Key messages.
-
‐
Measurement of sIgE might have additional utility in the diagnosis of asthma in adults.
-
‐
Sensitized patients who are examined for asthma may be more likely to have asthma.
To the Editor,
Physicians still have great difficulty in diagnosing asthma. Overdiagnosis and under diagnosis both occur for this lung disease from which around 300 million people suffer world‐wide.1 For diagnosing asthma, after careful history taking, lung function testing is considered the most relevant investigation. However, other parameters could also assist in completing the asthma jigsaw. Since atopy is one of the predisposing factors for asthma, measurement of specific IgE (sIgE) could be of assistance. This statement only applies to sIgE and not to total IgE measurement, which cannot be used as crucial evidence for allergy diagnosis.2
Currently, there is a lack of diagnostic accuracy data for sIgE to aeroallergens in relation to the diagnosis of asthma. In the Netherlands, measurement of sIgE to aeroallergens in relation to diagnosis of asthma is recommended by the national primary care asthma guideline.3 Therefore, the aim of this study was to investigate the sensitivity, specificity, positive predicted value (PPV) and negative predicted value (NPV) of sIgE to aeroallergens in primary care patients who were diagnosed with asthma confirmed by remote assessment of a pulmonologist in the asthma/COPD‐service and showing clear bronchodilator reversibility.
The study population of this retrospective cross‐sectional database study consists of primary care patients, who visited the asthma/COPD‐service for the first time between 2007 and 2016. According to Dutch regulations, ethical approval was not required because the data were used anonymously. Patients (aged 18–45 years and sIgE data available) who were examined for the diagnosis of asthma were included and divided into two groups. One group included asthma patients with a confirmed pulmonologist diagnosis of asthma and bronchodilator reversibility >12%. The other group included “control” patients where the pulmonologist indicated no obstructive lung disease and no asthma or COPD and no bronchodilator reversibility (Figure 1).
FIGURE 1.
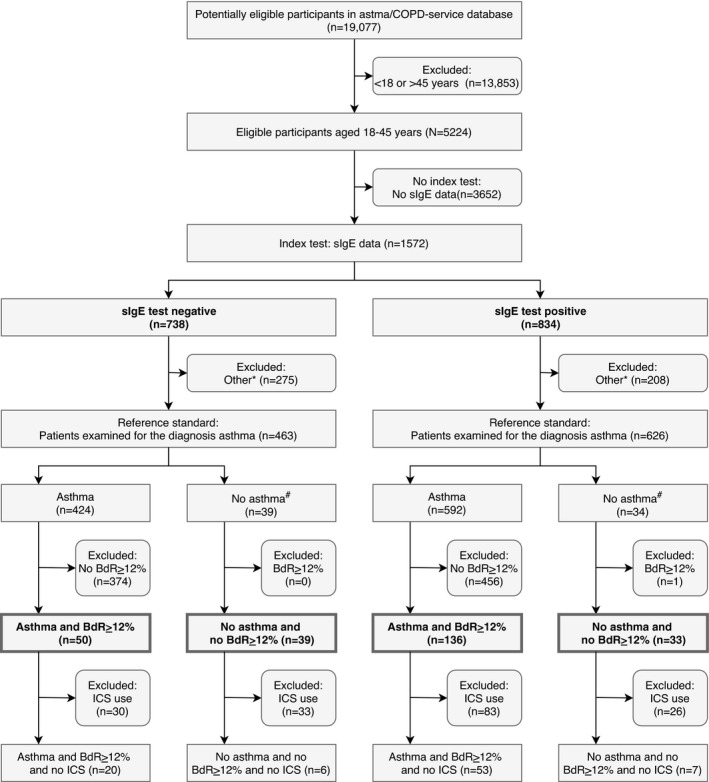
Flow chart patient inclusion. sIgE, Specific immunoglobulin E; BdR ≥ 12%, Increase in FEV1 of ≥12% and ≥200 mL compared to the FEV1 prior to bronchodilatation; ICS, inhaled corticosteroids; *Patients examined for diagnoses other than asthma; #“Control” patients where the pulmonologist indicated no obstructive lung disease and no asthma or COPD
The asthma/COPD‐service is an electronic service to support general practitioners (GPs) in the diagnosis and treatment of patients with suspicion of obstructive lung disease. Since 2007, over 19 000 patients have been assessed by this service.4 Variables obtained during the assessment of the asthma/COPD‐service include medical history, Asthma Control Questionnaire (ACQ), Clinical COPD Questionnaire (CCQ) and spirometry.
Measurement of specific sIgE is not part of the standard assessment within the asthma/COPD‐service. However, in a subset of patients sIgE test results were available by combining coded sIgE data with the coded asthma/COPD data. The Phadiatop test was performed for sIgE measurements, which includes common aeroallergens (grass pollen, tree pollen, house dust mite, cat dander, dog dander, moulds and weed pollen).5 The test was considered positive when a sIgE level of ≥0.35 kU/L was obtained (i.e. sensitization). The average time between sIgE screening and asthma diagnosis by the asthma/COPD‐service was 1.84 years (median 0.90 years, maximum 9.5 years).
The data were analysed using IBM SPSS Statistics for Mac, version 26.0 (IBM Corp., Armonk, NY). Sensitivity, specificity, PPV and NPV of sIgE test result in relation to the diagnosis of asthma were calculated using crosstabs. A sensitivity analysis was performed in a subsample of patients who had not been prescribed inhaled corticosteroids (ICS) at the moment they visited the asthma/COPD‐service for the first time (ICS naïve). The Standards for Reporting Diagnostic Accuracy (STARD) checklist was used for reporting.
A total of 258 patients who were examined for the diagnosis of asthma were included (Figure 1). Of the included patients, 186 (72%) patients were diagnosed with asthma (mean age 30.2 years (SD 8.2 years) and 68% female). In this group of asthma patients, 136 (73%) patients were sensitized. The sensitivity of being sensitized in relation to the diagnosis of asthma was 0.73. Of all sensitized patients (n = 169), 136 (80%) had asthma. The PPV of a positive sIgE test in this group of asthma patients was 0.80 (Table 1).
TABLE 1.
Crosstabs showing number of patients with positive or negative sIgE test results and asthma or no asthma diagnosis for all included patients (A) and ICS naïve patients (B)
| A | Patients examined for the diagnosis asthma | ||||
|---|---|---|---|---|---|
| Asthma and BdR≥12% | No asthma and no BdR≥12% | Total, n | |||
| sIgE, n | Positive | 136 (TP) | 33 (FP) | 169 | PPV = 0.80 (95% CI = 0.74–0.86) |
| Negative | 50 (FN) | 39 (TN) | 89 | NPV = 0.44 (95% CI = 0.34–0.54) | |
| Total, n | 186 | 72 | 258 | ||
| Sensitivity = 0.73 (95% CI = 0.66–0.79) | Specificity = 0.54 (95% CI = 0.43–0.65) | ||||
| B | ICS naïve patients examined for the diagnosis asthma | ||||
|---|---|---|---|---|---|
| Asthma and BdR ≥ 12% | No asthma and no BdR ≥ 12% | Total, n | |||
| sIgE, n | Positive | 53 (TP) | 7 (FP) | 60 | PPV = 0.88 (95% CI = 0.78–0.94) |
| Negative | 20 (FN) | 6 (TN) | 26 | NPV = 0.23 (95% CI = 0.11–0.42) | |
| Total, n | 73 | 13 | 86 | ||
| Sensitivity = 0.73 (95% CI = 0.61–0.82) | Specificity = 0.46 (95% CI = 0.23–0.71) | ||||
Sensitivity = TP/(TP + FN); Specificity = TN/(FP + TN); Positive predicted value (PPV) = TP/(TP + FP); Negative predicted value (NPV) = TN/(TN + FN); sIgE, Specific immunoglobulin E; BdR ≥ 12%, Increase in FEV1 of ≥12% and ≥200 mL compared to the FEV1 prior to bronchodilatation; ICS, inhaled corticosteroids; 95% CI, 95% confidence interval.
Of the included patients, 72 (27%) patients were classified as “control” patients (no asthma or COPD and no bronchodilator reversibility) (mean age 33.3 years (SD 8.5 years) and 67% female). In this group of “control” patients, 39 (54%) patients were not sensitized. The specificity of being not sensitized in relation to not having asthma was 0.54. Of all patients with a negative sIgE test (n = 89), 39 (44%) had no asthma. The NPV of a negative sIgE test in this group of “control” patients was 0.44 (Table 1).
Additionally, 86 out of 258 (33%) patients were ICS naïve. This subgroup of ICS naïve patients revealed a sensitivity of 0.73 in relation to the asthma diagnosis and a specificity of 0.46 in relation to not having asthma. PPV was 0.88 and NPV was 0.23 (Table 1).
This study found reasonable to high sensitivity and PPV for sIgE to aeroallergens in relation to the diagnosis of asthma and low specificity and NPV. Additional analysis showed comparable results in ICS naïve patients. These findings support the hypothesis that sensitized patients may be more likely to have asthma.
Although use of spirometry is recommended in most asthma guidelines, it has been reported that the sensitivity of spirometry for detecting asthma was 36% and the specificity was 75%.6 Given these results, spirometry seems to have a higher specificity whereas sIgE seems to have a higher sensitivity. Therefore, the combination of spirometry and measurement of sIgE to aeroallergens could assist in diagnosing asthma.
It has been reported that the greater the number of sensitizations in childhood, the greater the risk of developing asthma later on in life.7 This risk is assumed to be similar for adults up to the age of 40.8 Furthermore, there is a clear association between asthma and allergy for grass pollen, birch pollen and house dust mite and food allergy and asthma often coexist.9
It has been reported that sensitivity of sIgE in relation to the diagnosis of an allergy for grass pollen, birch pollen and house dust mite ranged from 70% to 100% and specificity ranged from 80% to 100%.5 This reported specificity range is higher than the specificity found in our study. Given the EAACI guidelines, the sensitivity of sIgE in relation to the diagnosis of a food allergy, including egg, wheat, soy and peanut, ranged from 70% to 100% and specificity ranged from 40% to 70%.10 These reported ranges of sensitivity and specificity are comparable with the results found in our study. Together these findings suggest that the diagnostic test accuracy of sIgE to aeroallergens in relation to asthma as found in our study is comparable to the diagnostic test accuracy of sIgE to food allergens in relation to food allergy. However, the diagnostic test accuracy of sIgE to aeroallergens in relation to allergy seems to be higher than in relation to asthma as found in our study.
The strengths of this study are the use of a very well‐structured dataset which includes high‐quality spirometry and the diagnosis of asthma was remotely conformed by a pulmonologist. Based on the Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool, the overall risk of bias of the study is judged as low. Limitations of this study are that sIgE measurement is no part of the standard assessment of the asthma/COPD‐service and that sIgE levels were measured in the past. However, it could be assumed that levels of sIgE are relatively stable over time when people are above 18 years. In addition, the population studied had a high prevalence of asthma diagnosis, which may limit the applicability of the NPV and PPV estimates to other populations.
In conclusion, this study showed that measurement of sIgE might have additional utility in the diagnosis of asthma in adults, as sensitized patients are more likely to have asthma. These data support the guidelines advising measurement of sIgE in panel of common aeroallergens for assisting in making the asthma jigsaw more complete towards the diagnosis of asthma, recognizing that such a panel of aeroallergens would be subject to regional variation.
CONFLICT OF INTEREST
HJHA, EvH, RAR and BMJFdB declare that they have no competing interests. JWHK reports grants, personal fees and non‐financial support from AstraZeneca, grants, personal fees and non‐financial support from Boehringer Ingelheim, grants and personal fees from Chiesi Pharmaceuticals, grants, personal fees and non‐financial support from GSK, grants and personal fees from Novartis, grants from MundiPharma, grants from TEVA, outside the submitted work, all paid to his institution; and JWHK holds 72.5% of shares in the General Practitioners Research Institute. RL reports grants and personal fees from GSK, grants and personal fees from AZ, grants and personal fees from Novartis, grants from Chiesi, outside the submitted work. IOA reports personal fees from Astrazeneca, grants and personal fees from Boehringuer Inguelheim, personal fees from GlaxoSmithKlein, personal fees from Novartis, personal fees from MSD, grants and personal fees from Chiesi, personal fees from Menarini, personal fees from TEVA, personal fees from BIAL, grants and personal fees from Mundipharma, outside the submitted work. DR reports grants from EAACI, grants from AUKCAR, personal fees from Regeneron, personal fees from MEDA, personal fees from AZ, personal fees from Chiesi, personal fees from BI, personal fees from Novartis, outside the submitted work. OSU reports grants and personal fees from astra zeneca, grants and personal fees from boehringer ingelheim, grants and personal fees from chiesi, grants and personal fees from glaxosmithkline, personal fees from napp, personal fees from mundipharma, personal fees from sandoz, personal fees from takeda, grants from edmond pharma, personal fees from cipla, personal fees from covis, personal fees from novartis, personal fees from mereobiopharma, personal fees from orion, personal fees from menarini, outside the submitted work. The authors report no other conflicts of interest in this work. The abstract of this letter was presented at the ERS Virtual Congress 2020 as E‐poster.
AUTHOR CONTRIBUTION
All authors made substantial contributions to conception and design (JWHK, RL, OSU and BFdB), acquisition of data (EvH, RAR), or analysis and interpretation of data (JWHK, HJHA, RL, IOA, DR, OSU and BFdB); took part in drafting the article (JWHK, HJHA and BFdB) or revising it critically for important intellectual content (EvH, RL, IOA, RAR, DR and OSU); all authors gave final approval of the version to be published.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Astma/COPD dienst, CERTE Laboratories, Groningen, the Netherlands. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the corresponding author Bertine Flokstra – de Blok, General Practitioners Research Institute, Groningen, the Netherlands with the permission of Astma/COPD dienst, CERTE Laboratories, Groningen, the Netherlands.
REFERENCES
- 1.Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121‐1131. 10.1503/cmaj.081332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang M‐L, Cui C, Liu Y‐H, Pei L‐C, Shao B. Analysis of total immunoglobulin E and specific immunoglobulin E of 3,721 patients with allergic disease. Biomed Rep. 2015;3(4):573‐577. 10.3892/br.2015.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NHG . NHG‐Standaard Astma bij volwassenen | NHG.
- 4.Metting EI, Riemersma RA, Kocks JH, Piersma‐Wichers MG, Sanderman R, Van Der Molen T. Feasibility and effectiveness of an Asthma/COPD service for primary care: A cross‐sectional baseline description and longitudinal results. NPJ Prim Care Respir Med. 2015;25(1):1‐7. 10.1038/npjpcrm.2014.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidal C, Gude F, Boquete O, et al. Evaluation of the PhadiatopTM test in the diagnosis of allergic sensitization in a general adult population. J Investig Allergol Clin Immunol. 2005;15(2):124‐130. [PubMed] [Google Scholar]
- 6.Gjevre JA, Hurst TS, Taylor‐Gjevre RM, Cockcroft DW. The American Thoracic Society’s spirometric criteria alone is inadequate in asthma diagnosis. Can Respir J. 2006;13(8):433‐437. 10.1155/2006/198940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubner FJ, Jackson DJ, Evans MD, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139(2):501‐507. 10.1016/j.jaci.2016.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallasaho P, Rönmark E, Haahtela T, Sovijärvi ARA, Lundbäck B. Degree and clinical relevance of sensitization to common allergens among adults: A population study in Helsinki, Finland. Clin Exp Allergy. 2006;36(4):503‐509. 10.1111/j.1365-2222.2006.02460.x [DOI] [PubMed] [Google Scholar]
- 9.Emons JAM, Gerth van Wijk R. Food Allergy and Asthma: Is There a Link? Curr Treat Options Allergy. 2018;5(4):436‐444. 10.1007/s40521-018-0185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraro A, Werfel T, Hoffmann‐Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy Eur J Allergy Clin Immunol. 2014;69(8):1008‐1025. 10.1111/all.12429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Astma/COPD dienst, CERTE Laboratories, Groningen, the Netherlands. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the corresponding author Bertine Flokstra – de Blok, General Practitioners Research Institute, Groningen, the Netherlands with the permission of Astma/COPD dienst, CERTE Laboratories, Groningen, the Netherlands.


