Abstract
Corals are experiencing unprecedented decline from climate change‐induced mass bleaching events. Dispersal not only contributes to coral reef persistence through demographic rescue but can also hinder or facilitate evolutionary adaptation. Locations of reefs that are likely to survive future warming therefore remain largely unknown, particularly within the context of both ecological and evolutionary processes across complex seascapes that differ in temperature range, strength of connectivity, network size, and other characteristics. Here, we used eco‐evolutionary simulations to examine coral adaptation to warming across reef networks in the Caribbean, the Southwest Pacific, and the Coral Triangle. We assessed the factors associated with coral persistence in multiple reef systems to understand which results are general and which are sensitive to particular geographic contexts. We found that evolution can be critical in preventing extinction and facilitating the long‐term recovery of coral communities in all regions. Furthermore, the strength of immigration to a reef (destination strength) and current sea surface temperature robustly predicted reef persistence across all reef networks and across temperature projections. However, we found higher initial coral cover, slower recovery, and more evolutionary lag in the Coral Triangle, which has a greater number of reefs and more larval settlement than the other regions. We also found the lowest projected future coral cover in the Caribbean. These findings suggest that coral reef persistence depends on ecology, evolution, and habitat network characteristics, and that, under an emissions stabilization scenario (RCP 4.5), recovery may be possible over multiple centuries.
Keywords: Caribbean, climate change, coral, Coral Triangle, eco‐evolutionary dynamics, metacommunity, modeling, Southwest Pacific
We used an eco‐evolutionary metapopulation model to simulate coral adaptation to warming across reef networks in the (a) Caribbean, (b) the Southwest Pacific, and (c) the Coral Triangle. We found that evolution is critical in preventing extinction and facilitating recovery in all regions. Furthermore, we found that the strength of immigration to a reef and current sea surface temperature robustly predicted reef persistence.
![]()
1. INTRODUCTION
Rapidly increasing temperatures are threatening coral populations around the world through mass bleaching events that have increased in frequency and severity in recent decades (Hughes et al., 2018). Although projections of coral persistence into the future are often dire (Hoegh‐Guldberg et al., 2017), recent work highlights potential evolutionary mechanisms that may facilitate coral adaptation to warming waters (Bay et al., 2017; Kleypas et al., 2016). This is particularly relevant in the context of networks or metapopulations because each reef's adaptive capacity is constrained by the balance between selection and migration (Lenormand, 2002). Subpopulations experience selection to adapt to their local environment, yet can also receive immigrants adapted to different environments. While there is an increasing recognition for the contribution of evolution to coral persistence under future conditions (Logan et al., 2014; Matz et al., 2018, 2020; Walsworth et al., 2019), there is less understanding of the interactions between evolutionary potential and reef characteristics on coral survival within regional‐scale reef networks.
From a demographic perspective, larval dispersal links coral reefs within a network. Connectivity matrices describe the probabilities of viable larvae reaching one reef from another through dispersal and are typically generated from ocean circulation models (Kool et al., 2011; Treml et al., 2008; Watson et al., 2010) or population genetics data (Davies et al., 2015; Galindo et al., 2010; Matz et al., 2018). An important metric of connectivity is destination strength, which is the sum of incoming connection probabilities into a particular patch and is associated with a greater probability of reef survival (McManus et al., 2020). While modeling studies have explored the ecological importance of particular sites within a metapopulation (Kininmonth et al., 2019; Watson et al., 2011), few papers examine the combined ecological and evolutionary consequences of connectivity that are likely to be important across metapopulations (but see Matz et al., 2020).
In addition, the relative importance of connectivity to coral persistence as compared to local factors such as baseline temperature and warming remains unclear. For example, bleaching records suggest that reefs that have experienced warm temperatures in the past are less likely to bleach in the future (Guest et al., 2012). Because temperatures are rising, coral populations in cooler conditions will likely benefit from receiving larvae that are pre‐adapted to warmer environments while relatively warm reefs are susceptible to the arrival of maladapted larvae (Norberg et al., 2012). In addition, temperature changes are likely to vary from one location to another (e.g., Ban et al., 2012). Generally, populations that experience a faster rate of environmental change are less likely to adapt (Lindsey et al., 2013), suggesting variation in extinction susceptibilities across reef networks. Due to evidence of thermal adaptation and heritability of heat tolerance in corals (Dixon et al., 2015; Dziedzic et al., 2019; Kirk et al., 2018), metrics that quantify the relative temperature of a patch and overall temperature change may be consequential determinants of individual reef persistence. In fact, in a recent modeling study of corals in the Indo‐West Pacific, both the proportion of recruits immigrating from warmer locations and the present‐day temperature were found to be useful factors in determining corals’ projected adaptive response (Matz et al., 2020). Therefore, from an evolutionary perspective, larval dispersal facilitates the exchange of traits across a network.
Climate change is impacting reefs across the world, but the rates of temperature change (McClanahan et al., 2019) and the structure of dispersal networks (Wood et al., 2014) differ substantially across regions. While most modeling work addressing coral adaptation has focused on the Indo‐West Pacific region (Kleypas et al., 2016; Matz et al., 2018, 2020), corals exist around the world, including throughout the Pacific and in the Caribbean (Veron, 1995). Comparisons among reef systems are important for understanding which results are general and which are sensitive to particular geographic contexts. Here, we implemented a dynamic eco‐evolutionary metacommunity model for years 1870–2300 on three spatially realistic reef networks: the Caribbean, Southwest Pacific (SWP), and Coral Triangle (CT). To simulate the response of a coral community to climate change, we modeled the dynamics of two competing coral types with contrasting life‐history strategies: a fast‐growing, temperature‐intolerant species (“fast coral”) and a slow‐growing, temperature‐tolerant species (“slow coral”; Baskett et al., 2014; Darling et al., 2012; Walsworth et al., 2019). In addition to important regional differences, we find that across all three regions, reefs with higher destination strength (larger numbers of immigrating larvae) and lower relative ocean temperatures were most likely to adapt successfully to future warming and that these metrics outperformed other potential predictors of reef persistence.
2. MATERIALS AND METHODS
2.1. General overview
We simulated the cover of two coral types through time during a historical period (1870–2018) and two future sea surface temperature (SST) projections (2018–2300) that followed either RCP 4.5, an emissions reduction and climate stabilization scenario, or RCP 8.5, a scenario without emissions reductions and with continuous and greater warming (Pachauri et al., 2015). Within each region, coral subpopulations exchanged larvae based on previously published biophysical model outputs (Schill et al., 2015; Thompson et al., 2018; Treml et al., 2008) and evolved in response to changing temperatures. To explore the role of ecological versus evolutionary dynamics on regional and local coral populations, we simulated metapopulation dynamics with different levels of standing genetic variation, which sets evolutionary potential (McManus et al., 2021; Norberg et al., 2012; Walsworth et al., 2019). Finally, we constructed general linear models to interpret the influence of patch‐level metrics on the minimum coral cover experienced on a reef. Doing so facilitated the identification of temperature and connectivity factors that constrain reef persistence under changing environmental conditions.
2.2. Eco‐evolutionary model
We applied an eco‐evolutionary model (McManus et al., 2021) forced with temperature projections and larval connectivity patterns in the Caribbean, SWP, and CT coral reef regions. Because reefs are typically considered as distinct habitat patches, we incorporated a novel extension to a continuous‐space metapopulation dynamics framework to allow for immigration during the larval phase according to a connectivity matrix D that quantifies dispersal connections in each region (described below). The connectivity matrix was based on output from biophysical simulations of coral larval dispersal. We simulated a coral reef metacommunity with the two competing coral types, “fast coral” and “slow coral” (Darling et al., 2012). Furthermore, growth and mortality rates of each species were affected by the difference between the experienced ocean temperature and an evolving trait called the optimal growth temperature.
On each reef patch a, change in coral cover and mean optimal growth temperature were given by
| (1) |
and
| (2) |
where Ni , a was the proportion of cover of coral species i at site a and zi , a was the optimum growth temperature (also referred to here as the “mean trait value”). The change in coral cover (Equation 1) for each species was affected by the local population's growth rate, gi , a, the proportion of free space on the patch, Fa , the larval input rate, li , a, and the additive genetic variance, V. The additive genetic variance is the heritable component of phenotypic variance and can be calculated as the product of narrow‐sense heritability and the total phenotypic variance (Falconer, 1996). While genetic variance can decrease due to strong selection (Klausmeier et al., 2020), we assume here that those effects are relatively small or counteracted by mutations and other processes that we did not explicitly model. As such, V can be considered roughly constant (Lande, 1976). In general, higher V enables rapid evolution but also increases genetic load, which is the reduction in the fitness of a population due to phenotypic variation around the mean trait value (Kirkpatrick & Barton, 1997). Thus, genetic load was greater at higher V and the corresponding term was either negative or zero due to the shape of the fitness curve. The change in optimum growth temperature (Equation 2) was also a function of the mean population‐weighted trait value of immigrants (Hanski et al., 2011), yi , a, and of qi , which reduced the effect of selection at very low coral cover (Norberg et al., 2012). The resident trait was subtracted from the mean trait of the immigrants and scaled by the fraction of cover represented by newly settled larvae, li , a/Ni , a, and by free space. Therefore, incoming larvae exerted a stronger effect on the average trait value if they represented a large fraction relative to the current cover of the species and if there was free space for settlement. Finally, stabilizing selection, , in Equation (2) acted to match the optimum growth temperature to the local temperature and this evolutionary potential was stronger with increasing V. Overall, this term was positive or negative depending on whether local temperature exceeded (positive) or was below (negative) the mean trait value, which led to an increase or decrease in the mean trait, respectively. At very low cover where we considered the population as functionally extinct (N min = 10−6), qi reduced the effect of selection and enhanced the numerical stability of our model (Equation 3).
| (3) |
In this framework, the local population growth rate or fitness (Equation 4) was determined by the intrinsic growth rate, ri , a, mortality rate, mi , a, and competitive interactions encoded in the species interaction matrix α, where ⍺ ij was the competitive effect of species j on species i. In our case, competitive interactions between the fast and slow coral were symmetric such that each species exerted the same effect on the other and intraspecific competition was stronger than interspecific competition (Table S1).
| (4) |
Intrinsic growth (Equation 5) and mortality (Equation 6) were Gaussian and exponential functions, respectively, of the local temperature Ta , the local average trait value, zi , a, the width of thermal tolerance, wi , and a growth scaling factor, r 0, i. In our formulation, 2/wi 2 is a measure of the strength of selection (Lande, 1976). In other words, selection strength is inversely proportional to the square of the thermal tolerance width and is greater in the fast coral than the slow coral. Following Walsworth et al. (2019) and the skewed shape of many thermal performance curves (Deutsch et al., 2008), we imposed additional mortality when the current local temperature exceeded the optimum growth temperature, Ta > zi , a (Equation 6).
| (5) |
| (6) |
In addition to population dynamics, change in coral cover through time was affected by the dispersal of coral larvae across the network. Our model differed from previous frameworks because we incorporated spatially explicit dispersal among patches (McManus et al., 2021) instead of a diffusion approximation (Norberg et al., 2012; Walsworth et al., 2019). Overall, there was higher settlement when there was more free space (i.e., Fa >> 0, see Equation 1) and a patch without free space (Fa = 0) had a settlement rate of zero. Free space was the portion of the patch not covered by coral (Equation 7). We note that because we tracked fractional cover, free space can also be interpreted as the total habitable space for both fast and slow corals (maximum free space = carrying capacity = 1).
| (7) |
Larval input (li , a) was calculated from the effective fecundity rate β and from the connectivity matrix D in which element Dab was the probability of reaching patch a from patch b. Here, the effective fecundity rate can be interpreted as the amount of new coral produced per unit time and per unit of existing coral (coral cover is dimensionless since we are tracking fractional cover). We also accounted for differences in area (Aa ) among patches such that a small fraction of the reproductive output from a large patch had a disproportionately large effect on a small receiving patch (assuming that there was free space in the latter):
| (8) |
In Equation (2), the change in mean optimal growth temperature was governed in part by gene flow (first term). The mean incoming trait value for each species (yi , a) was calculated as:
| (9) |
A summary of parameter definitions and values used for simulations is presented in Table S1.
2.3. Potential connectivity
Each region contained many spatially discrete reef patches (423 in Caribbean; 583 in SWP; 2083 in CT), as defined by three unique larval exchange connectivity matrices obtained from previously published biophysical simulations (Caribbean, Schill et al., 2015; Coral Triangle, Thompson et al., 2018; Southwest Pacific, Treml et al., 2008). All connectivity matrices were generated based on simulated larval dispersal for a coral‐like species. However, there were differences in the biological assumptions that underlay the creation of each matrix, including a longer pelagic larval duration in the SWP and the absence of larval mortality in the CT (see Table S2 for more details). Although beyond the scope of this study, differences in these key assumptions may have affected the final matrix output. For example, a longer pelagic duration and the lack of larval mortality will tend to increase the number of rare long‐distance dispersal events.
2.4. Sea surface temperatures
To simulate historical and future ocean warming, spatially explicit SST trajectories were obtained by applying the delta method (Fowler et al., 2007; Hay et al., 2000; Ramirez‐Villegas & Jarvis, 2010) to statistically downscale coarser (1 × 1 degree latitude and longitude) reconstructions from HadISST1 SST for years 1870–2018 (Rayner, 2003) and projections from GISS E2 H for years 2018–2300 (Schmidt et al., 2014) with a climatology created from the higher resolution (0.25 × 0.25 degree) historical NASA OISST V2 from 1982 to 2010 (Reynolds et al., 2007). We created SST trajectories for each region under RCP 4.5, an emissions reduction and climate stabilization scenario and RCP 8.5, a scenario without emissions reductions and with continuous and greater warming. Overall, the SST trajectories began in 1870 and ran to 2300, where the reconstruction and projection periods were 1870–2018 and 2018–2300, respectively. Each reef patch experienced a unique thermal environment based on the changing temperature at their location within a grid cell in these trajectories (Figure S1).
2.5. Simulations
Parameters (Table S1) were chosen to allow coexistence of the fast and slow coral species (Tekwa et al., 2020), which corresponds to empirical observations across the globe (Darling et al., 2012). To facilitate comparison within and among the three regions, all reefs had equivalent parameter values except for area, potential connectivity, and SST time series. To impose a trade‐off between the two corals, we set a relatively high growth rate and a narrow thermal tolerance for the fast coral while the slow coral had a lower growth rate and a wider thermal tolerance (Baskett et al., 2014; Darling et al., 2012). At the beginning of the hindcast run (1870), reefs were initialized such that each coral species started with a cover of 0.25 at every patch (Figure S2). To examine the effect of evolution on coral persistence, we tested three different levels of additive genetic variance to approximate zero (V = 0), low (V = 0.01) and high (V = 0.1) evolutionary potential. The analyses focused on simulations with an effective fecundity (β) of 0.5, although we also calculated trajectories for β = 0 and β = 0.05 as a sensitivity test (see Supporting Information; Álvarez‐Noriega et al., 2016).
2.6. Model summaries
We sought to understand whether relatively simple connectivity and temperature metrics could help identify patches that maintained high versus low coral cover. For each region, patch‐level explanatory variables included the change in SST over the 2018–2300 projection period (ΔSST), average SST from 2008 to 2018 (iSST), the probability of self‐connection relative to outgoing connections (local retention, LR), the probability of self‐connection relative to incoming connections (self‐recruitment, SR), the sum of all incoming connections (destination strength, DS), the difference in mean SST (2008–2018) between the source patches and destination patch of incoming larvae (initial temperature mismatch, ITM), the proportion of incoming connections from patches that are at least 0.5℃ warmer (pr05), and patch area (see Supporting Information for equations). We present a summary of hypothesized ecological and evolutionary effects of each metric in Table 1.
TABLE 1.
Description of hypothesized ecological and evolutionary effects of site characteristics on coral cover
| Covariate | Hypothesized ecological effects | Hypothesized evolutionary effects |
|---|---|---|
| Delta SST (ΔSST) | (−) Rising temperatures are associated with bleaching events and coral mortalitya (✓) | (−) Populations are less likely to adapt under faster rates of changeb (✓) |
| Initial SST (iSST) | N/A | (−) Cooler reefs receive beneficial warm‐adapted larvae while warmer reefs receive detrimental cold‐adapted larvaec (✓) |
| Local retention (LR) | (+) Higher LR increases population persistenced (✗) | N/A |
| Self‐recruitment (SR) | N/A | (+) High SR (i.e., low immigration) reduces the influence of gene flow and facilitates local adaptatione (✗) |
| Destination strength (DS) | (+) High levels of demographic input facilitate rescuef (✓) | (±) High DS facilitates beneficial gene flow if incoming larvae are pre‐adapted to the environmental optimum, and detrimental otherwise (✓) |
| Initial temperature mismatch (ITM) | N/A | (+) High ITM signifies the input of warmer‐adapted larvae that can facilitate adaptationc (✗) |
| Proportion of DS from locations that are at least 0.5℃ warmer (pr05) | N/A | (+) High pr05 signifies the input of warmer‐adapted larvae that can facilitate adaptation (like ITM)g (✓) |
| Area (m2) | (−) Smaller populations disproportionately benefit from immigration (Equation 8) (✓) | (±) Larger populations are less susceptible to gene swamping and are more likely to locally adapt but also benefit less from potentially favorable gene flowe (Equation 9) (✓) |
(+) and (−) indicate a positive or negative effect on coral cover, respectively. (✓) and (✗) indicate that the hypothesis was supported or unsupported, respectively.
Hoegh‐Guldberg et al. (2017).
Lindsey et al. (2013).
Norberg et al. (2012).
Botsford et al. (2009).
Lenormand (2002).
Brown and Kodric‐Brown (1977).
Matz et al. (2020).
To quantify the relative association of patch‐level characteristics with coral cover, we fit generalized linear models with binomial errors to the minimum fractional coral cover of each patch in a region between 2018 and 2300 (Burnham et al., 2002; Zuur et al., 2009). We applied a log transformation to LR, SR, DS, pr05, and area (log(x + 0.001)), and a log‐modulus transformation for ITM (sign(x)*log(|x| + 1)) to minimize skew. We centered and scaled all variables (mean = 0, SD = 1) after transformations. In addition to exploring the full statistical model, the model average, and the model with the lowest Akaike information criterion (AIC; Burnham & Anderson, 2004), we created statistical models for three hypotheses: (1) Connectivity‐only with LR, SR, and DS; (2) Temperature‐only with ΔSST and iSST; and (3) Warm‐adapted gene flow with pr05 and DS. Inferences regarding the influence of each of these variables on coral cover were made based on the coefficients of each explanatory variable in the full statistical model. To help avoid issues with applying statistical methods to simulated data (White et al., 2014), we limited the interpretation of these results to a qualitative comparison of effect size among our metrics and did not conduct statistical significance testing.
3. RESULTS
3.1. Future coral cover
Across all regions, we found that evolution was critical in maintaining coral cover through warming. Corals persisted under both mild (RCP 4.5) and severe (RCP 8.5) warming scenarios with high genetic variance (V = 0.1; Figure 1a–c; Table S3). With intermediate genetic variance (V = 0.01), corals managed to persist under mild warming (RCP 4.5) in the Caribbean and CT but not under strong warming (RCP 8.5), while in the SWP, corals maintained a small amount of cover even under severe warming (RCP 8.5; Figure S3). For moderate genetic variance (V = 0.01), warming was projected to cause coral declines until 2050, followed by slow recovery that did not reach historical levels by 2300 (Figure 1). In general, higher genetic variance (V) led to higher minimum and final coral cover across each region (Figure 1). The Coral Triangle retained the highest coral cover while the Caribbean had the lowest cover across all V.
FIGURE 1.

Mean total coral cover (sum of both functional types) (a–c) and mean trait values with sea surface temperatures (d–f) averaged across all reefs in the Caribbean, Southwest Pacific, and Coral Triangle under RCP 4.5 (solid lines) and RCP 8.5 (dashed lines). Trajectories are shown at three levels of additive genetic variance: V = 0 (violet), V = 0.01 (blue‐green), and V = 0.1 (yellow‐green). Error bounds refer to the 25th and 75th percentiles among reefs. Mean SSTs across each network are shown in gray (d–f)
Across regions, substantial spatial variation in coral cover was apparent under mild warming (Figure 2; Figures S4 and S5). In the Caribbean, the sites with the highest coral cover were found near the Bahamas and south of Cuba. In the Southwest Pacific, the high cover reefs were near American Samoa and the area between Papua New Guinea and the Solomon Islands. In the Coral Triangle, the highest cover reefs were near the Paracel Islands in the South China Sea and the Greater Sunda Islands in Indonesia. However, spatial patterns were different by the end of the more severe warming scenario (RCP 8.5) in the Caribbean and the Coral Triangle (Figure S4). In the Caribbean, only Bermuda and two sites off the coast of South America had surviving corals, while in the Coral Triangle, only sites near Hainan, China, and in the southern Great Barrier Reef maintained coral cover by the end of the projection (Figure S4 b,d,f). Sensitivity testing without larval fecundity or with lower fecundity (β = 0 or β = 0.05, respectively) revealed that higher fecundity (in effect, increased larval production) increased the variation of minimum coral cover values across each network (Figure S6).
FIGURE 2.
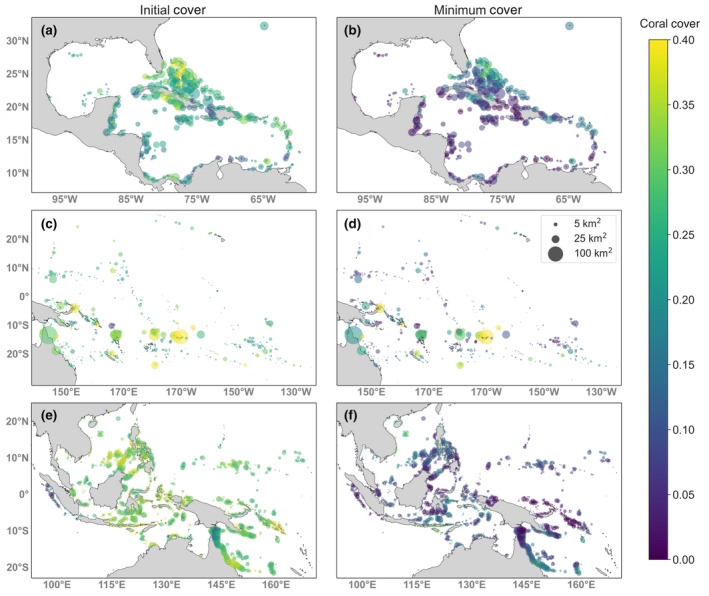
Fractional coral cover in 2018 (a, c, e) and minimum cover under RCP 4.5 (b, d, f) for the Caribbean (a, b), Southwest Pacific (c, d), and Coral Triangle (e, f). Reef sizes are proportional to areas. Results are shown for V = 0.01 and β = 0.5
We also found a strong temporal correlation in coral cover across all regions such that reefs with higher cover in 2018 tended to also have a higher minimum cover and a higher final cover. We found that initial and final coral cover was highly correlated with coefficients of 0.89, 0.95, and 0.85 for the Caribbean, SWP, and CT, respectively (Figure S7a). Initial and minimum cover (Figure S7b) and final and minimum cover (Figure S7c) were similarly highly correlated.
The simulations predicted time‐dependent shifts in coral composition in response to warming. Slow‐growing, temperature‐tolerant corals were relatively more abundant during the initial projection period and when coral cover was lowest across regions (Figure 3a–c). In contrast, fast‐growing corals with narrower thermal tolerance recovered faster and were more abundant later in the simulation period (Figure 3a–c; Table S3). Slow‐growing, stress‐tolerant corals had more variable cover among reefs for all regions throughout the time series, even when they were less abundant than fast‐growing corals (Table S3). Fast‐growing corals were able to adapt to local temperatures by 2300 across regions, whereas slow‐growing corals continued to have optimal temperatures that were lower than experienced temperatures (Figure 3d–f). In other words, the mismatch between optimal trait and temperature was better tolerated initially by slow‐growing corals, but higher evolutionary rates in fast‐growing corals were ultimately advantageous because they were able to match their optimal trait values to the local environment.
FIGURE 3.

Projected fractional coral cover through time under RCP 4.5 by species (fast = orange; slow = cyan) for the (a) Caribbean, (b) Southwest Pacific, and (c) Coral Triangle. (d–f) Mean sea surface temperature (gray) and trait value for each species. Additive genetic variance V = 0.01. Error bounds show the 25th and 75th percentiles among reefs
3.2. Predictors of patch‐scale coral cover
The strongest predictor of minimum coral cover across regions and levels of additive genetic variance was an individual reef's destination strength (DS; Figure 4a). This network metric, which corresponds to the sum of a reef's incoming dispersal links, was positively correlated with minimum cover across all regions, levels of V, and warming scenarios (Figure 4; Figures S8 and S9). Minimum coral cover was also negatively associated with the initial sea surface temperature (iSST) across all regions, levels of V, and warming scenarios (Figures S8 and S9). This latter pattern was in agreement with our evolutionary hypothesis that initially hot reefs generally received maladapted (colder adapted) larvae from connected reefs and were less able to adapt to warming temperatures (Table 1).
FIGURE 4.
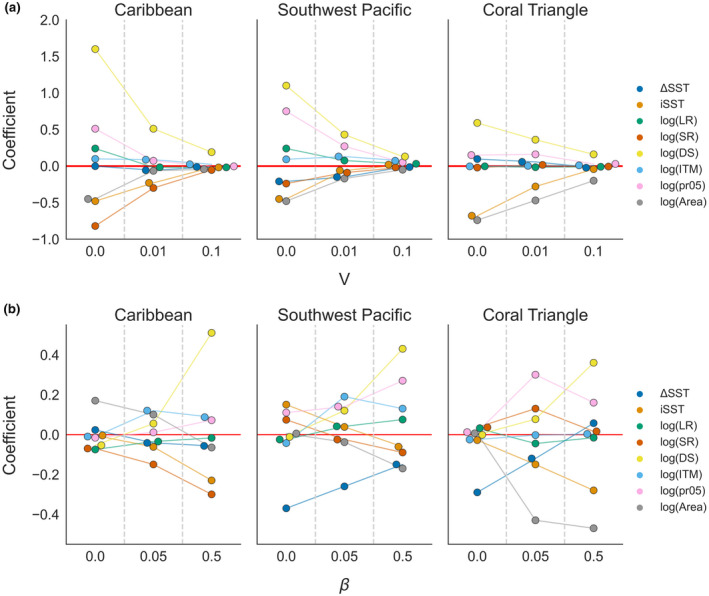
Standardized coefficient (effect size) of each covariate on minimum coral cover (a) across levels of genetic variance or (b) levels of fecundity in the Caribbean, Southwest Pacific, and Coral Triangle. ΔSST is the change in sea surface temperature over the projection period, iSST is the initial temperature at the start of the projection, LR is the local retention, SR is the self‐recruitment, DS is the destination strength, ITM is the initial temperature mismatch, and pr05 is the proportion of incoming links from sites that are at least 0.5℃ warmer (based on iSST). Results are for RCP 4.5 and β = 0.5 in part (a) and for V = 0.01 for part (b)
Certain predictors had a strong association with minimum cover in one region, but not in the others. For example, pr05 was strongly positively correlated with minimum coral cover in the SWP, moderately correlated in the Caribbean only with no additive genetic variance, and only weakly positively associated in the CT (Figure 4). In the Caribbean, self‐recruitment (SR) was strongly negatively correlated with minimum cover but was only weakly negatively correlated in the SWP and had a near‐zero effect size in the CT (Figure 4). Across all metrics, the effect sizes for all covariates decreased as V increased (Figure 4).
Some predictors differed in their frequency distributions across regions (Figure S10). For example, the Caribbean had higher local retention of larvae than the CT and SWP. The SWP had a wider range of ΔSST and iSST than the other two regions, and subsequently, a higher proportion of cold sites. The CT had substantially higher DS, and a greater number of rare, extremely small sites.
As a sensitivity test, we also performed simulations with no effective fecundity (β=0) which meant that there was no dispersal among reefs. In this scenario, only within‐reef ecological and evolutionary processes were important. Unsurprisingly, connectivity metrics had weak and inconsistent associations with minimum cover across regions without dispersal (Figure 4b). In addition, the negative iSST effect disappeared at low fecundity, supporting mechanistic hypotheses that the iSST effect was mediated by dispersal of larvae among sites, rather than being a result of within‐site dynamics (Table 1).
By comparing alternate statistical models based on three hypotheses, (1) Connectivity‐only with LR, SR, and DS; (2) Temperature‐only with ΔSST and iSST; and (3) Warm‐adapted gene flow with pr05 and DS), we found that the Warm‐adapted gene flow model performed better than the Temperature‐only and Connectivity‐only models across all regions based on △AIC and R 2 values (Tables S4–S6).
4. DISCUSSION
Reefs around the world are projected to experience frequent severe bleaching and mortality events during this century (Donner, 2009; Frieler et al., 2013; Logan et al., 2014; van Hooidonk et al., 2013). In this study, we implemented climate change projections across the Caribbean, Southwest Pacific, and Coral Triangle, and we explored a suite of temperature‐ and connectivity‐based reef patch characteristics to assess their relative impact on coral adaptive potential. Despite differences in dispersal and SST patterns among the regions, we find that several important general conclusions emerged. First, our model provides further evidence of corals’ capacity to evolutionarily adapt, but that such recovery would not be possible without evolution (Bay et al., 2017; Matz et al., 2020; Walsworth et al., 2019). Coral populations in our model collapsed with no additive genetic variance (V = 0), and we found that the declines were less severe under more rapid evolution. Second, differences in minimum coral cover were associated with both connectivity and temperature, including the proportion of incoming larvae and relative SST, which can help inform conservation strategies designed to maintain coral cover. Lastly, we explicitly modeled the dynamics of two coral life‐history strategies and found that an initial loss of fast‐growing corals over the coming century may be offset by their faster recovery if thermal conditions stabilize.
We examined whether temperature and larval connectivity characteristics were useful for explaining which reefs would persist with high cover and which would not. Analyses of past bleaching events have found that temperature‐based metrics, including mean SST, temporal variability of SST, and degree heating weeks (a measure of thermal stress) are strong predictors of past coral bleaching and mortality at specific sites (Hughes et al., 2018; McClanahan & Maina, 2003; Safaie et al., 2018; Sully et al., 2019; Welle et al., 2017). Studies that link larval connectivity patterns to marine population persistence typically focus on the network scale and calculate centrality metrics to identify sites which disproportionately contribute to metapopulation growth (Kininmonth et al., 2019; Treml & Halpin, 2012; Watson et al., 2011). Our work focused on integrating these two types of metrics with additional site‐specific connectivity measures such as destination strength (Thompson et al., 2018), self‐recruitment, and local retention (Burgess et al., 2014; Hastings & Botsford, 2006), as several previous studies have asserted that larval settlement and recruitment rates directly limit the local population size (Caley et al., 1996; Hughes, 1990; Menge et al., 2003). This comprehensive assessment not only highlighted the independent contributions of temperature and connectivity to coral persistence and adaptation but also the importance of their interactions for future reef persistence when there is potential for an evolutionary response.
While evolution has a broadly positive effect on coral persistence at all sites and across all regions, variation exists among sites’ resilience to ocean warming due to differences in temperature and connectivity. We found a consistently positive effect of a reef's destination strength and a consistently negative effect of initial SST on minimum cover during warming, regardless of region or level of genetic variance. The positive effect of destination strength likely operated through the ecological effects of larval immigration, implying that the demographic benefits of connectivity outweighed the potential negative evolutionary effects of gene swamping (Lenormand, 2002). Furthermore, the negative effect of initial SST was likely due to warmer reefs receiving cold‐adapted larvae: as the network warmed, cold‐adapted larvae arriving in relatively warm reefs counteracted evolutionary adaptation (McManus et al., 2021; Norberg et al., 2012).
Recently, Matz et al. (2020) found that pre‐warming SST and the fraction of recruits immigrating from sites that were at least 0.5℃ warmer (pr05) were strong predictors of reef persistence in the Indo‐West Pacific. Larvae that were pre‐adapted to warmer conditions strongly benefited cooler reefs, consistent with genetic theory (Norberg et al., 2012). Our results support some of Matz et al.’s findings: we found that initial SST and pr05 were relatively effective predictors in our model, although neither was as effective as destination strength. There are a few potential reasons for this discrepancy. Matz et al. (2020) used an individual‐based model based on forward genetic simulations, which was fundamentally different to our metapopulation approach with mean‐field local interactions, and also included fewer predictors in their statistical model. Matz et al. also explicitly included a “juvenile” coral stage where locally maladapted individuals may have experienced mortality before reproduction, whereas in our model, immigrants were immediately incorporated into the local population. Nevertheless, our study adds robust support for the importance of relative SST and the quantity and traits of incoming larvae on future coral cover, as these findings were consistent across (1) all three regions with different connectivity matrices (with different underlying assumptions) and unique SST trajectories and (2) multiple eco‐evolutionary models (this study and Matz et al., 2020). These ecological and evolutionary interactions highlight the importance of considering both sets of processes to understand future reef states.
Previous studies also projected spatial variation in coral declines. Couce et al. (2013) implemented a statistical habitat suitability model to project global coral habitat suitability in response to ocean warming and acidification for years 2010, 2040, and 2070. They found the greatest declines in the Western Pacific Warm Pool, which corresponds to much of the Coral Triangle and northern SWP in our model where we also found marked declines in cover. The regions projected to maintain high suitability for corals in the Couce et al. model also correspond to most of the regions which maintain cover in our model (e.g., Southern Great Barrier Reef in both the CT and SWP regions, Greater Sunda Islands, South China Sea in the CT, American Samoa, and the Solomon Islands in the SWP). However, the Couce et al.’s model projects that the entire Caribbean will maintain high suitability, or even increase in suitability, while our model projects severe declines in cover throughout much of the region. Differences in our results are expected due to the imperfect correlation between habitat suitability and abundance, as the former ignores all biological processes, as well as the coarser resolution of their model inputs (1 × 1 degrees). Matz et al. (2020) projected a similar spatial distribution of declines across the Coral Triangle as seen in our model and the Couce et al. results, with the highest declines occurring in near equatorial reefs and higher maintenance of coral cover in northwestern and southeastern reefs away from the equator. However, the Matz et al.’s study projected less severe coral cover declines in both warming scenarios as compared to our model. This could be attributed to differences in the way that reproduction, dispersal and genetic variation were specified in our two models, as well as the combination of parameter values used in the simulations. Overall, our work provides additional support for several projected geographic patterns of coral persistence that have been previously reported, despite the application of vastly different approaches.
Our results indicate that shifts in coral community composition in response to increasing temperatures should be expected and may be reversed during coral population recovery. In our simulations, the fast‐growing coral with a narrow temperature tolerance (with stronger strength of selection) experienced greater initial declines but also exhibited a higher capacity for adaptation relative to the slow‐growing species. Our fast‐growing coral closely resembles branching corals from the family Acroporidae (Darling et al., 2012). While this may imply that acroporid populations in the Caribbean can eventually recover when warming stabilizes, we note that the observed declines in real populations are primarily attributed to disease (Aronson & Precht, 2001), herbivore die‐offs (Lessios, 2016) and local stressors (Cramer et al., 2020), all of which we did not model, in addition to thermal stress (Hughes, 1994). We also do not model the possibility of range expansion in response to increases in temperature, which has been observed in the acroporid fossil record (Baird et al., 2012; Precht & Aronson, 2004; Yamano et al., 2011). Additionally, genomic analyses indicate that acroporids experienced a period of population decline following the Mid‐Pleistocene Transition (global cooling), and then a period of rapid diversification and population growth following the Northern Hemisphere Glaciation (global warming and sea‐level rise; Mao et al., 2018). While our model indicates that acroporids may be more sensitive to short‐term (years to decades) shifts in temperature than other scleractinian families, their fossil record and genome indicate that they may be poised for long‐term range expansion in a warmer climate, given their survival.
Several regional differences in population dynamics were apparent in our simulations. For example, there was higher initial coral cover, slower recovery, and more evolutionary lag (a larger mismatch between coral traits and local temperature) in the Coral Triangle, which has a larger number of reefs and greater rates of potential larval recruitment. The lowest minimum coral cover occurred in the Caribbean, which had fewer reefs and high local retention of larvae. The Caribbean also experienced the longest pre‐recovery period. The Southwest Pacific had the least evolutionary lag and correspondingly recovered to near‐historical levels of coral cover by 2300. The SWP experienced the least warming on average, had the greatest variation in temperature and warming across the network, and had a higher proportion of cool sites, all of which may have contributed to the region's recovery.
To our knowledge, no other study has modelled multiple regional coral networks under an eco‐evolutionary framework, and thus the regional differences suggested by our model stand to be tested. The observed regional differences in population dynamics are likely due to intrinsic differences in temperatures and dispersal among the regional networks, but may also be due, in part, to methodological differences in the models used to generate the connectivity relationships in our model (Schill et al., 2015; Thompson et al., 2018; Treml et al., 2008). These results suggest that future research and management should consider the unique characteristics of each region and that there is a need for additional regional comparison studies. While difficult to implement, consistent dispersal simulation approaches across regions would assist with these comparisons.
Although destination strength and initial SST had consistent effects across all regions, we also found that the types of reefs most likely to survive future warming differed among regions in important ways. For example, self‐recruitment had a stronger negative association with cover in the Caribbean, and area had a stronger negative effect in the Coral Triangle. Self‐recruitment is an indication of the “openness” of a reef, or the amount of larval input from the rest of the network relative to the contribution from the reef itself. In other words, reefs with high self‐recruitment received a lower proportion of larvae from locations other than their own. The Caribbean's prevailing surface currents tend to move larvae from warm sites to cold sites or other warm sites, but rarely from cold to warm (Carrillo et al., 2015; Chollett et al., 2012). Thus, open reefs in the Caribbean generally received evolutionarily neutral or beneficial larvae and only rarely received maladapted larvae. Relatively closed reefs in this region did not experience the synergistic benefits of demographic support and beneficial gene flow from warm‐adapted larvae. Next, the stronger negative association of area with minimum cover in the Coral Triangle was likely due to the wider range of reef areas in this region, including the presence of extremely small sites that were much less abundant in other regions. Therefore, this region was more likely to contain connections between sites with a large difference in area. Because the quantity of larvae dispersing in our model scaled with both reef area and coral cover, larger sites were more likely to demographically support smaller sites than vice versa, leading to a strong association between reef size and minimum cover in the CT. Again, these results suggest that future research and management would benefit from considering the unique characteristics of each region.
Evolution mitigated the impact of the environmental variation among sites in each regional network. As additive genetic variance decreased, the magnitudes of coefficients associated with all network factors increased. In other words, with reduced evolutionary capacity, the local temperature and connectivity of each site had a greater effect on its coral cover. In contrast, all sites tended to have higher cover if evolutionary capacity was high. Thus, the maintenance of genetic variation in coral networks would help support coral persistence across a range of environments. With little evolutionary potential, we can expect that well‐connected small reefs in colder microclimates are most likely to persist under warming.
Our projections contained a number of important assumptions. We assumed that within‐reef coral dynamics were identical among reefs and regions, except for temperature and network connectivity. This assumption allowed us to investigate evolutionary, network, and environmental effects on coral cover, holding other characteristics constant. In reality, coral communities in different regions are composed of different species (beyond our model of fast and slow corals) that have varied responses to environmental stress (Darling et al., 2012). We also assumed that the corals’ thermal optima and growth rate were not correlated to other traits which affect fitness; however, the rate of adaptation seen in our model may not be possible if thermal tolerance trades‐off with other traits affecting fitness (Etterson & Shaw, 2001). In addition, we only tracked coral cover in two dimensions, which ignored three‐dimensional reef structural complexity that plays a prominent role in ecosystem services (Darling et al., 2019). Some coral communities may also exhibit ecological alternative stable states when interactions with macroalgae are included (Mumby et al., 2007). In this study, we did not include non‐coral species and chose to parameterize the model to ensure coexistence, rather than alternative stable states between the corals (Tekwa et al., 2020). While this choice allowed for outcomes that were not as sensitive to initial conditions, one interesting avenue for future investigation would be the interaction of evolution with ecological alternative stable states. Eco‐evolutionary feedbacks have been linked to alternative community compositions in lake systems (Strauss, 2014; Walsh et al., 2012) and may have similar impacts on coral reefs (Mumby et al., 2007). Another caveat is that the timescale of initial decline in coral cover and subsequent recovery can only be interpreted qualitatively. Our results suggest that eventual recovery is possible with evolution, but it is not possible to infer from our model when recovery will occur. That is because recovery timescales are affected by both growth rates and additive genetic variance, which are difficult to measure and may vary greatly across species and regions (but see Anderson et al., 2017; Carilli et al., 2010; D’Croz & Maté, 2004; Edmunds, 2005; Jokiel & Coles, 1977 for estimates of coral growth rates; Dziedzic et al., 2019; Kirk et al., 2018; for estimates of additive genetic variance). Lastly, we note that our results are limited to assessing the response of individual reefs to warming; the impact of any particular reef on the network is beyond the scope of this work.
Based on our results, some general strategies are likely beneficial for coral conservation under warming. First, limiting greenhouse gas emissions and hence warming will facilitate coral adaptation, as demonstrated by the stark differences in coral cover between RCP4.5 and RCP8.5. Second, evolutionary potential is critical for mitigating coral loss and facilitating recovery of corals around the world, both during and after warming. Thus, policies that maintain genetic diversity are likely to have important long‐term benefits. For example, implementing protection across environmentally distinct sites can maintain relatively high additive genetic variance at the network scale by preserving populations that are locally adapted to different thermal regimes (Baums et al., 2019; Howells et al., 2013). Because higher genetic variance also leads to persistence at local scales, genotyping approaches to quantify local genetic variation can help inform conservation and restoration efforts (e.g., protecting sites with high diversity; Baums et al., 2019). Third, our results identify the characteristics of reefs that are likely to maintain coral cover versus those that will likely experience significant coral declines. For example, relatively cool reefs with high larval input may have a greater chance of coral cover maintenance or recovery in response to conservation measures that aim to mitigate external stressors such as reductions in local sedimentation (Bégin et al., 2016; Dubinsky & Stambler, 1996) and nutrient input (Dubinsky & Stambler, 1996). On the other hand, warm reefs with low larval input may benefit the most from larval supplementation (Cruz & Harrison, 2017) or restoration efforts (Baums et al., 2019; Ladd et al., 2018) since they are predicted to have less recovery potential overall. This finding also has implications for reef managers in terms of site selection criteria for management interventions. Managers could intentionally aim to include some cooler reefs and those with high larval settlement (resistant reefs), as well as some hotter reefs and those with low larval settlement (vulnerable reefs) within the managed network. Including a portfolio of reef types within a managed network helps to facilitate multiple means of adaptation to warming, including evolutionary and demographic rescue, in addition to local adaptation (Mumby et al., 2011; Walsworth et al., 2019).
Our projections suggest that a future for corals is possible if warming is limited. Maintaining evolutionary potential and habitat connectivity are both important for the continued existence of coral populations. While we predict a sharp decline in reef cover, we also expect recovery with sufficient genetic variability under a less severe warming scenario. In this work, we linked individual reef characteristics to coral cover response in three major reef networks. Future work can build on these results to investigate how conservation strategies could harness adaptive potential across the reef network as a whole under climate change.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge funding from the Gordon and Betty Moore Foundation and The Nature Conservancy. We also thank Lukas DeFilippo and members of the Pinsky laboratory for helpful discussions on earlier versions of this manuscript.
McManus, L. C., Forrest, D. L., Tekwa, E. W., Schindler, D. E., Colton, M. A., Webster, M. M., Essington, T. E., Palumbi, S. R., Mumby, P. J., & Pinsky, M. L. (2021). Evolution and connectivity influence the persistence and recovery of coral reefs under climate change in the Caribbean, Southwest Pacific, and Coral Triangle. Global Change Biology, 27, 4307–4321. 10.1111/gcb.15725
Lisa C. McManus and Daniel L. Forrest should be considered joint first author.
DATA AVAILABILITY STATEMENT
Code and data necessary to reproduce all results are available on GitHub (https://github.com/pinskylab/regional_coral_manuscript) and archived on Zenodo (https://doi.org/10.5281/zenodo.4784134).
REFERENCES
- Álvarez‐Noriega, M., Baird, A. H., Dornelas, M., Madin, J. S., Cumbo, V. R., & Connolly, S. R. (2016). Fecundity and the demographic strategies of coral morphologies. Ecology, 97(12), 3485–3493. 10.1002/ecy.1588 [DOI] [PubMed] [Google Scholar]
- Anderson, K. D., Cantin, N. E., Heron, S. F., Pisapia, C., & Pratchett, M. S. (2017). Variation in growth rates of branching corals along Australia’s Great Barrier Reef. Scientific Reports, 7(1), 2920. 10.1038/s41598-017-03085-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson, R. B., & Precht, W. F. (2001). White‐band disease and the changing face of Caribbean coral reefs. In Porter J. W. (Ed.), The ecology and etiology of newly emerging marine diseases (pp. 25–38). Springer. 10.1007/978-94-017-3284-0_2 [DOI] [Google Scholar]
- Baird, A. H., Sommer, B., & Madin, J. S. (2012). Pole‐ward range expansion of Acropora spp. along the east coast of Australia. Coral Reefs, 31(4), 1063. 10.1007/s00338-012-0928-6 [DOI] [Google Scholar]
- Ban, N. C., Pressey, R. L., & Weeks, S. (2012). Conservation objectives and sea‐surface temperature anomalies in the great barrier reef. Conservation Biology, 26(5), 799–809. 10.1111/j.1523-1739.2012.01894.x [DOI] [PubMed] [Google Scholar]
- Baskett, M. L., Fabina, N. S., & Gross, K. (2014). Response diversity can increase ecological resilience to disturbance in coral reefs. The American Naturalist, 184(2), E16–E31. 10.1086/676643 [DOI] [PubMed] [Google Scholar]
- Baums, I. B., Baker, A. C., Davies, S. W., Grottoli, A. G., Kenkel, C. D., Kitchen, S. A., Kuffner, I. B., LaJeunesse, T. C., Matz, M. V., Miller, M. W., Parkinson, J. E., & Shantz, A. A. (2019). Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications, 29(8). 10.1002/eap.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay, R. A., Rose, N. H., Logan, C. A., & Palumbi, S. R. (2017). Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Science Advances, 3. 10.1126/sciadv.1701413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bégin, C., Schelten, C. K., Nugues, M. M., Hawkins, J., Roberts, C., & Côté, I. M. (2016). Effects of protection and sediment stress on coral reefs in Saint Lucia. PLoS One, 11(2), e0146855. 10.1371/journal.pone.0146855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford, L. W., White, J. W., Coffroth, M. A., Paris, C. B., Planes, S., Shearer, T. L., Thorrold, S. R., & Jones, G. P. (2009). Connectivity and resilience of coral reef metapopulations in marine protected areas: Matching empirical efforts to predictive needs. Coral Reefs, 28(2), 327–337. 10.1007/s00338-009-0466-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. H., & Kodric‐Brown, A. (1977). Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology, 58(2), 445–449. 10.2307/1935620 [DOI] [Google Scholar]
- Burgess, S. C., Nickols, K. J., Griesemer, C. D., Barnett, L. A. K., Dedrick, A. G., Satterthwaite, E. V., Yamane, L., Morgan, S. G., White, J. W., & Botsford, L. W. (2014). Beyond connectivity: How empirical methods can quantify population persistence to improve marine protected area design: Supplementary Information. Ecological Society of America, 24(2), 8. 10.1890/13-0710.1 [DOI] [PubMed] [Google Scholar]
- Burnham, K. P., & Anderson, D. R. (2004). Multimodel inference: Understanding AIC and BIC in model selection. Sociological Methods & Research, 33(2), 261–304. 10.1177/0049124104268644 [DOI] [Google Scholar]
- Burnham, K. P., Anderson, D. R., & Burnham, K. P. (2002). Model selection and multimodel inference: A practical information‐theoretic approach (2nd ed.). Springer. [Google Scholar]
- Caley, M. J., Carr, M. H., Hixon, M. A., Hughes, T. P., Jones, G. P., & Menge, B. A. (1996). Recruitment and the local dynamics of open marine populations. Annual Review of Ecology and Systematics, 27(1), 477–500. 10.1146/annurev.ecolsys.27.1.477 [DOI] [Google Scholar]
- Carilli, J. E., Norris, R. D., Black, B., Walsh, S. M., & McField, M. (2010). Century‐scale records of coral growth rates indicate that local stressors reduce coral thermal tolerance threshold. Global Change Biology, 16(4), 1247–1257. 10.1111/j.1365-2486.2009.02043.x [DOI] [Google Scholar]
- Carrillo, L., Johns, E. M., Smith, R. H., Lamkin, J. T., & Largier, J. L. (2015). Pathways and hydrography in the Mesoamerican barrier reef system part 1: Circulation. Continental Shelf Research, 109, 164–176. 10.1016/j.csr.2015.09.014 [DOI] [Google Scholar]
- Chollett, I., Müller‐Karger, F. E., Heron, S. F., Skirving, W., & Mumby, P. J. (2012). Seasonal and spatial heterogeneity of recent sea surface temperature trends in the Caribbean Sea and southeast Gulf of Mexico. Marine Pollution Bulletin, 64(5), 956–965. 10.1016/j.marpolbul.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Couce, E., Ridgwell, A., & Hendy, E. J. (2013). Future habitat suitability for coral reef ecosystems under global warming and ocean acidification. Global Change Biology, 19(12), 3592–3606. 10.1111/gcb.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer, K. L., Jackson, J. B. C., Donovan, M. K., Greenstein, B. J., Korpanty, C. A., Cook, G. M., & Pandolfi, J. M. (2020). Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Science Advances, 6(17), eaax9395. 10.1126/sciadv.aax9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz, D. W. D., & Harrison, P. L. (2017). Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Scientific Reports, 7(1), 13985. 10.1038/s41598-017-14546-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Croz, L., & Maté, J. L. (2004). Experimental responses to elevated water temperature in genotypes of the reef coral Pocillopora damicornis from upwelling and non‐upwelling environments in Panama. Coral Reefs, 23(4), 473–483. 10.1007/s00338-004-0397-7 [DOI] [Google Scholar]
- Darling, E. S., Alvarez‐Filip, L., Oliver, T. A., McClanahan, T. R., & Côté, I. M. (2012). Evaluating life‐history strategies of reef corals from species traits. Ecology Letters, 15(12), 1378–1386. 10.1111/j.1461-0248.2012.01861.x [DOI] [PubMed] [Google Scholar]
- Darling, E. S., McClanahan, T. R., Maina, J., Gurney, G. G., Graham, N. A. J., Januchowski‐Hartley, F., Cinner, J. E., Mora, C., Hicks, C. C., Maire, E., Puotinen, M., Skirving, W. J., Adjeroud, M., Ahmadia, G., Arthur, R., Bauman, A. G., Beger, M., Berumen, M. L., Bigot, L., … Mouillot, D. (2019). Social–environmental drivers inform strategic management of coral reefs in the Anthropocene. Nature Ecology & Evolution, 3(9), 1341–1350. 10.1038/s41559-019-0953-8 [DOI] [PubMed] [Google Scholar]
- Davies, S. W., Treml, E. A., Kenkel, C. D., & Matz, M. V. (2015). Exploring the role of Micronesian islands in the maintenance of coral genetic diversity in the Pacific Ocean. Molecular Ecology, 24(1), 70–82. 10.1111/mec.13005 [DOI] [PubMed] [Google Scholar]
- Deutsch, C. A., Tewksbury, J. J., Huey, R. B., Sheldon, K. S., Ghalambor, C. K., Haak, D. C., & Martin, P. R. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of the United States of America, 105(18), 6668–6672. 10.1073/pnas.0709472105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, G. B., Davies, S. W., Aglyamova, G. A. V., Meyer, E., Bay, L. K., & Matz, M. V. (2015). Genomic determinants of coral heat tolerance across latitudes. Science, 348(6242), 1460–1462. 10.1126/science.1261224 [DOI] [PubMed] [Google Scholar]
- Donner, S. D. (2009). Coping with commitment: Projected thermal stress on coral reefs under different future scenarios. PLoS One, 4(6). 10.1371/journal.pone.0005712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubinsky, Z., & Stambler, N. (1996). Marine pollution and coral reefs. Global Change Biology, 2(6), 511–526. 10.1111/j.1365-2486.1996.tb00064.x [DOI] [Google Scholar]
- Dziedzic, K. E., Elder, H., Tavalire, H., & Meyer, E. (2019). Heritable variation in bleaching responses and its functional genomic basis in reef‐building corals (Orbicella faveolata). Molecular Ecology, 28(9), 2238–2253. 10.1111/mec.15081 [DOI] [PubMed] [Google Scholar]
- Edmunds, P. J. (2005). The effect of sub‐lethal increases in temperature on the growth and population trajectories of three scleractinian corals on the southern Great Barrier Reef. Oecologia, 146(3), 350–364. 10.1007/s00442-005-0210-5 [DOI] [PubMed] [Google Scholar]
- Etterson, J. R., & Shaw, R. G. (2001). Constraint to adaptive evolution in response to global warming. Science, 294(5540), 151–154. 10.1126/science.1063656 [DOI] [PubMed] [Google Scholar]
- Falconer, D. S. (1996). Introduction to quantitative genetics (4th ed.). Longman. [Google Scholar]
- Fowler, H. J., Blenkinsop, S., & Tebaldi, C. (2007). Linking climate change modelling to impacts studies: Recent advances in downscaling techniques for hydrological modelling: Advances in downscaling techniques for hydrological modelling. International Journal of Climatology, 27(12), 1547–1578. 10.1002/joc.1556 [DOI] [Google Scholar]
- Frieler, K., Meinshausen, M., Golly, A., Mengel, M., Lebek, K., Donner, S. D., & Hoegh‐Guldberg, O. (2013). Limiting global warming to 2°C is unlikely to save most coral reefs. Nature Climate Change, 3(2), 165–170. 10.1038/nclimate1674 [DOI] [Google Scholar]
- Galindo, H. M., Pfeiffer‐Herbert, A. S., McManus, M. A., Chao, Y., Chai, F., & Palumbi, S. R. (2010). Seascape genetics along a steep cline: Using genetic patterns to test predictions of marine larval dispersal. Molecular Ecology, 19(17), 3692–3707. 10.1111/j.1365-294X.2010.04694.x [DOI] [PubMed] [Google Scholar]
- Guest, J. R., Baird, A. H., Maynard, J. A., Muttaqin, E., Edwards, A. J., Campbell, S. J., Yewdall, K., Affendi, Y. A., & Chou, L. M. (2012). Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS One, 7(3), 1–8. 10.1371/journal.pone.0033353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski, I., Mononen, T., & Ovaskainen, O. (2011). Eco‐evolutionary metapopulation dynamics and the spatial scale of adaptation. The American Naturalist, 177(1), 29–43. 10.1086/657625 [DOI] [PubMed] [Google Scholar]
- Hastings, A., & Botsford, L. W. (2006). Persistence of spatial populations depends on returning home. Proceedings of the National Academy of Sciences of the United States of America, 103(15), 6067–6072. 10.1073/pnas.0506651103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, L. E., Wilby, R. L., & Leavesley, G. H. (2000). A comparison of delta change and downscaled GCM scenarios for three mountainous basins in the United States. Journal of the American Water Resources Association, 36(2), 387–397. 10.1111/j.1752-1688.2000.tb04276.x [DOI] [Google Scholar]
- Hoegh‐Guldberg, O., Poloczanska, E. S., Skirving, W., & Dove, S. (2017). Coral reef ecosystems under climate change and ocean acidification. Frontiers in Marine Science, 4, 158. 10.3389/fmars.2017.00158 [DOI] [Google Scholar]
- Howells, E. J., Berkelmans, R., Van Oppen, M. J. H., Willis, B. L., & Bay, L. K. (2013). Historical thermal regimes define limits to coral acclimatization. Ecology, 94(5), 1078–1088. 10.1890/12-1257.1 [DOI] [PubMed] [Google Scholar]
- Hughes, T. P. (1990). Recruitment limitation, mortality, and population regulation in open systems: A case study. Ecology, 71, 12–20. 10.2307/1940242 [DOI] [Google Scholar]
- Hughes, T. P. (1994). Catastrophes, phase shifts, and large‐scale degradation of a Caribbean coral reef. Science, 265, 1547–1551. 10.1126/science.265.5178.1547 [DOI] [PubMed] [Google Scholar]
- Hughes, T. P., Anderson, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., Baird, A. H., Baum, J. K., Berumen, M. L., Bridge, T. C., Claar, D. C., Eakin, C. M., Gilmour, J. P., Graham, N. A. J., Harrison, H., Hobbs, J.‐P., Hoey, A. S., Hoogenboom, M., Lowe, R. J., … Wilson, S. K. (2018). Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science, 359(6371), 80–83. 10.1126/science.aan8048 [DOI] [PubMed] [Google Scholar]
- Jokiel, P. L., & Coles, S. L. (1977). Effects of temperature on the mortality and growth of Hawaiian reef corals. Marine Biology, 43(3), 201–208. 10.1007/BF00402312 [DOI] [Google Scholar]
- Kininmonth, S., Weeks, R., Abesamis, R. A., Bernardo, L. P. C., Beger, M., Treml, E. A., Williamson, D., & Pressey, R. L. (2019). Strategies in scheduling marine protected area establishment in a network system. Ecological Applications, 29(1), 1–10. 10.1002/eap.1820 [DOI] [PubMed] [Google Scholar]
- Kirk, N. L., Howells, E. J., Abrego, D., Burt, J. A., & Meyer, E. (2018). Genomic and transcriptomic signals of thermal tolerance in heat‐tolerant corals (Platygyra daedalea) of the Arabian/Persian Gulf. Molecular Ecology, 27(24), 5180–5194. 10.1111/mec.14934 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick, M., & Barton, N. H. (1997). Evolution of a species’ range. The American Naturalist, 150(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Klausmeier, C. A., Osmond, M. M., Kremer, C. T., & Litchman, E. (2020). Ecological limits to evolutionary rescue. Philosophical Transactions of the Royal Society B: Biological Sciences, 375(1814), 20190453. 10.1098/rstb.2019.0453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas, J. A., Thompson, D. M., Castruccio, F. S., Curchitser, E. N., Pinsky, M., & Watson, J. R. (2016). Larval connectivity across temperature gradients and its potential effect on heat tolerance in coral populations. Global Change Biology, 22(11), 3539–3549. 10.1111/gcb.13347 [DOI] [PubMed] [Google Scholar]
- Kool, J. T., Paris, C. B., Barber, P. H., & Cowen, R. K. (2011). Connectivity and the development of population genetic structure in Indo‐West Pacific coral reef communities. Global Ecology and Biogeography, 20(5), 695–706. 10.1111/j.1466-8238.2010.00637.x [DOI] [Google Scholar]
- Ladd, M. C., Miller, M. W., Hunt, J. H., Sharp, W. C., & Burkepile, D. E. (2018). Harnessing ecological processes to facilitate coral restoration. Frontiers in Ecology and the Environment, 16(4), 239–247. 10.1002/fee.1792 [DOI] [Google Scholar]
- Lande, R. (1976). Natural selection and random genetic drift in phenotypic evolution. Evolution, 30(2), 314–334. 10.1111/j.1558-5646.1976.tb00911.x [DOI] [PubMed] [Google Scholar]
- Lenormand, T. (2002). Gene flow and the limits to natural selection. Trends in Ecology & Evolution, 17(4), 183–189. 10.1016/S0169-5347(02)02497-7 [DOI] [Google Scholar]
- Lessios, H. A. (2016). The great Diadema antillarum die‐off: 30 years later. Annual Review of Marine Science, 8(1), 267–283. 10.1146/annurev-marine-122414-033857 [DOI] [PubMed] [Google Scholar]
- Lindsey, H. A., Gallie, J., Taylor, S., & Kerr, B. (2013). Evolutionary rescue from extinction is contingent on a lower rate of environmental change. Nature, 494(7438), 463–467. 10.1038/nature11879 [DOI] [PubMed] [Google Scholar]
- Logan, C. A., Dunne, J. P., Eakin, C. M., & Donner, S. D. (2014). Incorporating adaptive responses into future projections of coral bleaching. Global Change Biology, 20(1), 125–139. 10.1111/gcb.12390 [DOI] [PubMed] [Google Scholar]
- Mao, Y., Economo, E. P., & Satoh, N. (2018). The roles of introgression and climate change in the rise to dominance of acropora corals. Current Biology, 28(21), 3373–3382.e5. 10.1016/j.cub.2018.08.061 [DOI] [PubMed] [Google Scholar]
- Matz, M. V., Treml, E. A., Aglyamova, G. V., & Bay, L. K. (2018). Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLoS Genetics, 14(4), 1–19. 10.1371/journal.pgen.1007220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz, M. V., Treml, E. A., & Haller, B. C. (2020). Estimating the potential for coral adaptation to global warming across the Indo‐West Pacific. Global Change Biology, 26(6), 3473–3481. 10.1111/gcb.15060 [DOI] [PubMed] [Google Scholar]
- McClanahan, T. R., Darling, E. S., Maina, J. M., Muthiga, N. A., ’agata, S. D., Jupiter, S. D., Arthur, R., Wilson, S. K., Mangubhai, S., Nand, Y., Ussi, A. M., Humphries, A. T., Patankar, V. J., Guillaume, M. M. M., Keith, S. A., Shedrawi, G., Julius, P., Grimsditch, G., Ndagala, J., & Leblond, J. (2019). Temperature patterns and mechanisms influencing coral bleaching during the 2016 El Niño. Nature Climate Change, 9(11), 845–851. 10.1038/s41558-019-0576-8 [DOI] [Google Scholar]
- McClanahan, T. R., & Maina, J. (2003). Response of coral assemblages to the interaction between natural temperature variation and rare warm‐water events. Ecosystems, 6(6), 551–563. 10.1007/s10021-002-0104-x [DOI] [Google Scholar]
- McManus, L. C., Tekwa, E. W., Schindler, D. E., Walsworth, T. E., Colton, M. A., Webster, M. M., Essington, T. E., Forrest, D. L., Palumbi, S. R., Mumby, P. J., & Pinsky, M. L. (2021). Evolution reverses the effect of network structure on metapopulation persistence. Ecology, e03381. 10.1002/ecy.3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus, L. C., Vasconcelos, V. V., Levin, S. A., Thompson, D. M., Kleypas, J. A., Castruccio, F. S., Curchitser, E. N., & Watson, J. R. (2020). Extreme temperature events will drive coral decline in the Coral Triangle. Global Change Biology, 26(4), 2120–2133. 10.1111/gcb.14972 [DOI] [PubMed] [Google Scholar]
- Menge, B. A., Lubchenco, J., Bracken, M. E. S., Chan, F., Foley, M. M., Freidenburg, T. L., Gaines, S. D., Hudson, G., Krenz, C., Leslie, H., Menge, D. N. L., Russell, R., & Webster, M. S. (2003). Coastal oceanography sets the pace of rocky intertidal community dynamics. Proceedings of the National Academy of Sciences of the United States of America, 100(21), 12229–12234. 10.1073/pnas.1534875100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby, P. J., Elliott, I. A., Eakin, C. M., Skirving, W., Paris, C. B., Edwards, H. J., Enríquez, S., Iglesias‐Prieto, R., Cherubin, L. M., & Stevens, J. R. (2011). Reserve design for uncertain responses of coral reefs to climate change: Reserve design for climate change. Ecology Letters, 14(2), 132–140. 10.1111/j.1461-0248.2010.01562.x [DOI] [PubMed] [Google Scholar]
- Mumby, P. J., Hastings, A., & Edwards, H. J. (2007). Thresholds and the resilience of Caribbean coral reefs. Nature, 450(7166), 98–101. 10.1038/nature06252 [DOI] [PubMed] [Google Scholar]
- Norberg, J., Urban, M. C., Vellend, M., Klausmeier, C. A., & Loeuille, N. (2012). Eco‐evolutionary responses of biodiversity to climate change. Nature Climate Change, 2(10), 747–751. 10.1038/nclimate1588 [DOI] [Google Scholar]
- Pachauri, R. K., Mayer, L. & Intergovernmental Panel on Climate Change . (Eds.). (2015). Climate change 2014: Synthesis report. Intergovernmental Panel on Climate Change. [Google Scholar]
- Precht, W. F., & Aronson, R. B. (2004). Climate flickers and range shifts of reef corals. Frontiers in Ecology and the Environment, 2(6), 307–314. https://doi.org/10.1890/1540‐9295(2004)002[0307:CFARSO]2.0.CO;2 [Google Scholar]
- Ramirez Villejas, J., & Jarvis, A. (2010). Downscaling global circulation model outputs: The delta method decision and policy analysis working paper no. 1. International Center for Tropical Agriculture (CIAT). Cali. CO. 18 p. https://hdl.handle.net/10568/90731
- Rayner, N. A. (2003). Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. Journal of Geophysical Research, 108(D14), 4407. 10.1029/2002JD002670 [DOI] [Google Scholar]
- Reynolds, R. W., Smith, T. M., Liu, C., Chelton, D. B., Casey, K. S., & Schlax, M. G. (2007). Daily high‐resolution‐blended analyses for sea surface temperature. Journal of Climate, 20(22), 5473–5496. 10.1175/2007JCLI1824.1 [DOI] [Google Scholar]
- Safaie, A., Silbiger, N. J., McClanahan, T. R., Pawlak, G., Barshis, D. J., Hench, J. L., Rogers, J. S., Williams, G. J., & Davis, K. A. (2018). High frequency temperature variability reduces the risk of coral bleaching. Nature Communications, 9(1), 1–12. 10.1038/s41467-018-04074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schill, S. R., Raber, G. T., Roberts, J. J., Treml, E. A., Brenner, J., & Halpin, P. N. (2015). No reef is an island: Integrating coral reef connectivity data into the design of regional‐scale marine protected area networks. PLoS One, 10(12), 1–24. 10.1371/journal.pone.0144199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, G. A., Kelley, M., Nazarenko, L., Ruedy, R., Russell, G. L., Aleinov, I., Bauer, M., Bauer, S. E., Bhat, M. K., Bleck, R., Canuto, V., Chen, Y.‐H., Cheng, Y. E., Clune, T. L., Del Genio, A., de Fainchtein, R. , Faluvegi, G., Hansen, J. E., Healy, R. J., … Zhang, J. (2014). Configuration and assessment of the GISS ModelE2 contributions to the CMIP5 archive. Journal of Advances in Modeling Earth Systems, 6(1), 141–184. 10.1002/2013MS000265 [DOI] [Google Scholar]
- Strauss, S. Y. (2014). Ecological and evolutionary responses in complex communities: Implications for invasions and eco‐evolutionary feedbacks. Oikos, 123(3), 257–266. 10.1111/j.1600-0706.2013.01093.x [DOI] [Google Scholar]
- Sully, S., Burkepile, D. E., Donovan, M. K., Hodgson, G., & van Woesik, R. (2019). A global analysis of coral bleaching over the past two decades. Nature Communications, 10(1), 1–5. 10.1038/s41467-019-09238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekwa, E. W., McManus, L. C., Greiner, A., Colton, M. A., Webster, M. S., & Pinsky, M. L. (2020). Geometric analysis of regime shifts in coral reef communities (Ecology) [Preprint]. bioRxiv. 10.1101/2020.01.10.899179 [DOI] [Google Scholar]
- Thompson, D., Kleypas, J., Castruccio, F., Curchitser, E., Pinsky, M., Jönsson, B., & Watson, J. (2018). Variability in oceanographic barriers to coral larval dispersal: Do currents shape biodiversity? Progress in Oceanography, 165(February), 110–122. 10.1016/j.pocean.2018.05.007 [DOI] [Google Scholar]
- Treml, E. A., & Halpin, P. N. (2012). Marine population connectivity identifies ecological neighbors for conservation planning in the Coral Triangle. Conservation Letters, 5(6), 441–449. 10.1111/j.1755-263X.2012.00260.x [DOI] [Google Scholar]
- Treml, E. A., Halpin, P. N., Urban, D. L., & Pratson, L. F. (2008). Modeling population connectivity by ocean currents, a graph‐theoretic approach for marine conservation. Landscape Ecology, 23(Suppl 1), 19–36. 10.1007/s10980-007-9138-y [DOI] [Google Scholar]
- van Hooidonk, R. , Maynard, J. A., & Planes, S. (2013). Temporary refugia for coral reefs in a warming world. Nature Climate Change, 3(5), 508–511. 10.1038/nclimate1829 [DOI] [Google Scholar]
- Veron, J. E. N. (1995). Corals in space & time. The biogeography & evolution of the scleractinia (Vol. xiii). Cornell University Press (Comstock). [Google Scholar]
- Walsh, M. R., DeLong, J. P., Hanley, T. C., & Post, D. M. (2012). A cascade of evolutionary change alters consumer‐resource dynamics and ecosystem function. Proceedings of the Royal Society B: Biological Sciences, 279(1741), 3184–3192. 10.1098/rspb.2012.0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsworth, T. E., Schindler, D. E., Colton, M. A., Webster, M. S., Palumbi, S. R., Mumby, P. J., Essington, T. E., & Pinsky, M. L. (2019). Management for network diversity speeds evolutionary adaptation to climate change. Nature Climate Change, 9(8), 632–636. 10.1038/s41558-019-0518-5 [DOI] [Google Scholar]
- Watson, J. R., Mitarai, S., Siegel, D. A., Caselle, J. E., Dong, C., & McWilliams, J. C. (2010). Realized and potential larval connectivity in the southern California bight. Marine Ecology Progress Series, 401, 31–48. 10.3354/meps08376 [DOI] [Google Scholar]
- Watson, J. R., Siegel, D. A., Kendall, B. E., Mitarai, S., Rassweiller, A., & Gaines, S. D. (2011). Identifying critical regions in small‐world marine metapopulations. Proceedings of the National Academy of Sciences of the United States of America, 108(43), E907–E913. 10.1073/pnas.1111461108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle, P. D., Small, M. J., Doney, S. C., & Azevedo, I. L. (2017). Estimating the effect of multiple environmental stressors on coral bleaching and mortality. PLoS One, 12(5), 1–15. 10.1371/journal.pone.0175018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. W., Rassweiler, A., Samhouri, J. F., Stier, A. C., & White, C. (2014). Ecologists should not use statistical significance tests to interpret simulation model results. Oikos, 123(4), 385–388. 10.1111/j.1600-0706.2013.01073.x [DOI] [Google Scholar]
- Wood, S., Paris, C. B., Ridgwell, A., & Hendy, E. J. (2014). Modelling dispersal and connectivity of broadcast spawning corals at the global scale. Global Ecology and Biogeography, 23(1), 1–11. 10.1111/geb.12101 [DOI] [Google Scholar]
- Yamano, H., Sugihara, K., & Nomura, K. (2011). Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophysical Research Letters, 38(L04601), 1–6. 10.1029/2010GL046474 [DOI] [Google Scholar]
- Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). GLM and GAM for absence‐presence and proportional data. In Zuur, A. F., Ieno, E. N., Walker, N., Saveliev, A. A., & Smith, G. M. (Eds.), Mixed effects models and extensions in ecology with R (pp. 245–259). Springer. 10.1007/978-0-387-87458-6_10 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Code and data necessary to reproduce all results are available on GitHub (https://github.com/pinskylab/regional_coral_manuscript) and archived on Zenodo (https://doi.org/10.5281/zenodo.4784134).


