Abstract
Oral semaglutide is the first oral glucagon‐like peptide‐1 receptor agonist for the treatment of type 2 diabetes, and showed significant benefits in glycaemic control and weight reduction versus active comparators in the PIONEER phase 3a randomized controlled trial programme. In this retrospective study, we present early data on the use of oral semaglutide in clinical practice, from the US IBM Explorys electronic health record database. In 782 patients prescribed oral semaglutide, 54.5% were women, and the mean age (SD) was 57.8 years (11.3); 66.0% of patients received their prescription from a primary care practitioner. Although prescribing information recommends increasing the dose to 7 mg after 30 days, 37.0% of patients received a prescription only for the initial 3 mg dose. Mean body mass index was 36.2 kg/m2 (7.6); mean HbA1c was 8.4% (1.8%). Mean HbA1c change from baseline to approximately 6 months after oral semaglutide initiation was −0.9% (95% CI: −1.1%; −0.6%), with greater reductions in patients with higher baseline HbA1c. These data indicate prevalent early adoption of oral semaglutide in primary care, show real‐world improvements in glycaemic control, and identify potential treatment gaps.
Keywords: antidiabetic drug, database research, GLP‐1, glycaemic control, observational study, type 2 diabetes
1. INTRODUCTION
Oral semaglutide (Rybelsus; Novo Nordisk) is the first glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) developed for oral administration for the treatment of type 2 diabetes (T2D), and it has been approved by the US Food and Drug Administration1 and the European Medicines Agency.2 The efficacy and safety of oral semaglutide were assessed in the Peptide InnOvatioN for Early diabEtes tReatment (PIONEER) programme of 10 phase 3a randomized controlled trials (RCTs), which included more than 9500 patients.3 This programme studied the incorporation of oral semaglutide at multiple stages of the T2D treatment pathway: early therapy, add‐on to oral therapy and addition to insulin. Oral semaglutide showed significant improvements in glycaemic control and weight reduction versus various comparator arms representing common treatment classes in T2D,4 is based on a molecule with a favourable cardiovascular (CV) risk‐benefit profile,5 and was shown to be non‐inferior to placebo in terms of CV safety in the PIONEER 6 trial.6 The US prescribing information for oral semaglutide specifies initiation with a 3 mg starting dose for 30 days, followed by escalation to the 7 mg treatment dose, with further escalation to 14 mg after another 30 days if needed for additional glycaemic control.7
Insights into the real‐world use of oral semaglutide are needed to further understand and support clinical decision‐making. The current study, InvestiGating New InitiaTors on oral semaglutidE in routine clinical practice (IGNITE), was designed to examine how trial data have been translated to US clinical practice during the early period of oral semaglutide availability, using electronic health record (EHR) data. We sought to evaluate the first patterns of routine clinical use of oral semaglutide, and to assess patients' clinical characteristics and glycaemic control.
2. METHODS
This was a retrospective, observational cohort study, using EHRs extracted from the IBM Explorys EHR database (IBM Watson Health, Armonk, NY, USA).8 Only de‐identified secondary data were used; therefore, approval by an ethics committee was not required. The study period began on 28 October 2019, with database lock on 15 December 2020. For inclusion, adult patients (aged ≥18 years) required a diagnosis of T2D (Table S1) and at least one prescription for oral semaglutide (index date; Figure S1). Patients with type 1 diabetes or gestational diabetes were excluded.
Demographic characteristics at index date, and baseline co‐morbidities and antidiabetic medication prescriptions (365 days preindex), were collected for the full cohort. Baseline HbA1c, weight and cholesterol measurements (90 days preindex) were recorded for patients with available data. HbA1c trajectory before and after index date was plotted (Figure S1); to ensure that the same patients were assessed longitudinally, only data from patients who had measurements available at three or more 3‐monthly time points were included (see the Figure 1 footnotes for full details).
FIGURE 1.
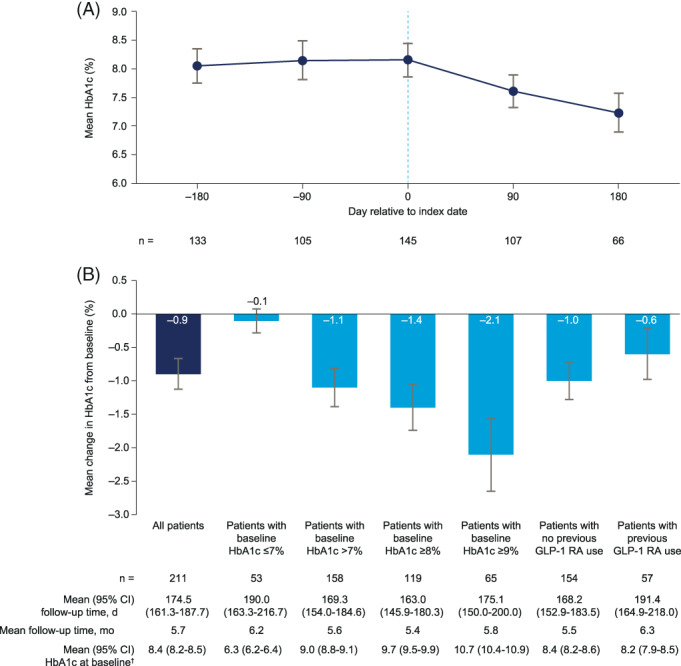
A, HbA1c trajectory and B, HbA1c changes from baseline. The HbA1c trajectory was constructed by extracting HbA1c measurements in five non‐overlapping time periods relative to the index date: day −180 +/−45 days, day −90 +/−45 days, day 0 (index date) −45/+14 days, day 90 +/−45 days and day 180 +/−45 days. For each patient and time period, the measurement closest to the target day was used. Only patients with data for three or more time points were included in the HbA1c trajectory dataset. The dotted line indicates index date. †Mean HbA1c for all patients in the study meeting this criterion. GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist
Changes from baseline in HbA1c were assessed in all patients with a baseline measurement, and at least one measurement at least 60 days postindex. For patients with more than one postindex HbA1c measurement, the most recent measurement was used. Descriptive statistics were then computed for the change from baseline in HbA1c and for follow‐up time. Change in HbA1c from baseline was also assessed in subgroups according to baseline HbA1c, and in patients with and without use of injectable GLP‐1 RAs in the 3 months preindex.
3. RESULTS
3.1. Study population and baseline characteristics
3.1.1. Full population
In total, 782 eligible patients were identified (Table 1). The mean age was 57.8 years (standard deviation [SD]: 11.3), and 426 patients (54.5%) were women. Most patients were Caucasian (558/782; 71.4%) and 181/782 (23.1%) were African American. In line with previous studies using the Explorys database,9 the majority of patients were from the US Midwest (503/782; 64.3%) or South (274/782; 35.0%). Overall, 516/782 patients (66.0%) were prescribed oral semaglutide by a primary care practitioner.
TABLE 1.
Baseline characteristics for the IGNITE study cohort
| Patients with a prescription for oral semaglutide | |
|---|---|
| Demographic characteristics (N = 782) | |
| Age at index date, mean (SD) | 57.8 (11.3) |
| Sex (men/women), % | 45.5/54.5 |
| Race, n (%) | |
| African American | 181 (23.1) |
| Asian | 21 (2.7) |
| Caucasian | 558 (71.4) |
| Other/unknown | 22 (2.8) |
| Region, n (%) | |
| Midwest | 503 (64.3) |
| South | 274 (35.0) |
| Other | 5 (0.6) |
| Provider speciality, n (%) | |
| Endocrinology | 135 (17.3) |
| Primary care practitioner | 516 (66.0) |
| Other/unknown | 131 (16.8) |
| Clinical characteristics a (baseline period: 3 mo) | |
| Weight, kg (N = 689) | 104.9 (24.0) |
| BMI (N = 687) | |
| Mean (SD), kg/m2 | 36.2 (7.6) |
| <30 kg/m2, n (%) | 154 (22.4) |
| 30‐<35 kg/m2, n (%) | 186 (27.1) |
| ≥35 kg/m2, n (%) | 347 (50.5) |
| HbA1c (N = 499) | |
| Mean (SD), % | 8.4 (1.8) |
| ≤7%, n (%) | 112 (22.4) |
| 7‐<10%, n (%) | 303 (60.7) |
| ≥10%, n (%) | 84 (16.8) |
| <8%, n (%) | 236 (47.3) |
| ≥8%, n (%) | 263 (52.7) |
| <9%, n (%) | 356 (71.3) |
| ≥9%, n (%) | 143 (28.7) |
| LDL cholesterol level (N = 297) | 91.4 (43.8) |
| ≥70 mg/dL, n (%) | 204 (68.7) |
| Time with T2D in the databaseb, years (N = 782) | 6.9 (4.8) |
| Co‐morbidities (N = 782; baseline period: 12 mo) | |
| Charlson co‐morbidity index scorea, mean (SD) | 2.43 (2.11) |
| Number of co‐morbidities, mean (SD) | 2.3 (1.5) |
| Any microvascular condition, n (%) | 232 (29.7) |
| Diabetic retinopathy, n (%) | 47 (6.0) |
| Diabetic neuropathy, n (%) | 150 (19.2) |
| Diabetic nephropathy, n (%) | 98 (12.5) |
| Established CVDc, n (%) | 224 (28.6) |
| Hypertension, n (%) | 573 (73.3) |
| Stroke, n (%) | 45 (5.8) |
| Acute MI, n (%) | 30 (3.8) |
| Antidiabetic medications (N = 782; baseline period: 12 mo) | |
| Number of glucose‐lowering agents | 2.1 (1.7) |
| No antidiabetic medications | 156 (19.9) |
| Baseline medication, n (%) | |
| Biguanides | 456 (58.3) |
| Sulphonylureas | 232 (29.7) |
| Thiazolidinediones | 61 (7.8) |
| DPP‐4is | 152 (19.4) |
| SGLT‐2is | 199 (25.4) |
| Any GLP‐1 RA | 179 (22.9) |
| Dulaglutide | 80 (10.2) |
| Liraglutide | 64 (8.2) |
| Injectable semaglutide | 48 (6.1) |
| Exenatide | 18 (2.3) |
| Lixisenatide | 6 (0.8) |
| Any insulin | 190 (24.3) |
| Long‐acting insulin | 168 (21.5) |
| Fast‐acting insulin | 114 (14.6) |
| Oral semaglutide dosing (N = 782) | |
| Initial oral semaglutide dose, n (%) | |
| 3 mg | 404 (51.7) |
| 3 and 7 mg | 112 (14.3) |
| 3, 7 and 14 mg | 5 (0.6) |
| 7 mg | 201 (25.7) |
| 7 and 14 mg | 8 (1.0) |
| 14 mg | 41 (5.2) |
| Unknown | 11 (1.4) |
| Highest oral semaglutide dose, n (%) | |
| 3 mg | 289 (37.0) |
| 7 mg | 379 (48.5) |
| 14 mg | 106 (13.6) |
Note: Data are mean (SD) except where otherwise stated.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; DPP‐4i, dipeptidyl peptidase‐4 inhibitor; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; LDL, low‐density lipoprotein; MI, myocardial infarction; SD, standard deviation; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitor; T2D, type 2 diabetes.
Last measurement or score available before index date.
Time with T2D in the database was the closest approximation of disease duration available from a data source such as this.
Included conditions were ischaemic heart disease, pulmonary embolism, transient cerebral ischaemic attacks and related syndromes, presence of cardiac and vascular implants and grafts, cardiomyopathy, cardiac arrest, atrial fibrillation, cardiac arrhythmia, heart failure, cerebrovascular disease, atherosclerosis, peripheral vascular disease, embolism and thrombosis.
The mean number of co‐morbidities in the 12 months preindex was 2.3 (SD: 1.5), and the mean Charlson co‐morbidity index score was 2.4 (SD: 2.1). In total, 573/782 patients (73.3%) had hypertension, 224/782 (28.6%) had established atherosclerotic cardiovascular disease and 232/782 (29.7%) had at least one microvascular complication of diabetes (neuropathy: 150/232; nephropathy: 98/232; retinopathy: 47/232) (Table 1).
On average, patients had prescriptions for two antidiabetic medications in the 12 months preindex (mean: 2.1 [SD: 1.7]). Most patients had a prescription for biguanides (456/782; 58.3%), and smaller proportions had prescriptions for sulphonylureas (232/782; 29.7%), sodium‐glucose co‐transporter‐2 inhibitors (199/782; 25.4%) and insulin (190/782; 24.3%). In total, 179/782 patients (22.9%) had prescriptions for another GLP‐1 RA (dulaglutide: 80/179; liraglutide: 64/179; injectable semaglutide: 48/179) (Table 1).
3.1.2. Subset with baseline clinical data
For patients with a measurement in the baseline period, mean weight was 104.9 kg (SD: 24.0; n = 689) and mean body mass index (BMI) was 36.2 kg/m2 (SD: 7.6). Most patients met the criteria for obesity: 77.6% had a BMI of 30 kg/m2 or higher. Mean HbA1c was 8.4% (SD: 1.8%; n = 499); 112/499 patients (22.4%) had HbA1c less than 7% and 84/499 (16.8%) had HbA1c of 10% or higher. Mean time with T2D noted in the Explorys database, used to estimate approximate disease duration, was 6.9 years (SD: 4.8; Table 1).
3.2. Oral semaglutide dosing
The majority of patients had a first prescription that included the 3 mg dose: 404/782 (51.7%) had a first prescription for 3 mg alone, 112/782 (14.3%) had prescriptions for 3 and 7 mg, and 5/782 (0.6%) had prescriptions for 3, 7 and 14 mg. However, 201/782 patients (25.7%) had an initial prescription recorded for 7 mg alone, 41/782 (5.2%) had a prescription for 14 mg alone, and 8/782 (1.0%) had a prescription for both 7 and 14 mg. Overall, 289/782 patients (37.0%) were prescribed 3 mg as their highest dose, of whom 36/289 received their prescription during the last 6 weeks of the study period (1 November to 15 December 2020). Only 106/782 patients (13.6%) were prescribed the 14 mg dose.
3.3. HbA1c trajectory
For patients with HbA1c measurements for at least three time points during baseline and follow‐up, mean HbA1c was 8.1% (95% confidence interval [CI]: 7.7%‐8.4%; n = 133) 6 months preindex, and 8.2% both at 3 months preindex (7.8%‐8.5%; n = 105) and at index date (7.9%‐8.5%; n = 145) (Figure 1A). Mean HbA1c was 7.6% (7.3%‐7.9%; n = 107) 3 months after oral semaglutide initiation and was 7.2% (6.9%‐7.6%) in the smaller group of patients with data 6 months after initiation (n = 66).
3.4. HbA1c changes from baseline
In patients with eligible baseline and follow‐up measurements (n = 211; 27.0% of full cohort; see Table S2 for baseline characteristics), the mean HbA1c change from baseline to follow‐up (mean: 5.7 months) was −0.9% (95% CI: −1.1%; −0.6%; Figure 1B). Greater HbA1c reductions were observed in patients with HbA1c more than 7% at baseline (−1.1% [−1.4%; −0.8%]), in patients with HbA1c of 8% or higher (−1.4% [−1.8%; −1.1%]), and in patients with HbA1c of 9% or higher (−2.1% [−2.6%; −1.5%]). HbA1c reductions from baseline were also observed in GLP‐1 RA‐naïve patients (−1.0% [−1.3%; −0.7%]; n = 154), and in patients who had switched to oral semaglutide from another GLP‐1 RA (−0.6% [−1.0%; −0.2%]; n = 57).
4. DISCUSSION
In this report, we describe the characteristics of some of the first patients prescribed oral semaglutide in US clinical practice, and present evidence of improvements in glycaemic control. This analysis also identifies strengths and gaps in translation of clinical innovations to care settings. The PIONEER programme showed the glycaemic efficacy of oral semaglutide across the full treatment continuum of T2D, and our data on baseline medications indicate adoption across the treatment spectrum. The majority of patients were prescribed oral semaglutide by a primary care practitioner. This is unusual for a newly available antidiabetic drug, which would typically be favoured by specialist practitioners. Obesity rates were generally higher in the study cohort than in the PIONEER programme; mean BMI was 36.2 kg/m2 in our study, compared with 31.8, 32.8, 32.5 and 31.5 kg/m2 in PIONEER 1,10 2,11 312 and 7,13 respectively. This may indicate that practitioners perceive a specific benefit of this class and therapy for patients with T2D and obesity. Baseline age and HbA1c were similar in our study and the PIONEER programme; however, a key difference was that 22.9% of patients had switched from another GLP‐1 RA. Future real‐world studies of oral semaglutide offer a valuable opportunity to assess this subgroup, which was not included in the PIONEER programme.
In total, 34% of patients had no prescription for the 3 mg starting dose. This could be attributable to switching from another GLP‐1 RA, which permits initiation at a higher dose,7 or to patients receiving oral semaglutide samples, which were not captured in this dataset. The latter possibility would cause underestimation of changes in HbA1c from baseline because initiation of oral semaglutide for these patients would be earlier than the recorded index date. It is also notable that HbA1c reductions were observed, even though 26.1% of this cohort did not have a dose escalation beyond the initial 3 mg dose, indicating that further improvements might be achieved with escalation to the highest recommended dose of 14 mg. This is supported by the results of the PIONEER 7 trial, a flexible dosing study comparing oral semaglutide with sitagliptin 100 mg. All patients initiated the 3 mg dose of oral semaglutide, with the potential for dose adjustment to 7 or 14 mg at subsequent eight‐weekly intervals, based on HbA1c and gastrointestinal tolerability. Overall, 59% of patients on oral semaglutide at week 52 were receiving the 14 mg dose, 30% were receiving 7 mg and only 9% were receiving 3 mg. Overall, mean HbA1c reduction in the oral semaglutide group at this time point was −1.3% (standard error [SE]: 0.1) for the treatment policy estimand and −1.4% (SE: 0.1) for the trial product estimand.13 Although it is not possible to gather comprehensive information on the rationale underlying clinical decision‐making in a retrospective study, the fact that some patients in the current study received prescriptions only for the 3 mg initial dose may indicate an opportunity for education on dose escalation in the clinical care setting. Finally, the higher HbA1c reductions observed for patients with higher baseline HbA1c levels are consistent with data from the PIONEER programme highlighting the glycaemic benefit of oral semaglutide against comparators,4 across the full range of baseline HbA1c.
At present, the sample available to assess the use of oral semaglutide is small and highly sensitive to variation, and with several factors affecting the generalizability of the data. Explorys data are captured mainly from large integrated delivery networks, which may bias the study population towards those with more severe disease. Similarly, follow‐up HbA1c measurements may be an indicator of closer monitoring because of greater disease severity, meaning that our glycaemic control data reflect this particular patient subgroup. To better assess the clinical impact and comparative effectiveness of oral semaglutide, and to overcome limitations of a prestudy/poststudy design, future studies including more patients will be designed to compare both HbA1c and weight in matched patient cohorts initiating different antidiabetic medications. Furthermore, throughout 2020, the coronavirus 2019 (COVID‐19) pandemic has imposed limitations on the uptake and availability of healthcare appointments,14 which is likely to be another source of bias in our study sample. This may partly explain delays in dose escalation; however, the extent to which the pandemic has affected these patients may never be fully understood. Finally, our analysis does not assess medication‐taking behaviour or adverse events (AEs) in patients receiving oral semaglutide. In future, the inclusion of administrative claims in a similar analysis can be used to assess persistence and adherence to medication. However, the limitations of the retrospective study design and data source mean that common AEs such as nausea are unlikely to be captured accurately and systematically, resulting in under‐reporting of AE incidence. Therefore, even once larger real‐world sample sizes are available, RCTs will continue to be the most reliable source of safety data for oral semaglutide.
Our analysis highlights patterns of early use of oral semaglutide in routine clinical practice. The first initiators of oral semaglutide have a high rate of obesity and other co‐morbidities, have diverse treatment backgrounds, and experience improved glycaemic control after treatment initiation. The comparatively large proportion of patients prescribed 3 mg as their highest dose suggests that, in patients for whom oral semaglutide proves to be well tolerated, dose escalation to 7 and 14 mg, in line with the clinical trial programme and prescribing information, may provide even greater improvements in glycaemic control. These data highlight an opportunity to bridge existing treatment and education gaps to fully realize the potential of oral GLP‐1 RA therapy. Continued evaluation of real‐world data will provide further insight on the translation, uptake and impact of such innovations in routine care.
CONFLICT OF INTEREST
V.R.A. has performed consultancy for Applied Therapeutics, Duke, Novo Nordisk, Pfizer and Sanofi, and has received research support via institutional contracts from Applied Therapeutics/Medpace, Eli Lilly, Premier/Fractyl, Novo Nordisk and Sanofi/Medpace. Her spouse has been employed at Janssen and Merck. M.F. is an employee and shareholder of Novo Nordisk A/S. S.L. is employed as a consultant by Novo Nordisk A/S. J.N. is an employee of Novo Nordisk. M.L.W. is an employee and shareholder of Novo Nordisk A/S. I.L. has received research funding, advisory/consulting fees and/or other support from AstraZeneca, Bayer, Boehringer Ingelheim, Duke CRI, Eli Lilly, GI Dynamics, Intercept, Intarcia, Janssen, Mannkind, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, TARGETPharma, Valeritas and Zealand Pharma.
AUTHOR CONTRIBUTIONS
V.R.A., M.F., M.L.W. and I.L. were investigators for the IGNITE study. All authors contributed to study design and data interpretation, and S.L. performed modelling and data analysis. All authors carried out critical review and revision of the manuscript. All authors have approved the final version for submission. M.F. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14453.
Supporting information
Table S1. Codes used to identify type 2 diabetes in the 2 years before index date
Table S2. Baseline characteristics for patients with baseline and follow‐up HbA1c measurements
Figure S1. Study design
ACKNOWLEDGEMENTS
The authors acknowledge the medical writing assistance of Caroline Freeman of Oxford PharmaGenesis, Oxford, UK (funded by Novo Nordisk A/S). Certain data used in this study were supplied by International Business Machines Corporation. Any analysis, interpretation or conclusion based on these data is solely that of the authors and not International Business Machines Corporation (IBM). This study was funded by Novo Nordisk A/S.
Aroda VR, Faurby M, Lophaven S, Noone J, Wolden ML, Lingvay I. Insights into the early use of oral semaglutide in routine clinical practice: The IGNITE study. Diabetes Obes Metab. 2021;23(9):2177–2182. 10.1111/dom.14453
DATA AVAILABILITY STATEMENT
Data supporting the findings of this study are under licence from IBM. Aggregate data are available from the authors upon reasonable request.
REFERENCES
- 1.Food and Drug Administration . Drug approval package: RYBELSUS. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/213051Orig1s000TOC.cfm. Accessed January 8, 2021.
- 2.European Medicines Agency . Rybelsus. Summary of product characteristics. 2020. https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf. Accessed January 8, 2021.
- 3.Rodbard HW, Dougherty T, Taddei‐Allen P. Efficacy of oral semaglutide: overview of the PIONEER clinical trial program and implications for managed care. Am J Manag Care. 2020;26:S335‐S343. [DOI] [PubMed] [Google Scholar]
- 4.Medicine Matters® Diabetes . A quick guide to the PIONEER trials. 2020. https://diabetes.medicinematters.com/en-GB/semaglutide/cardiovascular-outcomes/a-quick-guide-to-the-pioneer-trials/16877792. Accessed January 8, 2021.
- 5.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 6.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841‐851. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration . Highlights of prescribing information. RYBELSUS (semaglutide) tablets, for oral use. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf. Accessed January 8, 2021.
- 8.IBM Explorys EHR Solutions . What IBM explorys database & analytic tools can do for you. https://www.ibm.com/uk-en/marketplace/explorys-ehr-data-analysis-tools. Accessed March 4, 2020.
- 9.Sullivan SD, Bailey TS, Roussel R, et al. Clinical outcomes in real‐world patients with type 2 diabetes switching from first‐ to second‐generation basal insulin analogues: comparative effectiveness of insulin glargine 300 units/mL and insulin degludec in the DELIVER D+ cohort study. Diabetes Obes Metab. 2018;20:2148‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724‐1732. [DOI] [PubMed] [Google Scholar]
- 11.Rodbard HW, Rosenstock J, Canani LH, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42:2272‐2281. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieber TR, Bode B, Mertens A, et al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open‐label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7:528‐539. [DOI] [PubMed] [Google Scholar]
- 14.Wake DJ, Gibb FW, Kar P, et al. Endocrinology in the time of COVID‐19: remodelling diabetes services and emerging innovation. Eur J Endocrinol. 2020;183:G67‐G77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Codes used to identify type 2 diabetes in the 2 years before index date
Table S2. Baseline characteristics for patients with baseline and follow‐up HbA1c measurements
Figure S1. Study design
Data Availability Statement
Data supporting the findings of this study are under licence from IBM. Aggregate data are available from the authors upon reasonable request.


