Abstract
Objective
To evaluate the efficacy of lenvatinib in the treatment of radioiodine‐refractory thyroid carcinoma.
Background
Thyroid carcinoma is one of the top ten carcinomas worldwide. Clinically, thyroid cancers are managed with resections and adjuvant therapy with radioiodine. However, radioiodine is not effective for radioiodine‐refractory (RR) thyroid carcinoma in some patients.
Lenvatinib is a multi‐kinase inhibitor for the treatment of RR thyroid carcinoma. Several clinical trials showed its efficacy in prolonging progression‐free survival (PFS) and overall survival (OS).
Design, patients and measurements
A systematic search was done on databases (PubMed, Embase, MEDLINE, Cochrane) on 8 June 2020. Search keywords were lenvatinib, thyroid carcinoma and randomized controlled trials. Clinical trials fulfilling the SELECT protocol were selected to evaluate the efficacy of lenvatinib in terms of prolongation of PFS, OS and objective response rate (ORR). The risk ratio and distribution of grade 3 or above adverse events were documented.
Results
Of the 3997 patients of mean age 62.5 years in fifteen selected studies, lenvatinib is associated with prolonged PFS (hazard ratio 0.24, 95% CI, 0.19‐0.31, p < .001) and OS (hazard ratio 0.65, 95% CI, 0.52‐0.81, p < .001). Compared with placebo, the risk ratio of ORR and incidence of grade 3 or above adverse events are 35.41 (95% CI, 19.42‐64.58, p < .001) and 8.25 (95% CI, 6.50‐10.46, p < .001), respectively. Subgroup analysis shows that lenvatinib is effective for all patients with RR thyroid carcinoma, regardless of age, histological subtypes, radiological subtypes and mutation status.
Conclusion
Lenvatinib is effective in the treatment of RR thyroid carcinoma. Close monitoring of serious adverse events is recommended.
Keywords: lenvatinib, meta‐analysis, systematic review, thyroid carcinoma
1. INTRODUCTION
Thyroid carcinoma is a common endocrine malignancy globally. Its incidence is rapidly increasing in the United States, with estimation of 2,000 in 2020.1 After resection, radioactive iodine I‐131 (RAI) is one of the adjuvant treatments for high‐risk differentiated thyroid carcinoma (DTC). However, half of the metastatic DTC patients and 5% to 15% DTC without metastasis are resistant to RAI.2 The 5‐year survival rate of patients with RAI‐refractory (RR) thyroid cancer is 60% to 70%.3 Those with RR metastatic carcinoma have worse outcome, with a 10‐year survival rate of 10%.4
Lenvatinib is a multi‐kinase inhibitor for the treatment of several types of cancers, including thyroid cancer. It inhibits vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, VEGFR3, fibroblast growth factor receptor 1 (FGFR1), FGFR2, FGFR3, FGFR4, platelet‐derived growth factor receptor‐alpha (PDGFR‐α), c‐kit and the RET proto‐oncogene to exert its anti‐cancer effect.5 Several randomized controlled trials have proven its clinical efficacy in prolonging progression‐free survival (PFS) and overall survival (OS).6, 7, 8, 9, 10 It has been approved as a treatment for RR thyroid carcinoma since 2015.11 One meta‐analysis of three studies about thyroid cancers showed the efficacy of lenvatinib in its anti‐cancer effects.12 There is no meta‐analysis with specific focus on the use of lenvatinib to improve the prognosis of RR thyroid carcinoma.
This review included studies with high‐quality evidence to look into the use of lenvatinib in the treatment of RR thyroid carcinoma, focusing on its improvement in progression‐free survival, overall survival and overall response rate.
2. MATERIALS AND METHODS
2.1. Search strategy and criteria
A systematic search was done on electronic databases (PubMed, Embase, MEDLINE, Cochrane) on 8 June 2020. The keywords were lenvatinib, thyroid carcinoma and randomized controlled trials. All studies fitting the criteria were selected and analysed.
The inclusion criteria were clinical trials with specific focus on use of lenvatinib for the treatment of RR thyroid carcinomas. All studies fulfilled the criteria of “Study of (E7080) Lenvatinib in Differentiated Cancer of the Thyroid (SELECT) protocol",13 which is listed as follows: (a) age 18 years and older; (b) histologically or cytologically confirmed diagnosis of DTC; (c) measurable disease according to Response Evaluation Criteria in Solid Tumours version 1.1 and confirmed by central radiographic review; (d) with evidence of 131‐I‐refractory disease; (e) with evidence of progression within 12 months prior to enrolment; (f) prior treatment with 0 or 1 vascular endothelial growth factor (VEGF) or vascular endothelial growth factor receptor (VEGFR)–targeted therapy; and (g) adequate renal, liver, bone marrow and blood coagulation function, as defined in the protocol.
Exclusion criteria included the following: (a) anaplastic or medullary thyroid cancer; (b) 2 or more prior VEGF or VEGFR‐targeted therapies; and (c) received any anti‐cancer treatment within 21 days or any investigational agent within 30 days prior to the first dose of study drug.
Patients in treatment arm received lenvatinib 24 mg orally once daily until documentation of disease progression, development of unacceptable drug toxicity or withdrawal of consent. Patients in control arm received oral matching placebo 24 mg once daily continuously.
2.2. Data analysis
The primary aim of the review is to evaluate the improvement in prognosis of patients with RR thyroid carcinoma by measurement of hazard ratio of progression‐free survival (PFS) and overall survival (OS) after receiving lenvatinib. Progression‐free survival is defined as time from randomization to the first documentation of disease progression by independent radiological review or to death, in the intention to treat population (all patients who underwent randomization). Overall survival refers to the time from randomization until death from any cause. Secondary outcome is the measure of objective response rate (ORR). It is defined as the best objective response (complete or partial) according to response evaluation criteria in solid tumours (RECIST version 1.1).
The titles, abstracts and full articles were independently screened by two authors (ZY and CLL). Following the PRISMA guidelines in PRISMA flow diagram, the study profile is shown in Figure 1. Duplicate articles were removed, and reasons for exclusions were if the studies are reviews, conference abstracts or without primary therapeutic data.
FIGURE 1.
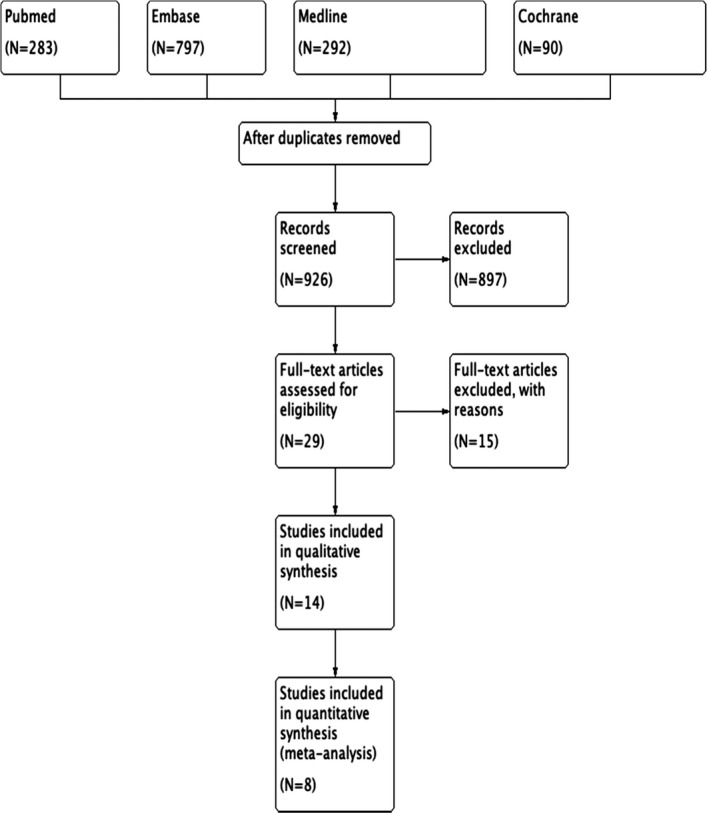
PRISMA flow diagram of study profile
Data extraction was performed by ZY and CLL with specific focus on study design, population demographics and therapeutic outcomes. Bias assessment was performed by Cochrane collaboration for randomized controlled trials (RCT). Bias or quality issues were minimized by cross‐checking between authors.
Review manager, version 5.3, and SPSS (IBM) were used in data analysis. Dichotomous data were pooled in random‐effect model as risk ratio with 95% confidence interval, while overall survival and progression‐free survival data were pooled as weighted hazard ratio using generic inverse‐variance method, random‐effect model with 95% confidence interval. Heterogeneity was assessed with chi‐square (χ2) test, with p‐value smaller than .1 as statistically significant. Its extent was measured with I 2‐test. As the number of studies included in each outcome measure was less than 10, Egger's test for funnel plot asymmetry could not be performed.
3. RESULTS
As of 8 June 2020, 1462 studies were retrieved from databases. After deleting duplicates and screening of titles and abstracts, 29 articles were identified for full‐text review. Eventually, 14 articles were selected for meta‐analysis. Articles with low evidence value were excluded due to case series (N = 5), case report (N = 3), cohort studies with focus not fitting inclusion criteria (N = 3) and reviews (N = 4).
Of the 3997 patients, the mean age was 62.5 years (excluding the studies where mean age was not reported, documented in Table 1). Lenvatinib increased the chance of prolonged PFS (hazard ratio 0.24, 95% CI, 0.19‐0.31, p < .001) and overall survival (hazard ratio 0.65, 95% CI, 0.52‐0.81, p < .001). All studies consistently showed that the median PFS was prolonged in lenvatinib group (ranging from 7.3 months to 33.1 months), compared with placebo group (ranging from 3 months to 12.9 months). Lenvatinib increased the chance of ORR (risk ratio 35.41, 95% CI, 19.42‐64.58, p < .001). Figure 2 shows the hazard ratio of PFS and OS in the pooled studies, and Figure 3 shows the risk ratio of ORR.
TABLE 1.
Summary of selected studies
| No. | References | Study design | Population | Mean age and sample size | Intervention, number of patients and mean follow‐up duration | Control, number of patients and mean follow‐up duration | Findings |
|---|---|---|---|---|---|---|---|
| 1 | Kiyota 2017 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63.0 years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients 17.1 months |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients 17.4 months |
‐Patients were stratified into 3 categories for comparison: ‘No radioactive iodine (RAI) uptake’, ‘Disease progression despite RAI avidity’ and ‘Extensive RAI exposure’ ‐Median progression‐free survival (PFS) in ‘No RAI uptake’ group was not reached (NR, 95% CI 14.8 to NR) and 3 months (95% CI 2.5‐5.3) in the lenvatinib and placebo arms, respectively. ‐Median PFS in ‘Disease progression despite RAI avidity’ was 16.5 months (95% CI 12.8 to NR) and 3.7 months (95% CI, 1.9‐5.4) in the lenvatinib and placebo arms, respectively ‐Median PFS in ‘Extensive RAI exposure’ group was 18.7 months (95% CI 10.7 to NR) and 3.6 months (95% CI 1.9‐5.5) in lenvatinib and placebo arms, respectively ‐The hazard ratio for progression of death compared with placebo arms was 0.21 (95% CI 0.15‐0.29; P < .001), 0.24 (95% CI 0.16‐0.36; P < .001) and 0.22 (95% CI, 0.10‐0.48; P < .001) ‐Overall response rates (ORRs) are 71.8%, 60.0% and 56.0% in patients receiving lenvatinib in the ‘no RAI uptake’, ‘disease progression despite RAI activity’ and ‘extensive RAI exposure’ groups, respectively. ORRs are 2.0%, 1.3% and 0% in the placebo arms of the above categories, respectively ‐Treatment‐related adverse events (of all grades) were experienced in more than 96% of patients receiving lenvatinib. 50% of patients experienced adverse events in placebo group ‐Incidence of treatment‐related adverse events (grade 3 or above) was experienced in more than 70% of patients receiving lenvatinib in 3 categories. The incidence rate is 0% to 10% in placebo arms of the 3 categories |
| 2 | Schlumberger 2015 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63.0 years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients 17.1 months (95% CI, 16.0 to 17.6; IQR, 14.4 to 20.4) |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients 17.4 months (95% CI, 15.9 to 19.0; IQR, 0.27; 14.8 to 20.4) |
‐Median progression‐free survival was 18.3 months (95% CI, 15.1 to not estimable) in lenvatinib group; the placebo group was 3.6 months (95% CI, 2.2 to 3.7). Hazard ratio for progression or death compared to placebo group, 0.21 99% CI 0.14 to 0.31; P < .001 ‐6‐month progression‐free survival rates were 77.5% and 25.4% in lenvatinib and placebo groups, respectively ‐Subgroup analysis of lenvatinib group, median progression‐free survival for patients without prior tyrosine kinase inhibitor (TKI) and with prior TKI was 18.7 months and 15.1 months, respectively ‐Progression‐free survival benefit maintained regardless of BRAF or RAS mutation status in patients ‐Significant improvement response rate in lenvatinib group (64.8%) vs placebo group (1.5%); odd ratio 28.87 (95% CI, 12.46 to 66.86; P < .001) ‐Overall survival (OS) was not significant (hazard ratio for death 0.73; 95% CI, 0.50 to 1.07, P = .10) ‐Treatment‐related adverse events (of all grades) in lenvatinib group were 97.3% and 59.5% in placebo group ‐Incidences of treatment‐related adverse events of grade 3 or higher were 75.9% and 9.9% in lenvatinib and placebo group, respectively |
| 3 | Brose 2017 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 62.0 years 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients 17.1 months |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients 17.1 months |
‐In patients younger than 65 years, median PFS was 20.2 months and 3.2 months in lenvatinib and placebo group, respectively (hazard ratio 0.19; 95% CI 0.13 to 0.27, P < .001) ‐In patients older than 65 years, median PFS was 16.7 months and 3.7 months in lenvatinib and placebo group, respectively (hazard ratio 0.27; 95% CI, 0.17 to 0.43; P < .001) ‐Median OS achieved in older placebo‐treated patients was 18.4 months; 95% CI 13.3 to 20.3 ‐Within lenvatinib group, longer OS was observed in patients less than 65 years, compared with those older (hazard ratio 0.53; 95% CI 0.31 to 0.9; P = .02) ‐Better ORR in lenvatinib group, compared with placebo group regardless of age (younger patients; odd ratio 45.7; 95% CI 14.8 to 141.0, P < .001 vs older patients, odd ratio 16.8, 95% CI 4.7 to 60.0, P < .001) ‐Among patients younger than 65 years, change of tumour size was −40.3% in lenvatinib group, compared with + 5.5% in placebo group ‐Among patients older than 65 years, change of tumour size was −41.5% in lenvatinib group, compared with + 0.6% in placebo group |
| 4 | Gianoukakis 2018 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | Average age unreported and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients 58 months |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients 36 months |
‐Median PFS was 19.4 months in lenvatinib group, compared with 3.7 months in placebo group (hazard ratio 0.24, 99% CI 0.17‐0.35; P < .001) ‐Median PFS was 33.1 months (95% CI, 27.8 to 44.6) in lenvatinib responders, compared with 7.9 months (95% CI 5.8‐10.7) in poor responders ‐ORR was 60.2% (95% CI, 54.2 to 66.1) for lenvatinib‐treated patients, compared with 2.3% (95% CI 0.0 to 4.9) for placebo‐treated patients ‐Median duration of response (DOR) was shorter in patients with heavier disease burden (tumour size less than 35 nm: 44.3 months; tumour size 35‐60 nm: 27.5 months; tumour size 60‐92 nm: 18.0 months; tumour size more than 92 nm: 15.7 months), patients with liver metastasis (yes: 15.7 months; no: 30.5 months) and patients with brain metastasis (yes: 9.3 months; no: 30.5 months) |
| 5 | Kiyota 2015 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 65.4 years and 40 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 30 patients |
Oral placebo once daily, continuously until toxicity or disease progression 10 patients |
‐Median progression‐free survival in lenvatinib group is 16.5 months (95% CI, 7.4‐not estimable), compared with 3.7 months (95% CI, 1.6‐9.1) in placebo group. Hazard ratio is 0.39 (99% CI, 0.10‐1.57, P = .067) ‐Median DOR is 16.6 months (95% CI 14.6‐NE) ‐Higher ORR in lenvatinib group (63.3%) compared with 0% for placebo (odd ratio 11.64; 95% CI 1.68‐80.82, P < .001) ‐83.3% clinical benefit rate in lenvatinib group, compared to 30.0% for placebo (OR, 5.68; 95% CI, 0.90‐35.92, P = .002) |
| 6 | Robinson 2016 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | Age unreported and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients |
‐Mean percentage change of tumour size is −45.9% for lenvatinib group vs 2.7% for placebo group (P < .001) in patients with lung metastasis ‐Mean percentage change of tumour size is −35.6% for lenvatinib group vs 5.1% for placebo group (P < .001) in patients with liver metastasis Mean percentage change of tumour size is −10.7% for lenvatinib group vs 6.5% for placebo group (P < .01) in patients with bone metastasis ‐Median maximum percentage change in tumour size was −42.9% for all patients receiving lenvatinib ‐Median time to first objective response was 2.0 months (95% CI 1.9‐3.5) ‐Responders to lenvatinib reduced median target lesion size by −51.9% (range, −100 to −30.3). ‐Non‐responders achieved median target lesion reduction by −20.3% (range, −37.8 to 65.6 ‐Lenvatinib‐induced tumour reduction was in two phases: phase one change of median average tumour size by −25.0% by 8 weeks, followed by phase two an average rate of reduction by 1.3% monthly ‐Median tumour size change was −22.1% by week 8, −29.6% at week 16 and greater than 50% by week 88 |
| 7 | Tahara 2017 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 61.9 years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients |
‐Lenvatinib PFS benefits observed in all subgroups regardless of tumour BRAF or RAS mutation status ‐BRAF mutation was associated with PFS in both univariate (P = .031) and multivariate (P = .0083) analyses in the placebo arm ‐Baseline Ang2 levels correlated with PFS (P = .0067) in the placebo arm and with maximum tumour shrinkage (MTS), ORR and PFS in the lenvatinib arm (P < .001 for each) ‐Baseline VEGF levels correlated significantly with MTS, ORR and PFS in the placebo in arm (P = .044, P = .038 and P = .037, respectively) and with MTS (P = .0082) and with ORR (P = .0009) in the lenvatinib group ‐High baseline thyroglobulin level may be a prognostic factor for poorer PFS in radioiodine‐refractory differentiated thyroid cancer ‐Thyroglobulin level decreased significantly in patients treated with lenvatinib, but increased in placebo patients at each treatment cycle |
| 8 | Tahara 2018 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63.2 years and 261 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression, but with dose interruption less than 0 134 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression, but with dose interruption more than 10%. 127 patients |
‐Median PFS for the shorter lenvatinib dose‐interruption group (less than 10%) was not yet reached, while the median PFS for the longer lenvatinib dose‐interruption group (more than 10%) was 12.8 months ‐Hazard ratio for mortality risk was 0.14 (95% CI 0.09‐0.20) in shorter lenvatinib dose‐interruption group, compared with placebo ‐Hazard ratio for mortality risk was 0.31 (95% CI 0.22‐0.43) in longer lenvatinib dose‐interruption group, compared with placebo ‐Patients in the shorter dose‐interruption group had a better objective response rate (ORR; 76.1%) than those in the longer dose‐interruption group (52.8%) |
| 9 | Wirth 2018 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63.0 years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients |
‐47% lenvatinib‐based patients experienced their first occurrence of treatment‐emergent hypertension (TE‐HTN) during cycle 1 of treatment compared with 6% of placebo‐treated patients ‐The first occurrence of worst‐grade TE‐HTN was also primarily in cycle 1 for both treatment groups (lenvatinib, 36%; placebo, 5%) ‐No difference in PFS improvement with lenvatinib between patients with and without baseline HTN (HR, 1.10; 95% CI, 0.75‐1.61; P = .6290) ‐Within lenvatinib group, PFS advantage was shown in patients with TE‐HTN than those without TE‐HTN (hazard ratio, 0.59; 95% CI, 0.39‐0.88, P = .0085) ‐Median PFS for lenvatinib‐treated patients was 18.8 months (95% CI, 16.5 months to not estimable) and 12.9 months (7.4 months to not estimable) in placebo (hazard ratio 0.59; 95% CI, 0.39 = 0.88; P = .0085) ‐Lenvatinib‐treated patients with TE‐HTN showed OS advantage compared with those without TE‐HTN (HR, 0.43, 95% CI, 0.27 to 0.69; P < .001) ‐ORR was 69% for lenvatinib‐treated patients with TE‐HTN and 56% for those without TE‐HTN (odds ratio, 1.72; 95% CI, 0.98‐3.01) ‐Within lenvatinib group, median change of tumour size for patients with TE‐HTN and without TE‐HTN is −45% and −40%, respectively |
| 10 | Haddad 2017 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | ? years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients |
‐Grade 3 treatment‐emergent adverse events (TEAEs) occurred in 72% of lenvatinib‐treated patients and 22% of placebo‐treated patients ‐Grade 4 TEAEs occurred in 12% in lenvatinib‐treated patients and 8% of placebo‐treated patients ‐Following hypertension, the most common TEAEs in lenvatinib‐treated patients vs placebo‐treated patients included diarrhoea (67% vs 17%), fatigue/asthenia/malaise (67% vs 35%), proteinuria (32% vs 3%), rash (23% vs 5%) and Palmar‐plantar erythrodysesthesia syndrome (PPES; 33% vs 1%) ‐Median time to first onset of any‐grade TEAEs was 12.1 weeks (IQR: 4.1‐23.7 weeks); for diarrhoea, 3.0 weeks (IQR 1.1‐7.0 weeks); for fatigue/asthenia/malaise, 6.1 weeks (IQR: 2.9‐16.3 weeks); for rash 7.3 weeks (IQR: 2.9‐16.3 weeks) and 5.9 weeks for PPES in lenvatinib‐treated patients |
| 11 | Nair 2015 | Phase 3, double‐blind, multicentre randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63 years and 392 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 261 patients |
Oral placebo once daily, continuously until toxicity or disease progression 131 patients |
‐Median PFS was 18.3 months (95% CI, 15.1‐not reached) in lenvatinib group, and it was 3.6 months (95% CI 2.2‐3.7). Hazard ratio 0.21 (95% CI 0.16‐0.28, P < .001) ‐Median OS was not reached (95% CI, 22.1‐not reached) in lenvatinib group, and it was not reached (95% CI, 20.3‐not reached) in placebo group. Hazard ratio 0.73 (95% CI, 0.50‐1.07, P = .10) ‐The ORRs in lenvatinib group and placebo group were 65% (95% CI, 59‐71) and 2% (95% CI, 0‐4). p‐value <.001. |
| 12 | Cabanillas 2015 | Phase 2, open‐labelled, multicentre single arm clinical trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 63 years and 58 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 58 patients |
No control |
‐The ORR was 50% (95% CI, 37%‐63%) ‐ORR was similar in patients who had received VEGFR‐targeted therapy and those without. ‐The median PFS was 12.6 months (95% CI, 9.9‐16.1 months) ‐The 6‐month PFS rate was 78% (95% CI, 64%‐87%, and the 12‐month PFS rate was 55% (95% CI, 40%‐67%) |
| 13 | Schlumberger 2016 | Phase 2, open‐labelled, multicentre single arm clinical trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 51.6 years and 59 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression 59 patients |
No control |
‐The ORR was 50% (95% CI, 37%‐63%) ‐The median duration of response for patients who had received prior VEGFR treatment and responded to lenvatinib therapy was 5.7 months (95% CI, 4.5‐not reached) and was not reached for lenvatinib responders without prior VEGFR treatment ‐The median PFS was 7.3 months (95% CI, 4.0‐not reached) in patients with prior VEGFR therapy and 12.9 months (95% CI, 7.1‐not reached) for patients without prior VEGFR therapy. ‐The median OS for patients with prior VEGFR therapy was 16.6 months (95% CI, not reached to not reached). Median OS was not reached for patients without prior VEGFR therapy ‐The disease control rate was 80% (95% CI, 67 to 89) ‐The 6‐month PFS rate was 67% (95% CI, 52%‐78%); the 12 month PFS rate was 46% (95% CI, 31%‐60%) |
| 14 | Takahashi 2019 | Phase 2, open‐labelled, multicentre non‐randomized controlled trial | Pathologically confirmed thyroid carcinoma with evidence of radioiodine‐refractory disease | 61.0 years and 51 patients |
Lenvatinib 24 mg once daily continuously until toxicity or disease progression, but with dose interruption less than 10% 51 patients |
No control |
‐Patients were classified into subtype for further investigations: radioiodine‐refractory differentiated thyroid cancer (RR‐DTC), medullary thyroid cancer (MTC) and anaplastic thyroid cancer (ATC) ‐The median PFS in patients with RR‐DTC, MTC and ATC was 25.8 months (95% CI: 18.4‐not reached), 9.2 months (95% CI: 1.8‐NR) and 7.4 months (95% CI: 1.7‐12.9) ‐The median OS in patients with RR‐DTC, MTC and ATC is 31.8 months (95% CI 31.8‐not reached), 12.1 months (95% CI: 3.8‐not reached) and 10.6 months (95% CI: 3.8‐19.8) ‐The objective response rate (ORR) in patients with RR‐DTC, MTC and ATC was 68%, 22% and 24% ‐The disease control rate in patients with RR‐DTC, MTC and ATC was 100%, 100% and 94%, respectively ‐The clinical benefit rate in patients with RR‐DTC, MTC and ATC was 84%, 78% and 71%, respectively |
FIGURE 2.
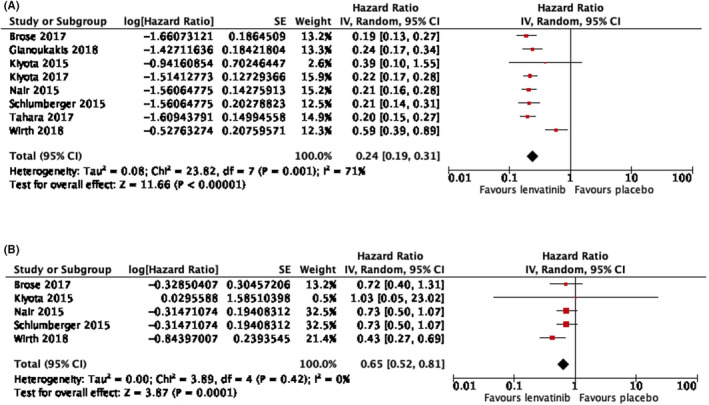
(A) Hazard ratio of progression‐free survival (PFS) of the pooled studies, 0.24 (95% CI, 0.19‐0.31; p < .001). (B) Hazard ratio of overall survival (OS) of the pooled studies, 0.65 (95% CI, 0.52‐0.81; p < .001)
FIGURE 3.
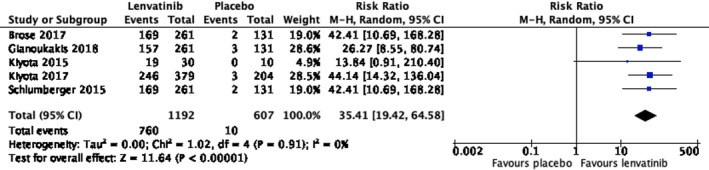
Risk ratio of objective response rate (ORR), 35.41 (95% CI, 19.42‐64.58; p < .001)
Grade 3 or above severe adverse events were documented in accordance with Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 for oncology drugs. Lenvatinib increases the risk of adverse events of grade 3 or above significantly (risk ratio 8.25, 95% CI, 6.50‐10.46, p < .001) as shown in Figure 4. The distribution of grade 3 or above adverse events was documented in Appendix S1.
FIGURE 4.
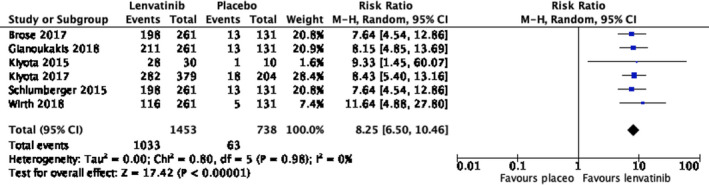
Risk ratio of grade 3 or above adverse events
Dose interruption was investigated with 10% as the cut‐off for comparison. Patients in the shorter dose‐interruption group (dose‐interruption time less than 10%) had a better outcome than patients in the longer dose‐interruption group (dose‐interruption time more than 10%).14 With a median follow‐up time of 16.9 months (95% CI, 15.5‐18.1) in shorter dose‐interruption group and 17.4 months (95% CI, 15.9‐19.0) in longer dose‐interruption group, the median PFS was not reached in shorter dose‐interruption group, while the median PFS was 12.8 months in the longer dose‐interruption group. Mortality risk was further reduced by half in the shorter dose‐interruption group. The hazard ratios for mortality risk in the shorter dose‐interruption group and the longer dose‐interruption group were 0.14 (95% CI, 0.09‐0.20) and 0.31 (95% CI, 0.22‐0.43) compared with placebo group, respectively.
Histological subtype analysis was performed. Patients with radioiodine‐refractory differentiated thyroid carcinoma (RR‐DTC) had similar outcomes as those with medullary thyroid carcinoma (MTC) and anaplastic thyroid cancer (ATC).15 The median PFS in patients with RR‐DTC, MTC and ATC was 25.8 months (95% CI, 18.4‐not reached), 9.2 months (95% CI, 1.8‐NR) and 7.4 months (95% CI, 1.7‐12.9), respectively. Similarly, the median OS in patients with RR‐DTC, MTC and ATC is 31.8 months (95% CI, 31.8‐not reached), 12.1 months (95% CI, 3.8‐not reached) and 10.6 months (95% CI, 3.8‐19.8), respectively. The objective response rate (ORR) in patients with RR‐DTC, MTC and ATC was 68%, 22% and 24%.
Within the lenvatinib groups, subgroup analysis based on radiological findings of radioiodine uptake showed that ‘no radioactive iodine (RAI) exposure’ group had better prognosis than patients with ‘disease progression despite RAI activity’ and ‘extensive RAI exposure’.8 With a median follow‐up time of 17.1 months in the lenvatinib group and 17.4 months in the placebo group, the median PFS in ‘no RAI exposure group’ was not reached (95% CI, 14.8‐not reached) in the lenvatinib and 3 months (95% CI, 2.5‐5.4) in the placebo group. The median PFS in ‘disease progression despite RAI activity’ was 16.5 months (95% CI, 12.8 to not reached) in the lenvatinib group and 3.7 months (95% CI, 1.9‐5.4) in the placebo group. The median PFS in patients with ‘extensive RAI exposure’ group was 18.7 months (95% CI, 10.7‐not reached) in the lenvatinib group and 3.6 months (95% CI, 1.9‐5.5) in the placebo group. The hazard ratio for progression of death the lenvatinib group compared with placebo group was similar: 0.21 (95% CI, 0.15‐0.29; p < .001), 0.24 (95% CI 0.16‐0.36; p < .001) and 0.22 (95% CI, 0.10‐0.48; p < .001) in ‘no RAI exposure’, ‘disease progression despite RAI activity’ and ‘no RAI exposure group’, respectively.
With 65 years old as cut‐off, subgroup analysis showed that younger patients (under 65 years old) have a similar therapeutic outcome as the older patients (over 65 years).7 In younger patient groups, the median PFS was 20.2 months and 3.2 months in the lenvatinib and the placebo group, respectively (hazard ratio 0.19; 95% CI, 0.13 to 0.27, p < .001). In patients older than 65 years, median PFS was 16.7 months and 3.7 months in the lenvatinib and the placebo group, respectively (hazard ratio 0.27; 95% CI, 0.17 to 0.43; p < .001). Within lenvatinib group patients, longer OS was observed in patients younger than 65 years, compared with those over 65 years (hazard ratio 0.53; 95% CI, 0.31 to 0.9; p = .02). The change of tumour size was similar, with reduction by around 40% in both treatment groups.
Patients receiving lenvatinib groups were further classified into responders and non‐responders based on RECIST version 1.1. Responders were defined as patients who had a complete response or partial response as their best overall response. The median PFS was in responder was 33.1 months (95% CI, 27.8‐44.6), whereas it was 7.9 months (95% CI, 5.8‐10.7) in non‐responders.9 The duration of response was 30.0 months (95% CI, 18.4‐36.7) in responders whose ORR was 60.2% (95% CI, 54.2‐66.1). The median time to response was 3.5 months (95% CI, 1.9‐3.7).
An analysis of baseline parameters showed that lenvatinib was beneficial to progression‐free survival. Lenvatinib prolonged progression‐free survival regardless of BRAF and RAS mutation.16 Baseline VEGF levels correlated significantly with maximum tumour shrinkage (MTS; p = .0082) and ORR (p = .0009). MTS was defined as the maximal percentage change from baseline in the sum of diameters of target lesions. Baseline Ang2 level correlated with MTS, ORR and PFS, with p < .001 for each. Thyroglobulin level decreased significantly in patients treated with lenvatinib, but increased in placebo patients in each treatment cycle.
Studies on the effect of prior treatment with anti‐VEGFR therapy on median PFS showed conflicting results. Cabanillas et al17 showed that the median PFS was similar in patients with prior VEGFR therapy exposure: 12.2 months (95% CI, 7.9‐not estimable) in the exposed group and 12.6 months (95% CI, 9.9‐not estimable) in the non‐exposed group. Nair et al11 also showed that the treatment effect of lenvatinib on PFS was similar among those who received prior anti‐VEGF therapy (hazard ratio 0.22, 95% CI, 0.12‐0.41) and those who did not (hazard ratio 0.20, 95% CI, 0.14‐0.27). However, Robinson et al18 showed that patients not receiving prior VEGFR‐targeted therapy were associated with prolonged PFS in univariate analysis (hazard ratio 0.75, 95% CI, 0.49‐1.14, p = .18) and multivariate analysis (hazard ratio = 0.86, 95% CI, 0.55‐1.34, p = .49). Similarly, Haddad et al19 also showed that patients without prior exposure had a higher likelihood of prolonged PFS than those with prior exposure (hazard ratio 0.86, 95% CI, 0.55‐1.33, p = .487). This was similarly observed in terms of OS (hazard ratio 0.68, 95% CI, 0.4‐1.15, p = .151).19 In a third study, Schlumberger et al20 also found that the median PFS was longer in patients without prior VEGFR therapy, 12.9 months (95% CI, 7.1 to not estimable) compared to those with prior VEGFR therapy, 7.3 months (95% CI, 4.0 to not estimable). The median OS for those without prior therapy was not reached and for patients with prior VEGFR therapy was 16.6 months (95% CI, not reached to not reached).
4. DISCUSSION
This systematic review and meta‐analysis evaluated the use of lenvatinib in the treatment of radioiodine‐refractory thyroid carcinoma. All studies included are clinical trials with high evidence values. One limitation of the study is the numerical value of median PFS and OS could not be obtained, since many patients did not reach the primary endpoints during the follow‐up period.
This study showed that lenvatinib was efficacious in treating patients with prolongation of PFS (hazard ratio 0.24, 95% CI 0.19‐0.31, p < .001) and OS (hazard ratio 0.65, 95% CI 0.52‐0.81, p < .001). The ORR also showed promising effect when compared with placebo (risk ratio 35.41, 95% CI 19.42‐64.58, p < .001). However, lenvatinib was associated with significant grade 3 or above adverse events compared with placebo (risk ratio 8.25, 95% CI 6.50‐10.46, p < .001).
The most common adverse event was treatment‐emergent hypertension (TE‐HTN). Wirth et al21 showed that TE‐HTN correlated significantly with improved PFS and OS for patients with RR‐DTC. TE‐HTN may be the outcome of an accumulated high‐dose exposure to lenvatinib. Dose interruptions and/or reductions may reduce adverse events. Tahara et al14 have shown that a reduction of less than 10% dose interruption was related with better prognosis.
Another common adverse event was proteinuria. Haddad showed that the median time to onset of proteinuria in patients receiving lenvatinib was 6.1 weeks (IQR 4.0‐15.6), and the median time to resolution was 8.8 weeks (IQR 4.0‐24.6).19 Proteinuria is considered to be a class effect of anti‐angiogenic treatments.22 Decision to interrupt medication or modification of dosage should be done on case‐by‐case analysis within the period of proteinuria.23
With a high objective response rate, lenvatinib significantly reduces tumour size. In a study by Robinson et al18 on the characterization of tumour size changes over time in 392 patients, the mean maximum change of tumour was significantly higher in the 4 categories of metastasis (lung: lenvatinib −45.9% vs placebo + 2.7%, p < .0001; liver: lenvatinib −35.6% vs placebo + 5.1%, p < .0001; lymph node: lenvatinib, −47.5% vs placebo −2.9%; bone: lenvatinib −10.7% vs placebo + 6.5%, p = .0021). The tumour reduction occurred in 2 phases, with the first 8 weeks of rapid reduction by an average of −25.2%, followed by a slower, continuous average rate of tumour size reduction by 1.3% per month.
Subgroup analysis showed that lenvatinib was effective for patients with RR thyroid carcinoma, regardless of age, histological subtypes and radiologically subtypes and mutation status. Tahara et al showed that lenvatinib was associated with improvement of PFS, independent of BRAF and RAS mutation status, which are the two common mutations in RR thyroid carcinoma. These two mutations have been associated with poor prognosis and thyroid carcinoma with aggressive nature.24, 25 The effect of prior VEGFR therapy on the clinical outcome of patients with RR thyroid carcinoma receiving lenvatinib is uncertain.
Combination therapy may be considered in patients with failed treatment or to achieve a higher efficacy. In a study of combination of anti‐PD‐1/PD‐L1 associated with lenvatinib by Gunda et al,26 anti‐PD‐1/PD‐L1 augmented the efficacy of lenvatinib by favourably altering the immune microenvironment. Lyer et al showed that pembrolizumab and lenvatinib might be effective salvage therapy at the time of progression of diseases in patients with thyroid carcinoma. In a phase 2 study of 34 patients receiving lenvatinib in combination with immune checkpoint inhibitor for the treatment of treatment‐resistant anaplastic thyroid cancer, seven of them had 22% to 63% tumour shrinkage. The median PFS and OS were 2.6 months and 2.9 months, respectively.27 Anaplastic thyroid cancer carries a very poor prognosis owing to its aggressive behaviour and resistance to cancer treatments.28 Further studies are required to explore the lenvatinib‐based combination of treating treatment‐resistant thyroid carcinoma.
5. CONCLUSION
Lenvatinib is beneficial for the treatment of radioiodine‐refractory thyroid carcinoma with prolongation of progression‐free survival and overall survival. In view of the high rate of adverse events, close clinical monitoring is recommended.
AUTHOR CONTRIBUTIONS
Literature search and study design were done by Zhipeng Yan and Ching‐Lung Lai. Figures, data collection, data analysis, data interpretation and manuscript writing were done by Zhipeng Yan, Ching‐Lung Lai and Ming Yang.
CONFLICT OF INTEREST
The authors Yan Zhipeng, Yang Ming and Lai Ching‐Lung have no declarable conflict of interest.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
None.
Yan Z, Yang M, Lai C‐L. Clinical efficacy of lenvatinib for the treatment of radioiodine‐refractory thyroid carcinoma: A systematic review and meta‐analysis of clinical trials. Clin Endocrinol (Oxf). 2021;95:478–488. 10.1111/cen.14479
Funding information
There is no funding to this research
Contributor Information
Zhipeng Yan, Email: u3537821@hku.hk.
Ching‐Lung Lai, Email: hrmelcl@hku.hk.
DATA AVAILABILITY STATEMENT
All the data were retrieved from online databases (PubMed, Embase, MEDLINE, Cochrane). All processed data were presented in figure, table and Appendix S1.
REFERENCES
- 1.American Cancer Society. Key statistics for thyroid cancer. 2020. https://www.cancer.org/cancer/thyroid‐cancer/about/key‐statistics.html Accessed June 12, 2020.
- 2.Aashiq M, Silverman DA, Na'ara S, Takahashi H, Amit M. Radioiodine‐refractory thyroid cancer: molecular basis of redifferentiation therapies, management, and novel therapies. Cancers. 2019;11(9):1382. 10.3390/cancers11091382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nixon IJ, Whitcher MM, Palmer FL, et al. The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid. 2012;22:884‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, et al. Long‐term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892‐2899. [DOI] [PubMed] [Google Scholar]
- 5.Drug Bank . Lenvatinib. https://www.drugbank.ca/drugs/DB09078. Accessed June 12, 2020.
- 6.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine‐refractory thyroid cancer. N Engl J Med. 2015;372:621‐630. [DOI] [PubMed] [Google Scholar]
- 7.Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine‐refractory differentiated thyroid cancer in the phase III SELECT Trial. J Clin Oncol. 2017;35:2692‐2699. [DOI] [PubMed] [Google Scholar]
- 8.Kiyota N, Robinson B, Shah M, et al. Defining radioiodine‐refractory differentiated thyroid cancer: efficacy and safety of lenvatinib by radioiodine‐refractory criteria in the SELECT Trial. Thyroid. 2017;27:1135‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianoukakis AG, Dutcus CE, Batty N, Guo M, Baig M. Prolonged duration of response in lenvatinib responders with thyroid cancer. Endocr Relat Cancer. 2018;25(6):699‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiyota N, Schlumberger M, Muro K, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine‐refractory differentiated thyroid cancer. Cancer Sci. 2015;106:1714‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair A, Lemery SJ, Yang J, et al. FDA approval summary: lenvatinib for progressive, radio‐iodine‐refractory differentiated thyroid cancer. Clin Cancer Res. 2015;21:5205‐5208. [DOI] [PubMed] [Google Scholar]
- 12.Zhu C, Ma X, Hu Y, et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta‐analysis. Oncotarget. 2016;7:44545‐44557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inc. E . A Multicenter, randomized, double‐blind, placebo‐controlled, trial of lenvatinib (E7080) in 131I‐refractory differentiated thyroid cancer (DTC) (SELECT). 2011. https://clinicaltrials.gov/ct2/show/NCT01321554. Accessed June 12, 2020.
- 14.Tahara M, Brose MS, Wirth LJ, et al. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine‐refractory differentiated thyroid cancer. Eur J Cancer. 2019;106:61‐68. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Kiyota N, Yamazaki T, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15:717‐726. [DOI] [PubMed] [Google Scholar]
- 16.Tahara M, Schlumberger M, Elisei R, et al. Exploratory analysis of biomarkers associated with clinical outcomes from the study of lenvatinib in differentiated cancer of the thyroid. Eur J Cancer. 2017;75:213‐221. [DOI] [PubMed] [Google Scholar]
- 17.Cabanillas ME, Schlumberger M, Jarzab B, et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine‐refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment. Cancer. 2015;121:2749‐2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson B, Schlumberger M, Wirth LJ, et al. Characterization of tumor size changes over time from the phase 3 study of lenvatinib in thyroid cancer. J Clin Endocrinol Metab. 2016;101:4103‐4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haddad RI, Schlumberger M, Wirth LJ, et al. Incidence and timing of common adverse events in lenvatinib‐treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017;56:121‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlumberger M, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (e7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22:44‐53. [DOI] [PubMed] [Google Scholar]
- 21.Wirth LJ, Tahara M, Robinson B, et al. Treatment‐emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT). Cancer. 2018;124:2365‐2372. [DOI] [PubMed] [Google Scholar]
- 22.Cabanillas ME, Hu MI, Durand JB, Busaidy NL. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res. 2011;2011:985780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabanillas ME, Takahashi S. Managing the adverse events associated with lenvatinib therapy in radioiodine‐refractory differentiated thyroid cancer. Semin Oncol. 2019;46:57‐64. [DOI] [PubMed] [Google Scholar]
- 24.Crispo F, Notarangelo T, Pietrafesa M, et al. BRAF Inhibitors in thyroid cancer: clinical impact, mechanisms of resistance and future perspectives. Cancers. 2019;11(9):1388. 10.3390/cancers11091388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabanillas ME, Habra MA. Lenvatinib: role in thyroid cancer and other solid tumors. Cancer Treat Rev. 2016;42:47‐55. [DOI] [PubMed] [Google Scholar]
- 26.Gunda V, Gigliotti B, Ashry T, et al. Anti‐PD‐1/PD‐L1 therapy augments lenvatinib's efficacy by favorably altering the immune microenvironment of murine anaplastic thyroid cancer. Int J Cancer. 2019;144:2266‐2278. [DOI] [PubMed] [Google Scholar]
- 27.Wirth LJ, Brose MS, Sherman EJ, et al. MON‐521 an open‐label, single‐arm, multicenter, phase 2 trial of lenvatinib (LEN) for the treatment of anaplastic thyroid cancer (ATC). J Endocr Soc. 2020;4:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu A‐H, Juan L‐Y, Yang A‐H, Chen H‐S, Lin H‐D. Anaplastic thyroid cancer with uncommon long‐term survival. J Chin Med Assoc. 2006;69:489‐491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
All the data were retrieved from online databases (PubMed, Embase, MEDLINE, Cochrane). All processed data were presented in figure, table and Appendix S1.


