Abstract
Aim
To explore the feasibility of screening for periodontitis by measuring biomarkers, namely total proteolytic activity (TPA), matrix metalloproteinase (MMP)‐8, chitinase, lysozyme or their combination, in saliva, oral rinse and gingival crevicular fluid (GCF).
Material and methods
Subjects were recruited among healthy/gingivitis individuals and untreated periodontitis patients in Academic Centre for Dentistry Amsterdam (ACTA). All participants donated samples of unstimulated whole saliva, oral rinse and GCF. The protein concentrations and MMP‐8 levels were determined by ELISA. Enzymatic activities were measured using appropriate fluorogenic substrates.
Results
In oral rinse samples, periodontitis patients (n = 19) exhibited significantly higher concentrations of MMP‐8 and TPA than controls (n = 20). MMP‐8 in combination with chitinase explained 88% of the variance and assigned a subject to control or periodontitis group, with best accuracy (87.2%) in oral rinse.
Conclusions
The combination of MMP‐8 and chitinase in the current oral rinse procedure has the potential to discriminate periodontitis from periodontal health/gingivitis.
Keywords: biomarkers, chitinase, gingival crevicular fluid, MMP‐8, modelling, oral rinse, periodontitis, principal component analysis (PCA), protease, saliva
Clinical Relevance.
Scientific rationale for the study: To investigate potential biomarkers in different oral fluids and the relative correlations with periodontal screening purposes.
Principal findings: In oral rinse, the combination of MMP‐8 and chitinase could assign best a subject to the control or periodontitis group.
Practical implications: Future studies could focus on the exploration of the combination of MMP‐8 and chitinase in oral rinse, which could potentially lead to developing a chair‐side test to support periodontal screening and periodontal diagnostics.
1. INTRODUCTION
Periodontitis is a biofilm‐induced chronic inflammatory disease with debilitating effects when left untreated and based on recent data, up to 50% of the population is affected by a form of periodontitis (Kassebaum et al., 2014; Wahlin et al., 2018). However, at least 10% of the subjects who are seen by general physicians do not visit the dentist (Ahdi et al., 2015), thus screening for periodontitis in a non‐dental medical professional setting could be a useful tool for early periodontal screening.
Oral fluids are considered to be potential diagnostic media containing valuable markers for periodontal inflammation. During the last two decades, salivary diagnostics have gained a lot of attention. Alternatively, a simple and non‐invasive oral rinse method was introduced to collect oral PMNs (Rijkschroeff et al., 2016). It has been shown that oral PMN counts correlate positively with pocket depth, number of sites with bleeding on probing and the overall severity of periodontitis (Landzberg et al., 2015). Thus, oral rinse samples could be used for screening periodontal inflammation as they provide valuable information regarding oral PMN‐derived markers. The third oral fluid considered for this study was GCF, which despite being more difficult to collect, provides a good approximation of the inflammatory condition of the periodontium (Barros et al., 2016).
Matrix metallo‐proteinases (MMPs) are host‐derived proteolytic enzymes, and MMP‐8 constitutes the main collagenolytic MMP detected in gingival tissues and oral fluids. Various studies have shown that salivary levels of MMP‐8 are significantly elevated as the severity of periodontal inflammation increased and subsequently decreased after periodontal treatment (Golub et al., 1994; Sorsa et al., 2004; Uitto, 2003; Uitto et al., 2003). However, MMP‐8 represents only a part of the total proteolytic activity (TPA) of a given sample. Several studies have shown that proteolytic enzymes are increased in saliva of patients with periodontitis (Nedzi‐Gora et al., 2014; Nizam et al., 2014; Sorsa et al., 2016). With TPA being host‐ and bacteria‐derived, measurements of TPA could shed more light on periodontal inflammation (Bikker et al., 2019; Sandholm, 1986).
Chitinase is an enzyme that breaks‐down glycosidic linkages in chitin (Overdijk & Van Steijn, 1994) and is secreted by activated macrophages and PMNs, as well as by salivary glands (Escott & Adams, 1995; Van Steijn et al., 1999). Periodontitis patients have an increased chitinase activity in their saliva compared to non‐periodontitis controls, and a successful periodontal treatment is accompanied by a concomitant decrease in chitinase activity (Van Steijn et al., 2002). Lysozyme cleaves glycosidic linkages in the peptidoglycan layer mainly of Gram‐positive bacteria and is secreted by macrophages and in relatively small amounts by salivary glands (Hansen & Karle, 1979; Venge, 1994). The protective role of lysozyme is important since insufficient levels of lysozyme can lead to an increased dental plaque accumulation (Jalil et al., 1992).
Considering the above, the aim of the current pilot study was to compare MMP‐8, TPA, chitinase, lysozyme and the combination of them, side by side with regard to their periodontal screening value when measured in saliva, oral rinse and GCF.
2. MATERIAL AND METHODS
2.1. Study population
Consecutive subjects referred to the Department of Periodontology of the Academic Centre for Dentistry Amsterdam (ACTA) for diagnosis and treatment from October 2016 to May 2017 were screened for eligibility to participate in the study. Eligible patients were asked to participate voluntarily after their initial consultation and before the initiation of the periodontal treatment. Control subjects were selected among non‐dentist staff members and subjects who visited the educational clinics of ACTA for regular dental check‐ups. A dentist performed the screening before inclusion.
Periodontitis cases were defined as proposed by (Tonetti & Claffey, 2005), namely presence of proximal attachment loss of ≥5 mm in ≥30% of teeth present. Bone loss was confirmed on recent periapical radiographs (<1 year old), and the number of teeth with bone loss >1/3 and >1/2 was counted. All the patients were classified as having periodontitis according to the new classification by (Papapanou et al., 2018) and specifically periodontitis Stage III, Grade B or C. For all the periodontitis patients, the full periodontal charts were retrieved from the electronical health record to calculate mean probing pockets depth (PPD) and mean bleeding on probing (BoP). Control subjects were included if they showed not more than one pocket of 4–5 mm, in the absence of proximal bone loss (confirmed on bitewing radiographs <1 year old), excluding third molars and have a minimum number of 28 teeth; no full mouth periodontal charting was performed. For all potential participants, exclusion criteria were previous periodontal treatment within the last 2 years, age of <18 years old, use of antibiotics the last 6 months and pregnancy.
For all participants, information about age, sex, ethnicity, smoking habits, level of education and use of medication was recorded by means of a questionnaire. Education was classified as yes or no beyond high school. Body Mass Index (BMI) was calculated dividing the weight (kg) by the square of the height (m).
All participants were informed about the purpose of this study, received verbal and written information and had given written informed consent prior to the start of the study. The protocol of the present study was approved by the Medical Ethical Committee of the VU Medical Center Amsterdam and following the principals of the Medical Research Involving Human Subjects Act (approval letter number 2016.470). The STROBE guidelines were used to ensure the proper reporting of this observational study.
2.2. Collection of saliva, oral rinse and gingival crevicular fluid
All oral fluids were collected between 9 AM and 12 AM and prior to any treatment, that is for the periodontitis patients, at a separate appointment within 2 weeks after the intake (day of diagnosis) and for the control/gingivitis group, at the beginning of the appointment of a regular dental visit. The participants were asked to refrain from tooth brushing, eating and drinking for at least one h prior to sample collection. Subsequently, unstimulated saliva was collected as follows (Prodan et al., 2015): the participants were asked to sit straight, not to speak or move, and then to drool in a collection tube (50 ml) for 5 min. Thereafter, oral rinse samples were collected by asking the participants to rinse their oral cavity thoroughly for 30 s with 10 ml of sterile saline solution (natrium chloride 0.9%, Versylene Fresenius, Zeist, The Netherlands) and to expectorate into a collection tube (50 ml) (Rijkschroeff et al., 2016). The procedure was repeated once after a 3.5 min intermission (Rijkschroeff et al., 2016). For the periodontitis patients, four GCF samples were taken, each from the deepest proximal buccal pockets per quadrant. More information regarding site‐specific clinical parameters is presented in Table S2. In control subjects, 4 GCF samples pooled, each originating from the mesio‐buccal surfaces of the first molars. The area to be sampled was isolated with cotton rolls, and the teeth were gently air‐dried for 5 s. The collection strips (PerioPaper strips, Oraflow Inc.) were placed for 30 s in the sulcus/pocket. When contamination with blood was observed, the strip was discarded and the sampling was attempted in the following deepest pocket. The PerioPaper strips (n = 4 per participant) were placed in a 2 ml Eppendorf tube (Eppendorf) and kept on ice until the end of the collection process. Albumin is not locally produced in the periodontium, so its concentration in GCF is assumed to be directly proportional to the volume of GCF secreted (Helmerhorst et al., 2018; Mantyla et al., 2003). In order to correct for different input volumes in GCF samples and to explore the influence of the leaked GCF in saliva, calculation of ratios of TPA, MMP‐8, chitinase and lysozyme to albumin in GCF and saliva was subsequently performed. The collected saliva, oral rinse and GCF samples were stored at −80°C until later analysis. The processing of samples and determination of biomarkers (TPA, MMP‐8, chitinase and lysozyme) were performed as describe before (Prodan et al., 2015) and are presented in the Supporting Information file.
2.3. Data analysis
Statistical analyses were carried out with SPSS software (v. 24.0, SPSS; IBM Statistics). Graph Prism (v. 5.0 for Windows; GraphPad Software) was used for the graphic representation of the data. Background variables were calculated as number or percentage of subjects and continuous variables as means ± standard deviation. Differences in categorical variables were assessed using the chi‐square test and for other variables with independent t tests. All targeted biomarkers (TPA, MMP‐8, chitinase, lysozyme and albumin) in the three oral fluids were at first tested for their normal distribution with the Kolmogorov–Smirnov test. Since the markers did not show normal distribution, the comparisons of the variables in saliva, oral rinse and GCF were performed with the Mann–Whitney U test. All data points are presented, and the medians are indicated in the figures. Correlations for the whole study population for the biochemical variables in saliva, oral rinse and GCF were calculated with Spearman's correlation coefficient. For additional explorative analyses, log‐transformed data were used in order to ordinate the distribution of the variables. The transformed values were used for principal component analyses (PCA) (v. 3.04 PAST software (Hammer et al., 2001)) to investigate whether the controls and periodontitis patients clustered together and which variables were the main determinants of the clusters. Assessment of differences between the periodontitis and control group was performed by one‐way permutational multivariate analysis of variance (PERMANOVA). A p < .05 was considered statistically significant. When appropriate, Bonferroni correction was applied. To explore whether the combination of MMP‐8 and chitinase has any potential as a diagnostic test, we utilized a receiver operating characteristic (ROC) curve analysis.
3. RESULTS
3.1. Study population
A total of 39 subjects (20 controls and 19 periodontitis patients) were recruited for this study. Demographic, anthropometrical, medical and clinical characteristics of the participants are presented in Table 1. Comparison between the two study groups revealed that periodontitis patients were older than controls (mean age 50.5 ± 10.6 vs 38.0 ± 13.6 in years, p = .003). In addition, the periodontitis group consisted of less Caucasian individuals (63.2% vs 95.0%, p = .014), more smokers (36.8% vs 5.0%, p = .014) and subjects with higher BMI (27.1 ± 5.4 vs 22.0 ± 3.2, p = .001) compared to the control group. Regarding sex distribution, educational level and medication use (antihypertensive, antidiabetic and anti‐hypercholesterolemia medication, anticoagulant and antiplatelet medication, corticosteroids, antihistamines, painkillers and vitamins), there were no statistically significant differences between the two study groups. The periodontitis group was characterized by a mean PPD of 3.9 ± 0.5 mm and a mean percentage of BOP of 63.0 ± 26.6%. The percentage of teeth with PPD ≥ 5 mm was 64.0 ± 15.9% on average. An average of 23.6 ± 14.2% and an average of 18.5 ± 12.6% of teeth exhibited bone loss extending to more than 33% and 50% of the length of the root, respectively. The average number of remaining teeth was 28.4 ± 2.7.
TABLE 1.
Background characteristics of the study population. Values are numbers (%) or means ± standard deviation
| Control | Periodontitis | p‐valuea | |
|---|---|---|---|
| (n = 20) | (n = 19) | ||
| Age | 38.0 ± 13.6 | 50.5 ± 10.6 | .003 |
| Males | 9 (45%) | 11(57.9%) | .873 |
| Caucasian | 19 (95.0%) | 12 (63.2%) | .014 |
| Smokers | 1 (5.0%) | 7 (36.8%) | .014 |
| BMI (kg/m2)b | 22.0 ± 3.2 | 27.1 ± 5.4 | .001 |
| Educational level (≥high school) | 16 (80.0%) | 11 (57.9%) | .135 |
| Medication usec (during the last year) | 2 (10.0%) | 4 (21.1%) | .339 |
| Mean PPDd (mm) | 3.9 ± 0.5 | ||
| Percentage of teeth with pockets ≥5 mm | 64.0 ± 15.9 | ||
| Percentage of sites with pockets ≥5 mm | 30.8 ± 11.8 | ||
| Percentage of teeth with ≥33% bone loss | 23.6 ± 14.2 | ||
| Percentage of teeth with ≥50% bone loss | 18.5 ± 12.6 | ||
| Mean BoPe (%) | 63.0 ± 26.6 | ||
| Mean number of remaining teeth | 28.4 ± 2.7 |
Independent t test for continuous variables, chi‐square test for categorical variables.
BMI: Body Mass Index.
Antihypertensive, antidiabetic and anti‐hypercholesterolemia medication, anticoagulant and antiplatelet medication, corticosteroids, antihistamines, painkillers and vitamins.
PPD: Probing pocket depth.
BoP: Bleeding on probing.
3.2. Biomarkers
Results from the biomarker analyses (TPA, MMP‐8, chitinase, lysozyme and albumin) in saliva, oral rinse and GCF are presented in Figures 1, 2, 3. Saliva from periodontitis patients exhibited higher TPA (p < .001) and higher concentrations of albumin (p = .03) in comparison with controls, while lysozyme activity was higher in the control group (p < .001; Figure 1). Also, in oral rinse samples, TPA was higher in periodontitis patients (p = .002) than in control samples. In addition, MMP‐8 concentrations in oral rinse samples from periodontitis patients were higher compared to controls (p < .001; Figure 2). Albumin levels were below the detection threshold in oral rinse samples. Similar to saliva and oral rinse samples, the GCF samples from periodontitis patients exhibited significantly higher values of TPA (p < .001), higher concentrations of MMP‐8 (p = .005), albumin (p = .02), higher chitinase (p = .01) and lysozyme activity (p = .003) compared to controls (Figure 3).
FIGURE 1.
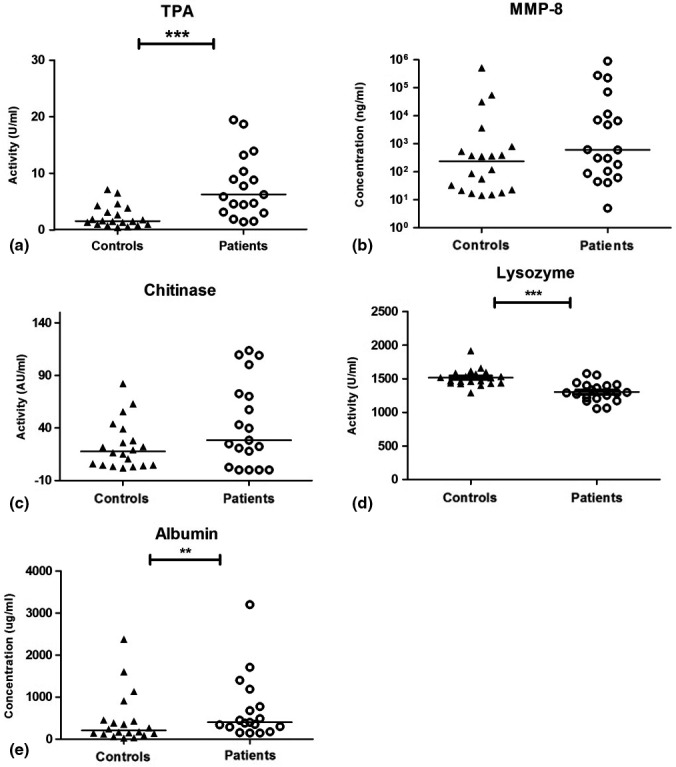
Results for unstimulated saliva samples of controls and periodontitis patients; total proteolytic activity (TPA) (a), matrix metalloproteinase‐8 (MMP‐8) concentrations (b), chitinase activity (c), lysozyme activity (d) and albumin concentrations (e). Horizontal lines represent median values for each group. ***p < .001, **p ≤ .01. Presentation of MMP‐8 values in logarithmic scale
FIGURE 2.
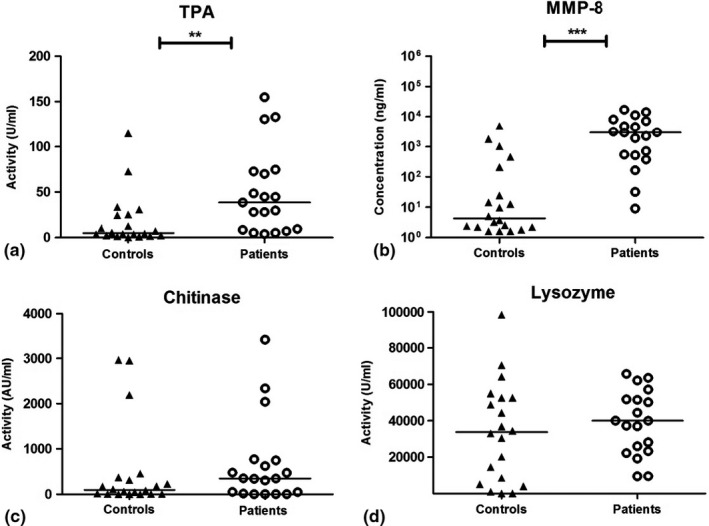
Results for oral rinse samples of controls and periodontitis patients; Total proteolytic activity (TPA) (a), matrix metalloproteinase‐8 (MMP‐8) concentrations (b), chitinase activity (c) and lysozyme activity (d). Albumin values were below detection level. Horizontal lines represent median values for each group. ***p < .001, **p ≤ .01. Presentation of MMP‐8 values in logarithmic scale
FIGURE 3.
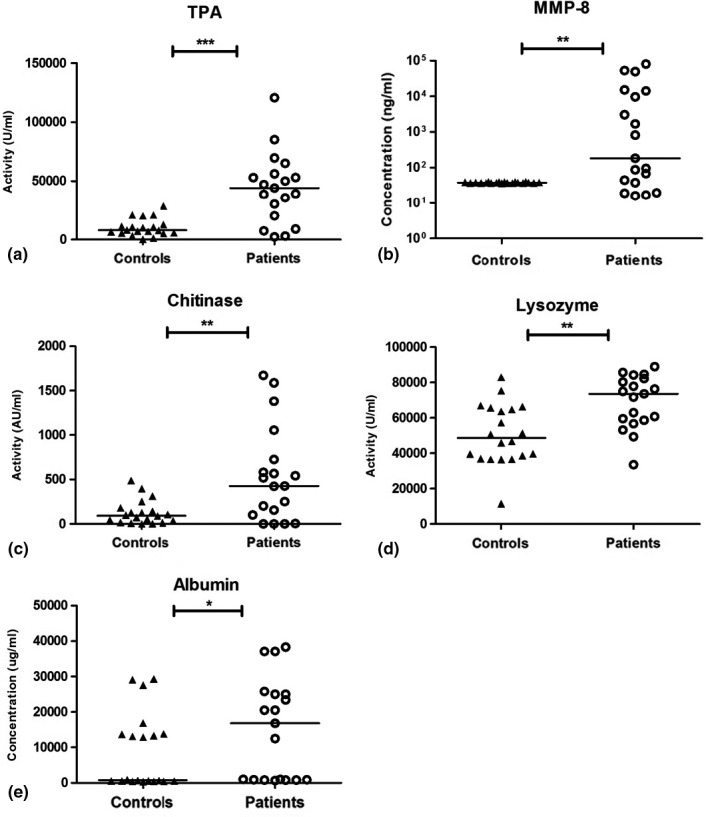
Results for gingival crevicular fluid samples of controls and periodontitis patients; Total proteolytic activity (TPA) (a), matrix metalloproteinase‐8 (MMP‐8) concentrations (b), chitinase activity (c), lysozyme activity (d) and albumin concentrations (e). Horizontal lines represent median values for each group. ***p < .001, **p ≤ .01, *p ≤ .05. Presentation of MMP‐8 values in logarithmic scale
Among the calculated ratios of TPA, MMP‐8, chitinase and lysozyme to albumin in GCF, the ratio MMP‐8/albumin was significantly higher in periodontitis group compared to control group (p = .04) (Table 2a). In saliva, the ratios of TPA, MMP‐8 and chitinase did not differ between the control and periodontitis group, whereas the ratio of lysozyme presented to be higher in the control group (Table 2b).
TABLE 2.
A. Ratio of total proteolytic activity (TPA), matrix metalloproteinase‐8 (MMP‐8), chitinase and lysozyme to albumin in gingival crevicular fluid. B. Ratio of total proteolytic activity (TPA), matrix metalloproteinase‐8 (MMP‐8), chitinase and lysozyme to albumin in saliva
| A. | Ratio TPA | Ratio MMP−8 | Ratio chitinase | Ratio lysozyme |
|---|---|---|---|---|
| Controls | 7.7 | 0.05 | 0.02 | 64.1 |
| (0–52) | (0–0.12) | (0–0.6) | (0.8–207) | |
| Periodontitis | 3.3 | 0.1 | 0.03 | 5.0 |
| (0.1–72) | (0.0–4.0) | (0.0–1.7) | (1.5–99.0) | |
| p‐Valuea | .399 | .04 | .623 | .286 |
| B. | Ratio TPA | Ratio MMP−8 | Ratio chitinase | Ratio lysozyme |
|---|---|---|---|---|
| Controls | 0.01 | 0.7 | 0.07 | 7.7 |
| (0–0.09) | (0.02–6133) | (0–0.9) | (0.6–68) | |
| Periodontitis | 0.018 | 0.8 | 0.1 | 3.3 |
| (0–6) | (0.0–788) | (0.0–0.4) | (0.05–10) | |
| p‐Valuea | .175 | .627 | .647 | .015 |
Values are medians (interquartile range).
Mann–Whitney U test.
Analysis of the correlations within the whole study population among the biomarkers, revealed that in saliva MMP‐8 correlated positively with TPA (ρ = .511, p < .003). The same pattern of correlation was noticed in GCF and oral rinse. Interestingly in oral rinse samples, MMP‐8 correlated positively with TPA (ρ = .762, p < .003) and chitinase activity (ρ = .505, p < .003) (Table S1).
PCA was performed for saliva, oral rinse and GCF. When all variables were included in one PCA analysis, MMP‐8 and chitinase in oral rinse dominated the PCA components, which could explain 52% of the variance of the samples and based on that, cluster a subject in control or periodontitis group (Figure 4a). The other 12 variances contributed less than <14% in PCA.
FIGURE 4.
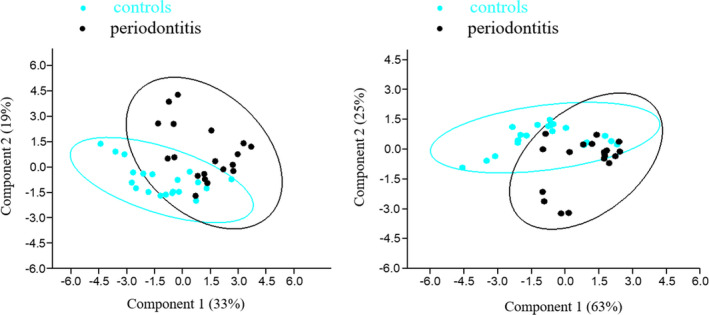
(a) Principal component analysis (PCA) plots of all oral samples (saliva, OR, GCF). Components 1 and 2 were mainly determined by matrix metalloproteinase‐8 (MMP‐8) and chitinase and explained 33% and 19% of variance, respectively. Controls and periodontitis patients differed significantly (PERMANOVA, F = 7.5, p = .0001). (b) PCA plots of oral rinse samples. Components 1 and 2 were mainly determined by matrix metalloproteinase‐8 (MMP‐8) and chitinase and explained 63% and 25% of variance, respectively. Controls and periodontitis patients differed significantly (PERMANOVA, F = 11, p = .0001)
In saliva samples, MMP‐8 and chitinase were the most discriminatory markers, explaining 82% of variance in principal component 1 (PC1) and PC2, with a statistical significant difference among periodontitis and control subjects (p = .04). The other 3 variances contributed less than <12% in PCA. In oral rinse samples, these markers explained 88% of the variation (p = .0001) (Figure 4b). The other 2 variances contributed less than <9% in PCA. In GCF samples, it was found that PC1 and PC2 were dominated by MMP‐8 and TPA, respectively, explaining 72% of the variation (p = .0001). The other 3 variances contributed less than <18% in PCA. After performing PCA for all oral fluids, including the background characteristics, the results remained similar, indicating that the contribution of the biomarkers was more indicative to assign each subject to one of the study groups.
The combination of MMP‐8 and chitinase in oral rinse samples showed to have a sensitivity of 84.2%, specificity of 90% and accuracy of 87.2%. The distinction between controls and periodontitis patients was significant with the area under the curve reaching a value of 0.929 (p < .001; 95% confidence interval 0.85–1.00 (Figure S1)).
4. DISCUSSION
The purpose of the current pilot study was to assess TPA, MMP‐8, chitinase and lysozyme in saliva, oral rinse and GCF samples in periodontitis patients and controls in order to investigate which sample type and which combination of these markers in these fluids could correctly assign a subject to the group of periodontitis patients or healthy controls. Such findings, after validation in a larger‐scale study, could eventually contribute to the development of a test based on an oral fluid to be used by non‐dental medical practitioners to identify patients with signs of periodontitis, to refer them for further periodontal examination and diagnosis.
A mouthrinse MMP‐8 chair‐side test kit has been already introduced and used to identify patients with signs of periodontitis (Heikkinen et al., 2016). Various studies have shown that MMP‐8 levels are elevated in saliva, mouthrinses and GCF of patients with gingivitis or periodontitis (Lauhio et al., 2016; Sorsa et al., ,2004, 2016; Sorsa, Mantyla, et al., 2011; Sorsa, Tervahartiala, et al., 2011). A recent systematic review concluded that MMP‐8 has a good capability, as a salivary biomarker, to detect periodontitis (Arias‐Bujanda et al., 2019). Thus, in the current study, MMP‐8 levels were included in the analysis and exploration of the potential biomarkers of periodontitis. The results of the current study are in accordance with previous studies since it is shown that periodontitis patients exhibited higher levels of MMP‐8 in saliva, oral rinse and GCF than controls. Recent studies with MMP‐8 chair‐side diagnostic tests evaluated the concentration of the activated form of MMP‐8 (Raisanen et al., 2018, 2019) and suggested that the activated and fragmented forms MMP‐8 are characteristics of, and specific to, active periodontitis and peri‐implantitis lesions, differentiating from gingivitis and healthy tissues (Gürsoy et al., 2018). However, it should be noted that in 2016, at the initiation of the current study, the importance of the activated MMP‐8 was insufficiently highlighted. The MMP‐8 measurement in our study does not discriminate between latent and activated MMP‐8 forms. On the other hand, we do not necessarily consider this aspect as shortcoming, since another assay employed here, namely the TPA assay, is measuring proteolytic activity in the given sample, both host‐ and bacteria‐derived and as such could probe a larger range of the proteolytic spectrum in periodontal inflammation. The results of the current investigation showed that in the three types of oral fluids, periodontitis patients presented with significantly higher levels of TPA than controls. Such findings are consistent with previous studies, which detected higher proteolytic activity in periodontitis patients (Nedzi‐Gora et al., 2014; Nizam et al., 2014; Sorsa et al., 2016).
Another currently investigated biomarker, chitinase, was found to be elevated in periodontitis patients in saliva, oral rinse and GCF. However, only in GCF samples, the differences between the two study groups reached statistical significant levels. Chitinase is an enzyme produced by salivary glands and secreted by PMNs (Escott & Adams, 1995; Van Steijn et al., 1999). High levels of chitinase in periodontitis patients may reflect the fact that this enzyme has an important contribution to the destruction of pathogens in the phagolysosomes of PMNs by cleaving the chitin of the cell wall.
Lysozyme was included in the analysis due to the fact that it constitutes an enzyme which is a part of the innate defence mechanism of the host. Interestingly, in the current study it was found that in unstimulated saliva, periodontitis‐free controls exhibited higher lysozyme activity than periodontitis patients. In contrast, in GCF samples, periodontitis patients presented with higher values of lysozyme activity compared to controls. These findings are in agreement with previous studies, in which lysozyme activity was significantly higher in GCF samples of periodontitis patients than of those with intact periodontium, whereas in unstimulated saliva lysozyme activity was less in periodontitis patients (Surna et al., 2009). A possible explanation for these results could be that a large number of leukocytes is concentrated in the gingival sulcus over a short period, and this could result in a decreased number of leukocytes in unstimulated saliva (Surna et al., 2009). Furthermore, lysozyme seems to have a protective role in saliva since it is more elevated in controls.
Very rarely a single biochemical marker can be used for reliable diagnosis of periodontal inflammation (Mantyla et al., 2003). Interestingly, in the current study, the combination of MMP‐8 and chitinase was found to explain a high percentage of the variation among the subjects and based on that, we could cluster a subject in the control or periodontitis group. The oral rinse sample, which was derived after centrifugation, appeared to be the sample type with the highest accuracy. The results of the current study suggest that oral rinse can be an alternative for a five min unstimulated saliva sample obtained by drooling, for screening periodontal biomarkers and MMP‐8 and chitinase may be a reliable combination of biomarkers for early screening purposes. Recently, a report on saliva biomarkers for periodontal screening purposes was published (Verhulst et al., 2019). They observed elevated chitinase and total proteolytic activity in oral rinse samples of patients with periodontitis compared to controls. However, their results cannot be compared to our current results due to differences in the processing method, as we used the cells and pellets of oral rinses. Future investigations could focus on enlarging the panel of PMN‐derived biomarkers for more accuracy. Moreover, an additional approach of study design is needed: a cross‐sectional population sample in which first the biomarkers are determined and subsequently the dental examination is performed. This will allow us to validate the current findings to discriminate periodontitis patients from controls on the basis of oral fluid biomarkers.
Among the oral fluids, saliva and oral rinse can be collected in an easy, non‐invasive, minimal‐time consuming manner with minimally trained personnel (Bolerazska et al., 2016). On the other hand, collection of GCF is much more difficult. First, the quantity of collectable GCF can be highly variable among different subjects. In periodontal healthy subjects, the amount of GCF to be retrieved can be very limited. Moreover, the collection of GCF requires trained personnel and their skills will be a determinant for the inter‐ and intra‐operator variability. GCF collection is also time‐consuming and contamination with saliva, plaque or blood is possible (Trindade et al., 2014). Thus, GCF does not seem the fluid of choice for a chair‐side test, especially outside the dental office. However, the reason for including GCF analyses in the current study was to observe the interrelations of the potential biomarkers in GCF and their discriminative power in periodontitis and compare them to those obtained in saliva and oral rinse. The GCF sampling protocol was derived on previously published protocols and the recommendations from a technical review paper on this topic (Wassall & Preshaw, 2016). Pooled GCF samples were selected in order to increase the reproducibility of the samples. The Periotron readings are subject to variation, and at low volumes, that is below 0.2 μl, as values for healthy gingiva often are, the variations become unacceptable (Chapple et al., 1995). To account for the variations between the input volumes of the sampled GCF, we included the measurement of albumin. As albumin is entirely serum‐derived, this could serve as a surrogate marker of GCF volume. Subsequently, ratio's between TPA, MMP‐8, chitinase and lysozyme values to albumin values were calculated, both for GCF and saliva. Of these ratio's, the MMP‐8/albumin in GCF was still higher in periodontitis than controls, suggesting that the MMP‐8 values are a strong biomarker, less dependent of GCF volumetric variations. In our study, it was shown that the values of the measured biomarkers in saliva were influenced by the amount of GCF in these samples, since after calculating the ratios of all biomarkers to albumin, only lysozyme remained different between control and periodontitis group. This confirms that the degree of inflammation determines the volume of GCF and its influence in the measured values in saliva.
The present study has several limitations. Since the study had been undertaken as a pilot study, is limited of size and no power calculation was performed. In addition, the population of patients and controls was not matched for age, ethnicity, smoking habits and BMI. Another point of discussion is the inclusion of subjects who were smokers, with co‐morbidities and use of medication. In a previous study, it has been shown that MMP‐8 levels in GCF of smokers were lower than in non‐smokers (Mantyla et al., 2003). In contrast, in another study, it was found that smoking and obesity contributed to increased levels of circulating MMP‐8 (Lauhio et al., 2016). These studies indicate the possible influence of health status and anthropometric characteristics on the investigated biomarkers. However, the intention of the current study was to explore whether a biomarker or combination of biomarkers could assign a subject to the control or periodontitis group regardless of the individual's lifestyle and medical status. For this purpose, PCA analysis was utilized in order to create a graphical representation of possible clusters formed from the subjects of this study, taking into account all the biomarkers and the background characteristics of them.
Within the limitations of the study, we conclude that in oral rinse samples, the combination of MMP‐8 and chitinase could assign a subject into the control or periodontitis group. Importantly, oral rinse presented the best accuracy for clustering a subject. On the basis of this outcome, future studies could try to discriminate different stages of periodontitis. Nevertheless, this study needs to be replicated on a larger scale with matched controls and periodontitis patients in terms of demographic characteristics, in order to confirm the current results and subsequently validated in a cohort of individuals with various degrees of periodontal disease.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in this study.
ETHICAL APPROVAL
The protocol of the present study was approved by the Medical Ethical Committee of the VU Medical Center Amsterdam and following the principals of the Medical Research Involving Human Subjects Act (approval letter number 2016.470).
Supporting information
Supplementary Material
Katsiki P, Nazmi K, Loos BG, et al. Comparing periodontitis biomarkers in saliva, oral rinse and gingival crevicular fluid: A pilot study. J Clin Periodontol. 2021;48:1250–1259. 10.1111/jcpe.13479
Panagiota Katsiki and Kamran Nazmi equally contributed to this manuscript.
Funding information
This study was supported by the authors’ institution ACTA Amsterdam and the University of Amsterdam for the research priority area ‘oral infection and inflammation’ and by a grant from the Dutch Society of Periodontology (NVvP).
DATA AVAILABILITY STATEMENT
The data sets generated during the current study are available from the corresponding author on reasonable request.
REFERENCES
- Ahdi, M., Teeuw, W. J., Meeuwissen, H. G., Hoekstra, J. B., Gerdes, V. E., Loos, B. G., & Meesters, E. W. (2015). Oral health information from the dentist to the diabetologist. European Journal of Internal Medicine, 26(7), 498–503. 10.1016/j.ejim.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Arias‐Bujanda, N., Regueira‐Iglesias, A., Balsa‐Castro, C., Nibali, L., Donos, N., & Tomas, I. (2019). Accuracy of single molecular biomarkers in saliva for the diagnosis of periodontitis: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 47(1), 2–18. 10.1111/jcpe.13202 [DOI] [PubMed] [Google Scholar]
- Barros, S. P., Williams, R., Offenbacher, S., & Morelli, T. (2016). Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000, 70(1), 53–64. 10.1111/prd.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikker, F. J., Nascimento, G. G., Nazmi, K., Silbereisen, A., Belibasakis, G. N., Kaman, W. E., Lopez, R., & Bostanci, N. (2019). Salivary total protease activity based on a broad‐spectrum fluorescence resonance energy transfer approach to monitor induction and resolution of gingival inflammation. Molecular Diagnosis & Therapy, 23(5), 667–676. 10.1007/s40291-019-00421-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolerazska, B., Marekova, M., & Markovska, N. (2016). Trends in laboratory diagnostic methods in periodontology. Acta Medica (Hradec Kralove), 59(1), 3–9. 10.14712/18059694.2016.47 [DOI] [PubMed] [Google Scholar]
- Chapple, I. L., Cross, I. A., Glenwright, H. D., & Matthews, J. B. (1995). Calibration and reliability of the Periotron 6000 for individual gingival crevicular fluid samples. Journal of Periodontal Research, 30(1), 73–79. 10.1111/j.1600-0765.1995.tb01255.x [DOI] [PubMed] [Google Scholar]
- Escott, G. M., & Adams, D. J. (1995). Chitinase activity in human serum and leukocytes. Infection and Immunity, 63(12), 4770–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub, L. M., Wolff, M., Roberts, S., Lee, H. M., Leung, M., & Payonk, G. S. (1994). Treating periodontal diseases by blocking tissue‐destructive enzymes. Journal of the American Dental Association, 125(2), 163–169, discussion 169–171. [DOI] [PubMed] [Google Scholar]
- Gürsoy, U. K., Könönen, E., Tervahartiala, T., Gürsoy, M., Pitkänen, J., Torvi, P., Suominen, A. L., Pussinen, P., & Sorsa, T. (2018). Molecular forms and fragments of salivary MMP‐8 in relation to periodontitis. Journal of Clinical Periodontology, 45(12), 1421–1428. 10.1111/jcpe.13024 [DOI] [PubMed] [Google Scholar]
- Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). Past: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 4, 9 pp. [Google Scholar]
- Hansen, N. E., & Karle, H. (1979). Elevated plasma lysozyme in Hodgkin's disease. An indicator of increased macrophage activity? Scandinavian Journal of Haematology, 22(2), 173–178. [DOI] [PubMed] [Google Scholar]
- Heikkinen, A. M., Nwhator, S. O., Rathnayake, N., Mantyla, P., Vatanen, P., & Sorsa, T. (2016). Pilot study on oral health status as assessed by an active matrix metalloproteinase‐8 chairside mouthrinse test in adolescents. Journal of Periodontology, 87(1), 36–40. 10.1902/jop.2015.150377 [DOI] [PubMed] [Google Scholar]
- Helmerhorst, E. J., Dawes, C., & Oppenheim, F. G. (2018). The complexity of oral physiology and its impact on salivary diagnostics. Oral Diseases, 24(3), 363–371. 10.1111/odi.12780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil, R. A., Ashley, F. P., & Wilson, R. F. (1992). The relationship between 48‐h dental plaque accumulation in young human adults and the concentrations of hypothiocyanite, ‘free’ and ‘total’ lysozyme, lactoferrin and secretory immunoglobulin A in saliva. Archives of Oral Biology, 37(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Kassebaum, N. J., Bernabé, E., Dahiya, M., Bhandari, B., Murray, C. J., & Marcenes, W. (2014). Global burden of severe periodontitis in 1990–2010: A systematic review and meta‐regression. Journal of Dental Research, 93(11), 1045–1053. 10.1177/0022034514552491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landzberg, M., Doering, H., Aboodi, G. M., Tenenbaum, H. C., & Glogauer, M. (2015). Quantifying oral inflammatory load: Oral neutrophil counts in periodontal health and disease. Journal of Periodontal Research, 50(3), 330–336. 10.1111/jre.12211 [DOI] [PubMed] [Google Scholar]
- Lauhio, A., Färkkilä, E., Pietiläinen, K. H., Åström, P., Winkelmann, A., Tervahartiala, T., Pirilä, E., Rissanen, A., Kaprio, J., Sorsa, T. A., & Salo, T. (2016). Association of MMP‐8 with obesity, smoking and insulin resistance. European Journal of Clinical Investigation, 46(9), 757–765. 10.1111/eci.12649 [DOI] [PubMed] [Google Scholar]
- Mantyla, P., Stenman, M., Kinane, D. F., Tikanoja, S., Luoto, H., Salo, T., & Sorsa, T. (2003). Gingival crevicular fluid collagenase‐2 (MMP‐8) test stick for chair‐side monitoring of periodontitis. Journal of Periodontal Research, 38(4), 436–439. [DOI] [PubMed] [Google Scholar]
- Nedzi‐Gora, M., Kostrzewa‐Janicka, J., & Gorska, R. (2014). Elastase and metalloproteinase‐9 concentrations in saliva in patients with chronic periodontitis. Central European Journal of Immunology, 39(3), 357–364. 10.5114/ceji.2014.45948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizam, N., Gumus, P., Pitkanen, J., Tervahartiala, T., Sorsa, T., & Buduneli, N. (2014). Serum and salivary matrix metalloproteinases, neutrophil elastase, myeloperoxidase in patients with chronic or aggressive periodontitis. Inflammation, 37(5), 1771–1778. 10.1007/s10753-014-9907-0. [DOI] [PubMed] [Google Scholar]
- Overdijk, B., & Van Steijn, G. J. (1994). Human serum contains a chitinase: Identification of an enzyme, formerly described as 4‐methylumbelliferyl‐tetra‐N‐acetylchitotetraoside hydrolase (MU‐TACT hydrolase). Glycobiology, 4(6), 797–803. [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N., Sanz, M., Buduneli, N., Dietrich, T., Feres, M., Fine, D. H., Flemmig, T. F., Garcia, R., Giannobile, W. V., Graziani, F., Greenwell, H., Herrera, D., Kao, R. T., Kebschull, M., Kinane, D. F., Kirkwood, K. L., Kocher, T., Kornman, K. S., Kumar, P. S., … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Periodontology, 89(Suppl 1), S173–s182. 10.1002/jper.17-0721 [DOI] [PubMed] [Google Scholar]
- Prodan, A., Brand, H. S., Ligtenberg, A. J. M., Imangaliyev, S., Tsivtsivadze, E., van der Weijden, F., Crielaard, W., Keijser, B. J. F., & Veerman, E. C. I. (2015). Interindividual variation, correlations, and sex‐related differences in the salivary biochemistry of young healthy adults. European Journal of Oral Sciences, 123(3), 149–157. 10.1111/eos.12182 [DOI] [PubMed] [Google Scholar]
- Räisänen, I., Heikkinen, A., Siren, E., Tervahartiala, T., Gieselmann, D.‐R., van der Schoor, G.‐J., van der Schoor, P., & Sorsa, T. (2018). Point‐of‐Care/Chairside aMMP‐8 analytics of periodontal diseases’ activity and episodic progression. Diagnostics (Basel), 8(4), 74. 10.3390/diagnostics8040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisanen, I. T., Sorsa, T., van der Schoor, G. J., Tervahartiala, T., van der Schoor, P., Gieselmann, D. R., & Heikkinen, A. M. (2019). Active matrix metalloproteinase‐8 point‐of‐care (PoC)/Chairside mouthrinse test vs. bleeding on probing in diagnosing subclinical periodontitis in adolescents. Diagnostics (Basel), 9(1), 34. 10.3390/diagnostics9010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkschroeff, P., Jansen, I. D., van der Weijden, F. A., Keijser, B. J., Loos, B. G., & Nicu, E. A. (2016). Oral polymorphonuclear neutrophil characteristics in relation to oral health: A cross‐sectional, observational clinical study. International Journal of Oral Science, 8(3), 191–198. 10.1038/ijos.2016.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm, L. (1986). Proteases and their inhibitors in chronic inflammatory periodontal disease. Journal of Clinical Periodontology, 13(1), 19–26. [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Gursoy, U. K., Nwhator, S., Hernandez, M., Tervahartiala, T., Leppilahti, J., Gursoy, M., Könönen, E., Emingil, G., Pussinen, P. J., & Mäntylä, P. (2016). Analysis of matrix metalloproteinases, especially MMP‐8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000, 70(1), 142–163. 10.1111/prd.12101 [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Mantyla, P., Tervahartiala, T., Pussinen, P. J., Gamonal, J., & Hernandez, M. (2011). MMP activation in diagnostics of periodontitis and systemic inflammation. Journal of Clinical Periodontology, 38(9), 817–819. 10.1111/j.1600-051X.2011.01753.x [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Tervahartiala, T., Leppilahti, J., Hernandez, M., Gamonal, J., Tuomainen, A. M., Lauhio, A., Pussinen, P. J., & Mäntylä, P. (2011). Collagenase‐2 (MMP‐8) as a point‐of‐care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non‐antimicrobial properties of tetracyclines. Pharmacological Research, 63(2), 108–113. 10.1016/j.phrs.2010.10.005 [DOI] [PubMed] [Google Scholar]
- Sorsa, T., Tjaderhane, L., & Salo, T. (2004). Matrix metalloproteinases (MMPs) in oral diseases. Oral Diseases, 10(6), 311–318. 10.1111/j.1601-0825.2004.01038.x [DOI] [PubMed] [Google Scholar]
- Surna, A., Kubilius, R., Sakalauskiene, J., Vitkauskiene, A., Jonaitis, J., Saferis, V., & Gleiznys, A. (2009). Lysozyme and microbiota in relation to gingivitis and periodontitis. Medical Science Monitor, 15(2), Cr66‐73. [PubMed] [Google Scholar]
- Tonetti, M. S., & Claffey, N. (2005). Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. Journal of Clinical Periodontology, 32(Suppl 6), 210–213. 10.1111/j.1600-051X.2005.00822.x [DOI] [PubMed] [Google Scholar]
- Trindade, F., Oppenheim, F. G., Helmerhorst, E. J., Amado, F., Gomes, P. S., & Vitorino, R. (2014). Uncovering the molecular networks in periodontitis. Proteomics Clinical Applications, 8(9–10), 748–761. 10.1002/prca.201400028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto, V. J. (2003). Gingival crevice fluid–an introduction. Periodontology 2000, 31, 9–11. [DOI] [PubMed] [Google Scholar]
- Uitto, V. J., Overall, C. M., & McCulloch, C. (2003). Proteolytic host cell enzymes in gingival crevice fluid. Periodontology 2000, 31, 77–104. [DOI] [PubMed] [Google Scholar]
- Van Steijn, G. J., Amerongen, A. V., Veerman, E. C., Kasanmoentalib, S., & Overdijk, B. (1999). Chitinase in whole and glandular human salivas and in whole saliva of patients with periodontal inflammation. European Journal of Oral Sciences, 107(5), 328–337. [DOI] [PubMed] [Google Scholar]
- Van Steijn, G. J., Amerongen, A. V., Veerman, E. C., Kasanmoentalib, S., & Overdijk, B. (2002). Effect of periodontal treatment on the activity of chitinase in whole saliva of periodontitis patients. Journal of Periodontal Research, 37(4), 245–249. [DOI] [PubMed] [Google Scholar]
- Venge, P. (1994). The monitoring of inflammation by specific cellular markers. Scandinavian Journal of Clinical and Laboratory Investigation, 219, 47–54. [DOI] [PubMed] [Google Scholar]
- Verhulst, M. J. L., Teeuw, W. J., Bizzarro, S., Muris, J., Su, N., Nicu, E. A., Nazmi, K., Bikker, F. J., & Loos, B. G. (2019). A rapid, non‐invasive tool for periodontitis screening in a medical care setting. BMC Oral Health, 19(1), 87. 10.1186/s12903-019-0784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin, A., Papias, A., Jansson, H., & Norderyd, O. (2018). Secular trends over 40 years of periodontal health and disease in individuals aged 20–80 years in Jonkoping, Sweden: Repeated cross‐sectional studies. Journal of Clinical Periodontology, 45(9), 1016–1024. 10.1111/jcpe.12978 [DOI] [PubMed] [Google Scholar]
- Wassall, R. R., & Preshaw, P. M. (2016). Clinical and technical considerations in the analysis of gingival crevicular fluid. Periodontology 2000, 70(1), 65–79. 10.1111/prd.12109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data sets generated during the current study are available from the corresponding author on reasonable request.


