Introduction
The increased axial coverage of long axial field-of-view (AFOV) PET scanners enable whole-body PET imaging in a single-bed position. The resultant marked increase in physical sensitivity produces images of higher quality than conventional PET scanners. These sensitivity gains can be leveraged in several oncologic applications, both in research and in the clinic.
The EXPLORER Consortium has developed two long axial field-of-view for human use, these being the uEXPLORER (currently installed at the University of California, Davis and at a number of sites in China), and the PennPET EXPLORER at the University of Pennsylvania. Performance testing of both devices and initial human studies have already been published [1–3]. Recent updates regarding development and/or application of these scanners are discussed in other chapters in this edition of the PET Clinics (Chapters 1 and 2 for the uEXPLORER and Chapter 3 for the PennPET EXPLORER). At the current time, the uEXPLORER is being utilized for both research and clinical imaging. The PennPET EXPLORER is being expanded to its final configuration for research investigations.
Human imaging on both of these long AFOV scanners have demonstrated many of the theoretical benefits of increased photon detection sensitivity: (i) total-body diagnostic quality images have been obtained in approximately one minute, increasing the tolerability of imaging; (ii) diagnostic quality images have been realized with significantly reduced quantities of radiotracer, decreasing the overall radiation exposure of patients; (iii) delayed acquisitions that may improve the visualization of tumor by increasing the ratio between tumor uptake and background; (iv) increased image quality has demonstrated sites of uptake previously unseen on conventional PET that may result in early detection of tumor/recurrence.
The implementation of this technology is not without risks. The interpretation of findings that were not visualized on conventional PET/CT scanners, or were visualized but have different quantitative levels of uptake, must be studied to avoid “overcalling.” New interpretation paradigms may be necessary as not to decrease the specificity of whole-body PET imaging.
This work reviews the advantages and challenges posed by the implementation of total body PET/CT in oncology. Exemplary whole-body PET images provide the motivation for this discussion. Early lessons and future directions are also discussed in this evolving field.
Shorter Scan Time
Exploiting the increased physical sensitivity of long AFOV, whole-body PET scanners can produce diagnostic images in as short as one minute. Initial human studies on the uEXPLORER and the PennPET EXPLORER explored the effects of decreased scan time by simulating shorter studies using listmode data.[2] [3] Both scanners demonstrated the ability to generate images with acceptable image quality in ~1/2 minute.
Fast acquisition time can benefit both adult and pediatric patients. The inherent stress of PET imaging often necessitates anesthesia for pediatric patients with inherent risks of the anesthesia itself, as well as the need for invasive monitoring. Further, anesthesia requires additional staff and equipment.[4] This increase in imaging complexity also adds to imaging cost and may even delay necessary imaging. Decreasing the anesthetic requirement or the elimination of anesthesia altogether could clearly benefit pediatric imaging. Additionally, shorter imaging time would aid in the imaging oncologic patients that may have difficulty and pain when lying flat on the scanner table, such as patients with widespread osseous metastatic disease. For these patients, lying flat for 20–30 minutes presents a significant barrier to imaging, and imaging may be degraded from motion if the patient is uncomfortable. Faster imaging on a long AFOV device may increase the accessibility for these patient populations.
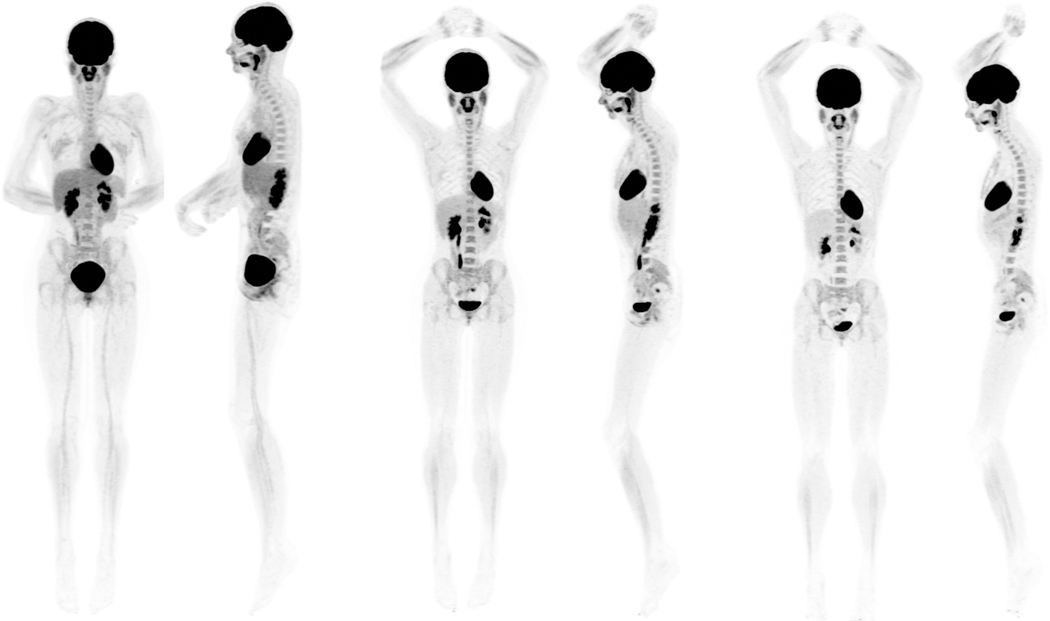
Shorter imaging frames may also benefit characterizing disease with radiotracers with time-varying kinetics. For example, 18F-fluciclovine uptake in tumors usually peaks early and decreases over time, whereas bladder uptake increases over time from urinary excretion.[5] Urinary excretion accumulated by layering in the dependent aspect of the urinary bladder over time (Figure 1). Urine activity can be misinterpreted as a recurrent tumor if localizing to the prostate bed, especially when confounded by motion artifact and/or suboptimal anatomic delineation from a low dose correlative CT. The use of a series of short time frame images, e.g. 2 minute frame duration, obtained from the 20-minute dataset may help avoid this pitfall and show that the bladder uptake follows the ureters uptake in time. However, uptake in the same region before radiotracer excretion may be suspicious for recurrent tumor.
Fig. 1.
A 43-year-old healthy female participant, injected with 18.5 MBq of 18F-FDG. Anterior and left lateral MIP views from three 20-minute datasets are presented: 40 to 60 minutes (left), 90 to 110 minutes (center), and 180 to 200 minutes (right). Images, scaled to the same window level, demonstrate the normal radiotracer biodistribution with high image quality. MIP, maximum intensity projection.
Fast acquisition on long AFOV PET imagers may enable breath-hold examinations. Even with state-of-the art PET scanners, imaging the chest occurs over minutes, precluding breath-hold imaging. Imaging in the setting of shallow respiration leads to respiratory motion artifact and partial volume averaging, both decreasing apparent uptake of lung nodules. Further, co-registration with the CT suffers. Breath-hold PET has been shown to mitigate these effects, leading to.[6 7] Prior simulation scans have demonstrated the possibility of pushing the limit of acquisition time below half a minute [1 3]. Breath-hold PET should be accompanied by breath-hold CT obtained in the same phase of respiration for anatomic registration and attenuation correction. This application may improve the characterization and detection of small lung nodules. The clinical implications of such an improvement would have to be studied in the context of current treatment paradigms.
Low Dose
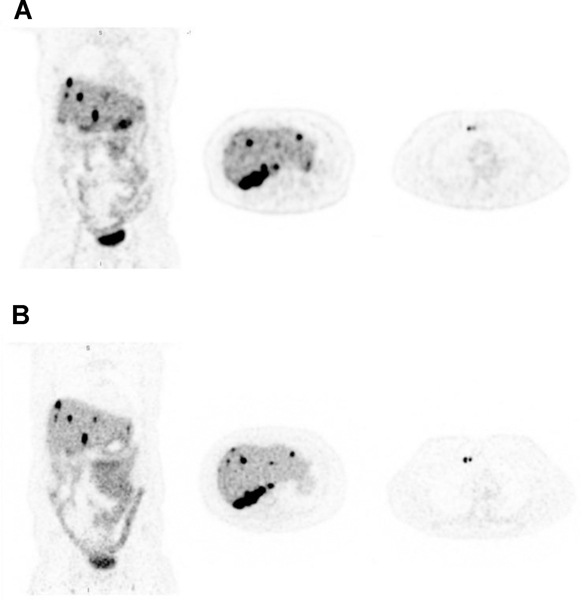
The increased sensitivity of whole-body PET can allow for a decrease in the injected activity while preserving image quality. On the uEXPLORER, images have been acquired with 18.5 MBq (0.5 mCi) of FDG, about 1/20th of the standard injected activity, that are considered to be of high quality. Examples of PET/CT images obtained using 18.5 MBq (0.5 mCi) are shown in Figure 2. As shown in this image, the expected biodistribution of FDG can be reliably traced even 3 hours after injection of such a small amount of activity. On the prototype PennPET Explorer with a 64 cm AFOV, 68Ga-DOTATATE PET of a patient with metastatic neuroendocrine tumor obtained 3.5 hours after injection of the radiotracer were comparable to images obtained on a standard-of-care PET scanner obtained 65 minutes after injection (Figure 3). At the time of scanning on the PennPET Explorer, the activity was one-fifth that at the time of the clinical scan and corresponded to an injected activity of 30 MBq if a standard uptake of an hour was utilized [2].
Fig. 2.
(A) 68Ga-DOTATATE PET images (coronal and transverse) of a subject with metastatic neuroendocrine tumor imaged on a standard- of-care PET scanner 65 minutes after radiotracer injection. (B) Coronal and transverse images from same subject on PennPETacquired 3.5 hours after injection (20-minute scan). Although the scan on the PennPET Explorer was obtained 2.5 hours after the standard-of-care commercial scan (greater than two-half lives), image quality is comparable. This research was originally published in JNM. Pan- tel et al. PennPET Explorer: Human Imaging on a Whole-Body Imager. 2020;61 ( 1 ): 144–51. © SNMMI.
Fig. 3.
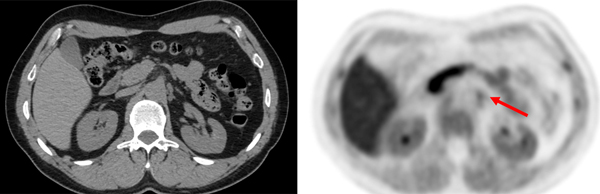
(A) Standard-of-care 18F-FDG PET/CT images (transverse and coronal) from a patient with metastatic colon cancer. (B) PennPET image acquired 2.75 and 4.2 hours after injection (10-minute scans) demonstrate better delineation of perihepatic disease (red arrow) compared with standard-of-care PET. An epiphrenic lymph node (yellow arrow) is also only seen on the delayed PennPET images. This research was originally published in JNM. Pantel et al. PennPET Explorer: Human Imaging on a Whole-Body Imager. 2020;61(1):144–51. © SNMMI.
Beyond merely a technologic achievement, imaging with less injected activity may have important clinical applications, especially in the pediatric, adolescent, and young adult population. Decreasing radiation dose is receiving increasing attention for these developing age groups. For example, lymphoma is the third most common childhood malignancy and PET/CT is widely accepted in its diagnosis-treatment paradigm.[8] Typically, a patient undergoes at least three FDG-PET/CTs. For example, a routine clinical protocol for Hodgkin lymphoma includes one PET/CT at staging, one after two cycles of chemotherapy, and one after completion of treatment. The patient may receive one or more additional PET/CT scans when there are concerns for persistent or recurrent disease. Decreasing the injected activity from 370 MBq (10 mCi) to 18.5 MBq (0.5 mCi) for 3 PET/CT studies decreases the exposure related to FDG injection from 21 mSv to approximately 1 mSv. Furthermore, decreasing the radiation dose per scan may increase the utilization of PET/CT in long-term follow-up of these patients to screen for recurrence, which is not generally routine practice at this time. [8] The role of total-body PET/CT for pediatric imaging is further explored in a review article by Nardo et al. [9]
In addition to reducing radiation exposure in pediatric imaging, unnecessary exposure to radiation in the adult population should also be avoided. Several campaigns in both the United States and Europe are dedicated to educating physicians about adhering to “as low as reasonably acceptable” (ALARA) principles, in the pediatric and adult population. For example, the “Image Wisely” campaign is a joint initiative supported by several radiological societies that aims to lower the amount of radiation used in medically necessary imaging studies and eliminate unnecessary procedures in adult imaging.[10] Similarly, “Image Gently” exists for pediatric radiology.[11] The use of decreased injected activity in total body PET/CT with low dose protocols is in keeping with the pledge that many radiologists have made to these campaigns. The implementation of low dose PET/CT may also allow PET/CT to be used as a screening tool for patients with hereditary cancer syndromes, such as a breast and ovarian cancer, Li-Fraumeni, or Lynch syndromes, where the risk of developing cancer is several times higher than that of the non-affected population.[12]
Imaging with lower activity can also be of benefit when using radiotracers with limited supply, for example as shown with 68Ga-DOTATATE on the PennPET Explorer study described above. Given production difficulty with 68Ga-DOTATATE, imaging each patient with a lesser dose may increase the availability of this study for patients.[2] Similarly, imaging with lower activity may benefit research radiotracers with difficult productions or dose limitations.
Delayed Imaging
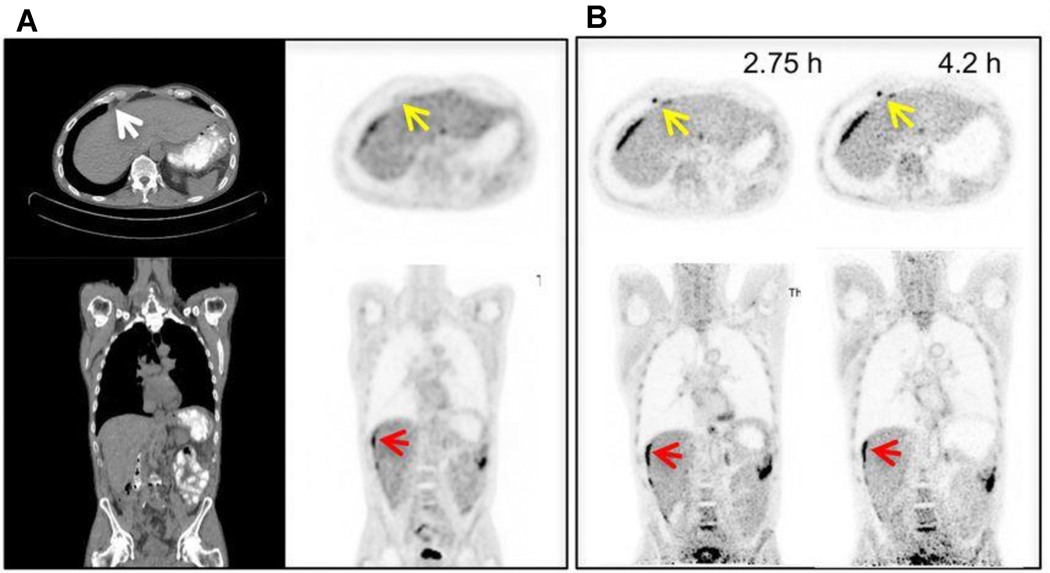
As an alternate to imaging with less injected activity at typical uptake times, the high sensitivity of long AFOV scanners enable imaging at delayed time points using the standard injected activity. Indeed, imaging on the PennPET EXPLORER was shown to be possible as late as 24 hours after the injection of 500 MBq (13.5 mCi) of FDG. Imaging at approximatively 4 hours demonstrated diagnostic image quality. [2] Depending on radiotracer kinetics, delayed imaging may prove valuable. For instance, given trapping of FDG in malignancy owing to increased hexokinase activity and washout of background tissue, lesion contrast increases over time. Imaging a clinical patient with metastatic colon cancer demonstrated this on the prototype PennPET Explorer with 64 cm AFOV (Figure 4). An epiphrenic lymph node was only seen on delayed imaging 2.75 and 4.2 after injection; this lymph node was not visualized by conventional PET 1 hour after injection. Washout of tracer from normal tissues increased contrast in this lymph node, which, in combination with increased sensitivity from the PennPET Explorer, enabled detection. [2] Several clinical studies have shown increased sensitivity with FDG in detecting disease by utilizing delayed imaging.[13 14] Delineation of normal tissue and tumor may also benefit from delayed imaging, as has been shown with FDG-PET in glioma imaging[15].
Fig. 4.
A 53-year-old healthy female subject, injected with 389 MBq of 18F-FDG. The shown images from a 20-min- ute acquisition obtained 90 minute after injection. The arrow indicates a small right submandibular lymph node with a tiny fatty hilum. This lymph node was approximativê 3 times more FDG avid than liver background. The finding was followed clinically and demonstrated to be a reactive lymph node.
Total Body Imaging
The 194 cm AFOV of the uEXPLORER scanner allows total body coverage in more than 90% of the population. This opens the door to performing total body dynamic imaging, providing simultaneous time activities curves for organs/lesions not contained within the view of conventional PET/CT. These datasets, inclusive of entire body kinetics, can be reconstructed and analyzed to create parametric images that may offer complementary information to typical SUV-scaled images. For example, utilization rate (Ki) images of FDG can be provided to the radiologist to visualize glucose utilization in the target lesion not confounded by non-specific uptake. These images are now available at UC Davis and their clinical value is being evaluated and compared to the routine static acquisition images. As expected, it is challenging to implement such protocols in clinical routine due to limited scanner time. However, if significant clinical utility is demonstrated, then workflows may be developed to allow these protocols to be routinely used.
Increased Detection Challenges
The improved image quality of total body PET may result in the detection of radiotracer uptake in small structures that cannot be seen on conventional PET/CT scanners. This ability arises from to the significant gain in signal, which allows reconstruction without the need for filtering to reduce noise, and from the excellent intrinsic spatial resolution of these devices. The visualization of such small structures presents challenges in the interpretation of total body PET images.
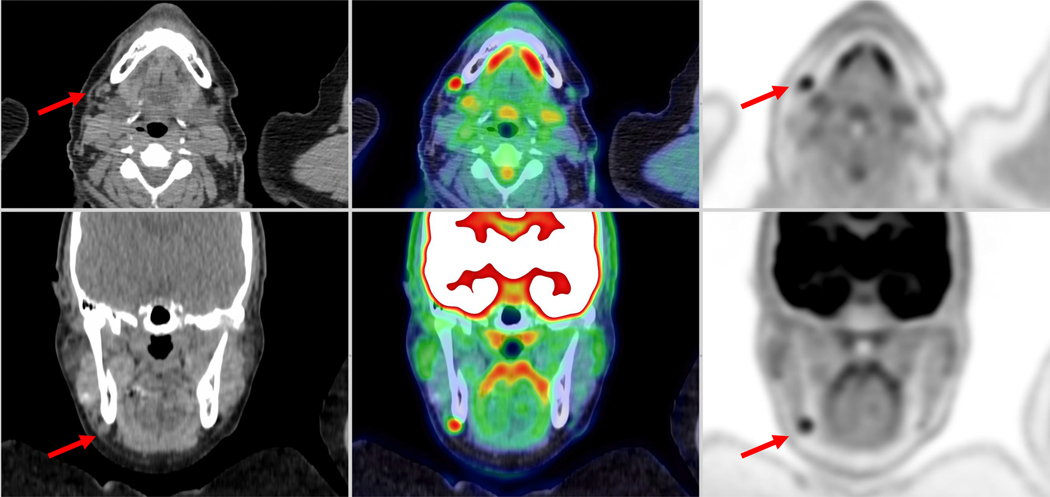
The interpretation of PET signal must be made in the context of disease pathophysiology versus normal physiology. Correlation with other imaging modalities is often helpful. For example, with total body PET/CT scanners, the visualization of a small lymph node with uptake measuring 2–3 times more than liver background may be interpreted as a normal finding rather than lymphoproliferative or metastatic disease (Figure 5). Mild reactive uptake in cervical lymph nodes, particular submandibular lymph nodes, is a common finding on conventional PET/CT and offers a challenge to the reader in terms of reporting the significance. Total body PET only accentuates these findings, although it is possible that delayed imaging may help. Normal anatomic features—e.g. preservation of the fatty hilum—may also support a benign etiology. In another context, though, radiotracer uptake in a small lymph node may be indicative of disease. For example, uptake in a small pelvic lymph node on a 18F-fluciclvoine study in a patient with biochemical recurrence of prostate cancer may likely represent a site of recurrent disease. In this case, total-body PET provides a clear advantage.
Fig. 5.
A 56-year-old man with biochemical recurrence of prostate cancer. Ten-minute images were reconstructed starting from 4-minute postinjection of 306 MBq of 18F-Fluci- clovine. The red arrow demonstrates uptake within the left celiac ganglia with radiotracer uptake similar or higher than bone marrow; this finding should not be misinterpreted
With increasing experience interpreting studies from total-body PET scanners, uptake in normal physiologic structures becomes apparent or more prominent. For example, the celiac ganglia and parasympathetic chain is seen more frequently in Total Body 18F-fluciclovine studies (Figure 5); on FDG studies, the adrenal glands, pituitary gland, and gray matter of the spinal cord are often prominent [16]. The radiologist must appreciate that these structures are normal as of these normal structures as misinterpreting these findings may lead to unnecessary follow-ups or procedures exposing the patients to further risk, such as radiation exposure.
The high spatial resolution of the uEXPLORER enables more accurate quantification of radiotracer uptake in small lesions. For example, ground glass opacities and small pulmonary nodule may now be visualized with level of uptake that are exceeding the radiologist expectations. These findings, though, may still correlate with benign findings.
Future directions
The long AFOV and technical advantages of the total body PET can be exploited for many research purposes in oncology, far beyond the applications described above.
Dosimetry studies of novel radiotracers can benefit from a long AFOV PET scanner. The ability to image lower activities afforded by increased sensitivity can be used to image at later time periods after radiotracer injection to better estimate the organ time-activity curve. For example, images on the PennPET Explorer with FDG were obtained out to 10 half-lives post-injection [2], permitting studies of the biodistribution of FDG over extended time-periods in healthy volunteers. Such increases in data could improve estimates of cumulative activity and thus the absorbed dose.
Total body PET also allows for the generation of vastly improved images when using radiotracers with unfavorable dosimetry such as those labeled with 89Zr, [17], which for safety reasons can only be injected in small quantities. This is particularly valuable when imaging antibodies, anti-body fragments and such, which must be labeled with long lived isotopes due to their slow kinetics. This has profound positive implications for immunoPET, which in turn may benefit oncologic science, drug development and personalized medicine [18]. PET imaging of cell-based therapies is a related application of interest. A critical concern when directly labeling cells for PET applications is toxicity to the cells, while a limiting factor when using reporter-gene approaches is detecting the cell signal over the background. In both cases, the sensitivity gain from total-body PET are likely to deliver important advantages.
Long AFOV PET can enable sequential imaging of two PET tracers. By imaging a lower dose of a first tracer and subsequently imaging a higher dose of a second tracer, two PET tracers may be imaged in one image session [19]. Such a protocol is analogous to ventilation-perfusion imaging with two 99mTc-based radiotracers where the counts from the second tracer overwhelm the first. Oncologic applications are numerous, with a simple example being the use of NaF followed by FDG [20]. The ability to image the first radiotracer on the PennPET Explorer—with doses as low as 18.5 −74 MBq (0.5 to 2 mCi) [21]—permits such a protocol. More details on this potential use of a long AFOV scanner are provided in Chapter 6.
Another unmet challenge in oncology is the ability to quantitatively understand, track and predict side-effects and off-target impacts of drug therapies, and impacts of concurrent symptoms such as cachexia. Total-body PET, by allowing simultaneous interrogation and parametric imaging of all organs, allows assessment of multi-organ function and multi-organ interactions. This facilitates a “systems biology” approach to understanding the pathophysiology of cancer and may open numerous doorways to future avenues of productive research. Related to this may be the possibility of better understanding the abscopal effect, whereby treatment of one site of disease leads to response in un-treated sites [22].
One of the earliest uses of PET was to study perfusion. Total-body PET offers the possibility, for the first time, of quantitatively measuring perfusion across the entire body, allowing measurement of perfusion for metastatic disease. This may have important applications in, for example, determining whether particular lesions may be expected to respond well to radiation therapy [23], or to track response to anti-angiogenic therapy [24].
Finally, there may be future applications of total-body PET in cancer prevention. By interrogating the mind-body connection with repeat studies at low dose in healthy subjects, there is the opportunity to understand how different kinds of stress (psychologic, dietary, etc) can impact the inflammatory and oncogenic milieu of the body. This in turn could lead to quantitative measurements of the mechanistic impacts of lifestyle changes which may reduce cancer risk.
Conclusion
The initial implementation of total body PET/CT at UC Davis and at the University of Pennsylvania has demonstrated improved image quality and opened the doors to many new applications in oncology. In the research setting, total body PET/CT can aid in the development of new radiopharmaceuticals and serve as a valuable tool to elucidate underlying biology. In the clinical setting, several new applications have been already successfully utilized, including low dose imaging, delayed imaging, faster imaging, and the use of parametric maps.
These applications, both in research and in the clinic, will drive the future development and adoption of long AFOV PET. Particularly, the ideal AFOV will likely depend on desired use. Both the PennPET Explorer, tested initially in a prototype configuration of 64 cm and more recently with 112 cm, and the uExplorer with a 2 m AFOV produce images superior to commercial PET scanners. As the AFOV increases beyond a 70 cm AFOV, though, the peak sensitivity at the center of the scanner does not markedly improve; rather the axial extent of maximal sensitivity increased.[25] For applications where maximum sensitivity must be maintained over a large area of the body, such as early biodistribution studies of novel radiotracers, a AFOV of 1.4 m or greater may be necessary. For targeted studies of particular body regions, such as imaging the abdomen/pelvis for prostate cancer or dynamic imaging of the breasts with novel radiotracers, scanners with an AFOV of 1 m or less may be most cost effective. As the indications for the scanners continue to evolve, so should the scanners themselves, pushing the current boundaries of modern PET imaging.
Key Points
Human imaging on the uEXPLORER at U.C. Davis and the PennPET EXPLORER at the University of Pennsylvania have demonstrated superb image quality.
The increased sensitivity of these instruments enables imaging with less injected activity or shorter scans, or delayed imaging. These new capabilities can uniquely benefit oncologic imaging.
The increased sensitivity of EXPLORER PET and new imaging paradigms create new challenges in imaging interpretation that must be overcome for optimal integration into the clinical workflow.
The ability to image lesser activities can be leveraged for research studies, including dosimetry and cell tracking applications, and even dual-tracer imaging.
Synopsis
New protocols for imaging cancer have been developed to take advantage of the improved imaging capabilities of long-axial field-of-view PET scanners. Both research and clinical applications have been pursued with encouraging early results. Clinical studies have demonstrated improved image quality and the ability to image with less injected activity or for shorter duration. With the increased sensitivity inherent in total-body PET scanners and new imaging paradigms, new challenges in image interpretation have emerged. New research applications have also emerged, including dosimetry, cell tracking, and dual-tracer applications.
Acknowledgments
Funding
NIH R01-CA206187, R01-CA113941, R01CA249422, R01-CA225874, and KL2TR001879.
Disclosure
Support for development of the PennPET EXPLORER was received from Philips Healthcare and from the Department of Radiology at the University of Pennsylvania.
Lorenzo Nardo has is principle investigator of a service agreement with United Imaging Healthcare
UC Davis has a revenue-sharing agreement with United Imaging Healthcare that is based on uEXPLORER sales.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Karp JS, Viswanath V, Geagan MJ, et al. PennPET Explorer: Design and Preliminary Performance of a Whole-Body Imager. J Nucl Med 2020;61(1):136–43 doi: 10.2967/jnumed.119.229997[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantel AR, Viswanath V, Daube-Witherspoon ME, et al. PennPET Explorer: Human Imaging on a Whole-Body Imager. J Nucl Med 2020;61(1):144–51 doi: 10.2967/jnumed.119.231845[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badawi RD, Shi H, Hu P, et al. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. J Nucl Med 2019;60(3):299–303 doi: 10.2967/jnumed.119.226498[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlachov Y, Ganatra RH. Sedation/anaesthesia in paediatric radiology. Br J Radiol 2012;85(1019):e1018–31 doi: 10.1259/bjr/28871143[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorensen J, Owenius R, Lax M, Johansson S. Regional distribution and kinetics of [18F]fluciclovine (anti-[18F]FACBC), a tracer of amino acid transport, in subjects with primary prostate cancer. Eur J Nucl Med Mol Imaging 2013;40(3):394–402 doi: 10.1007/s00259-012-2291-9[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 6.Torizuka T, Tanizaki Y, Kanno T, et al. Single 20-second acquisition of deep-inspiration breath-hold PET/CT: clinical feasibility for lung cancer. J Nucl Med 2009;50(10):1579–84 doi: 10.2967/jnumed.109.064246[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Balamoutoff N, Serrano B, Hugonnet F, Garnier N, Paulmier B, Faraggi M. Added Value of a Single Fast 20-second Deep-Inspiration Breath-hold Acquisition in FDG PET/CT in the Assessment of Lung Nodules. Radiology 2018;286(1):260–70 doi: 10.1148/radiol.2017160534[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 8.Gillman J, States LJ, Servaes S. PET in Pediatric Lymphoma. PET Clin 2020; 15(3):299–307 doi: 10.1016/j.cpet.2020.03.007[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 9.Nardo L, Schmall JP, Werner TJ, Malogolowkin M, Badawi RD, Alavi A. Potential Roles of Total-Body PET/Computed Tomography in Pediatric Imaging. PET Clin 2020;15(3):271–79 doi: 10.1016/j.cpet.2020.03.009[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayo-Smith WW, Morin RL. Image Wisely: The Beginning, Current Status, and Future Opportunities. J Am Coll Radiol 2017;14(3):442–43 doi: 10.1016/j.jacr.2016.11.027[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 11.Goske MJ, Applegate KE, Bulas D, et al. Image Gently: progress and challenges in CT education and advocacy. Pediatr Radiol 2011;41 Suppl 2:461–6 doi: 10.1007/s00247-011-2133-0[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 12.Tiwari R, Singh AK, Somwaru AS, Menias CO, Prasad SR, Katabathina VS. Radiologist’s Primer on Imaging of Common Hereditary Cancer Syndromes. Radiographics 2019;39(3):759–78 doi: 10.1148/rg.2019180171[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 13.Kubota K, Itoh M, Ozaki K, et al. Advantage of delayed whole-body FDG-PET imaging for tumour detection. Eur J Nucl Med 2001;28(6):696–703 doi: 10.1007/s002590100537[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 14.Mayerhoefer ME, Giraudo C, Senn D, et al. Does Delayed-Time-Point Imaging Improve 18F-FDG-PET in Patients With MALT Lymphoma?: Observations in a Series of 13 Patients. Clin Nucl Med 2016;41(2):101–5 doi: 10.1097/RLU.0000000000001005[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spence AM, Muzi M, Mankoff DA, et al. 18F-FDG PET of gliomas at delayed intervals: improved distinction between tumor and normal gray matter. J Nucl Med 2004;45(10):1653–9 [PubMed] [Google Scholar]
- 16.ECR 2020 Book of Abstracts. Insights into Imaging 2020;11(1):34 doi: 10.1186/s13244-020-00851-0[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckford Vera D, Schulte B, Henrich T, et al. First-in-human total-body PET imaging of HIV with 89Zr-VRC01 on the EXPLORER. J. Nucl. Med 2020;61(supplement 1):545 [Google Scholar]
- 18.Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET Imaging of T cells. Trends Cancer 2018;4(5):359–73 doi: 10.1016/j.trecan.2018.03.009[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mankoff DA, Pantel AR, Viswanath V, Karp JS. Advances in PET Diagnostics for Guiding Targeted Cancer Therapy and Studying In Vivo Cancer Biology. Curr Pathobiol Rep 2019;7(3):97–108 doi: 10.1007/s40139-019-00202-9[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin FI, Rao JE, Mittra ES, et al. Prospective comparison of combined 18F-FDG and 18FNaF PET/CT vs. 18F-FDG PET/CT imaging for detection of malignancy. European Journal of Nuclear Medicine and Molecular Imaging 2012;39(2):262–70 doi: 10.1007/s00259-011-1971-1[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 21.Viswanath V, Pantel A, Daube-Witherspoon M, et al. Quantifying bias and precision of kinetic parameter estimation on the PennPET Explorer, a long axial field-of-view scanner. IEEE Transactions on Radiation and Plasma Medical Sciences 2020;PP:1–1 doi: 10.1109/TRPMS.2020.3021315[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39(8):644–55 doi: 10.1016/j.it.2018.06.001[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capaldi DP, Banks TI, Hristov DH, Kidd EA. Parametric Response Mapping of Co-Registered PET and Perfusion CT to Identify Radioresistant Sub-Volumes in Locally Advanced Cervical Carcinoma. International Journal of Radiation Oncology, Biology, Physics 2019;105(1):S226 doi: 10.1016/j.ijrobp.2019.06.320[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 24.Bao X, Wang M-W, Luo J-M, Wang S-Y, Zhang Y-P, Zhang Y-J. Optimization of Early Response Monitoring and Prediction of Cancer Antiangiogenesis Therapy via Noninvasive PET Molecular Imaging Strategies of Multifactorial Bioparameters. Theranostics 2016;6(12):2084–98 doi: 10.7150/thno.13917[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surti S, Pantel AR, Karp JS. Total Body PET: Why, How, What for? Ieee Transactions on Radiation and Plasma Medical Sciences 2020;4(3):283–92 doi: 10.1109/Trpms.2020.2985403[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]