ABSTRACT
Background
Based on a variety of studies conducted in recent years, some of the factors that might contribute to the negative treatment responses of some TMD patients have been elucidated.
Methods
This paper describes known vulnerability factors that make individuals susceptible to developing temporomandibular disorders (TMDs), as well as those that contribute to the perpetuation of such problems. In addition, the topic of iatrogenesis is discussed as a major contributor to the negative outcomes that can be seen in this field.
Results
At the patient level, anatomical, psychosocial and genetic factors may contribute to individual vulnerability. The anatomy and pathophysiology of muscles, joints, disc and nerves may all be involved in predisposing to TMD symptoms, especially when the patients have pain elsewhere in the body. Among the psychosocial factors, some features may be elucidated by the DC/TMD axis II, while others (eg illness behaviour, Munchausen syndrome, lack of acceptance of non‐mechanical approaches) require careful evaluation by trained clinicians. Genetic predisposition to first onset TMDs and to chronification of symptoms has been identified for individuals with certain psychological traits, presence of comorbid conditions and certain abnormal clinical manifestations. Regarding iatrogenesis, sins of omission may influence the clinical picture, with the main ones being misdiagnosis and undertreatment. Joint repositioning strategies, occlusal modifications, abuse of oral appliances, use of diagnostic technologies, nocebo effect and complications with intracapsular treatments are the most frequent sins of commission that may contribute to chronification of TMDs. The patients who present with massive occlusal and jaw repositioning changes combined with persistent severe orofacial pain are not a rarity within TMD and orofacial pain canters; these patients are the most difficult ones to manage because of this horrific combination of negative factors.
Conclusions
The information presented in this paper will help clinicians to understand better why some individuals develop temporomandibular disorders, why some of them will progress to becoming chronic patients, and what the appropriate responses may be.
Keywords: chronic pain, Iatrogenesis, Temporomandibular Disorders, vulnerability
This paper describes known vulnerability factors that make individuals susceptible to developing temporomandibular disorders (TMDs), as well as those that contribute to the perpetuation of such problems. In addition, the topic of iatrogenesis is discussed as a major contributor to the negative outcomes that can be seen in this field.
STATEMENT OF CLINICAL RELEVANCE.
This paper helps clinicians to understand better why some individuals develop TMDs and why some of them progress to becoming chronic patients. Iatrogenic damage and overtreatment should be avoided as much as possible, because that simply adds to the unlucky burden borne by patients whose destiny is to become a chronic TMD patient because of their particular vulnerabilities.
1. INTRODUCTION
Despite all the advances in pain management in the 21st century, there are always some pain patients who do not respond successfully to even the best modern treatments. These individuals become chronic pain patients, and the management strategies for such patients are more challenging and more complex; still, they often do not produce totally positive outcomes. This phenomenon of chronification and treatment resistance has been reported for several regional conditions such as low back pain, headaches and several types of oro‐facial pain, as well as a number of widespread pain disorders like fibromyalgia.1, 2
Among the oro‐facial pain conditions, the most prominent ones are the temporomandibular disorders (TMDs).3 These disorders afflict about 10%–15% of the population at a clinically significant level, that is with symptoms severe enough to require professional treatment. A much larger segment of the population is found to have relatively minor signs and symptoms of TMDs (eg painless clicking, occasional functional jaw pain, limited or deviated jaw opening) when population survey studies are done, but they are not the subject of discussion here.4 Among the patients who do have initial onset of significant symptoms, a relatively high percentage (around 75%–80%) may respond positively to current biopsychosocially oriented conservative treatments5, 6; but patients with longstanding histories of untreated TMD pain usually prove to be quite difficult to treat.7, 8
Based on a variety of studies conducted in recent years, some of the factors that might contribute to the negative treatment responses of some TMD patients have been elucidated. Many of these factors are intrinsic to the individual patients and thus should be regarded as vulnerability factors. These include anatomical features (including central nervous system susceptibility), genetic variables, history of past and/or present pain disorders in other body sites and psychological issues.9, 10, 11, 12 Unfortunately, since the TMD field also includes a number of irreversible treatment procedures that can produce physical and psychological negative outcomes,13, 14, 15, 16 the topic of iatrogenesis becomes another major consideration in discussing possible pathways to the chronification of TMD symptoms.
In this paper, we intend to describe known vulnerability factors that make individuals susceptible to developing TMDs, as well as those that contribute to the perpetuation of such problems. In addition, the topic of iatrogenesis will be discussed as a major contributor to the negative outcomes that can be seen in this field. The patients who present with massive occlusal and jaw‐repositioning changes combined with persistent severe oro‐facial pain are not a rarity within TMD and oro‐facial pain centres; these patients are the most difficult ones to manage because of this horrific combination of negative factors.
2. PATIENT VULNERABILITY FACTORS
2.1. Anatomical factors
It is obvious that not all human beings are equally susceptible or resistant to developing various clinical medical problems. During their lifetime, some people will develop systemic disorders such as diabetes or auto‐immune disorders, while others will develop regional painful conditions (eg low back pain, headaches, tendinitis) or non‐painful conditions (eg tinnitus, vertigo, clicking joint). Within the orthopaedic domain, some people will have more injuries or disabilities, while others seem to be remarkably resistant to having such problems despite engaging in vigorous and strenuous repetitive activities. This tendency towards developing various clinical conditions is collectively described as ‘patient vulnerabilities’. A prominent role among those vulnerability factors belongs to a variety of anatomical factors that predispose people to certain problems.
Within the field of TMDs, the three main anatomical structures that are most commonly involved in clinical signs and symptoms of those disorders are the muscles, joints and discs. TMD patients are not always able to accurately describe which of these tissues are causing their pain and dysfunction, but careful examination and thorough history‐taking will generally reveal where the problems are located. In this section, the typical vulnerabilities of each tissue will be described briefly, concluding with a discussion about the role of the peripheral and central nervous system in the remission or progression of painful TMDs.
2.1.1. Muscles
The masticatory muscles as a whole are remarkably strong and capable of performing a multitude of functional tasks. The four major muscles (masseter, temporalis, pterygoids and suprahyoids) are comprised of various types of fast and slow‐acting fibres that enable the human jaw to carry out those tasks without usually developing fatigue or PAIN. However, it is not uncommon to hear some patients complain of various limitations, such as fatigue with activities like singing, cheerleading, chewing hard foods, playing musical instruments or even going through dental appointments. Similarly, while most sleep bruxism patients do not report pain upon awakening, certain individuals develop TMD symptoms as a consequence of their sleep‐time muscle activities.17, 18 On one hand, the amount and type of muscle work is emerging as a key factor to explain clinical symptoms; on the other hand, research is needed to get deeper into the possible role of individual muscle weakness and vulnerability.19, 20 As a general rule, it should be pointed out that patients who report muscular symptoms elsewhere in their bodies are more likely to develop pain in their masticatory muscles.21
2.1.2. Joints
The human TMJs are always loaded, and this load increases with certain oral behaviours, either during wakefulness or sleep—especially prolonged clenching and bracing. Some individuals report significant symptoms as a possible result of that increased load. Development of non‐painful internal derangements or painful osteoarthritis (OA) in the TMJ is not well understood in terms of aetiology,22 but these changes render people more susceptible to developing chronic symptoms in their TMJs.23, 24 Again, if those patients have arthritis in multiple joints (or more significantly, if they have systemic connective tissue disorders), the likelihood of developing soreness in their TMJs will be increased.25, 26
2.1.3. Discs
The human TMJ is somewhat unique in having a full‐sized articular disc interposed between the bony articular surfaces. It has been postulated that the ‘reason’ for having such a structure in this joint is to compensate for the incongruities of the articular surfaces (ie a ball on a hill that is both rotating and sliding). The main issue is that the human TMJ disc is remarkably capable of becoming displaced, generally to a more forward and medial position; this can be a totally benign situation, but in some cases it can evolve to become a significant clinical problem of pain and dysfunction.27, 28 These TMJ disc displacements are found in over 1/3 of the adult population, but fortunately they do not become seriously problematic in a large percentage of that group.29, 30
Many theories have been offered to explain why the TMJ disc slips forward in so many people.31 Weakness of the medial and lateral collateral ligaments certainly accounts for some of those displacements, and frictional resistance due to early OA changes on the bony surfaces probably contributes to that as well.32, 33 Many other anatomical theories have been postulated, but the bottom line is that TMJ disc displacement is a very common ‘vulnerability’ in the normal population that is usually clinically insignificant, but which can become quite a problem in some cases.
2.1.4. Nerves
In addition to the jaw muscles and joint components, there is another ‘tissue’ that must be considered in any discussion about pain, namely the nervous system that transmits pain signals from the periphery to the central nervous system (CNS). Ever since Melzack and Wall first presented the gate control theory in the 1960s,34 a number of operational features about how the human nervous system works have been elucidated. One of the most important features is the transition from acute pain to chronic pain, which can occur anywhere in the body; this transition is described as chronification.35, 36 The main difference between these two conditions is that acute pain represents transmission of nociceptive impulses from the site of injury or disease to the CNS that act as a warning system to prevent further damage, and therefore, it is biologically adaptive. However, chronic pain does not necessarily depend upon any nociceptive stimuli, so it is self‐sustaining even in the absence of the original injury or disease, if any, and therefore it has no biological adaptive purpose. This condition generally is maintained by the phenomenon of central sensitisation and belongs to the category of neuropathic pains.37 The most striking example is the phantom limb phenomenon, in which a body part is completely removed but the pain originally associated with it is still present. People often say that any pain lasting more than 6 months is chronic, but this is an arbitrary definition and assumes that no continuing pathology is present.
Regarding the TMJ and related structures, it is well known that for some patients an initial acute pain condition may be improved or resolved by treatment, but others may continue to report pain. Since central sensitisation within the trigeminal system is not uncommon, it is reasonable to assume that many of those patients are experiencing chronic neuropathic pain with symptoms localised in the TMJs and/or the jaw muscles.38, 39 In this paper, we will be considering this phenomenon as part of our analysis of transitioning from acute to chronic TMD pain.
2.2. Psychosocial factors
It is well accepted that establishing the physical diagnosis of a putative TMD patient should be integrated with an evaluation of the psychosocial status.40 This information‐gathering process is generally described as Axis I for the physical components and Axis II for the psychosocial components. The associations between various painful TMD conditions and several psychological issues (eg anxiety, depression, somatisation) and social (eg quality of life) features have been repeatedly described in many clinical studies.41 Such an association is not just an ancillary finding, but it has a strong influence on the clinical diagnosis, and therefore also on the treatment outcome. Within the framework of a biopsychosocial model of pain, the standard of reference for TMDs is represented by the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) guidelines, which offer a number of psychometric instruments to obtain an Axis II evaluation.42 As in all medical fields involving pain evaluation procedures, clinical experience and thoughtful questioning of the patient are critical factors for integrating and utilising this kind of assessment. In general terms, the more compromised the psychosocial axis, the more difficult the clinical management of the case will be.
Some of the risk factors for transitioning to chronic TMD can be identified by the use of biopsychosocial assessment.43, 44, 45 For example, high scores in the Characteristic Pain Intensity index and the presence of myofascial pain in the early phases are the strongest predictors for TMD chronification.46 Prospective studies on the efficacy of early biobehavioural interventions have shown that such strategies contributed a lot to reduce risks for chronification.47, 48 However, these findings came mainly from the same few groups of researchers and, more notably, they cannot help in providing individually tailored strategies for the management or prevention of chronic TMD pain. As a general remark, it must be borne in mind that this field of oro‐facial pain research intersects with the neurological and psychological areas and represents the real boundary line between acute, overload‐related, transient TMD symptoms and the more complex chronic oro‐facial pain conditions. Based on this premise, the psychosocial issues that play a potential vulnerability role in the transition from acute to chronic TMD pain can be summarised as follows:
2.2.1. Factors identified with the DC/TMD AXIS II
It is a common observation to all investigations on TMD epidemiology that patient populations have a higher prevalence of psychological distress than healthy individuals. In particular, chronic TMD pain populations generally produce the highest psychometric scores.49, 50 Early studies on depression and somatisation levels as measured by the SCL‐90R (included in the RDC/TMD Axis II) showed a link with the presence of pain.51 Catastrophism has also been depicted as a personality trait typical of patients at risk of developing chronic TMD pain.52 Anxiety evaluation has been included in the updated DC/TMD.42 The single and multiple variable analyses performed on data retrieved for the Orofacial Pain Prospective Evaluation and Risk Assessment (OPPERA) study showed that, among all psychological factors, measures of somatic symptom burden showed the strongest associations with the presence and number of chronic oro‐facial pain conditions. Additional psychological variables that showed significant associations with individual chronic oro‐facial pains and their overlap included negative mood, perceived stress and pain catastrophising.53, 54
In general, all these vulnerability factors may predispose to the development of chronic pain directly (eg lower mood, high tendency to worry) or by indirect mechanisms (eg abnormal stress sensitivity, tendency to develop hypervigilance, prolonged muscle bracing resulting in muscle fatigue and joint overload). For the treating doctor, it is important to recognise that when the presence of these factors is suspected or detected in the acute TMD pain patients, early referral to a proper professional is recommended to set an early psychosocial intervention and reduce the risk for symptom chronification.
2.2.2. Illness behaviour and Munchausen syndrome
Every individual copes with illness by developing strategies, in the form of actions or reactions, that aim to obtain physical or emotional relief from perceived or actual illness. Beginning in childhood, each person develops a specific combination of coping strategies; that combination of behaviours is called ‘illness behaviour’, and this phenomenon has been the target of several studies and psychological theories over the past decades.55 According to Mechanic, one of the pioneers of such studies, it involves the manner in which an individual monitors his/her body, defines and interprets the symptoms, takes remedial action and uses various sources of help as well as the more formal health‐care system. The deriving behaviour is modulated by variables that pre‐exist and are an intrinsic component of the patient's personality traits. It is quite intuitive to appreciate that these different perceptions, evaluations and responses to illness may have a strong impact on the extent to which symptoms interfere with usual life routines, chronicity, attainment of appropriate care and co‐operation of the patient in treatment.56 Pilowsky57 further elaborated the concept of abnormal illness behaviour by proposing the existence of clinical conditions characterised by a maladaptive mode of coping with illness.
The two extreme characteristics of illness behaviour are negative outlook and the sick role, both of which may be seen in some patients with TMD symptoms.58 Concerning the former, there is no specific literature that has addressed it in the TMD field; nevertheless, it is a factor that cannot be underestimated in the clinical setting. For instance, individuals who have mild pain or a clicking joint may attempt to utilise self‐coping strategies without asking for professional advice, thus potentially leading to a worsening and chronification of the clinical condition. Patients may avoid performing normal mandibular movements simply to avoid hearing the click or feeling pain. However, this is a strategy that, despite being seemingly protective and sensible, could actually lead to intra‐articular adhesions and/or to progressive functional limitation.
As for the sick role, it refers to inappropriately exaggerated over‐reaction to a physical condition.59 Secondary gain and certain cognitive factors are fundamental to explain this attitude, which sometimes produces the so‐called ‘Munchausen's syndrome’, viz., a psychological disorder where someone pretends to be ill or deliberately produces symptoms of illness in themselves.60 The main intention of these individuals is to assume the sick role so that people care for them, and they are the focus of attention. It is not uncommon for TMD practitioners to see individuals who have spent years travelling from doctor to doctor for a multitude of purported symptoms in the oro‐facial area and/or many other body regions. Due to the patient's willingness to receive care at any cost, overtreatment is a serious concern.
Another unfortunate variant of the sick role is the Munchausen syndrome by proxy, in which parents or relatives ‘want’ to have a family member sick in order to have a relationship with that person that is continuously dependent on them to seek ongoing care.61 This situation is not rare among TMD patient populations, especially in the case of some parents who do not accept easy explanations about the benign nature of clicking sounds or mild TMJ pain in their children or siblings. The influence of a parent's behaviour towards their child regarding injury and/or pain has a great impact on that person's presentation of pain behaviour and suffering in adolescent years and into adulthood. These individuals often dramatise every symptom of their proxy candidate and generally neglect their possible psychosocial origin. These two conditions are currently recognised in the DSM‐V as Factitious Disorder ‘imposed on self (FDIS)’ or ‘imposed on another (FDIA)’.62
2.2.3. Lack of acceptance of non‐mechanical approaches
There is now consensus that conservative approaches to initial TMD management, including regimens of cognitive‐behavioural approaches to reinforce counselling strategies and to control negative habits, are most appropriate for the initial management of TMDs.63 Symptom mildness, self‐remittance and fluctuations of pain are fundamental factors to explain treatment success in most cases.64, 65, 66 Controlling psychological reaction to daily stress and diminishing hypervigilance to anxiety stimuli are key factors to reduce jaw muscle tension and joint overload. However, given the psychological implications of such suggestions, which require self‐appraisal of some personality traits or personal problems that are potentially perceived as taboos, some patients may be reluctant to accept such counselling approaches and cognitive‐behavioural treatments.
As a result of this attitude, some individuals do not want to engage themselves in self‐care or cannot accept ‘easy’ solutions based on physical treatments or physiotherapy. In addition, they may be reluctant to take medications, because they instead want the doctor to ‘fix the problem’. Similarly, such patients are sceptical about the use of oral appliances that are recommended as simply being symptom‐relieving temporary crutches.67 Instead, they have read or heard about the more elaborate forms of splint therapy that will realign their displaced jaw and correct their occlusal ‘discrepancies’, and that is the kind of therapy they want.68
For practitioners working in tertiary centres, such patients are not rare. They generally have a combination of the two psychological traits discussed in the previous sections. Typically, they are in search for mechanical explanations of their problem due to belief systems implanted by previous dentists who emphasized mechanical concepts.69 Such patients were the majority in the middle decades of the past century, until the emergence of the first theories on the role of psychological factors.70, 71 This phenomenon is currently amplified by the number of self‐proclaimed ‘experts’ that are easily found on social network communications, who advocate dental occlusion‐based treatments for any sort of body and mind disease by taking advantage of the benign nature of most TMD conditions and the psychological frailty of some individuals. As a result of these biases and previous exposures, such patients are often hard to save from overtreatment.
2.3. Genetic factors
Genetic factors are part of any multiple variable predictive or analytical model that tries to explain the pathophysiology of specific medical conditions. In the field of TMDs and oro‐facial pain, genetic variables may influence a patient's vulnerability to develop a particular pain problem as well as the possibility of that pain becoming chronic. This can occur within two main frameworks: first, genetic factors determine many aspects of anatomical development (ie musculoskeletal features representing a structural weakness). Secondly, genetic factors play a major role in the development of CNS structure and function (ie neurological and psychological features that enhance the mechanisms outlined in the previous section). For instance, it is well known that bruxism partly runs in families.72, 73 Regarding the main issues being discussed in this paper, it has been shown that several genetic features are common in patients who are more at risk to develop oro‐facial pain or to be poor treatment responders, and these will be discussed below.
The recent large‐scale OPPERA study identified some genetic risk factors for first‐onset oro‐facial pain.74 Concerning the development of musculoskeletal pain in the oro‐facial area (ie TMDs), no specific single‐nucleotide polymorphism (SNP) was significantly associated with risk of initial TMD onset. On the other hand, a deeper analysis of more than 300 genes revealed twelve SNPs as risk factors for either the development of chronic TMD or for being associated with intermediate phenotypes for TMD. Examples of association with intermediate phenotypes include a serotonergic pathway in which multiple SNPs influenced risk of chronic TMD, as well as some gene‐environment interactions that have effects on stress‐related pain modulation by variation in the gene encoding catechol O‐methyltransferase (COMT).
The list of single‐nucleotide polymorphisms associated with intermediate phenotypes that are predictive of TMD onset is quite extensive, but some of the major ones are summarised here75, 76:
Non‐specific oro‐facial symptoms were associated with voltage‐gated sodium channel, type I, alpha subunit (SCN1A, rs6432860) and angiotensin I‐converting enzyme 2 (ACE2, rs1514280);
Global psychological symptoms were associated with prostaglandin‐endoperoxide synthase 1 (PTGS1, rs3842803);
Stress and negative affectivity were associated with amyloid‐β (A4) precursor protein (APP, rs466448);
Heat pain temporal summation was associated with multiple PDZ domain protein (MPDZ, rs10809907).
These findings are of importance to outline a big picture for future genetic studies. Based on them, we now know that those kinds of studies should be focussed on finding genetic markers and risk factors for intermediate phenotypes associated with TMD, because they are the key to understanding indirect genetic predisposition to developing those disorders.
In the clinical setting, this means that individuals with certain psychological traits, presence of comorbid conditions and certain abnormal clinical manifestations can be regarded as being genetically predisposed to first‐onset TMDs and to chronification of symptoms. The challenge will be to develop better tools for identifying all of these characteristics, thereby leading to improved clinical management.
3. SUMMARY OF VULNERABILITY FACTORS
The combination of potential TMD patient vulnerability factors as described above is fundamental to understanding why some individuals may develop one or more of the TMDs. Some of those factors also play an important role in determining who will develop a chronic version of their condition.
At the present time, there is no standard set of treatment modalities or approaches for any of the various TMD conditions that is universally agreed upon although there are widely recognised guidelines for appropriate management of most of these conditions.77 As a result, there is considerable controversy over the reasons for acute TMD patients to fail to respond to their initial treatment. Furthermore, the prevention or management of chronic TMD cases remains a significant challenge, as it does for most chronic pain conditions. Considering the vulnerability factors described in the previous sections, it is important to notice that, even if anatomical factors may not be directly changed, the clinician can at least try to adapt/reduce the load or overuse. In addition to that, psychosocial factors that make patients more vulnerable to develop chronic pain should be screened for and action taken whenever possible.
In the clinical setting, the situation is complicated by the possibility of iatrogenic treatment factors making things even worse. In the next section, we will discuss the many ways in which inappropriate treatments for TMDs may play a role in the failure to respond to initial care as well as in the development of chronic symptomatology. Because of the irreversible nature of many current treatments proposed for management of TMD patients, there are some extremely negative outcome scenarios that are comparable to ‘failed back syndrome’, due to the structural changes produced by those diverse radical treatments.
4. IATROGENESIS
According to most dictionary definitions, iatrogenesis is the causation of a disease, a harmful complication, or other ill effect by any medical activity, including diagnosis, intervention, error or negligence. The topic of iatrogenesis is quite complicated, mainly because the failure of a patient to respond to professional treatment may be due to multiple factors. For example, the compliance or non‐compliance of the patient can have significant effects on treatment outcomes. If a patient has a condition that requires medications, it is essential to take the correct dosages at the correct intervals, if a patient is told to restrict or perform certain activities (exercises, rest, relaxation procedures, etc.), those instructions must be followed; if a specific home self‐care regimen is recommended, the patient needs to be responsible in carrying out those procedures. Failure to do these things will probably reduce the chances for recovery, and the doctor may be inappropriately blamed. To a certain extend, iatrogenic problems may even start with the difficulties for patients to access proper information by searching through media outlets, Internet browsers and professional websites. Regarding this issue, an interesting paper pointed out that a patient's search for ‘expert’ opinions on the websites of self‐advertised TMD professionals via Google search was more likely than not to lead to practitioners who advocated irreversible treatments.78
In discussing the specific actions of a clinician, there are a number of possible iatrogenic ‘sins of omission’ as well as ‘sins of commission’. Beginning with the initial diagnostic visit, an oro‐facial pain problem could be due to one or more disorders—with TMD being among them. But if a non‐TMD patient is misdiagnosed as having a TMD, two problems are immediately created: (1) the correct diagnosis is not being addressed, and (2) the patient may be subjected to a series of TMD treatments that are either worthless, or potentially harmful if they are irreversible.
When care is initiated for a correctly diagnosed TMD patient (including the specific type of TM disorder as well as the presence of other comorbid pain conditions), it should be individually tailored to the specific case. A common mistake is the under‐treatment of the condition due to lack of knowledge about what should be done; for example, a patient who has myofascial pain may be told simply to take OTC analgesics every four hours, or a prescription may be given for a muscle relaxant that is too weak to be effective. Failure to explain that type of problem adequately and to instruct the patient about relevant home care is another aspect of under‐treatment, and it will not be surprising if the patient reports little or no improvement. Failure to control pain early in the process has been shown to be a major risk factor for progression to chronic pain.
4.1. Joint repositioning
Another major source of potential bad outcomes could be the ideological viewpoint of the treating dentist, especially if it is applied universally to diverse types of TMD patients. For example, the well‐known 20th century concepts of malpositioned condyles and/or occlusal disharmonies have been applied to both muscular and articular TMDs; even after many of those concepts were either disproved or found to be lacking supporting evidence, many dentists have continued to treat patients according to those theories. Because those aetiologic concepts generally required irreversible bite‐changing and jaw‐repositioning procedures, the potential for iatrogenic harm was increased accordingly. These problems have been addressed in a large number of papers in recent years, and current guidelines from the National Institute of Dental and Craniofacial Research (NIDCR),79 American Academy of Orofacial Pain (AAOP)77 and International Association for Dental Research (IADR)80, 81 all recommend a more conservative approach to managing TMD problems. Specifically, the paper by Greene and Obrez82 about the lack of medical necessity for jaw repositioning targeted this matter directly, and the more recent paper by the current authors about a ‘Third Pathway’ (ie the mechanistic theories of the 20th century) has argued for abandonment of that approach completely.14
4.2. Abuse of oral appliances
Perhaps the most important controversial element in the TMD field has been the oral appliance, also referred to by some as ‘occlusal splint’ or ‘orthotic device’. Several recent papers have been written about potentially valuable applications of those devices as part of a reversible and conservative treatment programme for certain types of TMDs.83, 84 However, many dentists still view such appliances as devices that can make the jaw muscles relax and settle into a more correct position, therefore being the first step of a two‐phase treatment programme. As a result, their use becomes the basis for major dental procedures for changing jaw positions and occlusal relationships. This means that the original occlusal maximum intercuspal position (MIP) will no longer coincide with the jaw position, and it is likely that a ‘corrective’ major dental procedure, a.k.a. ‘phase two’, will be performed independent of any curative effect on the original pain problem.
Some dentists use oral appliances as so‐called deprogrammers, which essentially guarantees that the mandible will slide somewhere along the articular eminence to a new position. Some clinicians even argue for such use of oral appliances for orthodontic diagnosis or for planning extensive prosthodontic treatments in a new centric relation. All those theories refer to some questionable philosophies derived from old gnathological principles, which have been criticised severely in previous publications and often contrast to each other.85, 86, 87
4.3. Use of ‘diagnostic’ technological instruments
Two groups involved in these mechanistic approaches deserve some special attention, namely those whose procedures are based on the search for either an ideal neuromuscular‐guided position or an ideal condylar‐guided position. These are two variants of the prosthodontic concept of an optimal jaw relationship. The adherents to these approaches often base their clinical diagnoses and treatments on the outcomes of using various electronic or mechanical devices such as electromyography (EMG), sonography, jaw tracking, muscle stimulators, condylography, axiography and even postural platforms. Regardless of what the specific TMD diagnosis might be, these protocols inevitably lead to some form of repositioning of the mandible as well as the establishment of a new vertical dimension. As the Figures 1 and 2 show, some amazingly bizarre oral appliances may be provided, and subsequent weird jaw relationships can be produced. In some cases, patients may experience pain relief for non‐specific reasons that are not related with the purported ideal mandible position, but the clinician is convinced that the ideal jaw position has been established. On the other hand, in many cases appliances are not successful in relieving the original pain, but the clinician still believes that the mandible is in the correct position anyway. In either case, when phase two treatment is provided following prolonged use of these kinds of appliances, irreversible bite changes are produced. Currently, there are no clear protocols for treating such patients who now have both chronic pain and a very distorted occlusal condition. Space does not permit a full discussion of the fallacies of following these instrumental approaches to the management of TMD conditions, so the reader is recommended to look at the cited papers to see what scientific investigations of this approach have shown.88, 89, 90, 91, 92, 93
FIGURE 1.
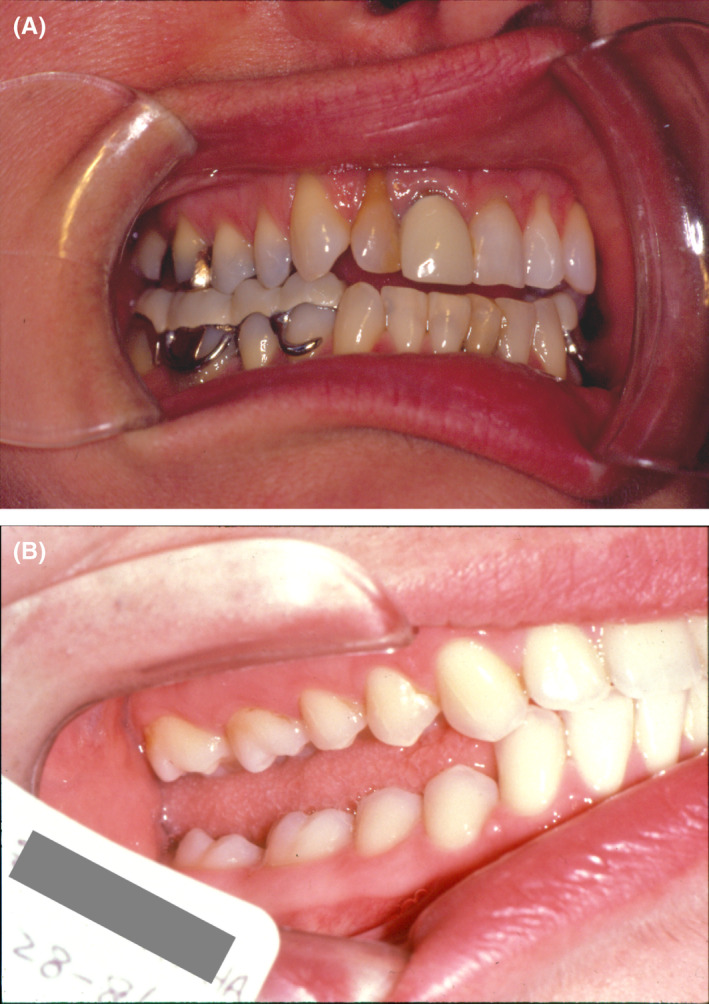
Back in the ‘80s, some practitioners used MORA appliances to manage TMDs (A). Those kind of devices carried the risk for iatrogenic posterior open bite (B)
FIGURE 2.
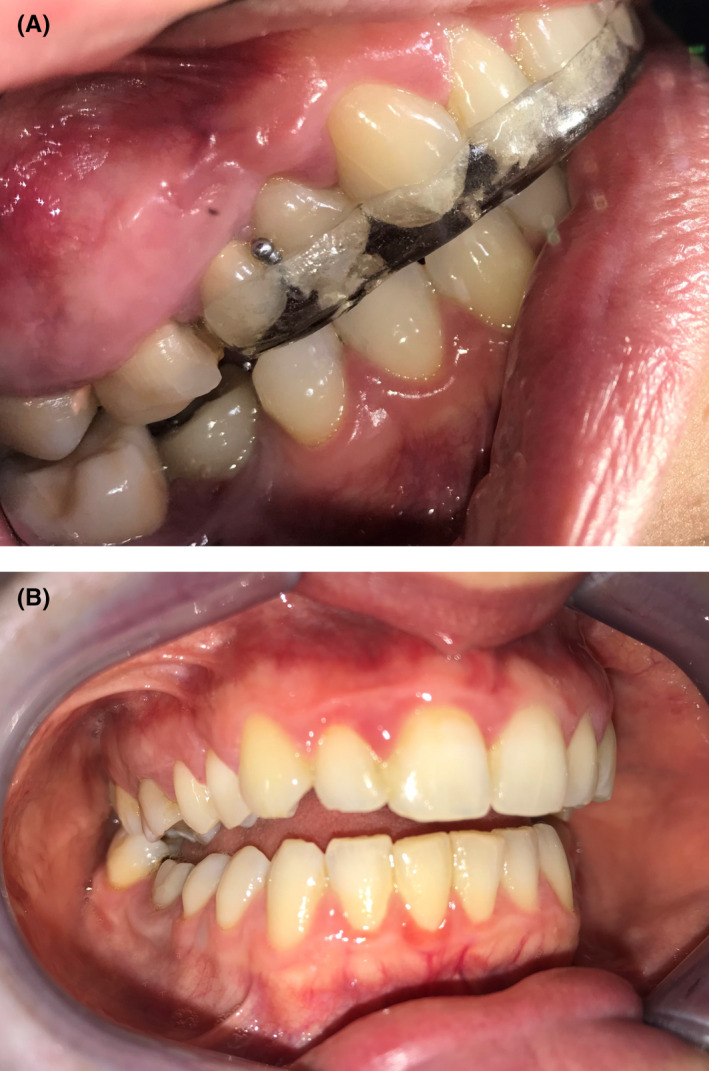
More recently, some other practitioners started recommending partial coverage devices (A), which carry the risk for iatrogenic anterior open bite (B)
4.4. Nocebo
Within the framework of unnecessary interventions that may be even harmful, the nocebo effect generated by the concepts and procedures of the patients’ previous treating doctors cannot be neglected.94 It is not uncommon among OFP specialists to receive patients who believe their mouth opening pattern is not correct, who think their biologically normal facial asymmetry has to do with their symptoms, or who insist their TMJ click sound must be solved by recapturing the disc at any cost to avoid a future closed lock. Others believe they have problems related with dental occlusion, just because of the purported diagnoses received from previous practitioners, and they are searching for the expert who can finally solve those occlusal problems. Patients who are focussed on their occlusion and use a lot of dental terminology can be very persistent despite a history of treatment failures. They still believe they have to find the right ‘specialist’ who will be able to finally solve their problems.
4.5. Perseverance with ‘one concept fits all’ approaches and patients’ disappointment
Among the commission mistakes, there is also the tendency for some clinicians to prosecute a certain favourite treatment protocol for all types of TMD cases. Regardless of whether this is a conservative treatment regimen or a more radical one as described above, a ‘one size fits all’ approach that fails to recognise the multifaceted nature of TMD problems and ignores continuing pain symptoms carries the risk of pain chronification.
Furthermore, it must be borne in mind that treatment failure can also affect the patient's psychological attitude, carrying the risk for entering a vicious mental loop, with catastrophising and a pessimistic attitude towards clinicians as a consequence of previous bad experiences. The failure to improve may produce that mental loop, even if the treatment is ‘modern and proper’, but of course it is much worse if bad treatment is being provided.
4.6. Intra‐capsular treatments
Many patients who have an intra‐capscular TMD problem may need to have something done inside the joint, similar to what is being done currently in other joints as well. For example, if the initial conservative treatment plan for a patient who has an articular problem is not sufficiently working, one possibility is to perform TMJ arthrocentesis with subsequent injection of various medications and compounds (eg steroids, hyaluronic acid, platelet‐rich plasma, etc.) that may reduce the inflammatory pain and facilitate restoration of function. There is little chance of iatrogenic damage with these procedures; rather, they may be helpful or they might not, and then other options can be considered. In general, the procedure of arthrocentesis carries little risk if done properly, and it has been shown to be quite effective in managing cases of ongoing arthritic pain and/or limited mobility due to disc interference.95
Historically, there were several surgical approaches to treat TMJ OA that frequently did not work out well and did produce iatrogenic damage in many cases. Procedures such as condylar shaving, partial condylectomy and condylotomy were in vogue at one time or another over 50 years ago, and the outcomes were often quite poor. However, when disc derangements became the focus of many clinicians starting in the 1970s, a number of new procedures were tried—usually based on the false assumption that an untreated anterior disc displacement (ADD) would eventually lead to severe OA and closed lock. Attempts were made to either reposition the displaced disc or to remove it entirely, with the latter procedure being followed by insertion of an alloplastic implant. It would not be an exaggeration to say that this period was one of the darkest in terms of iatrogenic damage, because the material used initially was a Proplast‐Teflon or Silastic sheet that turned out to be a very bad choice. Not only did many patients fail to improve, but as these implant sheets began to break down they released particles of that material into the bloodstream; eventually, this led to the Food and Drug Administration (FDA) in the USA recalling all such implants.96
Even today, the maxillofacial and oro‐facial pain literature includes plenty of articles describing case series of patients undergoing multiple (eg even more than 10) TMJ surgeries.97 In addition to possible pain chronification related with the repeating of various procedures, many cases of multiple surgeries are due to an erroneous initial diagnosis. A muscular TMD that is erroneously treated as being due to intra‐capsular problems, or neuropathic pains localised at the TMJ but misdiagnosed as articular disorders, are two examples of these situations.
In recent times, the concept of disc repositioning has made a comeback due to some new procedures for anchoring the disc to the condyle with some kind of pin. Unfortunately, this perspective ignores the tremendous accumulation of evidence that most ADD situations do not require any sort of disc repositioning procedure, and that a displaced disc has irreversible degeneration both at the micro‐level (ie histology) and macro‐level (ie morphology).16 Most patients can live successfully with a displaced TMJ disc—regardless of whether it is reducing or not upon opening. Nevertheless, it is not rare that an unfavourable surgical journey starts with an intervention aiming at disc repositioning, which is something that even lacks any clear indication to proceed98 (Figure 3).
FIGURE 3.
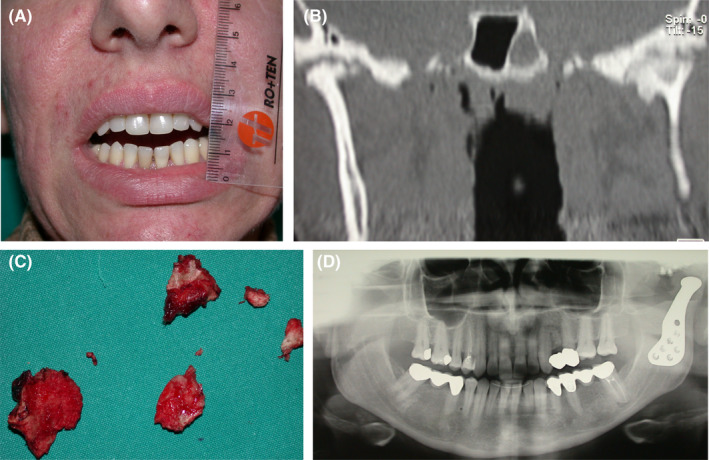
Frontal picture showing restricted mouth opening in a 42‐year‐old female patient who underwent three failed TMJ surgeries for disc repositioning (A). Computerised tomography shows ankylosis of the left TMJ, as a result of the previous surgeries (B). Surgical removal of the bone block (C) and the positioning of a full TMJ prosthesis (D) led to the restoration of mouth opening and resolution of the iatrogenic bony ankylosis (Surgery performed by Luca Guarda Nardini, MD, Hospital of Treviso, Treviso, Italy)
Finally, we now have artificial TMJ prostheses that can replace the entire TMJ bony complex.99 This is clearly a scientific advance overall, just as it is for other joints in the human body. However, a lot depends on proper diagnosis and excellent workmanship in order for these prosthetic joints to be successful. Equally important, the clinician must be prepared to deal with the chronic pain while treating such patients, since the prosthesis itself merely provides a good mechanical substitute for the original TMJ.
5. CONCLUSIONS
The combination of patient vulnerability factors and iatrogenic damage as described in this paper explains a lot about why some people develop TMD problems, with some of them progressing to chronic versions of those disorders. In the course of looking at the larger issue of risk factors, the OPPERA study found that there were four main conditions that were significantly present in first‐onset TMD cases: mental disorders, pain elsewhere in the body, sleep‐related disorders and local oro‐facial symptoms.100 In one sense, these constitute another aspect of vulnerability or susceptibility in determining who is most likely to become a TMD patient.
For the clinician, the question to be asked at this point is: what can we do with this information to prevent or mitigate the development of a TMD? Unfortunately, we have little or no control over the three main vulnerability factors described in this paper: anatomical, psychosocial history and genetics. However, the risk factors found in the OPPERA study can either heighten the clinician's awareness of potential TMD development, or in some cases they can be managed (eg mental disorders, sleep‐related disorders) not only for their own sake, but also to minimise the risk of developing a TMD condition. Finally, the progression of pain symptomatology in the trigeminal nervous system can often be avoided by aggressive early treatment of the pain associated with most TMDs.
On the other hand, the possibilities of iatrogenic damage can be reduced or prevented entirely by the choices made in each TMD case. Some people may be disturbed by the proposition that their favourite treatment concept has the potential to either fail in relieving the problem, or even to make it worse. However, the powerful counter‐argument to that viewpoint is the well‐documented successes reported by following the conservative TMD treatment philosophies embedded within the biopsychosocial framework. The key differentiating variable can be summarized in one simple question: Is the proposed treatment plan going to cross the line from reversible to irreversible, or not? As Reid and Greene13 pointed out in their paper on the ethical aspects of current TMD practices, every patient deserves to receive the least invasive treatment possible when there are competing theories within a given field of medicine. Clearly, this perfectly describes the situation in the TMD field, where we still see a lot of mechanistic treatment despite repeated calls to abandon the ‘Third Pathway’ of jaw repositioning and occlusion‐changing concepts and procedures. This is not merely an academic argument—it has clinical implications for both doctors and patients.
Therefore, the authors hope that the information presented in this paper will help clinicians to understand better why some individuals develop TMDs, why some of them will progress to becoming chronic patients, and what the appropriate responses may be. In addition, we hope to persuade readers to avoid causing iatrogenic damage as much as possible, because that simply adds to the unlucky burden borne by patients whose destiny is to become a chronic TMD patient because of their particular vulnerabilities.
CONFLICT OF INTEREST
None.
Greene CS, Manfredini D. Transitioning to chronic temporomandibular disorder pain: A combination of patient vulnerabilities and iatrogenesis. J Oral Rehabil. 2021;48:1077–1088. 10.1111/joor.13180
REFERENCES
- 1.Flynn DM. Chronic musculoskeletal pain: nonpharmacologic, noninvasive treatments. Am Fam Physician. 2020;102:465‐477. [PubMed] [Google Scholar]
- 2.Rajput K, Vadivelu N. Acute pain management of chronic pain patients in ambulatory surgery centres. Curr Pain Headache Rep. 2021;25:1. 10.1007/s11916-020-00922-3 [DOI] [PubMed] [Google Scholar]
- 3.Scrivani SJ, Keith DA, Kaban LB. Temporomandibular disorders. N Engl J Med. 2008;359:2693‐2705. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin SF, Huggins KH, Leresche L, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273‐281. [DOI] [PubMed] [Google Scholar]
- 5.Randolph CS, Greene CS, Moretti R, Forbes D, Perry HT. Conservative management of temporomandibular disorders: a post treatment comparison between patients from a university clinic and from a private practice. Am J Orthod Dentofacial Orthop. 1990;98:77‐82. [DOI] [PubMed] [Google Scholar]
- 6.Manfredini D. No significant differences between conservative interventions and surgical interventions for TMJ disc displacement without reduction. Evid Based Dent. 2014;15:90‐91. [DOI] [PubMed] [Google Scholar]
- 7.Jessri M, Sultan AS, Tavares T, Schug S. Central mechanisms of pain in orofacial pain patients: implications for management. J Oral Pathol Med. 2020;49:476‐483. [DOI] [PubMed] [Google Scholar]
- 8.McCloy K, Peck C. Common factors in the presentation and management of chronic temporomandibular disorders and chronic overlapping pain disorders. J Oral Pathol Med. 2020;49:454‐460. [DOI] [PubMed] [Google Scholar]
- 9.Naylor B, Boag S, Gustin SM. New evidence for a pain personality? A critical review of the last 120 years of pain and personality. Scand J Pain. 2017;17:58‐67. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin SF. Perspectives on the interaction of biological, psychological and social factors in TMD. J Am Dent Assoc. 1994;125:856‐863. [DOI] [PubMed] [Google Scholar]
- 11.Svensson P. Muscle pain in the head: overlap between temporomandibular disorders and tension‐type headaches. Curr Opin Neurol. 2007;20:320‐325. [DOI] [PubMed] [Google Scholar]
- 12.Manfredini D, Segù M, Arveda N, et al. Temporomandibular joint disorders in patients with different facial morphology: a systematic review of the literature. J Oral Maxillofac Surg. 2016;74:29‐46. [DOI] [PubMed] [Google Scholar]
- 13.Reid KI, Greene CS. Diagnosis and treatment of temporomandibular disorders: an ethical analysis of current practices. J Oral Rehabil. 2013;40:546‐561. [DOI] [PubMed] [Google Scholar]
- 14.Greene CS, Manfredini D. Treating temporomandibular disorders in the 21st century: can we finally eliminate the “Third Pathway”? J Oral Facial Pain Headache. 2020;34:206‐216. [DOI] [PubMed] [Google Scholar]
- 15.Israel HA. Internal derangement of the temporomandibular joint: new perspectives of an old problem. Oral Maxillofac Surg Clin North Am. 2016;28:313‐333. [DOI] [PubMed] [Google Scholar]
- 16.Guarda Nardini L, Meneghini M, Guido M, Bacioni F, Manfredini D. Histopathology of the temporomandibular joint disc: findings in 30 samples from joint with degenerative disease. J Oral Rehabil. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romprè PH, Daigle‐Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a slope bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837‐842. [DOI] [PubMed] [Google Scholar]
- 18.Manfredini D, Lobbezoo F. Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e26‐e50. [DOI] [PubMed] [Google Scholar]
- 19.Raphael KG, Janal MN, Sirois DA, et al. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 2013;40:883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manfredini D, Ahlberg J, Wetselaar P, Svensson P, Lobbezoo F. The bruxism construct: from cut‐off points to a continuum spectrum. J Oral Rehabil. 2019;46:991‐997. [DOI] [PubMed] [Google Scholar]
- 21.Stalnacke C, Ganzer N, Liv P, Wanman A, Lovgren A. Prevalence of temporomandibular disorder in adult patients with chronic pain. Scand J Pain. 2020;21(1):41‐47. 10.1515/sjpain-2020-0077 [DOI] [PubMed] [Google Scholar]
- 22.Milam SB. Pathogenesis of degenerative temporomandibular joint arthritides. Odontology. 2005;93:7‐15. [DOI] [PubMed] [Google Scholar]
- 23.Emshoff R, Puffer P, Rudisch A, Gassner R. Temporomandibular joint pain: relationship to internal derangement type, osteoarthritis, and synovial fluid mediator level of tumor necrosis factor‐alpha. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:442‐449. [DOI] [PubMed] [Google Scholar]
- 24.Manfredini D, Basso D, Arboretti R, Guarda‐Nardini L. Association between magnetic resonance signs of temporomandibular joint effusion and disk displacement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:266‐271. [DOI] [PubMed] [Google Scholar]
- 25.Wenneberg B, Kononen M, Kallenberg A. Radiographic changes in the temporomandibular joint of patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Craniomandib Disord. 1990;4:35‐39. [PubMed] [Google Scholar]
- 26.Sidebottom AJ, Salha R. Management of the temporomandibular joint in rheumatoid disorders. Br J Oral Maxillofac Surg. 2013;51:191‐198. [DOI] [PubMed] [Google Scholar]
- 27.Dolwick MF, Dimitroulis G. A re‐evaluation of the importance of disc position in temporomandibular disorders. Aust Dent J. 1996;41:184‐187. [DOI] [PubMed] [Google Scholar]
- 28.Stegenga B. Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain. 2001;15:193‐205. [PubMed] [Google Scholar]
- 29.Naeije M, Te Veldhuis AH, Te Veldhuis EC, Visscher CM, Lobbezoo F. Disc displacement within the human temporomandibular joint: a systematic review of a “noisy annoyance”. J Oral Rehabil. 2013;40:139‐158. [DOI] [PubMed] [Google Scholar]
- 30.Manfredini D, Guarda‐Nardini L, Winocur E, Piccotti F, Ahlberg J, Lobbezoo F. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiological findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:453‐462. [DOI] [PubMed] [Google Scholar]
- 31.Manfredini D. Etiopathogenesis of disk displacement of the temporomandibular joint: a review of the mechanisms. Indian J Dent Res. 2009;20:212‐221. [DOI] [PubMed] [Google Scholar]
- 32.Nitzan DW. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. J Oral Maxillofac Surg. 2001;59:36‐45. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzi Poluha R, De la Torre CG, Martins Costa Y, Grossmann E, Bonjardim LR, Conti PC. Temporomandibular joint disc displacement with reduction: a review of mechanisms and clinical presentation. J Appl Oral Sci. 2019;27:e20180433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971‐979. [DOI] [PubMed] [Google Scholar]
- 35.Heinricher MM. Pain modulation and the transition from a cute to chronic pain. Adv Exp Med Biol. 2016;904:105‐115. [DOI] [PubMed] [Google Scholar]
- 36.Pozek JPJ, Beausang D, Baratta JL, Viscusi ER. The acute to chronic pain transition: can chronic pain be prevented? Med Clin North Am. 2016;100:17‐30. [DOI] [PubMed] [Google Scholar]
- 37.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Brennan MT. Differential diagnosis of orofacial pain and temporomandibular disorder. Dent Clin North Am. 2013;57:419‐428. [DOI] [PubMed] [Google Scholar]
- 39.Balasubramaniam R, Klasser GD. Orofacial pain syndromes: evaluation and management. Med Clin North Am. 2014;98:1385‐1405. [DOI] [PubMed] [Google Scholar]
- 40.Ohrbach R, Dworkin SF. The evolution of TMD diagnosis: past, present, future. J Dent Res. 2016;95:1093‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De la Torre CG, Barbosa Camara‐Souza M, Munoz Lora VRM, et al. Prevalence of psychosocial impairment in temporomandibular disorder patients: a systematic review. J Oral Rehabil. 2018;45:881‐889. [DOI] [PubMed] [Google Scholar]
- 42.Schiffman E, Ohrbach R, Truelove E, et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28:6‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manfredini D, Borella L, Favero L, Ferronato G, Guarda‐nardini L. Chronic pain severity and depression/somatization levels in TMD patients. Int J Prosthodont. 2010;23:529‐534. [PubMed] [Google Scholar]
- 44.Meloto CB, Slade GD, Lichtenwalter RN, et al. Clinical predictors of persistent temporomandibular disorder in people with first‐onset temporomandibular disorder: a prospective case‐control study. J Am Dent Assoc. 2019;150:572‐581. [DOI] [PubMed] [Google Scholar]
- 45.Ohrbach R, Slade GD, Bair E, et al. Premorbid and concurrent predictors of TMD onset and persistence. Eur J Pain. 2020;24:145‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapos FP, Look JO, Zhang L, Hodges JS, Schiffman EL. Predictors of long‐term temporomandibular disorder pain intensity: an 8‐year cohort study. J Oral Facial Pain Headache. 2018;32:113‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gatchel RJ, Stowell AW, Wildenstein L, Riggs R, Ellis E 3rd. Efficacy of an early intervention for patients with acute temporomandibular disorder‐related pain: a one‐year outcome study. J Am Dent Assoc. 2006;137:339‐347. [DOI] [PubMed] [Google Scholar]
- 48.Wright AR, Gatchel RJ, Wildenstein L, Riggs R, Buschang P, Ellis E 3rd. Biopsychosocial differences between high‐risk and low‐risk patients with acute TMD‐related pain. J Am Dent Assoc. 2004;135:474‐483. [DOI] [PubMed] [Google Scholar]
- 49.Suvinen TI, Kemppainen P, Le Bell Y, Valjakka A, Vahlberg T, Forssell H. Research Diagnostic Criteria Axis II in screening and as a part of biopsychosocial subtyping of Finnish patients with temporomandibular disorder pain. J Orofac Pain. 2013;27:314‐324. [DOI] [PubMed] [Google Scholar]
- 50.De la Torre CG, Bonjardim LR, Poluha RL, et al. Correlation between physical and psychosocial findings in a population of temporomandibular disorder patients. Int J Prosthodont. 2020;33:155‐159. [DOI] [PubMed] [Google Scholar]
- 51.Dworkin SF, Massoth DL. Temporomandibular disorders and chronic pain: disease or illness? J Prosthet Dent. 1994;72:29‐38. [DOI] [PubMed] [Google Scholar]
- 52.Turner JA, Brister H, Huggins K, Mancl L, Aaron LA, Truelove EL. Catastrophizing is associated with clinical examination findings, activity interference, and health care use among patients with temporomandibular disorders. J Orofac Pain. 2005;19:291‐300. [PubMed] [Google Scholar]
- 53.Fillingim RB, Ohrbach R, Greenspan JD, et al. Association of psychologic factors with multiple chronic overlapping pain conditions. J Oral Facial Pain Headache. 2020;Suppl 34:s85‐s100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fillingim RB, Slade GD, Greenspan JD, et al. Long‐term changes in bio psychosocial characteristics related to temporomandibular disorder: findings from the OPPERA study. Pain. 2018;159:2403‐2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rau H, Williams P. Illness behaviour. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013. 10.1007/978-1-4419-1005-9_966 [DOI] [Google Scholar]
- 56.Mechanic D. Illness behaviour: an overview. In: McHugh S, Vallis TM, eds. Illness Behavior. Boston, MA: Springer; 1986:101–109. 10.1007/978-1-4684-5257-0_6 [DOI] [Google Scholar]
- 57.Pilowsky I. Aspects of abnormal illness behaviour. Psychother Psychosom. 1993;60:62‐74. [DOI] [PubMed] [Google Scholar]
- 58.Marbach JJ, Lipton JA. Aspects of illness behaviour in patients with facial pain. J Am Dent Assoc. 1978;96:630‐638. [DOI] [PubMed] [Google Scholar]
- 59.Twaddle AC. The concepts of the sick role and illness behaviour. Adv Psychosom Med. 1972;8:162‐179. [DOI] [PubMed] [Google Scholar]
- 60.Asher R. Munchausen's syndrome. Lancet. 1951;257:339‐341. [DOI] [PubMed] [Google Scholar]
- 61.Meadow R, Lennert T. Munchausen by proxy or Polle syndrome: which term is correct? Pediatrics. 1984;74:554‐556. [PubMed] [Google Scholar]
- 62.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, TX: American Psychiatric Association. 2013;324‐326. [Google Scholar]
- 63.Greene CS. Managing the care of patients with temporomandibular disorders: a new guideline for care. J Am Dent Assoc. 2010;141:1086‐1088. [DOI] [PubMed] [Google Scholar]
- 64.De Leeuw R, Boering G, van der Kuil B, Stegenga B. Hard and soft tissue imaging of the temporomandibular joint 30 years after diagnosis of osteoarthrosis and internal derangement. J Oral Maxillofac Surg. 1996;54:1270‐1280. [DOI] [PubMed] [Google Scholar]
- 65.Stohler CS. Phenomenology, epidemiology, and natural progression of the muscular temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:77‐81. [DOI] [PubMed] [Google Scholar]
- 66.Manfredini D, Favero L, Gregorini G, Cocilovo F, Guarda NL. Natural course of temporomandibular disorders with low pain‐related impairment: a 2‐ to 3‐year follow‐up study. J Oral Rehabil. 2013;40:436‐442. [DOI] [PubMed] [Google Scholar]
- 67.Dao TTT, Lavigne GJ. Oral splints: the crutches for temporomandibular disorders and bruxism? Crit Rev Oral Biol Med. 1998;9:345‐361. [DOI] [PubMed] [Google Scholar]
- 68.Porto F, Harrell R, Fuclher R, Gonzales T. Knowledge and beliefs regarding temporomandibular disorders among orthodontists. Am J Orthod Dentofacial Orthop. 2019;156:475‐484. [DOI] [PubMed] [Google Scholar]
- 69.Costen JB. Syndrome of ear and sinus symptoms, dependent on disturbed function of the TM joint. Ann Otol Rhinol Laryngol. 1934;43:1‐15. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz LL. Pain associated with the temporomandibular joint. J Am Dent Assoc. 1955;51:394‐397. [DOI] [PubMed] [Google Scholar]
- 71.Moulton RE. Emotional factors in non‐organic temporomandibular joint pain. Dent Clin North Am. 1966;10:609‐620. [PubMed] [Google Scholar]
- 72.Lobbezoo F, Visscher CM, Ahlberg J, Manfredini D. Bruxism and genetics: a review of the literature. J Oral Rehabil. 2014;41:709‐714. [DOI] [PubMed] [Google Scholar]
- 73.Ahlberg J, Piirtola M, Lobbezoo F, et al. Correlates and genetics of self‐reported sleep and awake bruxism in a nationwide twin cohort. J Oral Rehabil. 2020;47:1110‐1119. [DOI] [PubMed] [Google Scholar]
- 74.Slade GD, Ohrbach R, Greenspan JD, et al. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 2016;95:1084‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith SB, Maixner DW, Greenspan JD, et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 2011;12:T92‐T101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith SB, Mir E, Bair E, et al. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. J Pain. 2013;14(12 Suppl):T91‐T101.E1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Leeuw R, Klasser GD. Orofacial Pain. Guidelines for Assessment, Diagnosis, and Management, 6th ed. Chicago, IL: Quintessence Publishing; 2018. [Google Scholar]
- 78.Desai B, Alkandari N, Laskin DM. How accurate is information about diagnosis and management of temporomandibular disorders on dentist websites? Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:306‐309. [DOI] [PubMed] [Google Scholar]
- 79.National Institute of Health . TMJ Disorders Pamphlet 2017. https://www.nidcr.nih.gov/sites/default/files/2017‐12/tmj‐disorders.pdf. Accessed January 21, 2021.
- 80.Greene CS, Klasser GD, Epstein JB. Revision of the American Association of Dental Reseach's science information statement about temporomandibular disorders. J Can Dent Assoc. 2010;76:a115. [PubMed] [Google Scholar]
- 81.American Association for Dental Research (AADR) . Temporomandibular Disorders Policy Statement 2010. https://www.iadr.org/AADR/About‐Us/Policy‐Statements/Science‐Policy/Temporomandibular‐Disorders‐TMD. Accessed February 5, 2021.
- 82.Greene CS, Obrez A. Treating temporomandibular disorders with permanent mandibular repositioning: is it medically necessary? Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:489‐498. [DOI] [PubMed] [Google Scholar]
- 83.Klasser GD, Greene CS. Oral appliances in the management of temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:212‐223. [DOI] [PubMed] [Google Scholar]
- 84.Greene CS, Menchel HF. The use of oral appliances in the management of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2018;30:265‐277. [DOI] [PubMed] [Google Scholar]
- 85.Rinchuse DJ, Kandasamy S. Myths of orthodontic gnathology. Am J Orthod Dentofacial Orthop. 2009;136:322‐330. [DOI] [PubMed] [Google Scholar]
- 86.Greene CS. The “ball on the hill”: a new perspective on TMJ functional anatomy. Orthod Craniofac Res. 2018;21:170‐174. [DOI] [PubMed] [Google Scholar]
- 87.Manfredini D. Occlusal equilibration for the management of temporomandibular disorders. Oral Maxillofac Surg Clin North Am. 2018;30:257‐264. [DOI] [PubMed] [Google Scholar]
- 88.Manfredini D, Castroflorio T, Perinetti G, Guarda‐Nardini L. Dental occlusion, body posture and temporomandibular disorders: where we are now and where we are heading for. J Oral Rehabil. 2012;39:463‐471. [DOI] [PubMed] [Google Scholar]
- 89.Al‐Saleh MA, Armijo‐Olivo S, Flores‐Mir C, Thie NM. Electromyography in diagnosing temporomandibular disorders. J Am Dent Assoc. 2012;143:351‐362. [DOI] [PubMed] [Google Scholar]
- 90.Costantinides F, Parisi S, Tonni I, et al. Reliability of kinesiography vs magnetic resonance in internal derangement of TMJ diagnosis: a systematic review of the literature. Cranio. 2020;38:58‐65. [DOI] [PubMed] [Google Scholar]
- 91.Manfredini D, Cocilovo F, Favero L, Ferronato G, Tonello S, Guarda‐Nardini L. Surface electromyography of jaw muscles and kinesiographic recordings: diagnostic accuracy for myofascial pain. J Oral Rehabil. 2011;38:791‐799. [DOI] [PubMed] [Google Scholar]
- 92.Parlett K, Paesani D, Tallens RH, Hatala MA. Temporomandibular joint axiography and MRI findings: a comparative study. J Prosthet Dent. 1993;70:521‐531. [DOI] [PubMed] [Google Scholar]
- 93.Perinetti G, Contardo L. Posturography as a diagnostic aid in dentistry: a systematic review. J Oral Rehabil. 2009;36:922‐936. [DOI] [PubMed] [Google Scholar]
- 94.Colloca L, Barsky AJ. Placebo and nocebo effects. N Engl J Med. 2020;382:554‐561. [DOI] [PubMed] [Google Scholar]
- 95.Guarda‐Nardini L, Almeida AM, Manfredini D. Arthrocentesis of the temporomandibular joint. Systematic review and clinical implications of research findings. J Oral Facial Pain Headache. 2021;35(1):17‐29. [DOI] [PubMed] [Google Scholar]
- 96.Mercuri LG, Giobbie‐Hurder A. Long‐term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J Oral Maxillofac Surg. 2004;62:1088‐1096. [DOI] [PubMed] [Google Scholar]
- 97.Dolwick MF, Dimitroulis G. Is there a role for temporomandibular joint surgery? Br J Oral Maxillofac Surg. 1994;32:307‐313. [DOI] [PubMed] [Google Scholar]
- 98.Hoffman D, Puig L. Complications of TMJ surgery. Oral Maxillofac Surg Clin North Am. 2015;27:109‐124. [DOI] [PubMed] [Google Scholar]
- 99.Mercuri LG. Alloplastic temporomandibular joint replacement: rationale for the use of custom devices. Int J Oral Maxillofac Surg. 2012;41:1033‐1040. [DOI] [PubMed] [Google Scholar]
- 100.Bair E, Ohrbach R, Fillingim RB, et al. Multivariable modeling of phenotypic risk factors for first‐onset TMD: the OPPERA Prospective Cohort Study. J Pain. 2013;14(12 Suppl):T102‐T115. [DOI] [PMC free article] [PubMed] [Google Scholar]


