Abstract
Given the lack of head‐to‐head studies of systemic therapies in moderate‐to‐severe atopic dermatitis (AD), network meta‐analyses (NMAs) can provide comparative efficacy and safety data to inform clinical decision‐making. In this NMA, eligible randomized controlled trials (RCTs) published before 24 October 2019 were identified by a systematic literature review. Short‐term (12–16 weeks) efficacy (Investigator’s Global Assessment [IGA] and Eczema Area and Severity Index [EASI] responses), patient‐reported outcomes (PROs) and safety data from each trial were abstracted and analysed separately for monotherapy and combination therapy (systemic plus topical anti‐inflammatory therapy). RCTs were analysed in fixed‐effects and random‐effects Bayesian NMA models. Overall, 19 phase 2 and phase 3 RCTs of abrocitinib, baricitinib, dupilumab, lebrikizumab, nemolizumab, tralokinumab and upadacitinib were included. In monotherapy RCTs, upadacitinib 30 mg once daily (QD) had the numerically highest efficacy (83.6% achieved ≥50% improvement in EASI [EASI‐50 response]), followed by abrocitinib 200 mg QD (74.6%), upadacitinib 15 mg QD (70.5%), dupilumab 300 mg every 2 weeks (Q2W) (63.4%) and abrocitinib 100 mg QD (56.7%). Similar trends in EASI‐75 and EASI‐90 response were observed. In combination therapy RCTs, abrocitinib 200 mg QD had the highest EASI‐50 (86.6%), followed by dupilumab 300 mg Q2W (82.4%) and abrocitinib 100 mg QD (79.7%). Similar findings were observed for IGA response and PROs. In monotherapy and combination therapy RCTs, the probability of treatment‐emergent adverse events (TEAEs) was higher among all active treatments than with placebo (except for dupilumab 300 mg Q2W [odds ratio (OR), 0.96; 95% credible interval (CrI), 0.45–2.18] and abrocitinib 100 mg QD [OR, 0.95; 95% CrI, 0.35–2.66] in combination therapy RCTs), although active treatments did not significantly differ from one another. Abrocitinib, dupilumab and upadacitinib were consistently the most effective systemic therapies in adult and adolescent patients with AD, with no significant TEAE differences in short‐term RCTs.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by intense pruritus and eczematous lesions.1, 2 Affecting up to 20% of children and 10% of adults globally,3, 4 AD is significantly associated with decreased levels of health‐related quality of life (QoL),5, 6 increased work‐related impairment5, 7 and high healthcare costs.8, 9, 10
Few treatment options are approved for patients with moderate‐to‐severe AD who require systemic therapy.11 Dupilumab, an interleukin (IL)‐4 receptor antagonist that blocks both IL‐4 and IL‐13, is a subcutaneously administered biologic therapy available for children 6 years of age and older, adolescents, and adults with moderate‐to‐severe AD.12, 13 In Europe, baricitinib is approved for use in adults with moderate‐to‐severe AD who are candidates for systemic therapy,14 and cyclosporine is approved only in certain countries for severe AD but is associated with dose‐dependent nephrotoxicity.15 Systemic immunosuppressants (methotrexate, azathioprine and mycophenolate mofetil) are also used off‐label to treat moderate‐to‐severe AD, although their use is also associated with end‐organ toxicities.15
Treatments under the development for moderate‐to‐severe AD include oral Janus kinase (JAK) inhibitors (abrocitinib [JAK1], upadacitinib [JAK1] and baricitinib [JAK 1/2; outside of Europe]),16, 17, 18 IL‐13 antagonists (lebrikizumab19 and tralokinumab20) and an IL‐31 antagonist (nemolizumab21). Evaluating efficacy and safety of new interventions in comparison with existing treatments is important to assist healthcare decision‐makers and inform future clinical practice. Network meta‐analyses (NMAs) can be used to synthesize available evidence on multiple treatments not been directly compared in a randomized controlled trial (RCT)22, 23 and also allow indirect comparisons from sets of trials with a common treatment (e.g. placebo). NMA methodology combines all available comparative evidence while preserving within‐trial randomized treatment comparisons.
A recent NMA including data up to October 2019 examined the efficacy of systemic therapies in moderate‐to‐severe AD, although monotherapy and combination therapy trials were pooled and more recent trials were not included.24 Another recent NMA of systemic therapies reported robust evidence for baricitinib, dupilumab and ciclosporin‐A.25 The objective of this study was to provide an up‐to‐date analysis of the comparative efficacy and safety of systemic treatments for moderate‐to‐severe AD as monotherapy and in combination with topical therapies.
Methods
Eligibility criteria
Included RCTs were identified through a systematic literature review (SLR) in accordance with the guidelines and recommendations of The Cochrane Collaboration.26 Reporting of the SLR adhered to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses27 and A MeaSurement Tool to Assess Systematic Reviews28 (Methods S1). The search was limited to RCTs in adolescents (aged 12–17 years) or adults (aged ≥18 years) with moderate‐to‐severe AD, included identified systemic monotherapy or systemic therapy with topical anti‐inflammatory therapy (combination therapy) and reported efficacy and/or safety. Titles and abstracts of citations identified from searches and content of relevant full texts were evaluated. Screening was conducted by 2 investigators at each stage, with a third investigator resolving any disagreements (Tables S1–S4).
Data extraction and risk of bias assessment
Data on patient population, treatments, timepoints and available outcomes of interest were extracted by one investigator and validated for accuracy by a second, senior investigator. Risk of bias was evaluated using The Cochrane Collaboration’s Risk of Bias Assessment Tool.26
Outcomes of interest
Primary outcomes of interest included responder definitions of Eczema Area and Severity Index (EASI) and Investigator’s Global Assessment (IGA) measures.29, 30 Secondary outcomes included the SCORing Atopic Dermatitis (SCORAD) and the following patient‐reported outcomes (PROs): Peak Pruritus Numerical Rating Scale (PP‐NRS), Patient‐Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI), and Hospital Anxiety and Depression Scale (HADS).31, 32, 33, 34, 35 Rates of treatment‐emergent adverse events (TEAEs) and discontinuations owing to AEs were analysed.
Feasibility assessment
An assessment was conducted to evaluate the feasibility of an NMA for each outcome of interest, which includes determining whether sufficient data were available for generating an evidence network and whether clinical heterogeneity existed across studies on factors that could preclude a comparison or impact the results of analyses. The time range of interest was 12–16 weeks, with data being used from the latest timepoint available. Baseline characteristics of each trial were considered similar enough for comparison (Table S5). Baseline disease severity showed a small degree of variation across trials but was considered minor and did not warrant exclusion of any trials.
Statistical analysis
Information leveraged from each trial was maximized with a 4‐level efficacy variable based on the nested relationship among outcomes (e.g. a patient cannot achieve ≥90% improvement from baseline in EASI score [EASI‐90 response] without also achieving ≥75% and ≥50% improvement from baseline [EASI‐75 and EASI‐50 response, respectively]). The 4 response levels (no response, EASI‐50, EASI‐75, EASI‐90) represented the highest level of response achieved and were analysed using a Bayesian multinomial approach with methods from the National Institute for Health and Clinical Excellence Decision Support Unit and the literature.36, 37 Base‐case analysis assumed no treatment‐by‐threshold interaction effect (i.e. the pattern of results was assumed to be identical at each level of response). Model fit was not improved in analyses that relaxed this assumption; hence, the more parsimonious base‐case model is presented. Other efficacy and safety outcomes that do not exhibit hierarchical relationships were examined as binary (e.g. IGA response) or continuous outcomes (e.g. POEM mean change) in Bayesian NMAs.
All models for EASI and IGA response were adjusted for the absolute level of placebo rates (i.e. baseline risk models) unless model fit based on deviance information criterion was poorer after accounting for baseline risk.36 Additionally, the default approach was to implement random‐effects models, with prior for treatment heterogeneity selected to be vaguely informative (U[0,1] for the between‐trial standard deviation of probit or logit differences and U[0,7] for POEM mean change differences; median standard deviation in POEM pre/post change was used as the upper end of the distribution as the estimate of between‐study heterogeneity rarely exceeds the patient‐level standard deviation). However, a fixed‐effects alternative was used if the estimates of the random‐effects models were unstable because of sparse data. All NMAs were performed using OpenBUGS 3.2.3 and involved a 100 000 run‐in iteration phase and a 100 000‐iteration phase for parameter estimation. Although traditional concepts of statistical significance and null hypothesis testing do not exist in a Bayesian framework, we considered 95% credible intervals that do not overlap zero to represent statistically conclusive differences between treatments; no overlap corresponds to roughly a ≥97.5% probability that the better performing treatment has a superior effect relative to the worse performing treatment.
The networks included all doses investigated within each trial. However, to best inform healthcare decision‐making, results do not focus on off‐label dosing regimens of dupilumab or single administration of investigational biologic agents.
Results
Systematic review
972 unique records from electronic literature databases were identified. Following screening, 36 publications of 17 RCTs met inclusion criteria for the SLR and were considered eligible (Fig. 1).
Figure 1.
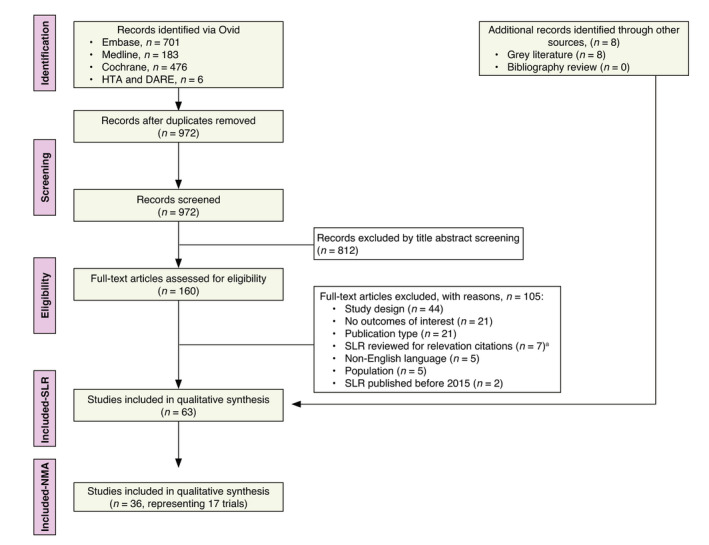
PRISMA study attrition diagram. aBibliographies were searched for relevant citations. DARE, database of abstracts of reviews of effects; HTA, health technology assessment; NMA, network meta‐analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses; SLR, systematic literature review.
Study characteristics
Trials included present data on treatment with abrocitinib,16, 38, 39 baricitinib,17, 40, 41 dupilumab,42, 43, 44, 45, 46 lebrikizumab,47 nemolizumab,21, 48 tralokinumab20 and upadacitinib49 (Table 1 and Fig. 2). All JAK inhibitors were provided orally once daily; dupilumab and tralokinumab were provided by subcutaneous injection every 2 weeks; and lebrikizumab and nemolizumab were provided by subcutaneous injection every 4 weeks. Large phase 3 study results were available for abrocitinib, baricitinib and dupilumab. Phase 2 results were available for other treatments. Eligible trials assessing systemic immunosuppressants were identified but did not connect to the network and were not included. Not all trials included all outcomes; therefore, included comparisons represent the outcomes reported in all trials of a given network.
Table 1.
Monotherapy and combination therapy trials included in the network meta‐analysis
| Trial name | Study phase | Study duration (weeks) | Interventions | Age (years) | EASI | IGA | PP‐NRS | POEM | DLQI | HADS | TEAE | DAE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monotherapy | Abrocitinib phase 2b | 2 | 12 | Abrocitinib, placebo | Adults (18–75) | X | X | X | X | ||||
| JADE MONO‐1 | 3 | 12 | Abrocitinib, placebo | Adolescents and adults (12+) | X | X | X | X | X | X | X | X | |
| JADE MONO‐2 | 3 | 13 | Abrocitinib, placebo | Adolescents and adults (12+) | X | X | X | X | X | X | X | X | |
| BREEZE‐AD1 | 3 | 16 | Baricitinib, placebo | Adults (unspecified) | X | X | |||||||
| BREEZE‐AD2 | 3 | 16 | Baricitinib, placebo | Adults (unspecified) | X | X | |||||||
| LIBERTY AD ADOL | 3 | 16 | Dupilumab, placebo | Adolescents (12–17) | X | X | X | X | X | ||||
| LIBERTY AD SOLO 1 | 3 | 16 | Dupilumab, placebo | Adults (18+) | X | X | X | X | X | X | X | ||
| LIBERTY AD SOLO 2 | 3 | 16 | Dupilumab, placebo | Adults (18+) | X | X | X | X | X | X | X | ||
| Dupilumab phase 2b | 2b | 16 | Dupilumab, placebo | Adults (18+) | X | X | |||||||
| XCIMA | 2 | 12 | Nemolizumab, placebo | Adults (18–65) | X | X | |||||||
| Upadacitinib phase 2b | 2b | 16 | Upadacitinib, placebo | Adults (18–75) | X | X | |||||||
| Combination therapy | JADE COMPARE | 3 | 16 | Abrocitinib, dupilumab placebo | Adults (18+) | X | X | X | X | X | X | X | X |
| BREEZE‐AD7 | 3 | 16 | Baricitinib, placebo | Adults (18+) | X | X | X | X | X | X | |||
| Baricitinib + TCS phase 2b | 2 | 16 | Baricitinib, placebo | Adults (18+) | X | X | X | X | X | ||||
| LIBERTY AD CAFE | 3 | 16 | Dupilumab, placebo | Adults (18+) | X | X | X | X | X | X | |||
| LIBERTY AD CHRONOS | 3 | 52 | Dupilumab, placebo | Adults (18+) | X | X | X | X | X | X | |||
| TREBLE | 2 | 12 | Lebrikizumab, placebo | Adults (18–75) | X | X | |||||||
| Nemolizumab phase 2b | 2b | 24 | Nemolizumab, placebo | Adults (18+) | X | X | X | ||||||
| Tralokinumab phase 2b | 2b | 12 | Tralokinumab, placebo | Adults (18–75) | X | X | X |
DAE, discontinuation due to an adverse event; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; HADS, Hospital Anxiety and Depression Scale; IGA, Investigator’s Global Assessment; POEM, Patient‐Oriented Eczema Measure; PP‐NRS, Peak Pruritus‐Numerical Rating Scale; TEAE, treatment‐emergent adverse events.
Figure 2.
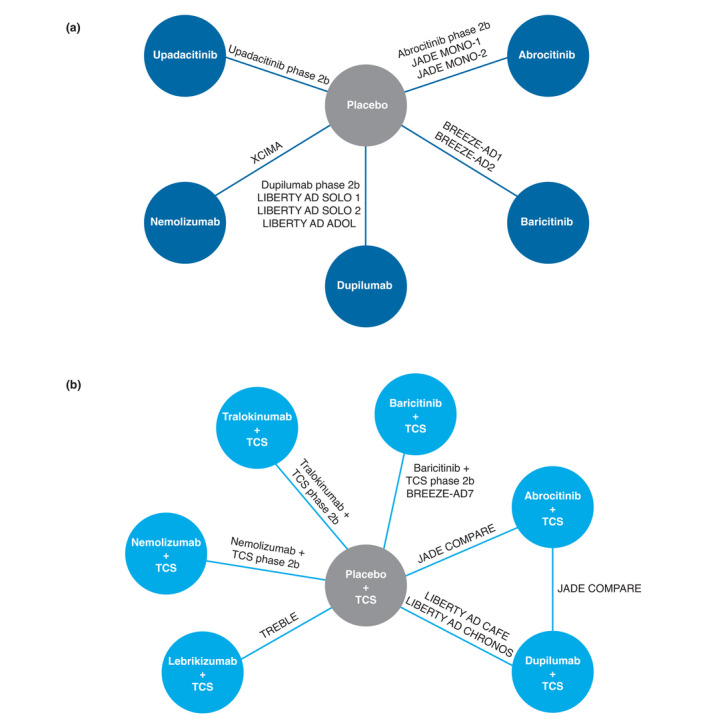
Network diagram for (a) monotherapy and (b) combination therapy trials. Not all outcomes were available for all trials. TCS, topical corticosteroids.
Risk of bias
Overall risk of bias was low, even when risk of blinding was unclear in most trials. All studies that met inclusion criteria were included in the NMA.
Statistical heterogeneity and inconsistency
Statistical heterogeneity, occurring when observed treatment effects vary more than expected due to sampling error, was calculated by examining I 2 values from direct, frequentist meta‐analyses. There was little evidence of detectable heterogeneity. Inconsistency could not be assessed as there were no independent sources of indirect and direct evidence for the same comparison.
Network meta‐analysis
Monotherapy
Clinical efficacy
With respect to EASI response (11 studies, N = 3339), highest numerical efficacy in EASI‐50 response was observed with upadacitinib 30 mg (84%), abrocitinib 200 mg (75%), upadacitinib 15 mg (70%), dupilumab 300 mg (63%) and abrocitinib 100 mg (57%). Both upadacitinib 30 mg and abrocitinib 200 mg exhibited a >97.5% probability of superiority over dupilumab 300 mg, both baricitinib doses and nemolizumab. Upadacitinib 30 mg was not statistically better than abrocitinib 200 mg (Table 2). Similar trends were observed with upadacitinib 30 mg, abrocitinib 200 mg, upadacitinib 15 mg, dupilumab 300 mg and abrocitinib 100 mg for EASI‐75 (70%, 58%, 53%, 45% and 39%, respectively) and EASI‐90 (52%, 39%, 34%, 28% and 22%, respectively) responses (Table 2).
Table 2.
Proportion of patients achieving each EASI threshold as estimated from the Bayesian network meta‐analysis model
| Treatment | Monotherapy (11 studies, N = 3339) | Combination therapy† (8 studies, N = 2193) | ||
|---|---|---|---|---|
| Median | 95% CrI | Median | 95% CrI | |
| EASI‐50 response | ||||
| Placebo | 0.21 | 0.20–0.21 | 0.47 | 0.43–0.51 |
| Baricitinib 2 mg QD | 0.39 | 0.29–0.50 | 0.61 | 0.52–0.70 |
| Baricitinib 4 mg QD | 0.46 | 0.35–0.56 | 0.67 | 0.58–0.75 |
| Dupilumab 200 mg Q2W | 0.48 | 0.32–0.66 | – | – |
| Dupilumab 200 or 300 mg Q2W | 0.70 | 0.54–0.84 | – | – |
| Dupilumab 300 mg Q2W | 0.63 | 0.56–0.70 | 0.82 | 0.79–0.86 |
| Lebrikizumab 125 mg Q4W | – | – | 0.76 | 0.60–0.88 |
| Nemolizumab 30 mg Q4W | – | – | 0.78 | 0.66–0.87 |
| Nemolizumab 0.5 mg/kg Q4W | 0.36 | 0.18–0.57 | – | – |
| Tralokinumab 300 mg Q2W | – | – | 0.72 | 0.58–0.84 |
| Upadacitinib 15 mg QD | 0.70 | 0.45–0.89 | – | – |
| Upadacitinib 30 mg QD | 0.84 | 0.61–0.95 | – | – |
| Abrocitinib 100 mg QD | 0.57 | 0.47–0.66 | 0.80 | 0.74–0.85 |
| Abrocitinib 200 mg QD | 0.75 | 0.66–0.82 | 0.87 | 0.82–0.90 |
| EASI‐75 response | ||||
| Placebo | 0.10 | 0.09–0.11 | 0.27 | 0.23–0.30 |
| Baricitinib 2 mg QD | 0.23 | 0.15–0.32 | 0.39 | 0.31–0.49 |
| Baricitinib 4 mg QD | 0.28 | 0.20–0.38 | 0.45 | 0.36–0.55 |
| Dupilumab 200 mg Q2W | 0.31 | 0.17–0.48 | – | – |
| Dupilumab 200 or 300 mg Q2W | 0.53 | 0.36–0.70 | – | – |
| Dupilumab 300 mg Q2W | 0.45 | 0.38–0.53 | 0.65 | 0.60–0.69 |
| Lebrikizumab 125 mg Q4W | – | – | 0.57 | 0.38–0.73 |
| Nemolizumab 30 mg Q4W | – | – | 0.59 | 0.45–0.72 |
| Nemolizumab 0.5 mg/kg Q4W | 0.20 | 0.09–0.39 | – | – |
| Tralokinumab 300 mg Q2W | – | – | 0.52 | 0.36–0.68 |
| Upadacitinib 15 mg QD | 0.53 | 0.28–0.78 | – | – |
| Upadacitinib 30 mg QD | 0.70 | 0.43–0.89 | – | – |
| Abrocitinib 100 mg QD | 0.39 | 0.29–0.48 | 0.61 | 0.54–0.68 |
| Abrocitinib 200 mg QD | 0.58 | 0.48–0.67 | 0.71 | 0.64–0.77 |
| EASI‐90 response | ||||
| Placebo | 0.04 | 0.03–0.05 | 0.11 | 0.09–0.14 |
| Baricitinib 2 mg QD | 0.11 | 0.07–0.17 | 0.20 | 0.14–0.27 |
| Baricitinib 4 mg QD | 0.15 | 0.09–0.22 | 0.24 | 0.17–0.32 |
| Dupilumab 200 mg Q2W | 0.16 | 0.08–0.30 | – | – |
| Dupilumab 200 or 300 mg Q2W | 0.34 | 0.20–0.52 | – | – |
| Dupilumab 300 mg Q2W | 0.28 | 0.22–0.35 | 0.42 | 0.37–0.47 |
| Lebrikizumab 125 mg Q4W | – | – | 0.34 | 0.18–0.51 |
| Nemolizumab 30 mg Q4W | – | – | 0.36 | 0.24–0.50 |
| Nemolizumab 0.5 mg/kg Q4W | 0.10 | 0.03–0.22 | – | – |
| Tralokinumab 300 mg Q2W | – | – | 0.30 | 0.17–0.45 |
| Upadacitinib 15 mg QD | 0.34 | 0.14–0.62 | – | – |
| Upadacitinib 30 mg QD | 0.52 | 0.26–0.78 | – | – |
| Abrocitinib 100 mg QD | 0.22 | 0.15–0.30 | 0.38 | 0.31–0.45 |
| Abrocitinib 200 mg QD | 0.39 | 0.30–0.49 | 0.49 | 0.41–0.56 |
Not all treatments have been assessed in both monotherapy and combination therapy; missing data are denoted with –.
CrI, credible interval; EASI, Eczema Area and Severity Index; QD, once daily; Q2W, once every 2 weeks; Q4W; once every 4 weeks.
In combination with topical therapy.
For IGA response (9 studies, N = 3188), the baseline risk model was used given its better fit (deviance information criterion [DIC] = 144.5); all treatments exhibited ≥99% probability of superiority to placebo (Fig. 3a). The greatest differentiation was observed for abrocitinib 200 mg (odds ratio [OR], 9.96; 95% credible interval [CrI], 7.77–12.59; >99.9% probability of superiority to placebo), dupilumab 300 mg (OR, 7.99; 95% CrI, 6.46–9.70; >99.9% probability) and abrocitinib 100 mg (OR, 5.11; 95% CrI, 3.89–6.57; >99.9% probability; Table 3). Abrocitinib 200 mg had a 92.5% probability of superiority to dupilumab 300 mg and >99.9% probability of superiority to either baricitinib dose. The greatest SCORAD‐50 responses (≥50% improvement from baseline; 4 studies, N = 990) were with upadacitinib 30 mg (OR, 24.34; 95% CrI, 5.50–144.75) and upadacitinib 15 mg (OR, 10.95; 95% CrI, 2.47–63.82; Table S6 and Fig. S4A).
Figure 3.
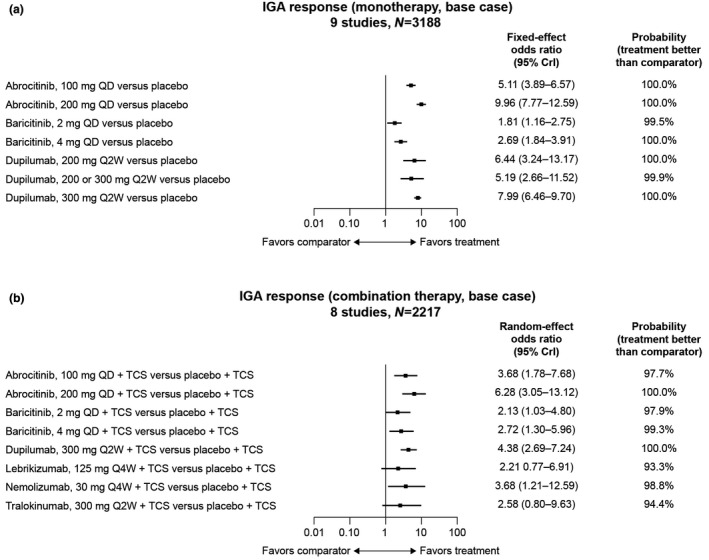
Odds ratios of achieving an IGA response compared with placebo in (a) monotherapy and (b) combination therapy. IGA response defined as clear (0) or almost clear (1) with ≥2‐point reduction from baseline. CrI, credible interval; IGA, Investigator’s Global Assessment; QD, once a day; Q2W, every 2 weeks; Q4W, every 4 weeks; TCS, topical corticosteroids.
Table 3.
League table comparing IGA response, PP‐NRS4 response and safety outcomes among monotherapy treatments
| Treatment | Odds ratio [95% CrI] | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Baricitinib 2 mg QD | Baricitinib 4 mg QD | Dupilumab 200 mg Q2W | Dupilumab 200 or 300 mg Q2W | Dupilumab 300 mg Q2W | Nemolizumab 0.5 mg/kg Q4W | Abrocitinib 100 mg QD | |
| IGA response (9 studies, N = 3188) | ||||||||
| Baricitinib 2 mg QD | 1.81 (1.16–2.75) | |||||||
| Baricitinib 4 mg QD | 2.69 (1.84–3.91) | 1.49 (0.88–2.54) | ||||||
| Dupilumab 200 mg Q2W | 6.44 (3.24–13.17) | 3.58 (1.65–7.89) | 2.40 (1.14–5.20) | |||||
| Dupilumab 200 or 300 mg Q2W | 5.19 (2.68–11.52) | 2.89 (1.38–6.58) | 1.94 (0.96–4.33) | 0.81 (0.34–2.02) | ||||
| Dupilumab 300 mg Q2W | 7.99 (6.46–9.70) | 4.41 (2.69–7.25) | 2.97 (1.88–4.61) | 1.24 (0.59–2.57) | 1.54 (0.64–3.14) | |||
| Abrocitinib 100 mg QD | 5.11 (3.89–6.57) | 2.83 (1.69–4.73) | 1.90 (1.18–3.03) | 0.79 (0.36–1.68) | 0.99 (0.41–2.04) | 0.64 (0.47–0.88) | ||
| Abrocitinib 200 mg QD | 9.96 (7.77–12.59) | 5.50 (3.33–9.16) | 3.71 (2.31–5.85) | 1.54 (0.71–3.24) | 1.92 (0.80–3.94) | 1.25 (0.92–1.70) | 1.95 (1.42–2.67) | |
| PP‐NRS4 response (6 studies, N = 1935) | ||||||||
| Dupilumab 200 or 300 mg Q2W | 12.48 (4.46–46.99) | |||||||
| Dupilumab 300 mg Q2W | 5.18 (3.63–7.49) | 0.41 (0.11–1.24) | ||||||
| Abrocitinib 100 mg QD | 4.06 (2.65–6.39) | 0.32 (0.08–1.00) | 0.79 (0.45–1.39) | |||||
| Abrocitinib 200 mg QD | 7.38 (4.81–11.68) | 0.59 (0.15–1.83) | 1.43 (0.81–2.52) | 1.82 (1.35–2.46) | ||||
| Treatment‐emergent AEs (5 studies, N = 1866) | ||||||||
| Dupilumab 200 or 300 mg Q2W | 1.13 (0.36–3.60) | |||||||
| Dupilumab 300 mg Q2W | 1.02 (0.49–2.15) | 0.91 (0.23–3.51) | ||||||
| Abrocitinib 100 mg QD | 1.56 (0.72–3.39) | 1.38 (0.34–5.54) | 1.53 (0.52–4.41) | |||||
| Abrocitinib 200 mg QD | 2.09 (0.96–4.54) | 1.85 (0.46–7.44) | 2.04 (0.69–5.94) | 1.34 (0.63–2.85) | ||||
| AEs leading to discontinuation (6 studies, N = 1748) | ||||||||
| Dupilumab 200 or 300 mg Q2W | 0.20 (0.00–7.25) | |||||||
| Dupilumab 300 mg Q2W | 0.84 (0.21–3.38) | 4.41 (0.09–2519.96) | ||||||
| Nemolizumab 0.5 mg/kg Q4W | 3.99 (0.32–109.95) | 24.53 (0.24–17747.5) | 4.76 (0.28–166.67) | |||||
| Abrocitinib 100 mg QD | 0.52 (0.22–1.22) | 2.63 (0.07–1464.11) | 0.61 (0.12–3.17) | 0.13 (0.00–1.81) | ||||
| Abrocitinib 200 mg QD | 0.52 (0.22–1.20) | 2.64 (0.07–1578.14) | 0.62 (0.12–3.14) | 0.13 (0.00–1.79) | 1.01 (0.43–2.34) | |||
IGA response defined as clear (0) or almost clear (1) with ≥2‐point reduction from baseline. PP‐NRS4 response defined as ≥4‐point improvement from baseline.
Cell numbers represent the odds ratio (95% CrI) of the row treatment meeting the response threshold (or experiencing the relevant AE) relative to the column treatment (e.g. baricitinib 2 mg QD has 1.81 the odds of achieving IGA response relative to placebo).
AE, adverse event; CrI, credible interval; IGA, Investigator’s Global Assessment; PP‐NRS, Peak Pruritus Numerical Rating Scale; QD, once daily; Q2W, once every 2 weeks; Q4W; once every 4 weeks.
Because efficacy evaluations were slightly different across included trials (12–16 weeks), post hoc sensitivity analyses limited to week 12 data were conducted to determine whether this would impact the results. Conclusions of week 12 results were similar to pooled results for weeks 12 to 16; therefore, the remaining analyses leveraged pooled results (Fig. S1). Adjustment for baseline risk was explored for response outcomes and determined justified only for IGA response due to improvement in DIC.
Patient‐reported outcomes
Abrocitinib and dupilumab had ≥99.9% probability of achieving a better clinically meaningful PP‐NRS4 response (≥4‐point improvement from baseline; 6 studies, N = 1935) than placebo (Fig. 4a). Abrocitinib 100 mg and 200 mg had 20.0% and 89.0% probability, respectively, of better PP‐NRS4 response than dupilumab 300 mg. The upadacitinib and nemolizumab trials had not reported numerical PP‐NRS4 response data at the time of our review. All treatments had ≥98.2% probability of better symptom/QoL improvement than placebo. Greatest reductions in POEM scores (6 studies, N = 1973) versus placebo were with upadacitinib 30 mg (mean difference, −10.69; 95% CrI, −15.65 to −5.52) and abrocitinib 200 mg (mean difference, −7.16; 95% CrI, −9.85 to −4.46; Table S6 and Fig. S2A). The greatest reductions in DLQI scores (5 studies, N = 1752) were with dupilumab 200 mg (mean difference, −5.86; 95% CrI, −9.33 to −2.44) and abrocitinib 200 mg (mean difference, −5.46; 95% CrI, −7.87 to −2.94; Table S6 and Fig. S3A). The greatest reductions in HADS total scores (5 studies, N = 1852) versus placebo were with dupilumab 300 mg (mean difference, −3.48; 95% CrI, −5.16 to −1.05) and abrocitinib 200 mg (mean difference, −2.75; 95% CrI, −5.05 to −0.42; Table S6 and Fig. S5A).
Figure 4.
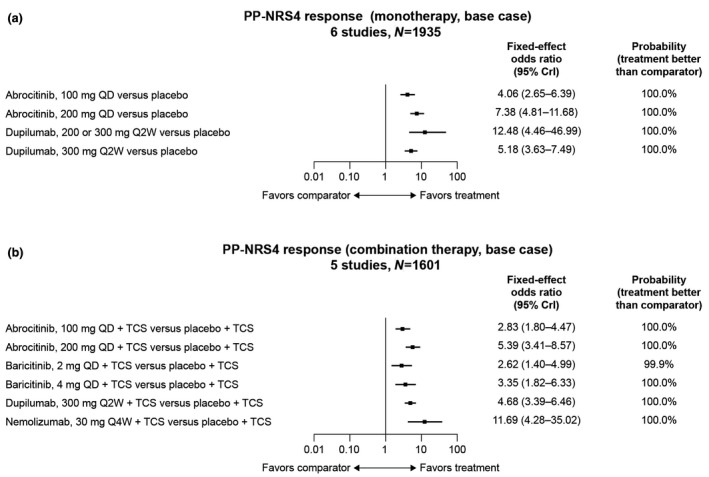
Odds ratios of achieving a PP‐NRS4 response with (a) monotherapy and (b) combination therapy compared with placebo. PP‐NRS4 response was defined as ≥4‐point improvement from baseline. CrI, credible interval; PP‐NRS, Peak Pruritus Numerical Rating Scale; QD, once a day; Q2W, every 2 weeks; Q4W, every 4 weeks; TCS, topical corticosteroids.
Safety
Probability of TEAEs (5 studies, N = 1866) was numerically higher for all active treatments than placebo, ranging from an OR of 1.02 (95% CrI, 0.49–2.15) for dupilumab 300 mg to 2.09 (95% CrI, 0.96–4.54) for abrocitinib 200 mg (Fig. 5a). Most active treatments had a numerically lower probability than placebo of having an AE leading to discontinuation (6 studies, N = 1748), including abrocitinib 100 mg (OR, 0.52; 95% CrI, 0.22–1.22), abrocitinib 200 mg (OR, 0.52; 95% CrI, 0.22–1.20) and dupilumab 300 mg (OR, 0.84; 95% CrI, 0.21–3.38; Fig. 6a). Patients treated with nemolizumab 0.5 mg/kg every 4 weeks were more likely to experience an AE leading to discontinuation (OR, 3.99; 95% CrI, 0.32–109.95; Table 3). No differences were statistically conclusive.
Figure 5.
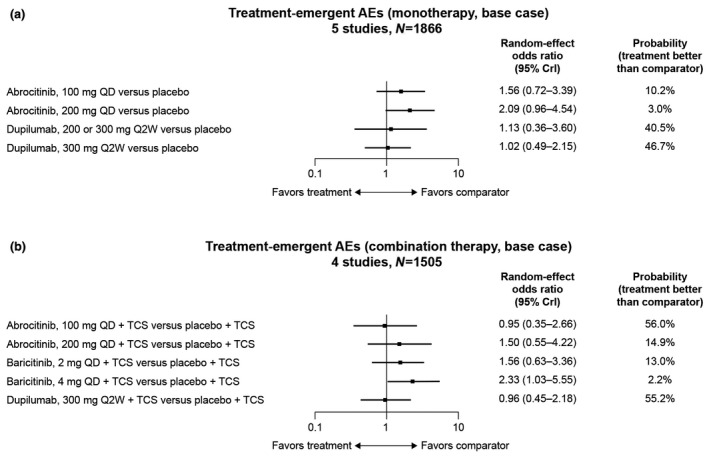
Odds ratios of experiencing a treatment‐emergent AE with (a) monotherapy and (b) combination therapy compared with placebo. AE, adverse event; CrI, credible interval; QD, once a day; Q2W, every 2 weeks; TCS, topical corticosteroids.
Figure 6.
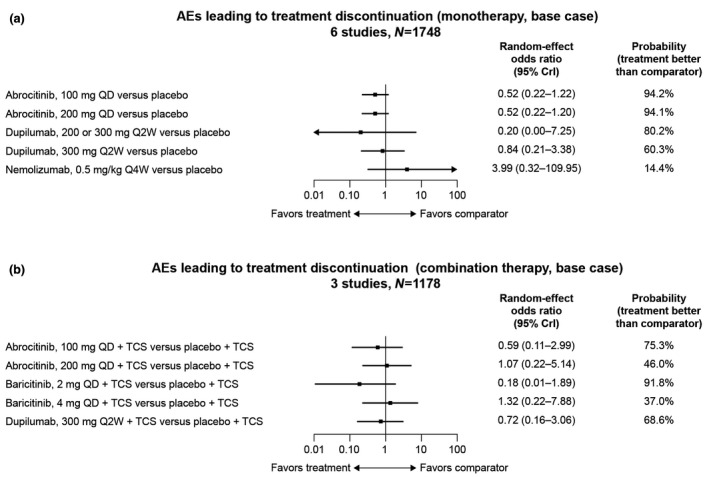
Odds ratios of experiencing an AE leading to discontinuation with (a) monotherapy and (b) combination therapy compared with placebo. AE, adverse event; CrI, credible interval; QD, once a day; Q2W, every 2 weeks; Q4W, every 4 weeks; TCS, topical corticosteroids.
Combination therapy
Clinical efficacy
For EASI response (8 studies, N = 2193), the highest numerical efficacy in EASI‐50 response was observed for abrocitinib 200 mg (87%), dupilumab 300 mg (82%), abrocitinib 100 mg (80%), nemolizumab 30 mg (78%) and lebrikizumab 125 mg (76%) (Table 2). Abrocitinib 200 mg exhibited a >99% probability of superiority over both baricitinib doses and 98.6% and 96% probability of superiority over tralokinumab 300 mg and dupilumab 300 mg, respectively. Similar trends for abrocitinib 200 mg, dupilumab 300 mg, abrocitinib 100 mg, nemolizumab 30 mg and lebrikizumab 125 mg were observed for EASI‐75 response (71%, 65%, 61%, 59% and 56%, respectively) and EASI‐90 response (49%, 42%, 38%, 36% and 34%, respectively; Table 2).
All treatments exhibited ≥93% probability of superiority to placebo in IGA response (8 studies, N = 2217; Fig. 3b). The greatest differentiation observed was for abrocitinib 200 mg (OR, 6.28; 95% CrI, 3.05–13.12; >99.9% probability of being more efficacious than placebo), dupilumab 300 mg (OR, 4.38; 95% CrI, 2.69–7.24; >99.9% probability), nemolizumab 30 mg (OR, 3.68; 95% CrI, 1.21–12.59, 98.8% probability) and abrocitinib 100 mg (OR, 3.68; 95% CrI, 1.78–7.68; 99.7% probability; Table 4). The greatest SCORAD‐50 responses (4 studies, N = 1225) compared with placebo were with abrocitinib 200 mg (OR, 4.80; 95% CrI, 3.12–7.50) and dupilumab 300 mg (OR, 4.73; 95% CrI, 3.30–6.88; Table S7 and Fig. S4B).
Table 4.
League table comparing IGA response, PP‐NRS4 response and safety outcomes among combination therapy
| Treatment | Odds ratio [95% CrI] | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Baricitinib 2 mg QD | Baricitinib 4 mg QD | Dupilumab 300 mg Q2W | Lebrikizumab 125 mg Q4W | Nemolizumab 30 mg Q4W | Tralokinumab 300 mg Q2W | Abrocitinib 100 mg QD | |
| IGA response (8 studies, N = 2217) | ||||||||
| Baricitinib 2 mg QD | 2.13 (1.03–4.80) | |||||||
| Baricitinib 4 mg QD | 2.72 (1.30–5.96) | 1.27 (0.62–2.52) | ||||||
| Dupilumab 300 mg Q2W | 4.38 (2.69–7.24) | 2.06 (0.79–4.94) | 1.61 (0.63–3.94) | |||||
| Lebrikizumab 125 mg Q4W | 2.21 (0.77–6.91) | 1.04 (0.26–4.04) | 0.82 (0.21–3.21) | 0.51 (0.16–1.74) | ||||
| Nemolizumab 30 mg Q4W | 3.68 (1.21–12.59) | 1.74 (0.44–7.03) | 1.36 (0.35–5.61) | 0.84 (0.25–3.11) | 1.67 (0.33–8.26) | |||
| Tralokinumab 300 mg Q2W | 2.58 (0.80–9.63) | 1.21 (0.29–5.47) | 0.96 (0.23–4.22) | 0.59 (0.16–2.37) | 1.17 (0.22–6.32) | 0.70 (0.13–4.00) | ||
| Abrocitinib 100 mg QD | 3.68 (1.78–7.68) | 1.72 (0.58–4.85) | 1.35 (0.47–3.82) | 0.84 (0.42–1.66) | 1.65 (0.44–6.03) | 1.01 (0.24–3.76) | 1.42 (0.32–5.79) | |
| Abrocitinib 200 mg QD | 6.28 (3.05–13.12) | 2.96 (0.98–8.18) | 2.31 (0.80–6.44) | 1.44 (0.72–2.84) | 2.82 (0.73–10.25) | 1.71 (0.41–6.39) | 2.42 (0.55–9.79) | 1.7 (0.82–3.58) |
| PP‐NRS response (5 studies, N = 1601) | ||||||||
| Baricitinib 2 mg QD | 2.62 (1.40–4.99) | |||||||
| Baricitinib 4 mg QD | 3.35 (1.82–6.33) | 1.28 (0.72–2.27) | ||||||
| Dupilumab 300 mg Q2W | 4.68 (3.39–6.46) | 1.79 (0.87–3.61) | 1.4 (0.68–2.80) | |||||
| Nemolizumab 30 mg Q4W | 11.69 (4.28–35.02) | 4.46 (1.36–15.78) | 3.49 (1.06–12.26) | 2.5 (0.88–7.87) | ||||
| Abrocitinib 100 mg QD | 2.83 (1.80–4.47) | 1.08 (0.49–2.33) | 0.84 (0.39–1.81) | 0.6 (0.40–0.90) | 0.24 (0.07–0.73) | |||
| Abrocitinib 200 mg QD | 5.39 (3.41–8.57) | 2.06 (0.94–4.50) | 1.61 (0.73–3.48) | 1.15 (0.77–1.73) | 0.46 (0.14–1.39) | 1.91 (1.24–2.95) | ||
| Treatment‐emergent AEs (4 studies, N = 1505) | ||||||||
| Baricitinib 2 mg QD | 1.56 (0.63–3.36) | |||||||
| Baricitinib 4 mg QD | 2.33 (1.03–5.55) | 1.50 (0.70–3.80) | ||||||
| Dupilumab 300 mg Q2W | 0.96 (0.45–2.18) | 0.62 (0.21–2.13) | 0.41 (0.13–1.31) | |||||
| Abrocitinib 100 mg QD | 0.95 (0.35–2.66) | 0.61 (0.18–2.45) | 0.41 (0.11–1.50) | 0.99 (0.36–2.64) | ||||
| Abrocitinib 200 mg QD | 1.5 (0.55–4.22) | 0.96 (0.28–3.88) | 0.64 (0.17–2.39) | 1.56 (0.56–4.18) | 1.58 (0.55–4.59) | |||
| AEs leading to discontinuation (3 studies, N = 1178) | ||||||||
| Baricitinib 2 mg QD | 0.18 (0.01–1.89) | |||||||
| Baricitinib 4 mg QD | 1.32 (0.22–7.88) | 7.29 (0.67–245.67) | ||||||
| Dupilumab 300 mg Q2W | 0.72 (0.16–3.06) | 3.97 (0.24–172.43) | 0.55 (0.05–5.35) | |||||
| Abrocitinib 100 mg QD | 0.59 (0.11–2.99) | 3.29 (0.18–144.89) | 0.44 (0.04–4.92) | 0.81 (0.17–4.04) | ||||
| Abrocitinib 200 mg QD | 1.07 (0.22–5.14) | 5.94 (0.35–254.93) | 0.82 (0.08–8.75) | 1.49 (0.35–6.87) | 1.83 (0.38–8.96) | |||
IGA response defined as clear (0) or almost clear (1) with ≥2‐point reduction from baseline. PP‐NRS4 response defined as ≥4‐point improvement from baseline.
Cell numbers represent the OR (95% CrI) of the row treatment meeting the response threshold (or experiencing the relevant AE) compared with the column treatment (e.g. baricitinib 2 mg QD in combination with topical therapy has 2.13 the odds of achieving IGA response compared with placebo in combination with topical therapy).
AE, adverse event; IGA, Investigator’s Global Assessment; PP‐NRS, Peak Pruritus‐Numerical Rating Scale; QD, once daily; Q2W, once every 2 weeks; Q4W; once every 4 weeks.
Patient‐reported outcomes
All treatments had ≥95% probability of superiority to placebo in achieving PP‐NRS4 response (5 studies, N = 1601; Fig. 4b), with the largest differences observed for nemolizumab 30 mg (OR, 11.69; 95% CrI, 4.28–35.02; >99.9% probability of superiority to placebo), abrocitinib 200 mg (OR, 5.39; 95% CrI, 3.41–8.57; >99.9% probability) and dupilumab 300 mg (OR, 4.68; 95% CrI, 3.39–6.46; >99.9% probability; Table 4). All treatments had ≥99.9% probability of greater symptom/QoL improvement than placebo. The greatest reductions in POEM scores (4 studies, N = 1798) were with abrocitinib 200 mg (mean difference, −8.21; 95% CrI, −13.15 to −3.06) and dupilumab 300 mg (mean difference, −7.04; 95% CrI, −10.25 to −3.81; Table S7 and Fig. S2B). The greatest reduction in DLQI scores (6 studies, N = 2026) was with abrocitinib 200 mg (mean difference, −5.52; 95% CrI, −7.35 to −3.75) and dupilumab 300 mg (mean difference, −4.62; 95% CrI, −5.84 to −3.46; Table S7 and Fig. S3B). The greatest reductions in HADS total scores (4 studies, N = 1472) were with abrocitinib 200 mg (mean difference, −3.06; 95% CrI, −7.53 to −1.47) and dupilumab 300 mg (mean difference, −2.30; 95% CrI, −5.23–0.54; Table S7 and Fig. S5B).
Safety
Numerically higher probabilities of TEAEs (4 studies, N = 1505) were observed with both baricitinib doses (2 mg: OR, 1.56; 95% CrI, 0.63–3.36; 4 mg: OR, 2.33; 95% CrI, 1.03–5.55) and abrocitinib 200 mg (OR, 1.50; 95% CrI, 0.55–4.22) compared with placebo, but dupilumab 300 mg (OR, 0.96; 95% CrI, 0.45–2.18) and abrocitinib 100 mg (OR, 0.95; 95% CrI, 0.35–2.66) had numerically lower probabilities (Fig. 5b). A higher probability of AEs leading to discontinuation (3 studies, N = 1178) was observed with baricitinib 4 mg (OR, 1.32; 95% CrI, 0.22–7.88) and abrocitinib 200 mg (OR, 1.07; 95% CrI, 0.22–5.14) compared with placebo; other therapy/doses reported lower probabilities (Fig. 6b). These differences were not statistically conclusive (Table 4).
Discussion
Because direct head‐to‐head studies among systemic therapies in moderate‐to‐severe AD are not available, NMAs are an important means of examining comparative data of various treatments simultaneously. Although the focus and methods of this study vary from previous NMAs,24, 25 the broad conclusions are similar. Results suggest that EASI response was highest for abrocitinib 200 mg and upadacitinib 30 mg; although not statistically different from one another, EASI responses were meaningfully different (probability of superiority ≥97.5%) from all other treatments including dupilumab 300 mg and both baricitinib doses. Similar findings were observed for IGA.
Results of this NMA highlight the potential of JAK1 inhibition as a potent treatment for moderate‐to‐severe AD and suggest important clinical differentiation from other treatments. Clinical data on IL‐13 inhibition are limited; however, results of this study also preliminarily suggest a trend towards a more efficacious profile for dual IL‐4 and IL‐13 inhibition by targeting IL‐4 receptor alpha rather than IL‐13 inhibition alone, although more research is needed.
The pattern of PROs was similar to that of clinical efficacy, yet the differentiation among treatments was reduced. The only meaningful separation at week 12/week 16 was between active treatments and placebo, in part because of inconsistent inclusion of PROs between trials. More research is needed to understand differences in PROs across treatments, but these results suggest that abrocitinib, upadacitinib and dupilumab consistently improved symptoms and disease‐specific QoL in patients compared with placebo.
Assessing safety outcomes was challenging in this NMA because of the variability in types of information reported. Our analyses were limited to incidence of TEAEs and AEs that led to treatment discontinuation in short‐term studies. No meaningful statistical differences were observed. Future research, including specific events of interest and trials that have longer follow‐up times, is necessary to evaluate safety profiles of these treatments.
There are several limitations to this NMA. The analyses did not include change in EASI score; however, previous analyses showed that percentage change from baseline in EASI score is sensitive to outliers. EASI response thresholds are more stable, and thus, these were the focus of this analysis. In addition, since not all outcomes were consistently reported between trials, some treatment comparisons were not possible. It is possible that some between‐trial differences could have influenced results, including abrocitinib monotherapy efficacy being assessed at 12 weeks rather than at 16 weeks for other treatments, differences in baseline characteristics in trials (e.g. HADS exclusion criteria) and differences in the use of background therapy in combination trials. Moreover, data for some treatments were available from only a single trial, thus potentially limiting the generalizability of findings. Finally, rapidity of response and long‐term efficacy were beyond the scope of this NMA because of data availability limitations.
In conclusion, results of this NMA highlight that efficacy outcomes of JAK1 inhibitors (abrocitinib and upadacitinib) were consistently higher than those of dupilumab and baricitinib in moderate‐to‐severe AD. No meaningful statistical differences in safety‐related outcomes were observed.
Supporting information
MaterialS1. Data sources and search strategy.
Table S1. Embase search strategies.
Table S2. MEDLINE search strategies.
Table S3. CENTRAL and CDSR search strategies.
Table S4. DARE and HTA database search strategies.
Table S5. Baseline demographic and clinical characteristics of studies included in the network meta‐analysis.
Table S6. League table comparing mean differences of POEM, mean differences of DLQI, SCORAD‐50 response, and mean differences of HADS among monotherapies.
Table S7. League table comparing mean differences of POEM, mean differences of DLQI, SCORAD‐50 response, and mean differences of HADS among combination therapies.
Figure S1. Proportion of patients achieving each EASI threshold as estimated from the Bayesian network meta‐analysis model at week 12.
Figure S2. Mean differences in POEM for (a) monotherapy (b) combination therapy.
Figure S3. Mean differences in DLQI for (a) monotherapy and (b) combination therapy.
Figure S4. Mean differences in SCORAD‐50 response for (a) monotherapy and (b) combination therapy.
Figure S5. Mean differences in HADS for (a) monotherapy and (b) combination therapy.
Acknowledgments
Editorial/medical writing support under the guidance of authors was provided by Marianna Johnson, PhD, and Juan Sanchez‐Cortes, PhD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–464).
Funding sources
This study was sponsored by Pfizer, Inc.
Conflict of interests
J.I. Silverberg has served as an investigator for Celgene, Eli Lilly, F. Hoffmann‐LaRoche, Menlo Therapeutics, Realm Therapeutics, Regeneron and Sanofi; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron and Sanofi; and as a speaker for Regeneron and Sanofi. J.P. Thyssen is an advisor/investigator or speaker for Pfizer Inc., AbbVie, Eli Lilly, LEO Pharma, Regeneron, and Sanofi Genzyme. K. Fahrbach and K. Mickle are employees of Evidera Inc., which received research funding from Pfizer Inc. J.C. Cappelleri, W. Romero, M.C. Cameron, D.E. Myers, C. Clibborn, and M. DiBonaventura are employees and shareholders of Pfizer Inc.
References
- 1.Boguniewicz M, Fonacier L, Guttman‐Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol 2018; 120: 10–22.e12. [DOI] [PubMed] [Google Scholar]
- 2.Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet 2020; 396: 345–360. [DOI] [PubMed] [Google Scholar]
- 3.DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc 2012; 33: 227–234. [DOI] [PubMed] [Google Scholar]
- 4.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population‐based study. J Allergy Clin Immunol 2013; 132: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 5.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health‐related quality of life and productivity in adults in the United States: An analysis using the National Health and Wellness Survey. J Am Acad Dermatol 2017; 77: 274–279.e273. [DOI] [PubMed] [Google Scholar]
- 6.Ring J, Zink A, Arents BWMet al. Atopic eczema: burden of disease and individual suffering ‐ results from a large EU study in adults. J Eur Acad Dermatol Venereol 2019; 33: 1331–1340. [DOI] [PubMed] [Google Scholar]
- 7.Nørreslet LB, Ebbehoj NE, Ellekilde Bonde JP, Thomsen SF, Agner T. The impact of atopic dermatitis on work life ‐ a systematic review. J Eur Acad Dermatol Venereol 2018; 32: 23–38. [DOI] [PubMed] [Google Scholar]
- 8.Drucker AM, Qureshi AA, Amand Cet al. Health care resource utilization and costs among adults with atopic dermatitis in the united states: a claims‐based analysis. J Allergy Clin Immunol Pract 2018; 6: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 9.Eichenfield LF, DiBonaventura M, Xenakis Jet al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb) 2020; 10: 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha S, Miao R, Wang L, Chao J, Yuce H, Wei W. Burden of atopic dermatitis in the United States: Analysis of healthcare claims data in the commercial, Medicare, and Medi‐Cal databases. Adv Ther 2017; 34: 1989–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wollenberg A, Barbarot S, Bieber Tet al. Consensus‐based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol 2018; 32: 850–878. [DOI] [PubMed] [Google Scholar]
- 12.Regeneron . Dupixent. Regeneron Pharmaceuticals Inc., Tarrytown, NY, 2018. [Google Scholar]
- 13.Sanofi‐Aventis Groupe . Dupixent. Sanofi‐Aventis Groupe, Paris, France, 2017. [Google Scholar]
- 14.Olumiant . Prescribing information. Eli Lilly, Indianapolis, IN, 2020. [Google Scholar]
- 15.Sidbury R, Davis DM, Cohen DEet al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol 2014; 71: 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gooderham M, Forman S, Bissonnette Ret al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol 2019; 155: 1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guttman‐Yassky E, Silverberg JI, Nemoto Oet al. Baricitinib in adult patients with moderate‐to‐severe atopic dermatitis: a phase 2 parallel, double‐blinded, randomized placebo‐controlled multiple‐dose study. J Am Acad Dermatol 2019; 80: 913–921.e919. [DOI] [PubMed] [Google Scholar]
- 18.Guttman‐Yassky E, Thaçi D, Pangan ALet al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16‐week results from a randomized, placebo‐controlled trial. J Allergy Clin Immunol 2020; 145: 877–884. [DOI] [PubMed] [Google Scholar]
- 19.Guttman‐Yassky E, Blauvelt A, Eichenfield LFet al. Efficacy and safety of lebrikizumab, a high‐affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol 2020; 156: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wollenberg A, Howell MD, Guttman‐Yassky Eet al. Treatment of atopic dermatitis with tralokinumab, an anti‐IL‐13 mAb. J Allergy Clin Immunol 2019; 143: 135–141. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg JI, Pinter A, Pulka Get al. Phase 2B randomized study of nemolizumab in adults with moderate‐to‐severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol 2020; 145: 173–182. [DOI] [PubMed] [Google Scholar]
- 22.Dias S, Ades A, Welton N, Jansen J, Sutton A. Network Meta‐Analysis for Decision‐Making. John Wiley & Sons Ltd., Chichester, UK, 2018. [Google Scholar]
- 23.Sutton AJ, Abrams KR. Bayesian methods in meta‐analysis and evidence synthesis. Stat Methods Med Res 2001; 10: 277–303. [DOI] [PubMed] [Google Scholar]
- 24.Drucker AM, Ellis AG, Bohdanowicz Met al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta‐analysis. JAMA Dermatol 2020; 156: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegels D, Heratizadeh A, Abraham Set al. Systemic treatments in the management of atopic dermatitis: a systematic review and meta‐analysis. Allergy 2020; 76: 1053–1076. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman DG, Gotzsche PCet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutton B, Salanti G, Caldwell DMet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med 2015; 162: 777–784. [DOI] [PubMed] [Google Scholar]
- 28.Shea BJ, Reeves BC, Wells Get al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: Many options, no standards. J Am Acad Dermatol 2016; 74: 288–294. [DOI] [PubMed] [Google Scholar]
- 30.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18. [DOI] [PubMed] [Google Scholar]
- 31.Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997; 195: 10–19. [DOI] [PubMed] [Google Scholar]
- 32.Phan NQ, Blome C, Fritz Fet al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol 2012; 92: 502–507. [DOI] [PubMed] [Google Scholar]
- 33.Charman CR, Venn AJ, Williams HC. The patient‐oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Arch Dermatol 2004; 140: 1513–1519. [DOI] [PubMed] [Google Scholar]
- 34.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 36.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Med Decis Making 2013; 33: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity–subgroups, meta‐regression, bias, and bias‐adjustment. Med Decis Making 2013; 33: 618–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverberg JI, Simpson EL, Thyssen JPet al. Efficacy and safety of abrocitinib in patients with moderate‐to‐severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020; 156: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson EL, Sinclair R, Forman Set al. Efficacy and safety of abrocitinib in adults and adolescents with moderate‐to‐severe atopic dermatitis (JADE MONO‐1): a multicentre, double‐blind, randomised, placebo‐controlled, phase 3 trial. Lancet 2020; 396: 255–266. [DOI] [PubMed] [Google Scholar]
- 40.Reich K. Efficacy and Safety of Baricitinib in Combination with Topical Corticosteroids in Moderate to Severe Atopic Dermatitis: results of a Phase 3 Randomized, Double‐Blind, Placebo‐Controlled 16‐week Trial (BREEZE‐AD7). European Academy of Dermatology and Venereology, Madrid, Spain, 2019. [Google Scholar]
- 41.Simpson EL, Lacour JP, Spelman Let al. Baricitinib in patients with moderate‐to‐severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020; 183: 242–255. [DOI] [PubMed] [Google Scholar]
- 42.Blauvelt A, de Bruin‐Weller M, Gooderham Met al. Long‐term management of moderate‐to‐severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1‐year, randomised, double‐blinded, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 43.de Bruin‐Weller M, Thaçi D, Smith CHet al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 44.Simpson EL, Paller AS, Siegfried ECet al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol 2020; 156: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thaçi D, L. Simpson E, Deleuran Met al. Efficacy and safety of dupilumab monotherapy in adults with moderate‐to‐severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019; 94: 266–275. [DOI] [PubMed] [Google Scholar]
- 46.Thaci D, Simpson EL, Beck LAet al. Efficacy and safety of dupilumab in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo‐controlled, dose‐ranging phase 2b trial. Lancet 2016; 387: 40–52. [DOI] [PubMed] [Google Scholar]
- 47.Simpson EL, Flohr C, Eichenfield LFet al. Efficacy and safety of lebrikizumab (an anti‐IL‐13 monoclonal antibody) in adults with moderate‐to‐severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo‐controlled phase II trial (TREBLE). J Am Acad Dermatol 2018; 78: 863–871.e811. [DOI] [PubMed] [Google Scholar]
- 48.Ruzicka T, Hanifin JM, Furue Met al. Anti‐interleukin‐31 receptor a antibody for atopic dermatitis. N Engl J Med 2017; 376: 826–835. [DOI] [PubMed] [Google Scholar]
- 49.Reich K, Guttman‐Yassky E, Beck LA, Hu X, Pangan AL, Teixeira HD. Early Response to Upadacitinib in Moderate‐to‐Severe Atopic Dermatitis: Results from a Phase 2B Randomized, Placebo‐Controlled trial. European Academy of Allergy and Clinical Immunology, Munich, Germany, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MaterialS1. Data sources and search strategy.
Table S1. Embase search strategies.
Table S2. MEDLINE search strategies.
Table S3. CENTRAL and CDSR search strategies.
Table S4. DARE and HTA database search strategies.
Table S5. Baseline demographic and clinical characteristics of studies included in the network meta‐analysis.
Table S6. League table comparing mean differences of POEM, mean differences of DLQI, SCORAD‐50 response, and mean differences of HADS among monotherapies.
Table S7. League table comparing mean differences of POEM, mean differences of DLQI, SCORAD‐50 response, and mean differences of HADS among combination therapies.
Figure S1. Proportion of patients achieving each EASI threshold as estimated from the Bayesian network meta‐analysis model at week 12.
Figure S2. Mean differences in POEM for (a) monotherapy (b) combination therapy.
Figure S3. Mean differences in DLQI for (a) monotherapy and (b) combination therapy.
Figure S4. Mean differences in SCORAD‐50 response for (a) monotherapy and (b) combination therapy.
Figure S5. Mean differences in HADS for (a) monotherapy and (b) combination therapy.


