MZL of mucosa‐associated lymphoid tissue (MALT) and 117 as non‐MALT MZL. We analyzed the most commonly used first‐line and second‐line chemo(immuno)therapies between 2009 and 2016 and examined objective response rate (ORR), progression‐free survival (PFS), overall survival (OS) and prognostic factors for survival. Compared to patients with MALT MZL, those with non‐MALT MZL more often presented with bone marrow involvement (43% vs. 14%), Ann Arbor stage III/IV (72% vs. 57%) and were slightly less often in good general condition (ECOG = 0; 41% vs. 47%). In German routine practice, rituximab‐bendamustine for a median of 6 cycles was the most frequently used first‐line (76%) and second‐line treatment (36%), with no major differences between MZL subtypes. The ORR for patients encompassing any positive response was 81%. For patients with MALT and non‐MALT MZL, respectively, 5‐years PFS was 69% (95% CI 52%–81%) and 66% (95% CI 56%–75%), 5‐years OS 79% (95% CI 65%–89%) and 75% (95% CI 66%–83%). Cox proportional hazards models showed a significantly increased risk of mortality for higher age in all patient groups. Our prospective real world data give valuable insights into the management and outcome of non‐selected patients with MZL requiring systemic treatment and can help optimize therapy recommendations.
Keywords: disease management, marginal zone B‐cell lymphoma, mucosa‐associated lymphoid tissue lymphoma, outpatients, prognosis, progression‐free survival, registries
1. INTRODUCTION
Marginal zone lymphomas (MZL) are derived from B‐lymphocytes of the marginal zone as the external part of the secondary lymphoid follicles1 and account for approximately 5%–15% of all non‐Hodgkin's lymphomas in the Western world.2, 3 MZL has an orphan disease designation with an age‐standardized incidence per 100,000 population reported to range from 0.5–2.6.4 The median age at diagnosis is close to 67 years but may differ by MZL subtype.5 According to the World Health Organization classification, there are three different MZL subtypes with specific diagnostic criteria, genetic features, clinical course and therapeutic implications: extranodal MZL of mucosa‐associated lymphoid tissue (MALT), splenic MZL (SMZL) and nodal MZL (NMZL)6, 7, 8 representing 50%–70%, 20% and 10% of all MZL, respectively.3, 9, 10 MALT MZL can arise at any extranodal site with the stomach as the most common, followed by ocular/adnexal, lung, skin and salivary glands.5 They are usually associated with chronic immune stimulation due to infections (e.g., Helicobacter pylori in gastric MALT MZL) or autoimmunity (e.g., Hashimoto's and Sjogren's syndrome).3 Based on population‐based data from SEER (Surveillance, Epidemiology, and End Results) and the UK's Haematological Malignancy Research Network (HMRN), respectively, the 5‐years overall survival (OS) has been reported to be 61%11–84%12 for overall MZL over the past 2 decades, ranging from 75%11, 13 to 87%12 for MALT MZL, 68%13 to 80%12 for SMZL and 64%13 to 78%12 for NMZL. A greater understanding of the disease biology has broadened the therapeutic landscape of MZL over the past decade with the development of novel therapeutic approaches targeting signaling pathways.9, 14, 15 However, owing to its rarity and disease heterogeneity, only few phase III randomized clinical trials (RCTs) have focused on MZL so far.16 Thus, MZL remains largely understudied.5
Since management of patients with disseminated MZL requiring systemic treatment may vary, prospectively collected routine data are of great interest to identify treatment patterns and survival of patients with distinct MZL subtypes. To our knowledge, no larger data on treatment and survival of patients with MZL in Germany have been published so far. Here, we present data on 175 patients, classified into MALT and non‐MALT MZL subtypes, documented within the prospective clinical cohort study TLN (Tumour Registry Lymphatic Neoplasms) which had recruited patients with indolent17, 18, 19, 20 or aggressive NHL21 requiring systemic therapy and who were treated by office‐ and hospital‐based hematologists across Germany. We show choice of first‐line and second‐line treatment and outcome of patients by analyzing best response, progression‐free survival (PFS) and OS. Furthermore, we present a multivariate regression model identifying factors influencing survival.
2. METHODS
2.1. Data source
The TLN was an open, longitudinal, multicentre, observational, prospective cohort study collecting data on systemic treatment of patients with lymphoid B‐cell neoplasms between 2009 and 2019. The study had been approved by the responsible ethics committee and is registered at ClinicalTrials.gov (NCT00889798). Patients were treated according to physicians' choice. There were no specifications as to the timing, frequency or criteria of tumor assessment. Further details on the methodology of the TLN have been previously described elsewhere.17, 18, 19, 20, 21
2.2. Cohort definition
A total of 3,795 patients with lymphoid B‐cell neoplasms had been recruited into the TLN (Figure 1). Of 1,187 patients diagnosed with indolent NHL (excluding chronic lymphocytic leukemia and multiple myeloma), 1,049 had been enrolled at start of their first‐line systemic chemo(immuno) therapy. Among them, 175 patients with MZL recruited in 71 office‐ and hospital‐based medical oncology/hematology centers across Germany between May 2009 and January 2014 were included in this analysis. Patients registered as MALT‐lymphoma and patients registered as MZL with documented extranodal involvement such as gastrointestinal tract, lung, breast, eye/orbita, breast, kidney and other were categorized as (extranodal) MALT MZL (n = 58, 33%). Since no clear specification between SMZL and NMZL had been recorded in the TLN, the remaining patients were classified within the non‐MALT MZL subgroup (n = 117, 67%). Data cut‐off for this analysis was 31 August 31 2019.
FIGURE 1.
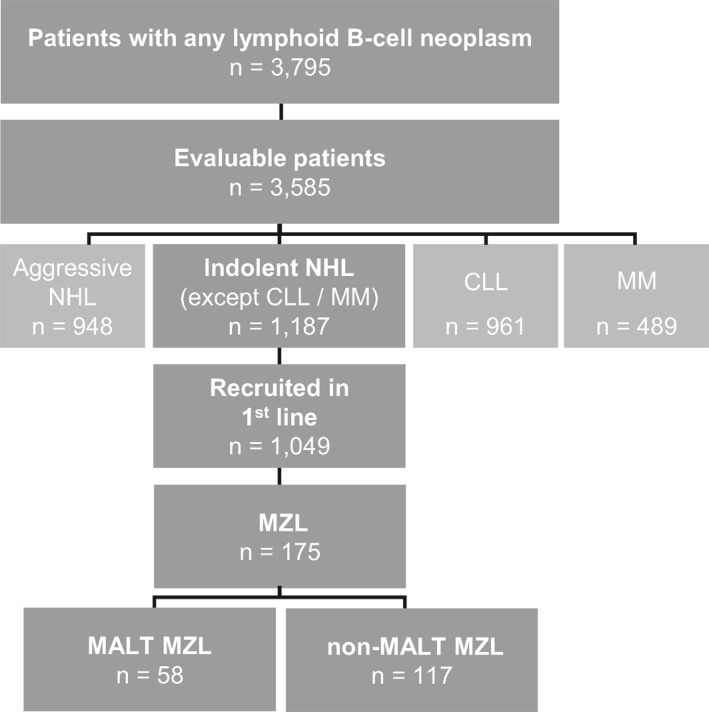
Cohort definition. Number of patients enrolled in the TLN from April 2009 until August 2014, split up according to different types of lymphoid B‐cell neoplasms. Of all evaluable patients with indolent NHL (other than CLL or MM), 175 patients with MZL who had been prospectively enrolled at the start of their first‐line treatment were included into this analysis. Among them, 58 patients presented with MALT MZL and 117 patients with non‐MALT MZL. Data cut‐off for this analysis was 31 August 2019. CLL, chronic lymphocytic leukemia; MALT, mucosa‐associated lymphoid tissue; MM, multiple myeloma; MZL, marginal zone lymphoma; NHL, non‐Hodgkin's lymphoma
2.3. Statistical analysis
Time‐to‐events was analyzed by the Kaplan‐Meier method. PFS was defined as the interval between start of first‐line treatment and date of progression or death prior to start of second‐line treatment; patients without such an event were censored at either the start of second‐line treatment or at the last documented contact. OS was defined as the interval between start of first‐line treatment until death from any cause. Data of patients alive or lost to follow‐up were censored at the last documented contact. The median observation time was calculated using the reverse Kaplan‐Meier estimate.22 Confidence limits for the survivor function were calculated employing a log‐log transformation.23 Confidence intervals (CI) for median survival were calculated as described by Brookmeyer and Crowley.24 All analyses were performed using SAS software, Version 9.4 of the SAS System for Windows. Copyright © 2002‐2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA.
To identify potential independent prognostic factors for survival, separate Cox proportional hazards models were fitted for each MZL subtype. The following pre‐defined independent variables were examined: age, bone marrow involvement, LDH level, presence of B symptoms, spleen involvement and stage. Backwards selection by minimizing Akaike information criterion (AIC) was chosen for variable selection. CI for the regression coefficients were based on the Wald statistics. All presented p values are two‐sided and p‐values smaller than 5% will be interpreted as significant. There were no multiplicity adjustments to the level of significance.
3. RESULTS
3.1. Patient demographics and clinical characteristics
Table 1 shows the demographics and clinical characteristics of patients with MZL included in this analysis (n = 175). Half of patients were male (51%, n = 89). The median age at start of first‐line treatment was 69 years. Patients with MALT MZL slightly more often presented in good general condition (ECOG = 0; 47%, n = 27 vs. 41%, n = 48). At least one concomitant disease was present in 67% of all patients (n = 118) at start of first‐line treatment with arterial hypertension as the most common (38%, n = 66). Presence of B symptoms, lower hemoglobin and elevated LDH levels had been more frequently documented in patients with non‐MALT MZL (35%, n = 41 vs. 16%, n = 9; 45%, n = 53 vs. 31%, n = 18 and 33%, n = 38 vs. 26%, n = 15, respectively). Furthermore, patients with non‐MALT MZL more often presented with Ann Arbor stage of III/IV (72%, n = 84 vs. 57%, n = 33) and more often had bone marrow involvement (43%, n = 50 vs. 14%, n = 8). For patients with MALT MZL, lung (33%, n = 19) and gastrointestinal tract (26%, n = 15), respectively, were the most frequently affected organs. Prior radiotherapy had been more frequently documented in patients with MALT MZL (9%, n = 5 vs. 3%, n = 3).
TABLE 1.
Patient demographics and clinical characteristics at start of first‐line treatment
| Characteristic | MALT MZL n = 58 | Non‐MALT MZL n = 117 | Overall MZL n = 175 |
|---|---|---|---|
| Age in years, median (25%–75% quartile) | 66.5 (56.7–75.1) | 69.6 (61.2–76.2) | 69.0 (59.4–75.9) |
| ≥70 years | 25 (43.1%) | 56 (47.9%) | 81 (46.3%) |
| BMI in kg/m2, mean (StD) | 26.4 (4.8) | 26.5 (5.3) | 26.5 (5.1) |
| Missing | 1 (1.7%) | 5 (4.3%) | 6 (3.4%) |
| Sex | |||
| Female | 30 (51.7%) | 56 (47.9%) | 86 (49.1%) |
| Male | 28 (48.3%) | 61 (52.1%) | 89 (50.9%) |
| Performance status | |||
| ECOG = 0 | 27 (46.6%) | 48 (41.0%) | 75 (42.9%) |
| ECOG = 1 | 22 (37.9%) | 49 (41.9%) | 71 (40.6%) |
| ECOG ≥2 | 3 (5.3%) | 7 (6.0%) | 10 (5.7%) |
| Unknown | 5 (8.6%) | 13 (11.1%) | 18 (10.3%) |
| Missing | 1 (1.7%) | 0 (0.0%) | 1 (0.6%) |
| Patients with comorbidity | |||
| Any comorbiditya | 39 (67.2%) | 79 (67.5%) | 118 (67.4%) |
| CCI = 0b | 38 (69.5%) | 91 (77.8%) | 129 (73.7%) |
| CCI ≥1b | 20 (34.5%) | 26 (22.2%) | 46 (26.3%) |
| Arterial hypertension | 21 (36.2%) | 45 (38.5%) | 66 (37.7%) |
| Cardiac disordersc | 12 (20.7%) | 30 (25.6%) | 42 (24.0%) |
| Diabetes | 16 (10.3%) | 14 (12.0%) | 20 (11.4%) |
| Chronic pulmonary disease | 4 (6.9%) | 13 (11.1%) | 17 (9.7%) |
| Autoimmune disordersd | 4 (6.9%) | 8 (6.8%) | 12 (6.9%) |
| B symptomse | |||
| Present | 9 (15.5%) | 41 (35.0%) | 50 (28.6%) |
| Unknown | 7 (12.1%) | 6 (5.1%) | 13 (7.4%) |
| Hemoglobin | |||
| <12 g/dL | 18 (31.0%) | 53 (45.3%) | 71 (40.6%) |
| LDH | |||
| >ULN | 15 (25.9%) | 38 (32.5%) | 53 (30.3%) |
| Unknown | 6 (10.3%) | 4 (3.4%) | 10 (5.7%) |
| Ann Arbor stagef | |||
| I | 6 (10.3%) | 4 (3.4%) | 10 (5.7%) |
| II | 15 (25.9%) | 11 (9.4%) | 26 (14.9%) |
| III/IV | 33 (56.9%) | 84 (71.8%) | 117 (66.9%) |
| Ambiguous | 4 (6.9%) | 18 (15.4%) | 22 (12.6%) |
| Lymph node regions, median (25%–75% quartile) | 2 (0–4) | 3 (1–4) | 2 (1–4) |
| = 0 | 13 (22.4%) | 13 (11.1%) | 26 (14.9%) |
| = 1–2 | 14 (24.1%) | 23 (19.7%) | 37 (21.1%) |
| = 3–4 | 10 (17.2%) | 23 (19.7%) | 33 (18.9%) |
| ≥5 | 6 (10.3%) | 18 (15.4%) | 24 (13.7%) |
| Unknown | 15 (25.9%) | 40 (34.2%) | 55 (31.4%) |
| Extranodal sides | |||
| Present | 48 (82.8%) | 65 (55.6%) | 113 (64.6%) |
| Unknown | 5 (8.6%) | 10 (8.5%) | 15 (8.6%) |
| Affected organsg | |||
| Bone marrow | 8 (13.8%) | 50 (42.7%) | 58 (33.1%) |
| Spleen | 5 (8.6%) | 31 (26.5%) | 36 (20.6%) |
| Lung | 19 (32.8%) | 2 (1.7%) | 21 (12.0%) |
| Gastrointestinal tract | 15 (25.9%) | 0 (0.0%) | 15 (8.6%) |
| Skin | 6 (10.3%) | 0 (0.0%) | 6 (3.4%) |
| Liver | 2 (3.4%) | 3 (2.6%) | 5 (2.9%) |
| Breast | 4 (6.9%) | 0 (0.0%) | 4 (2.3%) |
| Eye/orbita | 3 (5.2%) | 1 (0.9%) | 4 (2.3%) |
| Other | 5 (8.6%) | 7 (6.0%) | 12 (6.9%) |
| Prior radiotherapy | 5 (8.6%) | 3 (2.6%) | 8 (4.6%) |
| Splenectomy | 1 (1.7%) | 8 (6.8%) | 9 (5.1%) |
Note: Data are n (%), unless otherwise stated. Some percentages might not add up to 100% due to rounding.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; StD, standard deviation; ULN, upper limit of normal.
At least one comorbidity according to Charlson and/or additional concomitant diseases.
Charlson comorbidity index (CCI) according to Quan et al.25, 26; MZL (2 points) was not counted as a comorbidity.
Heart insufficiency, myocardial infarction, coronary artery disease and other cardiac disorders.
Autoimmune hemolytic anemia, arthritis, collagen disorder, immune thrombocytopenic purpura, rheumatic disorder, systemic lupus erythematosus and potential autoimmune disorders: hypothyroidism or other thyroid disorder, Basedow's disease, polyneuropathy; of all autoimmune disorders presented, n = 4 potential.
Fever, night sweats, loss of weight.
As documented by study sites.
Patients may be affected by multiple organ involvement. Other: Bone, Kidney, Pleura and some other rarely documented organ sites.
3.2. Choice of systemic treatment
3.2.1. First‐line treatment
Figure 2 displays the most frequently used first‐line treatments between 2009 and 2014, clustered by substance groups, for the overall MZL cohort (n = 175, Figure 2A) and for patients with either MALT (n = 58) or non‐MALT MZL (n = 117, Figure 2B).
FIGURE 2.

Choice of systemic first‐line treatment in marginal zone lymphoma. First‐line treatments/treatment combinations between 2009 and 2014 sorted by relative frequency for (A) all patients included (n = 175) and (B) classified into either MALT MZL (n = 58) or non‐MALT MZL (n = 117). Bendamustine‐based: bendamustine (±prednisone), bendamustine + rituximab (±prednisone)a, bendamustine + vincristine (±prednisone/ rituximab); chlorambucil‐based: chlorambucil (mono), chlorambucil + rituximab; fludarabine‐based: fludarabine + cyclophosphamide (±rituximab); CHOP‐based: CHOP or COP + rituximab; other: R‐CHO(P)/R‐DHAP, dexamethasone + methotrexate, trofosfamide + rituximab + prednisone. Note: Percentages may not add up to 100% due to rounding. CHO(P), cyclophosphamide/doxorubicin/vincristine/(prednisone); COP, cyclophosphamide/vincristine/prednisone; DHAP, dexamethasone/high‐dose cytarabine (Ara‐C)/cisplatin; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; R, rituximab.a One patient switched to bendamustine + rituximab after one cycle of R‐CHOP
Bendamustine‐based therapies were the most common first‐line treatments (81%, n = 141), with no differences between patients with MALT and non‐MALT MZL and with rituximab‐bendamustine (±prednisone) used most frequently (76%, n = 133). In these patients, bendamustine was used for a median of 6 cycles (interquartile range [IQR] 2.0) and rituximab for a median of 6 cycles (IQR 1.0) as well.
First‐line treatments based on cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP) were applied to 10% of all patients (n = 18), slightly more frequently for patients with MALT than with non‐MALT MZL (14%, n = 8 vs. 9%, n = 10). Two patients with MALT and 4 patients with non‐MALT MZL (both 3%) received rituximab monotherapy as first‐line treatment. Fludarabine‐ or chlorambucil‐based regimens as well as other treatments were applied only rarely (each 2%).
3.2.2. Second‐line treatment
At the time of this analysis, second‐line treatment was documented for 28 patients (16%): 9 patients (16%) with MALT and 19 patients (16%) with non‐MALT MZL. 25 patients (14%) had died prior to receiving a second‐line treatment. From 2010 to 2016, the most common second‐line treatments were bendamustine‐based (39%, n = 11), mainly rituximab‐bendamustine (±prednisone; 36%, n = 10). For patients with MALT MZL, bendamustine‐based therapy was used as often as rituximab monotherapy (each 33%, n = 3) as second‐line therapy. Two patients (22%) received second‐line DHAP combined with rituximab, and one patient (11%) received fludarabine in combination with cyclophosphamide and rituximab. For patients with non‐MALT MZL, bendamustine‐based therapy was used most frequently (42%, n = 7), followed by rituximab‐CHOP (R‐CHOP; 21%, n = 4) and fludarabine‐based therapy (16%, n = 3). Two patients (11%) were treated with rituximab monotherapy. One patient received ibrutinib, another patient chlorambucil (5% both).
3.3. Sequential treatment
The three most commonly used sequences for all patients proceeding from first‐line to second‐line treatment (n = 28) were: bendamustine‐based treatment followed by (a) a bendamustine‐based (32%, n = 9), or (b) a CHOP‐based (14%, n = 4), or (c) a fludarabine‐based therapy (14%, n = 4).
3.4. Best response, progression‐free survival and overall survival
Outcome data are presented in Figure 3 and shown in Table 2. The objective response rate (ORR) for patients encompassing any positive response was 81% for the overall cohort, with 76% documented in MALT and 84% in non‐MALT MZL. Stable disease had been reported in 5% (n = 9) of all patients, while there was no disease progression documented during first‐line treatment. However, in 14% of patients (n = 24), the status of response had been documented as unknown, with a higher proportion of unknown responses in the MALT MZL subgroup (19%, n = 11 vs. 11%, n = 13).
FIGURE 3.
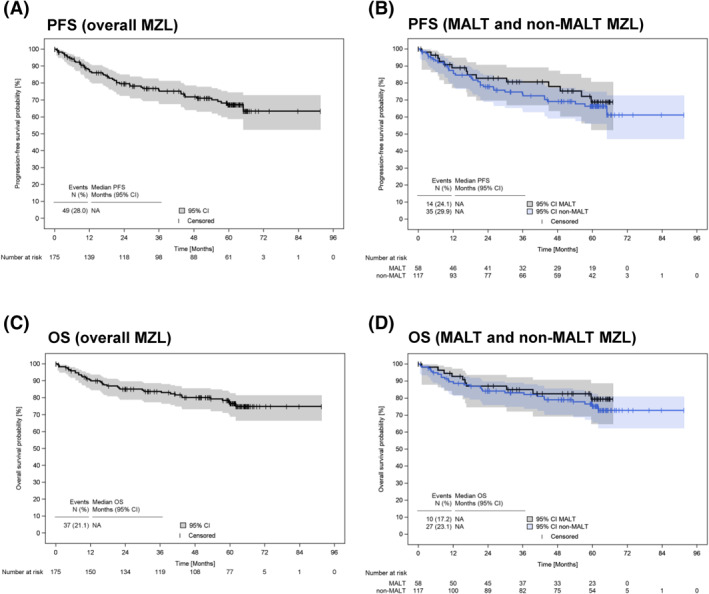
Progression‐free and overall survival of patients with marginal zone lymphoma who had been enrolled at the beginning of their first‐line treatment. (A) PFS since start of first‐line treatment for all patients included (n = 175) and (B) classified into either MALT MZL (n = 58) or non‐MALT MZL (n = 117); C) OS since start of first‐line treatment for all patients included (n = 175) and (D) classified into either MALT MZL (n = 58) or non‐MALT MZL (n = 117). CI, confidence interval; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; OS, overall survival; PFS, progression‐free survival
TABLE 2.
OS, PFS and best response since start of first‐line treatment
| Characteristic | MALT MZL n = 58 | Non‐MALT MZL n = 117 | Overall MZL n = 175 |
|---|---|---|---|
| Best response | |||
| CRua | 20 (34.5%) | 37 (31.6%) | 57 (32.6%) |
| PR | 24 (41.4%) | 61 (52.1%) | 85 (48.6%) |
| SD | 3 (5.2%) | 6 (5.1%) | 9 (5.1%) |
| PD | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Unknown | 11 (19.0%) | 13 (11.1%) | 24 (13.7%) |
| Progression‐free survival | |||
| Events | 14 (24.1%) | 35 (29.9%) | 49 (28.0%) |
| Median PFS, months (95% CI)b | NA | NA | NA |
| Survival rate, percent (95% CI) | |||
| 12 months | 88.9 (76.9–94.8) | 87.4 (79.7–92.4) | 87.9 (81.9–92.0) |
| 24 months | 82.8 (69.5–90.7) | 77.9 (68.8–84.6) | 79.5 (72.4–85.0) |
| 36 months | 80.6 (66.9–89.1) | 73.6 (64.1–81.0) | 75.9 (68.4–81.9) |
| 48 months | 77.9 (63.5–87.2) | 69.1 (59.1–77.1) | 71.9 (63.9–78.4) |
| 60 months | 68.8 (52.3–80.7) | 66.3 (56.0–74.8) | 67.2 (58.6–74.3) |
| Overall survival | |||
| Events | 10 (17.2%) | 27 (23.1%) | 37 (21.1%) |
| Median OS, months (95% CI) | NA | NA | NA |
| Survival rate, percent (95% CI) | |||
| 12 months | 92.7 (81.7–97.2) | 89.5 (82.3–93.9) | 90.6 (85.1–94.1) |
| 24 months | 86.9 (74.5–93.6) | 84.1 (75.9–89.7) | 85.0 (78.6–89.6) |
| 36 months | 84.9 (72.1–92.2) | 83.1 (74.8–88.9) | 83.7 (77.1–88.5) |
| 48 months | 82.6 (69.1–90.6) | 79.0 (70.1–85.6) | 80.1 (73.0–85.5) |
| 60 months | 79.4 (64.6–88.6) | 75.2 (65.5–82.5) | 76.5 (68.7–82.6) |
| Median duration of observation, months (95% CI) | 59.7 (51.5–61.5) | 60.4 (59.9–61.7) | 60.3 (59.9–61.3) |
Note: Data are n (%), unless otherwise stated. Some percentages might not add up to 100% due to rounding.
Abbreviations: CI, confidence interval; CR, complete response; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; NA, not applicable; OS, overall survival; PD, progression; PFS, progression‐free survival; PR, partial response; SD, stable disease.
Assessment by study sites, no evaluation by the criteria used in clinical trials.
Assessment by study sites and not according to response criteria used in clinical trials.
Median duration of observation was 60.3 months (95% CI 59.9–61.3). 5‐years PFS was 69% (95% CI 52%–81%) for MALT and 66% (95% CI 56%–75%) for non‐MALT MZL (Table 2, Figure 3A–B). 5‐years OS was 79% (95% CI 65%–89%) for MALT and 75% (95% CI 66%–83%) for non‐MALT MZL (Table 2, Figure 3C–D).
At data cut‐off, 37 patients (21%) had died, 25 (14%) were still being observed, 34 (19%) were lost to follow‐up. Another 80 patients (46%) were alive at the end of the individual 5‐years observation period which was defined as the maximum observation time in the TLN registry.
3.5. Factors influencing survival
Results of multivariate regression analyses are shown in Table 3. There are three Cox models for both mortality and disease progression, that is one model for each MZL subgroup (overall, MALT, non‐MALT MZL). Due to the relatively small patient numbers, models fitting best to the data have been selected and are presented here. Thus, the subset of predictors included varies between models. Regarding OS, age was the only variable associated with a significantly increased risk of mortality for the whole MZL cohort and both subgroups. For disease progression, age was also found to be a statistically significant predictor in the overall cohort. For the MALT MZL group, absence of B‐symptoms was associated with a significantly decreased risk of progression, while for the non‐MALT MZL group, age, and absence of bone marrow involvement and absence of LDH elevation were linked with a significantly increased risk of progression.
TABLE 3.
Multivariate regression analysis – Cox proportional hazards models for progression‐free and overall survival in MZL
| Progression‐free survival | |||
|---|---|---|---|
| Variable | Patients n (%) | HR (95% CI) | p‐value |
| Overall MZL (n = 175) | |||
| Age (10‐years increments) | ‐ | 1.64 (1.23–2.20) | <0.001* |
| B symptoms | |||
| Present | 50 (28.6%) | Reference | ‐ |
| Not present | 112 (64.0%) | 0.60 (0.34–1.07) | 0.083 |
| Unknown | 13 (7.4%) | 0.19 (0.03–1.44) | 0.109 |
| MALT MZL (n = 58) | |||
| B symptoms | |||
| Present | 9 (15.5%) | Reference | ‐ |
| Not present | 42 (72.4%) | 0.29 (0.10–0.88) | 0.029* |
| Unknown | 7 (12.1%) | 0.00 (0.00–NA) | 0.993 |
| Non‐MALT MZL (n = 117) | |||
| Age (10‐years increments) | ‐ | 1.90 (1.33–2.72) | <0.001* |
| B symptoms | |||
| Present | 41 (35.0%) | Reference | |
| Not present | 70 (59.8%) | 0.57 (0.29–1.14) | 0.113 |
| Unknown | 6 (5.1%) | 0.22 (0.03–1.77) | 0.155 |
| Increased LDH | |||
| Yes | 38 (32.5%) | Reference | ‐ |
| No | 75 (64.1%) | 2.36 (1.03–5.38) | 0.042* |
| Unknown | 4 (3.4%) | 0.00 (0.00–NA) | 0.992 |
| Spleen involvement | |||
| Yes | 31 (26.5%) | Reference | ‐ |
| No | 86 (73.5%) | 0.41 (0.17–1.00) | 0.051 |
| Bone marrow involvement | |||
| Yes | 50 (42.7%) | Reference | ‐ |
| No | 67 (57.3%) | 2.65 (1.14–6.14) | 0.023* |
| Overall survival | |||
|---|---|---|---|
| Variable | Patients n (%) | HR (95% CI) | p ‐value |
| Overall MZL (n = 175) | |||
| Age (10‐years increments) | ‐ | 1.99 (1.38–2.85) | <0.001* |
| MALT MZL (n = 58) | |||
| Age (10‐years increments) | ‐ | 2.44 (1.17–5.08) | 0.017* |
| Bone marrow involvement | |||
| Yes | 8 (13.8%) | Reference | ‐ |
| No | 50 (86.2%) | 0.33 (0.08–1.31) | 0.115 |
| Non‐MALT MZL (n = 117) | |||
| Age (10‐years increments) | ‐ | 1.94 (1.28–2.96) | 0.002* |
| Increased LDH | |||
| Yes | 38 (32.5%) | Reference | ‐ |
| No | 75 (64.1%) | 2.33 (0.91–5.96) | 0.077 |
| Unknown | 4 (3.4%) | 0.00 (0.00–NA) | 0.992 |
| Spleen involvement | |||
| Yes | 31 (26.5%) | Reference | ‐ |
| No | 86 (73.5%) | 0.37 (0.13–1.02) | 0.055 |
| Bone marrow involvement | |||
| Yes | 50 (42.7%) | Reference | ‐ |
| No | 67 (57.3%) | 2.16 (0.83–5.62) | 0.114 |
Note: Backwards selection by minimizing Akaike information criterion (AIC) was chosen for variable selection. Models fitting best to the data have been selected and are presented here. Some percentages might not add up to 100% due to rounding.
Abbreviations: CI, confidence interval; HR, hazard ratio; y, year; LDH, lactate dehydrogenase; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; NA, not applicable; OS, overall survival; PFS, progression‐free survival.
*Significant results (p < 0.05).
4. DISCUSSION
The present study shows prospective real world data on treatment and outcome of patients with MZL requiring systemic therapy offered in German routine practice. Rituximab‐bendamustine was the most frequently applied first‐line and second‐line regimen. Survival rates tended to be slightly higher for patients with MALT MZL which might be related to differences in patient characteristics. Age significantly increased the risk of mortality in both patient groups.
Strengths of this study are the prospective, longitudinal design and the non‐selected participation of hematologists/oncologists across Germany recruiting into a large study cohort which allows the analysis of smaller subsets including the MZL patient population. Our study is limited by the absence of central pathology review and the sole enrollment of patients with MZL receiving systemic therapy. Therefore, patients with a watch‐and‐wait approach or patients receiving only local treatment were not included. In case of localized disease, the WHO classification distinguishes between three MZL subtypes. However, when patients present with disseminated disease, it can be difficult to determine the correct subtype. The classification of MALT and non‐MALT MZL used for this work represents an approximation and characteristics of our cohort may thus not be representative of the general MZL patient population. In the TLN, there were no specifications as to the timing, frequency or criteria of tumor assessment. Thus, clinical PFS data should be considered as the best clinical approximation, but might not be identical to the PFS determined in controlled clinical trials.
According to our data, median 6 cycles rituximab‐bendamustine was by far the most frequently used first‐ and second‐line treatment for patients with MALT and non‐MALT MZL. The combination of bendamustine with rituximab has been widely adopted as first‐line regimen for indolent B‐cell lymphomas including MZL (in general27 and for advanced MZL of any subtype5). Two phase III RCTs, the BRIGHT study and the StiL (Study group indolent Lymphomas) study enrolling treatment‐naive patients with indolent NHL or mantle cell lymphoma, have reported improved PFS and lower toxicity of rituximab‐bendamustine compared with R‐CHOP or R‐CVP (rituximab/cyclophosphamide/vincristine/prednisolone), while there was no difference in OS between the treatment groups.28, 29, 30 In both studies, however, the proportion of patients with MZL was low: 12% in BRIGHT,28 14% in StiL.29 In two phase II studies, rituximab‐bendamustine has proven to be a very effective first‐line regimen alongside a favorable toxicity profile in patients with MALT MZL at any site or stage even at 4 cycles (MALT2008‐0131 , 32) and in SMZL (BRISMA/IELSG3633), respectively. In the IELSG‐19 study, the combination of rituximab plus chlorambucil versus either rituximab or chlorambucil monotherapy was assessed as first‐line treatment in 401 patients with MALT MZL, subsequently determining early progression of disease (POD) within two years from initial systemic treatment as predictive for inferior OS. Patients with high‐risk MALT‐IPI (based on age ≥70 years, elevated LDH and Ann Arbor stage III/IV) were more likely to have early POD in the IELSG19 study.10, 34
In patients with recurrent MZL, rituximab alone or in combination has also shown to provide high response rates,13, 35 with rituximab‐bendamustine as the most common second‐line treatment as revealed by our data. Notably, it has to be taken into account that, at the time of this analysis and beyond, there were no comprehensive European guidelines on the diagnosis/management of MZL. While guidelines of the European Society for Medical Oncology (ESMO) only focused on gastric MALT MZL,36 solely few national guidelines existed.16 Usually, treatments used for follicular lymphoma have also been applied for MZL.9 Most recently, the first clinical practice guideline of MZL has become available by the ESMO.3 In the presence of disseminated symptomatic disease, chemoimmunotherapy such as rituximab‐bendamustine or rituximab‐chlorambucil is currently recommended for all MZL subtypes.
Since this study focused on MZL treatment between 2009 and 2016, data from more recent studies exploring the impact of newer drugs including lenalidomide and ibrutinib9, 37, 38, 39, 40, 41 or small molecules targeting pathways in MZL15 were still limited. In the TLN, more recently approved drugs had been documented for only a single patient (ibrutinib).
The results of this analysis showing a 5‐years OS of 77% for the overall MZL cohort are in line with those reported from other population‐based studies revealing 5‐years OS rates/estimates of 61% (UK's HMRN, 2004–2014),11 72% (SEER, 1995–2009)13 and 84% (SEER, 2009–2017),12 respectively. With 69 years in median and 46% of patients ≥70 years, the age and age distribution of our cohort is almost identical to that from SEER including patients diagnosed between 1995 and 2009 (median age of 68 years; 45% of patients ≥70 years),13 while patients from the later SEER cohort (2009–2017) are younger (median age of 64 years)12 and those from the UK's HMRN study older (median age of 72 years).11 Of note, the proportion of patients presenting with stage III/IV is almost twice as high in the overall cohort of our analysis compared to that from the SEER cohorts (67% vs. 34%12, 13), which might be explained by the fact that within SEER also patients receiving local therapy (surgery or radiotherapy) have been enrolled, while the proportion of patients requiring systemic treatment is not clear.
From SEER and the UK's HMRN study, no data on the type of systemic treatment are available. In a most recent retrospective Italian study on 65 patients with untreated MZL receiving median 6 cycles bendamustine‐rituximab, the ORR was 89%, the estimated 5‐years PFS approximately 70% and the OS at 68 months was 85%.42 Although the 5‐years OS and ORR (81%) revealed by our data are lower, the 5‐years PFS of 67% is similar and almost identical to the 66% reported from the BRIGHT trial.30 The ORR reported from the existing RCTs ranged from 91% to 100%.29, 32, 33 Any direct comparisons of such results, however, are biased: for example, with 66 years in median, patients from the Italian study are younger as compared to those from our cohort. Also, the Italian data resulted from retrospective analysis,42 while we present analyses from prospectively collected data. In addition, differences in patient characteristics between non‐selected patients from routine practice and selected patients from RCTs are much more pronounced, so that a direct comparison of both patient populations is methodically not appropriate.
Regarding the different MZL subtypes, in SEER, 5‐years OS was higher for MALT MZL (75%13/87%12) than for NMZL (64%13/78%12) and SMZL (68%13/80%12). According to our results, there was also a tendency towards a higher 5‐years OS for MALT compared to non‐MALT MZL (79% vs. 75%). However, as also reported from SEER, differences in patient demographics and clinical characteristics between the subgroups must be considered,13 for example presence of B symptoms, bone marrow involvement, lower hemoglobin levels, elevated LDH levels and Ann Arbor stage III/IV being more frequently documented in patients with non‐MALT MZL.
In the current analysis, higher age was found to significantly increase the risk of mortality (for all subgroups) confirming the important role of age as prognostic factor for MZL revealed by retrospective and prospective studies.12, 13, 34, 43, 44
5. CONCLUSION
These prospective data provide important insights into the management and survival of patients with MZL treated in German routine practice. At a time when comprehensive guidelines were missing, our findings show that rituximab‐bendamustine was the most commonly used first‐line and second‐line treatment for MALT and non‐MALT MZL. Survival rates of patients with MALT MZL tended to be slightly higher compared with those of patients with non‐MALT MZL. Age was found to be a significant prognostic factor for mortality confirming the results of previous retrospective and prospective studies. When only few clinical phase III trials are available, real world data can help optimize treatment recommendations.
CONFLICTS OF INTEREST
Wolfgang Abenhardt, Michael Koenigsmann, Christoph Maintz, Susanne Tech, Mark‐Oliver Zahn, Roland Schnell, Anja Kaiser‐Osterhues and Leonora Houet declare no conflict of interest concerning the topic of this publication. Wolfgang Knauf has received honoraria from Mundipharma and Roche for talks and the attendance of conferences. Reiner Sandner has received travel support/financial support from Roche, Teva and ribosepharm for the attendance of conferences and holds iOMEDICO shares. Norbert Marschner is Chief Executive Officer of iOMEDICO and holds shares of this company.
ETHICS STATEMENT
All experiments comply with the current laws in Germany, where they were performed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments. This study was approved by the responsible ethics committee and is registered at ClinicalTrials.gov (NCT00889798).
AUTHORS CONTRIBUTIONS
Wolfgang Knauf, Wolfgang Abenhardt, Leonora Houet, Susanne Tech, Anja Kaiser‐Osterhues and Norbert Marschner designed the research study. Wolfgang Knauf, Wolfgang Abenhardt, Michael Koenigsmann, Christoph Maintz, Reiner Sandner, Mark‐Oliver Zahn, Roland Schnell and Norbert Marschner performed the research. Susanne Tech, Leonora Houet and Anja Kaiser‐Osterhues analyzed the data. Anja Kaiser‐Osterhues wrote the paper. All the authors critically reviewed and approved the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2868.
ACKNOWLEDGEMENTS
The authors would like to thank all patients, physicians and study teams participating in the TLN. We thank Renate Grugel (iOMEDICO) for support during project management and data analysis, Dr. Martina Jänicke (iOMEDICO) for critical comments on the design of the research study and review of the manuscript, Marie Merling (iOMEDICO) and Natalie Wetzel (iOMEDICO) for their support during data analysis. The TLN is designed, managed and analyzed by iOMEDICO and has received continuous financial support from CelgeneGmbH, Mundipharma GmbH, Onkovis GmbH and Roche Pharma AG. None of the funding companies had any role in study design, data collection and analysis, interpretation of results, decision to publish, or preparation of the manuscript. The TLN‐Group collaborates with the Arbeitskreis Klinische Studien in onkologischen und hämatologischen Praxen e.V. (AKS) and the Kompetenznetz Maligne Lymphome (KML). The authors confirm that this work has not been published previously. Parts of it have been presented at the German annual meeting of the DGHO German Society for Hematology and Medical Oncology eV, 2019 (poster presentation).
Knauf W, Abenhardt W, Koenigsmann M, et al. Rare lymphomas in routine practice – Treatment and outcome in marginal zone lymphoma in the prospective German Tumour Registry Lymphatic Neoplasms. Hematol Oncol. 2021;39(3):313‐325. 10.1002/hon.2868
[Correction added on 18 May 2021, after first online publication: Peer review history statement has been added.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127(17):2082‐2092. 10.1182/blood-2015-12-624304 [DOI] [PubMed] [Google Scholar]
- 2.Al‐Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non‐Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the national cancer data base from 1998 to 2011. Am J Hematol. 2015;90(9):790‐795. 10.1002/ajh.24086 [DOI] [PubMed] [Google Scholar]
- 3.Zucca E, Arcaini L, Buske C, et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2020;31(1):17‐29. 10.1016/j.annonc.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 4.Monga N, Nastoupil L, Garside J, et al. Burden of illness of follicular lymphoma and marginal zone lymphoma. Ann Hematol. 2019;98(1):175‐183. 10.1007/s00277-018-3501-8 [DOI] [PubMed] [Google Scholar]
- 5.Sindel A, Al‐Juhaishi T, Yazbeck V. Marginal zone lymphoma: state‐of‐the‐art treatment. Curr Treat Options Oncol. 2019;20(12):90. 10.1007/s11864-019-0687-5 [DOI] [PubMed] [Google Scholar]
- 6.Campo E, Pileri SA, Jaffe ES, et al. Nodal marginal zone B‐cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised. 4th ed. Lyon, France: IARC Press; 2017:263‐265. [Google Scholar]
- 7.Cook JR, Isaacson PG, Chott A, et al. Extranodal marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue (MALT lymphoma). In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised. 4th ed. Lyon, France: IARC Press; 2017:259‐262. [Google Scholar]
- 8.Piris MA, Isaacson PI, Swerdlow S, et al. Splenic marginal zone lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised. 4th ed. Lyon, France: IARC Press; 2017:223‐225. [Google Scholar]
- 9.Denlinger NM, Epperla N, William BM. Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res. 2018;10:615‐624. 10.2147/CMAR.S133291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conconi A, Thieblemont C, Cascione L, et al. Early progression of disease predicts shorter survival in MALT lymphoma patients receiving systemic treatment. Haematologica. 2020;105(11):2592‐2597. 10.3324/haematol.2019.237990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith A, Crouch S, Lax S, et al. Lymphoma incidence, survival and prevalence 2004–2014: sub‐type analyses from the UK's haematological malignancy research network. Br J Canc. 2015;112(9):1575‐1584. 10.1038/bjc.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughn JL, Pinheiro LC, Olszewski A, Epperla N. Survival of patients with marginal zone lymphoma in the United States: a population‐based cohort study (2000 to 2017). Am J Hematol. 2021;96(4):E123‐E126. 10.1002/ajh.26103 [DOI] [PubMed] [Google Scholar]
- 13.Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma. Cancer. 2013;119(3):629‐638. 10.1002/cncr.27773 [DOI] [PubMed] [Google Scholar]
- 14.Bertoni F, Rossi D, Zucca E. Recent advances in understanding the biology of marginal zone lymphoma. F1000Res. 2018;7:406. 10.12688/f1000research.13826.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertoni F, Rossi D, Raderer M, Zucca E. Marginal zone lymphomas. Canc J 2020;26(4):336‐347. 10.1097/PPO.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 16.Ladetto M, Dreyling M. EHA endorsement of ESMO clinical practice guidelines for marginal zone lymphomas. Hemasphere. 2020;4(2):e351. 10.1097/HS9.0000000000000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knauf W, Abenhardt W, Aldaoud A, et al. Treatment of non‐transplant patients with multiple myeloma: routine treatment by office‐based haematologists in Germany ‐ data from the prospective tumour registry lymphatic neoplasms (TLN). Oncol Res Treat. 2014;37(11):635‐644. 10.1159/000368315 [DOI] [PubMed] [Google Scholar]
- 18.Knauf W, Abenhardt W, Dörfel S, et al. Routine treatment of patients with chronic lymphocytic leukaemia by office‐based haematologists in Germany‐data from the prospective tumour registry lymphatic neoplasms. Hematol Oncol. 2015;33(1):15‐22. 10.1002/hon.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knauf W, Aldaoud A, Aldaoud A, et al. Survival of non‐transplant patients with multiple myeloma in routine care differs from that in clinical trials‐data from the prospective German tumour registry lymphatic neoplasms. Ann Hematol. 2018;97(12):2437‐2445. 10.1007/s00277-018-3449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knauf W, Abenhardt W, Slawik HR, et al. Rare lymphomas in routine practice‐Treatment and outcome in Waldenström's macroglobulinaemia in the prospective German tumour registry lymphatic neoplasms. Hematol Oncol. 2020;38(3):344‐352. 10.1002/hon.2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauf W, Abenhardt W, Mohm J, et al. Similar effectiveness of R‐CHOP‐14 and ‐21 in diffuse large B‐cell lymphoma‐data from the prospective German tumour registry lymphatic neoplasms. Eur J Haematol. 2019;103(5):460‐471. 10.1111/ejh.13295 [DOI] [PubMed] [Google Scholar]
- 22.Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Contr Clin Trials. 1996;17(4):343‐346. [DOI] [PubMed] [Google Scholar]
- 23.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 24.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29‐41. 10.2307/2530286 [DOI] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 27.Olszewski AJ, Butera JN, Reagan JL, Castillo JJ. Outcomes of bendamustine‐ or cyclophosphamide‐based first‐line chemotherapy in older patients with indolent B‐cell lymphoma. Am J Hematol. 2020;95(4):354‐361. 10.1002/ajh.25707 [DOI] [PubMed] [Google Scholar]
- 28.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet. 2013;381(9873):1203‐1210. 10.1016/S0140-6736(12)61763-2 [DOI] [PubMed] [Google Scholar]
- 29.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944‐2952. 10.1182/blood-2013-11-531327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flinn IW, van der Jagt R, Kahl B, et al. First‐line treatment of patients with indolent non‐hodgkin lymphoma or mantle‐cell lymphoma with bendamustine plus rituximab versus R‐CHOP or R‐CVP: results of the BRIGHT 5‐year follow‐up study. J Clin Oncol. 2019;37(12):984‐991. 10.1200/JCO.18.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salar A, Domingo‐Domenech E, Panizo C, et al. First‐line response‐adapted treatment with the combination of bendamustine and rituximab in patients with mucosa‐associated lymphoid tissue lymphoma (MALT2008‐01): a multicentre, single‐arm, phase 2 trial. Lancet Haematol. 2014;1(3):e104‐e111. 10.1016/S2352-3026(14)00021-0 [DOI] [PubMed] [Google Scholar]
- 32.Salar A, Domingo‐Domenech E, Panizo C, et al. Long‐term results of a phase 2 study of rituximab and bendamustine for mucosa‐associated lymphoid tissue lymphoma. Blood. 2017;130(15):1772‐1774. 10.1182/blood-2017-07-795302 [DOI] [PubMed] [Google Scholar]
- 33.Iannitto E, Bellei M, Amorim S, et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: results from the phase II BRISMA/IELSG36 study. Br J Haematol. 2018;183(5):755‐765. 10.1111/bjh.15641 [DOI] [PubMed] [Google Scholar]
- 34.Thieblemont C, Cascione L, Conconi A, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409‐1417. 10.1182/blood-2017-03-771915 [DOI] [PubMed] [Google Scholar]
- 35.Conconi A, Martinelli G, Thiéblemont C, et al. Clinical activity of rituximab in extranodal marginal zone B‐cell lymphoma of MALT type. Blood. 2003;102(8):2741‐2745. 10.1182/blood-2002-11-3496 [DOI] [PubMed] [Google Scholar]
- 36.Zucca E, Copie‐Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2013;24(Suppl 6):vi144‐vi148. 10.1093/annonc/mdt343 [DOI] [PubMed] [Google Scholar]
- 37.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open‐label, phase 2 trial. Lancet Oncol. 2014;15(12):1311‐1318. 10.1016/S1470-2045(14)70455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiesewetter B, Troch M, Dolak W, et al. A phase II study of lenalidomide in patients with extranodal marginal zone B‐cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica. 2013;98(3):353‐356. 10.3324/haematol.2012.065995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiesewetter B, Willenbacher E, Willenbacher W, et al. A phase 2 study of rituximab plus lenalidomide for mucosa‐associated lymphoid tissue lymphoma. Blood. 2017;129(3):383‐385. 10.1182/blood-2016-06-720599 [DOI] [PubMed] [Google Scholar]
- 40.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224‐2232. 10.1182/blood-2016-10-747345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiesewetter B, Lamm W, Neuper O, Mayerhoefer ME, Simonitsch‐Klupp I, Raderer M. Prolonged follow‐up on lenalidomide‐based treatment for mucosa‐associated lymphoid tissue lymphoma (MALT lymphoma)‐Real‐world data from the Medical University of Vienna. Hematol Oncol. 2019;37(4):345‐351. 10.1002/hon.2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morigi A, Argnani L, Lolli G, et al. Bendamustine‐rituximab regimen in untreated indolent marginal zone lymphoma: experience on 65 patients. Hematol Oncol. 2020;38(4):487‐492. 10.1002/hon.2773 [DOI] [PubMed] [Google Scholar]
- 43.Florindez JA, Alderuccio JP, Reis IM, Lossos IS. Splenic marginal zone lymphoma: a US population‐based survival analysis (1999–2016). Cancer. 2020;126(21):4706‐4716. 10.1002/cncr.33117 [DOI] [PubMed] [Google Scholar]
- 44.Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127(17):2064‐2071. 10.1182/blood-2015-12-624296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


