ABSTRACT
Background
Levodopa‐carbidopa intestinal gel (LCIG) is administered directly to the small intestine of patients with advanced Parkinson's disease (APD) to help maintain stable plasma levodopa levels.
Objective
The objective of this study was to investigate the effect of LCIG in reducing polypharmacy for the treatment of APD.
Methods
The COmedication Study assessing Mono‐ and cOmbination therapy with levodopa‐carbidopa inteStinal gel (COSMOS) is a large, real‐world, multinational observational study investigating comedication use with LCIG. All enrolled patients had used LCIG for ≥12 months and data were collected cross‐sectionally (study visit) and retrospectively. The primary endpoint was the percentage of patients using LCIG as monotherapy (without add‐on PD medications) at initiation and at 3, 6, 9, and 12 months thereafter.
Results
Overall, 409 patients were enrolled from 14 countries and were treated with LCIG for a mean of 35.8 ± 23.2 months. A total of 15.2% of patients initiated LCIG as monotherapy and 31.7% were receiving monotherapy at 12 months after initiation. The mean duration of LCIG monotherapy was 39.3 ± 25.6 months. Use of add‐on medications decreased over time with all LCIG regimens. From LCIG initiation to the patient visit, mean off time decreased by 3.8, 4.6, and 3.9 hours/day for LCIG monotherapy, LCIG daytime monotherapy, and LCIG polytherapy groups, respectively, while duration of dyskinesia decreased by 1.7, 2.0, and 1.9 hours/day, respectively. Adverse events likely related to study treatment occurred in 112 patients (27.4%) during LCIG treatment.
Conclusions
LCIG is an effective long‐term monotherapy option with a positive risk–benefit profile and contributes to reduced polypharmacy for patients with APD. © 2021 The AbbVie Inc. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: levodopa‐carbidopa intestinal gel; monotherapy; Parkinson's disease; drug polytherapy; observational studies
Despite proven efficacy and widespread use of levodopa for the treatment of Parkinson's disease (PD), the chemical properties of orally administered levodopa include a short plasma half‐life that leads to intermittent receptor stimulation and subsequent fluctuations in symptom control.1 As PD progresses, the therapeutic window of levodopa treatment narrows and patients experience intermittent motor and nonmotor symptoms that require addition of drugs such as dopamine agonists, catechol‐O‐methyl‐transferase (COMT) inhibitors, monoamine oxidase‐B (MAO‐B) inhibitors, or amantadine.2 Poor treatment adherence is common among patients with PD, possibly because of side effects and/or drug–drug interactions from comedications to help manage PD, other comorbidities, or aspects of PD disease progression such as cognitive decline and dysphagia.3, 4, 5 Therefore, the complex oral drug regimens that are often used and frequently result in inadequate symptom control can be particularly burdensome to patients with advancing PD, creating a paradoxical need to simplify drug regimen while the disease progresses.6, 7
Levodopa‐carbidopa intestinal gel (LCIG) allows individualized doses of levodopa to be infused continuously into the small intestine to maintain physiological dopamine levels.8 Evidence to date suggests LCIG is clinically superior to oral polypharmacy in patients with advanced PD (APD),9, 10 and leads to clinically significant improvements in dyskinesias, motor and nonmotor fluctuations, and health‐related quality of life (HRQoL) compared with immediate‐release levodopa.1, 8, 10, 11, 12, 13, 14 Such evidence suggests LCIG has the potential to be administered as monotherapy, thereby reducing pill burden, drug–drug interactions, side effects, and poor treatment adherence. However, pivotal trials investigating LCIG required patients to take LCIG monotherapy, which can affect real‐world outcomes.10, 11
The effect of LCIG on reducing medication burden has not been thoroughly investigated. In many studies, LCIG titration and oral drug management is left to the judgment of neurologists, which contribute to a lack of standardization and reproducibility.10, 11 Results from 1 phase 3 study demonstrated LCIG treatment reduced the percentage of patients using COMT inhibitors, dopamine agonists (DA), MAO‐B inhibitors, and amantadine over 12 months compared with baseline usage; however, in that study, patients were required to stop all treatments other than LCIG for the first 4 weeks of the trial.11 Data from the Global LOng‐term Registry on efficacy and safety of LCIG In patients with APD in routine care (GLORIA) demonstrated decreased use of COMT inhibitors, DAs, MAO‐B inhibitors, and amantadine over 2 years of LCIG treatment compared with use before LCIG initiation. Results showed 37% of patients were taking LCIG monotherapy at 24 months, whereas 57% who initiated LCIG as monotherapy were taking LCIG monotherapy at 24 months, and 23% of patients used LCIG monotherapy exclusively over the entire 24‐month period.15, 16
Other device‐aided therapies (DATs) include continuous subcutaneous apomorphine infusion (CSAI) therapy and deep brain stimulation (DBS), which have shown promise as therapeutic options.17, 18 However, despite their effectiveness, results on their efficacy and safety as monotherapies are limited.17, 18, 19, 20
The COmedication Study assessing Mono‐ and cOmbination therapy with levodopa‐carbidopa inteStinal gel (COSMOS) is a large, multinational study, and the first dedicated to investigating comedication use with LCIG and the potential usability of LCIG as a monotherapy by generating relevant data from routine clinical practice. Here, we report medication usage patterns over 12 months in patients with APD who were treated with LCIG and compare attributes of LCIG therapy such as use of concomitant medications versus monotherapy, duration of LCIG monotherapy, and patient‐ and physician‐based predictors for achieving long‐term LCIG monotherapy.
Methods
Study Design and Setting
COSMOS (Clinicaltrials.gov identifier: NCT03362879) was a multinational, retrospective, and cross‐sectional, post‐marketing observational study of patients with APD who were treated with LCIG in routine clinical care. Data were collected retrospectively from patient medical records covering a period of at least 12 months since LCIG initiation and cross‐sectionally during a clinic visit.
Participants
Patients were eligible for inclusion if they were diagnosed with APD and received ongoing LCIG treatment for ≥12 months and for ≥80% of days in the year preceding the study. Patients who had used LCIG therapy as part of a previous or concurrent interventional trial or were unable to complete study questionnaires were excluded. Written informed consent was obtained by each patient or legal authorized representative before any data collection.
Variables and Data Sources
All clinical data obtained at the patient visit or from medical records were entered into a web‐based electronic data capture system for analysis.
Patient Demographics and Disease Characteristics
During the study visit, the physician collected current patient demographic information and socio‐demographic information both current to the visit and from before initiation of LCIG. These data included patient PD status based on Unified Parkinson's Disease Rating Scale (UPDRS) categories I–V, and Mini‐Mental State Examination (MMSE) scores. Patients or caregivers were asked to state their overall preference for LCIG as a monotherapy compared with LCIG with add‐on PD medication. Medical history was recorded based on available information in patient medical records and included PD phenotype, disease and symptom chronology, time spent in the “off” state and the “on” state with troublesome/nontroublesome dyskinesia, presence of motor and nonmotor PD symptoms, UPDRS scores, modified Hoehn and Yahr stage (UPDRS part V) ratings, and comorbidities related to PD as judged by the physician at the time the record was taken. Information on PD‐related treatment (medication or DATs) from just before LCIG initiation was also collected.
Treatments
Patients were stratified by LCIG treatment regimen into 3 groups based on their treatment regimen at 12 months of therapy; (1) “LCIG monotherapy,” defined as use of LCIG only with no add‐on PD medications; (2) “LCIG daytime monotherapy,” defined as LCIG with add‐on PD medications used in the evening after the daily LCIG infusion hours are completed; and (3) “LCIG polytherapy,” defined as LCIG with add‐on medications at any time, including during LCIG infusion hours.
Primary Endpoint
The primary endpoint was the percentage of patients receiving LCIG monotherapy immediately after LCIG initiation (after permanent system placement) and at 3, 6, 9, and 12 months after LCIG initiation.
Secondary Analyses
Secondary objectives included describing LCIG treatment settings, analyzing patterns of add‐on medication use during LCIG treatment, assessing clinical outcomes between treatment groups, and examining predictors of LCIG monotherapy regimen versus LCIG with add‐on medication. Clinical characteristics such as duration of dyskinesia and off time from the day before the study visit, and UPDRS, nonmotor symptom scale (NMSS), PDSS‐2, and 8‐item PD Questionnaire (PDQ‐8) scores at the patient visit were also analyzed.
Safety
Data from safety assessments previously collected from healthcare professionals for other purposes were used to document adverse events (AEs) that had a reasonable possibility of being causally related to the treatment drug or device.
Statistical Methods
Sample size was determined based on predefined precision of the estimators, expressed as the maximum length of the corresponding two‐sided 95% confidence intervals (CIs). A sample of least 385 evaluable patients was considered necessary to achieve a precision of ±5% (ie, CIs ≤10%) and considering an underlying percentage of 50% (ie, the percentage that leads to the largest sample size).
Data collected from patient medical records and the single‐study visit are presented using descriptive statistics. All results are expressed as mean ± SD unless otherwise stated. Two‐sided 95% CIs were calculated for primary and secondary endpoints defined by proportions. Logistic regression was applied to investigate the impact of potential prognostic factors for LCIG monotherapy, including demographic variables, baseline disease characteristics at LCIG initiation, and physician and study site characteristics. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Participants
A total of 409 patients from 49 sites in 14 countries were included in the analysis. These sites treat a mean of 730.3 ± 768.8 patients with PD/APD annually; the average frequency of routine visits for patients with APD who were receiving DAT was 4.3 visits per year. A total of 74 neurologists participated in the study and 71.4% of the 56 physicians who stated a preference “preferred LCIG as monotherapy versus polytherapy.”
Most patients were male (65.3%) with a mean age of 66.5 ± 7.8 years, UPDRS total score of 57.7 ± 24.5, and MMSE score of 27.5 ± 2.8 before LCIG initiation (Table 1). Baseline characteristics were generally well matched between treatment groups (Table 1; Supplementary Table S1), although the UPDRS Part III score and time between PD diagnosis and LCIG initiation were significantly increased in the LCIG polytherapy group compared with the LCIG monotherapy group (Table 1). The primary reasons for LCIG initiation were disabling motor fluctuations (n = 375; 91.7%) and decreased quality of life (n = 231; 56.5%) (Supplementary Table S2). At the patient visit, the mean duration of LCIG therapy among patients was 35.8 ± 23.2 months (Supplementary Table S3).
TABLE 1.
Demographics and baseline characteristics
| LCIG monotherapya N = 120 | LCIG daytime monotherapya N = 94 | LCIG polytherapya N = 164 | Total population N = 409 | |||||
|---|---|---|---|---|---|---|---|---|
| Description | n | % | n | % | n | % | n | % |
| Sex | ||||||||
| Female | 45 | 37.5 | 28 | 29.8 | 60 | 36.6 | 142 | 34.7 |
| Male | 75 | 62.5 | 66 | 70.2 | 104 | 63.4 | 267 | 65.3 |
| n | mean ± SD | n | mean ± SD | n | mean ± SD | n | mean ± SD | |
| Age, at LCIG initiation, y | 118 | 66.4 ± 8.2 | 94 | 67.8 ± 7.4 | 164 | 65.9 ± 7.8 | 389 | 66.5 ± 7.8 |
| Duration of “off” period,b h | 89 | 5.7 ± 3.3 | 66 | 6.7 ± 4.4 | 110 | 6.1 ± 3.3 | 277 | 6.1 ± 3.6 |
| Duration of dyskinesia,b h | 85 | 3.3 ± 2.9 | 59 | 4.6 ± 3.5 | 110 | 3.8 ± 3.4 | 268 | 3.7 ± 3.4 |
| Time from PD diagnosis toc: | ||||||||
| LCIG initiation, y | 118 | 12.0 ± 5.1 | 94 | 12.7 ± 4.8 | 164 | 13.5 ± 5.8d | 390 | 12.8 ± 5.4 |
| Motor fluctuation onset, y | 119 | 7.3 ± 3.4 | 92 | 7.1 ± 3.6 | 156 | 7.5 ± 4.1 | 396 | 7.3 ± 3.7 |
| Morning akinesia, y | 68 | 7.6 ± 3.9 | 62 | 7.2 ± 4.0 | 124 | 8.0 ± 4.1 | 268 | 7.7 ± 4.1 |
| Wearing off, y | 105 | 7.5 ± 3.5 | 87 | 7.2 ± 3.6 | 150 | 7.8 ± 4.3 | 364 | 7.5 ± 4.0 |
| Dyskinesia, y | 89 | 8.1 ± 3.5 | 82 | 8.0 ± 3.9 | 135 | 9.0 ± 4.6 | 326 | 8.4 ± 4.2 |
| MMSE total score | 74 | 27.4 ± 3.1 | 46 | 26.6 ± 2.7 | 80 | 28.0 ± 2.7 | 222 | 27.5 ± 2.8 |
| UPDRS total score | 35 | 58.2 ± 22.9 | 33 | 55.5 ± 26.6 | 45 | 60.8 ± 24.7 | 118 | 57.7 ± 24.5 |
| Part I | 34 | 4.5 ± 2.5 | 31 | 4.1 ± 2.9 | 44 | 4.6 ± 2.5 | 112 | 4.4 ± 2.6 |
| Part II | 43 | 17.0 ± 8.3 | 36 | 15.4 ± 6.9 | 51 | 17.5 ± 8.1 | 135 | 16.6 ± 7.8 |
| Part III | 54 | 26.8 ± 12.5 | 48 | 28.3 ± 12.9 | 82 | 33.9 ± 17.8e | 190 | 30.1 ± 15.3 |
| Part IV | 43 | 8.2 ± 5.1 | 37 | 8.5 ± 4.6 | 62 | 9.9 ± 6.0 | 147 | 8.8 ± 5.4 |
| PD medication before LCIG initiation, n (%) | ||||||||
| Any medication | 100 | 83.3 | 79 | 84.0 | 141 | 86.0 | 329 | 80.4 |
| Levodopaf | 91 | 75.8 | 78 | 83.0 | 132 | 80.5 | 309 | 75.6 |
| DA | 40 | 33.3 | 41 | 43.6 | 55 | 33.5 | 139 | 34.0 |
| MAO‐B inhibitors | 27 | 22.5 | 22 | 23.4 | 25 | 15.2 | 74 | 18.1 |
| COMT inhibitors | 13 | 10.8 | 17 | 18.1 | 25 | 15.2 | 56 | 13.7 |
| NMDA antagonists | 13 | 10.8 | 19 | 20.2 | 16 | 9.8 | 51 | 12.5 |
| APO injection | 0 | 0 | 0 | 0 | 7 | 4.3 | 7 | 1.7 |
| APO CI | 1 | 0.8 | 1 | 1.1 | 1 | 0.6 | 3 | 0.7 |
| Anticholinergics | 1 | 0.8 | 2 | 2.1 | 6 | 3.7 | 9 | 2.2 |
| Other | 0 | 0 | 0 | 0 | 6 | 3.7 | 6 | 1.5 |
| Symptoms at LCIG initiation, n (%) | ||||||||
| Hallucination | 1 | 0.8 | 2 | 2.1 | 4 | 2.4 | 7 | 1.7 |
| Sleep disorders | 29 | 24.2 | 22 | 23.4 | 38 | 23.2 | 91 | 22.2 |
| Any impulsive‐compulsive disorder | 21 | 18.9 | 18 | 20.5 | 31 | 20.9 | 71 | 18.9 |
| Previous deep brain stimulation | 2 | 1.7 | 1 | 1.1 | 7 | 4.3 | 11 | 2.7 |
Patients were grouped by their treatment regimen at 12 months, including 31 patients with inconclusive/missing treatment regimen data.
Hours during the day before the clinical visit as reported by the patient.
Restricted to patients with these conditions.
P < 0.05 vs. LCIG monotherapy.
P < 0.05 vs. before LCIG initiation.
Levodopa use included levodopa/carbidopa, levodopa/carbidopa/entacapone, and/or levodopa/benserazide.
Abbreviations: APO, apomorphine; CI, continuous infusion; COMT, catechol‐O‐methyl transferase; DA, dopamine agonist; LCIG, levodopa‐carbidopa intestinal gel; MAO‐B, monoamine oxidase‐B; MMSE, Mini‐Mental State Examination; NMDA, N‐methyl‐D‐aspartate; PD, Parkinson's disease; SD, standard deviation; UPDRS, Unified Parkinson's Disease Rating Scale.
Study patients were receiving LCIG monotherapy (n = 120), LCIG daytime monotherapy (n = 94), or LCIG polytherapy (n = 164) 12 months after LCIG initiation. Patients in the LCIG monotherapy group were receiving monotherapy for a mean of 30.7 ± 20.4 months and were receiving any LCIG regimen for 39.3 ± 25.6 months, which was significantly longer than the duration of any LCIG regimen for patients in the LCIG daytime monotherapy group (31.8 ± 22.3 months; P < 0.05), but not in the LCIG polytherapy group (35.7 ± 21.9 months; P = 0.428). LCIG titration was achieved most rapidly for patients in the LCIG monotherapy group (6.0 ± 3.4 days), which was significantly decreased compared with LCIG daytime monotherapy patients (8.8 ± 6.2 days; P < 0.001; Supplementary Table S3).
Primary Endpoint
The percentage of patients treated with LCIG monotherapy increased from 15.2% (n = 54/356) at LCIG initiation to 31.7% (n = 120/378) at 12 months, whereas 13.2% (n = 47/356) of patients initiated treatment with LCIG daytime monotherapy, which increased to 24.9% (n = 94/378) at 12 months (Fig. 1A). Therefore combined, the LCIG monotherapy group and LCIG daytime monotherapy group comprised 28.4% of patients at baseline and 56.6% of patients at month 12. LCIG polytherapy was most common, with 71.6% of patients (n = 255/356) initiating LCIG therapy with this regimen; however, usage decreased notably by month 3 to 42.8% of patients (n = 158/369), then remained stable until month 12 (43.4% [n = 164/378]; Fig. 1A).
FIG 1.
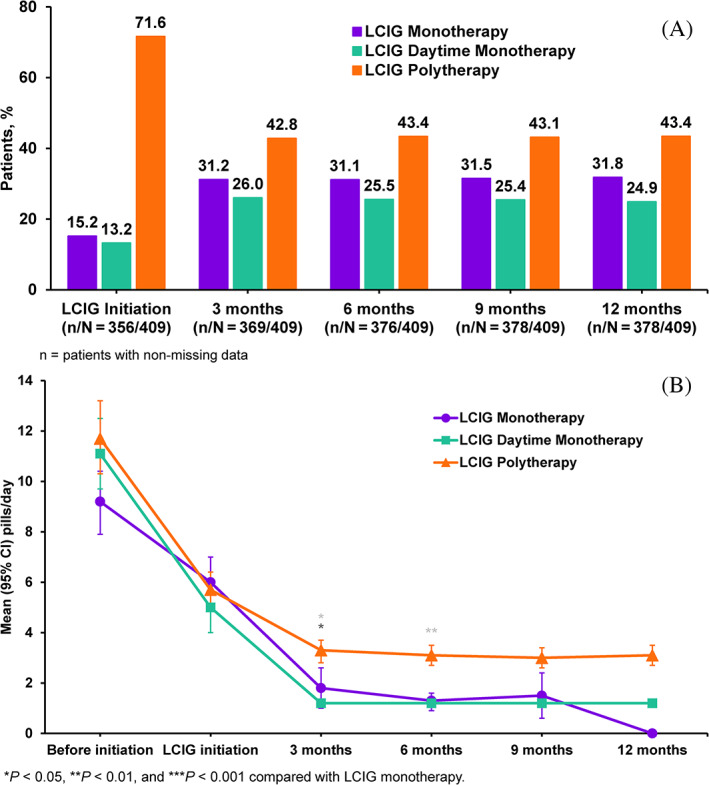
Percentages of patients taking levodopa‐carbidopa intestinal gel (LCIG) monotherapy at indicated time points (A). Number of pills per day of add‐on Parkinson's disease medication by treatment group over time (B).
Secondary Analyses
Add‐on PD Medication Use by Class
The percentages of patients using add‐on PD medication by class and the LCIG treatment regimen are presented in Figure 2 and Supplementary Table S4. The numbers of patients using DA, COMT inhibitor, and MAO‐B inhibitors decreased over time in both the LCIG monotherapy and LCIG daytime monotherapy groups, whereas only COMT inhibitors decreased among patients in the LCIG polytherapy group. As expected, LCIG initiation was the most common reason for discontinuation of add‐on medications (73.3% of patients; not shown). Most patients in the LCIG monotherapy group (93.8%) and LCIG daytime monotherapy group (73.8%) discontinued DA, MAO‐B, and COMT inhibitor use within 3 months of LCIG initiation. No percentage increases in DA, MAO‐B, and COMT inhibitor use were observed in any treatment group after 3 months of LCIG therapy (Fig. 2). Before LCIG initiation, the mean numbers of pills per day of all add‐on medications were 9.2 ± 6.6 for LCIG monotherapy, 11.1 ± 6.7 for LCIG daytime monotherapy, and 11.7 ± 9.2 for LCIG polytherapy groups. At month 12, the corresponding values were 0, 1.2 ± 0.4, and 3.1 ± 2.6 pills per day, respectively, with a statistically significant difference from baseline in all groups (Fig. 1B).
FIG 2.
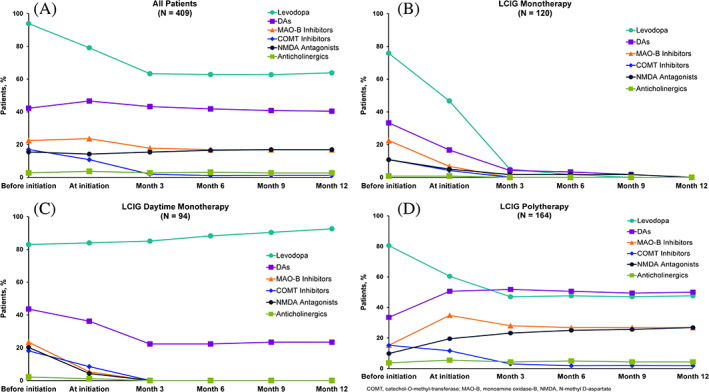
Percentages of patients with reported Parkinson's disease medication usage before initiation of levodopa‐carbidopa intestinal gel (LCIG) and as add‐on therapy over time by class. All patients (A), LCIG monotherapy (B), LCIG daytime monotherapy (C), and LCIG polytherapy (D). “Levodopa” indicates oral formulations. COMT, catechol‐O‐methyl‐transferase; MAO‐B, monoamine oxidase‐B, NMDA, N‐methyl D‐aspartate.
Overall Medication Needs
At LCIG initiation, the levodopa equivalent daily dose (LEDD) for the LCIG daytime monotherapy group (2583.0 ± 922.6) and LCIG polytherapy group (2630.8 ± 1099.4) was significantly higher than the LEDD in the LCIG monotherapy group (2266.6 ± 929.7; P < 0.05; Supplementary Fig. S1A). Similar results occurred at 12 months when the LEDD of the LCIG daytime monotherapy and LCIG polytherapy groups were 2227.4 ± 614.5 and 2331.0 ± 879.7, respectively, versus 1834.6 ± 793.1 for LCIG monotherapy group (both P < 0.0001). The LEDD for add‐on PD medications decreased from initiation to month 12 overall; however, the LEDD remained significantly increased for the LCIG daytime monotherapy and LCIG polytherapy groups compared with the LCIG monotherapy group at all time points after LCIG initiation (Supplementary Fig. S1B).
For all patients, the daily LCIG dose was similar at 12 months versus at initiation (1384 ± 520 mg/day and 1312 ± 498 mg/day, respectively). Yet, patients in the LCIG daytime monotherapy group had higher daily doses of LCIG than did patients in the LCIG monotherapy group at initiation (1388 ± 444 mg/day vs. 1242 ± 506 mg/day; P < 0.01) and at 12 months (1458 ± 414 mg/day vs. 1326 ± 568 mg/day; P < 0.01) (Supplementary Fig. S1C).
Motor and Nonmotor Symptoms
In all groups, off time and dyskinesia duration were reduced at the patient visit compared with LCIG initiation, with no significant differences between groups (Table 2). The mean number of motor symptoms decreased from 6.6 ± 2.1 at LCIG initiation to 5.8 ± 2.4 at the patient visit for all patients (P < 0.0001). The proportion of patients who experienced rigidity, tremor, dystonia/cramps, gait impairment, balance problems, hypophonia, dysphagia, nocturnal/morning akinesia, and freezing of gait showed significant decline at the patient visit compared with LCIG initiation in all treatment groups (P < 0.0001). Motor symptoms were similarly reduced from the initiation of LCIG to the patient visit in all treatment groups, although patients in the LCIG monotherapy group showed significantly greater reductions in nocturnal/morning akinesia versus those in the LCIG daytime monotherapy group (20.8% reduction vs. 11.7% reduction; P = 0.0099) and freezing of gait versus those in the LCIG polytherapy group (14.2% reduction vs. 0% reduction; P = 0.0036; Supplementary Fig. S2). The percentage of patients who experienced nonmotor symptoms of anxiety, pain, depression, and constipation decreased significantly from the initiation of LCIG to the patient visit, whereas the percentages of patients who experienced cognitive impairment, apathy, fatigue, urinary symptoms, and orthostatic hypotension increased significantly over that time (all P < 0.001; Supplementary Fig. S2). The total NMSS score, or any domain scores, were not significantly different between treatment groups, and no significant differences between treatment groups were observed in UPDRS total score, PDSS‐2 total score, Questionnaire for Impulsive‐Compulsive Disorders in Parkinson's Disease Rating Scale total score, and PDQ‐8 summary index scores (Table 2).
TABLE 2.
Unified Parkinson's Disease Rating Scale, Non‐Motor Symptoms Scale, Parkinson's Disease Sleep Scale 2, Questionnaire for Impulsive‐Compulsive Disorders in Parkinson's Disease Rating Scale, and 8‐item Parkinson's Disease Questionnaire scores at the patient visit
| Mean ± SD | LCIG monotherapy N = 120 | LCIG daytime monotherapy N = 94 | LCIG polytherapy N = 164 | Total population N = 409 | ||||
|---|---|---|---|---|---|---|---|---|
| n | Result | n | Result | n | Result | n | Result | |
| UPDRS total score | 120 | 54.8 ± 24.5a | 94 | 54.8 ± 20.3 | 164 | 57.2 ± 23.0 | 408 | 56.2 ± 23.6 |
| Part I | 120 | 3.6 ± 2.9 | 94 | 3.4 ± 2.6 | 164 | 3.6 ± 2.4a | 409 | 3.5 ± 2.6 |
| Part II | 120 | 16.8 ± 8.3 | 94 | 17.3 ± 7.4 | 164 | 17.6 ± 7.9 | 409 | 17.4 ± 8.0 |
| Part III | 120 | 29.2 ± 13.6 | 94 | 28.2 ± 12.2 | 164 | 30.1 ± 13.6 | 408 | 29.7 ± 13.7 |
| Part IV | 120 | 5.2 ± 3.6b | 94 | 5.9 ± 3.1b , d | 164 | 5.9 ± 3.5c | 409 | 5.6 ± 3.4c |
| Dyskinesias and dystonia, score | 120 | 2.5 ± 2.4 | 94 | 2.9 ± 2.0 | 164 | 2.8 ± 2.3 | 409 | 2.7 ± 2.3 |
| Motor fluctuations, score | 120 | 1.8 ± 1.5 | 94 | 2.0 ± 1.3 | 164 | 2.3 ± 1.4d | 409 | 2.0 ± 1.5 |
| Other complications, score | 120 | 0.9 ± 0.9 | 94 | 1.0 ± 0.9 | 164 | 0.8 ± 0.9 | 409 | 0.8 ± 0.9 |
| Dyskinesias duration during the day, h | 120 | 1.6 ± 2.6 | 93 | 2.5 ± 2.8d | 163 | 1.8 ± 2.4d | 404 | 1.8 ± 2.5 |
| Change from pre‐LCIG initiation | 85 | −1.7 ± 2.9 | 59 | −2.0 ± 4.4 | 110 | −1.9 ± 3.8 | 267 | −1.8 ± 3.6 |
| “Off” time during waking day, h | 120 | 2.1 ± 3.6 | 92 | 1.9 ± 2.2 | 162 | 2.3 ± 3.1a | 403 | 2.1 ± 3.1 |
| Change from pre‐LCIG initiation | 89 | −3.8 ± 4.4 | 64 | −4.6 ± 4.3 | 110 | −3.9 ± 4.2 | 275 | −4.0 ± 4.3 |
| Duration of dyskinesias or “off” statee during waking day, h | 120 | 3.6 ± 5.0 | 92 | 4.4 ± 4.1d | 162 | 4.1 ± 4.7 | 402 | 4.0 ± 4.6 |
| Change from pre‐LCIG initiation | 83 | −5.8 ± 4.9 | 56 | −6.6 ± 6.1 | 105 | −6.0 ± 6.4 | 255 | −6.0 ± 5.7 |
| NMSS total score | 120 | 58.2 ± 45.4 | 94 | 60.8 ± 38.1 | 160 | 57.5 ± 41.4 | 404 | 57.6 ± 42.2 |
| Cardiovascular including falls | 120 | 2.2 ± 3.4 | 94 | 1.7 ± 3.4 | 164 | 1.9 ± 3.1 | 409 | 1.9 ± 3.2 |
| Sleep/fatigue | 120 | 9.7 ± 8.7 | 94 | 9.4 ± 8.3 | 164 | 9.6 ± 8.2 | 409 | 9.5 ± 8.4 |
| Mood/cognition | 120 | 11.8 ± 14.7 | 94 | 11.5 ± 14.9 | 164 | 9.1 ± 11.0 | 409 | 10.5 ± 13.3 |
| Perceptual problems/hallucinations | 120 | 2.2 ± 4.0 | 94 | 1.4 ± 3.1 | 164 | 1.9 ± 4.0 | 409 | 1.8 ± 3.7 |
| Attention/memory | 120 | 6.8 ± 8.7 | 94 | 6.5 ± 7.2 | 164 | 6.9 ± 8.5 | 409 | 6.6 ± 8.2 |
| Gastrointestinal tract | 120 | 5.0 ± 6.1 | 94 | 5.7 ± 7.0 | 164 | 6.1 ± 7.4 | 409 | 5.6 ± 6.8 |
| Urinary | 120 | 9.0 ± 8.9 | 94 | 11.3 ± 9.2 | 164 | 10.7 ± 10.0 | 409 | 10.3 ± 9.4 |
| Sexual function | 120 | 4.5 ± 7.3 | 94 | 4.5 ± 6.6 | 160 | 3.2 ± 5.8 | 404 | 3.7 ± 6.4 |
| Miscellaneous | 120 | 7.1 ± 8.5 | 94 | 8.8 ± 7.9d | 164 | 8.4 ± 9.3 | 409 | 8.0 ± 8.7 |
| QUIP‐RS total score | 112 | 12.6 ± 13.9 | 91 | 11.4 ± 11.8 | 154 | 11.2 ± 14.5 | 387 | 11.5 ± 13.4 |
| Gambling | 117 | 0.7 ± 1.9 | 94 | 0.6 ± 1.7 | 163 | 0.8 ± 2.2 | 404 | 0.7 ± 1.9 |
| Sex | 116 | 1.8 ± 2.9 | 93 | 1.9 ± 3.2 | 160 | 1.9 ± 2.8 | 400 | 1.8 ± 2.9 |
| Buying | 117 | 1.6 ± 2.7 | 94 | 1.8 ± 2.9 | 162 | 1.5 ± 2.6 | 404 | 1.6 ± 2.7 |
| Eating | 117 | 2.3 ± 3.2 | 94 | 2.1 ± 2.8 | 160 | 2.0 ± 3.2 | 402 | 2.1 ± 3.1 |
| Hobbyism/punding | 116 | 4.9 ± 6.2 | 93 | 3.9 ± 4.5 | 158 | 3.8 ± 5.2 | 398 | 4.1 ± 5.3 |
| Medication use | 116 | 2.4 ± 3.4 | 92 | 1.3 ± 2.2 | 161 | 1.8 ± 3.5d | 400 | 1.8 ± 3.1 |
| Total ICD score | 114 | 5.9 ± 7.3 | 93 | 6.4 ± 7.3 | 158 | 6.0 ± 8.6 | 395 | 6.0 ± 7.8 |
| PDSS‐2 total score | 112 | 19.6 ± 11.0 | 90 | 19.5 ± 10.6 | 152 | 21.6 ± 10.8 | 383 | 20.6 ± 10.8 |
| PDQ‐8 summary index scores | 119 | 41.5 ± 17.7 | 93 | 36.5 ± 18.1 | 161 | 40.2 ± 18.3 | 404 | 40.3 ± 18.2 |
P < 0.05.
P < 0.001.
P < 0.0001 vs. baseline.
P < 0.05 vs. LCIG monotherapy.
Change of the duration of dyskinesias or “off” state duration should be considered additional “on time.”
Abbreviations: ICD, impulsive‐compulsive disorder; LCIG, levodopa‐carbidopa intestinal gel; NMSS, Non‐Motor Symptoms Scale; PD, Parkinson's disease; PDSS‐2, PD Sleep Scale 2; PDQ‐8, 8‐item PD Questionnaire; QUIP‐RS, Questionnaire for Impulsive‐Compulsive Disorders in PD Rating Scale; UPDRS, Unified PD Rating Scale.
Predictors of Combined LCIG Monotherapy
Significant predictors of LCIG monotherapy use at 12 months after initiation were treatment with DA before LCIG initiation versus no prior DA treatment (odds ratio [OR], 1.743 [95% CI, 1.094, 2.777]; P = 0.0194), patient visits to their physician ≥3 versus <3 times/year while receiving DAT (OR, 2.297 [95% CI, 1.178, 4.478]; P = 0.0146), and approximate number of patients with APD treated with LCIG annually by the physician (OR, 1.019 [95% CI, 1.000, 1.038]; P = 0.0489; Supplementary Table S5). Other variables that predicted use of LCIG monotherapy were lower number of motor symptoms (OR, 0.828 [95% CI, 0.714, 0.960]; P = 0.0125), and lower gross national income of the treatment site location (OR, 0.976 [95% CI, 0.958, 0.994]; P = 0.0101).
Safety
A total of 274 AEs likely related to study treatment occurred in 112 patients (27.4%) from LCIG initiation to the patient visit. The most common AEs reported were stoma site infection (n = 11; 2.7%), dyskinesia (n = 9; 2.2%), and device malfunction (n = 8; 2.0%). Neuropathies occurred in 6 patients (1.5%): polyneuropathy in 4 patients (1.0%), and 1 patient (0.2%) each was reported with demyelinating polyneuropathy and peripheral neuropathy. A decrease in vitamin B6 was reported for 1 patient (0.2%) and decreased weight for 5 patients (1.2%).
Discussion
In this observational study, the percentages of patients with APD treated with LCIG monotherapy (either with no add‐on medications or with add‐on medications outside LCIG infusion hours) increased between treatment initiation and month 3 and remained relatively stable through month 12. LCIG with add‐on medications (LCIG polytherapy) was the most frequently used treatment regimen, although the frequency of prescribing add‐on medications decreased considerably between LCIG initiation and month 3, after which usage remained stable. Titration of LCIG was achieved most rapidly in the LCIG monotherapy group, potentially because most were able to discontinue DA, MAO‐B, and COMT inhibitor therapy quickly (within 3 months of LCIG therapy, without increase after 3 months of LCIG therapy), possibly because part of the titration was performed during the naso‐jejunal test phase. Moreover, during the 12‐month observation period, COMT inhibitor use decreased within all LCIG treatment groups. All groups demonstrated a significant improvement in off time and dyskinesia, and we did not observe any clinically significant differences between groups in symptomatic outcomes, as rates of off time and dyskinesia decreased by month 12 compared with LCIG initiation in all treatment groups. Use of LCIG monotherapy was predicted by previous DA usage, more frequent physician visits (≥3 times in a year), and number of motor symptoms, suggesting advancing disease may predict use of LCIG monotherapy; this supports the notion that DAs are less tolerated in patients who need more frequent evaluations. Most physicians preferred to prescribe LCIG monotherapy to simplify the medication regimen after a DAT was implemented and to reduce the pill burden.
This primary analysis of the COSMOS study represents the first investigation dedicated to add‐on PD medication use and monotherapy before and during LCIG therapy. Our findings are supported by results of a post hoc analysis from the multinational GLORIA registry study that monitored LCIG therapy over 24 months in patients with APD. In that study, between 36% and 40% of patients were treated with LCIG monotherapy at all time points, and at the end of 24 months, 23% of patients (n = 59) had used LCIG monotherapy exclusively over the full observation period.16 In the GLORIA study, the proportion of patients who used oral levodopa, DAs, COMT inhibitors, MAO‐B inhibitors, and the N‐methyl‐D‐aspartate receptor antagonist amantadine decreased during LCIG therapy compared with baseline.15 Other results that support our findings include those from a 12‐month, phase 3, single‐arm trial, where percentages of patients using add‐on DA decreased from 55.4%–12.7%, COMT inhibitors from 28.2%–3.7%, MAO‐B inhibitors from 12.7%–1.5%, and amantadine from 29.9%–9.6%; whereas the 26.6% of patients who were taking monotherapy at baseline increased to 76.5%, this increased use included those patients who received LCIG with or without oral supplementation.11 However, it is notable that patients enrolled in that study were required to stop all non‐LCIG PD medications for the first 4 weeks of LCIG treatment, which does not occur in clinical practice, suggesting rates of add‐on medication use in the COSMOS and GLORIA studies more closely represent real‐world clinical treatment than medication dosing in a phase 3 clinical trial. Both the COSMOS and GLORIA studies presented favorable data on symptomatic outcomes, supporting the feasibility of using long‐term LCIG monotherapy in selected patients.16
Poor treatment adherence is a pervasive issue among patients treated for PD, for whom up to 67% take <80% of their prescribed medications.7 Lack of medication adherence often increases over time for several likely reasons. First, patients with PD are susceptible to high pill burden. In addition to standard dopaminergic medications that may be needed up to 10 times per day, more than half of patients have multiple prescriptions for nonmotor symptoms and comorbidities.7 Unfortunately, neurodegenerative processes that create the need for higher quantities and more frequent medications underlie progressive cognitive deficits that render the patient less capable of managing increasingly complex regimens. Further, patients with advancing PD can experience problems such as difficulty swallowing and irregular gastric emptying that negatively affect the ingestion and absorption of oral medications.5, 21 Poor treatment adherence in PD may negatively impact patients both in terms of motor and nonmotor complications, and in HRQoL.22 Overall, in patients with PD, the fewer medications prescribed, the better the treatment adherence.23
A few studies have been performed to investigate the need for add‐on medications with DATs. Results from investigations of CSAI revealed that, although use of this treatment approach could significantly reduce the need for oral levodopa, most patients continue to require oral levodopa to achieve a full clinical effect.24, 25 Similar findings were observed in studies on DBS, which can significantly reduce oral anti‐PD medications for improving motor function, yet evidence is lacking to support DBS as long‐term monotherapy.26, 27 In our study, even though nearly one‐third of patients were taking LCIG monotherapy at 12 months of LCIG treatment, no clinically significant differences in symptomatic outcomes (off time and dyskinesia) were apparent between groups receiving LCIG monotherapy and groups that used LCIG and add‐on medications. These data suggest LCIG monotherapy was as effective as LCIG plus add‐on medication in controlling symptoms, which could not be explained by any remarkable differences in demographic or PD history between groups. Therefore, when possible, LCIG monotherapy is a valid solution for many patients with APD to achieve similar symptom control as when using add‐on medications, while reducing PD‐related pill burden.
A major strength of this study was use of real‐world, multinational, large‐cohort clinical data on LCIG monotherapy and add‐on PD medication use. The study was limited by partially retrospective data collection and purely observational data, which resulted in missing data for some analyses. Further, patients were grouped by their status at month 12, so lack of randomization precluded meaningful comparisons between groups. Choice of medication regimen may have correlated with disease severity, although no major differences in disease characteristics between groups were apparent. We noted in our study that patients with more severe disease tended to remain on polytherapy. Further detailed analysis may be required to clarify this observation. Finally, our study only included patients treated with ongoing LCIG and able to sustain LCIG treatment for at least 12 months; therefore, results are not representative of all patients who initiate LCIG. This requirement, in addition to limiting AEs to those related to LCIG treatment, likely contributed to the low frequency of AEs in this study versus the rates of AEs in phase 3 studies.28
Conclusion
The data from this observational, real‐world study in patients with APD provided evidence that LCIG monotherapy is a feasible and effective long‐term treatment option for symptoms of APD. Patients who reduced their pill intake by receiving LCIG as monotherapy experienced similar clinical benefits as patients who continued to take add‐on medications. Yet patients with preserved combination therapy also experienced considerable reductions in comedication use. The safety results were consistent with the known profile of LCIG. These results reveal LCIG monotherapy is an effective means to manage symptoms while reducing pill burden.
Author Roles
All authors had access to the data and participated in the development, review, critique, and approval of the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. All named authors meet the ICMJE criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.F.: 1A, 1B, 1C, 2C, 3A, 3B.
T.G.: 1C, 2C, 3A, 3B.
R.J.: 1C, 2C, 3A, 3B.
N.K.: 1C, 2C, 3A, 3B.
P.S.: 1C, 2C, 3A, 3B.
J.S.: 1C, 2C, 3A, 3B.
J.C.P.: 1A, 1B, 1C, 2A, 2C, 3A, 3B.
L.B.: 1A, 1B, 1C, 2C, 3A, 3B.
A.J.: 1A, 1B, 1C, 2C, 3A, 3B.
O.S.S.: 1A, 1B, 1C, 2A, 2C, 3A, 3B.
Z.T.: 1B, 2A, 2B, 2C, 3A, 3B.
L.V.D.: 1C, 2C, 3A, 3B.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.AbbVie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html
Supporting information
Appendix S1 Supporting Information
Acknowledgments
AbbVie participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving this manuscript for publication. AbbVie funded the research for this study and provided writing support for this manuscript. Medical writing assistance was provided by Nate Connors, PhD, CMPP, Kersten Reich, MPH, CMPP, and Lamara D. Shrode, PhD, CMPP, of JB Ashtin, who developed the first draft based on an author‐approved outline and assisted in implementing author revisions throughout the editorial process. JB Ashtin adheres to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. The authors would like to express their gratitude to the patients and all study investigators who made this study possible.
Relevant conflicts of interest/financial disclosures: A.F. is a study investigator and an external study consultant who has served as an advisor for AbbVie, and a consultant for Abbott, UCB Pharma, Medtronic, Boston Scientific, and AbbVie. He has received research support from Medtronic, Boston Scientific, University of Toronto, The Michael J. Fox Foundation for Parkinson's Research, and honoraria for serving as a speaker from UCB, Medtronic, Novartis, Chiesi, Boston Scientific, AbbVie, and Teva. T.G. was a study investigator. She has served as an advisor for Cytora, Synnerva, Teva, AbbVie, and Allergan, and has received honoraria from AbbVie and Neuroderm; research support from Parkinson's Foundation, University Tel Aviv, and the Israel innovation authority; and travel support for her team and herself from AbbVie, Medison, Medtronic, and Allergan. R.J. was a study investigator and received honoraria from AbbVie, Medtronic, Ipsen, Allergan, and Cardion for consultancies and lectures. N.K. was a study investigator and has received honorarium from UCB, AbbVie, Medtronic, Boston Scientific, Abbott, KRKA, Teva Boehringer‐Ingelheim, and GSK Pharmaceuticals for lecturing at symposia. He has been a consultant for AbbVie, Abbott, TEVA, and KRKA. He has received research funding from the Hungarian National Research, Development and Innovation Office, University of Pécs, Medtronic, and Abbott. J.S. was a study investigator and received compensation from AbbVie, Novartis, UCB, Boehringer‐Ingelheim, GSK, Ever, Lundbeck, Teva, and Pfizer for consultancies and speaker activities. P.S. was a study investigator and received compensation from AbbVie, Shire, and Sanofi‐Genzyme for speaker activities. L.V.D. was a study investigator and received honoraria for educational presentations and advice from AbbVie, Zambon, Teva, and Bial. L.B., A.J., O.S.S., J.C.P., and Z.T. are employees of AbbVie, and may hold AbbVie stock and/or stock options. This study was funded by AbbVie. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the manuscript for publication.
Funding agency: AbbVie Inc.
References
- 1.Wang L, Li J, Chen J. Levodopa‐carbidopa intestinal gel in Parkinson's disease: a systematic review and meta‐analysis. Front Neurol 2018;9:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser RA. Levodopa: past, present, and future. Eur Neurol 2009;62:1–8. [DOI] [PubMed] [Google Scholar]
- 3.Morin L, Johnell K, Laroche ML, Fastbom J, Wastesson JW. The epidemiology of polypharmacy in older adults: register‐based prospective cohort study. Clin Epidemiol 2018;10:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daley DJ, Myint PK, Gray RJ, Deane KH. Systematic review on factors associated with medication non‐adherence in Parkinson's disease. Parkinsonism Relat Disord 2012;18:1053–1061. [DOI] [PubMed] [Google Scholar]
- 5.Suttrup I, Warnecke T. Dysphagia in Parkinson's disease. Dysphagia 2016;31:24–32. [DOI] [PubMed] [Google Scholar]
- 6.Davis KL, Edin HM, Allen JK. Prevalence and cost of medication nonadherence in Parkinson's disease: evidence from administrative claims data. Mov Disord 2010;25:474–480. [DOI] [PubMed] [Google Scholar]
- 7.Malek N, Grosset DG. Medication adherence in patients with Parkinson's disease. CNS Drugs 2015;29:47–53. [DOI] [PubMed] [Google Scholar]
- 8.Wirdefeldt K, Odin P, Nyholm D. Levodopa‐carbidopa intestinal gel in patients with Parkinson's disease: a systematic review. CNS Drugs 2016;30:381–404. [DOI] [PubMed] [Google Scholar]
- 9.Nyholm D, Nilsson Remahl AI, Dizdar N, Constantinescu R, Holmberg B, Jansson R, et al. Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 2005;64:216–223. [DOI] [PubMed] [Google Scholar]
- 10.Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, et al. Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double‐blind, double‐dummy study. Lancet Neurol 2014;13:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, et al. Levodopa‐carbidopa intestinal gel in advanced Parkinson's disease: final 12‐month, open‐label results. Mov Disord 2015;30:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slevin JT, Fernandez HH, Zadikoff C, Hall C, Eaton S, Dubow J, et al. Long‐term safety and maintenance of efficacy of levodopa‐carbidopa intestinal gel: an open‐label extension of the double‐blind pivotal study in advanced Parkinson's disease patients. J Parkinsons Dis 2015;5:165–174. [DOI] [PubMed] [Google Scholar]
- 13.Standaert DG, Rodriguez RL, Slevin JT, Lobatz M, Eaton S, Chatamra K, et al. Effect of levodopa‐carbidopa intestinal gel on non‐motor symptoms in patients with advanced Parkinson's disease. Mov Disord Clin Pract 2017;4:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhmann C, Hilker R, Lingor P, Schrader C, Schwarz J, Wolz M, et al. Levodopa/carbidopa intestinal gel (LCIG) infusion as mono‐ or combination therapy. J Neural Transm (Vienna) 2017;124:1005–1013. [DOI] [PubMed] [Google Scholar]
- 15.Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, et al. Levodopa‐carbidopa intestinal gel in advanced Parkinson's: final results of the GLORIA registry. Parkinsonism Relat Disord 2017;45:13–20. [DOI] [PubMed] [Google Scholar]
- 16.Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A. Levodopa‐carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy and safety. J Parkinsons Dis 2019;9:531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzenschlager R, Hughes A, Evans A, Manson AJ, Hoffman M, Swinn L, et al. Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson's disease: a prospective study using single‐dose challenges. Mov Disord 2005;20:151–157. [DOI] [PubMed] [Google Scholar]
- 18.Deuschl G, Schade‐Brittinger C, Krack P, Volkmann J, Schäfer H, Bötzel K, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 19.Kimber TE, Fang J, Huddy LJ, Thompson PD. Long‐term adherence to apomorphine infusion in patients with Parkinson disease: a 10‐year observational study. Intern Med J 2017;47:570–573. [DOI] [PubMed] [Google Scholar]
- 20.Antonini A, Isaias IU, Rodolfi G, Landi A, Natuzzi F, Siri C, et al. A 5‐year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol 2011;258:579–585. [DOI] [PubMed] [Google Scholar]
- 21.Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, et al. Gastric emptying time and gastric motility in patients with Parkinson's disease. Mov Disord 2001;16:1041–1047. [DOI] [PubMed] [Google Scholar]
- 22.Straka I, Minár M, Škorvánek M, Grofik M, Danterová K, Benetin J, et al. Adherence to pharmacotherapy in patients with Parkinson's disease taking three and more daily doses of medication. Front Neurol 2019;10:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosset D, Antonini A, Canesi M, Pezzoli G, Lees A, Shaw K, et al. Adherence to antiparkinson medication in a multicenter European study. Mov Disord 2009;24:826–832. [DOI] [PubMed] [Google Scholar]
- 24.Cenci MA, Ohlin KE, Odin P. Current options and future possibilities for the treatment of dyskinesia and motor fluctuations in Parkinson's disease. CNS Neurol Disord Drug Targets 2011;10:670–684. [DOI] [PubMed] [Google Scholar]
- 25.Grandas F. Subcutaneous infusions of apomorphine: a reappraisal of its therapeutic efficacy in advanced Parkinson's disease. Expert Rev Neurother 2013;13:1343–1353. [DOI] [PubMed] [Google Scholar]
- 26.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol 2005;62:554–560. [DOI] [PubMed] [Google Scholar]
- 27.Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, et al. Long‐term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson's disease. Mov Disord 2010;25:578–586. [DOI] [PubMed] [Google Scholar]
- 28.Zadikoff C, Poewe W, Boyd JT, Bergmann L, Ijacu H, Kukreja P, et al. Safety of levodopa‐carbidopa intestinal gel treatment in patients with advanced Parkinson's disease receiving ≥2000 mg daily dose of levodopa. Parkinsons Dis 2020;2020:9716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.AbbVie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html


