Abstract
Severe asthma is a heterogeneous disease with different phenotypes based on clinical, functional or inflammatory parameters. In particular, the eosinophilic phenotype is associated with type 2 inflammation and increased levels of interleukin (IL)‐4, IL‐5 and IL‐13). Monoclonal antibodies that target the eosinophilic inflammatory pathways (IL‐5R and IL‐5), namely mepolizumab, reslizumab, and benralizumab, are effective and safe for severe eosinophilic asthma. Eosinophils threshold represents the most indicative biomarker for response to treatment with all three monoclonal antibodies. Improvement in asthma symptoms scores, lung function, the number of exacerbations, history of late‐onset asthma, chronic rhinosinusitis with nasal polyposis, low oral corticosteroids use and low body mass index represent predictive clinical markers of response. Novel Omics studies are emerging with proteomics data and exhaled breath analyses. These may prove useful as biomarkers of response and non‐response biologics. Moreover, future biomarker studies need to be undertaken in paediatric patients affected by severe asthma. The choice of appropriate biologic therapy for severe asthma remains challenging. The importance of finding biomarkers that can predict response continuous an open issue that needs to be further explored. This review describes the clinical effects of targeting the IL‐5 pathway in severe asthma in adult and paediatric patients, focusing on predictors of response and non‐response.
1. INTRODUCTION
Asthma is a common chronic disease and affects approximately 315 million people worldwide, with an estimated 3%–10% of asthma patients suffering from the severe form of the disease.1 Severe asthma is defined as asthma that remains uncontrolled despite adherence with GINA steps 4–5 treatment (high‐dose ICS and LABA or leukotriene modifier) and optimal treatment of contributing factors or asthma that worsens when high‐dose treatment is decreased.2
Due to severe and difficult to control symptoms and numerous medication side effects, severe asthma represents a substantial burden for affected patients, increasing the risk of frequent exacerbations, hospital admissions and resulting in high healthcare costs and a decreased quality of life of patients and their families.3, 4
Severe asthma is a heterogeneous disease with different phenotypes based on clinical, functional or inflammatory parameters.5 Among them, the eosinophilic phenotype represents a well‐recognized condition that involves T‐helper 2 and innate lymphoid cells activation and leads to abnormal production of type 2 cytokines (Interleukin (IL)‐4, IL‐5 and IL‐13).6, 7 For this reason, most of the new biological treatments target eosinophilic inflammatory pathways and particularly IL‐5, the main mediator of eosinophilic inflammation.
IL‐5 exerts its effect by binding the alpha chain of its specific receptor (IL‐5R), regulating eosinophils promotion, migration, maturation and survival.13, 14 Upon IL‐5 activation, eosinophils degranulate and release cytotoxins with antimicrobial effects inducing damage to surrounding cells and tissue.15 Targeting IL‐5 or IL‐5R with monoclonal antibodies has a prominent role in the pathogenic evolution of severe asthma, it reduces eosinophilia, and it is an effective alternative in patients with severe asthma and uncontrolled symptoms. These drugs offer a new perspective for patients with severe asthma, who are not fully responsive to standard treatments.
This review aims to summarize the clinical effects of treatments that target the IL‐5 pathway in severe asthma, to describe predictors of response, and to discuss knowledge gaps in non‐response mechanisms.
2. CLINICAL EFFECTS OF ANTI‐IL5 TREATMENTS
Eosinophilic asthma in adults is a generally a well‐characterized phenotype. The presence of eosinophilic inflammation in the airways is associated with disease severity, increased risk of exacerbations, airway hyper‐responsiveness and worsening of symptom control.16, 17, 18 Once eosinophils migrate into the lungs, they have a pivotal effect on the airway type‐2 inflammation, by promoting the activation of the innate and adaptive immune response.15 There are currently three biologics approved for severe asthma treatment, targeting specifically IL‐5 and IL5‐Rα: mepolizumab and reslizumab are both monoclonal antibodies targeting IL‐5, while benralizumab targets IL‐5Ra (Figure 1).
FIGURE 1.
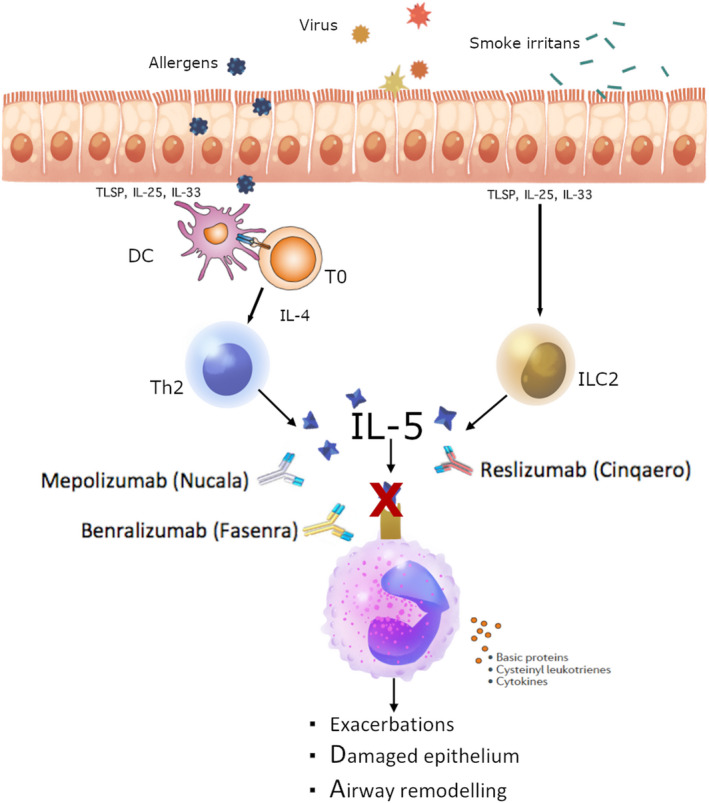
Anti‐IL5/IL5Rα mechanism of action. With permission from NSAN (www.nordstar‐NSAN.com). Monoclonal antibodies inhibit eosinophils functions directly neutralizing IL‐5 (Mepolizumab and Reslizumab) or targeting and blocking the IL‐5 receptor on eosinophils surface (Benralizumab)
Biological treatments targeting IL‐5 and IL‐5Rα have recently been approved for paediatric severe asthma; therefore, it is important to include this patient group as well. Since molecular pathways underlying severe asthma seem to differ between the paediatric population and adults, clinical effects and predictors or response might vary. For example, in contrast to the adult population, blood eosinophils or airway eosinophils might not always equal type‐2 inflammation9 in the paediatric population, and features of type‐2 inflammation seem to be age‐dependent.10 In children, airway Th2 cytokines, including interleukin (IL)‐5 and IL‐13, have been difficult to detect and levels vary between patients.8, 11, 12
2.1. Mepolizumab
The FDA approved mepolizumab in 2014 for the treatment of severe eosinophilic asthma. Mepolizumab prevents the binding of IL‐5 to IL‐5Rα, consequently inhibiting eosinophils maturation in the bone marrow and their activation and mobilization into the lungs.19, 20 Mepolizumab has been approved for severe asthmatics over 6 years old, with an eosinophil concentration of 150/μl at screening, or 300/μl over the previous 12 months. For adults, It is administered subcutaneously (SC) every 4 weeks in a fixed dose of 100 mg,21 for children 40 mg every 4 weeks. A multi‐centre double‐blind, placebo‐controlled trial, The Dose Ranging Efficacy And safety with Mepolizumab (DREAM) study,22 showed that intravenous Mepolizumab was effective and tolerable, especially for patients with severe eosinophilic asthma, but there was no improvement in lung function and symptoms score. Efficacy was measured by the reduction in the frequency of exacerbations, while safety was assessed by the number of adverse events.
Consequently, the steroid reduction with mepolizumab study (SIRIUS),23 a randomized double‐blind clinical trial that included severe asthma patients from 12 years old, confirmed that mepolizumab was able to reduce exacerbations by 32%, to improve asthma symptoms of 52% as well as in reducing the use of oral corticosteroids.
The mepolizumab as adjunctive therapy in patients with severe asthma (MENSA) study was the first randomized, double‐blind trial to prove that the administration of mepolizumab subcutaneously or intravenously, is linked to a reduction in asthma exacerbations and to an improvement of asthma control markers,24 and it also showed some moderate clinical effect in improving lung function (the mean increase from baseline FEV1 after bronchodilation was +146 and +138 ml in the intravenous mepolizumab group and in the subcutaneous mepolizumab group, respectively). In these studies, that enrolled 567 patients among teenagers and adults, the main factors influencing the overall number of exacerbations were as follows: blood eosinophil count at screening, numbers of exacerbations in the year prior to screening, and baseline maintenance oral corticosteroid use. Later on, the open‐label extension study COSMOS25 confirmed the safety and efficacy of subcutaneous mepolizumab in patients with severe eosinophilic asthma. In this study, mepolizumab was administered for 52 weeks, to the same patients who had completed the MENSA and SIRIUS trials. Safety assessment included the registration of adverse events, while efficacy included the annualized exacerbation rate and durability of response (defined as the reduction in frequency of exacerbations and the reduction in oral corticosteroids use). Subsequently, the MUSCA trial, a phase IIIb randomized, double‐blind, placebo‐controlled, parallel group, multi‐centre trial26 was designed to demonstrate the efficacy of mepolizumab as an add‐on therapy in patients with severe eosinophilic asthma. Markers of asthma control were based on mean changes from baseline in St. George's Respiratory Questionnaire (SGRQ) score and Asthma Control Questionnaire (ACQ‐5) score, and mean change from baseline in clinic pre‐bronchodilator forced expiratory volume in one second (FEV1). Mepolizumab was associated with significant improvements in quality of life of patients with severe eosinophilic asthma and had a safety profile comparable to placebo.
In the paediatric population, mepolizumab is now indicated for children aged 6 years and older with severe eosinophilic asthma. The approval is based on the MUSCA, SIRIUS and MENSA trial and a 12‐week pharmacokinetic and pharmacodynamics study with 36 participants (aged 6–12 years).27 However, studies reporting on safety and efficacy data for the paediatric population remain scarce in number. In MENSA, MUSCA, DREAM and SIRIUS combined, only 34 (1.8%) adolescent patients were included (out of 1878 participants).28 This post hoc analysis of adolescent population provided evidence for comparable efficacy and safety of mepolizumab for adolescents compared with overall trial population. Moreover, preliminary data of a comparison of efficacy in children, adolescents and adults by Gupta et al.29 showed that mepolizumab in children (aged >12) with severe eosinophilic asthma results in similar efficacy (exacerbations and ACQ‐5) when compared to adults after 12 weeks (27.8%). More recently, a long‐term (52‐weeks) safety study reported a positive benefit‐risk profile for mepolizumab in 30 children (aged 6–12) with severe asthma and an eosinophilic phenotype. Compared with baseline values, mepolizumab treatment reduced blood eosinophil counts and asthma exacerbations and improved asthma control across all treatment groups.
2.2. Reslizumab
The other approved anti‐IL5 is reslizumab, a humanized monoclonal antibody that binds circulating IL‐5 and prevents its binding to the IL‐5Rα receptor. Reslizumab has been approved in 2017 for the treatment of severe eosinophilic asthma patients over 18 years old. It is administered intravenously every 4 weeks, and it is dosed based on bodyweight (3 mg/kg) with an eosinophil cut‐off value of ≥400 cells/μl.30 Castro et al.31 conducted a randomized, double‐blind, placebo‐controlled trial to evaluate the efficacy of reslizumab in patients with eosinophilic asthma defined by 3% or more of eosinophils in sputum and poor symptom control (ACQ ≥ 2). Reslizumab was able to induce a significant reduction in eosinophils in sputum and blood (95.4% and 38.7% in reslizumab and placebo group, respectively), and reduce exacerbations (8% of patients in the reslizumab group had an exacerbations compared to 19% in the placebo group). Modest improvements in asthma control symptoms were observed, even though the analysis of a subgroup of patients with nasal polyposis showed a significant response to reslizumab in terms of ACQ score improvement when compared to placebo. Two other duplicate, multi‐centre, double‐blind, randomized, phase 3 trials,32 enrolled 953 severe asthma patients who had blood eosinophils of ≥400 cells/μl, confirmed the reduction in the annual rate of clinical asthma exacerbations, the improvement of pulmonary function and asthma control in patients with severe eosinophilic asthma treated with reslizumab, while the same effects were not observed in patients with baseline eosinophils <400 cells/μl.33 A post hoc analysis of the same trial showed that reslizumab was effective and well tolerated in patients with more severe and refractory disease and with high eosinophilia.34
2.3. Benralizumab
Benralizumab is the approved monoclonal antibody against IL5‐Rα, a humanized monoclonal antibody that binds the alpha subunit of the interleukin‐5 receptor (IL5‐Rα), which is expressed on the surface of, among other cell types, eosinophils and basophils. It has been approved by the FDA to treat paediatric severe asthma patients of 12 years and older with an eosinophilic phenotype, but the EMA has approved this biologic only for the treatment of adults. Benralizumab induces apoptosis of eosinophils and basophils through enhanced antibody‐dependent cell‐mediated cytotoxicity (ADCC), binding the FcγRIII receptors on the surface of immune effectors cells such as natural killer (NK).35 Benralizumab is administered subcutaneously at the dose of 30 mg, initially every four weeks, and then every eight weeks after three injections. Eight weeks is the largest interval among all biological treatments and it is a primary benefit of benralizumab therapy. Patients who are considered eligible for benralizumab medication have severe eosinophilic asthma, with an eosinophil cut‐off value of ≥150 cells/μl.36 In the SIROCCO and CALIMA trials,37 subjects with severe uncontrolled asthma were enrolled, and both studies demonstrated that benralizumab 30 mg reduced asthma exacerbations rate by 36% with 4 weekly dosing and 28% with 8 weekly dosing,38 improved lung function and asthma symptom scores. Again adolescents have been underrepresented in these trial populations. In the SIROCCO and CALIMA trials, only 108 (4.3%) out of 2510 participants were <18 years. Additionally, the more recent ZONDA trial showed that the add‐on therapy with benralizumab reduced the need for oral corticosteroids and controlled asthma symptoms without effect on forced FEV1 compared with placebo.39 Benralizumab long‐term safety and efficacy was confirmed by the BORA extension phase 3 trial of SIROCCO and CALIMA of 1 year of duration40 (Table 1).
TABLE 1.
Main Phase II/III studies before the global approval of anti‐IL5/IL5R
| First author (publication year) | Study design | Study population | Regimen | Outcome |
|---|---|---|---|---|
| Mepolizumab | ||||
| Pavord I. D. (2012) | Phase II | N = 621 (Age 12–65 years) | Intravenous mepolizumab (75, 250, or 750 mg) vs. placebo every 4 weeks | Efficacy and safety in terms of reduction in number of exacerbations/year OCS sparing effect, improvement of pre‐ and post‐bronchodilator FEV1, improvement in ACQ score |
| Bel E. H. (2014) | Phase III | N = 135 (Age 16–74 years) | 100 mg subcutaneously vs. placebo every 4 weeks | Degree of reduction in the glucocorticoid dose, reduction in asthma exacerbations, improvement in asthma control, safety |
| Ortega H. G. (2014) | Phase III | N = 576 (Age 12–82 years) | 75‐mg intravenous dose or a 100‐mg subcutaneous dose vs placebo every 4 weeks | Reduction in exacerbations, improvement FEV1, scores SGRQ and ACQ‐5 and safety |
| Lugogo N. (2016) | Phase III | N = 651 (Age 12–82 years) | 100 mg subcutaneous every 4 weeks | Long‐term safety and efficacy (annualized exacerbation rate and durability of response) |
| Chupp G. L. (2017) | Phase IIIb | N = 556 (Age >12 years) | 100 mg subcutaneous every 4 weeks | Mean change from baseline in the SGRQ and ACQ‐5 scores, mean change in pre‐bronchodilator FEV1 |
| Reslizumab | ||||
| Castro M. (2015) | Phase III | N = 953 (Age 12–75 years) | Intravenous reslizumab (30 mg/kg) vs. placebo every 4 weeks | Reduction in the annual frequency of asthma exacerbations and safety |
| Corren J. (2016) | Phase III | N = 496 (Age 18–65 years) | Intravenous reslizumab (30 mg/kg) vs. placebo every 4 weeks | FEV1 improvement, improvement in ACQ‐7 scores, reduction in SABAs, and improvement in FVC |
| Christian Virchow J. (2020) | Post hoc analysis of two phase 3 trials (NCT01287039 and NCT01285323) | N = 953 (Age 12–75 years) | Intravenous reslizumab (30 mg/kg) vs. placebo every 4 weeks | Steroid sparing effect, serious exacerbations reduction, FEV1 improvement, AQLQ, ACQ and ASUI improvement, safety |
| Benralizumab | ||||
| Bleecker E. R. (2016) | Phase III | N = 1205 (Age 12–75 years) | Subcutaneous benralizumab 30 mg (Q4W) or (Q8W) vs. placebo | Reduction in annual exacerbation rate, improvement in pre‐bronchodilator FEV1 and asthma symptom score for patients with blood eosinophil counts of at least 300 cells per μl |
| FitzGerald J. M. (2016) | Phase III | N = 1306 (Age 12–75 years) | Subcutaneous benralizumab 30 mg (Q4W) or (Q8W) vs. placebo | Reduction in annual exacerbation rate, improvement in pre‐bronchodilator FEV1 and asthma symptom score for patients with blood eosinophil counts of at least 300 cells per μl |
| Nair P. (2017) | Phase III | N = 220 (Age 18–75 years) | Subcutaneous benralizumab 30 mg (Q4W) or (Q8W) vs. placebo | Reduction in the oral glucocorticoid dose from baseline, reduction in annual asthma exacerbation rates, improvement FEV1, improvement in asthma symptom score and ACQ‐6, safety |
| Busse W. W. (2019) | Phase III | N = 1926 (Age 12–75 years) | Subcutaneous benralizumab 30 mg (Q4W) or (Q8W) | Safety and tolerability |
Abbreviations: ACQ, asthma control questionnaire; AQLQ, asthma quality of life questionnaire; ASUI, asthma symptoms utility index; OCS, oral corticosteroids; Q4W, every 4 weeks; Q8W, every 8 weeks; SABA, short‐acting β‐agonists; SGRQ, St. George respiratory questionnaire.
It is also important to notice that benralizumab, compared with the others anti‐IL5 therapies, is able to reduce eosinophils faster and near‐completely including eosinophil‐lineage committed progenitor cells in blood and sputum41 suggesting a greater effectiveness in severe eosinophilic asthma over mepolizumab and reslizumab.42
Even though all the anti‐IL5/IL5R report relevant clinical efficacy and safety profiles in severe asthma patients with the evidence of eosinophilic inflammation, information on biomarkers able to predict a better response of these biologics compared with the others are still lacking and need to be clarified.
3. PREDICTORS OF RESPONSE
Clinical trials have primarily assessed the efficacy and safety of anti‐IL5/IL‐5Rα mAbs. The evaluation of patients’ variable responses to treatment is still challenging and difficult to determine before market approval. The latest GINA recommendations evaluate biomarkers that may predict significant responses to monoclonal antibodies for personalized treatments.43 Even if several biomarkers have been explored in patients with T2 severe asthma, there is still a gap on their feasibility in clinical practice and there are not enough data on their ability to predict treatment response.44 We briefly summarized the state of the art of clinical predictors of positive or poor response to monoclonal antibodies and the explored biomarkers (Tables 2 and 3).
TABLE 2.
Studies with predictive variables of response to mepolizumab, reslizumab and benralizumab
| Study (first author, publication year) | Study population | Anti‐eosinophilic monoclonal antibody | Variables used to determine response | Predictive variables of good response |
|---|---|---|---|---|
| Kavanagh J.E. (2020) | 99 | Mepolizumab |
Nasal polyposis ACQ‐6 BMI OCS use |
History of nasal polyposis Low baseline ACQ‐6 Low BMI Low prednisolone dosage at baseline |
| Albers F.C. (2019) | 936 | Mepolizumab | Baseline blood eosinophil count (<150, ≥150, ≥300, ≥400, ≥500, ≥750, ≥1000, ≥150–<300, or ≥300–<500 cells/μl) |
All threshold: Reduction in annual clinically significant exacerbations ≥150 cells/μl Reduction in annual clinically significant exacerbations; Improvement FEV1 Improvement ACQ‐5 and SGRQ |
| Ortega H.G. (2016) | 1192 | Mepolizumab |
Baseline eosinophil counts (≥150 cells per μl, ≥300 cells per μl, ≥400 cells per μl, and ≥500 cells per μl) Baseline blood eosinophil ranges (<150 cells per μl, ≥150 cells per μl to <300 cells per μl, ≥300 cells per μl to <500 cells per μl, and ≥500 cells per μl) |
≥150 cells/μl Reduction in annual clinically significant exacerbations |
| Albers F.C. (2019) | 936 | Mepolizumab |
Body weight (≤60, >60–75, >75–90, >90, <100, ≥100 kg) BMI (≤25, >25–30, >30, <36, ≥36 kg/m2) |
Reduction in exacerbations and improvements in SGRQ and ACQ‐5 scores were seen across all categories <90 kg of weight improvements in lung function |
| Drick N. (2018) | 42 | Mepolizumab |
FEV1 (≥12% or ≥200 ml) Blood eosinophils (<150/μl or <80% from baseline) VAS and ACT |
Increase in FEV1 Increase in oxygenation Improvement VAS scale and ACT Reduction in the exacerbation rate |
| Wechsler M. (2018) | 477 | Reslizumab |
FEV1 Number of exacerbations ACQ‐6 |
Late onset of asthma Higher baseline ACQ‐6 Lower BMI History of nasal polyps |
| Bateman E. D. (2019) | 321 | Reslizumab |
ACQ and AQLQ FEV1 Number of exacerbations |
These measures were evaluated in a mathematical model for their ability to predict the response at 52 weeks |
| FitzGerald J.M. (2018) | 2295 | Benralizumab |
Eosinophils blood levels Number of exacerbations |
High eosinophils blood levels (≥300 cells/μl) High rate of exacerbations in the previous year |
| Bleecker E.R. (2018) | 2295 | Benralizumab |
Eosinophils blood levels Number of exacerbations OCS use Nasal polyposis Pre‐bronchodilator FEV1 FVC Age‐onset |
≥300 eosinophils/μl (Q8W): Reduction in annual exacerbation rate Improvement in pre‐bronchodilator FEV1 <300 eosinophils/μl (Q8W): Reduction in OCS use History of nasal polyposis FVC <65% of predicted Reduction in exacerbation rates |
| Chipps B.E. (2018) | 2295 | Benralizumab |
IgE (≥150 kU/L; <150 kU/L) History of atopy Blood eosinophils |
≥300 eosinophils/μL: Reduction exacerbations Increase FEV1 No correlation with history of atopy and serum IgE |
Abbreviations: ACQ, asthma control questionnaire; ACT, asthma control questionnaire; AQLQ, asthma quality of life questionnaire; BMI, body mass index; OCS, oral corticosteroids; SGRQ, St. George respiratory questionnaire; VAS, visual analogue scale.
TABLE 3.
Studies with predictive variables of non‐response to mepolizumab, reslizumab and benralizumab
| Study (first author, publication year) | Study population | Anti‐eosinophilic monoclonal antibody | Variables used to determine response | Predictive variables of poor response |
|---|---|---|---|---|
| Harvey E.S. (2020) | 309 | Mepolizumab |
Blood eosinophils ACQ‐5 and HRQoL FEV1 Number of exacerbations OCS use |
Lower ACQ‐5 score Male sex High BMI |
| Mukherjee M. (2020) | 250 | Mepolizumab or Reslizumab |
ACQ OCS use Number of exacerbations Sputum eosinophils Blood eosinophils FEV1 |
Late‐onset asthma Sinus diseases Requirement of maintenance OCS Anti‐eosinophil peroxidase immunoglobulin (Ig)G Increase in sputum of C3c Deposition of C1q‐bound/IL‐5‐bound IgG. |
| Eger K. (2020) | 114 | Mepolizumab, Benralizumab or Reslizumab |
OCS use ACQ FEV1% of predicted levels FeNO Comorbidities control |
Lower ACQ Decreased FEV1 Increased OCS use Higher FeNO Sinonasal disease Atopic disease Adrenal insufficiency |
| Condreay L. (2017) | 492 | Reslizumab |
FEV1 and FVC ACQ‐7 Use of SABAs Blood eosinophils |
Eosinophils <400 cells/μl showed no significant improvement in FEV1 and ACQ‐7 |
| Shrimanker R. (2019) | 606 | Mepolizumab |
Blood eosinophils count (≥150 cells/μl; <150 cells/μl) FeNO (≥25 ppb; <25 ppb) Number of exacerbations (requiring OCS) Pre‐bronchodilator FEV1 |
High blood eosinophils High FeNO Increase number of exacerbations requiring OCS |
Abbreviations: ACQ, asthma control questionnaire; BMI, body mass index; HRQoL, Health Related Quality of Life; OCS, oral corticosteroids; SABA, short‐acting β‐agonists.
3.1. Predictors of response to mepolizumab
Level of blood eosinophils is the best‐established indicator of anti‐IL‐5/IL‐5Rα mAbs efficacy. The optimal eosinophils threshold in response to mepolizumab has been explored in two post hoc analyses, the DREAM and MENSA studies.45 Both clinical trials demonstrated that patients with an eosinophil count of 150 cells/μl or more at baseline respond better to mepolizumab treatment, especially in terms of exacerbation rate reduction (yet, a sensitivity analyses for adolescents is lacking and would probably be underpowered with the low patient numbers). Afterwards, the MENSA and MUSCA trials46 confirmed the clinical benefits of mepolizumab in patients with a baseline of blood eosinophil counts ≥150 cells/μl. Differences in baseline characteristics between responders and super‐responders have been explored in a total of 99 severe asthma patients treated with mepolizumab.47 In this retrospective study, severe asthma patients under mepolizumab treatment were classified as responders if they reported ≥50% reduction in the annualized exacerbation rate, while for those who required maintenance OCS dosage (mOCS) response was defined as ≥50% reduction in daily prednisolone (or equivalent) dose. Patients who did not respond to treatment and did not complete a full year of treatment were classified as non‐responders. Additionally, a subgroup of responders’ patients who did no longer need mOCS therapy for asthma and did not report exacerbations within a year of follow‐up, were included as “super‐responders.” Baseline characteristics significantly associated with responder status included a lower BMI, the presence of nasal polyposis, lower baseline ACQ‐6. Twenty‐eight percent were classified as super‐responders, and an additional trend towards significance was also observed for FEV1 (% predicted), with better lung function observed in the super‐responders. These responses seem to be also influenced by and correlated with age.
Conversely, a meta‐analysis of the MENSA and MUSCA RCT data48, 49 demonstrated that mepolizumab was able to reduce exacerbations rate independently from the baseline percentage predicted FEV1 (considering a value of <60%, >60%, <80% and >80% predicted FEV1 at screening). A different meta‐analysis of the MENSA and MUSCA trials showed the efficacy of mepolizumab in terms of reduction in exacerbation rate across different body weight categories and BMI thresholds (thresholds: <36 and ≥36 kg/m2), suggesting that mepolizumab dose does not need to be adjusted in relation to patient's weight. Another analysis explored the clinical efficacy of IL‐5 therapy with mepolizumab and potential predictors for treatment response; in this case, mepolizumab response to treatment was assessed measuring a positive response after 6 months of treatment with an improvement of FEV1 ≥ 12% or 200 ml, a reduction in blood eosinophils up to 150/μl or ≤80% from baseline and better symptoms control. Non‐responders were determined when patients reported a lack of response that could not be explained by respiratory infections, worsening of symptoms and exacerbations. There was no comparison between responders and non‐responders due to the small sample size (76% responders, 24% non‐responders); nevertheless, the analysis showed that improved lung function, decreased eosinophils blood level and improved symptoms may be considered as potential markers of treatment response to mepolizumab in clinical practice.50 Data evaluating the response to mepolizumab in the paediatric population is lacking.
3.2. Predictors of response to reslizumab
Similarly, clinical, functional and inflammatory parameters have been selected to address responses to reslizumab in adults; post hoc analyses of 477 patients from two phase 3 trials compared clinical and functional characteristics in non‐responders, moderate, high and super‐responders. Eighty‐seven percent were responders within 35% defined as moderate responders, 35% as high‐responders and 17% as super‐responders. In this case, response was stratified taking into account an improvement of at least 10% of FEV1 or ≥5% percent predicted FEV1, the absence of exacerbations or ACQ‐6 improvement. The analysis showed that super‐responders tend to have a higher age of onset, higher baseline ACQ, lower BMI and a history of chronic rhinosinusitis with nasal polyps (CRSwNP), with no significant differences in age, gender, baseline lung function and medications.51 Another analysis of two clinical trials used a mathematical model to predict long‐term response and non‐response in patients with severe asthma, after 16 weeks of reslizumab treatment. The algorithm was based on clinical indicators as a change from baseline to 16 weeks in clinical scores (ACQ and AQLQ), lung function FEV1, and number of asthma exacerbations. It resulted in 95.4%–95.5% sensitivity and 40.6%–54.1% specificity, and it was successful at predicting long‐term response at 52 weeks, but was not predictive of long‐term non‐response.52
To the best of our knowledge, no other new biomarkers have been explored for personalized treatment with reslizumab.
3.3. Predictors of response to benralizumab
As far as benralizumab is concerned, several clinical and functional baseline characteristics that might influence its efficacy were evaluated in two pooled analyses of the SIROCCO and CALIMA trials. Patients with severe eosinophilic asthma, treated with benralizumab every 8 weeks, with high eosinophils blood levels (≥300 cells/μl) and high rate of exacerbations in the previous year, seemed predictive of a better efficacy,53 as opposed to patients with <300 eosinophils/μl, OCS use, nasal polyposis and FVC <65%, who were associated with greater benralizumab responsiveness in terms of exacerbation rate reduction.54 Another pooled analysis based on the SIROCCO and CALIMA evaluated the atopic status and its possible connection with anti‐IL5R response. Patients were stratified according to IgE blood concentrations (high ≥150 kU/L or low <150 kU/L), and history of atopy, finding that Benralizumab decreased exacerbations and improved lung function regardless of serum IgE concentrations and atopy status. More recently, a similar analysis of both trials performed by Jackson et al.,55 demonstrated that baseline eosinophils, but not serum IgE levels, are predictor of exacerbation risk in patients with severe eosinophilic asthma under benralizumab treatment.
4. PREDICTORS OF POOR RESPONSE
At present, there are limited data on the long‐term effects of anti‐IL5/IL‐5R therapy and so far have focused less on the predictors of response. A small proportion of patients who are treated with biologics does not seem to be responsive to the therapy and sometimes report worsening of symptoms. Although their therapeutic effect is widely recognized, it still remains unclear is why a reduction in IL‐5 signalling does not result in an improvement of symptoms for some patients.47, 56, 57, 58
A recent multi‐centre study enrolled 309 patients aged ≥12 years with severe eosinophilic asthma under treatment with mepolizumab, to assess its efficacy and safety prior to and post‐commencement.58 The response to mepolizumab was evaluated based on blood eosinophils reduction, improvement of symptoms (ACQ‐5 reduction and improvement in HRQoL), lung function improvement, reduction in the exacerbation annual rate and OCS sparing effect. During a 12‐month period, 14.6% of the patients were considered non‐responders based on failure of the ACQ response or clinical decision, and their treatment was suspended. Lower ACQ‐5 score after 6 months of treatment, male gender and high BMI were indicative of a poor response. A greater improvement in ACQ‐5 has been reported for those patients who had higher blood eosinophils threshold and late age of asthma onset, with fewer comorbidities. Twenty‐five percent of patients were considered super‐responders based on ACQ‐5 score improvement. This group was composed of mostly females, with a low BMI, never smokers, with a short duration of asthma higher baseline eosinophils, ACQ and FeNO levels, and history of nasal polyps, and no need of OCS maintenance therapy. These findings are in line with the MENSA and MUSCA trials, and they increase mepolizumab clinical evidence as a promising targeted therapy in real‐world clinical practice.58 Moreover, these results are comparable to a study by Mukherjee et al.,57 where 250 patients with moderate‐to‐severe asthma who were treated with mepolizumab and reslizumab were enrolled to evaluate possible autoimmune predictors of non‐response to anti‐IL‐5 mAbs, focusing on effects that may cause a suboptimal response. Non‐response to therapy was determined taking into account at least one of the three main clinical criteria (failure in OCS sparing effect, failure to reduce ACQ ≤1.5, failure to reduce exacerbations by 50%) and including the persistence of sputum eosinophils >3% or blood eosinophils ≥400 cells/μl after 4 months of therapy. Reduction in FEV1 by at least 25% from baseline and/or any increase in maintenance corticosteroid and/or increase in ACQ by 0.5 (minimal clinically important difference) was also considered to be representative of a suboptimal response. About 42.8% of patients treated with mepolizumab or reslizumab were considered non‐responders and the strongest predictors of low response were as follows: late‐onset asthma, chronic rhinosinusitis without nasal polyps (CRSsNP) and the requirement of maintenance OCS. In addition, anti‐eosinophil peroxidase immunoglobulin (Ig)G, the increase in sputum of C3c (marker of complement activation) and the deposition of C1q‐bound/IL‐5‐bound IgG were considered potential markers of a worse response.
This study suggests that other potential biomarkers like autoimmune mediators other than clinical characteristics may contribute to predict less response to anti‐IL5 mAbs.
A double‐centre study that included severe eosinophilic asthma patients under treatment with anti‐IL5/IL5R mAbs, assessed the prevalence of “super‐responders,” “partial‐responders” and “non‐responders” in a long‐term period.59 “Super‐responders,” “partial‐responders” and “non‐responders” were defined according to the OCS assumption, ACQ score, FEV1% of predicted levels, FeNO levels and comorbidities control. Non‐responders were 11% of the total number (n = 114) and were considered those who reported clinical worsening with either increased symptoms, decreased FEV1, or increased OCS use. Partial‐responders and super‐responders were 69% and 14%, respectively, and reported OCS reduction, improvement in the ACQ score, improvement in FEV1 and reduction in FeNO levels. Super‐responder also reported better control of comorbidities such as nasal polyps, rhinosinusitis and atopic dermatitis. After 2 years of treatment, the most common residual disease manifestations included impaired lung function (59%), uncontrolled sinus disease (58%) and unstable asthma symptoms (48%). Super‐responder predictors were as follows: recent and adult‐onset asthma, higher FEV1, and tended to be associated with the absence of CRSwNP and lower BMI, encouraging an early start of treatment in order to improve the benefits of anti‐IL5/IL5R mAbs.
As previously mentioned, eosinophils blood ranges have been explored to evaluate which threshold may be indicative of a less response to reslizumab. In a phase 3 trial, a cut‐off value of eosinophils blood count of <400 cells/μl was also correlated to a lower response to reslizumab, without any improvement in asthma outcomes such as lung function or symptoms control.33
Other biomarkers, such as FeNO levels, have been investigated to better understand the response to mepolizumab. A post hoc analysis of the DREAM trial explored the predictive power of combining FeNO and blood eosinophils levels.60 The study reports that in patients with severe eosinophilic asthma, high blood eosinophil counts and high FeNO, who were treated with placebo, the rate of severe exacerbations requiring oral corticosteroid treatment was increased when compared to the placebo group with low or discordant FeNO and eosinophils. These results may be of additional value to the traditional risk assessment.
Additionally, IL‐5 has been considered a potential molecular biomarker for prediction of response to therapy. In a small study with only preliminary results, the concentration of systemic IL‐5 was evaluated in patients treated with mepolizumab, and it was shown that non‐responders had increased IL‐5 levels compared with responders. Non‐response was assessed considering the need for a high corticosteroids dosage and high frequency of exacerbations.61
5. DISCUSSION
The introduction of new anti‐IL‐5/IL‐5R drugs in severe eosinophilic asthmatics requires possible factors that can be predictive of response to treatment. This manuscript focussed on studies addressed on the identification and the search of new variables that will provide clinicians more instruments to find the correct treatment target among different biologics nowadays available. A new theoretical model for the action of biologics shows that the target molecule is part of a causal network of different inflammatory and non‐inflammatory markers that influence reciprocally.62 Therefore, many factors may contribute to mAbs response or non‐response in patients with severe eosinophilic asthma, but with the studies currently available, there are still pitfalls that need further investigations to find out whether patients will receive more or less benefits from the biological therapy.
5.1. Heterogeneous definition of response and non‐response
The evaluation of efficient treatment strategies is still a challenge and key points remain to be addressed, such as the heterogeneous definition of response and non‐response among studies and the underrepresentation of children and adolescents in clinical trials. A European experts consensus statement63 defined “a traffic light system” as the process to assess response after 4 months of treatment with anti‐eosinophilic therapies and then after a year, with the purpose to guide clinicians’ decisions to stop or continue treatments.
Clearly, a 4–6‐month period appears to be the current consensus for evaluating optimal or suboptimal response and consider the possibility of switching between biologicals. However, defining the main clinical characteristics of responders or partial/non‐responders is still considered an open challenge in the clinical field. In this regard, the National Institute for health and Care Excellence (NICE) suggested the interesting approach to target overall systemic steroid exposure.64 Recently, an “algorithm” which describes the current common sense, prioritizing patients’ preferences in real‐life has been proposed according to previous findings,65 but real‐life studies and registries, in which well‐defined severe asthma patients are followed prospectively, are still needed better define new specific characteristics.
5.2. The overlap among endotypes
Response may be influenced by the fact that the underlying molecular mechanisms (also referred to as endotypes) of eosinophilic pathways, which are addressed by anti‐IL5/IL5R therapies, overlap with other asthma endotypes.66 This condition is one of the reasons that possibly explain the switch to other biological therapies if the optimal therapeutic effect is not obtained with the first choice. A consistent overlap among eosinophilic and atopic asthma has been investigated, showing that a proportion of patients with eosinophilic asthma can also be classified as atopic, especially for those patients who report a higher blood eosinophil cut‐off. Conversely, a lower proportion of atopic asthma can be classified as eosinophilic.67 Even though there are no guidelines available that provide the efficacy and safety profile of switching among different monoclonal antibodies, a recent review of Papaioannou et al.,68 provides a general overview of different possibilities of switching. Thereby taking into account a summary of clinical, functional and laboratory biomarkers now available, in order to guide clinicians to choose to switch biologics when it is needed and obtain a more efficient response.
5.3. The need of new biomarkers
Clinical, functional and inflammatory predictors have been analysed in several studies in order to identify possible predictors of a better response to treatment. All these studies have primarily focussed on treatment efficacy and safety, but there is still a lack of information regarding the potential mechanisms behind non‐response to anti‐IL5/IL5R monoclonal antibodies therapies. Additionally, long‐term response will need to be further explored considering that biomarkers, which guide clinicians to the best treatment options, may also vary over time.
To date, blood eosinophils levels seem to represent the most indicative biomarker in determining response to anti‐IL5/IL5R mAbs even though there is still a debate of its role in real‐life studies.69 Higher eosinophils levels, the presence of late‐onset asthma, history of nasal polyposis and frequent exacerbations might further increase the chance of good response.43 Nonetheless, further studies are still needed to validate selected biomarkers of treatment response to provide information to clinicians in order to choose the right biological among different anti‐IL5 mAbs.
Interestingly, Mukherjee et al.57 recently demonstrated that the currently approved indications for anti‐IL5 mAbs are not a sufficient indication to prove an optimal response in real‐life clinical practice that other factors, like the developing of autoimmune phenomena, may be relevant and requires attention.
The need to recognize the clinical relevance of phenotypes and biomarkers, both those currently available and those to be expected, is one of the main debates in the evaluation of treatment response.
5.4. Real‐life cohorts
Because of the heterogeneity and complexity of the disease, there is the need of a different approach from the current practice to the disease management, accounting for the variability of multiple factors involved in the development of this disease.
For example, an accurate evaluation of patients’ characteristics in clinical trials may help with the selection of patients that could benefit from the treatment. A large proportion of patients, in a real‐life setting, is currently treated with protocols based on clinical trials for which they would have not been eligible.70 This proportion increases in elderly patients with comorbidities,71 whether age can influence response to monoclonal antibodies is still controversial. A recent meta‐analysis72 shows how clinical factors, such as age, do not influence the efficacy of anti‐IL‐5/IL‐5R, which highlights how these medications could be effective and safe also within the geriatric population, even if less represented.73 In some studies, it is reported how age may directly be a positive response predictor, but further studies are needed to prove its reliability as a clinical marker and what this means for response in the paediatric population. Given the different clinical needs and different underlying mechanisms driving the disease in adults and children, it is crucial to design trials for children.74 Due to the challenge to recruit sufficient study participants, there is a need for real‐life studies and international consortia (such as SPACE and PERMEABLE and SHARP).75, 76, 77 Treatments targeting IL‐5/IL‐5R have not been investigated in younger patients (<6 years) with severe asthma. Prolonged effectiveness and the impact of such treatments on the natural history of the disease should also be studied. The possibility to prevent the evolution of severe asthma in children has been also considered as a point of interest. Currently, the preventing asthma in high risk kids (PARK) study is the first to attempt to answer such a research question. This study aims to explore whether a two‐year treatment with omalizumab (anti‐IgE) of pre‐schoolchildren aged 2–3 years at high risk for asthma will prevent the progression to childhood asthma.
5.5. The new frontier of “omic sciences”
Understanding the impact of different asthma endotypes in treatment response may help physicians with choosing a personalized treatment, even if limited data are available for biomarkers prediction of treatment response to biologicals in severe asthma.78, 79, 80 Especially for children, it is important to develop non‐invasive techniques to phenotype paediatric patients and guide treatment at an early phase. New frontiers labelled as “omics sciences” such as genomics, transcriptomics, proteomics, pharmacogenomics and metabolomics (which includes breathomics), are fascinating cutting‐edge technique that needs to be further explored in severe asthma, considered promising tools for the identification of novel predictive biomarkers related to good or poor response. Over the past 3 years, the number of independent asthma‐associated genetic loci has increased to 128 according to well‐powered genetic studies.81 In a post hoc analysis of the DREAM and MENSA study, genetic markers were tested in patients with severe asthma and found no genetic associations related to response to mepolizumab, even though it cannot be excluded the possibility of the existence of rare genetic variants not yet explored predictive of response.80 Further studies are needed to establish the functional significance of gene variants associated with asthma and potential genetic biomarkers indicators of response to anti‐IL5/IL5R mAbs still need to be explored. The inclusion of omic sciences to understand the mechanisms of biologic therapies may be improved using big data consortium and harmonizing biobanking procedures; an analysis of the European U‐BIOPRED network evaluated urinary eicosanoids metabolites to phenotype T2 asthma using a non‐invasive approach, but little is known regarding its potential role specifically for severe asthmatics under anti‐IL5/IL5R mAbs.82 Recent studies have also considered the role of airway dysbiosis and microbial colonization in severe asthmatic patients.83, 84 The association of specific microbiota that may modulate inflammatory processes in patients with severe asthma may be considered an additional feature during the phenotyping process. However, further understanding of how microbiota functionally mediates asthma development will require better integration of advanced scientific and analytic tools and well‐designed clinical studies.
The analysis of exhaled breath can be focussed on the study of breath patterns and compositions of volatile organic compounds (VOCs), seems attractive for the purpose of diagnoses and monitoring of asthma and other respiratory diseases. Exhaled breath samples can be obtained using a non‐invasive and easy to use tool, the electronic nose (eNose), which has already proven to be predictive of loss of asthma control85 and distinguish between asthma and other chronic pulmonary diseases.86 Although it seems to be a promising method and a group of severe asthma experts defined it as one of the most important potential biomarkers for the future,87 larger studies including breath samples are needed to confirm these findings. Considering how it is important for the paediatric asthma patients to develop non‐invasive techniques to guide treatment and to characterize different asthma phenotypes, the analyses of exhaled breath may be promising also for severe asthmatic children.
This review summarizes the current state of knowledge on anti‐IL5/IL5Rα targeted therapies in severe asthma. Although these studies provide some insights, there are several topics that still need to be elucidated and will require additional evidence. Extended studies that include real‐world data and big data prospective studies are needed. Moreover, new tools to identify predictive biomarkers may be considered valuable in the future. Harmonization of data processing and collection of specimens may definitely improve biomarkers identification and reliability. The correct choice of biological therapies in severe asthma is still challenging, and the importance of finding parameters to predict response stays an open issue that needs to be further explored.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors participated in drafting and writing the manuscript and approved the manuscript.
ACKNOWLEDGEMENTS
The authors wish to thank and acknowledge all the partners, in particular all the members of the 3TR (Taxonomy, Treatment, Targets and Remission) Consortium.
Principe S, Porsbjerg C, Bolm Ditlev S, et al. Treating severe asthma: Targeting the IL‐5 pathway. Clin Exp Allergy. 2021;51:992–1005. 10.1111/cea.13885
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Publ Health. 2012;12:204. 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343‐373. 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 3.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S‐12S. 10.1378/chest.130.1_suppl.4S [DOI] [PubMed] [Google Scholar]
- 4.Sadatsafavi M, Lynd L, Marra C, et al. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17(2):74‐80. 10.1155/2010/361071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groot JC, Storm H, Amelink M, et al. Clinical profile of patients with adult‐onset eosinophilic asthma. ERJ Open Res. 2016;2(2):00100‐2015. 10.1183/23120541.00100-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung KF. Targeting the interleukin pathway in the treatment of asthma. Lancet. 2015;386(9998):1086‐1096. 10.1016/S0140-6736(15)00157-9 [DOI] [PubMed] [Google Scholar]
- 7.Schleich FN, Chevremont A, Paulus V, et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J. 2014;44(1):97‐108. 10.1183/09031936.00201813 [DOI] [PubMed] [Google Scholar]
- 8.Bossley CJ, Fleming L, Gupta A, et al. Pediatric severe asthma is characterized by eosinophilia and remodeling without TH2 cytokines. J Allergy Clin Immunol. 2012;129(4):974‐982.e13. 10.1016/j.jaci.2012.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush A. Pathophysiological mechanisms of asthma. Front Pediatr. 2019;7:68. 10.3389/fped.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah SP, Grunwell J, Shih J, Stephenson S, Fitzpatrick AM. Exploring the utility of noninvasive type 2 inflammatory markers for prediction of severe asthma exacerbations in children and adolescents. J Allergy Clin Immunol Pract. 2019;7(8):2624‐2633.e2. 10.1016/j.jaip.2019.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown SD, Brown LA, Stephenson S, et al. Characterization of a high TNF‐α phenotype in children with moderate‐to‐severe asthma. J Allergy Clin Immunol. 2015;135(6):1651‐1654. 10.1016/j.jaci.2014.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang TS, Lemanske RF, Mauger DT, et al. Childhood asthma clusters and response to therapy in clinical trials. J Allergy Clin Immunol. 2014;133(2):363‐369. 10.1016/j.jaci.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73‐S80. 10.1016/j.jaci.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menzies‐Gow A, Flood‐Page P, Sehmi R, et al. Anti‐IL‐5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111(4):714‐719. 10.1067/mai.2003.1382 [DOI] [PubMed] [Google Scholar]
- 15.McBrien CN, Menzies‐Gow A. The biology of eosinophils and their role in asthma. Front Med. 2017;4:93. 10.3389/fmed.2017.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218‐224. 10.1164/rccm.200711-1754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033‐1039. 10.1056/NEJM199010113231505 [DOI] [PubMed] [Google Scholar]
- 18.Louis R, Lau L, Bron A, et al. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9‐16. 10.1164/ajrccm.161.1.9802048 [DOI] [PubMed] [Google Scholar]
- 19.Walsh GM. An update on emerging drugs for asthma. Expert Opin Emerg Drugs. 2012;17(1):37‐42. 10.1517/14728214.2012.657625 [DOI] [PubMed] [Google Scholar]
- 20.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709‐750. 10.1111/j.1365-2222.2008.02958.x [DOI] [PubMed] [Google Scholar]
- 21.Choy MS, Dixit D, Bridgeman MB. Mepolizumab (Nucala) for severe eosinophilic asthma. P T. 2016;41(10):619‐622. [PMC free article] [PubMed] [Google Scholar]
- 22.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380(9842):651‐659. 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 23.Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189‐1197. 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 24.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198‐1207. 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 25.Lugogo N, Domingo C, Chanez P, et al. Long‐term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi‐center, open‐ label, phase IIIb study. Clin Ther. 2016;38(9):2058‐2070.e1. 10.1016/j.clinthera.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 26.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add‐on therapy on health‐related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double‐blind, placebo‐controlled, parallel‐group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5(5):390‐400. 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 27.Gupta A, Pouliquen I, Austin D, et al. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr Pulmonol. 2019;54(12):1957‐1967. 10.1002/ppul.24508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancey SW, Ortega HG, Keene ON, Bradford ES. Efficacy of add‐on mepolizumab in adolescents with severe eosinophilic asthma. Allergy Asthma Clin Immunol. 2019;15:53. 10.1186/s13223-019-0366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A, Steinfeld J, Price R, Azmi J, Bradford E, Yancey S. Mepolizumab for severe eosinophilic asthma: a comparison of efficacy in children, adolescents, and adults. Eur Respir J. 2018;52(62):PA5447. [Google Scholar]
- 30.Hom S, Pisano M. Reslizumab (Cinqair): an interleukin‐5 antagonist for severe asthma of the eosinophilic phenotype. P T. 2017;42(9):564‐568. [PMC free article] [PubMed] [Google Scholar]
- 31.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo‐controlled study. Am J Respir Crit Care Med. 2011;184(10):1125‐1132. 10.1164/rccm.201103-0396OC [DOI] [PubMed] [Google Scholar]
- 32.Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double‐blind, randomised, placebo‐controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355‐366. 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 33.Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150(4):799‐810. 10.1016/j.chest.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 34.Christian Virchow J, McDonald M, Garin M, Korn S. Reslizumab as add‐on therapy in patients with refractory asthma. BMJ Open Respir Res. 2020;7(1):e000494. 10.1136/bmjresp-2019-000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolbeck R, Kozhich A, Koike M, et al. MEDI‐563, a humanized anti‐IL‐5 receptor α mAb with enhanced antibody‐dependent cell‐mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125(6):1344‐1353.e2. 10.1016/j.jaci.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Dávila González I, Moreno Benítez F, Quirce S. Benralizumab: a new approach for the treatment of severe eosinophilic asthma. J Investig Allergol Clin Immunol. 2019;29(2):84‐93. 10.18176/jiaci.0385 [DOI] [PubMed] [Google Scholar]
- 37.FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2128‐2141. 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 38.Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388(10056):2115‐2127. 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 39.Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448‐2458. 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 40.Busse WW, Bleecker ER, FitzGerald JM, et al. Long‐term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1‐year results from the BORA phase 3 extension trial. Lancet Respir Med. 2019;7(1):46‐59. 10.1016/S2213-2600(18)30406-5 [DOI] [PubMed] [Google Scholar]
- 41.Sehmi R, Lim HF, Mukherjee M, et al. Benralizumab attenuates airway eosinophilia in prednisone‐dependent asthma. J Allergy Clin Immunol. 2018;141(4):1529‐1532.e8. 10.1016/j.jaci.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 42.Hillas G, Fouka E, Papaioannou AI. Antibodies targeting the interleukin‐5 signaling pathway used as add‐on therapy for patients with severe eosinophilic asthma: a review of the mechanism of action, efficacy, and safety of the subcutaneously administered agents, mepolizumab and benralizumab. Expert Rev Respir Med. 2020;14(4):353‐365. 10.1080/17476348.2020.1718495 [DOI] [PubMed] [Google Scholar]
- 43.Difficult‐to‐treat & severe asthma in adolescent and adult patients – diagnosis and management. Global Initiative for Asthma; 2018. www.ginasthma.org
- 44.Kim H, Ellis AK, Fischer D, et al. Asthma biomarkers in the age of biologics. Allergy, Allergy Asthma Clin Immunol. 2017;13:48. 10.1186/s13223-017-0219-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortega HG, Yancey SW, Mayer B, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med. 2016;4(7):549‐556. 10.1016/S2213-2600(16)30031-5 [DOI] [PubMed] [Google Scholar]
- 46.Albers FC, Licskai C, Chanez P, et al. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir Med. 2019;159:105806. 10.1016/j.rmed.2019.105806 [DOI] [PubMed] [Google Scholar]
- 47.Kavanagh JE, Hearn AP, Dhariwal J, et al. Real world effectiveness of benralizumab in severe eosinophilic asthma. Chest. 2021;159(2):496‐506. 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 48.Silver J, Menzies‐Gow A, Smith S, et al. Baseline percent predicted fev1 does not predict a response to mepolizumab in patients with severe eosinophilic asthma: meta‐analysis from two phase 3 trials. Chest. 2019;156(4):A459‐A460. 10.1016/j.chest.2019.08.482 [DOI] [Google Scholar]
- 49.Albers FC, Papi A, Taillé C, et al. Mepolizumab reduces exacerbations in patients with severe eosinophilic asthma, irrespective of body weight/body mass index: meta‐analysis of MENSA and MUSCA. Respir Res. 2019;20(1):169. 10.1186/s12931-019-1134-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drick N, Seeliger B, Welte T, Fuge J, Suhling H. Anti‐IL‐5 therapy in patients with severe eosinophilic asthma – clinical efficacy and possible criteria for treatment response. BMC Pulm Med. 2018;18(1):119. 10.1186/s12890-018-0689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wechsler M, McDonald M, Garin MC. Reslizumab high‐responder and super‐responder asthma patients. Am J Respir Crit Care Med. 2018;197:A1375. https://www.atsjournals.org/doi/abs/10.1164/ajrccm‐conference.2018.197.1_MeetingAbstracts.A1375 [Google Scholar]
- 52.Bateman ED, Djukanović R, Castro M, et al. Predicting responders to reslizumab after 16 weeks of treatment using an algorithm derived from clinical studies of patients with severe eosinophilic asthma. Am J Respir Crit Care Med. 2019;199(4):489‐495. 10.1164/rccm.201708-1668OC [DOI] [PubMed] [Google Scholar]
- 53.FitzGerald JM, Bleecker ER, Menzies‐Gow A, et al. Predictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir Med. 2018;6(1):51‐64. 10.1016/S2213-2600(17)30344-2 [DOI] [PubMed] [Google Scholar]
- 54.Bleecker ER, Wechsler ME, FitzGerald JM, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J. 2018;52(4):1800936. 10.1183/13993003.00936-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson DJ, Humbert M, Hirsch I, Newbold P, Garcia GE. Ability of serum IgE concentration to predict exacerbation risk and benralizumab efficacy for patients with severe eosinophilic asthma. Adv Ther. 2020;37(2):718‐729. 10.1007/s12325-019-01191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight‐adjusted intravenous reslizumab in severe asthma with inadequate response to fixed‐dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197(1):38‐46. 10.1164/rccm.201707-1323OC [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee M, Forero DF, Tran S, et al. Suboptimal treatment response to anti‐IL‐5 monoclonal antibodies in severe eosinophilic asthmatics with airway autoimmune phenomena. Eur Respir J. 2020;56(4):2000117. 10.1183/13993003.00117-2020 [DOI] [PubMed] [Google Scholar]
- 58.Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super‐responders in severe asthma. Eur Respir J. 2020;55(5):1902420. 10.1183/13993003.02420-2019 [DOI] [PubMed] [Google Scholar]
- 59.Eger K, Kroes JA, ten Brinke A, Bel EH. Long‐term therapy response to anti–IL‐5 biologics in severe asthma—a real‐life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194‐1200. 10.1016/j.jaip.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 60.Shrimanker R, Keene O, Hynes G, Wenzel S, Yancey S, Pavord ID. Prognostic and predictive value of blood eosinophil count, fractional exhaled nitric oxide, and their combination in severe asthma: a post hoc analysis. Am J Respir Crit Care Med. 2019;200(10):1308‐1312. 10.1164/rccm.201903-0599LE [DOI] [PubMed] [Google Scholar]
- 61.Skrgat S, Sušanj PG, Stojkovič UB. Increase in systemic IL‐5 is associated with mepolizumab treatment failure in patients with severe asthma. Eur Respir J. 2018;52:1132. https://www.ers‐education.org/lr/show‐details/?idP=209297 [Google Scholar]
- 62.Hyland ME, Masoli M, Lanario JW, Jones RC. A possible explanation for non‐responders, responders and super‐responders to biologics in severe asthma. Explor Res Hypothesis Med. 2019;4(2):35‐38. 10.14218/ERHM.2019.00008 [DOI] [Google Scholar]
- 63.Buhl R, Humbert M, Bjermer L, et al. Severe eosinophilic asthma: a roadmap to consensus. Eur Respir J. 2017;49(5):1700634. 10.1183/13993003.00634-2017 [DOI] [PubMed] [Google Scholar]
- 64.Asthma: diagnosis, monitoring and chronic asthma management. London: National Institute for Health and Care Excellence (UK); 2020. Feb. [PubMed] [Google Scholar]
- 65.Papadopoulos NG, Barnes P, Canonica GW, et al. The evolving algorithm of biological selection in severe asthma. Allergy. 2020;75(7):1555‐1563. 10.1111/all.14256 [DOI] [PubMed] [Google Scholar]
- 66.Zervas E, Samitas K, Papaioannou AI, et al. An algorithmic approach for the treatment of severe uncontrolled asthma. ERJ Open Res. 2018;4(1):00125‐2017. 10.1183/23120541.00125-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2‐high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37‐42. 10.1016/j.anai.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 68.Papaioannou AI, Fouka E, Papakosta D, Papiris S, Loukides S. Switching between biologics in severe asthma patients. When the first choice is not proven to be the best. Clin Exp Allergy. 2021;51(2):221‐227. 10.1111/cea.13809 [DOI] [PubMed] [Google Scholar]
- 69.Bagnasco D, Massolo A, Bonavia M, et al. The importance of being not significant: blood eosinophils and clinical responses do not correlate in severe asthma patients treated with mepolizumab in real life. Allergy. 2020;75(6):1460‐1463. 10.1111/all.14135 [DOI] [PubMed] [Google Scholar]
- 70.Richards LB, van Bragt JJMH, Aarab R, et al. Treatment eligibility of real‐life mepolizumab‐treated severe asthma patients. J Allergy Clin Immunol Pract. 2020;8(9):2999‐3008.e1. 10.1016/j.jaip.2020.04.029 [DOI] [PubMed] [Google Scholar]
- 71.Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real‐life outpatients? Respiration. 2015;89(5):383‐389. 10.1159/000375314 [DOI] [PubMed] [Google Scholar]
- 72.Principe S, Benfante A, Calzetta L, Rogliani P, Scichilone N. Age does not affect the efficacy of anti‐IL‐5/IL‐5R in severe asthmatics. World Allergy Organ J. 2019;12(11):100081. 10.1016/j.waojou.2019.100081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benfante A, Principe S, Battaglia S, Scichilone N. Are biological drugs effective and safe in older severe asthmatics? Expert Opin Drug Saf. 2019;18(5):369‐380. 10.1080/14740338.2019.1607838 [DOI] [PubMed] [Google Scholar]
- 74.Treating severe paediatric asthma: a randomised controlled trial of mepolizumab and omalizumab (TREAT trial). (CPMS 44294, IRAS 252084). Report No.: ISRCTN12109108. http://www.isrctn.com/ISRCTNISRCTN12109108
- 75.van Bragt JJMH, Hansen S, Djukanovic R, et al. SHARP: Enabling generation of real‐world evidence on a pan‐European scale to improve the lives of individuals with severe asthma. ERJ Open Res. 2021;7(2):00064‐2021. 10.1183/23120541.00064-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golebski K, Kabesch M, Melén E, et al. Childhood asthma in the new omics era: challenges and perspectives. Curr Opin Allergy Clin Immunol. 2020;20(2):155‐161. 10.1097/ACI.0000000000000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu NM, van Aalderen W, Carlsen KCL, et al. Severe paediatric asthma collaborative in Europe (SPACE): protocol for a European registry. Breathe. 2018;14(2):93‐98. 10.1183/20734735.002018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kroes JA, Zielhuis SW, van Roon EN, ten Brinke A. Prediction of response to biological treatment with monoclonal antibodies in severe asthma. Biochem Pharmacol. 2020;179:113978. 10.1016/j.bcp.2020.113978 [DOI] [PubMed] [Google Scholar]
- 79.van Bragt JJMH, de Vries R, Sterk PJ, Maitland‐van der Zee AH. Exhaled Breath Analysis for Prediction of Responders to Mepolizumab in Patients with Severe Asthma. American Thoracic Society; 2019:A2673.
- 80.Condreay L, Chiano M, Ortega H, et al. No genetic association detected with mepolizumab efficacy in severe asthma. Respir Med. 2017;132:178‐180. 10.1016/j.rmed.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 81.El‐Husseini ZW, Gosens R, Dekker F, Koppelman GH. The genetics of asthma and the promise of genomics‐guided drug target discovery. Lancet Respir Med. 2020;8(10):1045‐1056. 10.1016/S2213-2600(20)30363-5 [DOI] [PubMed] [Google Scholar]
- 82.Kolmert J, Gómez C, Balgoma D, et al. Urinary leukotriene E4 and prostaglandin D2 metabolites increase in adult and childhood severe asthma characterized by type‐2 inflammation. Am J Respir Crit Care Med. 2021;203(1):37‐53. 10.1164/rccm.201909-1869OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874‐884. 10.1016/j.jaci.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chun Y, Do A, Grishina G, et al. Integrative study of the upper and lower airway microbiome and transcriptome in asthma. JCI Insight. 2020;5(5):e133707. 10.1172/jci.insight.133707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brinkman P, van de Pol MA, Gerritsen MG, et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin Exp Allergy. 2017;47(9):1159‐1169. 10.1111/cea.12965 [DOI] [PubMed] [Google Scholar]
- 86.de Vries R, Dagelet YWF, Spoor P, et al. Clinical and inflammatory phenotyping by breathomics in chronic airway diseases irrespective of the diagnostic label. Eur Respir J. 2018;51(1):1701817. 10.1183/13993003.01817-2017 [DOI] [PubMed] [Google Scholar]
- 87.Pavord I, Bahmer T, Braido F, et al. Severe T2‐high asthma in the biologics era: European experts’ opinion. Eur Respir Rev. 2019;28(152):190054. 10.1183/16000617.0054-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


