Abstract
A wide range of mental illnesses show high rates of addiction comorbidities regardless of their genetic, neurodevelopmental and/or adverse-environmental etiologies. Understanding how the spectrum of mental illnesses produce addiction vulnerability will be key to discovering more effective preventions and integrated treatments for adults with addiction and dual diagnosis comorbidities. A population of N=131 rats containing a spectrum of etiological mental illness models and degrees of severity was experimentally generated by crossing neonatal ventral hippocampal lesions (NVHL; n=68) or controls (SHAM-operated; n=63) with adolescent rearing in environmentally/socially enriched (ENR; n=66) or impoverished (IMP; n=65) conditions. This population was divided into 2 experiments: first, examining NVHL and IMP effects on novelty and mild stress-induced locomotion across 3 adolescent ages; second, looking at initial cocaine reactivity and long-term cocaine behavioral sensitization in adulthood. NVHL and IMP-environmental conditions independently produced remarkably similar and robustly significant abnormalities of hyper-reactivity to novelty, mild stress, and long-term cocaine sensitization. The combined NVHL-IMP groups showed the most severe phenotypes across the board, so that the mental illness and addiction vulnerability phenotypes increased together in severity in a consistent step-wise progression from the healthiest rats to those with the greatest loading of etiological models. These findings add weight to our understanding of mental illness and addiction vulnerability as brain disorders that are biologically and developmentally unified in ways that transcend etiological causes, and yet co-intensify with increased loading of etiological conditions. Combining neurodevelopmental and adverse-environmental models of mental illness may provide an approach to identifying and therapeutically targeting cortical-striatal-limbic network mechanisms that generate addiction and dual diagnosis diseases.
Keywords: Addiction, Dual Diagnosis, Mental illness, Cocaine, Ventral Hippocampus, Environmental Deprivation
INTRODUCTION
Mental illnesses and drug addictions often co-occur in humans [1,2]. A growing body of basic and clinical neuroscience indicates that the brain pathologies of these disorders are anatomically overlapping, and causally bidirectional, involving shared genetic and environmental risk factors [3–7]. Furthering our understanding of how these two major classes of brain diseases interact biologically and developmentally to produce ‘dual diagnosis’ clinical presentations will be crucial to designing better prevention and treatment strategies [8]. Toward this goal, two decades of animal modeling of dual diagnosis has implemented a neurodevelopmental rodent model of schizophrenia, produced by neonatal ventral hippocampal lesions (NVHLs), across a range of addiction paradigms [9–12]. This body of work has been fruitful in not only replicating the development and clinical phenomenology of mental illness and addictions comorbidities routinely observed in humans, but in characterizing neural substrates in mental illness that involuntarily increase risk and severity of addiction disease [13–15].
This study examines the independent and combined effects of 1) early neurodevelopmental abnormalities that lead to adult mental illness (as modeled by NVHLs), and 2) impoverished adolescent rearing experience, on endophenotypes core to both mental illness and addiction vulnerability. After its introduction in 1993 [16], the NVHL model has been characterized in over a hundred studies, as reproducing most of the major behavioral, developmental, neurobiological and pharmacological features of human schizophrenia [17]. The model’s schizophrenia-like positive symptom set, including behavioral hyper-reactivity to novel or stressful stimuli, or dopamine receptor stimulation, shows post-adolescent worsening and is reducible with neuroleptics. In contrast, NVHL negative-like symptoms and cognitive impairments are not neuroleptic responsive and do not show peri-adolescent onset. NVHLs also produce a wide range of behavioral and motivational hypersensitivities to addictive drugs, indicating that the mental illness syndrome and addiction vulnerability are unified disease processes in the brain. Specifically, while cocaine and amphetamine produce hyperactive locomotor responses in NVHL rats upon first exposure [16, 18]. NVHL rats also show abnormal elevations in short and long-term behavioral sensitization after repeated-chronic exposures to cocaine, nicotine and alcohol [18–20]. Suggestive of how these abnormal sensitization patterns reflect mental illness-induced amplification of neuroadaptative processes that underpin addiction [21, 13], NVHL rats also show elevated vulnerabilities and severities of addicted phenotypes in self-administration of cocaine, methamphetamine, nicotine, alcohol [9, 10, 22, 12], and multidrug self-administration (e.g. with nicotine and alcohol) [23].
Prolonged living in environments that are relatively deprived of complex stimuli and social contacts has long been known to produce a range of behavioral abnormalities in mammals, as reported in laboratory settings, and in various non-research contexts such as zoos and prisons [24, 25]. In parallel with the NVHL model, these environments can have a profound biological impact on neurocircuits and behavior that generate both psychiatric symptoms and addiction vulnerability [26–30] including augmentation of cocaine self-administration [31, 32]. The present study is the first to examine the independent and integrated effects of these two forms of brain pathogenesis, on fundamental behavioral measures of mental illness and addiction vulnerability, through adolescence and early adulthood. Using this combined mental illness model approach, we could thus generate a population of subjects that contain multiple etiologies of mental illness, and a wider range of mental illness severities, for observing if and how addiction vulnerability phenotypes vary according to mental illness condition. In the first experiment we measured how NVHLs and/or adverse-impoverished (IMP) rearing impact novelty and mild-stress locomotor reactivity, tested at three ages from early adolescence to young adulthood. In a second experiment, we examined how the mental illness model groups differentially respond to initial and chronic (behaviorally sensitizing) doses of cocaine in early adulthood. Characterization of how these neurodevelopmental and environmentally-induced neuropathologies compare and interact to raise addiction risk could thus contribute to a deeper understating of the multifactorial nature of addiction disease, and how it is worsened in the brain by different etiologies and degrees of severity of mental illness.
METHODS AND MATERIALS
Subjects and Neonatal Surgeries
Sprague Dawley pups born from females arriving at 14-17 days gestation (Harlan, Indianapolis) were culled to males on post-natal day (PD)-3 in preparation for surgeries on PD7. A total of 147 pups weighing 16-19 grams were randomized to NVHL vs. SHAM surgeries in ratios of about 4:3 (to anticipate inaccurate lesion attrition) as detailed in [33]. Briefly, ibotenic acid (3.0 μg; Sigma) in 0.3 μL artificial CSF (or aCSF only) was delivered to the ventral hippocampus bilaterally (A/P - 3.0 mm, M/L ± 3.5mm, DV −5.0mm, bregma) under hypothermic anesthesia. Pups were returned to their litters and reared under standard conditions until weaning (PD21). Surgical and experimental procedures were conducted in line with the NIH Guide for the Care and Use of Laboratory Animals and Indiana University IACUC-approved protocols.
Enriched (ENR) vs Impoverished (IMP) Housing
In Experiment 1, on PD21, SHAM and lesioned rats were further randomized to ENR vs IMP environments for 1 week (Group 1A), 3 weeks (Group 1B) vs 5 weeks (Group 1C). ENR vs IMP conditions were intended to be on different extremes in terms of allowing for social interaction, exercise, and a complex/stimulating environment (Figure 1). In ENR housing, rats lived in groups of 8 in a large wire cage complex, with climbable walls comprising 2 towers (76.2 x 45.7 x 91.4 cm high each), connected by an elevated bridge (30.5 x 15.5 x 15.2 cm). The towers had 4 levels linked by 3 ramps and contained numerous toys, a running wheel, and hammocks. This provided a total living space of 643,618.5 cm3, with 21,293.8 cm2 of floor area (including all levels and ramps) for 8 rats, making 80,452.1 cm3 (space) and 2661.7 cm2 (floor) per rat. This contrasted with IMP housing in which rats lived in solitary confinement in plastic cages with no toys, and no climbable walls (36.6 x 30.5 x 19.1 cm high), providing 21,321.3 cm3 (space) and 1,116.3 cm2 (floor) per rat. In both ENR and IMP housing, the same fine woodchip bedding was used (approximately 6 cm) deep, and all rats lived under the same standard feeding (ad lib), lighting, and cage changing conditions.
Figure 1.
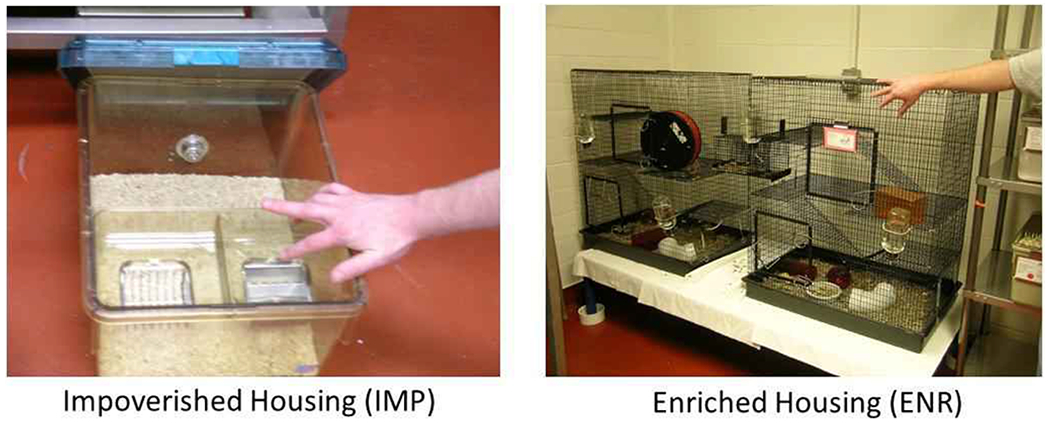
Housing Conditions. Rats raised in Impoverished Housing (IMP) lived alone in a small, bland space. Rats raised in Enriched Housing (ENR) lived in groups of 8 in a large complex environment with ramps and toys. Food, water, and lighting conditions were the same and both housing conditions were in the same room.
In Experiment 2, rats lived with their litter mates (but without their mothers) after weening on PD21, before randomization to ENR vs. IMP housing on PD28. Group 2A rats lived in ENR vs IMP housing for 4 weeks until PD56, and Group 2B for 8 weeks until PD84.
Behavioral Testing
Behavioral paradigms, subgroup numbers, lesion status (after verification), and housing randomizations used in the experimental designs are summarized in Figure 2. In Experiment 1, Groups 1A, 1B and 1C underwent 2 hour testing in locomotor arenas at PD28, PD42 and PD56 respectively (first and only time for each group). Locomotor activity was tested in arenas with clear plastic walls and white floors (43.2 x 43.2 x 30.5 cm) equipped with x,y,z-dimension infrared photo beam arrays with 16 beams spaced 2.5 cm apart (Med Associates, St. Albans, VT). Horizontal distance traveled was measured with Activity Monitory 5.0 Software (Med Associates) under red lighting in a separate room from the rat’s housing, during 2 hour sessions. For all rats, their first hour in the arena was their first ever experience in this environment (or anywhere outside their housing) so that the locomotor activity during this hour was a robust measure of novel environment reactivity. In Experiment 1, however, rats received an injection of saline (1 ml/kg i.p.) in between the first and second hours, whereas in Experiment 2 they all received cocaine (NIDA: 15 mg/kg i.p., dissolved in saline injected at 1 mg/kg). Thus, the second hour in Experiment 1, measured locomotor reactivity to a mild stressor (the saline injection) at different stages of adolescent development. In Experiment 2, the second hour measured reactivity to cocaine for the first time in PD56 rats (Group 2A), or, after a history of a chronic-sensitizing cocaine injection series (15 mg/kg i.p. x 10 days PD56-68, delivered in home housing) in PD84 rats (Group 2B).
Figure 2.
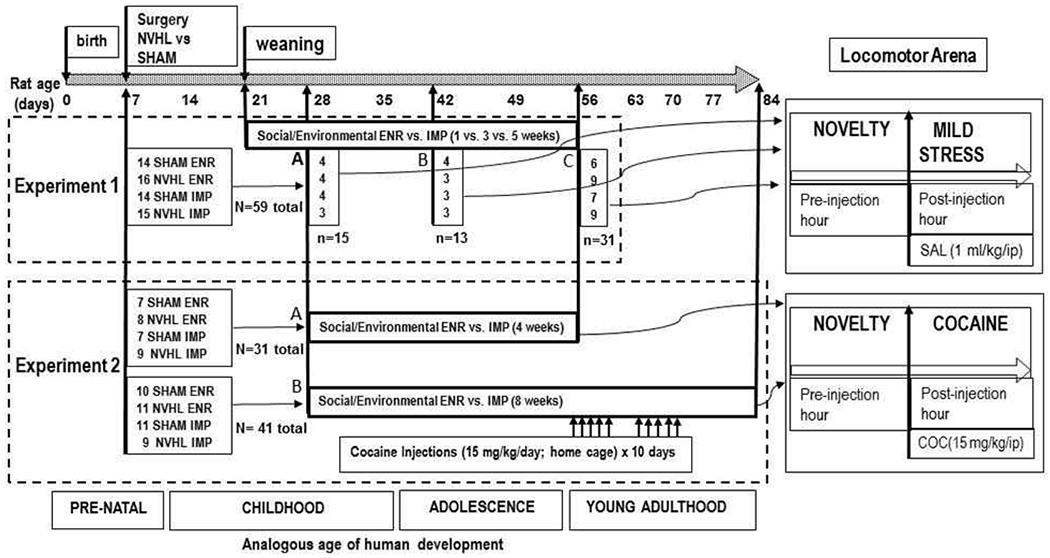
Experimental Design. In Experiment 1, NVHL and SHAM rats were randomized to ENR vs. IMP conditions. 1A rats (PD28-SAL) were tested in the novel arena before and after saline (SAL) injections at Postnatal Day 28 (PD28); 1B (PD42-SAL) were tested at PD42; and 1C (PD56-SAL) were tested at PD56. 2A (PD56-COC) and 2B (PD84-SENS) rats were all tested in the novel arena before and after cocaine (COC) injections, but with 2A rats tested at PD 56 with no prior cocaine history, and 2B rats tested at PD84 after having experienced as sensitizing series of 10 cocaine injections (PD56-PD68).
Histological Lesion Verification
After locomotor testing and sacrifice, brains were cryostat cut into 40 μM coronal sections through the hippocampus. Sections were fixed and 0.5% thionin stained and microscopically examined blind to behavioral data [33]. Figure 3 shows representative histologies. Rats showing bilateral evidence in at least one section of tissue atrophy, paucity or misshapen nuclei, and cellular disarray in the ventral hippocampus with lateral ventricular enlargement were included. Brains with unilateral or dorsal-only hippocampal damage, or direct damage to structures beyond the hippocampus (e.g. striatum, amygdala, cortex, thalamus) were excluded from analysis. For Experiment 1, n=28 SHAMs and n=31 NVHLs were included (3 lesioned rats excluded) for a lesion success rate of 91%. In Experiment 2, the lesion success rate was 74% with n=35 SHAMs and n=37 NVHLs included (13 lesioned rats excluded). The overall lesion success rate, 81%, was similar to our prior large N studies [34, 14]
Figure 3.
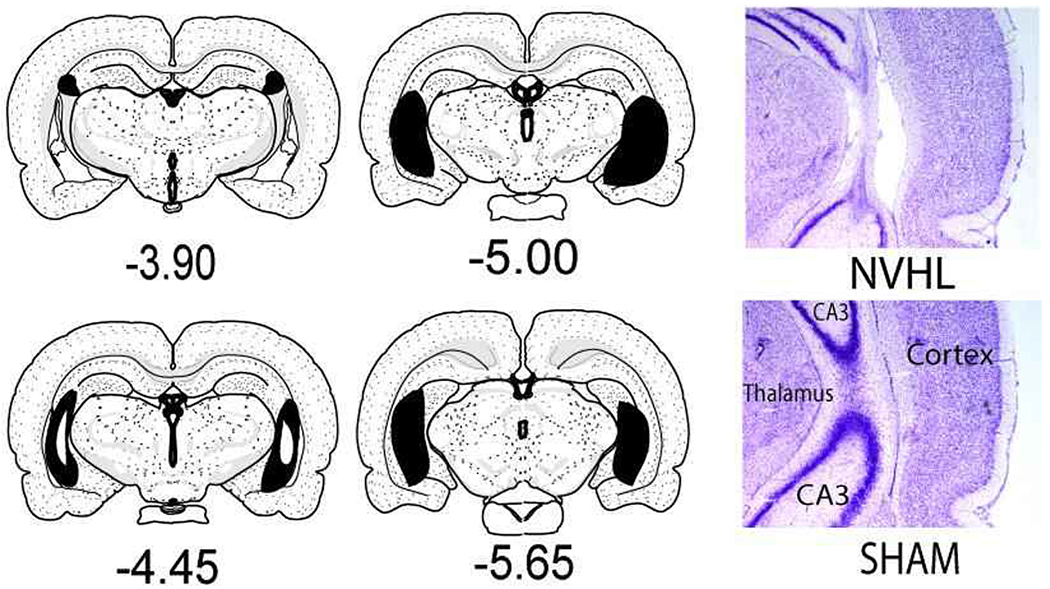
Brain maps of Lesions. Representative, maps on left show the range of gross tissue impact across all NVHL rats (N=68) included in the study (Maps adapted from Swanson, LW (2004) Brain Maps: Structure of the Rat Bain, 3rd ed., New York, Elsevier; sections mm AP relative to bregma). Largest lesions are shown in black with smallest lesions represented as white insets (e.g. at −4.40). The micrographs on the right show representative intermediate NVHL damage under higher magnification, at the primary target zone where the ventral and dorsal blades of CA3 intersect (e.g. at about −5.00 mm bregma) compared to a SHAM rat with no damage.
Data Analysis
Given the different experimental groups and group sizes in this study, in which all subjects were only tested once for dependent measures (pre- and post-injection locomotion in the novel arenas), we applied Two-way or Three-way ANOVAs with lesion, environmental condition and age-cohort, as the main independent factors, across several subgroup comparisons. Post-hoc analyses were reserved for clarifying statistical interactions between main effects using secondary lower order ANOVAs as needed. Significant statistical results are assumed at p<0.05, and informative near significant p<0.10, and non-significant p>0.10 are reported, with group means ±SEMS throughout text and figures.
RESULTS
Novelty Activation (Experiment 1: Pre-injection hour)
In Experiment 1 (N=59), all 3 main effects showed highly significant effects on novelty locomotion with NVHLs and IMP increasing activity (lesion: (F(1,47)=71.2, p<0.001); environment: (F(1,47)= 210.4, p<0.001)), and older age decreasing it: age (F(2,47)=22.0, p<0.001) (Figure 4A). NVHLs and age interacted (lesion x age: F(2,47)=7.1, p<0.01), so that lesion effects were significantly more pronounced in the PD28 group (lesion: F(1,13)=6.9, p<0.05), but not in the older groups as indicated by post-hoc testing (one-way ANOVA on lesion at each age) (Figure 4B). NVHLs and IMP also showed an interaction (lesion x environment: F(1,47)=4.6, p<0.05) in which NVHL effects were stronger under IMP rearing (F(1,28)=16.3,p<0.001), compared to ENR (F(1,28)=14.3, p<0.01) by post-hoc testing (one-way ANOVA at each environmental condition) (Figure 4C).
Figure 4.
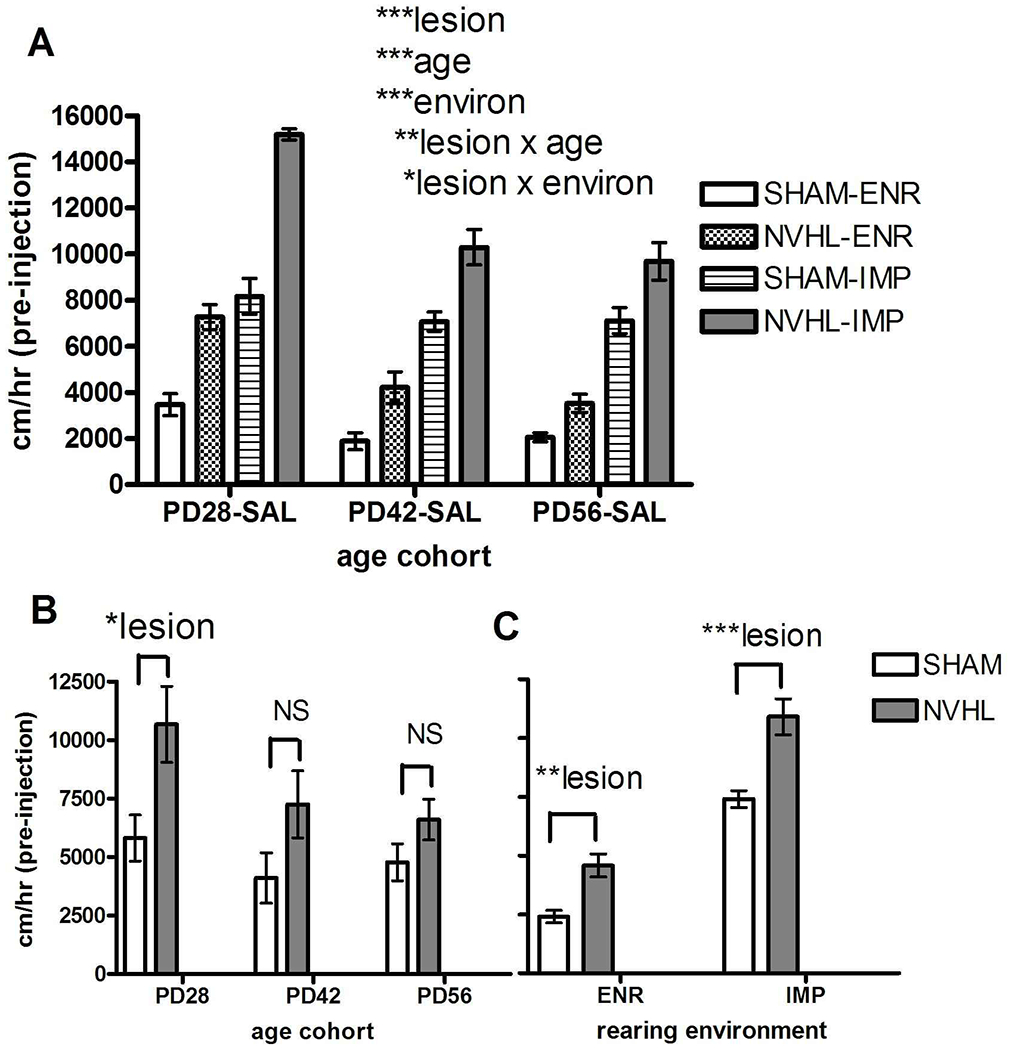
Experiment 1: Novelty responding across rat groups tested at PD28, PD42 and PD56. NVHL/SHAM rats crossed with ENR/IMP housing comprised groups with numbers as shown in Figure 3. A) Main effects of lesion, age and environmental housing (3-Way ANOVA) and significant interactions were followed by post-hoc testing (One way ANOVAs) examining lesion effects at each age (B) or at each environmental condition (C). All bars reflect means ±SEMS. All asterisks represent degree of statistical significance (*p<0.05; **p<0.01; ***p< 0.001).
Mild Stress Activation (Experiment 1: Post-injection hour)
Locomotion in the arena after the saline injection (second hour) showed much less activity overall (2271.1 ± 217.7 cm) compared to the pre-injection hour (6349.5 ± 486.2 cm) for all rats, and fewer and weaker effects compared to the novel first hour, with only the main effects of lesion: (F(1,47)=4.5, p<0.05) and IMP rearing: (F(1,47)=53.4, p<0.001) increasing activity (Figure 5). Unlike for novelty responding, increasing age did not significantly impact postinjection activity.
Figure 5.
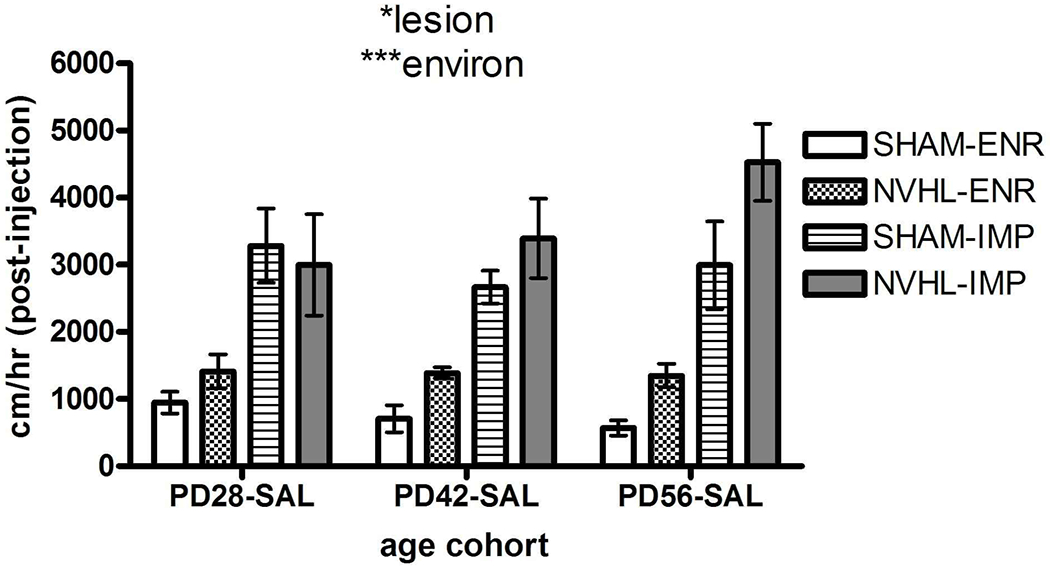
Experiment 1: Mild-Stress reactivity induces by saline injections across rat groups tested at PD28, PD 42 and PD56. NVHL/SHAM rats crossed with ENR/IMP housing comprised groups with numbers as shown in Figure 3. Main effect of lesion and environment are shown for the 3-way ANOVA. All bars reflect means ± SEMS. All asterisks represent degree of statistical significance (*p<0.05; ***p< 0.001).
Initial Cocaine Activation (Experiment 1 and 2, PD56 aged rats)
For examining lesion and environmental effects on an initial cocaine reactivity in early adulthood, we analyzed the n=31 rats from the Experiment 1C group (tested at PD56 in the novel arena, with saline injections (PD56-SAL)) together with the n=31 rats from Experiment 2A group (tested at PD56 with cocaine injections (PD56-COC)) (Figure 6). Notably, this analysis assumes that the rearing conditions of the Experiment 1C and 2A groups were the same, which is not quite the case because the 1C rats had 5 weeks of IMP vs. ENR rearing (PD21 to PD56), whereas the 2A rats had 4 weeks of IMP vs. ENR rearing (PD28 to PD56). Regardless, the assumption that these minor rearing differences were close enough in functional impact at PD56 to allow them to be treated as the same in this testing was born out by the fact that the drug-cohort comparison between 1C vs 2A groups produced no differences in novelty-induced locomotion (F(1,54)=1.9, p=NS), or interactions with lesion or environment (Figure 6A). Thus, the different mean subgroup activity profiles and error bars produced by lesion and environmental effects happening in the 1C rats (PD56-SAL) looked nearly identical to the 2A rats (PD56-COC) in the novel pre-injection hour (Figure 6A). At the same time, both NVHL (F(1,54)=25.9, p<0.001) and IMP rearing (F(1,54)=136.1, p<0.001) produced strong noninteracting effects to increase activity in the novel arena regardless of cohort (Figure 6A). In contrast, in the post-injection phase, there was a strong drug-cohort effect: the 2A group rats (PD56-COC) that received cocaine showed a more robust degree of locomotor activation compared to the 1C (PD56-SAL) rats (drug-cohort:(F(1,54) =18.1, p<0.001)(Figure 6B). Rats receiving saline injections (PD56-SAL) had declines in locomotion (from 5839.3 ±614.8 cm preinjection to 2491.0±355.6 cm post-injection), whereas rats receiving cocaine had increases (6560±752.7 cm to 9415.1+1627.9 cm). In the post-injection phase, only NVHLs (but not environment) increased locomotion (F(1,54)= 5.8, p<0.05) in association with a relatively strong but non-significant lesion x drug-cohort interaction (F(1,54)=2.8, p<0.10).
Figure 6.
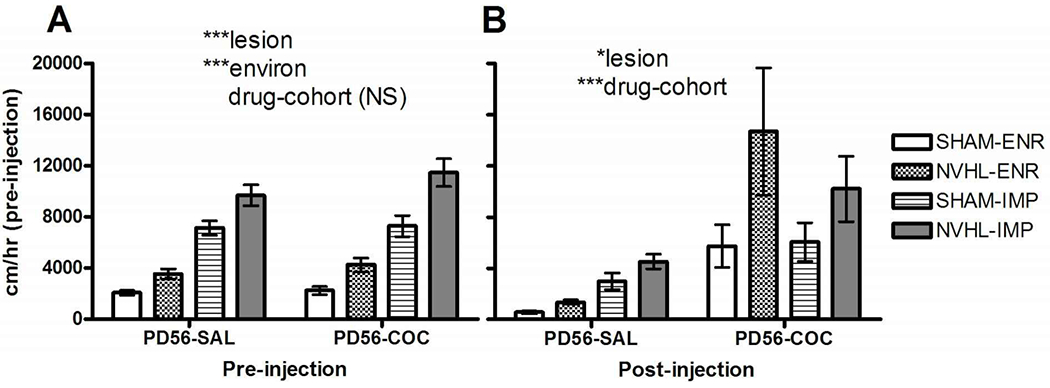
Acute cocaine effects assessed in comparison of Experiment 1C and 2A rats tested at PD56, given either saline (SAL) or cocaine (COC) injections. NVHL/SHAM rats crossed with ENR/IMP housing comprised groups with numbers as shown in Figure 3. A) Robust main effects of lesion and environment are similar for the locomotor response to the novel arena in both PD56-SAL and PD56-COC groups (only drug-cohort is non-significant in the 3-way ANOVA). B) With either saline injections (PD56-SAL) or cocaine (PD56-COC) a strong drug effect emerged together with a persistent main effect of lesion, but with no environmental effect. All bars reflect means ± SEMS. All asterisks represent degree of statistical significance (*p<0.05; ***p< 0.001).
Cocaine Sensitization (Experiment 2)
For analysis of NVHL and/or environmental effects on long-term cocaine sensitization, we examined Experiment 2A (n=31) and 2B (n=41) rats in one analysis (Figure 7). Notably in this testing, the 2B rats (PD84-SENS) differed from the 2A rats (PD56-COC) by not only having a sensitizing history of cocaine (10 days of cocaine (15 mg/kg/day) in home cages, PD 56 to PD70) but by being 4 weeks older than the 2A rats (at the time of behavioral testing), and having lived in the IMP or ENR conditions for 8 weeks (PD28-PD84) as compared to 4 weeks (PD28-PD56) in the 2A rats. In the pre-injection hour, NVHLs and IMP again amplified locomotor activation in response to the novel environment (lesion: F(1, 64)=10.4, p<0.01; environment: F(1,64)=78.3, p<0.001)), but in contrast to what happened with the younger rats (Experiment 1), the older age group with cocaine history (PD84-SENS) also showed a greater overall response to the novel arena (age-cohort: (F(1,64)=18.2, p<0.001) (Figure 7A). After cocaine injection, there was still an overall amplification of locomotor activation due to NVHLs (F(1,64)=12.3, p<0.01), but now with only a non-significant main trend of environment (F(1,64)=3.1, NS (p=0.08)) (Figure 7B). Age-cohort also significantly elevated post-cocaine activation (F(1,64)=8.5, p<0.01), suggestive of a cocaine sensitization effect (and/or older age) in increasing acute cocaine-induced activation. In fact, while cocaine injections generally elevated post-injection activity, the highest levels were seen in the PD84-SENS rats. PD56-COC rats increased from 6561.0±752.7 cm pre-injection to 9415.1±1627 cm post-injection, while PD84-SENS rats changed from 9827.1+1009.8 cm to 14978.0+1647.6 cm. This main effect was accompanied by an age-cohort x environment interaction (F(1,64)=7.6, p<0.01). As shown by post-hoc testing (one-way ANOVAs performed on age-cohort effect) under each environmental condition, a significant increase in cocaine-induced activation was specific only to the older rats that had undergone cocaine sensitization F(1,34)=16.8, p<0.001) (Figure 7C).
Figure 7.
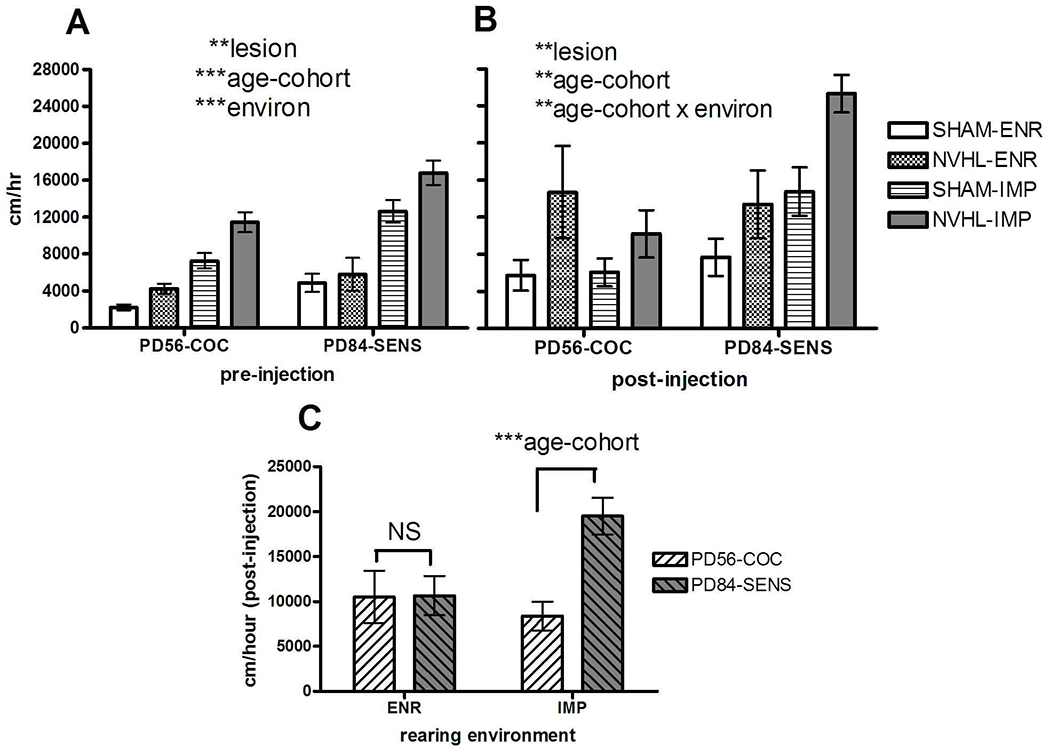
Experiment 2: Differential effects of cocaine sensitization. NVHL/SHAM rats crossed with ENR/IMP housing comprised groups with numbers as shown in Figure 3. A) As in other comparisons, the lesion and environmental main effects (3 -way ANOVA) to increase reactivity to the novel environment were similar in both PD56-COC and PD84-SENS rats, but also there was a significant effect of PD84-SENS rats (tested at an older age and with a sensitizing cocaine history) to show greater novelty reactivity overall. B) Cocaine injections delivered to all rats produced reactivity where lesion main effects remained strong, but with a loss of environment main effects. However, differences in acute cocaine reactivity based on age and sensitization history were strong and did show a significant age-cohort x environment interaction. C) Post-hoc testing (1-way ANOVA) at each environmental condition, showed that cocaine reactivity differences between the PD56-COC and PD84-SENS) groups were strongly emergent only after IMP but not ENR housing. All bars reflect means ± SEMS. All asterisks represent degree of statistical significance (*p<0.05; **p<0.01; ***p< 0.001).
A summary analysis of novelty and post-injection locomotor responding across the different age and drug history cohorts is shown in Figure 8. Here, the responses of Experiment 1 rats (that only received saline injections, PD28-SAL, PD56-SAL groups) and Experiment 2 rats (that received cocaine injections, either without (PD56-COC) or with a prior history of chronic cocaine exposure (PD84-SENS)) are shown with summary results of 2-way ANOVAs (lesion, environmental condition) performed at each age group. As shown in Figure 8A, both NVHLs and IMP consistently increased novelty-induced locomotion (albeit in a pattern that tended to decrease in statistical strength with increasing age for both factors), creating a 4-group separation across ages indicative of comparable and compounding effects between these factors. In contrast (Figure 8B), post-injection locomotion due to NVHLs tended to gain effect strength with increasing age and more cocaine exposure, whereas post-injection IMP tended to be statistically weaker compared to IMP-novelty responding, and not to increase in strength with age and cocaine exposure.
Figure 8.
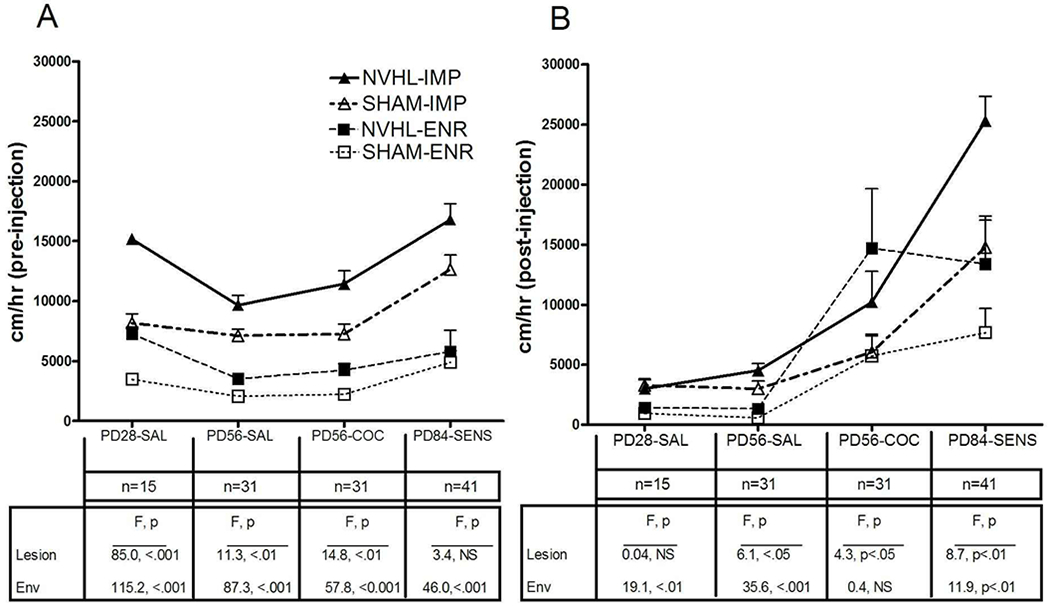
Summary analysis of locomotor reactivity of select groups from Experiments 1 and 2. In this analysis 2-way ANOVAs examining lesion and environment main effects were conducted independently for each age/drug history) cohort as tested in response to (A) the novel environment, and after (B) saline or cocaine injections. Overall, age and more cocaine allow greater expression of NVHL effects, while IMP-environmental reading has greater statistical expression at younger age and with respect to novelty responding. All bars reflect means ± SEMS.
DISCUSSION
This study demonstrates that early developmental ventral hippocampal circuit disruption and impoverished social/environmental rearing independently produce similar abnormalities in behavioral reactivity to novelty, mild stress, acute cocaine exposure and cocaine sensitization. Moreover, when combined in the same animals, these two brain pathogenesis models produce compounding phenotypic effects across these measures. When considered as a population study of subjects containing a diversity of etiologies and degrees of mental illness symptom severity, these experiments comport with broad clinical and epidemiological data suggesting there is a positive relationship between addiction risk and severity, and mental illness severity [2, 35, 8].
Our findings replicate Alquicer et al, who showed this same step-wise pattern in similarly prepared adult rats (NVHL/isolation rearing) of tested in the novel field [27]. Examining mild stress responsivity and acute and chronic cocaine effects allowed us build on this data to show for the first time that regardless of the mental illness model etiology, both the mental illness and addiction risk phenotypic severities tend to track together, and in proportion to the overall loading of causal etiological conditions that are generating the mental illness syndrome. These findings add weight to understanding addiction and mental illness as interconnected diseases, while illustrating how the phenomena of both equifinality and multifinality [36] may be concurrently in play in the pathogenesis of dual diagnosis disorders. On one hand, both early neurodevelopmental damage and adverse environmental experience are shown to produce similar phenotypes (equifinality). On the other hand, each one of these etiological pathways produced abnormal behaviors that relate to multiple diagnostic domains (multifinality across novelty responding, stress reactivity, and behavioral sensitization to an addictive drug). These animal modeling observations are congruent clinical-epidemiological evidence indicating that both genetic and adverse environment experiences conspire in a multifactorial, dose-dependent like manner in populations to generate both mental illness and addiction disease severity [5, 37, 6, 7]. Additionally, our findings agree with evidence that adverse environmental experiences can exacerbate a wide range of different mental illness diagnostic categories (e.g. spanning psychosis, mood, trauma and personality and substance use disorders) via multifinality even though these conditions likely have differential genetic contributions [38]. Together with this literature (and see [39]), our findings cast doubt on the utility of novel clinical testing modalities that attempt to pinpoint the pathogenesis or diagnoses of dual diagnosis patients based on limited sets of either genetic markers or specific environmental events.
The extent to which seemingly different etiological models—NVHLs vs. impoverished environmental rearing— produced such quite similar phenotypic abnormalities is remarkable and prompts a neuroscience-based interpretation. But first, it should be noted that the NVHL and IMP effects we observed were not exactly the same. For example, IMP environment consistently produced statistically stronger effects than NVHLs on novelty-induced locomotion, whereas NVHLs, but not environment, interacted with age to alter novelty-induced locomotion. Also, NVHLs were more hyper-responsive to initial cocaine in early adulthood, whereas IMP had no impact. Then, differing from IMP, the phenotypic impact of NVHLs increased in statistical strength in post-injection locomotion testing, as rats aged out of adolescence and accumulated more cocaine exposure.
Despite these nuanced differences, the overall similarity of NVHLs and IMP rearing, and the way they fairly consistently compound one another when occurring together, suggests that both of these etiological interventions produce comparable biological effects on common brain systems that are more additive and/or synergistic, rather than antagonistic or disruptive. In fact, precisely this kind of brain impact has been observed in Golgi-staining studies of prefrontal cortical (PFC) neurons in rats that have been reared in IMP-environments and in NVHL rats [26, 40]. When healthy rats are directly compared to NVHL vs. IMP-environment reared rats vs. combined NVHL-IMP rats, pyramidal neurons of the medial PFC show a step-wise progression in the loss of dendritic lengths and synaptic spine counts [27] that mirrors the same pattern of step-wise worsening of phenotypes we found across measures of novelty, mild-stress and cocaine sensitization. Similar but less pronounced trends have been observed within in the Nucleus Accumbens involving dendritic length of medium spiny neurons, although a more complex interaction between NVHLs and IMP-rearing was noted with regard to synaptic spine densities [27]. Regardless, an overarching theme of worsening prefrontal cortical-ventral striatal neural network connectivity disruption (as induced regardless of the specific mental illness model etiology or combination) seems to be proportionately generative of both mental illness and addiction risk phenotypic severity. Together with the Golgi findings, our behavioral findings hint that addiction vulnerability and pathogenesis, especially as exacerbated by mental illness, is perhaps best understood as a pathogenesis of neural network connectivity and information processing, involving a wide range of molecular and cellular elements and etiological ingredients, rather than reflecting abnormalities in a limited set of genes, transmitters or receptors. Consistent with this view, we have previously ruled out changes in presynaptic dopamine neurotransmission as occurring in, or being correlated with changes in behavioral sensitization due to NVHLs [41]. However, we have identified a wide spectrum of changes in gene expression [42] and corresponding alterations in neural network activation levels [13] in prefrontal cortical-striatal circuits due to both NVHLs and sensitizing cocaine regimens, that integrate biologically to generate more extreme addiction-related phenotypes. Evidence for disruptions in dopamine-glutamate signaling in the prefrontal cortex of NVHL rats [43], and abnormal patterns of striatal neuronal excitably in socially isolated rodents (both with [30], and without cocaine exposure [32]) together with the Golgi-staining studies, point to the capacity for NVHL and IMP to work independently and in concert to damage connectivity and information processing capacity in prefrontal-cortical striatal circuits. These conditions might allow the acute and long-term neuroadaptative effects of addictive drugs on prefrontal cortical-striatal circuits (post-synaptic to DA signaling) to have a greater impact on pathologically altering motivated behavior [44, 45].
These experiments should be considered initial forays in exploring the complex etiologies and neural network basis of addiction vulnerability and dual diagnosis disorders; future studies are needed to address the limitations inherent in the designs implemented here. Further experiments should implement more precise controls to disentangle the effects of developmental ages and timing of drug exposures and behavioral testing. Whereas the IMP-environment living context we used was quite severe, involving impoverishment of both social contacts and physical environments, future studies should attempt to tease out the differential effects of these two components on the NVHL syndrome. Assessment of a wider range of mental illness behavioral phenotypes (e.g. examining PFC-dependent cognitive function) are needed, and of course, more sophisticated addiction models, including drug self-administration, should be conducted. Notably, our results did not quite replicate original characterizations of the NVHL model showing that abnormal novelty responding follows a post-adolescent-onset pattern. However, we observed that the IMP condition robustly increased novelty responding in early adolescence in particular, with or without the NVHL being present, and this effect is significantly interactive with the NVHL throughout adolescence. Thus, in some respects, IMP rearing likely alters the way the NVHL syndrome expresses developmentally, or as a matter of degree of severity, in which the whole result is not entirely explainable as a simple summation of NVHL plus IMP-environmental conditions. These phenomena may have relevance to understanding early onset forms of schizophrenia and the wide variances in other mental illness comorbidities and pharmacological treatment responses seen in these patients. Future studies examining how variations in genetic background and/or brain gene expression patterns may interact with NVHL/IMP-environmental effects would also be informative.
The broader clinical-translational implications of this study spring largely from the main observation that the IMP-environmental rearing condition has a profound and long-lasting impact that is at least as potent, and of a similar quality, as what early hippocampal lesions produce on endophenotypes relevant to both mental illness and addiction. Moreover, this abnormal environmental experience combines with the neurodevelopmental damage to produce a particularly extreme endophenotypes of dual diagnosis psychopathology. In parallel to our findings, humans growing up in poverty, as a correlate of IMP-environmental rearing, show structural-neurodevelopmental deficits in frontal cortical-hippocampal circuits that correspond to poor cognitive outcomes [46]. While illustrating the fallacy of discounting environmental experience as being biologically minor in the pathogenesis and treatment of mental disorders and addictions in concrete terms [39], these data provide a neuroscience-based warning about the unintended consequences of national policies that utilize mass-incarceration and financial punishment strategies to combat addictions, and as a solution to deficient psychiatric and addiction treatment infrastructure [47]. Conversely, these findings suggest the importance of integrating psychotherapies, therapeutic experiences and medications—all as important biological interventions in the prevention and recovery from addiction and mental illness comorbidities [39, 14, 48].
ACKNOWLEDGEMENT
The authors would like to thank the animal husbandry team with the IU department of psychiatry for their enthusiastic support and extra effort in maintaining and managing the rats in the Enriched Environmental housing system.
FUNDING SOURCES
This work was supported by grants from National Institutes of Health (NIDA-K08 DA019850, (RAC as PI), and NIAAA-1RC2AA019366 (RAC, as co-I). These funding agencies had no role in the design or conduct of this research other than to support the costs of the project.
Footnotes
STATEMENT OF ETHICS
All housing, procedures and experimental conditions involving the rodent subjects in this study were approved by the IACUC of IU and were conducted in accordance with the APA Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research.
DISCLOSURE STATEMENT
RAC has advisory and/or creative contracts with Indigobio (Bioanalytics Technology and Software, Indianapolis, IN) and Proniras (Pharmaceutical Research Firm, Seattle, WA). Neither author has any conflicts of interest to declare that relate to this study.
REFERENCES
- 1.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drugs of abuse. Journal of the American Medical Association. 1990;264(19):2511–18. [PubMed] [Google Scholar]
- 2.RachBeisel J, Scott J, Dixon L. Co-occurring severe mental illness and substance use disorders: a review of recent research. Psychiatric Services. 1999;50(11):1427–34. [DOI] [PubMed] [Google Scholar]
- 3.Chambers RA, Krystal JK, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers RA, Bickel WK, Potenza MN. A scale-free systems theroy of motivation and addiction. Neurosciene and Biobehavioral Reviews. 2007;31:1017–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey CE, Agrawal A, Bucholz KK, Hartz SM, Lynskey MT, Nelson EC, et al. Associations between Polygenic Risk for Psychiatric Disorders and Substance Involvement. Front Genet. 2016;7:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018February09;359(6376):693–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarse E, Neff M, Yoder R, Hulvershorn L, Chambers J, Chambers R. The adverse childhood experiences scale: Two decades of research on childhood trauma as a primary cause of adult mental illness, addiction and medical diseases. Cogent Medicine. 2019February13, 2019;6(1581447). [Google Scholar]
- 8.Chambers RA. The 2 x 4 model : a neuroscience-based blueprint for the modern integrated addiction and mental health treatment system. First edition. ed. New York, NY: Routledge; 2018. [Google Scholar]
- 9.Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27(6):889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady AM, McCallum SE, Glick SD, O’ Donnell P. Enhanced methamphetamine selfadministration in a neurodevelopmental rat model of schizophrenia. Pschopharmacology. 2008;200:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson RM, Kircher DM, Shaham Y, O’Donnell P. Exaggerated cue-induced reinstatement of cocaine seeking but not incubation of cocaine craving in a developmental rat model of schizophrenia. Psychopharmacology. 2013March;226(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeanblanc J, Balguerie K, Coune F, Legastelois R, Jeanblanc V, Naassila M. Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addict Biol. 2014April13. [DOI] [PubMed] [Google Scholar]
- 13.Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical-striatal integration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol Psychiatry. 2010April15;67(8):788–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao KN, Sentir AM, Engleman EA, Bell RL, Hulvershorn LA, Breier A, et al. Toward early estimation and treatment of addiction vulnerability: radial arm maze and N-acetyl cysteine before cocaine sensitization or nicotine self-administration in neonatal ventral hippocampal lesion rats. Psychopharmacology (Berl). 2016December;233(23-24):3933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khokhar JY, Todd TP. Behavioral predictors of alcohol drinking in a neurodevelopmental rat model of schizophrenia and co-occurring alcohol use disorder. Schizophr Res. 2018April;194:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9(1):67–75. [DOI] [PubMed] [Google Scholar]
- 17.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009December7;204(2):295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers RA, Taylor JR. Animal modeling dual diagnosis schizophrenia: sensitization to cocaine in rats with neonatal ventral hippocampal lesions. Biol Psychiatry. 2004;56(5):308–16. [DOI] [PubMed] [Google Scholar]
- 19.Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacology Biochemistry & Behavior. 2007;86:386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–91. [DOI] [PubMed] [Google Scholar]
- 22.Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2014November;19(6):1020–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sentir AM, Bell RL, Engleman EA, Chambers RA. Polysubstance addiction vulnerability in mental illness: Concurrent alcohol and nicotine self-administration in the neurodevelopmental hippocampal lesion rat model of schizophrenia. Addict Biol. 2018December28. [DOI] [PubMed] [Google Scholar]
- 24.Harlow HF. Total Social Isolation: Effects on Macaque Monkey Behavior. Science. 1965April30;148(3670):666. [DOI] [PubMed] [Google Scholar]
- 25.Ridley RM. The psychology of perseverative and stereotyped behavior. Progress in Neurobiology. 1994;44:221–31. [DOI] [PubMed] [Google Scholar]
- 26.Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003September05;983(1-2):128–36. [DOI] [PubMed] [Google Scholar]
- 27.Alquicer G, Morales-Medina JC, Quirion R, Flores G. Postweaning isolation enhances morphological changes in the neonatal ventral hippocampal lesion rat model of psychosis. J Chem Neuroanatomy. 2008;35:179–87. [DOI] [PubMed] [Google Scholar]
- 28.Fabricius K, Helboe L, Fink-Jensen A, Wortwein G, Steiniger-Brach B. Pharmacological characterization of social isolation-induced hyperactivity. Psychopharmacology. 2011May;215(2):257–66. [DOI] [PubMed] [Google Scholar]
- 29.Garcia EJ, Haddon TN, Saucier DA, Cain ME. Differential housing and novelty response: Protection and risk from locomotor sensitization. Pharmacol Biochem Behav.2017March;154:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang XQ, Yu ZP, Ling Y, Zhao QQ, Zhang ZY, Wang ZC, et al. Enduring effects of juvenile social isolation on physiological properties of medium spiny neurons in nucleus accumbens. Psychopharmacology (Berl). 2019June13. [DOI] [PubMed] [Google Scholar]
- 31.Schenk S, Lacelle G, Gorman K, Amit Z. Cocaine self-administration in rats influenced by environmental conditions: implications for the etiology of drug abuse. Neurosci Lett. 1987October16;81(1-2):227–31. [DOI] [PubMed] [Google Scholar]
- 32.Howes S, Dalley JW, Morrison CH, Robbins TW, Everitt BJ. Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracllular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal Fos expression. Psychopharmacology. 2000;151:55–63. [DOI] [PubMed] [Google Scholar]
- 33.Chambers RA, Lipska BK. A Method to the Madness: Producing the neonatal ventral hippocampal lesion rat model of schizophrenia. In: O’Donnell P, editor. Animal Models of Schizophrenia and Related Disorders. Totowa, N.J.: Humana Press; 2011. [Google Scholar]
- 34.Berg SA, Sentir AM, Bell RL, Engleman EA, Chambers RA. Nicotine effects in adolescence and adulthood on cognition and alpha(4)beta(2)-nicotinic receptors in the neonatal ventral hippocampal lesion rat model of schizophrenia. Psychopharmacology (Berl). 2015May;232(10):1681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler RC. The epidemiology of dual diagnosis. Biological Psychiatry. 2004;56:730–37. [DOI] [PubMed] [Google Scholar]
- 36.Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996Fal;8(4):597–600. [Google Scholar]
- 37.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016September19;17(10):652–66. [DOI] [PubMed] [Google Scholar]
- 38.Teicher MH, Samson JA. Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. American Journal of Psychiatry. 2013October;170(10):1114–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat Neurosci. 2012April15;15(5):663–8. [DOI] [PubMed] [Google Scholar]
- 40.Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–70. [DOI] [PubMed] [Google Scholar]
- 41.Chambers RA, Sentir AM, Engleman EA. Ventral and dorsal striatal dopamine efflux and behavior in rats with simple vs. co-morbid histories of cocaine sensitization and neonatal ventral hippocampal lesions. Pschopharmacology. 2010;212(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers RA, McClintick JN, Sentir AM, Berg SA, Runyan M, Choi KH, et al. Cortical-striatal gene expression in neonatal hippocampal lesion (NVHL)-amplified cocaine sensitization. Genes Brain Behav. 2013July;12(5):564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biol Psychiatry. 2007October1;62(7):730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chambers RA. Impulsivity, dual diagnosis, and the structure of motivated behavior in addiction. Behavioral and Brain Sciences. 2008;31(4):443–44. [Google Scholar]
- 45.Chambers RA. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend. 2013June1;130(1-3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of Child Poverty, Brain Development, and Academic Achievement. JAMA Pediatr. 2015September;169(9):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grecco GG, Andrew Chambers R. The Penrose Effect and its acceleration by the war on drugs: a crisis of untranslated neuroscience and untreated addiction and mental illness. Transl Psychiatry. 2019November28;9(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers RA, Wallingford SC. On Mourning and Recovery: Integrating Stages of Grief and Change Toward a Neuroscience-Based Model of Attachment Adaptation in Addiction Treatment. Psychodyn Psychiatry. 2017Winter;45(4):451–73. [DOI] [PMC free article] [PubMed] [Google Scholar]


