Abstract
Background
In this feasibility study we aimed to evaluate the value of previously reported molecular tumor biomarkers associated with lymph node metastasis in oral squamous cell carcinoma (OSCC) to optimize neck strategy selection criteria.
Methods
The association between expression of cortactin, cyclin D1, FADD, RAB25, and S100A9 and sentinel lymph node status was evaluated in a series of 87 (cT1‐2N0) patients with OSCC treated with primary resection and SLNB procedure.
Results
Tumor infiltration depth and tumor pattern of invasion were independent prognostic markers for SLN status, while none of the tumor makers showed a better prognostic value to replace SLNB as neck staging technique in the total cohort. However, in the subgroup of patients with pT1N0 OSCC, cortactin expression (OR 16.0, 95%CI 2.0–127.9) was associated with SLN classification.
Conclusions
Expression of cortactin is a promising immunohistochemical tumor marker to identify patients at low risk that may not benefit from SLNB or END.
Keywords: lymph node classification, molecular marker, oral cancer, oral squamous cell carcinoma, sentinel lymph node biopsy
1. INTRODUCTION
A positive lymph node classification (N‐classification) is one of the most important prognostic factors for shorter disease‐specific and disease‐free survival in oral squamous cell carcinoma (OSCC).1 In OSCC, metastatic cells will drain to regional lymph nodes located in cervical neck levels.2 Despite a clinically negative neck, patients with early stage OSCC (cT1‐2N0) have a reported 23%–37% risk for occult (preoperatively undetected) lymph node metastasis.3, 4, 5
The sentinel lymph node biopsy (SLNB) has been introduced in the last decade as diagnostic technique for the detection of occult metastasis, in order to select patients with early stage OSCC for a neck dissection or a clinical follow‐up strategy.1 In the Netherlands, patients with a metastasis‐positive sentinel lymph node (SLN) currently receive a modified radical neck dissection (MRND) in a second surgical procedure.6, 7 A meta‐analysis reported a high pooled sensitivity (87%) and negative predictive value (94%) for detecting occult metastasis using the SLNB procedure.8 Advantages of the SLNB are individual drainage pattern assessment9 and more comprehensive histological assessment of the lymph node allowing the detection of micrometastasis (0.2–2 mm) and isolated tumor cells (ITCs, metastasis size <0.2 mm).7 Despite these advantages, the SLNB is still an invasive staging procedure. Preoperative selection of patients at risk for occult metastasis using molecular tumor profiling might lead to a more individualized approach with a watchful waiting strategy for low‐risk patients,10 a SLNB for intermediate‐risk patients and a neck dissection for patients with a high risk.11 The additional and more detailed information on individual drainage patterns, micrometastasis, and ITCs provided by the SLNB might have impact on the clinical value of molecular biomarkers that have previously been associated with N‐classification.12 Currently, only few studies reported the value of clinicopathological tumor markers to predict N‐classification in a cohort of patients with early stage OSCC who underwent neck staging using the SLNB procedure12, 13, 14 with clinical follow‐up as reference for the SLNB negative patients. Only one study validated the clinical value of CD44 as a molecular tumor biomarker, but did not find an association with N‐classification.11
Before the introduction of the SLNB procedure, patients with a pT1cN0 tumor and a tumor infiltration depth <4 mm were selected for a watchful waiting strategy instead of an elective neck dissection.15 Tumor profiling using molecular biomarkers to predict N‐classification in OSCC has been studied before. One such a well‐studied genetic alteration in head and neck squamous cell carcinomas that has been associated with N‐classification is amplification of chromosome 11q13.16, 17, 18, 19 Three genes (CTTN, CCND1, and FADD) located in this commonly amplified region have previously been validated for N‐classification in a well‐defined early stage OSCC cohort with neck staging by END. A negative predictive value (NPV) of 80%–84% was reported.17 Other promising markers associated with N‐classification in OSCC were RAB25 and S100A9 hypermethylation and expression levels, markers selected using a genome wide methylation assay20 (Clausen, S100A9, manuscript in preparation).
These molecular biomarkers (cortactin, cyclin D1, FADD, RAB25, and S100A9) have been reported as potential prognostic and predictive tumor markers in early stage OSCC, but have not been studied in relation to SLNB‐staged patients. The aim of this study was to analyze the additional clinical value of combining classical prognostic factors (tumor stage and tumor infiltration depth) with molecular tumor biomarkers in order to select patients for a watchful waiting or SLNB procedure as neck strategy using preoperative tumor biopsies of patients with early stage OSCC who underwent neck staging using the SLNB procedure.
2. MATERIAL AND METHODS
2.1. Patients and treatment
Patients diagnosed with cT1‐2N0 and pT1‐2 OSCC (7th TNM classification), treated by primary surgical resection and neck staging with SLNB between 2008 and 2017, were selected for analysis. These patients were treated in the University Medical Center Groningen (UMCG) (n = 101) and the Medical Center Leeuwarden (MCL) (n = 12). Most of these patients (n = 91) were part of a clinical study assessing the accuracy of the SLNB procedure.7 Clinical and histopathological data were retrospectively collected from the electronical patient files (Table 1). Pathological reports are standardized in our centers and contain data on tumor infiltration depth, tumor pattern of invasion, extranodal extension, perineural invasion, lymphovascular invasion, and N‐classification. In case of missing histopathological data, tumors or lymph nodes were reassessed by a pathologist (BvdV). Eighty‐seven of the 113 tumors were suitable for biomarker analysis (see tissue microarray construction below). Twenty‐six (30%) of these 87 patients had a positive SLN and received a modified radical neck dissection in a second operation. SLNB negative patients (70%) had a clinical follow‐up of the neck. Four SLN negative patients were diagnosed with a regional recurrence without local disease during follow‐up (false negatives) after 8, 9, 18, and 22 months. Ten patients received postoperative radiotherapy, which was combined with chemotherapy in two patients indicated by involved surgical resection margins, multiple positive lymph nodes, or extranodal extension. The median follow‐up time was 35 months (IQR 20–49 months). Thirteen (15%) patients deceased during the follow‐up of which two died as a result of the OSCC.
TABLE 1.
Patient and tumor characteristics and true N‐classification
| True N negative | True N positive | p‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Total | 57 (66) | 30 (34) | |
| Sex | |||
| Male | 30 (53) | 12 (40) | 0.367 |
| Female | 27 (47) | 18 (60) | |
| Age at first treatment | |||
| Median (IQR) | 64 (57–71) | 66 (59–75) | 0.302 |
| Site | |||
| Tongue | 31 (54) | 21 (70) | 0.209 |
| Floor of mouth | 19 (33) | 4 (13) | |
| Cheek | 3 (5) | 4 (13) | |
| Others | 4 (7) | 1 (3) | |
| pT status (7th TNM) | |||
| 1 | 50 (88) | 19 (63) | 0.012 |
| 2 | 7 (12) | 11 (37) | |
| Tumor infiltration depth (mm) | |||
| Median (IQR) | 3.5 (2.0–5.0) | 5.0 (3.6–7.0) | 0.007 |
| Tumor infiltration depth | |||
| <4.0 mm | 31 (54) | 9 (30) | 0.042 |
| ≥4.0 mm | 26 (46) | 21 (70) | |
| Perineural invasion | |||
| No | 55 (97) | 24 (80) | 0.018 |
| Yes | 2 (3) | 6 (20) | |
| Lymphovascular invasion | |||
| No | 53 (93) | 25 (83) | 0.265 |
| Yes | 4 (7) | 5 (17) | |
| Tumor grade | |||
| Well | 19 (33) | 6 (20) | 0.222 |
| Moderate | 38 (67) | 24 (80) | |
| Tumor pattern of invasion | |||
| Pushing | 42 (64) | 11 (37) | 0.001 |
| Infiltrative | 15 (25) | 19 (60) | |
| Cortactin | |||
| Low expression | 43 (75) | 18 (60) | 0.148 |
| High expression | 14 (25) | 12 (40) | |
| Cyclin D1 | |||
| Low expression | 26 (46) | 11 (37) | 0.497 |
| High expression | 31 (54) | 19 (63) | |
| FADD | |||
| Low expression | 39 (70) | 15 (50) | 0.101 |
| High expression | 17 (30) | 15 (50) | |
| RAB25 | |||
| Low expression | 23 (40) | 8 (27) | 0.245 |
| High expression | 34 (60) | 22 (73) | |
| S100A9 nuclear | |||
| Low expression | 17 (30) | 10 (33) | 0.809 |
| High expression | 40 (70) | 20 (67) | |
| S100A9 cytoplasmic | |||
| Low expression | 23 (40) | 10 (33) | 0.643 |
| High expression | 34 (60) | 20 (67) | |
Note: True N‐classification is determined by the combination of postoperative pathological lymph node classification (pN) combined with regional recurrence (false negatives). Four patients with a negative SLNB (pN0) were diagnosed with a regional recurrence and counted as true N positives. Molecular expression was dichotomized for cortactin, cyclin D1, RAB25, and S100A9 using a ROC‐analysis. FADD was semiquantitatively scored. In bold: significant different variables (p ≤ 0.05).
Abbreviations: IQR, interquartile range; TNM, American Joint Committee of Cancer TNM classification.
The SLNB protocol used was described before.7 Briefly, 1‐day before surgery a radioactive tracer (99mTc‐nannocolloid) was peritumorally injected, followed by dynamic and static lymphoscintigraphy and Single Photon Emission Computed Tomography (SPECT)–CT scanning. The next day during surgery, SLNs were located and harvested using a handheld gamma‐probe. Postoperatively, the SLNs were histopathological assessed with step‐serial‐sectioning with an interval of 500 μm resulting in an average of six slides. Slides from each level were stained for hematoxylin and eosin (HE) and additional cytokeratin (CK AE1‐ AE3, clone AE1/AE3, Ventana Medical Systems, Tucson, AZ).
2.2. Tissue microarray construction
All HE slides of the primary tumor were revised by a dedicated head and neck pathologist to assess tumor cell presence. Twenty‐six of the 113 tumors were too small or had no redundant tissue available for TMA construction. Five other tumors were not suitable for inclusion in the TMA as a result of limited tumor size, but could be assessed using whole tumor slide sections. Finally, 87 cases were available for analysis (82 tumors on two TMA blocks and five whole tumor slide sections). The TMA construction procedure in our center has been described earlier.21 Briefly, tumors were marked on the HE slides by a pathologist, after which three 0.6 mm cores were taken from the corresponding donor FFPE blocks and added to the recipient TMA FFPE block using a Manual Tissue Arrayer I (Beecher Instruments, Sun Prairie, WI). Both TMA blocks had a unique lay‐out with normal tissue samples of liver, testis, cervix, colon and placenta incorporated into the TMAs as negative and positive control tissues. Three micrometer thick sections were cut from the TMA blocks for the immunohistochemical staining of the markers. For IHC staining, the first and last sections were HE stained to confirm presence of tumor in the cores of TMA sections.
2.3. Immunohistochemistry
The staining procedures and antibodies have been described before20, 22, 23 and are summarized in Table 2. Cyclin D1 was automatically stained using the Ventana Benchmark Ultra (Ventana Medical Systems, Tucson, AZ). For the other markers: after deparaffinization in xylene and rehydration in a graded alcohol series, sections were treated for antigen retrieval followed by endogenous peroxidase blockage by incubating in 0.3% peroxide solution. Slides were then incubated for 1 h with the primary Mouse anti human antibody, followed by 30 min incubation of a Rabbit anti Mouse (RAMpo) secondary antibody and 30 min incubation of a Goat anti Rabbit tertiary antibody (GARpo). All antibodies were Horseradish Peroxidase conjugated and diluted in 1% BSA–PBS serum (primary antibody) or diluted in 1:100 1% BSA–PBS–1% AB serum (RAMpo and GARpo). Slides were developed with 3,3‐di‐amniobenzidine (DAB) chromogen solution (DAKO, Glostrup, Denmark) and counterstained with hematoxylin. HE, cyclin D1, and FADD stained glass slides were digitized using the NanoZoomer 2.0 HT with a 40× magnification lens and the NDP.view 2 Viewing software U12388‐01 (Hamamatsu Photonics, Hamamatsu City, Shizuoku, Japan). Cortactin, RAB25, and S100A9 slides were digitized using the IntelliSite Ultra Fast Scanner with the IntelliSite Image Management System software (Phillips Electronics, Best, the Netherlands).
TABLE 2.
Antibody information
| Antibody | Clone | Company | Dilution |
|---|---|---|---|
| Cortactin | 30 | BD BIOSCIENCES | 1:1000 |
| Cyclin D1 | SP4‐R | Roche | |
| FADD | A66‐2 | BD Biosciences | 1:100 |
| RAB25 | 3F12F3 | Santa Cruz Biotechnology | 1:50 |
| S100A9 | S36.48 | BMA biomedicals | 1:100 |
All molecular biomarkers were scored as described earlier17, 20, 22, 23 (Clausen, S100A9, in preparation). Cortactin and cyclin D1 were scored as the percentage of tumor cells with a higher cytoplasmic expression (cortactin) or nuclear expression (cyclin D1) compared to normal epithelium or surrounding tissue cells. FADD was scored semi‐quantitatively as tumor cells with negative (0), weakly positive (+), positive (++), or strong positive (+++) cytoplasmic expression. RAB25 was scored as percentage of tumor cells with negative (−), moderate positive (+), or strong positive (++) cytoplasmic expression. S100A9 was scored for both percentage of tumor cells with any nuclear (−/+) expression or percentage of any cytoplasmic (−/+) expression. Markers were independently scored by two observers (KB with MM or LSM). Discordances were discussed with a dedicated head and neck pathologist (BvdV) until consensus was reached. Except for FADD, optimal cut‐offs between high and low expression of the markers and N‐classification were determined using a ROC curve and the average expression of the cores per tumor. The cut‐offs were 46% of cytoplasmic expression for cortactin, 73% of nuclear expression for cyclin D1, 4% of any expression (+, ++) of RAB25 and 17% of nuclear expression and 55% of cytoplasmic expression for S100A9. For FADD, cases with at least one core scored as ++ or +++ were defined as high expression. Representative examples of low and high expression detected by immunohistochemical staining are shown in Figure 1.
FIGURE 1.
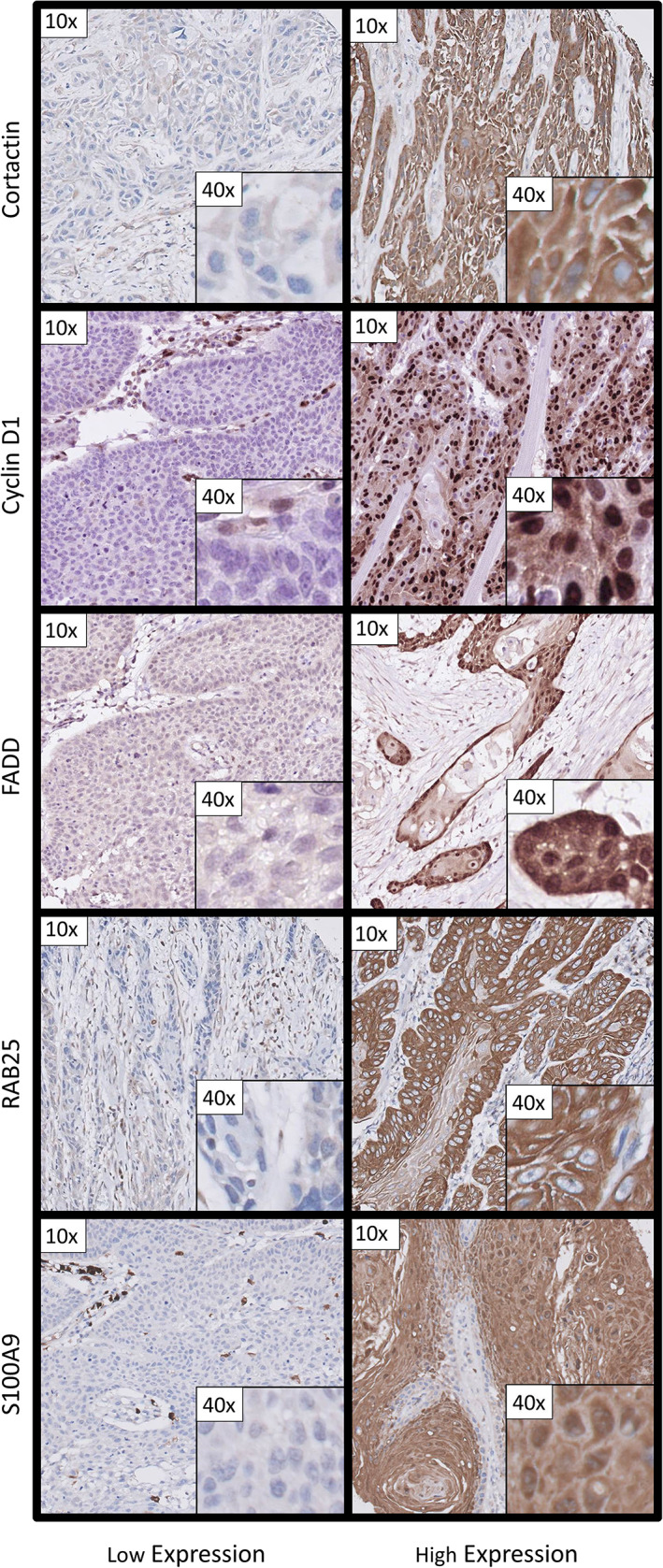
Representative examples of low and high expression on tissue microarryas constructed of OSCC primary tumor tissue for the biomarkers used in this study. Examples are given with a 10× and 40× magnification [Color figure can be viewed at wileyonlinelibrary.com]
2.4. Ethical considerations
This study used retrospective data and leftover tumor tissue which was collected during treatment according to the national guidelines for oral cavity cancer. Therefore, this research was not a clinical study with human subjects as meant in the Medical Research Involving Human Subjects Act as was concluded by the local Medical Ethics Review Board of the University Medical Center Groningen (M18.225755) and no further approval was required.
2.5. Statistics
Numbers (n) with corresponding percentages, mean with standard deviation (SD) and median with interquartile range (IQR) are given for respectively categorical data, normally distributed data and skewed distributed data. Associations between categorical data were tested using the Fisher's exact test or chi‐square test. The Student's t test (normally distribution) or Mann–Whitney U test (skewed distribution) was used for the associations between groups with continuous data. True N‐classification positive (N+) was defined as patients with histological proven metastasis in their SLN (pN+) Also, the four patients with a negative SLNB (pN0) and a regional recurrence without local disease during their follow‐up were considered as true N‐classification positive. Associations with true N‐classification were multivariable tested using a stepwise binary logistic regression model in which variables with a p‐value ≤0.1 from an univariable analysis were included. The homogeneity of the tumor markers was tested by calculating the intraclass correlation coefficient (ICC) using only cases with three scorable TMA cores. An ICC of 0.61 (substantial correlation) or higher was used as cut‐off between a homogeneous or heterogeneous expression of the marker.17, 24 In case of homogeneity (ICC > 0.61) also cases with one of two cores were included. In 88% (FADD) or more (other biomarkers) of the tumors on the TMAs were two or three cores available (Data S1, Supporting Information). The ICC analysis using cases with three available cores showed the lowest ICC of 0.66 for RAB25 and the highest of 0.86 for FADD, representing respectively a substantial and almost perfect reproducibility of measurements (Data S1). Therefore, all molecular tumor markers were considered as homogeneous and cases with one or two cores on the TMA were included for further analyses. The threshold for significant differences was a p‐value ≤0.05. All statistical analyses were performed using IBM SPSS Statistics 23 (Statistical Package for the Social Sciences, Inc., Chicago, IL).
3. RESULTS
Twenty‐six patients had a positive SLN. Four patients with a negative SLNB were diagnosed with a regional recurrence during follow‐up. These 30 (34%) patients were considered as true N positives. The other 57 (66%) were considered as true N negatives (Table 1).
A univariable analysis of associations between true N‐classification and clinicopathological and molecular tumor biomarkers showed significant associations for pT‐classification (p = 0.012), tumor infiltration depth (p = 0.007), perineural invasion (p = 0.018), and infiltrative tumor pattern of invasion (p = 0.001) (Table 1). In a multivariable analysis with tumor infiltration depth as dichotomous variable (4 mm cut‐off), pT‐classification (OR 3.6, 95%CI 1.1–11.3) and infiltrative tumor pattern of invasion (OR 4.4, 95%CI 1.7–11.7) were found to be independent factors for true N‐classification. When tumor infiltration depth is treated as continuous variable in a multivariable analysis, both tumor infiltration depth (OR 1.3, 95%CI 1.1–1.6) and tumor pattern of invasion (OR 5.0, 95%CI 1.8–13.5) were independent variables. Expression levels of the molecular tumor biomarkers cortactin, cyclin D1, FADD, RAB25, and S100A9 were not associated with true N‐classification in the total cohort of patients with cT1‐2N0 OSCC with SLNB neck staging (Table 1).
Today, patients with OSCC with a low risk of occult metastasis, defined by pT1cN0 and a tumor infiltration depth <4 mm, surgical excision of the tumor is followed by a watchful waiting strategy of the neck. In a subgroup of 33 patients with both a pT1cN0 and a tumor infiltration depth <4 mm, SLNB revealed that 6 (18%) had a positive true N‐classification, whereas 24 (44%) of the 54 patients with pT1 or pT2 tumors and a tumor infiltration depth ≥4 mm were truly N‐classification positive (p = 0.019). To investigate whether these same biomarkers were predictive in this subgroup with a low risk of occult metastasis, we found that cortactin (OR 16.0, 95%CI 2.0–127.9) and FADD (OR 8.8, 95%CI 1.2–62.2) expression were strongly associated with true N‐classification. Cortactin and FADD showed overexpression in the same four patients with a positive true N‐classification. Cortactin expression showed a sensitivity of 67% and NPV of 92% as predictive marker for true N‐classification in these 33 low‐risk patients (Table 3). Both cortactin and FADD had a false negative rate of 33% (2/2 + 4). In other words, in the 6 out of 33 low‐risk patients with true positive lymph nodes identified by SLNB, 4 cases were also found to be overexpressing cortactin on the same tumor specimen used to determine tumor infiltration depth and would have had an incorrect neck staging strategy with watchful waiting only (Figure 2). On the other hand, cortactin expression would only identify 2 of 26 patients in the low‐risk group with pT1cN0 and tumor infiltration depth <4 mm as false negative lymph node classification identified by SLNB (Figure 2). None of these 33 patients had a regional recurrence after pN0 neck staging by SLNB resulting in a NPV of 100% for SLNB in these low‐risk group.
TABLE 3.
Molecular biomarkers in pT1cN0‐staged OSCC with a tumor infiltration depth <4 mm
| True N negative | True N positive | p‐value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Cortactin | |||
| Low expression | 24 (89) | 2 (33) | 0.011 |
| High expression | 3 (11) | 4 (67) | |
| Cyclin D1 | |||
| Low expression | 13 (48) | 2 (33) | 0.665 |
| High expression | 14 (52) | 4 (67) | |
| FADD | |||
| Low expression | 22 (82) | 2 (43) | 0.034 |
| High expression | 5 (18) | 4 (57) | |
| RAB25 | |||
| Low expression | 15 (56) | 4 (67) | 1.000 |
| High expression | 12 (44) | 2 (33) | |
| S100A9 nuclear | |||
| Low expression | 18 (67) | 4 (67) | 1.000 |
| High expression | 9 (33) | 2 (33) | |
| S100A9 cytoplasmic | |||
| Low expression | 14 (52) | 4 (67) | 0.665 |
| High expression | 13 (48) | 2 (33) | |
Note: True N‐classification is determined by the combination of postoperative pathological lymph node classification (pN) combined with regional recurrence (false negatives). Patients with a negative SLNB (pN0) and diagnosed with a regional recurrence were counted as true N positives. Cortactin and FADD expression were significantly associated with true N‐classification in pT1cN0‐staged OSCC and a tumor infiltration depth <4 mm with the highest sensitivity of 67% [4/(4 + 2)] and negative predictive value of 92% [24/(24 + 2)] for cortactin. In bold: significant different variables (p ≤ 0.05).
FIGURE 2.
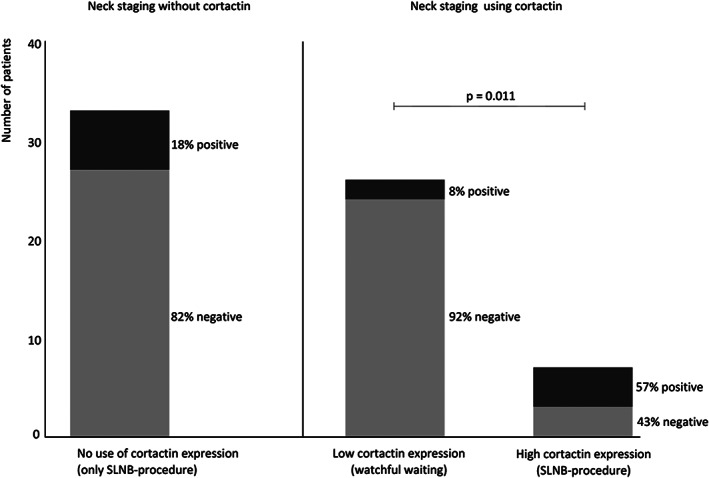
Differences between neck staging using only the sentinel lymph node biopsy procedure or combined with cortactin expression levels. The differences in selection of neck staging procedures in 33 patients with a pT1cN0 OSCC and a tumor infiltration depth <4 mm with and without using cortactin expression. These 33 patients had neck staging using SLNB, while 82% had no neck lymph node involvement (negative true N‐classification). If these patients were selected for a watchful waiting procedure using cortactin expression, 8% out of 26 patients with a low cortactin expression would have been false negatives (positive true N‐classification) and 43% out of 7 patients with a high cortactin expression and without lymph node involvement (negative true N‐classification) would have had neck staging using SLNB procedure
4. DISCUSSION
SLNB is nowadays used as neck staging strategy in early stage (cT1‐2N0) OSCC. Although the SLNB procedure is less invasive compared to the conventional END, it is still an invasive staging technique while in ~70% of these early stage OSCCs no lymph node involvement is observed. In this study we analyzed the additional clinical value of molecular tumor biomarkers to select patients with early stage OSCC with a low risk of occult metastasis for a watchful waiting strategy instead of performing a SLNB or END procedure.
In the total cohort of cT1‐2N0, in univariable analysis true N‐classification was significantly associated with pT classification, tumor infiltration depth, perineural invasion and tumor pattern of invasion, as was reported previously.25, 26 In multivariable analysis tumor infiltration depth and tumor pattern of invasion were independent predictors for true N‐classification. In the total cohort, none of the molecular markers was associated with true N‐classification as determined by SLNB. An earlier study, with 78 patients with OSCC with SLNB neck staging, also reported a significant association between tumor pattern of invasion and N‐classification; however, they did not find an association for tumor infiltration depth as categorical variable.14 Recently, the same research group reported a significant association between tumor infiltration depth as continuous variable and regional recurrences in 92 patients with early stage OSCC staged by SLNB.11 Moreover, they stated that tumor infiltration depth ≤2 mm might be used to select patients for watchful waiting, 2–5 mm for a SLNB and >5 mm for an END.11 Similar to our findings, Morand et al. did not find an association between another lymph node associated tumor marker (CD44 expression) and SLNB status.11 In a large cohort with data of 199 patients with early stage OSCC with SLNB neck staging from two Dutch head and neck centers, a cut‐off of 3.4 mm was reported as most optimal (sensitivity 83%, specificity 47%) between tumor infiltration depth and N‐classification based on a ROC‐curve analysis.12 Because a 15% risk for metastasis below the cutoff, the authors stated that tumor infiltration depth could not serve as an optimal predictive marker to select patients for a watchful waiting strategy instead of a SLNB procedure.12 In a randomized clinical trial studying END versus watchful waiting and only performing MRND in case of conversion of the neck, it was found that survival rates were better for END.27 In that study depth of invasion of the primary tumor was the only factor that was significantly associated with node positivity. A marked increase in cumulative lymph‐node positivity was observed with increasing depth of invasion from 3 mm (5.6%) to 4 mm (16.9%).
Although individual clinical, histopathological and molecular markers are poor predictors for regional metastasis in OSCC compared to SLNB, a combination of these types of markers might have clinical value to select low‐risk patients for watchful waiting instead of an invasive procedure. In a subgroup of patients at low risk for lymph node metastasis, defined by pT1cN0 and tumor infiltration depth <4 mm who were conventionally selected for a watchful waiting strategy instead of an END,15 six patients were found with SLNB as true lymph node positive and of these four were identified by cortactin and FADD overexpression. Of the true lymph node negative patients, 24 out of 27 were associated with low cortactin expression (92% NPV). No association was found between any of the molecular markers and the true N‐classification in the total cohort or between the molecular markers and pT1 or pT2 tumors with a tumor infiltration depth ≥4 mm. Our data suggests that using cortactin expression as a predictive marker on tumor specimen, the number of patients with an incorrect negative neck classification and consequently should have been treated more aggressively, decreases from 6/33 (18%) to 2/26 (8%). A false omission rate (FOR = 1‐NPV) of 8% (1%–92%) in the lymph node negative classified low‐risk patients using cortactin expression is similar compared to reported FOR of 6% for early stage OSCC (cT1‐2N0) in SLNB negative patients in a meta‐analysis of 66 studies8 and to the reported FOR of 7% for both SLNB and END in a large multicenter retrospective study.28 However, the FNR of 33% in this study for cortactin in low‐risk OSCC is much higher compared to FNR of 19% for SLNB and 16% for END in the large retrospective study28 and compared to a 100% SLNB accuracy in these 33 patients. Although SLNB had a very high accuracy in these low‐risk patients, it is still an invasive technique of which many hospitals around the world lack equipment and knowledge.29, 30 Especially for those hospitals, cortactin expression is a low cost and easy to perform technique and therefore might contribute to select patients with low‐risk OSCC for watchful waiting instead of an END. The relatively high FNR rate for cortactin could be explained by the low numbers in this subgroup and indicate that independent studies are needed to confirm our data indicating that cortactin expression is a reliable and noninvasive alternative for neck staging in patients with low‐risk OSCC.
In 2012, a diagnostic algorithm for early stage OSCC using gene‐expression profiling of tumor biopsy specimens to select low‐risk patients for a watchful waiting strategy instead of a SLNB procedure has been proposed.10 An 89% NPV for selecting patients for a watchful waiting strategy instead of SLNB was observed. In that study, 11% false negatives in patients selected as N‐classification negative by gene expression profile but with lymph node involvement was found.10 S100A9 was the only molecular biomarker of the current study which was also part of that gene expression profile. The difference with our study is that we used cortactin in combination with a tumor infiltration depth cut‐off of 4 mm defined in a large cohort of OSCC to predict N‐classification.15 Without cortactin, the risk for positive N‐classification is 18% in pT1cN0 and with cortactin that risk lowered to 8%. If we used the 3.4 mm cut‐off as reported by den Toom12 in combination with or without cortactin in our pT1cN0 patients, the metastasis risk lowered from 18% to 9% (data not shown). These data show that infiltration depth between 3 and 5 mm did not significantly affect the risk showing the potential additional value of cortactin to select patients with a low metastases risk for a watchful waiting strategy instead of a SLNB.
Tumor infiltration depth is not routinely measured preoperatively nowadays, a recent meta‐analysis showed high potential of ultrasound for preoperative tumor thickness measurement of clinically T1‐T2‐staged tongue tumors (Pearson correlation 0.82, p < 0.001) with an overestimation of 0.5 mm for ultrasound compared to histopathological measurement.31 Although there is a difference between tumor infiltration depth and tumor thickness, that meta‐analysis stated that tumor thickness is in general larger than its infiltration depth thereby preventing these patients from under‐treatment of the neck.31
Cortactin and FADD were both significantly associated with true N‐classification in pT1 patients with a tumor infiltration depth <4 mm and both genes are located at the 11q13 chromosome which is frequently amplified in HNSCC.19, 32 Cortactin had the highest predictive value in this study and is a candidate driver gene of the 11q13 amplification.23 Cortactin has many binding sites and interactions with other proteins which play an important role in metastasis as was reviewed in 2019.16 Cortactin plays a key role in the regulation of protrusive structures such as invadopodia and lamellipodia that enable respectively the invasion and migration of tumor cells by changing the actin cytoskeleton.16, 33, 34, 35 Also in vitro studies showed that cell migration of OSCC cell lines was regulated by cortactin expression.35, 36, 37 The pooled data of nine studies with patients with OSCC showed an association between N‐classification or expression of CTTN/cortactin with an OR 2.78 (95%CI 1.68–4.60) (reviewed in Ramos‐Garcia et al.38). FADD expression was also associated with lymph node classification in low‐risk patients. The main biological function of the phosphorylated‐isoform of FADD (pFADD) is controlling cell cycle by regulating many cellular processes involving apoptosis, cell survival, division, tumor progression, necrosis, and autophagy.39 Expression of pFADD in HNSCC is associated with local control upon radiotherapy in early‐stage glottic carcinomas40 in line with this biological function. In a cohort of HNSCC we demonstrated that expression of the (nonphosphorylated) FADD was associated with higher incidence of lymph node metastasis at presentation22 and FADD expression was associated with lymph node status in early stage (cT1‐2N0) OSCC.17
In the current study, we have shown that especially cortactin expression has additional clinical value in pT1cN0 patients by identifying a subset of watchful waiting patients with lower risk for lymph node metastases with a NPV of 92%. This is similar to the pooled NPV of 94% for the SLNB procedure in a meta‐analysis.8 Our study is based on a relatively small cohort, but our findings warrant further research with large prospective data to validate the selection of low‐risk patients for a watchful waiting strategy using cortactin expression in tumor biopsy specimens of OSCC tumors.
This is one of the first studies using SLNB data for the validation of molecular tumor biomarkers associated with lymph node classification. Although this study is limited by a relatively small number of patients compared to non‐SLNB OSCC studies,15, 25, 41 it shows that cortactin expression might have additional value in preoperative neck strategy decisions in a subgroup of patients with early stage OSCC. Independent studies are needed to confirm these observations.
5. CONCLUSION
None of the analyzed clinical, histopathological, or molecular markers were better prognostic markers to replace SLNB as neck staging technique overall. However, this study showed the potential association between cortactin and lymph node classification in patients with pT1cN0 OSCC with a tumor infiltration depth <4 mm and SLNB neck staging who were conventionally selected for a watchful waiting strategy instead of an END. A combination of a tumor size smaller than 20 mm, a tumor infiltration depth smaller than 4 mm, and low cortactin expression might have clinical value to select patients for a watchful waiting strategy instead of subjecting patients to a SLNB procedure. Further prospective research is needed to confirm the clinical value of cortactin as a predictive marker next to the SLNB procedure in one neck staging protocol in early stage OSCC.
Supporting information
Supplementary data 1 Number of cores per tumor for each marker and corresponding intraclass correlation coefficient.
Boeve K, Mastik MF, Slagter‐Menkema L, et al. Cortactin expression assessment improves patient selection for a watchful waiting strategy in pT1cN0‐staged oral squamous cell carcinomas with a tumor infiltration depth below 4 mm. Head & Neck. 2021;43(9):2688–2697. 10.1002/hed.26746
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.de Bree R, Nieweg OE. The history of sentinel node biopsy in head and neck cancer: from visualization of lymphatic vessels to sentinel nodes. Oral Oncol. 2015;51:819‐823. [DOI] [PubMed] [Google Scholar]
- 2.de Bree R, Takes RP, Shah JP, et al. Elective neck dissection in oral squamous cell carcinoma: past, present and future. Oral Oncol. 2019;90:87‐93. [DOI] [PubMed] [Google Scholar]
- 3.Schilling C, Stoeckli SJ, Haerle SK, et al. Sentinel European Node Trial (SENT): 3‐year results of sentinel node biopsy in oral cancer. Eur J Cancer. 2015;51:2777‐2784. [DOI] [PubMed] [Google Scholar]
- 4.Broglie MA, Haile SR, Stoeckli SJ. Long‐term experience in sentinel node biopsy for early oral and oropharyngeal squamous cell carcinoma. Ann Surg Oncol. 2011;18:2732‐2738. [DOI] [PubMed] [Google Scholar]
- 5.Ganly I, Patel S, Shah J. Early stage squamous cell cancer of the oral tongue—clinicopathologic features affecting outcome. Cancer. 2012;118:101‐111. [DOI] [PubMed] [Google Scholar]
- 6.Den Toom IJ, Heuveling DA, Flach GB, et al. Sentinel node biopsy for early‐stage oral cavity cancer: the VU University Medical Center experience. Head Neck. 2015;37:573‐578. [DOI] [PubMed] [Google Scholar]
- 7.Boeve K, Schepman KP, van der Vegt B, et al. Lymphatic drainage patterns of oral maxillary tumors: Approachable locations of sentinel lymph nodes mainly at the cervical neck level. Head & Neck. 2017;39:486‐491. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Wang SJ, Yang X, Peng H. Diagnostic efficacy of sentinel lymph node biopsy in early oral squamous cell carcinoma: a meta‐analysis of 66 studies. PLoS One. 2017;12:e0170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Toom IJ, Boeve K, van Weert S, et al. High rate of unexpected lymphatic drainage patterns and a high accuracy of the sentinel lymph node biopsy in oral cancer after previous neck treatment. Oral Oncol. 2019;94:68‐72. [DOI] [PubMed] [Google Scholar]
- 10.Leusink FK, van Es RJ, de Bree R, et al. Novel diagnostic modalities for assessment of the clinically node‐negative neck in oral squamous‐cell carcinoma. Lancet Oncol. 2012;13:e554‐e561. [DOI] [PubMed] [Google Scholar]
- 11.Morand GB, Ikenberg K, Vital DG, et al. Preoperative assessment of CD44‐mediated depth of invasion as predictor of occult metastases in early oral squamous cell carcinoma. Head Neck. 2019;41:950‐958. [DOI] [PubMed] [Google Scholar]
- 12.Toom IJ, Janssen LM, RJJ vE et al. Depth of invasion in patients with early stage oral cancer staged by sentinel node biopsy. Head & Neck. 2019;41:2100‐2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goda H, Okamoto M, Nakashiro KI, Hino S, Murase R, Hamakawa H. Prognostic impact of preoperative serum interleukin‐6 levels in patients with early‐stage oral squamous cell carcinoma, defined by sentinel node biopsy. Oncol Lett. 2017;14:7965‐7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goerkem M, Braun J, Stoeckli SJ. Evaluation of clinical and histomorphological parameters as potential predictors of occult metastases in sentinel lymph nodes of early squamous cell carcinoma of the oral cavity. Ann Surg Oncol. 2010;17:527‐535. [DOI] [PubMed] [Google Scholar]
- 15.Melchers LJ, Schuuring E, van Dijk BA, et al. Tumour infiltration depth ≥4 mm is an indication for an elective neck dissection in pT1cN0 oral squamous cell carcinoma. Oral Oncol. 2012;48:337‐342. [DOI] [PubMed] [Google Scholar]
- 16.Ramos‐Garcia P, Gonzalez‐Moles MA, Gonzalez‐Ruiz L, et al. An update of knowledge on cortactin as a metastatic driver and potential therapeutic target in oral squamous cell carcinoma. Oral Dis. 2019;25:949‐971. [DOI] [PubMed] [Google Scholar]
- 17.Noorlag R, Boeve K, Witjes MJ, et al. Amplification and protein overexpression of cyclin D1: predictor of occult nodal metastasis in early oral cancer. Head Neck. 2017;39:326‐333. [DOI] [PubMed] [Google Scholar]
- 18.Gibcus JH, Menkema L, Mastik MF, et al. Amplicon mapping and expression profiling identify the Fas‐associated death domain gene as a new driver in the 11q13.3 amplicon in laryngeal/pharyngeal cancer. Clin Cancer Res. 2007;13:6257‐6266. [DOI] [PubMed] [Google Scholar]
- 19.Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes—a review. Gene. 1995;159:83‐96. [DOI] [PubMed] [Google Scholar]
- 20.Clausen MJ, Melchers LJ, Mastik MF, et al. RAB25 expression is epigenetically downregulated in oral and oropharyngeal squamous cell carcinoma with lymph node metastasis. Epigenetics. 2016;11:653‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melchers LJ, Bruine de Bruin L, Schnell U, et al. Lack of claudin‐7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2013;49:998‐1005. [DOI] [PubMed] [Google Scholar]
- 22.Pattje WJ, Melchers LJ, Slagter‐Menkema L, et al. FADD expression is associated with regional and distant metastasis in squamous cell carcinoma of the head and neck. Histopathology. 2013;63:263‐270. [DOI] [PubMed] [Google Scholar]
- 23.Gibcus JH, Mastik MF, Menkema L, et al. Cortactin expression predicts poor survival in laryngeal carcinoma. Br J Cancer. 2008;98:950‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinn S. Statistics in respiratory medicine. 2. Repeatability and method comparison. Thorax. 1991;46:454‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen SR, Johansen J, Sorensen JA, Krogdahl A. The prognostic significance of histological features in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38:657‐662. [DOI] [PubMed] [Google Scholar]
- 26.Chinn SB, Spector ME, Bellile EL, et al. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013;149:893‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node‐negative oral cancer. N Engl J Med. 2015;373:521‐529. [DOI] [PubMed] [Google Scholar]
- 28.den Toom IJ, Boeve K, Lobeek D, et al. Elective neck dissection or sentinel lymph node biopsy in early stage oral cavity cancer patients: the Dutch experience. Cancers (Basel). 2020;12:1783. 10.3390/cancers12071783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schilling C, Shaw R, Schache A, et al. Sentinel lymph node biopsy for oral squamous cell carcinoma. Where are we now? Br J Oral Maxillofac Surg. 2017;55:757‐762. [DOI] [PubMed] [Google Scholar]
- 30.Cramer JD, Sridharan S, Ferris RL, Duvvuri U, Samant S. Sentinel lymph node biopsy versus elective neck dissection for stage I to II oral cavity cancer. Laryngoscope. 2019;129:162‐169. [DOI] [PubMed] [Google Scholar]
- 31.Klein Nulent TJW, Noorlag R, Van Cann EM, et al. Intraoral ultrasonography to measure tumor thickness of oral cancer: a systematic review and meta‐analysis. Oral Oncol. 2018;77:29‐36. [DOI] [PubMed] [Google Scholar]
- 32.Noorlag R, van Kempen PM, Stegeman I, Koole R, van Es RJ, Willems SM. The diagnostic value of 11q13 amplification and protein expression in the detection of nodal metastasis from oral squamous cell carcinoma: a systematic review and meta‐analysis. Virchows Arch. 2015;466:363‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Artym VV, Zhang Y, Seillier‐Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034‐3043. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15‐33. [DOI] [PubMed] [Google Scholar]
- 35.Schuuring E, Verhoeven E, Litvinov S, Michalides RJ. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v‐src substrate and is located in cell‐substratum contact sites. Mol Cell Biol. 1993;13:2891‐2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Damme H, Brok H, Schuuring‐Scholtes E, Schuuring E. The redistribution of cortactin into cell‐matrix contact sites in human carcinoma cells with 11q13 amplification is associated with both overexpression and post‐translational modification. J Biol Chem. 1997;272:7374‐7380. [DOI] [PubMed] [Google Scholar]
- 37.Rothschild BL, Shim AH, Ammer AG, et al. Cortactin overexpression regulates actin‐related protein 2/3 complex activity, motility, and invasion in carcinomas with chromosome 11q13 amplification. Cancer Res. 2006;66:8017‐8025. [DOI] [PubMed] [Google Scholar]
- 38.Ramos‐Garcia P, Gonzalez‐Moles MA, Ayen A, Gonzalez‐Ruiz L, Ruiz‐Avila I, Gil‐Montoya JA. Prognostic and clinicopathological significance of CTTN/cortactin alterations in head and neck squamous cell carcinoma: systematic review and meta‐analysis. Head Neck. 2019;41:1963‐1978. [DOI] [PubMed] [Google Scholar]
- 39.Tourneur L, Chiocchia G. FADD: a regulator of life and death. Trends Immunol. 2010;31:260‐269. [DOI] [PubMed] [Google Scholar]
- 40.Schrijvers ML, Pattje WJ, Slagter‐Menkema L, et al. FADD expression as a prognosticator in early‐stage glottic squamous cell carcinoma of the larynx treated primarily with radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 41.Woolgar JA, Beirne JC, Vaughan ED, Lewis‐Jones HG, Scott J, Brown JS. Correlation of histopathologic findings with clinical and radiologic assessments of cervical lymph‐node metastases in oral cancer. Int J Oral Maxillofac Surg. 1995;24:30‐37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1 Number of cores per tumor for each marker and corresponding intraclass correlation coefficient.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


