Abstract
Purpose:
The compositional, structural, and functional relationships of meibum may provide insights into the loss of tear film stability. Although the conformation of meibum lipids has been studied rigorously, that of tear lipids has not.
Methods:
Tear lipids (TLHSCT) and meibum (MHSCT) from patients who had hematopoietic stem cell transplantation were pooled prospectively. The infrared spectra of meibum from donors with (MMGD) and without (Mn) meibomian gland dysfunction were retrospectively analyzed to measure the lipid composition and structure. The infrared CH stretching region was used to measure the relative content of CH3 and CH2 moieties in the meibum.
Results:
The 3 major findings of the current study are as follows: 1) compared with Mn, MHSCT and MMGD had 18% fewer CH3 moieties; 2) compared with MHSCT, the phase transition temperature, cooperativity, and order were approximately 20% greater for TLHSCT; and 3) compared with Mn and MMGD, MHSCT and TLHSCT contained fewer double bonds.
Conclusions:
Tear lipids are more ordered than meibum lipids, which could have functional consequences. The human meibum peak height ratio of the CH3/CH2 bands is not a factor related to tear film stability with age or sex. The amount of CH3 moieties relative to CH2 moieties and saturation could contribute to a higher meibum lipid order associated with a younger age, meibomian gland dysfunction, and dry eye from hematopoietic stem cell transplantation. Therefore, the hydrocarbon order may be a marker of or contribute to an unstable tear film layer.
Keywords: age, dry eye, FTIR, sex, lipid, meibum, meibomian gland dysfunction
Meibum produced by the meibomian glands is the major source of lipids on the surface of tears. The thin1,2 tear film lipid (TFL) layer is responsible for the stability of tears.3–8 The compositional, structural, and functional relationships of meibum may provide insights into the loss of tear film stability with age (references in Mudgil et al9) and dry eye. The lipid order is related to the lipid structure and pertains to their conformation, how the molecules are arranged in space. The lipid structural order may be thought of as lipid fluidity; however, fluidity may have an additional mobility component. When lipids are ordered, as in butter, the hydrocarbon chains pack tightly together. This maximizes van der Waals interactions between hydrocarbon chains (Fig. 1D), and the carbons align in the trans rotamer conformation. When lipids are disordered, as in olive oil, there are more gauche rotamers than trans rotamers. In addition, hydrocarbon chain packing becomes less tight, thus minimizing van der Waals interactions between hydrocarbon chains.
FIGURE 1.
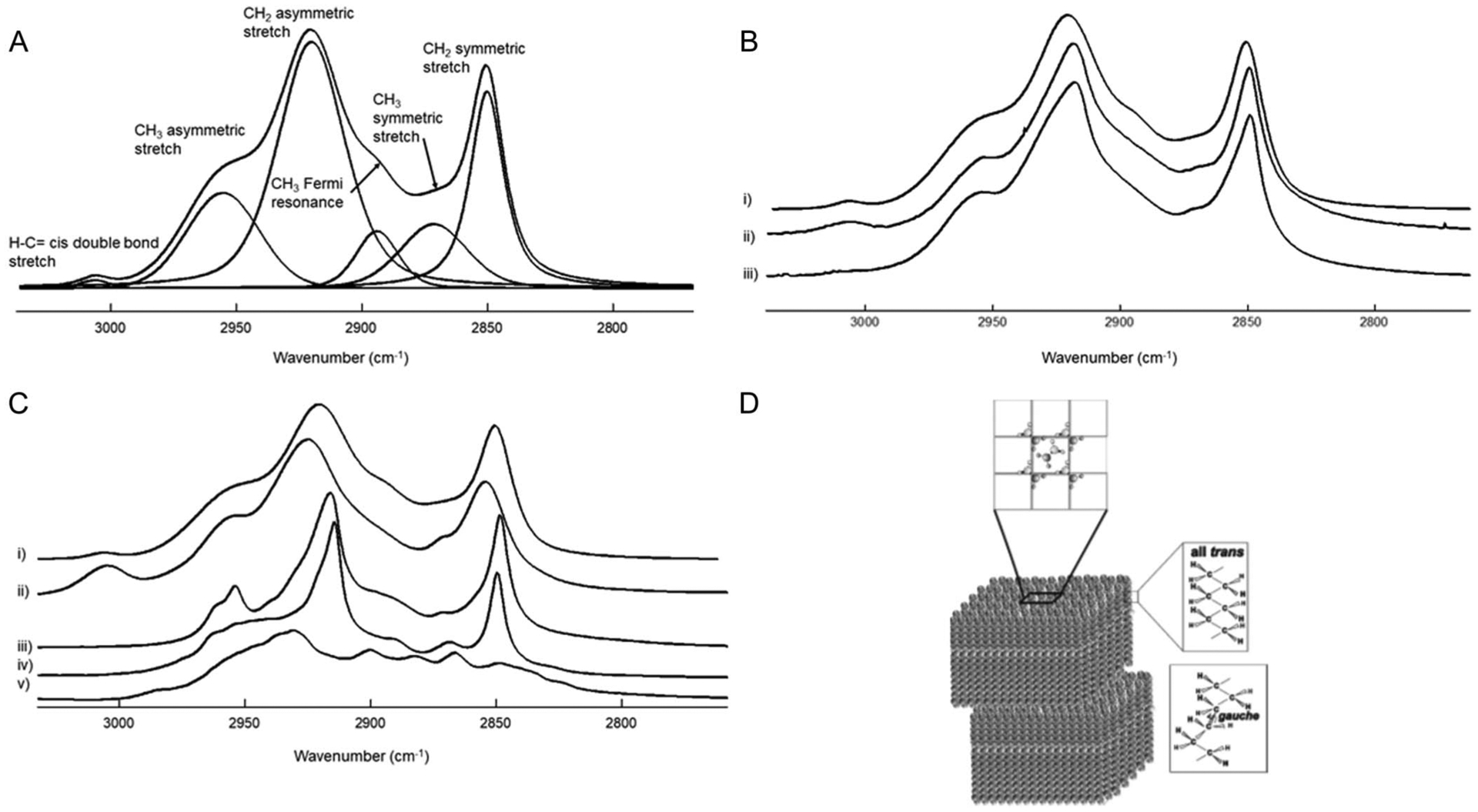
A, Curve fit of the average infrared CH stretching region spectra of meibum from donors without dry eye. B, Average infrared CH stretching region spectra of (i) meibum from donors without dry eye; (ii) meibum from donors with dry eye due to meibomian gland dysfunction; and (iii) meibum from donors who have had hematopoietic stem cell transplantation and are susceptible to severe dry eye. C, Infrared CH stretching region spectra of (i) meibum from donors without dry eye; (ii) oleyl oleate; (iii) stearyl palmitate; (iv) cholesteryl behenate; and (v) cholesterol. D, Schematic of wax structure and conformation.
In relation to the eye, infrared spectroscopy has been used to measure the lipid order of human meibum,9–23 the rod outer segment,24 and lens membranes.25–29 Saturation plays a major-role in determining the lipid order in these tissues,16,18,21 but cholesterol,29 cholesteryl esters,30 and hydrocarbon chain branching31–33 can also contribute to hydrocarbon chain order. The human meibum order increases with age between 0 and 25 years,9 meibomian gland dysfunction,12,22 and with donors who have had hematopoietic stem cell transplantation.20,22,23 Interestingly, tear film stability also increases in the same order: meibum from donors without dry eye (Mn) between the ages 0 and 25 years,9 meibum from donors with meibomian gland dysfunction (MMGD), and meibum from donors who have had hematopoietic stem cell transplantation (MHSCT). Correlation does not necessitate cause, but the relationship between the hydrocarbon chain order and tear film stability is intriguing, and, at best, the lipid order is a marker for age and dry eye, with a positive correlation of 93%.11
Evidence suggests that more ordered lipids can contribute to formation of a discontinuous patchy TFL layer, which in turn results in deteriorated spreading21 and decreased surface elasticity. One may speculate that a more ordered lipid layer results in an attenuated capability to restore the TFL layer structure between blinks. A detailed review on this topic has been published.8 Too much lipid order may also keep meibum from flowing out of the meibomian glands.
Although the conformation of meibum lipids has been studied rigorously,9–23,30 that of tear lipids has not.19 The major source of TFL is the meibomian glands7; however, other sources may include lipocalin-bound or free phospholipids34–39 and eyelid sebum.15,40,41 The composition4,10,34–44 and physical properties10,19,43,44,45 of tear lipids are slightly different than those of meibum lipids.
In the current study, we compared the structural order and phase transition parameters of MHSCT with tear lipids from patients who had hematopoietic stem cell transplantation (TLHSCT). Furthermore, we used the infrared CH stretching region to measure the relative content of CH3 and CH2 moieties of Mn, MMGD, and MHSCT.
MATERIALS AND METHODS
Tear lipids and meibum from 44 patients who had undergone hematopoietic stem cell transplantation were grouped into 5 pools, respectively. Meibum from 8 younger (average age of 1.7 ± 0.2 years) and 8 older (average age of 38 ± 0.6 years) donors without dry eye were each prospectively grouped into 2 pools. The infrared CH stretching region was used to measure the relative content of CH3 and CH2 moieties in the meibum. Human meibum peak height ratios (PHRs) of the CH3/CH2 bands (2955 cm−1/2919 cm−1) and lipid saturation were retrospectively calculated from the infrared spectra of meibum from 42 donors with meibomian gland dysfunction, 45 donors without dry eye, and 19 patients who had undergone hematopoietic stem cell transplantation.9–23
Because the CH stretching bands become smaller and broader with disorder at temperatures above the phase transition temperature (27–40°C), which changes the PHR, in the current study, we measured the PHR of meibum at temperatures below the phase transition temperature (~13°C) and comparable levels of order.
Written informed consent was obtained from all donors. Protocols and procedures were reviewed by the University of Louisville Institutional Review Board and the Robley Rex Veterans Affairs Institutional Review Board. All procedures were in accordance with the Declaration of Helsinki. Protocols and procedures for the current retrospective study were approved by the University of Louisville Institutional Review Board (# 11.0319, August 2016).
As a portion of this study was a retrospective study from published methods, collection and processing of human meibum, infrared spectral acquisition parameters, and clinical diagnoses can be found in citations.19–24 Subjects were recruited from the Kentucky Lions Eye Center, the Robley Rex Veterans Affairs Medical Center, and the James Graham Brown Cancer Center, all in Louisville, KY. Briefly, meibomian gland expression was done by compressing the eyelid between cotton-tipped applicators, with strict attention to avoid touching the eyelid margin during expression. All 4 eyelids were expressed, and approximately 1 mg of meibum was collected per individual for direct spectroscopic study. To collect MHSCT, the meibomian glands were expressed by lightly compressing the eyelids, with strict attention to avoid touching the eyelid margin during expression. All 4 eyelids were expressed, and approximately 0.5 mg of meibum was collected per individual. The secretion was collected with a platinum spatula and dissolved in 600 μL of chloroform.
Clinical Diagnosis
Clinical diagnosis was made on the same cohorts as published previously12,20 and copied below. Subjects were recruited from the Kentucky Lions Eye Center and the Robley Rex Veterans Affairs Medical Center in Louisville, KY. Normal status was assigned when the subject’s meibomian gland orifices showed no evidence of keratinization or plugging with turbid or thickened secretions, and no dilated blood vessels were observed on the eyelid margin.
The diagnosis of meibomian gland dysfunction was made according to the criteria of Foulks and Bron.46 Plugging of the meibomian glands of at least 5 of 10 orifices in the central portion of the upper eyelid was required for the diagnosis of meibomian gland dysfunction. The secretion expressed by the meibomian gland had to be turbid, turbid with clumps, or paste-like. Inflammation of the eyelid margin, as evidenced by both swelling of the eyelid margin and 2+ vascular engorgement of the posterior eyelid margin, was necessary for diagnosis. The presence of telangiectasia of the posterior eyelid margin confirmed chronic disease, but it was not required for entry. Tear stability was determined by instillation of sodium fluorescein into the tear film. The tear breakup time was less than 5 seconds for all subjects with meibomian gland dysfunction.
The participants classified as “without dry eye” did not recall having dry eye symptoms. Patients with graft-versus-host disease were diagnosed by medical oncologists. Patients who had undergone hematopoietic stem cell transplantation underwent full ophthalmic eye examination using slit-lamp biomicroscopy. The tear ebreakup time was measured using a slit lamp after instillation of 1 fluorescein drop. The diagnosis of dry eye was based on the clinical examination results, including fluorescein stain uptake of the cornea or conjunctiva, an irregular tear film, a low tear meniscus, and symptoms. Symptoms that were considered positive for dry eye included foreign body sensation, excessive tearing, excessive blinking, burning of eyes, and blurry vision. The Schirmer test was performed on all patients by placing a standard strip in the lower conjunctival sac without anesthesia for 5 minutes. Meibomian gland orifices, eyelid changes at the mucocutaneous junction, and expression of meibum by gentle pressure were all evaluated for the diagnosis of meibomian gland dysfunction.
Collection and Extraction of Tear Lipids
Tear lipids were collected from Schirmer strips, but the amount collected was too low to measure phase transition parameters. Therefore, tear lipids had to be pooled from 8 to 16 donors. For comparison, meibum samples from the same individuals in the cohort were also pooled.
The leading tip (5 mm) of the Schirmer strip was bent at a 90-degree angle and subsequently placed over the edge of the lower eyelid for 5 minutes. The lower portion (5 mm) of the strip was cut off, and the upper and lower portions were each placed in a vial filled with argon to prevent oxidation. Care was taken not to contaminate the lower end of the test strip with finger lipids.
Lipids were extracted from the pools of Schirmer strips that were placed in the 2 glass vials using 5 mL of methanol, which had been bubbled with argon for 5 minutes. The strips and methanol were sonicated with a Sonifier 1 cell disrupter microprobe (Branson Ultrasonics, Danbury, CT) 3 times for 15 seconds each, with a 2-minute cooling period in between sonication. The methanol was decanted and centrifuged at 10,000 rpm for 15 minutes to remove methanol-insoluble impurities. The methanol was decanted again, and care was taken to not disturb the pellet. The steps above were repeated with CHCl3 and then again with benzene instead of methanol. The CHCl3 and benzene lipid extracts were added to the methanol lipid extracts, and the solvents were evaporated under a stream of argon. CDCl3 (1 mL) was added to each sample.
Curves were fit using SigmaPlot 10 software (Systat Software, Inc, Chicago, IL), and the confidence levels were obtained from a critical value table of the Pearson product–moment correlation coefficient. Significance between cohorts was tested using the Student t test. P < 0.05 was considered statistically significant. Data are reported as mean ± standard error.
Measurement of Lipid Phase Transitions Using Fourier Transform Infrared Spectroscopy
Lipid phase transitions were measured as described previously.9,18 Approximately 500 μL of sample in CDCl3 was applied to a AgCl infrared window. The solvent was evaporated under a stream of argon gas, and the window was placed in a lyophilizer for 4 hours to remove all traces of solvent. Infrared spectra were measured using a Fourier transform infrared spectrometer (Nicolet 5000 Magna Series; Thermo Fisher Scientific, Inc, Waltham, MA). Lipids on the AgCl window were placed in a temperature-controlled infrared cell. The cell was jacketed by an insulated water coil connected to a circulating water bath (model R-134A; Neslab Instruments, Newton, NH). The sample temperature was measured and controlled by a thermistor touching the sample cell window. The water bath unit was programmed to measure the temperature at the thermistor and to adjust the bath temperature so that the sample temperature could be set to the desired value. The rate of heating or cooling (1°C/15 minutes) of the sample was also adjusted by the water bath unit. Temperatures were maintained within ±0.01°C. Exactly 100 interferograms were recorded and averaged. Spectral resolution was set to 1.0 cm−1. Infrared data analysis was then performed (GRAMS/386 software; Galactic Industries, Salem, NH). The frequency of the symmetric CH2 stretching band near 2850 cm−1 (ṽsym) was used to estimate the content of trans and gauche rotamers in the hydrocarbon chains. ṽsym was calculated by first baseline leveling the OH–CH stretching region between 3500 and 2700 cm−1. The center of mass of the CH symmetric stretching band was calculated by integrating the top 10% of the intensity of the band. The baseline for integrating the top 10% of the intensity of the band was parallel to the OH–CH region baseline. The change in ṽsym versus temperature was used to characterize the lipid phase transitions, as described previously.5 Because rotamers are either in the trans or gauche conformation, phase transitions were fit to a 2-state sigmoidal equation using SigmaPlot 10 software (Systat Software, Inc, Chicago IL) as follows:
where ṽsym is the frequency of the symmetric CH2 stretching band near 2850 cm−1 and Tc is the phase transition temperature.
The lipid order at 33.4°C and 36°C, the temperatures at the ocular surface and of the meibomian glands, respectively,47 was calculated by extrapolating the ṽsym at 33.4°C and 36°C, from the fit of the phase transition and then converting ṽsym to the percentage of trans rotamers, a measure of lipid conformational order. The data for the percentage of trans rotamers were used to calculate the phase transition enthalpy and entropy from the slopes of the Arrhenius plots.
RESULTS
The infrared CH stretching region bands of meibum from 134 donors were examined in the largest infrared spectroscopic study of meibum.20 The donor demographics are presented in Table 1. Of the patients with hematopoietic stem cell transplants, 28% had no graft-versus-host disease, 50% had chronic graft-versus-host disease, and 22% had acute graft-versus-host disease. However, because of the small sample size, there was no statistical difference (P > 0.4) between the phase transition parameters of meibum from patients with or without graft-versus-host disease or between meibum from patients with graft-versus-host disease classified as acute or chronic.20 There was a trend toward an increased order with the severity of graft-versus-host disease.20
TABLE 1.
Cohort Demographics and Data
| Number | Average Age (y) | PHR (2955 cm−1/2919 cm−1) | P | |
|---|---|---|---|---|
| Cohort without dry eye | ||||
| Total | 45 | 22.3 ± 0.2 | 0.46 ± 0.01 | |
| Female | 14 | 20.9 ± 0.5 | 0.43 ± 0.02 | |
| Male | 31 | 23.3 ± 0.4 | 0.47 ± 0.02 | 0.2, vs. females |
| White | 35 | 22.1 ± 0.3 | 0.46 ± 0.03 | |
| Asian | 5 | 23 ± 1 | 0.47 ± 0.05 | 0.8, vs. whites |
| Black | 4 | 30 ± 3 | 0.49 ± 0.04 | 0.7, vs. whites |
| Hispanic | 1 | 29 | 0.49 | |
| Cohort with meibomian gland dysfunction | ||||
| Total | 42 | 65 ± 2 | 0.39 ± 0.01 | 0.0003, vs. without dry eye |
| Female | 13 | 58.9 ± 0.9 | 0.38 ± 0.03 | |
| Male | 29 | 63.8 ± 0.2 | 0.39 ± 0.02 | 0.8, vs. females |
| White | 34 | 67.2 ± 0.2 | 0.39 ± 0.02 | |
| Black | 4 | 56 ± 2 | 0.40 ± 0.06 | 0.7, vs. whites |
| Hispanic | 1 | 50 | 0.34 | |
| Cohort that had hematopoietic stem cell transplantation | ||||
| Total | 19 | 23 ± 2 | 0.39 ± 0.01 | 0.0017, vs. without dry eye |
| Female | 8 | 20.9 ± 0.5 | 0.43 ± 0.05 | |
| Male | 11 | 23.3 ± 0.4 | 0.35 ± 0.02 | 0.09, vs. females |
| White | 15 | 43.5 ± 0.6 | 0.39 ± 0.03 | |
| Asian | 1 | 13 | 0.33 | |
| Black | 3 | 42 ± 4 | 0.36 ± 0.09 | 0.7, vs. whites |
| Tear lipids from the cohort that had hematopoietic stem cell transplantation | ||||
| Total | 5 pools from 44 donors | 23 ± 2 | 0.0387 ± 0.004 | <0.001, vs. without dry eye |
Average ± standard error of the mean. Data for meibum, except where indicated.
Infrared CH stretching region spectra of meibum samples were measured at average temperatures of 11 ± 5°C, 11 ± 1°C, and 14 ± 2°C for Mn, MHSCT, and MMGD, respectively. Five bands were resolved in the infrared CH stretching region of Mn and MMGD (Fig. 1A). Four bands were resolved in the infrared CH stretching region of MHSCT as the =CH band at 3010 cm−1 (Fig. 1B.iii). The infrared bands for the wax oleyl oleate (Fig. 1C.ii) were similar to those of Mn (Fig. 1C.i), but they were broader than those for the wax stearyl palmitate (Fig. 1C.iii) and cholesteryl behenate (Fig. 2C.iv). Approximately 7 infrared bands were poorly resolved for cholesterol (Fig. 1C.v).
FIGURE 2.
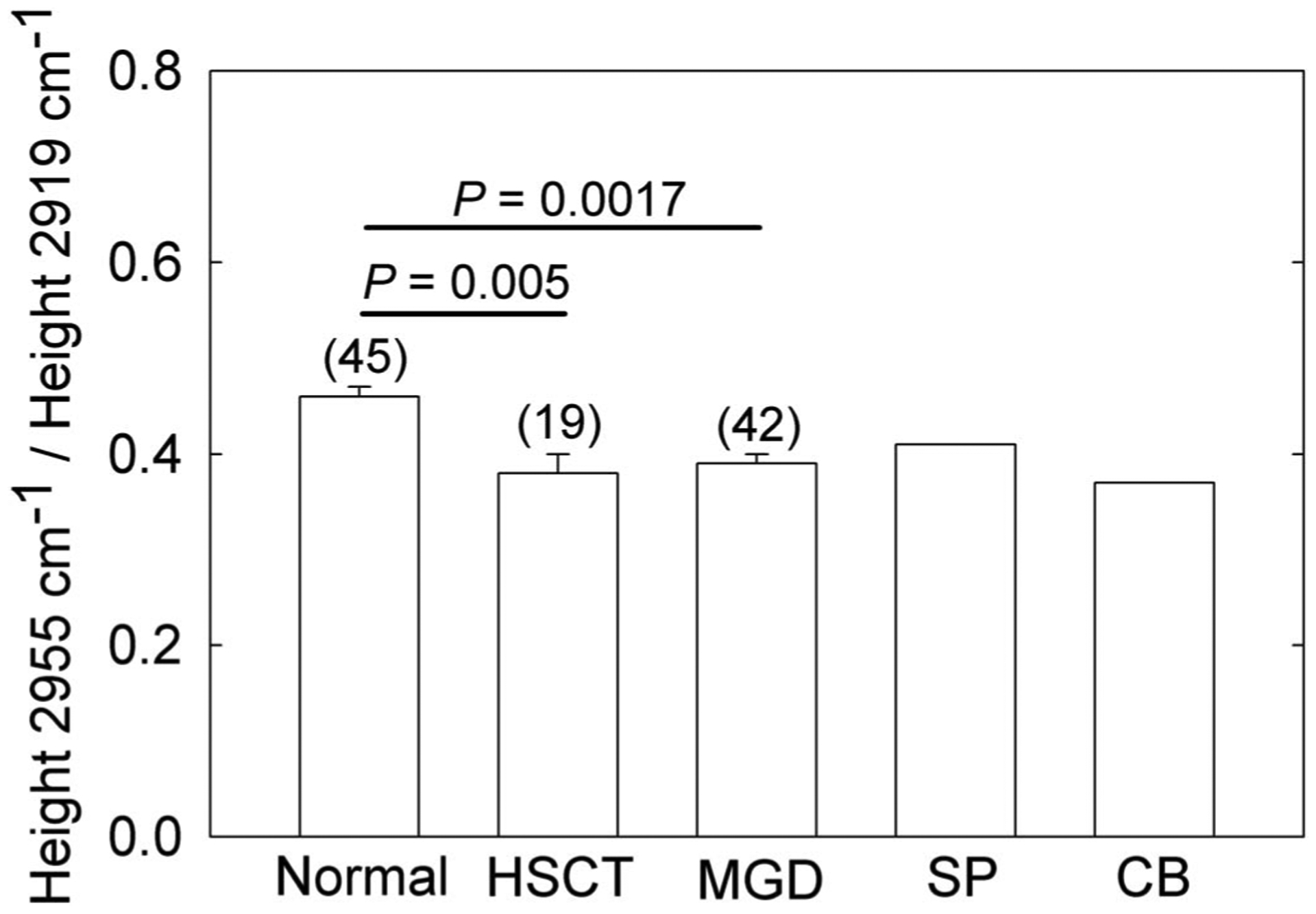
The PHR, on the y axis, is a relative measure of CH3 to CH2 moieties in the hydrocarbon chains of meibum. The ratio changes with hydrocarbon saturation, branching, and chain length. Mn, meibum from donors without dry eye; MMGD, meibum from donors with dry eye due to meibomian gland dysfunction; MHSCT, meibum from donors who have had hematopoietic stem cell transplantation and are susceptible to severe dry eye; SP, stearyl palmitate; CB, cholesteryl behenate.
The PHRs for MHSCT and MMGD were significantly lower than the PHR for Mn (15%, P = 0.0017 and 0.0003, respectively, Table 1, Fig. 2). The PHRs for TLHSCT and MHSCT were nearly identical (P > 0.05, Table 1).
There were no statistical differences in the meibum PHR in terms of sex or race. Similarly, the PHR did not change with age: r = 0.03, P = 0.85; r = 0.009, P = 0.97; r = 0.12, P = 0.45 for Mn, MMGD, and MHSCT, respectively.
The phase transition parameters from pooled samples were compared with the average values obtained from the same individual samples (Table 2). Although some of the phase transition parameters were significantly different, the differences were relatively small and inconsistent, so we conclude that pooling did not affect the phase transition parameters compared with the parameters measured individually.
TABLE 2.
Phase Transition Parameters of Human Meibum That Was Pooled or Calculated Individually
| Sample | Minimum Wavenumber (cm−1) | Maximum Wavenumber (cm−1) | Phase Transition Temperature (°C) | Cooperativity (Relative) | Age (yr) | n |
|---|---|---|---|---|---|---|
| Pooled, normal, younger* | 2849.39 ± 0.09 | 2853.93 ± 0.09 | 23.9 ± 0.4 | 4.3 ± 0.3 | 1.7 ± 0.2 | 1 pool from 8 donors |
| Individual, normal, younger | 2849.71 ± 0.07 | 2853.6 ± 0.9 | 27 ± 5 | 8 ± 3 | 1.7 ± 0.2 | 8 |
| P | <0.05† | >0.05 | >0.05 | <0.05† | ||
| Pooled, older, normal* | 2850.0 ± 0.1 | 2854.0 ± 0.1 | 29.6 ± 0.4 | 10 ± 1 | 38 ± 0.6 | 1 pool from 8 donors |
| Individual, older, normal | 2849.7 ± 0.1 | 2853.7 ± 0.2 | 30.3 ± 0.8 | 7.9 ± 0.8 | 35 ± 4 | 35 |
| P | <0.05† | >0.05 | >0.05 | <0.05† | ||
| Pooled HSCT | 2849.1 ± 0.3 | 2854.1 ± 0.5 | 34 ± 4 | 6 ± 2 | 45 ± 5 | 5 pools from 44 donors |
| Individual HSCT | 2849.3 ± 0.1 | 2854.0 ± 0.2 | 35.4 ± 0.6 | 4.3 ± 0.6 | 45 ± 5 | 19 |
| P | >0.05 | >0.05 | >0.05 | >0.05 |
Average ± 95% confidence interval. Age is presented as mean ± standard error of the mean.
HSCT is the cohort that had hematopoietic stem cell transplantation. P is for the comparison of pooled versus individual samples.
±Experimental error, n = 1 experiment.
Significantly different.
Tear lipids were collected from Schirmer strips, but the amount collected was too low to measure the phase transition parameters, so the tear lipids had to be pooled. For comparison, meibum samples from the same individuals in the cohort were also pooled. The phase transition parameters for 5 pools of tear lipids and 5 pools of meibum from donors who had hematopoietic stem cell transplantation are listed in Table 3. Compared with MHSCT, the phase transition temperature, cooperativity, and order were significantly greater for TLHSCT (P < 0.05, Fig. 3).
TABLE 3.
Phase Transition Parameters of Lipids From Pooled Meibum and Tears From the Same 44 Donors Who Had Hematopoietic Stem Cell Transplantation
| Phase Transition Parameter | Pooled Meibum | Pooled Tears |
|---|---|---|
| Minimum wavenumber (cm−1) | 2849.1 ± 0.1 | 2849.0 ± 0.1 |
| Maximum wavenumber (cm−1) | 2854.1 ± 0.2 | 2854.4 ± 0.3 |
| Phase transition temperature (°C) | 34 ± 2 | 40 ± 4 |
| Cooperativity (relative) | 6 ± 1 | 7 ± 1 |
| Order at 33.4°C (% trans) | 52 ± 5 | 64 ± 7 |
| Order at 36°C (% trans) | 46 ± 4 | 57 ± 8 |
| Δ Enthalpy (kcal/mol) | 131 ± 17 | 122 ± 12 |
| Δ Entropy (kcal/mol/degree) | 0.4 ± 0.1 | 0.39 ± 0.04 |
| No. of pools | 5 | 5 |
Data are presented as mean ± standard error of the mean.
FIGURE 3.
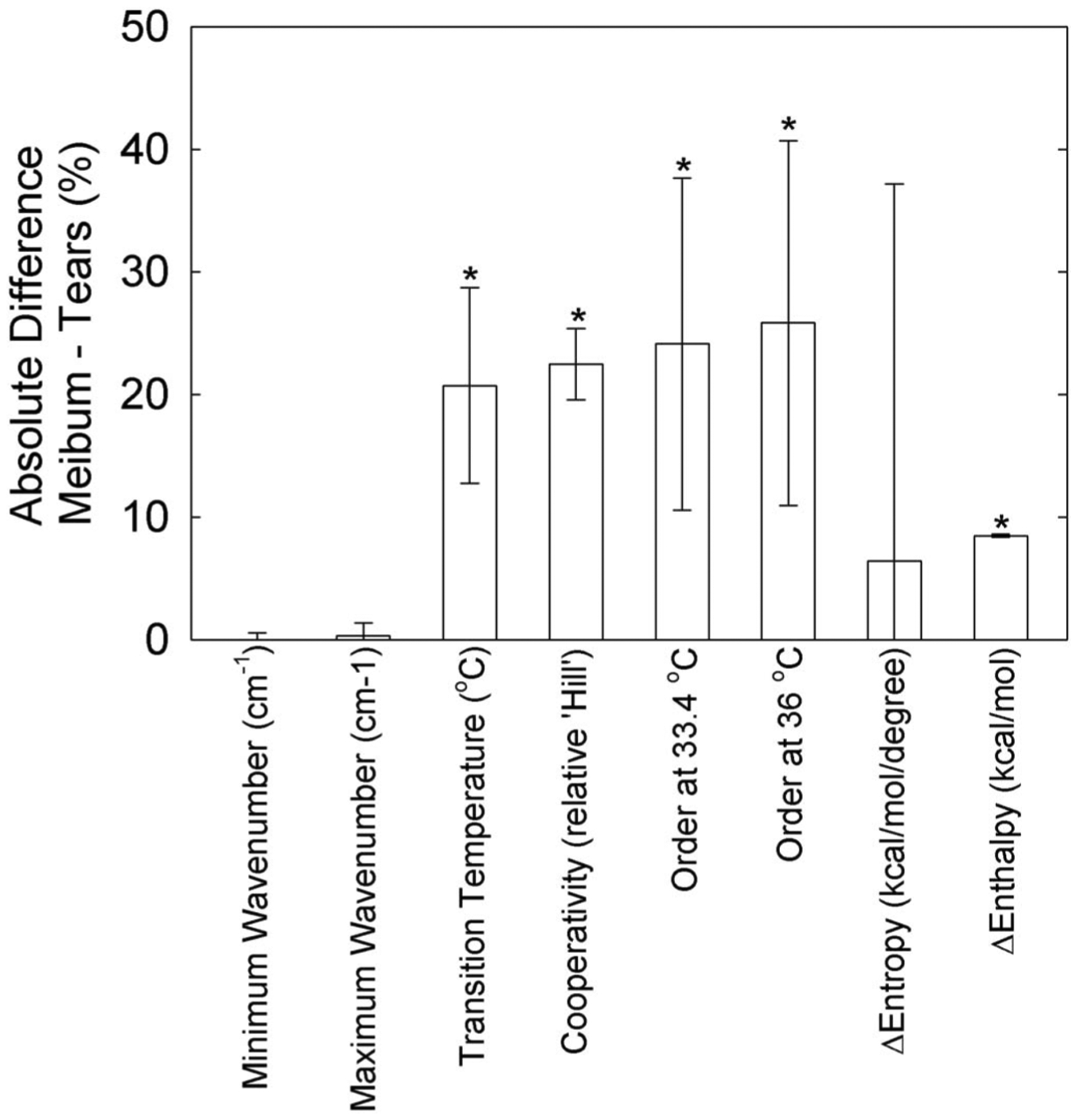
The absolute percentage difference (meibum – tears) for lipid phase transition parameters. The difference was positive for the minimum wavenumber, Δ enthalpy, and Δ entropy. Data are presented as mean ± 95% confidence limit. * indicates a significant difference, P < 0.05.
DISCUSSION
The compositional, structural, and functional relationships of meibum may provide insights into the loss of tear film stability with age and dry eye. The 3 major findings of the current study are as follows: 1) compared with the PHR for Mn, the PHRs for MHSCT and MMGD were significantly lower (15%; P = 0.0017 and 0.003, respectively); 2) compared with MHSCT, the phase transition temperature, cooperativity, and order were significantly greater for TLHSCT (23%–48%, P < 0.05); and 3) compared with Mn and MMGD, MHSCT and TLHSCT contained fewer double bonds (all samples of MHSCT and TLHSCT had no detectable double bonds in the infrared spectra). A similar result was obtained for 1 sample of Mn and tear lipids from a donor without dry eye.19 The trend for the magnitude change in order and phase transition temperature for meibum remained the same for tear lipids: Mn < MHSCT and tear lipids from donors without dry eye < TLHSCT; but the order and phase transition temperature of tear lipids were greater than those of meibum. The pooled sample data from the current study confirm the published individual sample data,22 so pooling was unlikely to alter the results. The functional consequences of a more ordered lipid layer are discussed earlier.
In the current study, the PHRs of Mn, MMGD, and MHSCT were measured using infrared spectroscopy. The PHR is related to the tear lipid and meibum composition, as discussed in the Introduction. The infrared CH stretching region spectra of meibum were measured well below the phase transition temperature so that the hydrocarbon chain structural differences did not contribute to the PHR values. The major finding of the current study is that the PHRs for MHSCT and MMGD were significantly less than that for Mn (15%; P = 0.0017 and 0.003, respectively). This observation is in agreement with a qualitative infrared spectroscopy study using principal component analysis.11 The PHR could be a marker for dry eye, and the compositional differences implied by differences in the PHR could perhaps contribute to it. The moieties responsible for the differences between the PHRs are speculative. Branched hydrocarbon chains contain fewer CH2 moieties and more CH3 moieties compared with straight chain hydrocarbons. As Mn cholesteryl esters contain more branched chains compared with Mn wax esters,30 fewer cholesterol esters in MMGD compared with Mn30 could contribute to the difference in the PHRs observed in the current study. The cholesterol ester content of MHSCT has yet to be determined.
No age or sex differences were noted between the PHRs for Mn, MMGD, and MHSCT, in agreement with a nuclear magnetic resonance spectroscopic study of human meibum.31 Because of the small sample size, the lack of differences in the PHRs with race should be viewed cautiously. Therefore, the PHR is not a factor related to tear film stability with age or sex. The finding that the PHRs of tear lipids and meibum are nearly identical implies that the higher order of tear lipids compared with meibum is not due to differences in hydrocarbon chain branching.
In the current study, in addition to having a lower PHR, MHSCT contained fewer double bonds compared with Mn and MMGD. Fewer double bonds could also contribute to more CH2 moieties and a smaller PHR for MHSCT compared with Mn. Fewer double bonds could also contribute to the very high order16,18 of MHSCT.23 Patients who have had hematopoietic stem cell transplantation have a higher incidence of dry eye, which is often very severe.48
van der Waals interactions between CH2 moieties are responsible for lipid ordering. CH3 moieties sterically inhibit CH2–CH2 interchain interactions and cause lipids to be more disordered, as observed with anteiso-branching.32 More CH2 moieties (fewer CH3 moieties) observed with MMGD and MHSCT compared with Mn could contribute to the higher order of MMGD and MHSCT compared with Mn.20,23 As double bonds also contribute to lipid disorder,16,18 fewer double bonds in MHSCT compared with Mn and MMGD, as observed in the current study, could also contribute to a more ordered MHSCT compared with Mn and MMGD.20,23
As stated earlier, a more ordered TFL layer could contribute to the formation of a discontinuous patchy TFL layer, which in turn results in deteriorated spreading21 and decreased surface elasticity.8 In addition to lipid order, amphiphilic lipids found in tears such as phospholipids and (O-acyl)-ω-hydroxy fatty acids, which have surfactant properties, may contribute significantly toward the structure and function of the tear film lipid layer at the tear interface region.8,29 In addition, the amount and quality of the protein content in the meibum may play a crucial role in its surface film structure and properties.8 Langmuir film studies involving proteins show that they can penetrate deeply into the tear film lipid layer, which can modify its performance (reviewed in citation8).
In conclusion, tear lipids are more ordered than meibum lipids, which could have functional consequences. The PHR is not a factor related to tear film stability with age or sex. The PHR and saturation can contribute to a higher meibum lipid order associated with a younger age, meibomian gland dysfunction, and dry eye from hematopoietic stem cell transplantation. Therefore, the hydrocarbon order may be a marker of or contribute to an unstable tear film layer.
ACKNOWLEDGMENTS
Major support was obtained from the National Institutes of Health (EYORO126180 to D.B.) and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY (GN151619B). Henry Collins received a Medical School Student fellowship from the “Summer Research Scholar Program” at the University of Louisville, Louisville, KY. Varun Ramakrishnan was supported by a “Summer Vision Sciences Training Program” fellowship (NIH/NEI EY026509, Co I, D.B.).
Footnotes
The authors have no funding or conflicts of interest to disclose
REFERENCES
- 1.King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interfereometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci. 2010;51:2418–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King-Smith PE, Fink BA, Fogt N, et al. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41:3348–3359. [PubMed] [Google Scholar]
- 3.Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pucker AD, Nichols JJ. Analysis of meibum and tear lipids. Ocul Surf. 2012;10:230–250. [DOI] [PubMed] [Google Scholar]
- 5.Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murube J The origin of tears: III. The lipid component in the XIX and XX centuries. Ocul Surf. 2013;10:200–209. [DOI] [PubMed] [Google Scholar]
- 8.Georgiev GA, Eftimov P, Yokoi N. Structure-function relationship of tear film lipid layer: a contemporary perspective. Exp Eye Res. 2017;163: 17–28. [DOI] [PubMed] [Google Scholar]
- 9.Mudgil P, Borchman D, Ramasubramanian A. Insights into tear film stability from babies and young adults; a study of human meibum lipid conformation and rheology. Int J Mol Sci. 2018;19:E3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr Eye Res. 2010;35:778–786. [DOI] [PubMed] [Google Scholar]
- 11.Borchman D, Foulks GN, Yappert MC. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp Eye Res. 2010;91:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faheem S, Kim S, Nguyen J, et al. Wax-tear and meibum protein, wax-β-carotene interactions in vitro using infrared spectroscopy. Exp Eye Res. 2012;100:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter M, Bhola R, Yappert MC, et al. Pilot study of the influence of eyeliner cosmetics on the molecular structure of human meibum. Ophthalmic Res. 2015;53:131–135. [DOI] [PubMed] [Google Scholar]
- 15.Mudgil P, Borchman D, Gerlach D, et al. Sebum/meibum surface film interactions and phase transitional differences. Invest Ophthalmol Vis Sci. 2016;57:2401–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sledge S, Henry C, Borchman D, et al. Human meibum age, lipid-lipid interactions and lipid saturation in meibum from infants. Int J Mol Sci. 2017;18:E1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthal Res. 2010;44:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mudgil P, Borchman D, Yappert MC, et al. Human meibum saturation and lipid order. Exp Eye Res. 2013;116C:7985. [DOI] [PubMed] [Google Scholar]
- 19.Borchman D, Foulks GN, Yappert MC, et al. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers. 2007;87:124–133. [DOI] [PubMed] [Google Scholar]
- 20.Ramasubramanian A, Blackburn R, Sledge SM, et al. Structural differences in meibum from donors after hematopoietic stem cell transplantations. Cornea. 2019. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nencheva Y, Ramasubramanian A, Eftimov P, et al. Effects of lipid saturation on the surface properties of human meibum films. Int J Mol Sci. 2018;19:E2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borchman D. The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf. 2019;17:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramasubramanian A, Borchman D. Structural differences in meibum from teenage donors with and without dry eye induced by allogeneic hemopoetic stem cell transplantations. J Ped Hematol Oncol. 2019. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamba OP, Borchman D, O’Brien PJ. Fourier transform infrared study of the rod outer segment disk and plasma membranes of vertebrate retina. Biochemistry. 1994;33:1704–1712. [DOI] [PubMed] [Google Scholar]
- 25.Borchman D, Yappert MC, Afzal M. Lens lipids and maximum lifespan. Exp Eye Res. 2004;79:761–768. [DOI] [PubMed] [Google Scholar]
- 26.Borchman D, Yappert MC. Lipids and the ocular lens. J Lipid Res. 2010;51:2473–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borchman D, Stimmelmayr R, George JC. Whales, lifespan, phospholipids, and cataracts. J Lipid Res. 2017;58:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stimmelmayr R, Borchman D. Lens Lipidomes among phocids and odobenidae. Aquat Mammals. 2018;44:496–508. [Google Scholar]
- 29.Borchman D, Cenedella RI, Lamba OP. Role of cholesterol in the structural order of lens lipids. Exp Eye Res. 1996;62:191–197. [DOI] [PubMed] [Google Scholar]
- 30.Borchman D, Ramasubramanian A, Foulks GN. Human meibum cholesteryl and wax ester variability with age, gender and meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2019;60:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536. [PubMed] [Google Scholar]
- 32.Borchman D, Ramasubramanian A. Human meibum chain branching variability with age, gender and meibomian gland dysfunction. Ocul Surf. 2019;17:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butovich IA, McMahon A, Wojtowicz JC, et al. Dissecting lipid metabolism in meibomian glands of humans and mice: an integrative study reveals a network of metabolic reactions not duplicated in other tissues. Biochim Biophys Acta. 2016;1861:538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam SM, Tong L, Duan X, et al. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55:289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown SH, Kunnen CM, Duchoslav E, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci. 2013; 54:7417–7424. [DOI] [PubMed] [Google Scholar]
- 36.Dean AW, Glasgow BJ. Mass spectrometric identification of phospholipids in human tears and tear lipocalin. Invest Ophthalmol Vis Sci. 2012; 53:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rantamäki AH, Seppänen-Laakso T, Oresic M, et al. Human tear fluid lipidome: from composition to function. PLoS One. 2011;6:e19553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saville JT, Zhao Z, Willcox MD, et al. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp Eye Res. 2011;92:238–240. [DOI] [PubMed] [Google Scholar]
- 39.Wollensak G, Mur E, Mayr A, et al. Effective methods for the investigation of human tear film proteins and lipids. Graefes Arch Clin Exp Ophthalmol. 1990;228:78–82. [DOI] [PubMed] [Google Scholar]
- 40.Borchman D, Yappert MC, Milliner SE, et al. Confirmation of the presence of squalene in human eyelid lipid by heteronuclear single quantum correlation spectroscopy. Lipids. 2013;48:1269–1277. [DOI] [PubMed] [Google Scholar]
- 41.Robosky LC, Wade K, Woolson D, et al. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J Lipid Res. 2008;49:686–692. [DOI] [PubMed] [Google Scholar]
- 42.Pucker AD, Haworth KM. The presence and significance of polar meibum and tear lipids. Ocul Surf. 2015;13:26–42. [DOI] [PubMed] [Google Scholar]
- 43.Borchman D, Foulks GN, Yappert MC, et al. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102. [DOI] [PubMed] [Google Scholar]
- 44.Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008; 49:3779–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagyová B, Tiffany JM. Components responsible for the surface tension of human tears. Curr Eye Res. 1999;19:4–11. [DOI] [PubMed] [Google Scholar]
- 46.Foulks GN, Bron AJ. Meibomian-gland dysfunction: a clinical scheme for description, diagnosis, classification and grading. Ocul Surf. 2003;1:17–36. [DOI] [PubMed] [Google Scholar]
- 47.Abreau K, Callan C, Kottaiyan R, et al. Temperatures of the ocular surface, lid, and periorbital regions of Sjögren’s, evaporative, and aqueous-deficient dry eyes relative to normals. Ocul Surf. 2016;14:64–73. [DOI] [PubMed] [Google Scholar]
- 48.Ogawa Y, Kuwana M. Dry eye as a major complication associated with chronic graft-versus-host disease after hematopoietic stem cell transplantation. Cornea. 2003;22:19–27. [DOI] [PubMed] [Google Scholar]


