Abstract
Recent studies suggest a therapeutic effect of psychiatric service dogs for military veterans with posttraumatic stress disorder (PTSD), but are limited by self-report biases. The current study assessed the effect of PTSD service dogs on the salivary cortisol awakening response (CAR) and arousal-related functioning in a population of military veterans with PTSD. Participants included 73 post-9/11 military veterans with PTSD including 45 with a service dog and 28 on the waitlist to receive one. Saliva samples were collected on two consecutive weekday mornings at awakening and 30 min later to quantify the cortisol awakening response (CAR) and its area under the curve (AUCi) in addition to standardized survey measures of anxiety, anger, sleep quality and disturbance, and alcohol abuse. There was a significant main effect of having a service dog on both the CAR and the AUCi, with individuals with a service dog exhibiting a higher CAR and AUCi compared to those on the waitlist. Results also revealed that those with a service dog reported significantly lower anxiety, anger, and sleep disturbance as well as less alcohol abuse compared to those on the waitlist, with medium to large effect sizes. Although those with a service dog reported significantly less PTSD symptom severity, CAR was not significantly associated with PTSD symptoms within or across group. In conclusion, results indicate that the placement of a PTSD service dog may have a significant positive influence on both physiological and psychosocial indicators of wellbeing in military veterans with PTSD. Although clinical significance cannot be confirmed, a higher CAR/AUCi among those with a service dog may indicate better health and wellbeing in this population. Future within-subject, longitudinal research will be necessary to determine potential clinical significance and impact of individual differences.
Keywords: Posttraumatic stress disorder, PTSD, Military veterans, Service dogs, Human-animal interaction, Cortisol awakening response
1. The effect of a service dog on salivary cortisol awakening response in a military population with posttraumatic stress disorder (PTSD)
Approximately 5–20% of US military veterans returning from post-9/11 deployments have posttraumatic stress disorder (PTSD; Ramchand et al., 2010). PTSD is an anxiety and stress-related disorder characterized by persistent and intense symptoms related to intrusion, avoidance, negative alterations in cognition and mood, and alterations in arousal and reactivity (American Psychiatric Association, 2013). PTSD is often associated with high rates of comorbidity with substance use, depression, and suicidal ideation (Brady et al., 2000; Brown and Wolfe, 1994; Jakupcak et al., 2009).
Within the military population, PTSD can often be difficult to treat. A recent meta-analysis of nine randomized clinical trials of psychotherapy, a common treatment for PTSD, found that up to 72% of military patients maintained their pre-treatment PTSD diagnoses (Steenkamp et al., 2015). In addition, military members infrequently take advantage of mental health services (Hoge et al., 2014). Among those who do seek professional help, dropout rates during PTSD treatment can be as high as 50% (Schottenbauer et al., 2008). Therefore, there is a critical need for alternative and complementary treatments for PTSD that maintain efficacy while encouraging treatment retention in military patient populations (Bomyea and Lang, 2012).
One complementary treatment is the placement of specially trained PTSD service dogs. PTSD service dogs are specifically trained to perform tasks that are thought to mitigate symptoms of PTSD. For example, the dog may position itself behind the individual to “watch their back” and alert to approaching strangers (a task intended to decrease hypervigilance in crowds). PTSD service dogs can also be trained to be attentive to an individual’s behavior and provide a redirection of attention during an episode of re-experiencing or distress. Qualitative evidence suggests that PTSD service dogs can confer unique benefits to military veterans that address PTSD symptomology, especially hyperarousal (Crowe et al., 2017; Olmert et al., 2015; Taylor et al., 2013; Yount et al., 2012). Beyond anecdotal reports, quantitative studies have documented significant effects of PTSD service dogs on self-reports of PTSD symptoms, depression, quality of life, emotional health, and interpersonal relationships (Kloep et al., 2017; O’Haire and Rodriguez, 2018; Vincent et al., 2017; Yarborough et al., 2017). While these findings offer promising preliminary evidence of the therapeutic efficacy of PTSD service dogs, there remains a need for empirical, objective research to advance our understanding of the potential mechanisms of the effects of PTSD service dogs, particularly with respect to hyperarousal and stress (Krause-Parello et al., 2016; O’Haire et al., 2015).
Stress in humans can be measured via the hormone cortisol, a product of the hypothalamic-pituitary-adrenocortical (HPA) axis (Stratakis and Chrousos, 1995). Cortisol concentrations can be assayed from saliva samples upon awakening and shortly thereafter to estimate the cortisol awakening response (CAR; Pruessner et al., 1997b). In healthy individuals without PTSD, the CAR is characterized by a 50–75% increase of cortisol (Pruessner et al., 1997a). Studies consistently link individual differences in the magnitude of this increase to both acute and chronic stress (Chida and Steptoe, 2009).
In contrast to healthy individuals, individuals with PTSD tend to experience hyperarousal-induced dysregulation of HPA activity leading to atypical cortisol profiles (Yehuda et al., 1996). In particular, cortisol output can often attenuate over time among individuals with PTSD; this is thought to be induced by the continuous “fight or flight” response which may create a negative feedback within the HPA axis (Clow et al., 2010). Recent meta-analyses investigating the relationship between PTSD and cortisol output have found that PTSD is significantly associated with lower morning cortisol output (Boggero et al., 2017; Morris et al., 2012). Studies have also examined how cortisol activity and reactivity may change as a result of PTSD treatment (Ryan et al., 2016). Of the three studies that have quantified pre and post-treatment CAR, two found evidence of a decreased CAR as a result of PTSD treatment (Bergen-Cico et al., 2014; Rapcencu et al., 2017) while one found no significant difference in the CAR among treatment and controls (Pacella et al., 2014).
There is evidence that the HPA-axis and cortisol activity are sensitive to human-canine interaction. For example, when positively interacting with pet dogs, healthy adults secrete significantly less cortisol (Odendaal and Meintjes, 2003), and the presence of a dog during a stressful situation leads to significantly less salivary cortisol output than the presence of a friendly human or a toy dog (Beetz et al., 2011; Polheber and Matchock, 2013). However, to our knowledge, only one study to date has directly examined the effect of human-canine interaction on the CAR. A study of 42 children with autism found that a service dog in the home was related to a decreased CAR compared to when the service dog was briefly removed from the home (Viau et al., 2010). In this regard, the effects of the service dog’s presence and companionship may be an important contextual state-related factor contributing to variance in HPA-axis activity and the CAR (Law et al., 2013).
1.1. Present study
Although several self-report and anecdotal accounts have suggested that PTSD service dogs can reduce hyperarousal, to the best of our knowledge no studies have quantified the effect of service dogs on cortisol secretion or the CAR in the context of PTSD (Krause-Parello et al., 2016; O’Haire et al., 2015). In this exploratory study, we attempt to fill this gap. The main goal was to investigate the effect of a service dog on the CAR among military veterans with PTSD; secondary outcomes included subjective functioning of anxiety, sleep quality, anger, and alcohol use. Although previous findings are mixed regarding the relationship between PTSD treatment outcomes and HPA axis activity, we hypothesized that compared to a waitlist control group, participants placed with a PTSD service dog while receiving treatment as usual would exhibit a significantly altered CAR profile as well as higher functioning in the areas of anger, anxiety, sleep quality, and alcohol abuse. Additionally, we explored the relationship between individual CAR profiles and PTSD severity and symptoms.
2. Methods
2.1. Participants
Participants were recruited between November 2015 and February 2016 from a national sample of 304 military members and veterans who applied for and were approved to receive a PTSD service dog from the organization K9s For Warriors. Inclusion criteria for organizational approval consisted of: (1) military service after September 11, 2001, (2) a community diagnosis of PTSD or meeting the clinical cutoff of 50 on the validated PTSD Checklist (PCL) (3) honorable discharge or current honorable service, (4) no current or past substance abuse, (5) no conviction of any crime against animals, and (6) no more than two pet dogs currently in the home.
2.1.1. Waitlist group
Participants on the waitlist had been accepted by the organization, but had not yet received a service dog. Waitlist participants had been on the waitlist anywhere from 2.5 months to 1.59 years (M = 0.64 years, SD = 0.33) and were awaiting a scheduled date to receive a service dog while receiving their usual care. The service dog provider does not suggest nor discourage engagement with any specific treatments for PTSD outside of the placement of a service dog, thus, both those placed with a service dog and those on the waitlist to receive a service dog had unrestricted access to usual care determined by individual preference.
2.1.2. Service dog group
Participants in the service dog group had been placed with PTSD service dogs from the organization anywhere from 1 month to 4 years (M = 1.71 years, SD = 1.12). Participants had attended a three-week team training program in which they were taught by K9 s For Warriors personnel how to interact with, care for, and maintain ongoing training with their service dog with a group of 6–10 recipients of the same sex. Service dogs were primarily acquired from animal shelters and selected and screened for physical and temperamental characteristics (e.g. 24 inches at the shoulder, no past or current aggression). All service dogs were trained for a minimum of 120 hours over at least 6 months for basic obedience and a variety of commands specifically trained to mitigate veterans’ PTSD symptoms. Examples of tasks include “cover” (positioning backwards to alert the veteran of approaching people and provide comfort in public), “block” (standing sideways in front of the veteran to assist with creating personal space), and alerting to agitation or anxiety to prevent or distract from rising panic.
2.2. Procedure
The study protocol was approved by the Purdue University Human Research Protection Program Institutional Review Board (IRB Protocol 1504015973). No interactions occurred between researchers and service dogs during the study, therefore a waiver was obtained from the Institutional Animal Care and Use Committee (IACUC).
Individuals from the K9s For Warriors database (either on the waitlist or already placed with a dog) were sent a study packet in the mail which included detailed study information, consent forms, and $20 cash as compensation for reviewing the materials. Participants could earn an additional $20 for participating in a self-report survey and completing saliva collections following voluntary informed consent.
2.3. Saliva collection and determination of cortisol
Participants collected four passive drool, whole saliva samples on two consecutive weekdays both at awakening (S1) and 30 min after awakening (S1 + 30; Pruessner et al., 1997b). All participants were provided with detailed print material and an instructional video on how to collect samples. Participants were advised to collect their first sample immediately upon awakening and to not eat, drink anything other than water, or brush their teeth 30-min prior to sampling (Stalder et al., 2016).
An SMS texting software (EZ Texting, Callfire Inc.) aided in collection compliance; participants registered their time zone and wake times from their personal cell phone, allowing the software to send personalized reminders on collection days. As a marker of compliance and a timestamp for collection, the participant was required to reply to these reminder texts. If the participant did not text in, he/she was sent a reminder to complete the sample collection. In the rare cases in which sampling was disrupted or if a participant accidentally ate or drank, the participant’s sampling day was rescheduled and new saliva collection materials were sent in the mail.
After successful completion of sampling, participants were instructed to store their samples in their home freezers before overnighting their samples back to the research team in a pre-paid shipping envelope. Samples were kept frozen at −80°C until assay, and assayed in duplicate for salivary cortisol using a commercially available enzyme immunoassay without modification to the manufacturers recommended protocol (Salimetrics, Carlsbad, CA, USA). The test volume was 25 μl, lower limit of sensitivity was 0.007 μg/dL, and intra- and inter-assay coefficients of variation were less than 10 and 15%, respectively. Duplicate values were averaged to represent the cortisol levels used in all statistical analyses and are reported in ug/dL. Cortisol samples showed substantial deviation from normality and outliers greater than three standard deviations from the group mean were winsorized. After winsorizing the outliers, all cortisol variables were within an acceptable range (Tabachnick et al., 2001).
The cortisol awakening response (CAR) was computed based on the absolute difference between S1 and S1 + 30 (Pruessner et al., 1997b). As a second measure of the CAR, the area under the curve with respect to increase (AUCi) was calculated (the distance of both samples from zero; Chida and Steptoe, 2009). As the peak cortisol awakening response occurs within a narrow time window (20–45 min after awakening; Chida and Steptoe, 2009; Clow et al., 2010), samples were excluded from CAR and AUCi analyses if the time between samples fell outside of 20–45 min after awakening. In addition, we conservatively excluded samples in which the time between samples was unknown (Stalder et al., 2016).
2.4. Survey assessments
Participants completed a questionnaire consisting of basic demographic questions (employment and relationship status) and a series of standardized self-report measures of mental health and wellbeing. By consenting to participate in the survey, veterans also allowed the research team to access their initial application to the service dog provider. Application demographic variables included date of birth, sex, BMI, diagnosis, use of mobility aids, and either the date of service dog placement or date of being approved for those on the waitlist.
2.4.1. Diagnostic and demographic measures
2.4.1.1. PTSD symptoms.
Verification of clinician-reported PTSD diagnosis was assessed using the PTSD Checklist (PCL), a 17-item scale based on the DSM-IV diagnosis (Weathers et al., 1993). The three symptom cluster subscales are re-experiencing (subscale B), avoidance (subscale C), and arousal (subscale D) with higher scores indicating greater overall symptom severity (Forbes et al., 2001). A clinical cutoff of 50 on a scale of 17–85 indicates the presence of a PTSD diagnosis (Forbes et al., 2001). Cronbach’s α’s in the current sample was 0.92 for the total score and 0.96, 0.93, and 0.93 for the three subscales, respectively.
2.4.1.2. Medication use.
A medication questionnaire asked the participant to list all current medication names and their uses (either daily or as needed). Following Granger and colleagues (Granger et al., 2009), medications were coded for their potential to impact salivary cortisol synthesis or secretion. Using this method, a total score of the number of medications influencing cortisol was created for each participant.
2.4.1.3. Physical health.
The Physical Component Score (PCS) of the VR-12 was used to assess general physical health. The six PCS items correspond to general health perceptions, physical functioning, role limitations due to physical problems, and bodily pain.
2.4.2. Behavior measures
2.4.2.1. Patient-Reported outcome measurement information system (PROMIS).
PROMIS is a system of highly reliable, precise measures of patient-reported health status for physical, mental, and social wellbeing (Cella et al., 2010). The PROMIS adult short forms of Anxiety (8A), Anger (5A), Alcohol Use (7A), and Sleep Disturbance (8A) were used with higher scores indicating greater severity. Reliability was high for all subscales (Cronbach’s α ranged from 0.90–0.95).
2.4.2.2. Sleep quality.
6.0.4.2.1 The PSQI is a 19-item scale of self-reported sleep quality over the past one month. Scoring is based on seven components of (1) subjective sleep quality, (2) sleep latency, (3) sleep duration, (4) habitual sleep efficiency, (5) sleep disturbance, (6) use of sleep medication, and (7) daytime dysfunction. The summary score is a continuous variable with lower scores indicating better overall sleep quality.
2.5. Data analysis strategy
Data cleaning, descriptive statistics, and correlations among all study variables were conducted prior to all analyses. Next, possible group differences in demographic characteristics were examined. To examine possible differences in CAR profile between those with and without a service dog, we examined a two-level mixed model controlling for demographic and physical health variables. Mixed-effects modeling, also known as hierarchical or multi-level modeling, was selected to account for both within- and between-participant variation (Raudenbush and Bryk, 2002). This technique is also recommended by the CAR expert consensus guidelines (Stalder et al., 2016) to handle the continuous dynamics of time, missing values, heteroscedasticity, and autocorrelations in the error structure of cortisol data.
In the mixed model, the levels represent repeated measures over time (Level 1) nested within individuals (Level 2); thus, day and individual were included as random factors. The primary outcome of interest was having a service dog, included as a fixed factor. Additional fixed factors were included as covariates to address relevant state and trait covariates as recommended by the CAR expert consensus guidelines (Stalder et al., 2016). These fixed factors included demographic variables (age, sex), physical health (VR-12 PCS, use of a mobility aid, BMI), medication and substance use (total number of medications taken that may influence cortisol, PROMIS Alcohol Use), sleep quality (PROMIS Sleep Disturbance), salivary cortisol at awakening, and time of awakening.
3. Results
3.1. Preliminary analyses
Prior to analyses, all data were screened. A total of 85 participants provided saliva samples, including 52 with a service dog and 33 on the waitlist to receive a service dog (participation rate of 27.96%). A total of 10 participants failed to use the text messaging system; due to unknown sampling times, these participants were conservatively excluded from analyses. Two participants that provided saliva samples had sample quantities insufficient for assay on both days, and were also excluded from analyses. On one of the two days of sampling, an additional three participants had sample quantities insufficient while 10 participants were noncompliant (sample times outside of the 20–45 min CAR window); for these participants, mean values reflect only one sample day rather than two.
The final sample analyzed included 73 participants, including 45 with a service dog and 28 on the waitlist. Descriptive statistics were examined for all variables of interest by group (Table 1). Participants were an average of 37.03 years old (SD = 7.91), mostly male (80.82%), and predominantly married or cohabitating with a partner (84.93%). Participants did not statistically differ in whether they were currently receiving PTSD treatment nor the frequency in which they attended treatment (p’s > 0.15). There were significant group differences in PTSD symptom severity, which is expected from the service dog intervention. However, on average, participants across both groups had symptom severity above the clinical cutoff of 50 on the PCL (waitlist group M = 69.00, SD = 11.13; service dog group M = 57.38, SD = 13.40). Participants’ S1 and S1 + 30 samples were significantly correlated across days, providing support for the decision to create average values across the two days (S1 r (69) = 0.30, p < 0.01; S1 + 30 r (69) = 0.34, p < 0.01).
Table 1.
Demographic and clinical characteristics of participants across groups.
| Group | Group difference | |||
|---|---|---|---|---|
| Waitlist (n = 28) | Service Dog (n = 45) | t or χ 2 | p | |
| Age, M (S.D.), years | 37.29 (7.26) | 36.87 (8.36) | −0.22 | 0.83 |
| Gender, n (%) male | 21 (75.00) | 38 (84.44) | 0.99 | 0.32 |
| Relationship status, n (%) married/cohabitating | 22 (78.57) | 40 (88.88) | 1.44 | 0.23 |
| Employed, n (%) | 11 (39.29) | 9 (20.00) | 2.82 | 0.09 |
| BMI, M (S.D.) | 29.57 (5.81) | 30.13 (4.41) | 0.46 | 0.65 |
| Using a mobility aid, n (%) | 13 (46.43) | 15 (33.33) | 1.25 | 0.26 |
| Education, n (%) | 5.96 | 0.20 | ||
| High School or GED | 2 (7.14) | 7 (15.56) | ||
| Some College | 13 (46.43) | 28 (62.22) | ||
| Bachelor’s Degree | 7 (25.00) | 5 (11.11) | ||
| Postgraduate Degree | 6 (21.43) | 5 (11.11) | ||
| Military branch, n (%) | 1.32 | 0.73 | ||
| Air Force | 3 (10.71) | 6 (13.33) | ||
| Army | 15 (53.57) | 26 (57.78) | ||
| Marines | 5 (17.86) | 4 (8.89) | ||
| Navy | 5 (17.86) | 9 (20.00) | ||
| Traumatic Brain Injury (TBI) comorbidity n (%) | 8 (28.57) | 9 (20.00) | 0.71 | 0.40 |
| Receiving PTSD treatment, n (%) | 23 (82.14) | 35 (77.78) | 1.96 | 0.38 |
| Treatment sessions per year, M (S.D.)a | 39.85 (34.61) | 27.68 (33.53) | −1.45 | 0.15 |
Note: M, Mean; S.D., Standard deviation; BMI, Body mass index; PTSD, Posttraumatic stress disorder.
Among those receiving PTSD treatment.
3.2. Effect of PTSD service dog on survey assessments
Descriptive statistics of self-report survey measures for both those with a service dog and on the waitlist are displayed in Table 2. As hypothesized, those with a service dog reported significantly less anxiety, anger, and sleep disturbance than those on the waitlist (PROMIS Anxiety t (68) = −3.98, p < 0.001, d = 0.96; PROMIS Anger t (68) = −2.95, p < 0.01, d = 0.73; PROMIS Sleep Disturbance t (69) = −2.71, p < 0.01, d = 0.67). There was no significant group difference in sleep quality, although scores trended in the hypothesized direction (PSQI t (67) = −1.74, p = 0.09, d = 0.43). Although there was no significant group difference in the proportion of individuals who self-reported having consumed alcohol in the past 30 days (46.67% of those with a service dog, and 50.00% of those on the waitlist), those with a service dog who were current users of alcohol reported fewer symptoms of alcohol abuse than those on the waitlist (PROMIS Alcohol t (33) = −2.38, p < 0.05, d = 0.87). Among those with a service dog, sleep disturbance was significantly positively correlated with time since receiving the dog (r (45) = 0.37, p < 0.05); among those on the waitlist, both anger (r (28) = 0.33, p < 0.01) and anxiety (r (28) = 0.26, p < 0.05) were significantly positively correlated with time since being approved for the waitlist.
Table 2.
Comparison of behavior measures between groups.
| Measure | Group | Group difference | |||
|---|---|---|---|---|---|
| Waitlist (n = 28) M (S.D.) | Service Dog (n = 45) M (S.D.) | t | p | d | |
| PTSD Checklist (PCL) | 69.00 (11.13) | 57.38 (13.40) | −3.80 | < 0.001 | 0.94 |
| Re-experiencing (B) Subscale | 19.36 (4.34) | 16.19 (4.23) | −3.04 | < 0.01 | 0.75 |
| Avoidance (C) Subscale | 27.54 (5.12) | 22.55 (6.47) | −3.42 | < 0.001 | 0.84 |
| Arousal (D) Subscale | 22.11 (3.05) | 18.64 (4.27) | −3.96 | < 0.001 | 0.94 |
| Pittsburgh Sleep Quality Index (PSQI) | 16.26 (3.55) | 14.76 (3.45) | −1.74 | 0.09 | 0.43 |
| PROMIS Alcohol Use 7Aa | 53.14 (8.91) | 39.88 (19.51) | −2.38 | 0.02 | 0.87 |
| PROMIS Anxiety 8Aa | 71.31 (7.28) | 64.50 (6.83) | −3.98 | < 0.001 | 0.96 |
| PROMIS Anger 5Aa | 73.24 (8.49) | 66.54 (9.81) | −2.95 | <0.01 | 0.73 |
| PROMIS Sleep Disturbance 8Aa | 66.31 (7.62) | 60.65 (9.19) | −2.71 | <0.01 | 0.67 |
Note: M, mean; S.D., standard deviation; t, t statistic, d, Cohen’s d effect size; PCL, Posttraumatic stress disorder checklist; PROMIS, Patient Reported Outcome Measurement Information System.
All PROMIS instrument scores are represented at normalized t-score metrics in which the general population mean is 50 with a standard deviation of 10.
3.3. Effect of PTSD service dog on CAR
Table 3 contains model output describing the effect of having a service dog on cortisol via the CAR and AUCi. As hypothesized, there was a significant main effect of having a service dog on the salivary cortisol awakening response via both the AUCi (ß = −1.13, p < 0.05) and the CAR (ß = −0.08, p < 0.05). Specifically, after controlling for several covariates that may influence cortisol output, including both state (e.g. medication and alcohol use, sleep quality, cortisol at awakening, and time of awakening) and trait variables (e.g. age, sex, physical health attributes, BMI), individuals with a service dog exhibited both a higher CAR and a higher AUCi compared to those without a service dog while on the waitlist (Table 3; Fig. 1). In addition to having a service dog, both age and S1 were significant predictors of AUCi and CAR while sex was a significant predictor of AUCi, but not CAR. A post-hoc analysis of S1 revealed that there was no significant effect of having a service dog on waking cortisol (ß = −0.04, p = 0.18), and no covariates were significant predictors of S1 (all p’s > 0.18). Among participants with and without a service dog, neither time since receiving the service dog nor time since being approved for the waitlist were significant correlates with AUCi (r’s < 0.15) or CAR (r’s < 0.17; Table 4).
Table 3.
Summary of mixed model analysis of AUCi and CAR.
| AUCi | CAR | |||||
|---|---|---|---|---|---|---|
| Variable | Estimate | p | 95% CI | Estimate | p | 95% CI |
| Intercept | 6.36 | 0.02 | (0.87, 11.85) | 0.40 | 0.03 | (0.05, 0.76) |
| Service Dog Group (Reference: Waitlist) | −1.13 | 0.03 | (−2.15, −0.12) | −0.08 | 0.02 | (−0.15, −0.02) |
| Age | −0.08 | 0.01 | (−0.15, −0.02) | 0.00 | 0.02 | (−0.01, 0.00) |
| Sex (Reference: Female) | 0.99 | 0.12 | (−0.27, 2.24) | 0.06 | 0.12 | (−0.02, 0.14) |
| Use of Mobility Aid (Reference: No) | −0.68 | 0.21 | (−1.74, 0.38) | −0.05 | 0.18 | (−0.11, 0.02) |
| BMI | −2.78 | 0.33 | (−8.42, 2.87) | −0.18 | 0.31 | (−0.55, 0.18) |
| VR-12 PCS | 0.03 | 0.22 | (−0.02, 0.07) | 0.00 | 0.25 | (0.00, 0.00) |
| PROMIS Alcohol Use | 0.05 | 0.09 | (−0.01, 0.11) | 0.00 | 0.11 | (0.00, 0.01) |
| PROMIS Sleep Disturbance | 0.04 | 0.32 | (−0.04, 0.11) | 0.00 | 0.29 | (0.00, 0.01) |
| Cortisol-Influencing Medication | 0.21 | 0.17 | (−0.09, 0.50) | 0.01 | 0.23 | (−0.01, 0.03) |
| Waking Cortisol (S1) | −5.25 | < 0.001 | (−8.02, −2.47) | −0.34 | < 0.001 | (−0.52, −0.17) |
| Wake Time | 0.00 | 0.33 | (0.00, 0.00) | 0.00 | 0.33 | (0.00, 0.00) |
Note: AUCi, Area under the curve with respect to increase; CAR, Cortisol awakening response in μg/dL; CI, confidence interval; BMI, Body Mass Index; VR-12 PCS, Veteran’s Rand 12 item Health Survey- Physical Health Component Score; PROMIS, Patient-Reported Outcome Measurement Information System; Waking cortisol (S1), cortisol at awakening in μg/dL.
Fig. 1.
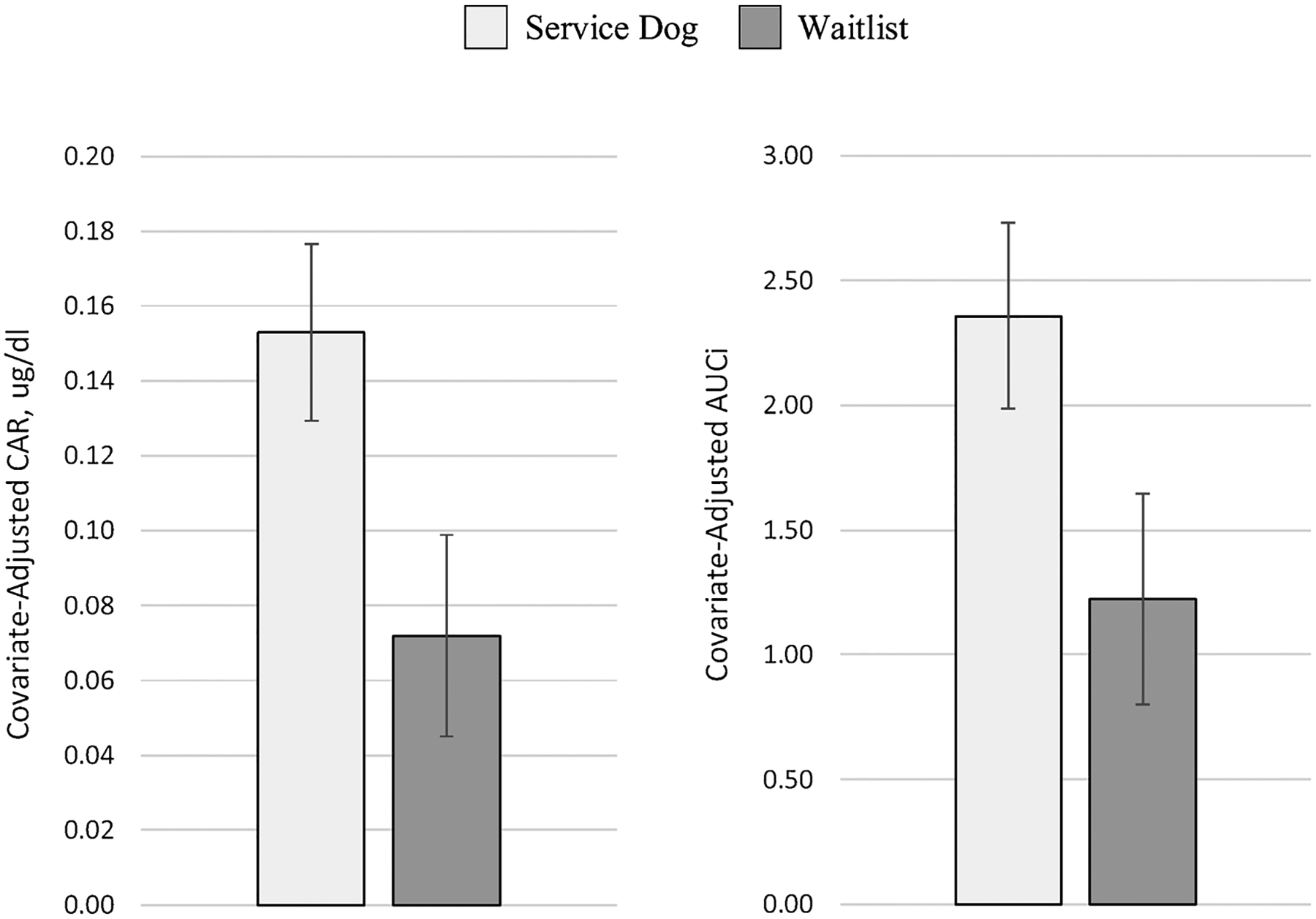
Graphic display of CAR and AUCi by group.
Note: Covariate-adjusted CAR and AUCi are displayed as least square means (LSM) from mixed model output, controlling for age, sex, use of a mobility aid, body mass index (BMI), physical health (VR-12 PCS), alcohol use, sleep disturbance, cortisol-influencing medication, waking cortisol value, and wake time.
CAR, Cortisol awakening response in μg/dL; AUCi, Area under the curve with respect to increase.
Table 4.
Pearson’s r bivariate correlation matrix among study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 AUCi | 1.00*** | −0.23 | −;0.13 | 0.01 | 0.12 | −;0.10 | −;0.12 | −;0.06 | 0.15 | |
| 2 CAR | 1.00*** | −;0.24 | −;0.15 | 0.01 | 0.09 | −;0.11 | −;0.13 | −;0.08 | 0.17 | |
| 3 S1 | −0.24 | −0.24 | −;0.14 | 0.07 | 0.2 | 0.11 | 0.29 | 0.11 | −;0.25 | |
| 4 PTSD Checklist | −0.05 | −0.04 | −0.01 | 0.51 ** | −;0.09 | 0.64 *** | 0.38 * | 0.42 * | −;0.09 | |
| 5 PSQI | −0.13 | −0.13 | −0.04 | 0.29 | 0.24 | 0.34 | 0.36 | 0.64 *** | 0.01 | |
| 6 Alcohol Usea,b | 0.14 | 0.14 | 0.00 | 0.24 | 0.11 | 0.00 | 0.34 | 0.25 | −;0.05 | |
| 7 Anxietya | −0.09 | −0.09 | −0.21 | 0.60*** | 0.05 | 0.17 | 0.3 | 0.22 | −;0.27 | |
| 8 Angera | −0.11 | −0.10 | 0.02 | 0.59*** | 0.13 | 0.36* | 0.53*** | 0.58 ** | 0.00 | |
| 9 Sleep Disturbancea | 0.18 | 0.19 | −0.16 | 0.49** | 0.63*** | 0.27 | 0.07 | 0.17 | −;0.03 | |
| 10 Timec | −0.06 | −0.04 | 0.17 | 0.31* | 0.11 | 0.10 | 0.15 | 0.09 | 0.35* |
Note: Correlations for those with a service dog are displayed below the diagonal, while correlations for those on the waitlist are displayed above the diagonal in bolded text; S.D., Standard deviation; AUCi, Average of area under the curve with respect to increase; CAR, Average of cortisol awakening response; S1; Average of awakening cortisol; PTSD, Posttraumatic Stress Disorder as quantified through the PTSD Checklist (PCL); PSQI, Pittsburgh Sleep Quality Index;
p < 0.001,
p < 0.01,
p < 0.05.
Patient Reported Outcome Measurement Information System (PROMIS) short forms Alcohol Use 7A, Anxiety 8A, Anger 5A, and Sleep Disturbance 8A.
Alcohol Use questionnaire only filled out by those who indicated that they had consumed alcohol in the past 30 days, which consisted of n = 21 (46.7%) with a service dog and n = 14 (50.0%) on the waitlist.
For participants with a service dog, this continuous variable represents time elapsed since receiving a service dog; For participants on the waitlist, this variable represents time since applying and being placed on the waitlist to receive a service dog.
3.4. Correlational analyses with PTSD severity
Correlations between outcome variables and PTSD symptom severity for both those with a service dog and on the waitlist are displayed in Table 4. Among those with a service dog, PTSD symptomology as measured through the PCL was not significantly correlated with CAR or with AUCi, but was significantly positively correlated with anxiety, anger, and sleep disturbance (Table 4). Among those on the waitlist, PTSD symptomology was again not significantly correlated with CAR or with AUCi and was similarly positively correlated with anxiety, anger, and sleep disturbance as well as sleep quality. Among both groups neither CAR nor AUCi were significantly correlated with any behavioral outcomes or with S1 (Table 4).
Further analyses indicated that none of the three PCL subscales (re-experiencing, avoidance, and arousal) among those with and without a service dog were significantly correlated with AUCi (all p’s > 0.20) or CAR (all p’s > 0.18). Correlations between PTSD symptom severity and cortisol outcomes were even less after conducting partial correlation analyses controlling for age, gender, medication use, and physical health (all p’s > 0.24).
4. Discussion
The purpose of this study was to examine the potential physiological and arousal-modulating effects of the placement of a specially trained PTSD service dog on a population of military members and veterans with PTSD compared to a usual care, waitlisted control group. To our knowledge, this research represents the first study to assess the effects of service dogs on military members and veterans with PTSD using an objective, physiological measure of stress and arousal. Results indicated that after controlling for demographic and physical health covariates, having a PTSD service dog was significantly associated with a higher morning cortisol awakening response, supporting the main hypothesis. Compared to those on the waitlist, participants with a service dog also self-reported significantly lower anxiety, anger, sleep disturbance, and alcohol abuse supporting the secondary hypothesis. Findings suggest that in combination with usual care, service dogs may confer therapeutic psychological and physiological effects on military veterans with PTSD.
4.1. CAR and AUCi findings
After controlling for several state and trait variables known to influence HPA-axis activity, participants with a service dog exhibited a significantly higher CAR and AUCi compared to a waitlisted control group. While the presence of a service dog was not a significant predictor of waking cortisol values, having a service dog was specifically associated with a larger magnitude of the awakening response. While it has been debated whether a smaller or larger CAR is indicative of a healthier state, especially in individuals with histories of traumatic exposure and/or psychosocial dysfunction (Chida and Steptoe, 2009), the results from this study provide support that, in this specific population, a higher CAR may be indicative of better health and wellbeing. Specifically, both results from this study and additional data show that those with a service dog exibit significantly lower PTSD symptomology than those on the waitlist in addition to greater psychosocial wellbeing, less depressive symptoms, higher social functioning, and better overall quality of life (O’Haire and Rodriguez, 2018).
The result of a significantly higher CAR among the treatment group in this study mirror that of studies that have examined the effects of PTSD treatment on cortisol outcomes other than the CAR. Specifically, results are supported by findings of a 2007 study in which n = 21 civilians with PTSD receiving a form of cognitive behavioral therapy exhibited increased post-treatment A.M. basal cortisol (Olff et al., 2007). Similarly, a 2014 study reported increased 24-hour urinary cortisol output among n = 14 survivors of the 9/11 World Trade Center terrorist attack with PTSD who responded to prolonged exposure treatment (Yehuda et al., 2014). In this regard, successful treatment of PTSD symptoms via a service dog or psychotherapies may correspond with increases in HPA-activity and thus, in circulating cortisol. However, among the studies published to date that have examined the CAR in particular as an outcome variable in PTSD treatment studies, the results of this study contribute to mixed findings likely due to methodological and population differences. Specifically, Pacella et al. (2014) found no pre- and post-treatment difference in CAR or AUCi among civilians with PTSD receiving prolonged exposure therapy or anti-depressant medication. However, saliva was only collected for a single day introducing a potential for low validity (Clow et al., 2004). Bergen-Cico et al. (2014) found that veterans with PTSD who completed a mindfulness-based treatment had a reduced CAR and AUCi compared to baseline, but results were limited by small sample size (n = 9). Future research is necessary to better understand how cortisol biomarkers respond to PTSD treatment in military veteran and civilian populations.
4.2. Survey assessment findings
Participants with a service dog reported significantly better psychosocial functioning than those on the waitlist including exhibiting lower anger, anxiety, and alcohol abuse symptoms, all of which are common symptoms and comorbidities of a PTSD diagnosis. These findings are consistent with the emerging literature providing evidence of a significant effect of the placement of a service dog on standardized measures of PTSD and psychosocial functioning (e.g. Kloep et al., 2017; Vincent et al., 2017; Yarborough et al., 2017). As individuals with a service dog reported less severe PTSD symptomology than those on the waitlist, it is logical that group differences would also be observed in these symptom areas with medium to large effect sizes (PROMIS Anger, d = 0.73; PROMIS Anxiety, d = 0.96; PROMIS Alcohol Use, d = 0.87, PTSD Checklist, d = 0.94).
Sleep quality and sleep disturbance, other areas commonly affected by PTSD, had small to medium effect sizes across group (PSQI sleep quality, d = 0.44; PROMIS sleep disturbance, d = 0.67). Although sleep quality via the PSQI was not significantly different across group in this study, recent findings have shown a significant improvement in PSQI scores (d = 0.82) among military veterans with a PTSD who have had a service dog for 3 months, compared to before they received the service dog (Vincent et al., 2017). Because our study design surveyed individuals with a service dog for varying amounts of time, it is possible that this variation may have contributed to the discrepancy in effect sizes across studies.
Our findings also indicated a significant positive correlation with sleep disturbance and time since receiving a service dog (r = 0.35; but not overall sleep quality, r = 0.11). In addition, there was a significant positive correlation with overall PTSD symptoms and time since receiving a service dog (r = 0.31). Thus, although those with a service dog had significantly lower levels of sleep disturbance (d = 0.67) and PTSD symptoms (d = 0.94) on average compared to those on the waitlist, there may be change in symptomology over time that is unexplained by current findings. While these correlations were small in magnitude, they warrant future research and replication using objective measures of sleep (e.g. actigraphy) and a longitudinal design.
4.3. PTSD and the CAR
While results did indicate a significant main effect of having a service dog on the CAR, there was no significant relationship between the CAR profile and PTSD symptomology (despite a large effect size difference in PTSD severity among those with a service dog compared to those on the waitlist). This unsupported relationship between the CAR and PTSD symptoms among our sample suggests that the potential physiological stress-buffering effects from the service dog on the CAR were independent of the service dog’s subjective effect on PTSD symptomology. Further, this result also suggests that perceived hyperarousal symptoms (the subscale of the PTSD checklist that exhibited the greatest group difference) may be similarly independent of the PTSD service dog’s psychophysiological effect on arousal via the HPA axis. While this is an interesting preliminary finding, further research will require a within-subject longitudinal design to investigate the potential interaction effects between individual differences in the CAR and PTSD symptomology following the placement of a service dog.
4.4. Limitations
Outcomes from this preliminary, cross-sectional study should be interpreted with several important limitations. First, the use of a cross-sectional design means that causation and directionality cannot be determined. Although findings suggest a higher CAR and AUCi in those with a service dog compared to those on the waitlist, it is unknown how these physiological measures may change over time and how observed variance in the CAR/AUCi may be attributable to individual differences. For example, Pacella et al. (2014) found that PTSD treatment responders had a higher AUCi at baseline than non-responders (d = 1.12). Future, within-subject studies are needed to determine how pre-treatment CAR (i.e. having a decreasing, flat, or increasing CAR) and waking cortisol as well as latent, trait-specific effects on HPA activity may predispose an individual to experience either decreases or increases in morning cortisol output following pairing with a PTSD service dog.
Another limitation of the study was the use of a simplified sampling design assessing only two samples across two days to minimize participant burden instead of a more time-intensive design (i.e. 15, 30, and 45 min after awakening across 2+ days). Although the assessment of CAR with two awakening samples is a common approach, some reliability in the measurement of the CAR may have been lost (Clow et al., 2004). Specifically, many individuals in the sample may not have exhibited a peak in cortisol secretion right at 30 min, preventing us from obtaining a precise measurement of the CAR across individuals. In addition, while our mixed model analyses did control for covariates suggested by expert guidelines (Stalder et al., 2016), there may be unaccounted for confounds that could have impacted the CAR. In particular, the presence of other pets in the home, especially pet dogs, may be a key confounding factor that will be useful to examine in future, longitudinal research.
An additional limitation of the study is that both the waitlisted control group as well as the treatment group were receiving unrestricted access to PTSD treatment as usual, current treatments engaged in at the time of surveying were unknown. Although the distribution of those receiving treatment and number of sessions per year were not statistically different across groups, there is a possibility that those on the waitlist may have been engaging in different types of treatment for PTSD than those with a service dog. However, our goal was to conduct an ecologically valid and preliminary assessment of the potential physiological differences across those with and without a service dog. Future, more resource-intensive studies will benefit from carefully distinguishing the effects of the placement of a PTSD service dog from other evidence-based treatments individuals may be receiving. Additionally, because allocation to the treatment group was not randomized, it remains unclear if the observed differences in HPA axis activity among those with a PTSD service dog were simply due to the passage of time during treatment. While it was not possible to randomize treatment in this preliminary study, this is a future direction to be addressed in a large-scale, clinical trial.
A final limitation is the systematic biases that may have been present in our sample. Participants had volunteered for the treatment and were thus amenable to being placed with a service dog, therefore our findings may not generalize to the average military veteran with PTSD. Additionally, consent bias may have been present such that individuals who participated in the study may not have been representative of the true sample.
4.5. Future research
While the findings from this research suggest that the HPA axis may be sensitive to the effects of a PTSD service dog, precise mechanisms for these effects remain speculative and will be an important future direction of research. While anecdotal and qualitative reports consistently state that one of the most helpful and therapeutic aspects of a PTSD service dog is their calming and stress-reducing abilities (Crowe et al., 2017; Taylor et al., 2013; Yount et al., 2013) and studies on the therapeutic efficacy of service dogs for PTSD continue to find evidence of reduced self-reported hyperarousal symptoms (Kloep et al., 2017; O’Haire and Rodriguez, 2018; Vincent et al., 2017; Yarborough et al., 2017), it remains unclear how changes in PTSD symptoms and hyperarousal relate to changes in the HPA-activity. Specifically, this study was not able to determine if changes to the CAR are psychophysiological in nature (increasing as a result of these positive psychological benefits experienced by the PTSD service dog’s presence) or potentially either preceding or even moderating these perceived outcomes. Despite growing knowledge in the study of animal-assisted intervention for trauma including PTSD (O’Haire et al., 2015), future research will benefit from incorporating physiological measurement using longitudinal designs to address this gap in the knowledge base (Dreschel and Granger, 2016).
Future research may also help in determining the specific aspects of the HPA-axis that a service dog may impact by collecting more cortisol samples throughout the day. For example if only the CAR, rather than diurnal secretion of cortisol, differs across group this may indicate that the therapeutic effects of the PTSD service dog are CAR-specific rather than applying to the HPA-axis more generally. It may also be helpful to measure the CAR across more days (including weekend days) as a measure of state variation and CAR flexibility, which can also be predictive of individual differences in psychosocial wellbeing (Law et al., 2013; Mikolajczak et al., 2010). In addition, as Elder et al. (2016) points out, both waking cortisol levels and the magnitude of the CAR are potentially sensitive to daily variation; measuring the CAR across 3–4 days instead of two days would greatly aid in the interpretation of results (Elder et al., 2016).
Finally, future research will benefit from examining canine-specific traits, training, and relationship factors that may impact the physiological activity of their handlers. In particular, specific trained commands (e.g. calming and distracting from anxiety or intrusive thoughts), behavioral profiles (e.g. non-reactive temperaments), or relationship factors (owner-dog attachment) may be key moderators of psychophysiological activity (Schöberl et al., 2015). In addition, examining the co-regulation or attunement between the cortisol outputs of both a military veteran participant and their service dog may be beneficial for examining the dyadic factors underlying physiological change (Haubenhofer and Kirchengast, 2007; Dreschel and Granger, 2016).
4.6. Conclusion
In conclusion, this novel and preliminary study quantified the therapeutic effects of PTSD service dogs using both subjective measurement and objective, physiological measurement of stress and arousal. Results provide initial evidence that, compared to usual care alone, military members and veterans placed with PTSD service dogs exhibit lower PTSD symptomology, better psychosocial wellbeing, and higher morning cortisol output quantified via the CAR and AUCi. However, PTSD severity and cortisol outcomes were not significantly related, suggesting that the psychosocial and physiological effects of a service dog may be independent of each other. Future longitudinal research will contribute to a more precise understanding in how within-subject change in cortisol activity is related to psychosocial functioning and hyperarousal symptoms in military veterans with PTSD. If replicated with a longitudinal design, the findings from this study could have clinical implications suggesting that the CAR is an effective biomarker for capturing psychophysiological change following receipt of a service dog.
Acknowledgements
This research was funded by the Human-Animal Bond Research Institute (HABRI) and Morris Animal Foundation grant #D15HA-031 and Bayer Animal Health. This publication was made possible with partial support from Grant # KL2TR001106 and UL1TR001108 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to acknowledge K9s For Warriors for their assistance in conducting this study.
Footnotes
Conflict of interest statement
In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC and these relationships are managed by the policies of the committees on conflict of interest at Johns Hopkins University School of Medicine and the University of California at Irvine.
References
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-V, 5th ed. Author, Washington, DC. [Google Scholar]
- Beetz A, Kotrschal K, Turner DC, Hediger K, Uvn√§s-Moberg K, Julius H, 2011. The effect of a real dog, toy dog and friendly person on insecurely attached children during a stressful task: an exploratory study. Anthrozoös 24, 349–368. [Google Scholar]
- Bergen-Cico D, Possemato K, Pigeon W, 2014. Reductions in cortisol associated with primary care brief mindfulness program for veterans with PTSD. Med. Care 52, S25–S31. [DOI] [PubMed] [Google Scholar]
- Boggero IA, Hostinar CE, Haak EA, Murphy ML, Segerstrom SC, 2017. Psychosocial functioning and the cortisol awakening response: meta-analysis, P-curve analysis, and evaluation of the evidential value in existing studies. Biol. Psychol 129, 207–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomyea J, Lang AJ, 2012. Emerging interventions for PTSD: future directions for clinical care and research. Neuropharmacology 62, 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Killeen TK, Brewerton T, Lucerini S, 2000. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J. Clin. Psychiatry [PubMed] [Google Scholar]
- Brown PJ, Wolfe J, 1994. Substance abuse and post-traumatic stress disorder comorbidity. Drug Alcohol Depend 35, 51–59. [DOI] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, 2010. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol 63, 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2009. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol. Psychol 80, 265–278. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F, 2004. The awakening cortisol response: methodological issues and significance. Stress 7, 29–37. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L, 2010. The cortisol awakening response: more than a measure of HPA axis function. Neurosci. Biobehav. Rev 35, 97–103. [DOI] [PubMed] [Google Scholar]
- Crowe TK, Sánchez V, Howard A, Western B, Barger S, 2017. Veterans transitioning from isolation to integration: a look at veteran/service dog partnerships. Disabil. Rehabil 1–9. [DOI] [PubMed] [Google Scholar]
- Elder GJ, Ellis JG, Barclay NL, Wetherell MA, 2016. Assessing the daily stability of the cortisol awakening response in a controlled environment. BMC Psychol 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D, Creamer M, Biddle D, 2001. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav. Res. Ther 39, 977–986. [DOI] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH, 2009. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology 34, 1437–1448. [DOI] [PubMed] [Google Scholar]
- Haubenhofer DK, Kirchengast S, 2007. ‘Dog handlers’ and dogs’ emotional and cortisol secretion responses associated with animal-aassisted therapy sessions. Soc. Anim 15, 127–150. [Google Scholar]
- Hoge CW, Grossman S, Auchterlonie J, Riviere L, Milliken C, Wilk J, 2014. PTSD treatment for soldiers after combat deployment: low utilization of mental health care and reasons for dropout. Psychiatr. Serv 65, 997–1004. [DOI] [PubMed] [Google Scholar]
- Jakupcak M, Cook J, Imel Z, Fontana A, Rosenheck R, McFall M, 2009. Posttraumatic stress disorder as a risk factor for suicidal ideation in Iraq and Afghanistan war veterans. J. Trauma. Stress 22, 303–306. [DOI] [PubMed] [Google Scholar]
- Kloep ML, Hunter RH, Kertz SJ, 2017. Examining the effects of a novel training program and use of psychiatric service dogs for military-Related PTSD and associated symptoms. Am. J. Orthopsychiatry [DOI] [PubMed] [Google Scholar]
- Krause-Parello CA, Sarni S, Padden E, 2016. Military veterans and canine assistance for post-traumatic stress disorder: a narrative review of the literature. Nurse Educ. Today (Advance online publication). [DOI] [PubMed] [Google Scholar]
- Law R, Hucklebridge F, Thorn L, Evans P, Clow A, 2013. State variation in the cortisol awakening response. Stress 16, 483–492. [DOI] [PubMed] [Google Scholar]
- Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillée C, de Timary P, 2010. Cortisol awakening response (CAR)’s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology 35, 752–757. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J, 2012. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev 32, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME, Rodriguez K, 2018. Preliminary efficacy of service dogs as a complementary treatment for posttraumatic stress disorder in military members and veterans. J. Consult. Clin. Psychol 86, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Haire ME, Guérin NA, Kirkham AC, 2015. Animal-assisted intervention for trauma: a systematic literature review. Front. Psychol 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendaal J, Meintjes R, 2003. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J 165, 296–301. [DOI] [PubMed] [Google Scholar]
- Olff M, de Vries GJ, Güzelcan Y, Assies J, Gersons BP, 2007. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology 32, 619–626. [DOI] [PubMed] [Google Scholar]
- Olmert MD, Nordstrom M, Peters M, St Laurent M, Yount R, 2015. Canine connection therapy: finding purpose and healing through the training of service dogs. In: Ritchie EC (Ed.), Posttraumatic Stress Disorder and Related Diseases in Combat Veterans Springer International Publishing, Cham, pp. 197–209. [Google Scholar]
- Pacella ML, Feeny N, Zoellner L, Delahanty DL, 2014. The impact of PTSD treatment on the cortisol awakening response. Depress. Anxiety 31, 862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polheber J, Matchock R, 2013. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J. Behav. Med 1–8. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Wolf O, Hellhammer D, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C, 1997a. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61, 2539–2549. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, Kaspers F, Kirschbaum C, 1997b. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci 61, 2539–2549. [DOI] [PubMed] [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, Caldarone LB, 2010. Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. J. Trauma. Stress 23, 59–68. [DOI] [PubMed] [Google Scholar]
- Rapcencu A, Gorter R, Kennis M, van Rooij S, Geuze E, 2017. Pre-treatment cortisol awakening response predicts symptom reduction in posttraumatic stress disorder after treatment. Psychoneuroendocrinology 82, 1–8. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, 2002. Hierarchical Linear Models: Applications and Data Analysis Methods Sage. [Google Scholar]
- Ryan R, Booth S, Spathis A, Mollart S, Clow A, 2016. Use of salivary diurnal cortisol as an outcome measure in randomised controlled trials: a systematic review. Ann. Behav. Med 50, 210–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöberl I, Beetz A, Solomon J, Gee N, Kotrschal K, 2015. Social factors influencing cortisol modulation in dogs during a strange situation procedure. J. Vet. Behav.: Clin. Appl. Res [Google Scholar]
- Schottenbauer MA, Glass CR, Arnkoff DB, Tendick V, Gray SH, 2008. Nonresponse and dropout rates in outcome studies on PTSD: review and methodological considerations. Psychiatry 71, 134–168. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH, 2016. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. [DOI] [PubMed] [Google Scholar]
- Steenkamp MM, Litz BT, Hoge CW, Marmar CR, 2015. Psychotherapy for military-related ptsd: a review of randomized clinical trials. JAMA 314, 489–500. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Chrousos GP, 1995. Neuroendocrinology and pathophysiology of the stress system. Ann. N. Y. Acad. Sci 771, 1–18. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, Osterlind SJ, 2001. Using Multivariate Statistics
- Taylor MF, Edwards ME, Pooley JA, 2013. Nudging them back to reality: toward a growing public acceptance of the role dogs fulfill in ameliorating contemporary veterans’ PTSD symptoms. Anthrozoos 26, 593–611. [Google Scholar]
- Viau R, Arsenault-Lapierre G, Fecteau S, Champagne N, Walker C-D, Lupien S, 2010. Effect of service dogs on salivary cortisol secretion in autistic children. Psychoneuroendocrinology 35, 1187–1193. [DOI] [PubMed] [Google Scholar]
- Vincent C, Belleville G, Gagnon D, Dumont F, Auger E, Lavoie V, Besemann M, Champagne N, Lessart G, 2017. Effectiveness of service dogs for veterans with PTSD: preliminary outcomes. Stud. Health Technol. Inform 242, 130. [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T, 1993. The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. Annual convention of the international society for traumatic stress studies 462. [Google Scholar]
- Yarborough BJH, Owen-Smith Ashli A., Stumbo Scott P., Yarborough Micah T., Perrin Nancy A., Green Carla A., 2017. 2017.an observational study of service dogs for veterans with posttraumatic stress disorder. Psychiatr. Serv (0, appi.ps.201500383.). [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ, 1996. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol. Psychiatry 40, 79–88. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Pratchett L, Elmes M, Lehrner A, Daskalakis N, Koch E, Makotkine I, Flory J, Bierer L, 2014. Glucocorticoid-related predictors and correlates of post-traumatic stress disorder treatment response in combat veterans. Interface Focus 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount RA, Olmert MD, Lee MR, 2012. Service dog training program for treatment of posttraumatic stress in service members. U.S. Army Med. Department J 63–69. [PubMed] [Google Scholar]
- Yount RA, Ritchie EC, Laurent MS, Chumley P, Olmert MD, 2013. The role of service dog training in the treatment of combat-related PTSD. Psychiatr. Ann 43, 292–295. [Google Scholar]


