Abstract
Background
Persistent motor or vocal tic disorder (PMVT) has been hypothesized to be a forme fruste of Tourette syndrome (TS). Although the primary diagnostic criterion for PMVT (presence of motor or vocal tics, but not both) is clear, less is known about its clinical presentation.
Objective
The goals of this study were to compare the prevalence and number of comorbid psychiatric disorders, tic severity, age at tic onset, and family history for TS and PMVT.
Methods
We analyzed data from two independent cohorts using generalized linear equations and confirmed our findings using meta‐analyses, incorporating data from previously published literature.
Results
Rates of obsessive–compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) were lower in PMVT than in TS in all analyses. Other psychiatric comorbidities occurred with similar frequencies in PMVT and TS in both cohorts, although meta‐analyses suggested lower rates of most psychiatric disorders in PMVT compared with TS. ADHD and OCD increased the odds of comorbid mood, anxiety, substance use, and disruptive behaviors, and accounted for observed differences between PMVT and TS. Age of tic onset was approximately 2 years later, and tic severity was lower in PMVT than in TS. First‐degree relatives had elevated rates of TS, PMVT, OCD, and ADHD compared with population prevalences, with rates of TS equal to or greater than PMVT rates.
Conclusions
Our findings support the hypothesis that PMVT and TS occur along a clinical spectrum in which TS is a more severe and PMVT a less severe manifestation of a continuous neurodevelopmental tic spectrum disorder. © 2021 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: chronic tics, meta‐analysis, severity, Tourette
Persistent motor or vocal tic disorder (PMVT) is hypothesized to be a forme fruste (milder version) of Tourette syndrome (TS).1, 2 This formulation is reflected in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), where the diagnostic criteria differ only in the requirement that either motor or vocal tics be present in PMVT and that both be present in TS.3 During the planning phase, the DSM‐5 committee on TS and tics considered merging these disorders, citing lack of evidence of distinct etiologies.4 Ultimately, the distinct classifications were retained to facilitate research of TS and PMVT separately to provide insights about their phenomenology.3, 4
Although tics are the distinguishing feature of TS, 76–90% of those who present for clinical care also have one or more comorbid psychiatric illnesses,5, 6, 7, 8 which contribute to the observed phenotypic heterogeneity of the disorder and have a strong influence on clinical outcomes.9 Attention deficit hyperactivity disorder (ADHD) and obsessive–compulsive disorder (OCD) are the two most common psychiatric comorbidities, and their presence increases the likelihood of potentially impairing symptoms, such as social disinhibition and self‐injurious behaviors, other psychiatric disorders, and the use of or need for pharmacological treatment.10, 11, 12
Despite its close clinical similarity to TS and higher population prevalence, less work has been done to characterize PMVT. In particular, it is unknown whether its clinical presentation, outside of the manifestation of tics, is similar to that of TS, or whether it has different patterns of comorbidities, tic severity, age of onset, or family history. To our knowledge, only two publications have directly compared the clinical manifestations of PMVT and TS, both of which concluded that, in terms of tic severity and psychiatric comorbidities, PMVT can be thought of as a less severe (milder) form of TS.13, 14 In the first study, PMVT and TS were compared in a sample of patients with OCD,13 while in the second, the characteristics of participants with primary tic disorders were examined.14 These differences in ascertainment strategy make direct comparison of these studies difficult and necessitate additional work to more fully elucidate the similarities and differences between PMVT and TS.
The goals of this study were thus to evaluate the clinical characteristics of PMVT and to compare them with TS. Specifically, we aimed to compare the prevalence and patterns of comorbid psychiatric disorders, tic severity, and age of onset in PMVT versus TS in two independent samples and to corroborate our findings using meta‐analysis in all available published and unpublished datasets. We also examined and compared rates of TS, PMVT, OCD, and ADHD in first‐degree relatives of probands with TS and those with PMVT. We hypothesized that PMVT would appear clinically to be a milder form of TS, with similar patterns but lower prevalence of comorbidities, lower tic symptom severity, and later mean age at onset for both tics and comorbid psychiatric disorders. We also hypothesized that rates of all disorders would be elevated in family members compared with reported population rates, and TS would be more common than PMVT in first‐degree relatives of TS probands and similar in first‐degree relatives of PMVT probands.
Patients and Methods
Participants and Assessments
The first (Tourette Association of America International Consortium for Genetics [TAAICG]) sample consisted of 2098 individuals with TS and 298 parents or siblings with PMVT who were recruited in parallel for genetic studies through the TAAICG.8 Details regarding recruitment, assessment, and clinical and demographic sample characteristics have been previously published.8 In brief, lifetime psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM‐IV in adults and the Kiddie Schedule for Affective Disorders and Schizophrenia in children.8 Consensus lifetime diagnoses and ages of onset were established for each diagnosis.8 The motor and vocal subscales of the Yale Global Tic Severity Scale (YGTSS) were used to assess tic severity at the worst‐ever time point during the participant's lifetime (see Supporting Information for details).15
The second (OCGAS) sample consisted of 87 individuals with PMVT and 84 with TS who were recruited for genetic studies of OCD by the OCD Collaborative Genetics Association Study (OCGAS).16 All participants had OCD, with symptoms beginning before the age of 18 years. Details regarding enrollment, assessment, and patients' characteristics can be found elsewhere.16 There was no overlap between the OCGAS and TAAICG samples. Clinical assessments, including lifetime history of tic disorders, were conducted using an extended version of the Structured Clinical Interview for DSM‐IV.16 Tic severity was measured using the YGTSS, and age of onset of symptoms was reported for each diagnosis.
Data Analysis
Age at interview, sex, and prevalence of lifetime history of comorbid psychiatric disorders were compared between PMVT and TS using t tests, Pearson chi‐square tests, or Fisher exact tests. The following comorbid disorders were examined (see Supporting Information for details): ADHD, OCD, mood, anxiety, disruptive behavior, eating and psychotic disorders, substance misuse, and elimination disorders.
Comparison of rates of lifetime psychiatric comorbidities, worst‐ever tic severity, and ages at onset of all diagnoses was next conducted using generalized estimating equations covarying for sex, age at time of interview, ADHD, OCD, and familial clustering. Family history of TS, PMVT, OCD, and ADHD was assessed and compared with expected rates under the null hypothesis. Rates of individual disorders in family members of TS probands were then compared with those in family members of PMVT probands (see Supporting Information for details).
Meta‐analysis
A meta‐analysis using all available published data was conducted to corroborate findings identified in the two primary datasets. To identify all available published data, we performed a manual search of cited literature in relevant TS articles, followed by a systematic search of the literature in MEDLINE (PubMed) (see Supporting Information for details). Inclusion criteria were: (1) data for both PMVT and TS available, and (2) data available for psychiatric comorbidities and/or ages at tic onset and/or tic severity (YGTSS Motor only) for PMVT and TS separately. Publications with interventions (ie, pharmacological), non‐PMVT or TS tic disorders, data for TS only, and non‐English manuscripts were excluded. Authors of identified articles were subsequently contacted for additional information as needed.
Seven studies were identified in the initial manual search (Supporting Information Fig. S1). In the systematic review, 40 full‐text studies were screened out of 863 abstracts identified. Twelve studies met inclusion criteria, of which four were also identified in the manual search. One study included separate data for TS and PMVT and was retained17; the authors of the seven remaining studies were contacted for additional information.18, 19, 20, 21, 22, 23, 24 For these seven studies, either separate data for TS and PMVT were not available or the authors did not respond, and these studies were thus excluded. The final meta‐analysis included data from eight published studies,2, 13, 14, 17, 25, 26, 27, 28 plus the TAAICG and OCGAS samples. One study17 reported data on a privately insured sample and a publicly insured sample, which were considered separately, for a total of 11 samples (Table 1).
TABLE 1.
Studies included in the meta‐analysis
| First Author | Publication Year | Diagnostic Criteria | Core Dx | Ascertainment | Assessment | Mean Age (y) | Total Number of PMVT Cases | Total Number of TS Cases | Na | Number (%) of Males in Total Sample | Types of Comorbidities Reported |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAAICG | This study | DSM‐5 | TS/PMVT | Clinical | Structured interview and best estimate | 21.3 | 298 (12.3%) | 2129 (87.7%) | 2427 | 1706 (72.3%) | ADHD, OCD, DB, mood, others |
| OCGASb | This study | DSM‐5 | OCD | Clinical | Structured interview and best estimate | 27.3 | 87 (50.9%) | 84 (49.1%) | 171 | 89 (52.0%) | ADHD, OCD, DB, mood, others |
| Müller‐Vahl et alc 14 | 2019 | DSM‐IV | TS/PMVT | Clinical | Semistructured interview | 21.0 | 40 (3.9%) | 978 (96.1%) | 1018 | 771 (77.6%) | ADHD, OCD, mood, and DB |
| Diniz et alb 13 | 2006 | DSM‐IV | OCD | Clinical | Structured interview and best estimate | 30.0 | 31 (50.8%) | 30 (49.2%) | 61 | N/A | OCD and mood |
| Khalifa et al25 | 2006 | DSM‐IV | TS/PMVT (split in MT/VT) | Community | Parent‐completed questionnaire | 10.3 | 58 (69.9%) | 25 (30.1%) | 83 | N/A | ADHD, OCD, mood, DB |
| Amiri et al26 | 2012 | DSM‐IV‐TR | TS/PMVT (split in MT/VT) | Community | Structured interview | 9.5 | 161 (88%) | 22 (12%) | 183 | N/A | ADHD |
| Kraft et alc 27 | 2012 | DSM‐IV‐TR | TS/PMVT (MT only) | Community | Tics screening and structured interview | 10.0 | 37 (52.9%) | 33 (47.1%) | 70 | 53 (75.7%) | ADHD, DB |
| Scharf et al28 | 2012 | DSM‐IV‐TR | TS/PMVT (split in MT/VT) | Community | Parent‐completed questionnaire | 13.0 | 61 (45.5%) | 73 (54.5%) | 134 | 127 (70.6%) | ADHD, OCD |
| Spencer et al2 | 1995 | DSM‐III‐R | TS/PMVT | Clinical | Structured interview | 11.4 | 39 (54.9%) | 32 (45.0%) | 71 | 64 (90.1%) | ADHD, OCD, DB, mood, others |
| Olfson et al17 | 2011 | ICD‐9 | TS/PMVT | Clinical | ICD codes in EHR | Children (4–18 y) | 2432 (15.3%) | 13,491 (84.7%) | 15,923 | 12,984 (81.5%) | ADHD, OCD, DB, mood, others |
Dx, diagnosis; PMVT, persistent motor or vocal tic disorder; TS, Tourette syndrome; TAAICG, Tourette Association of America International Consortium for Genetics; DSM, Diagnostic and Statistical Manual of Mental Disorders; ADHD, attention deficit hyperactivity syndrome; OCD, obsessive–compulsive disorder; DB, disruptive behavior disorder; OCGAS, OCD Collaborative Genetics Association Study; MT, motor tics; VT, vocal tics; ICD‐9, International Diagnostic Classification, Ninth Revision; EHR, Electronic Health Record; N/A, not available.
Total number (N) does not include individuals without tics or individuals with transient tics.
All participants had OCD.
Did not find any case of VT or did not target individuals with VT.
The reported prevalence rates of psychiatric comorbidities and age at tic onset were examined between PMVT and TS across the studies by comparing the odds ratios (ORs) of each group using a random‐effects model performed with STATA 16.1.29 The proportion of individuals with only motor tics in the PMVT samples was also examined. A heterogeneity test was performed to determine whether there were significant differences in the reported values across studies. Metaregressions and leave‐one‐out analyses were conducted to identify sources of heterogeneity for those meta‐analyses demonstrating high heterogeneity.29, 30 Metaregressions included sample size, ascertainment type, mean age at interview, proportion of male participants, proportion of individuals with OCD, and proportion with ADHD as covariates. Leave‐one‐out analyses examined the change in heterogeneity by excluding one study at a time from the meta‐analyses. Although not a formal test of influence, this approach can be used to identify studies exerting excessive influence on heterogeneity.30
Results
Prevalence of Psychiatric Comorbidities
Baseline characteristics of participants in the TAAICG and OCGAS samples are summarized in Table 2. PMVT participants were older and more likely to be female than were TS participants in TAAICG. These differences were not seen in OCGAS.
TABLE 2.
Baseline characteristics of participants with PMVT and TS in the TAAICG and OCGAS samples
| Cohort | PMVT, n/Total (%) with Available Data | TS, n/Total (%) with Available Data | χ2 (P) or t (P) |
|---|---|---|---|
| TAAICG | |||
| Age groups | |||
| <18 y, n (%) | 49/279 (17.6) | 1373/2032 (67.6) | |
| ≥18 y, n (%) | 230/279 (82.4) | 659/2032 (32.4) | 259.16 (<0.001) |
| Mean age at interview, y | 36.6 ± 14.8 | 19.2 ± 13.8 | 18.42 (<0.001) |
| Sex | |||
| Female, n (%) | 126/293 (43.0) | 527/2066 (25.5) | |
| Male, n (%) | 167/293 (57.0) | 1539/2066 (74.5) | 39.23 (<0.001) |
| Comorbid disorders, n (%) | |||
| ADHD | 62/291 (21.3) | 1099/2034 (54.0) | 109.06 (<0.001) |
| OCD | 126/296 (42.6) | 1377/2098 (65.6) | 59.07 (<0.001) |
| Mood disorders | 35/116 (30.2) | 256/985 (26.0) | 0.93 (0.33) |
| Anxiety disorders | 37/114 (32.5) | 280/982 (28.5) | 0.77 (0.38) |
| Disruptive behavior disorders | 7/28 (25.0) | 194/661 (29.3) | 0.24 (0.62) |
| Eating disorders | 4/113 (3.5) | 19/973 (1.9) | 1.23 (0.27) |
| Psychotic disorders | 0/112 (0) | 6/967 (0.6) | 0.69 (0.40) |
| Substance use disorders | 15/117 (12.8) | 54/992 (5.4) | 9.76 (<0.01) |
| Elimination disorders | 4/25 (16.0) | 128/723 (17.7) | 0.05 (0.83) |
| Score, mean ± SD (no. of individuals) | Score, mean ± SD (no. of individuals) | t (P) | |
| Tic severity | |||
| Motor tics | 10.6 ± 4.6 (110) | 17.2 ± 4.7 (1329) | −14.40 (<0.001) |
| Vocal tics | 5.4 ± 3.0 (11) | 12.9 ± 5.3 (1326) | −8.10 (<0.001) |
| OCGAS | |||
| Age groups, n (%) | |||
| <18 y | 30/87 (34.5) | 33/84 (39.3) | |
| ≥18 y | 57/87 (65.5) | 51/84 (60.7) | 0.4 (0.52) |
| Mean age at interview, y | 28.3 ± 15.7 | 26.3 ± 15.0 | 0.09 (0.93) |
| Sex, n (%) | |||
| Female | 43/87 (52.4) | 39/84 (47.6) | |
| Male | 44/87 (49.4) | 45/84 (50.6) | 0.15 (0.69) |
| Comorbid disorders, n (%) | |||
| ADHD | 18/87 (20.7) | 20/84 (23.8) | 0.24 (0.62) |
| OCD | 87/87 (100) | 84/84 (100) | N/A |
| Mood disorders | 58/87 (66.7) | 55/84 (65.5) | 0.03 (0.87) |
| Anxiety disorders | 69/87 (79.3) | 60/84 (71.4) | 1.43 (0.23) |
| Disruptive behavior disorders | 5/87 (5.8) | 7/84 (8.33) | 0.44 (0.51) |
| Eating disorders | 5/87 (5.8) | 3/84 (3.6) | 0.45 (0.50) |
| Psychotic disorders | 1/87 (1.2) | 0/84 (0) | 0.97 (0.32) |
| Substance use disorders | 13/87 (14.9) | 21/84 (25.0) | 2.71 (0.1) |
| Elimination disorders | N/A | N/A | |
| Score, mean ± SD (no. of individuals) | Score, mean ± SD (no. of individuals) | t test | |
| Tic severity | |||
| Motor tics | 9.54 ± 3.06 (46) | 14.64 ± 5.37 (64) | −5.78 (<0.001) |
| Vocal tics | 9.21 ± 2.96 (29) | 11.86 ± 4.04 (61) | −3.16 (<0.01) |
PMVT, persistent motor and vocal tics; TS, Tourette syndrome; TAAICG, Tourette Association of America International Consortium for Genetics; OCGAS, OCD Collaborative Genetics Association Study; ADHD, attention deficit hyperactivity disorder; OCD, obsessive–compulsive disorder; SD, standard deviation; N/A, not available.
PMVT and TS had similar prevalences of co‐occurring psychiatric disorders except ADHD, OCD, and substance use disorders in both samples (Table 2). Nearly half (52.8%) of PMVT participants in TAAICG had either ADHD or OCD, and 20.7% had both, compared with 80.5% of TS participants with either OCD or ADHD and 42.1% with both (Supporting Information Table S1). To determine whether the observed differences between TS and PMVT were due to age effects, the samples were stratified by age. These results (Supporting Information Tables S1 and S2) indicated that, although ADHD prevalence decreased with age and OCD prevalence increased with age, TS participants in TAAICG still had significantly higher rates of ADHD than those with PMVT across the age strata, whereas ADHD rates were only slightly higher for TS compared with PMVT in OCGAS. Prevalence rates for all psychiatric disorders were similar when the TAAICG sample was limited to those who also had co‐occurring OCD to be directly comparable with OCGAS (data not shown).
We next evaluated the relationship between TS and PMVT with each psychiatric comorbidity, adjusting for age, sex, OCD and ADHD, and familial clustering. These results confirmed the unadjusted results: there were no differences for psychiatric comorbidities in either sample other than OCD and ADHD, both of which were half as likely to occur in PMVT than in TS (Supporting Information Table S3). Rates of mood, anxiety, substance use, and disruptive behavior disorders were positively associated with presence of ADHD and OCD. Rates of most psychiatric comorbidities also differed by age and sex for both PMVT and TS (Supporting Information Tables S1–S4).
Number of Psychiatric Comorbidities
Approximately 50% of individuals with PMVT or TS in TAAICG and 20% in OCGAS had no psychiatric comorbidities other than ADHD and OCD (Supporting Information Fig. S2). PMVT/TS status was not associated with number of psychiatric comorbidities in either sample after controlling for age, sex, age at tic onset, and co‐occurring OCD and ADHD (TAAICG: coefficient [coeff] = −0.13, 95% confidence interval [CI] = −0.43 to 0.17, P = 0.39; OCGAS: coeff = −0.03, 95% CI = −0.64 to 0.58, P = 0.92), suggesting that the higher rate of individuals with psychiatric comorbidities in OCGAS was secondary to co‐occurring OCD.
Tic Severity
Motor and vocal tic severity, as measured by the YGTSS Motor Tic and YGTSS Vocal Tic Scores, respectively, were significantly lower in individuals with PMVT than in those with TS in both samples (Fig. 1). Because the number of individuals who endorsed vocal tics only was very small, additional analyses were conducted for motor tic severity only. Differences in YGTSS Motor Tic Scores between PMVT and TS persisted after adjusting for familial clustering, age at interview, age at tic onset, ADHD, OCD, and sex (Supporting Information Table S5).
FIG. 1.
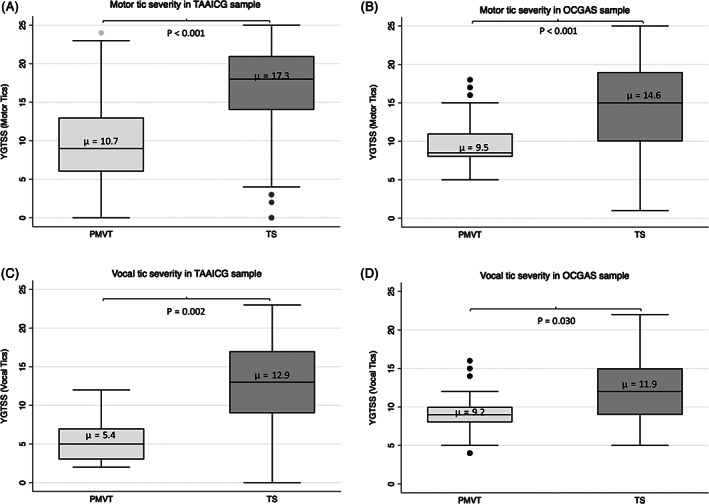
Yale Global Tic Severity Scale (YGTSS) Motor and YGTSS Vocal tic severity subscale scores in individuals with persistent motor and vocal tics (PMVT) versus Tourette syndrome (TS) in the Tourette Association of America International Consortium for Genetics (TAAICG) (A,B) and (OCGAS) (C,D) samples. Note that only PMVT participants with motor tics were included in the YGTSS Motor tic severity subscale analysis, and only PMVT participants with vocal tics were included in the YGTSS Vocal tic severity subscale analyses.
Age of Onset
The mean age of tic onset in PMVT was approximately 2.5 years later than in TS for both samples, after controlling for sex and age at interview (Supporting Information Table S6). In the TAAICG PMVT sample, mean age of tic onset was similar for motor and vocal tics (8.4 ± 3.6 and 8.8 ± 3.7 years, respectively). In the OCGAS PMVT sample, the mean age of motor tic onset was slightly later than for vocal tics (11.4 ± 6.6 and 9.0 ± 4.3 years, respectively). There were no significant differences for either sample in the mean ages at onset for any of the comorbid psychiatric disorders, including OCD and ADHD, after controlling for sex and age at interview (Supporting Information Table S6 and Fig. 2). The patterns of symptom onset for the psychiatric comorbidities were similar for PMVT and TS, with ADHD and disruptive behavior disorders beginning earlier, followed by tics, OCD, anxiety and mood disorders, and substance misuse at a later age.
FIG. 2.
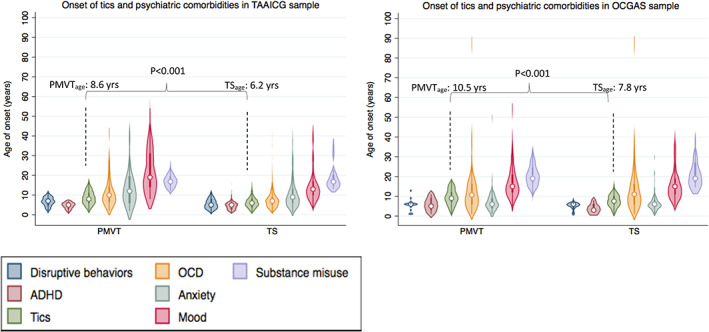
Ages of onset of tics and comorbid psychiatric disorders in persistent motor and vocal tics (PMVT) versus Tourette syndrome (TS). Ages of onset are arranged in chronological order. Left panel, Tourette Association of America International Consortium for Genetics (TAAICG) sample. Right panel, OCD Collaborative Genetics Association Study (OCGAS) sample. ADHD, attention deficit hyperactivity disorder; OCD, obsessive–compulsive disorder. [Color figure can be viewed at wileyonlinelibrary.com]
Family History
Rates of TS, PMVT, OCD, and ADHD were substantially higher in first‐degree relatives of probands compared with reported population prevalences (Supporting Information Tables S7 and S8). About 45.7% of TAAICG probands with TS or PMVT had a family member with TS, compared with 18.1% with PMVT. About 13.8% of OCGAS probands with TS or PMVT had a family member with TS, compared with 6.3% with PMVT. Bilineality (both parents with the disorder) was also elevated in the TAAICG sample, which consisted disproportionately of TS probands. TS and PMVT rates were consistently higher in fathers than in mothers, consistent with the known male predominance of tic disorders.1,2 There were no significant differences in family member rates of PMVT or TS in TS versus PMVT participants, with one exception: in mothers, TS was more common in TS probands, and PMVT was more common in PMVT probands, although this difference was significant in the OCGAS sample only (Supporting Information Table S8).
Meta‐analyses
Because the two primary study samples differed in ascertainment and characteristics, meta‐analyses were conducted to confirm and extend the primary findings. Of the 11 datasets, 4 were clinically ascertained, 4 were community based, and 3 were registry based (Table 1). Two samples were composed solely of individuals with OCD and co‐occurring tic disorders (OCGAS and Diniz et al13). Consistent with our primary findings, the meta‐analyses suggested that odds of OCD and ADHD were both lower in PMVT than in TS (Fig. 3A,B). In particular, the odds of comorbid OCD were more than 50% lower in PMVT compared with TS (OR = 0.37, 95% CI, 0.31–0.44, z = −11.49, P < 0.001), with low heterogeneity across studies (n = 7; I2 = 0%, P = 0.771). The odds of ADHD were 51% lower in PMVT when compared with TS, but this meta‐analysis showed high heterogeneity (n = 10, I2 = 89.8%, P < 0.001; Fig. 3B). Examination of the forest plots indicated that studies with either small (<100) or large (>1000) sample sizes showed the strongest evidence of heterogeneity. A metaregression including sample size, mean age, proportion of males with tics, and the proportion of OCD did not explain the high heterogeneity (data not shown), and leave‐one‐out analyses suggested that the TAAICG study was a relative outlier, although none of the estimated ORs fell outside the CIs for the combined analysis (Supporting Information Fig. S3).
FIG. 3.
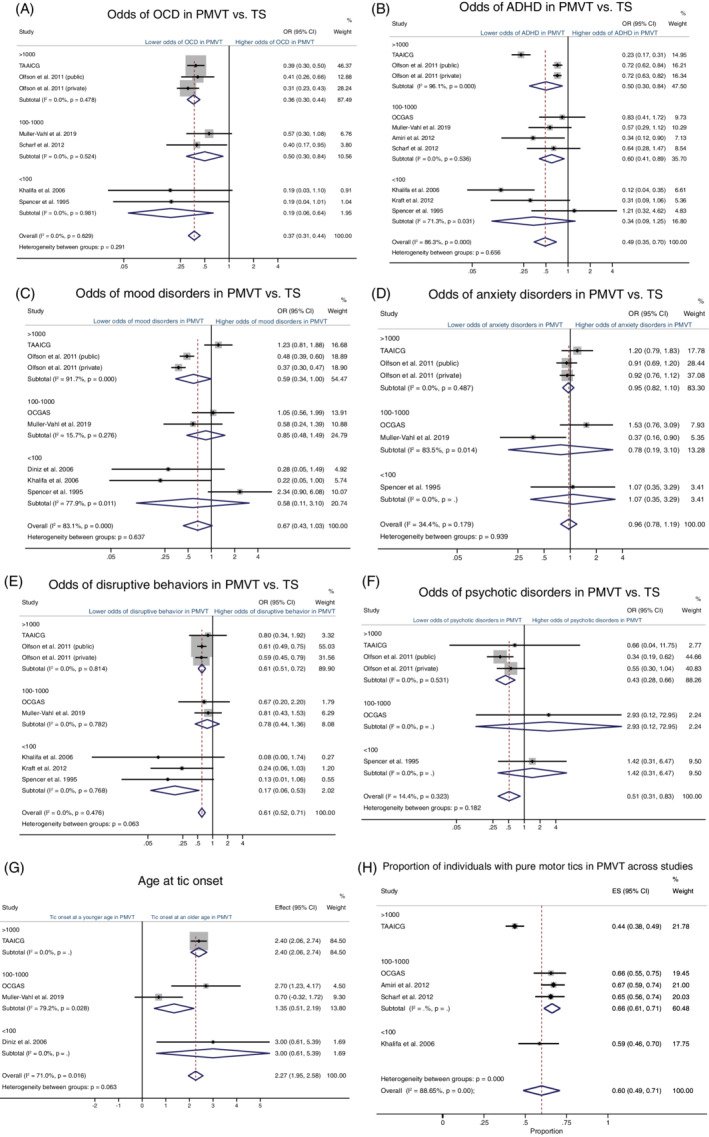
Forest plots comparing the prevalence of psychiatric comorbidities (A–F), age of tic onset (G) in individuals with persistent motor and vocal tics (PMVT) and Tourette syndrome (TS), and proportion of individuals with pure motor tics (H) stratified by sample size. ADHD, attention deficit hyperactivity disorder; CI, confidence interval; OCD, obsessive–compulsive disorder; OR, odds ratio. [Color figure can be viewed at wileyonlinelibrary.com]
Metaregression was also suggestive of lower odds of mood disorders for PMVT than for TS (OR = 0.67; 95% CI, 0.43–1.03; z = −1.63; P = 0.104; Fig. 3C), although this finding was not statistically significant, and there was high heterogeneity between studies (n = 8; I2 = 87.4%; P < 0.001). Metaregression suggested that this heterogeneity was explained by the mean age of the cohort (coeff = 0.50; 95% CI, <0.01–1.00; z = 1.96, P = 0.050) and the proportion of individuals with ADHD (coeff = 0.02; 95% CI, <0.01–0.05; z = 2.13; P = 0.033), with older age and higher ADHD rates contributing to higher odds of mood disorders. Leave‐one‐out analyses did not identify excessive influence on the results from any single study (Supporting Information Fig. S3). There were no differences between PMVT and TS in the odds of comorbid anxiety (n = 6; OR = 0.95; 95% CI, 0.82–1.09; z = −0.74; P = 0.461). As hypothesized, the presence of anxiety was associated with the mean age of the cohort (coeff = 0.70; 95% CI, 0.13–1.27; z = 2.42; P = 0.016) and proportion of individuals with OCD (coeff = −0.14; 95% CI, −0.26 to −0.22; z = −2.31; P = 0.021). Odds of disruptive behaviors (n = 8; OR = 0.60; 95% CI, 0.52–0.71; z = − 6.19; P < 0.001) and psychotic disorders (n = 5; OR = 0.53; 95% CI, 0.31–0.90, z = −2.35; P = 0.019) were lower in PMVT than in TS, and between‐study heterogeneity was low (Fig. 3). Metaregressions suggested that co‐occurring ADHD and OCD were not associated with disruptive behaviors (OCD: coeff = 0.06, 95% CI, −0.05 to 0.18, z = 1.08, P = 0.282; ADHD: coeff = 0.02, 95% CI, −0.02 to 0.07, z = 1.10, P = 0.269) or psychotic disorders (OCD: coeff = −0.02, 95% CI, −0.17 to 0.12, z = −0.29, P = 0.771; ADHD: coeff = −0.05, 95% CI, −0.28 to 0.18, z = −0.43, P = 0.666 for ADHD). Secondary analyses excluding the OCGAS and Diniz et al13 samples did not substantially change the results of the meta‐analyses (Supporting Information Fig. S4).
Tics presented approximately 2 years later in PMVT than in TS (n = 4; mean difference = 2.27; 95% CI: 1.95–2.58 years; z = 14.26; P < 0.001), but these results were highly heterogenous (I2 = 71.0%, P = 0.016), primarily because of study differences in sample size (coeff = 0.001; 95% CI, <0.001–0.002; z = 3.01; P = 0.003) and mean age at interview (coeff = 0.42; 95% CI, 0.136–0.696; z = 2.91, P = 0.004) (Fig. 3G). YGTSS Motor tic severity was lower for PMVT than TS (n = 2; mean difference = −5.30 YGTSS; 95% CI, −8.13 to −2.47; z = −3.67; P < 0.001), but we did not find published studies that reported these scores in PMVT and TS separately, thus limiting our analysis to TAAICG and OCGAS samples. Almost 60% of those with PMVT had motor tics (Fig. 3H). However, the findings for tic severity and proportion of individuals with pure motor tics showed high heterogeneity (I2 = 87.9%, P = 0.004 and I2 = 88.7%, P < 0.001, respectively), and it was not possible to verify the source of heterogeneity using metaregression analyses. Secondary analyses excluding the OCD samples resulted in a similar age of onset estimate but a substantially higher proportion of motor tics in PMVT compared with vocal tics (Supporting Information Fig. S4).
Discussion
The results of this study support the concept that, rather than being clinically distinct disorders, TS and PMVT may be more accurately construed as different levels of severity of the same disorder. Although there was some heterogeneity between studies, the overall results of this study suggest that, as hypothesized, individuals with PMVT had lower tic severity, later age of tic onset, strong family histories, and lower or similar rates of psychiatric comorbidities among individuals when compared with those with TS. Ages of onset for the psychiatric comorbidities did not differ after controlling for age and comorbid OCD and/or ADHD, nor did total number of psychiatric comorbidities (eg, total psychiatric burden). The prevalence of motor and vocal tics was split nearly evenly in the PMVT samples, with just more than half of individuals presenting with purely motor tics.
As with TS, individuals with PMVT presented with high rates of psychiatric comorbidities, most commonly OCD and ADHD, which occurred in between 20% and 60% of participants across studies. Although the odds of comorbid OCD and ADHD were generally lower in PMVT than in TS, the rates of these disorders were still approximately 10 times higher than would be expected based on general population rates (approximately 2% and 6%, respectively).31 ADHD and OCD are known to be the most common psychiatric comorbidities in patients with TS, and they have been identified as core components of the syndrome, with shared genetic etiologies,32, 33, 34, 35, 36 such that many clinicians who treat TS colloquially refer to the co‐occurrence of these three disorders as “TS‐plus.”37
Co‐occurring OCD and ADHD were also independent predictors of the presence of additional psychiatric comorbidities, irrespective of type of tic disorder. This finding is particularly relevant because the combination of ADHD and OCD in individuals with a tic disorder is associated with greater global impairment, higher need for psychiatric medications, family history of tics, and greater tic severity.7 Similarly, individuals with TS and comorbid ADHD have more maladaptive behaviors, lower cognitive skills, and a greater disability burden because of motor and executive control deficits.34, 38 Although the question has never been formally examined to our knowledge, the same may be true of individuals with PMVT, albeit to a lesser degree.
The results of this study suggest that clinically these disorders are more similar than they are different, and they likely represent a single entity manifested along a severity spectrum where TS is the most severe manifestation. Similarly, although we cannot examine the underlying etiology of these disorders in this study, results from previously published genetic studies suggest that TS and PMVT fall along a single etiological spectrum.36 In support of this concept, we found that first‐degree relatives, in particular parents, of TS and PMVT probands had elevated rates of both TS and PMVT, as would be expected if these disorders were due to the same underlying genetic factors. Although sample sizes were small for the family analysis, TS rates were higher in family members than were PMVT rates, regardless of whether the proband had TS or PMVT, suggesting that environmental or epigenetic factors, in combination with underlying genetic susceptibility, may also partly determine whether TS or PMVT develops in a given individual.
Significance and Clinical Implications
Elucidating the clinical manifestations of neuropsychiatric disorders is critical for understanding their etiologies, predicting the likely course of illness, providing effective treatment, and maximizing functioning and quality of life. This study, which quantifies the prevalence of psychiatric comorbidities, age of onset, tic severity, and family history of tic disorders in PMVT, resulted in several key findings that are of potential relevance to clinicians. First, the clinical presentation of PMVT is, in most respects, similar to but less severe than that of TS. Second, despite this, the psychiatric burden in PMVT is still higher than in the general population; in particular, either comorbid OCD or ADHD will develop in between 20% and 60% of individuals with PMVT, and both will develop in a substantial proportion. Individuals with PMVT are also at risk for the development of other psychiatric comorbidities and, as with TS, the risk for these comorbidities is higher in those with OCD and/or ADHD. Third, comorbid psychiatric disorders in PMVT are expected to develop at ages similar to those seen among individuals with TS. Clinicians should regularly assess for the development of OCD, ADHD, mood, anxiety, and disruptive behavior disorders in their patients with PMVT, because data from the TS literature suggest that these disorders are more likely to cause functional impairment than are the tics themselves.34
Limitations
This study has several limitations. First, all data are retrospective, and thus may be subject to recall bias. Second, the study samples differed in mean age, presence of comorbid OCD, and ascertainment. Furthermore, the rates of psychotic disorders were very low in individual samples, and disruptive behavior disorders were assessed primarily in children, all of which could potentially confound the results. We addressed these potential confounds to the best of our ability by controlling for these factors in our analyses, and we do see evidence that mean age at ascertainment and comorbid ADHD and/or OCD contribute to some, but not all, of the observed differences between studies. We do not have data on the severity or impact of comorbid psychiatric disorders or on treatment outcomes. Additional prospectively designed studies that eliminate these potential sources of bias would be useful to replicate and extend this work.
Financial Disclosures
The authors have received funding from the following sources in the past 12 months:
Karla Claudio‐Campos, PhD – Employment: University of Florida; Grants: NIH T32 HG008958.
Daniel Stevens, MD – Employment: Johns Hopkins University.
Sang‐Wahn Koo, MD – Employment: University of Florida.
Alexa Valko, BS – Employment: University of Florida.
Oscar Joseph Bienvenu, MD – Employment: Johns Hopkins University; Contracts: clinical contract with the Bureau of Alcohol, Tobacco, Firearms, and Explosives; Royalties: Oxford University Press.
Cathy B. Budman, MD – Employment: private practice; Advisory Board: Tourette Association of America Long Island Chapter.
Danielle C. Cath, MD, PhD – Grants: from local Dutch funding, unrelated to Tourette's disorder; Zorgondersteuningsfonds, Fonds Espria; Advisory Boards: Dutch Tourette Association, European Sociatey on the Study of Tourette Syndrome; Employment: Utrecht University.
Sabrina Darrow, PhD – Contracts: City and County of San Francisco Department of Public Health (not related to the present work); Grants: UCSF Resource Allocation Program/ Clinical and Translational Science Institute grant number UL1 TR001872 (not related to the present work), American Foundation for Suicide Prevention (not related to the present work); Employment: University of California, San Francisco.
Daniel Geller, MD; Employment: Massachusetts General Hospital; Grants: from the pharmaceutical industry and philanthropy, both unrelated to Tourette syndrome.
Fernando S. Goes, MD – Grants: from Janssen Pharmaceuticals for a study of at risk youths for bipolar disorder; Employment: Johns Hopkins University.
Marco A. Grados, MD, MPH – Advisory Boards: Cornelia de Lange Foundation; Employment: Johns Hopkins University; Contracts: Freespira, Inc.
Benjamin D. Greenberg, MD – Grants: VA – N9228‐C, I01 RX002450; NIH P50 MH106435 NIMH P20 GM130452 NIGMS; Employment: Butler Hospital and Providence VA Medical Center.
Erica Greenberg, MD – Advisory Board: Tourette Association of America; Honoraria Tourette Association of America; Employment: Massachusetts General Hospital.
Matthew E. Hirschtritt, MD MPH – Contract: from the California Mental Health Services Oversight and Accountability Commission (19MHSOAC064) outside of the current work; Employment: past – University of California San Francisco, present – The Permanente Medical Group, Inc.
Cornelia Illmann, PhD – Employment: Massachusetts General Hospital.
Franjo Ivankovic, BS ‐ Employment: University of Florida; Grants: T32NS082168, University of Florida Graduate Student Fellowship
Robert A. King, MD – Royalties: Cambridge University Press; Employment: Yale University.
James A. Knowles, MD, PhD – Grants: R01MH112904 and R01MH123775; Co‐PI on R01MH103657 and Co‐I on R01MH104964. Employment: SUNY Downstate Health Sciences University.
Janice Krasnow, PhD – Employment: Johns Hopkins University.
Paul C. Lee, MD – Employment: Tripler Army Medical Center.
Gholson J. Lyon, MD, PhD – Advisory Boards: Cornelia de Lange Foundation, KBG Syndrome Foundation, Omicia, Inc., re‐named Fabric Genomics; Employment: New York State OPWDD; Grants: NIGMS R35.
James T. McCracken, MD – Consultant: Tris Pharma, GW Biosciences; Grants: NIH P50 HD055784, U54 HD087101, P50 HD103557, T32 MH073517; Employment: University of California Los Angeles.
Mary M. Robertson, MD – Advisory Boards: European Society for the Study of Tourette Syndrome.
Lisa Osiecki, BS – Employment: Massachusetts General Hospital.
Mark A. Riddle, MD – Employment: Johns Hopkins University.
Guy Rouleau, PhD – Advisory Boards: AL‐S Pharma; Grants: Canadian Institutes of Health; Employment: McGill University.
Paul Sandor, MD – Advisory Board: National Canadian Association for Equality; Shares in Neurozone MSH; Contract: Youthdale Treatment Centers; Employment: University of Toronto.
Gerald Nestadt, MD – Grants: MH118261; Employment: Johns Hopkins University.
Jack Samuels, MD – Grants: MH118261 and James Marshall OCD Foundation; Employment: Johns Hopkins University.
Jeremiah M. Scharf, MD PhD – Advisory Boards: Tourette Association of America; Honoraria Tourette Association of America; Employment: Massachusetts General Hospital; Grants: National Institutes of Health NS105746, MH117114, NS102371, Rosen Family Foundation.
Carol A. Mathews, MD – Advisory Boards: Tourette Association of America, International OCD Foundation; Employment: University of Florida, Honoraria National Institutes of Health; Royalties: W.W. Norton Inc; Grants: National Institutes of Health NS105746, MH117114, NS102371, AT009988, Rosen Family Foundation.
Author Roles
Karla Claudio‐Campos, PhD: Statistical analysis: design, execution; Manuscript: writing of the first draft. Daniel Stevens, MD: Statistical analysis: design and execution; Manuscript: review and critique. Sang‐Wahn Koo, MD: Research project: execution; Manuscript: writing of the first draft. Alexa Valko, BS: Research project: execution; Manuscript: review and critique. Oscar Joseph Bienvenu, MD: Research project: execution; Manuscript: review and critique. Cathy B. Budman, MD: Research project: execution; Manuscript: review and critique. Danielle C. Cath, MD, PhD: Research project: execution; Manuscript: review and critique. Sabrina Darrow, PhD: Research project: execution; Manuscript: review and critique. Daniel Geller, MD: Research project: execution; Manuscript: review and critique. Fernando S. Goes, MD: Research project: execution; Manuscript: review and critique. Marco A. Grados, MD, MPH: Research project: execution; Manuscript: review and critique. Benjamin D. Greenberg, MD: Research project: execution; Manuscript: review and critique. Erica Greenberg, MD: Research project: execution; Manuscript: review and critique. Matthew E. Hirschtritt, MD, MPH: Research project: execution; Manuscript: review and critique. Cornelia Illmann PhD: Research project: organization and execution; Manuscript: review and critique. Franjo Ivankovic, BS: Research project: execution; Manuscript, review and critique. Robert A. King, MD: Research project: execution; Manuscript, review and critique. James A. Knowles, MD, PhD: Research project: execution; Manuscript: review and critique. Janice Krasnow, PhD: Research project: execution; Manuscript: review and critique. Paul C. Lee, MD: Research project: organization and execution; Manuscript: review and critique. Gholson J. Lyon, MD PhD: Research project: execution; Manuscript: review and critique. James T. McCracken, MD: Research project: execution; Manuscript, review and critique. Mary M. Robertson, MD: Research project: execution; Manuscript: review and critique. Lisa Osiecki, BS: Research project: organization and execution; Manuscript: review and critique. Mark A. Riddle, MD: Research project: execution; Manuscript: review and critique. Guy Rouleau, PhD: Research project: execution; Manuscript: review and critique. Paul Sandor, MD: Research project: execution; Manuscript: review and critique. Gerald Nestadt, MD: Research project: organization and execution; Statistical analysis: design; Manuscript: review and critique. Jack Samuels, MD: Research project: organization and execution; Statistical analysis: design; Manuscript: review and critique. Jeremiah M. Scharf, MD, PhD: Research project: conceptualization, organization, and execution; Statistical analysis: design, review, and critique; Manuscript: review and critique. Carol A. Mathews, MD: Research project: conceptualization, organization, and execution; Statistical analysis: design, review, and critique; Manuscript: review and critique.
Supporting information
Appendix S1: Supporting Information
Acknowledgments
This work was funded by National Institute of Neurological Disorders and Stroke grants to J.M.S. and C.A.M. (NS102371 and NS105746) and by the Rosen Family Foundation. K.C.‐C. received support for this work by National Human Genome Research Institute grant T32 HG008958. F.I. received support for this work by National Institute of Neurological Disorders and Stroke grant T32 NS082168. The OCGAS was supported by the National Institutes of Mental Health (MH50214, MH071507, MH079488, MH079494) and by the James E. Marshall OCD Foundation. The use of Covidence for the systematic review was supported by the University of Florida Clinical and Translational Science Institute, which is funded in part by the National Institutes of Health National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge the participants and families who contributed their information so that this study could be conducted, and Dongmei Yu for providing feedback and support for the statistical analyses.
Relevant conflicts of interest/financial disclosures: C.A.M. and J.M.S. serve on the scientific advisory board for the Tourette Association of America (TAA), and E.G. serves on the medical advisory board of the TAA. D.C.C. serves on the scientific advisory board of the Dutch Tourette syndrome association. F.S.G. has received funding from Janssen Pharmaceuticals for a study of bipolar disorder unrelated to this work. The other coauthors report no relevant conflicts of interest.
Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Robertson MM, Eapen V, Singer HS, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers 2017;3:16097. 10.1038/nrdp.2016.97 [DOI] [PubMed] [Google Scholar]
- 2.Spencer T, Biederman J, Harding M, et al. The relationship between tic disorders and Tourette's syndrome revisited. J Am Acad Child Adolesc Psychiatry 1995;34(9):1133–1139. 10.1097/00004583-199509000-00009 [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association., American Psychiatric Association. DSM‐5 Task Force . Diagnostic and statistical manual of mental disorders: DSM‐5. 5th ed. Arlington, VA, Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 4.Walkup JT, Ferrão Y, Leckman JF, et al. Tic disorders: some key issues for DSM‐V. Depress Anxiety 2010;27(6):600–610. 10.1002/da.20711 [DOI] [PubMed] [Google Scholar]
- 5.Freeman RD, Fast DK, Burd L, et al. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol 2000;42(7):436–447. 10.1111/j.1469-8749.2000.tb00346.x [DOI] [PubMed] [Google Scholar]
- 6.Mol Debes NMM, Hjalgrim H, Skov L. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol 2008;23(9):1017–1027. 10.1177/0883073808316370 [DOI] [PubMed] [Google Scholar]
- 7.Grados MA, Mathews CA. Tourette syndrome association international consortium for G. latent class analysis of gilles de la tourette syndrome using comorbidities: clinical and genetic implications. Biol Psychiatry 2008;64(3):219–225. 10.1016/j.biopsych.2008.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschtritt ME, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette Syndrome Comorbid psychiatric disorders in Tourette Syndrome Comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiat 2015;72(4):325–333. 10.1001/jamapsychiatry.2014.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groth C, Skov L, Lange T, et al. Predictors of the clinical course of Tourette syndrome: a longitudinal study. J Child Neurol 2019;34(14):913–921. 10.1177/0883073819867245 [DOI] [PubMed] [Google Scholar]
- 10.Hirschtritt ME, Darrow SM, Illmann C, et al. Social disinhibition is a heritable subphenotype of tics in Tourette syndrome. Neurology 2016;87(5):497–504. 10.1212/WNL.0000000000002910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews CA, Waller J, Glidden D, et al. Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry 2004;75(8):1149–1155. 10.1136/jnnp.2003.020693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debes NM, Hjalgrim H, Skov L. The presence of comorbidity in Tourette syndrome increases the need for pharmacological treatment. J Child Neurol 2009;24(12):1504–1512. 10.1177/0883073808331363 [DOI] [PubMed] [Google Scholar]
- 13.Diniz JB, Rosario‐Campos MC, Hounie AG, et al. Chronic tics and Tourette syndrome in patients with obsessive–compulsive disorder. J Psychiatr Res 2006;40(6):487–493. 10.1016/j.jpsychires.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Müller‐Vahl KR, Sambrani T, Jakubovski E. Tic disorders revisited: introduction of the term “tic spectrum disorders”. Eur Child Adolesc Psychiatry 2019;28(8):1129–1135. 10.1007/s00787-018-01272-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leckman JF, Riddle MA, Hardin MT, et al. The Yale global tic severity scale: initial testing of a clinician‐rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28(4):566–573. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 16.Mattheisen M, Samuels JF, Wang Y, et al. Genome‐wide association study in obsessive‐compulsive disorder: results from the OCGAS. Mol Psychiatry 2015;20(3):337–344. 10.1038/mp.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olfson M, Crystal S, Gerhard T, et al. Patterns and correlates of tic disorder diagnoses in privately and publicly insured youth. J Am Acad Child Adolesc Psychiatry 2011;50(2):119–131. 10.1016/j.jaac.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 18.de Alvarenga PG, de Mathis MA, Dominguez Alves AC, et al. Clinical features of tic‐related obsessive‐compulsive disorder: results from a large multicenter study. CNS Spectr 2012;17(2):87–93. 10.1017/S1092852912000491 [DOI] [PubMed] [Google Scholar]
- 19.Byler DL, Chan L, Lehman E, et al. Tourette syndrome: a general pediatrician's 35‐year experience at a single center with follow‐up in adulthood. Clin Pediatr 2015;54(2):138–144. 10.1177/0009922814550396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storch EA, Hanks CE, Mink JW, et al. Suicidal thoughts and behaviors in children and adolescents with chronic tic disorders. Depress Anxiety 2015;32(10):744–753. 10.1002/da.22357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlander M, Schwarz O, Rothenberger A, et al. Tic disorders: administrative prevalence and co‐occurrence with attention‐deficit/hyperactivity disorder in a German community sample. Eur Psychiatry 2011;26:370–374. [DOI] [PubMed] [Google Scholar]
- 22.Weingarden H, Scahill L, Hoeppner S, et al. Self‐esteem in adults with Tourette syndrome and chronic tic disorders: the roles of tic severity, treatment, and comorbidity. Compr Psychiatry 2018;84:95–100. 10.1016/j.comppsych.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernández de la Cruz L, Rydell M, Runeson B, et al. Suicide in Tourette's and chronic tic disorders. Biol Psychiatry 2017;82(2):111–118. 10.1016/j.biopsych.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 24.Cubo E, Trejo J, Ausín V, et al. Association of tic disorders with poor academic performance in Central Spain: a population‐based study. J Pediatr 2013;163(1):217–23.e1‐3. 10.1016/j.jpeds.2012.12.030 [DOI] [PubMed] [Google Scholar]
- 25.Khalifa N, VONK AL. Psychopathology in a Swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry 2006;45(11):1346–1353. 10.1097/01.chi.0000251210.98749.83 [DOI] [PubMed] [Google Scholar]
- 26.Amiri S, Fakhari A, Golmirzaei J, et al. Tourette's syndrome, chronic tics, and comorbid attention deficit/hyperactivity disorder in elementary students. Arch Iran Med 2012;15(2):76–78. [PubMed] [Google Scholar]
- 27.Kraft JT, Dalsgaard S, Obel C, et al. Prevalence and clinical correlates of tic disorders in a community sample of school‐age children. Eur Child Adolesc Psychiatry 2012;21(1):5–13. 10.1007/s00787-011-0223-z [DOI] [PubMed] [Google Scholar]
- 28.Scharf JM, Miller LL, Mathews CA, et al. Prevalence of Tourette syndrome and chronic tics in the population‐based Avon longitudinal study of parents and children cohort. J Am Acad Child Adolesc Psychiatry 2012;51(2):192–201.e5. 10.1016/j.jaac.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.StataCorp . Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. 2019. [Google Scholar]
- 30.Thomas S.METANINF: Stata module to evaluate influence of a single study in meta‐analysis estimation. Statistical Software Components S419201, Boston College Department of Economics. 2001.
- 31.Harvard Medical School . National Comorbidity Survey (NCSSC). 2017, August 21. https://www.hcp.med.harvard.edu/ncs/index.php
- 32.Gorman DA, Thompson N, Plessen KJ, et al. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry 2010;197(1):36–44. 10.1192/bjp.bp.109.071050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson BS, Pine DS, Cohen P, et al. Prospective, longitudinal study of tic, obsessive‐compulsive, and attention‐deficit/hyperactivity disorders in an epidemiological sample. J Am Acad Child Adolesc Psychiatry 2001;40(6):685–695. 10.1097/00004583-200106000-00014 [DOI] [PubMed] [Google Scholar]
- 34.Denckla MB. Attention deficit hyperactivity disorder: the childhood co‐morbidity that most influences the disability burden in Tourette syndrome. Adv Neurol 2006;99:17–21. [PubMed] [Google Scholar]
- 35.Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed 2012;97(5):166–175. 10.1136/archdischild-2011-300585 [DOI] [PubMed] [Google Scholar]
- 36.Yu D, Sul JH, Tsetsos F, et al. Interrogating the genetic determinants of Tourette's syndrome and other tic disorders through genome‐wide association studies. Am J Psychiatry 2019;176(3):217–227. 10.1176/appi.ajp.2018.18070857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packer LE. Social and educational resources for patients with Tourette syndrome. Neurol Clin 1997;15(2):457–473. 10.1016/S0733-8619(05)70326-1 [DOI] [PubMed] [Google Scholar]
- 38.Rizzo R, Curatolo P, Gulisano M, et al. Disentangling the effects of Tourette syndrome and attention deficit hyperactivity disorder on cognitive and behavioral phenotypes. Brain Dev 2007;29(7):413–420. 10.1016/j.braindev.2006.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


