ABSTRACT
Background
Anemia is a worldwide concern. Nutritional deficiencies and inflammation are considered main contributors, but zinc deficiency has only recently been associated with anemia.
Objectives
In this study we assessed associations between zinc status and hemoglobin (Hb) concentrations and anemia in preschool children 6–59 mo old (PSC) and nonpregnant women of reproductive age 15–49 y old (WRA) in population-based nutrition surveys.
Methods
Cross-sectional data from 13 (PSC) and 12 (WRA) countries within the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project were used. Multivariable linear models were constructed that included zinc status (plasma/serum zinc concentrations), Hb concentrations and anemia, iron status, age, sex, and inflammation (C-reactive protein and α-1-acid glycoprotein). Zinc was adjusted for inflammation in PSC according to the BRINDA algorithm.
Results
Data were available for 18,658 PSC and 22,633 WRA. Prevalence of anemia ranged from 7.5% to 73.7% and from 11.5% to 94.7% in PSC and WRA, respectively. Prevalence of zinc deficiency ranged from 9.2% to 78.4% in PSC and from 9.8% to 84.7% in WRA, with prevalence of zinc deficiency >20% in all countries except Azerbaijan (PSC), Ecuador (PSC), and the United Kingdom (WRA). Multivariable linear regression models showed that zinc concentrations were independently and positively associated with Hb concentrations in 7 of 13 countries for PSC and 5 of 12 countries for WRA. In the same models, ferritin concentration was also significantly associated with Hb among PSC and WRA in 9 and 10 countries, respectively. Zinc deficiency was significantly associated with anemia in PSC and WRA in 5 and 4 countries respectively.
Conclusions
Zinc deficiency was prevalent in most countries and associations between zinc and Hb in roughly half of the countries examined suggesting that strategies to combat zinc deficiency may help reduce anemia prevalence. More research on mechanisms by which zinc deficiency is associated with anemia and the reasons for the heterogeneity among countries is warranted.
Keywords: anemia, zinc deficiency, inflammation, preschool children, women of reproductive age
Introduction
Anemia is a major public health concern worldwide. Kassebaum and colleagues estimated that in 2010, one-third of the world population was anemic (1), with almost 90% of the global burden of anemia residing in developing countries (2). Moreover, it was estimated that iron deficiency anemia was 1 of the 5 leading causes of years lived with disability in 2016, with 1.24 billion cases globally (3). Anemia is associated with a variety of health conditions, including maternal death, reduced physical capacity, and impaired cognitive development in children (4). The etiology of anemia is multifactorial, with both nutritional and nonnutritional causes contributing to overall anemia prevalence. Iron deficiency is regarded as the most important cause of anemia, with the WHO estimating that approximately half of all anemia is due to iron deficiency (5). However, using data from 23 national micronutrient surveys, Petry and colleagues came to a much lower estimate, with 25% of the anemia in children and 37% of the anemia in nonpregnant women being associated with iron deficiency (6). Other analyses conducted by the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project found that the proportion of anemia associated with iron deficiency ranged from 30% to 58% in preschool children (PSC) (7) and 35% and 71% in nonpregnant women of reproductive age (WRA) (8), with the contribution of iron deficiency to overall anemia being dependent on the infection burden in the population (7, 8).
In addition to iron deficiency, there are many other factors associated with anemia, including other micronutrient deficiencies, inflammation, malaria, hemoglobinopathies, and hookworm infection, with some of these factors acting synergistically. To guide policies on programs aimed at reducing anemia prevalence, it is important to identify the underlying factors causing the anemia in a specific setting. Programs trying to improve iron status to reduce anemia prevalence will fail to achieve their targets if iron deficiency is not a key determinant of the anemia in the target population. In a recent national micronutrient survey in Cambodia, the prevalence of iron deficiency was very low, and was not a significant correlate of anemia, while at the same time, the prevalence of anemia was above 40% in both PSC and WRA. In contrast, zinc deficiency was associated with anemia in the Cambodian setting (9). Zinc deficiency has not been recognized as a primary cause of anemia, but zinc deficiency was also associated with anemia in a recent study in New Zealand (10). Indirect mechanisms by which zinc deficiency could affect hemoglobin (Hb) concentrations, and hence anemia prevalence, include increased prevalence of infection in zinc deficiency, as systemic inflammation depresses erythropoiesis (11). Given the high prevalence of zinc deficiency in developing countries (12), and the high proportion of anemia that cannot be attributed to iron deficiency or other known risk factors for anemia, more information is needed on how zinc deficiency may be implicated in the etiology of anemia. Therefore, we explored associations between zinc status, Hb concentrations, and anemia among PSC and nonpregnant WRA, using the BRINDA dataset.
Methods
As described in more detail previously, data for the BRINDA project were requested from representative surveys that included at least Hb or a micronutrient biomarker and ≥1 biomarker for acute inflammation, either C-reactive protein (CRP) or α-1-acid glycoprotein (AGP) (13). The BRINDA project primarily aims to improve the interpretation of nutrient biomarkers in the context of inflammation by accounting for the effects of inflammation on the concentrations of nutrient biomarkers (www.BRINDA-nutrition.org). Since 2014, the BRINDA project has been collecting national representative databases on anemia, micronutrient status, and biomarkers for inflammation. While all countries provided data on Hb concentrations, the provided data varied on other biomarkers of micronutrient status and inflammation, such as serum or plasma concentrations of ferritin, soluble transferrin receptor (sTfR), retinol or retinol-binding protein, and zinc concentrations. The selection criteria for inclusion in the BRINDA project have been described in detail by Namaste et al (13). For the present study, datasets were included that measured at least zinc status (plasma or serum zinc concentrations), Hb concentrations, iron status (ferritin and/or sTfR concentrations) and ≥1 marker for inflammation (CRP or AGP). This resulted in an overall dataset representing PSC in 13 countries and WRA in 12 countries. All countries except the United Kingdom (WRA) were classified as low- and middle-income countries.
Laboratory analysis
The laboratory analyses of the different surveys, including zinc status, have been described in detail elsewhere (14). In brief, venous blood was obtained in all surveys, except in Mongolia, which collected a combination of capillary and venous blood (based on cluster location). Plasma zinc concentrations (PZCs) were measured in plasma (4 surveys) or serum (8 surveys). Children and women were mostly in a nonfasting state during blood drawing, or no data on fasting state were available. Subjects were in a fasting state only in Mexico and the United Kingdom, and some but not all in a fasting state in Cameroon and Ecuador. Hb concentrations were measured by Hemocue (different models). Hb concentrations were corrected for altitude and smoking, according to WHO guidelines (15) where data were available. Altitude data were available in Afghanistan, Azerbaijan, Colombia, Ecuador, the United Kingdom, Malawi, and Mexico; smoking data were available in Colombia, Ecuador, and Mexico. Laboratory analysis of zinc concentrations was performed in 8 different laboratories. CRP and AGP were measured using a variety of methods, including sandwich ELISAs, immunoassays, turbidimetry, or nephelometry. Details on sample collection, time of collection of the blood sample, and laboratory methods are described elsewhere (14).
Definitions
Anemia was defined by Hb concentrations of <110 g/L for children and <120 g/L for women, adjusted for altitude and/or smoking (13). Inflammation was defined as a CRP concentration >5 mg/L or an AGP concentration >1 g/L, or both. According to Thurnham et al. (16, 17), the inflammatory status was defined as being apparently healthy (CRP ≤5 mg/L, AGP ≤1 g/L), in the incubation phase of inflammation (CRP >5 mg/L, AGP ≤1 g/L), in early convalescence (CRP >5 mg/L, AGP >1 g/L), or in late convalescence (CRP ≤5 mg/L, AGP >1 g/L). Ferritin concentrations were adjusted for inflammation according to the formula developed by the BRINDA project:
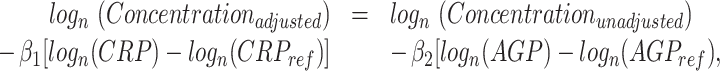 |
(1) |
where logn(CRPref) and logn(AGPref) were, respectively, equal to −2.26 and −0.52 for children and to −1.83 and −0.63 for women; β1 is the CRP regression coefficient and β2 is the AGP regression coefficient from survey-specific datasets (18). Deficient iron stores were defined as an adjusted ferritin concentration <12 μg/L for children and <15 μg/L for women (5). Transferrin concentrations were adjusted according to the same formula but only for AGP (19). Body iron stores (BIS; mg/kg) were calculated according to the formula developed by Cook et al. (with adjusted concentrations of sTfR and ferritin in mg/L and μg/L, respectively) (20):
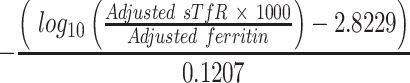 |
(2) |
This indicator can also yield negative values, indicating that a subject is in negative iron balance.
For each country, when a consistent negative association between zinc concentration and CRP or AGP was observed, zinc concentration was adjusted for AGP and CRP according to the formula developed by the BRINDA project (14). These associations were assessed using Spearman correlations and decile analyses. Zinc concentrations were significantly associated with biomarkers of inflammation in PSC but not in WRA; hence, zinc concentrations were only adjusted for inflammation in PSC, as described earlier (14). Zinc deficiency was defined according to International Zinc Nutrition Consultative Group recommendations (21). More specifically, in PSC, cutoffs of <65 μg/dL and <57 μg/dL were used to define zinc deficiency depending on whether the blood sample was obtained during the morning or afternoon, respectively. In PSC, a nonfasting state was always presumed. In WRA, 3 cutoffs of <70 μg/dL, <66 μg/dL, and <59 μg/dL were used depending on whether the blood sample was drawn 1) in the morning when women were known to be in an overnight fasting state, 2) in the morning, with no information on the fasting or nonfasting state, and 3) in the afternoon when a nonfasting state was presumed, respectively. In addition, we defined severe zinc deficiency as a plasma or serum zinc concentration <50 μg/dL among all subjects. The latter cutoff was found to have the highest sensitivity and specificity in predicting clinical signs of zinc deficiency (22).
Statistical analyses
Data analysis was performed using R software version 3.4.0 (The R Foundation for Statistical Computing). All analyses were disaggregated by country. Dichotomous variables were expressed as percentages and continuous variables were expressed as arithmetic means, except for adjusted ferritin, which was expressed as geometric means due to its lognormal distribution. CIs (95% CI) for these percentages and means were also computed.
The hypothesized relations between Hb concentration (or anemia) and zinc concentration (or deficiency) were tested using linear and logistic regression models with Hb and anemia as response variables, respectively. Models also included zinc concentration or zinc deficiency as the predictor and inflammation (CRP and/or AGP concentrations), age, and child's sex as potential covariates. Zinc concentrations in PSC were adjusted to inflammation as described below, whereas no adjustment for inflammation was made in WRA.
To further explore associations between zinc and Hb concentrations, iron status was also added to the models. First, iron status was defined as inflammation-adjusted ferritin, then as BIS (BIS <0 mg/kg body weight).
Finally, inflammation might have impacted Hb and zinc concentrations independently, thereby creating a superficial association. To explore this possibility that infection and/or inflammation might have affected the association between zinc and Hb concentrations, we disaggregated analyses by inflammatory status, as described by Thurnham et al, using the cutoffs for AGP and CRP (17), and reran the analyses for only “apparently healthy” subjects in a subanalysis.
Hb concentrations were adjusted to altitude and smoking, whereas ferritin and zinc concentrations in PSC were adjusted for inflammation according to the BRINDA methodology. As reported elsewhere, in models including both markers of inflammation, zinc concentrations in PSC were significantly associated with either CRP and/or AGP in 8 of the 13 countries, whereas in WRA, the association between zinc concentration and CRP and/or AGP was weak (14). Therefore, zinc concentrations were adjusted for inflammation using the formula developed by BRINDA only in the PSC in the countries where the association was significant, as recently recommended (14). BIS was calculated with inflammation-corrected ferritin concentrations. To help with the interpretation of results, concentrations of Hb, zinc, and ferritin, as well as BIS and age, were transformed to z-scores, centered around a mean of zero for each country separately. Associations were assessed through ANCOVA and using coefficients associated with the z-scores and ORs associated with qualitative variables. Adjusted prevalence ratios (aPRs) were computed using Poisson models including anemia as outcome and zinc deficiency, ferritin deficiency, or negative BIS, sex, country, and inflammation (CRP and/or AGP concentrations) and age as covariates. The type 1 error rate was set at 0.05. All analyses took the survey design into account (stratification, clustering, and sampling), using the R package survey version 3.34.
Results
Description of the population
Data on Hb and zinc concentrations were available for 18,658 PSC from 13 country surveys (Supplemental Table 1) and for 22,633 nonpregnant WRA from 12 country surveys (Supplemental Table 2). Additional data on iron status (either ferritin or sTfR concentrations) were available for 18,331 PSC and 22,263 WRA (Figure 1).
FIGURE 1.
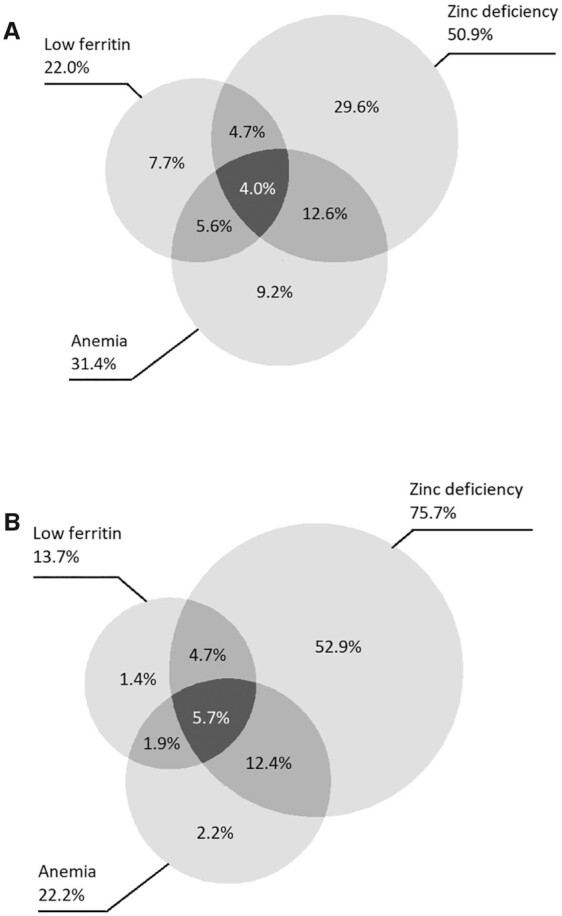
Venn diagrams representing the overlapping prevalence of anemia, zinc deficiency, and low ferritin among 18,331 PSC (A) and 22,263 nonpregnant women of childbearing age (B) from a pooled analysis of representative surveys, the BRINDA project. Low ferritin was defined as inflammation-adjusted ferritin concentration <12 μg/L for children and <15 μg/L for women [using BRINDA, Namaste et al. (23)]. Zinc deficiency was defined as inflammation-adjusted serum zinc <65 μg/dL (morning collection) of <57 μg/dL (afternoon collection) for PSC [using BRINDA, McDonald et al. (14)]. and in nonpregnant women, zinc deficiency was not inflammation adjusted and the cutoffs were <70 μg/dL, <66 μg/dL, or <50 μg/dL for morning collection in a fasting state, morning collection in a nonfasting state, or afternoon collection in a nonfasting state, respectively. BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; PSC, preschool children.
The median Hb concentration in PSC was 115 g/L (range 99.2–128.2 g/L, data not shown) across surveys, while the prevalence of anemia ranged from 7.5% (Vietnam) to 73.7% (Burkina Faso, Supplemental Table 1). Among WRA, the median Hb concentration was 127 g/L (range 99.5–131.5 g/L, data not shown) across surveys, with anemia prevalence ranging from 11.5% (Vietnam and United Kingdom) to 94.7% (India, Supplemental Table 2). The prevalence of zinc deficiency was high in some cases, with the prevalence of zinc deficiency ranging from 9.2% in Azerbaijan to 78.4% in Mongolia in PSC (zinc concentrations were adjusted for inflammation in most countries). In WRA, the prevalence of zinc deficiency ranged from 9.8% (United Kingdom) to 84.7% (Cameroon, Supplemental Table 2). Adjustment for inflammation in the 8 surveys for PSC where the association between zinc status and biomarkers for inflammation significantly affected the estimates of zinc deficiency, with prevalence of deficiency being lower from 3.5 to 24.9 percentage points (Supplemental Table 1). Zinc concentrations were positively associated with age but not with gender in the children and with age in WRA (data not shown).
Prevalence of inflammation, as indicated by elevated concentrations of CRP or AGP, varied and was high in some cases for PSC (Supplemental Table 3), ranging from 9.3% in Mexico (based on CRP only) to 93% in Burkina Faso (based on elevated CRP or AGP). Prevalence of inflammation was lower in WRA than PSC (Supplemental Table 4), ranging from 6.6% (CRP only) in Vietnam to 78.2% in Burkina Faso (both CRP and AGP). In PSC, as expected, the prevalence of elevated AGP was higher than the prevalence of elevated CRP, as AGP concentrations remain elevated for a much longer period after the initial start of the acute phase response than CRP concentrations (23). However, this was not always the case in the WRA, where the prevalence of elevated CRP was higher than that of elevated AGP in Afghanistan and Cameroon (13.6% compared with 12.1% and 18.3% compared with 7.3%, respectively).
The prevalence of iron deficiency indicated by inflammation-adjusted low ferritin ranged from 5.3% to 51.1% in PSC (Supplemental Table 5). In the 6 countries with data available on both ferritin and transferrin receptor concentrations, negative BIS (adjusted for inflammation) prevalence ranged from 8.2% to 37.0%. In WRA, prevalence of inflammation-adjusted low ferritin ranged from 3.4% to 57.0% (Supplemental Table 6). For the 8 countries with available data on BIS, the prevalence of negative body iron values (adjusting for inflammation) ranged from 1.6% to 43.3%.
Considering countries for which data were available on zinc, Hb, and ferritin concentrations (n = 18,331 PSC, n = 22,263 WRA), the proportion of children with zinc deficiency and anemia (16.6%) was higher than that for children with deficient iron stores and anemia (9.6%) (Figure 1). Four percent of the children had anemia, low ferritin concentrations, and zinc deficiency simultaneously. A similar pattern was observed for WRA, with 18.1% being zinc deficient and anemic and 7.6% being anemic with low ferritin concentrations. Almost 6% of the WRA had anemia, ferritin deficiency, and zinc deficiency simultaneously.
Association of Hb concentration and anemia with zinc status
In children, zinc concentrations (adjusted for inflammation, sex, and age) were significantly and positively associated with Hb concentrations in 7 of the 13 countries, with standardized β values for these countries ranging from 0.04 (Mexico) to 0.47 (Mongolia, Table 1). In Bangladesh, the association was borderline significant (β = 0.23, P = 0.058). In the WRA, zinc concentrations were significantly and positively associated with Hb concentrations in 5 of the 12 countries, with standardized β values for these countries ranging from 0.10 (Vietnam and the United Kingdom) to 0.24 (Ecuador, Table 1). In Cameroon, the association was borderline significant (β = 0.14, P = 0.06).
TABLE 1.
Associations between zinc and Hb concentrations in PSC and nonpregnant WRA, BRINDA project1
| PSC | WRA | |||||
|---|---|---|---|---|---|---|
| Country | n | β zinc (95% CI) | P value | n | β zinc (95% CI) | P value |
| Afghanistan | 600 | −0.03 (−0.26, 0.20) | 0.79 | 953 | 0.02 (−0.09, 0.13) | 0.66 |
| Azerbaijan | 1015 | 0.03 (−0.05, 0.11) | 0.41 | |||
| Bangladesh | 298 | 0.23 (−0.01, 0.47) | 0.06 | 719 | 0.14 (0.06, 0.23) | <0.001 |
| Burkina Faso | 118 | 0.23 (0.04, 0.43) | 0.02 | 125 | 0.20 (−0.05, 0.45) | 0.12 |
| Cambodia | 327 | 0.09 (−0.05, 0.23) | 0.22 | 427 | −0.02 (−0.14, 0.10) | 0.75 |
| Cameroon | 729 | 0.25 (0.09, 0.42 | 0.003 | 736 | 0.14 (−0.01, 0.28) | 0.06 |
| Colombia | 3569 | 0.02 (−0.01, 0.05) | 0.18 | |||
| Ecuador | 2017 | 0.14 (0.08, 0.20) | <0.001 | 7267 | 0.24 (0.19, 0.30) | <0.001 |
| India | 323 | 0.06 (−0.07, 0.18) | 0.37 | |||
| Malawi | 1063 | 0.11 (0.04, 0.18) | 0.001 | 751 | 0.21 (0.12, 0.31) | <0.001 |
| Mexico | 1163 | 0.04 (0.00, 0.09) | 0.035 | 1679 | −0.05 (−0.13, 0.03) | 0.26 |
| Mongolia | 227 | 0.47 (0.47, 0.47) | <0.001 | |||
| Pakistan | 7158 | −0.01 (−0.04, 0.02) | 0.56 | 7129 | 0.01 (−0.02, 0.04) | 0.44 |
| United Kingdom | 838 | 0.10 (0.03, 0.16) | 0.003 | |||
| Vietnam | 374 | 0.14 (0.04, 0.24) | 0.01 | 1478 | 0.10 (0.05, 0.16) | <0.001 |
Zinc concentrations in preschool children were adjusted for inflammation in the following countries: Afghanistan, Azerbaijan, Burkina Faso, Cambodia, Cameroon, Ecuador, India, Malawi, and Vietnam. Zinc concentrations were not adjusted for inflammation in WRA. Hb concentrations were adjusted for altitude and smoking, where those data were available (altitude data were available in Afghanistan, Azerbaijan, Colombia, Ecuador, United Kingdom, Malawi, and Mexico; smoking data were available in Colombia, Ecuador, and Mexico). Zinc and Hb concentrations and age were centered around zero and reduced to an SD = 1 for each country separately. BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; Hb, hemoglobin; PSC, preschool children; WRA, women of reproductive age.
To explore associations between zinc and Hb concentrations further, we added iron status, defined as ferritin concentration, to the models (Table 2). In the PSC, iron status was significantly associated with Hb concentrations in 9 of the 13 countries (β values from 0.10 to 0.22, Table 2). Zinc concentrations remained associated with Hb concentrations in 6 of the 7 countries described above in Table 1. Only in Mexico was the association no longer statistically significant (P = 0.09). In contrast, the association between zinc and Hb concentrations became statistically significant in Bangladesh ( = 0.049) when iron was added to the model (P value without iron in model = 0.06).
TABLE 2.
Associations between zinc and Hb concentrations in PSC with ferritin concentrations added to the model, BRINDA project1
| Country | n | Zinc concentrations, βzinc (95% CI) | P value | Ferritin concentrations, βfer (95% CI) | P value |
|---|---|---|---|---|---|
| Afghanistan | 600 | −0.04 (−0.24, 0.17) | 0.73 | 0.12 (0.05, 0.19) | <0.001 |
| Azerbaijan | 1015 | 0.04 (−0.04, 0.12) | 0.31 | 0.20 (0.13, 0.27) | <0.001 |
| Bangladesh | 297 | 0.22 (0.00, 0.45) | 0.05 | 0.21 (0.08, 0.34) | 0.002 |
| Burkina Faso | 118 | 0.22 (0.03, 0.42) | 0.02 | 0.16 (−0.05, 0.37) | 0.14 |
| Cambodia | 327 | 0.06 (−0,07, 0,19) | 0.37 | 0.20 (0.05, 0.35) | 0.008 |
| Cameroon | 729 | 0.24 (0.07, 0.40) | 0.005 | 0.18 (0.09, 0.27) | <0.001 |
| Colombia | 3569 | 0.02 (−0.01, 0.05) | 0.18 | 0.02 (−0.04, 0.09) | 0.49 |
| Ecuador | 2017 | 0.13 (0.08, 0.18) | <0.001 | 0.20 (0.13, 0.27) | <0.001 |
| Malawi | 1063 | 0.10 (0.04, 0.17) | 0.002 | −0.06 (−0.16, 0.03) | 0.21 |
| Mexico | 1162 | 0.03 (−0.01, 0.08) | 0.09 | 0.10 (0.04, 0.17) | 0.002 |
| Mongolia | 227 | 0.38 (0.13, 0.64) | 0.003 | 0.22 (0.12, 0.32) | <0.001 |
| Pakistan | 6833 | −0.01 (−0.03, 0.02) | 0.65 | 0.19 (0.16, 0.22) | <0.001 |
| Vietnam | 374 | 0.14 (0.05, 0.24) | 0.004 | 0.07 (−0.01, 0.14) | 0.07 |
Ferritin and zinc concentrations were corrected for inflammation according to the method developed by BRINDA. Zinc concentrations in preschool children were adjusted for inflammation in the following countries: Afghanistan, Azerbaidjan, Burkina Faso, Cambodia, Cameroon, Ecuador, India, Malawi, and Vietnam. Age, sex, and country were included in the model as covariates. Hb was adjusted for altitude where those data were available (Afghanistan, Azerbaijan, Colombia, Ecuador, United Kingdom, Malawi, and Mexico). Zinc, ferritin and Hb concentrations were centered around zero and reduced to an SD = 1. BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; Hb, hemoglobin; PSC, preschool children.
In the WRA, ferritin concentrations were significantly associated with Hb concentrations in all but 2 countries (β values from 0.17 to 0.62, Table 3). Zinc concentrations remained significantly associated with Hb concentrations (β values from 0.09 to 0.19) in the same 5 countries that had significant associations between zinc and Hb without iron in the models.
TABLE 3.
Associations between zinc and Hb concentrations in nonpregnant WRA with ferritin concentrations added to the model, BRINDA project1
| Country | n | Zinc concentrations, βzinc (95% CI) | P value | Ferritin concentrations,βfer (95% CI) | P value |
|---|---|---|---|---|---|
| Afghanistan | 953 | 0.02 (−0.09, 0.12) | 0.71 | 0.24 (0.15, 0.32) | <0.001 |
| Bangladesh | 707 | 0.14 (0.05, 0.22) | 0.001 | 0.17 (0.05, 0.28) | 0.004 |
| Burkina Faso | 125 | 0.20 (−0.05, 0.44) | 0.11 | −0.03 (−0.21, 0.15) | 0.74 |
| Cambodia | 427 | −0.01 (−0.13, 0.10) | 0.82 | 0.19 (−0.10, 0.49) | 0.20 |
| Cameroon | 736 | 0.11 (−0.02, 0.24) | 0.08 | 0.62 (0.48, 0.75) | <0.001 |
| Ecuador | 7266 | 0.18 (0.14, 0.21) | <0.001 | 0.44 (0.41, 0.48) | <0.001 |
| India | 321 | −0.05 (−0.15, 0.06) | 0.38 | 0.47 (0.34, 0.60) | <0.001 |
| Malawi | 751 | 0.19 (0.10, 0.28) | <0.001 | 0.34 (0.24, 0.44) | <0.001 |
| Mexico | 1674 | −0.04 (−0.12, 0.04) | 0.31 | 0.34 (0.25, 0.42) | <0.001 |
| Pakistan | 6980 | 0.01 (−0.01, 0.04) | 0.30 | 0.29 (0.26, 0.33) | <0.001 |
| United Kingdom | 836 | 0.09 (0.03, 0.15) | 0.003 | 0.24 (0.18, 0.30) | <0.001 |
| Vietnam | 1478 | 0.09 (0.04, 0.15) | <0.001 | 0.24 (0.19, 0.28) | <0.001 |
Ferritin and zinc concentrations were corrected for inflammation according to the method developed by BRINDA. Zinc concentrations in preschool children were adjusted for inflammation in the following countries: Afghanistan, Azerbaidjan, Burkina Faso, Cambodia, Cameroon, Ecuador, India, Malawi, and Vietnam. Hb was adjusted for altitude and smoking, where those data were available (Altitude data were available in Afghanistan, Azerbaijan, Colombia, Ecuador, United Kingdom, Malawi, and Mexico; smoking data were available in Colombia, Ecuador, and Mexico). Models controlled for age and country. Zinc, ferritin and Hb concentrations and age were centered around zero and reduced to an SD = 1. BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; Hb, hemoglobin; WRA, women of reproductive age.
Next, we explored associations between anemia (binary rather than continuous Hb), zinc deficiency, and iron deficiency, defined as inflammation-adjusted low ferritin concentrations. In the PSC, zinc deficiency was significantly associated with anemia in 5 of the 13 countries, with aPRs ranging from 1.25 in Cameroon to 1.75 in Mongolia (Table 4). In the same model, iron deficiency was associated with anemia in 10 of the 13 countries, with aPRs ranging from 1.36 to 2.47.
TABLE 4.
aPRs for anemia in PSC with zinc deficiency or deficient iron stores, BRINDA project1
| Country | n | Zinc deficiency, aPR (95%CI) | P value | Deficient iron stores, aPR (95% CI) | P value |
|---|---|---|---|---|---|
| Afghanistan | 600 | 1.33 (1.01, 1.75) | 0.045 | 1.44 (1.07, 1.95) | 0.022 |
| Azerbaidjan | 1015 | 1.09 (0.74, 1.59) | 0.66 | 2.07 (1.56, 2.75) | <0.001 |
| Bangladesh | 297 | 0.99 (0.65, 1.53) | 0.98 | 1.77 (1.03, 3.04) | 0.042 |
| Burkina Faso | 118 | 1.19 (0.99, 1.42) | 0.15 | 1.19 (0.92, 1.54) | 0.28 |
| Cambodia | 327 | 1.44 (1.09, 1.90) | 0.012 | 1.37 (1.08, 1.73) | 0.013 |
| Cameroon | 729 | 1.25 (1.10, 1.42) | 0.001 | 1.36 (1.17, 1.58) | 0.000 |
| Colombia | 3569 | 0.97 (0.78, 1.20) | 0.78 | 1.24 (0.96, 1.61) | 0.10 |
| Ecuador | 2017 | 1.32 (1.07, 1.61) | 0.010 | 1.44 (1.13, 1.84) | 0.004 |
| Malawi | 1063 | 1.16 (0.98, 1.38) | 0.09 | 1.19 (0.96, 1.47) | 0.11 |
| Mexico | 1162 | 1.09 (0.77, 1.55) | 0.62 | 1.36 (1.01, 1.85) | 0.045 |
| Mongolia | 227 | 1.75 (0.80, 4.59) | <0.001 | 2.47 (1.39, 4.57) | <0.001 |
| Pakistan | 6833 | 0.99 (0.95, 1.03) | 0.70 | 1.43 (1.36, 1.49) | <0.001 |
| Vietnam | 374 | 0.94 (0.44, 2.00) | 0.87 | 2.10 (1.05, 4.20) | 0.039 |
All models were controlled for age, sex, and country. Zinc deficiency was defined as <65 μg/dL (morning sample) or <57 μg/dL (afternoon sample). Deficient iron stores was defined as inflammation-adjusted ferritin concentrations <12 μg/L. Ferritin and zinc concentrations were corrected for inflammation according to the method developed by BRINDA [Namaste et al. (13)]. Zinc concentrations in preschool children were adjusted for inflammation in the following countries: Afghanistan, Azerbaijan, Burkina Faso, Cambodia, Cameroon, Ecuador, India, Malawi, and Vietnam. aPR, adjusted prevalence ratio; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; PSC, preschool children.
Zinc deficient WRA were also more likely to be anemic than WRA without zinc deficiency in 4 of the 12 countries (aPR ranging from 1.43 to 1.66, Table 5). Iron deficiency was a significant risk factor for anemia in WRA in all but 2 countries, with aPRs ranging from 1.58 to 5.40. The same approaches were undertaken using body iron stores as the marker of iron status, which showed similar results (data not shown).
TABLE 5.
aPR for anemia in nonpregnant WRA with zinc deficiency or deficient iron stores, BRINDA project1
| Country | n | Zinc deficiency, aPR (95% CI) | P value | Deficient iron stores, aPR (95% CI) | P value |
|---|---|---|---|---|---|
| Afghanistan | 953 | 1.10 (0.89, 1.36) | 0.39 | 1.71 (1.37, 2.13) | <0.001 |
| Bangladesh | 707 | 1.66 (1.16, 2.36) | 0.006 | 2.45 (1.46, 4.14) | 0.001 |
| Burkina Faso | 125 | 1.63 (0.93, 2.84) | 0.16 | 1.57 (0.91, 2.70) | 0.18 |
| Cambodia | 427 | 0.82 (0.63, 1.05) | 0.12 | 2.01 (1.63, 2.47) | <0.001 |
| Cameroon | 736 | 1.16 (0.82, 1.64) | 0.41 | 2.46 (2.08, 2.92) | <0.001 |
| Ecuador | 7266 | 1.58 (1.38, 1.80) | <0.001 | 5.40 (4.33, 6.74) | <0.001 |
| India | 321 | 1.03 (0.96, 1.10) | 0.44 | 1.02 (0.95, 1.10) | 0.53 |
| Malawi | 751 | 1.54 (1.05, 2.26) | 0.029 | 3.21 (2.33, 4.42) | <0.001 |
| Mexico | 1674 | 0.93 (0.62, 1.40) | 0.72 | 3.78 (2.11, 6.78) | <0.001 |
| Pakistan | 6989 | 1.01 (0.96, 1.06) | 0.77 | 1.58 (1.49, 1.67) | <0.001 |
| United Kingdom | 836 | 1.67 (0.88, 3.17) | 0.12 | 2.46 (1.46, 4.15) | <0.001 |
| Vietnam | 1478 | 1.43 (1.03, 1.98) | 0.034 | 4.74 (3.62, 6.20) | <0.001 |
All models were controlled for age and country. Zinc deficiency was defined according to International Zinc Nutrition Consultative Group recommendations: for women 3 cutoffs (<70 μg/dL, <66 μg/dL, and <59 μg/dL) were used depending on whether the blood sample was drawn 1) in the morning when women were known to be in an over-night fasting state, 2) in the morning, with no information on the fasting state or stated to be nonfasting, and 3) in the afternoon when a nonfasting state was presumed, respectively. Deficient iron stores were defined as inflammation-adjusted ferritin concentrations <15 μg/L using BRINDA methodology [Namaste et al. (13)]. aPR, adjusted prevalence ratio; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; WRA, women of reproductive age.
When children were stratified according to inflammatory status, in children with no evidence of recent inflammation (“apparently healthy”), that is, no elevated acute-phase protein concentrations, zinc concentrations remained significantly and positively associated with Hb concentrations in 6 of 13 countries, and borderline significant in 3 countries (Malawi, Bangladesh, and Cambodia, P = 0.05, P = 0.06, and P = 0.08 respectively). In WRA with no evidence of recent inflammation, zinc concentrations were significantly associated with Hb concentrations in 6 of 12 countries (data not shown).
Discussion
Although the association between zinc status and anemia was previously examined in studies of Cambodian school children (9), school children in New Zealand (10), and pregnant Ethiopian women (24), our study presents the first multicountry analysis using PSC and WRA data from >13 countries. The association between zinc and Hb concentrations appears to be independent of iron status in some countries, as addition of iron status to the predictive models had little impact on the strength of association between zinc and Hb concentrations. In roughly half of countries, zinc and Hb concentrations were associated in PSC and in WRA, even when controlling for iron status. Zinc deficiency was associated with a higher prevalence of anemia among PSC in 5 countries (of 13) and among WRA in 4 (of 12) countries, respectively.
The heterogeneity among countries on whether zinc status is associated with Hb concentrations warrants further reflection. For the countries where data were available for both PSC and WRA, there was a strong correlation between the regression coefficient β values for PSC and WRA (n = 10, Pearson's r = 0.67, P = 0.035), suggesting a common etiology for the association between zinc and Hb concentrations in PSC and WRA in those countries. However, there were no clear regional patterns across Africa, Asia, or Latin America, with marked differences between neighboring countries such as Vietnam and Cambodia, or between Mexico and Ecuador. The contribution of iron deficiency to anemia prevalence has been shown to be lower in countries where the prevalence of anemia is >40% (6). To assess whether the same pattern holds for zinc deficiency in our analyses, we plotted the β values for the association between zinc and Hb concentrations in each country against the anemia prevalence. In PSC, there was no association, whereas in WRA, there was a tendency towards a negative association (n = 11, Pearson's r = −0.47, P = 0.06), meaning that the association between zinc and Hb concentrations became stronger when anemia prevalence in a country was lower. Perhaps the contribution of zinc deficiency to overall anemia prevalence becomes more important if nonnutritional causes of anemia, for example, are less prevalent. However, for the moment, a possible causality between zinc deficiency and anemia remains to be proven, as from our data, we can only access associations.
Chronic inflammation is well known to lead to anemia, partly through mechanisms involving hepcidin and reduced iron uptake (25), but also through reduced erythropoiesis (11). Earlier studies have also shown associations between markers of acute inflammation (CRP, AGP) and anemia (26). Zinc concentrations are thought to be influenced by the acute phase response too, although the extent to which zinc concentrations are affected remains to be determined, and the impact of inflammation on zinc concentrations is perhaps less strong than initially thought (14, 27). We included 2 biomarkers for acute inflammation, CRP and AGP, to correct for possible effects of inflammation on zinc concentrations. CRP is thought to be a fast-rising acute-phase protein, whose concentrations increase within 48 h of the onset of the inflammation and return to normal values within 2 wk. In contrast, AGP concentrations rise more slowly after the onset of inflammation, but can remain elevated for weeks (23). In our dataset, more than half of the children had evidence for inflammation, underscoring again the importance of infection control in childhood in low- and middle-income countries. Nevertheless, the association between Hb and zinc concentrations remained significant even in subjects considered as “apparently healthy” (subanalysis using Thurnham categorization, data not shown), suggesting that the association was not mediated by inflammatory status.
Zinc was shown to play a role in erythropoiesis in animal models, with zinc supplementation stimulating erythropoiesis in anemic rats (28, 29). And chronic zinc deficiency in rats led to a significant decrease in erythropoiesis (30). In humans, while no direct relation has been found between zinc concentration and production of red blood cells, zinc is directly involved in erythroid differentiation and development (31). Also, zinc is present in erythrocytes where it mainly participates in metalloenzyme activities or is bound to metallothionein (32). However, zinc is a cofactor in hundreds of metabolic processes, making it hard to pinpoint a specific potential role for zinc deficiency in the etiology of anemia. Other examples where zinc could play a role are erythrocyte membrane integrity and fragility, with zinc deficiency associated with increased cell water content and higher osmotic fragility of erythrocyte membranes (33, 34). Thus, impaired cell function and/or red blood cell fragility could be envisaged as factors that could contribute to the association of zinc status and anemia. Houghton et al. reported that in New Zealand, zinc deficiency was the only factor associated with anemia in school-aged children (10). Interestingly, the effect of selenium deficiency on risk for anemia was mediated through zinc status, suggesting a role for multiple micronutrients in the etiology of anemia, in a high-income country setting.
The prevalence of zinc deficiency was >20% in most countries, and alarmingly high in some countries, both in the children and in the WRA, making the overall contribution of zinc deficiency to anemia burden potentially substantial. Even though the aPRs that link zinc deficiency with anemia need to be interpreted with caution given the multifactorial etiology of anemia, the relative importance of zinc deficiency for anemia approached that of iron deficiency, specifically in PSC. This finding underscores the necessity of considering approaches to improve zinc status as well as iron status when combating nutritional anemia. One limitation of the current study is that an individual patient data meta-analysis was not performed due to uncertainties around specific study designs. Another limitation is that all data are cross-sectional, so causality cannot be determined. Finally, we have only taken iron deficiency into account in our analyses, and no other potential nutritional deficiencies associated with anemia, such as vitamin B12 or folate deficiency, because including these vitamins would have reduced our sample size considerably (vitamin B12 and folate were not often measured in these surveys). However, acknowledging the multiple factors that can contribute to the occurrence of anemia is essential when designing programs to reduce anemia prevalence, and zinc deficiency should be considered as a potential factor. Further research is warranted to clarify the etiology of zinc deficiency in anemia.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—VG, RG, FR, AW, MFY, JPW, MAD, PSS, and FTW: were responsible for study hypothesis and study design; VG, SF, AW, MFY, JO, JPW, RLL, CMM, PSS, and FTW: were responsible for data collection and analyses; all authors: were involved in interpretation of the results; VG, SF, JB, and FTW: wrote the first draft; RG, AW, MFY, JPE, and PSS: made substantial contributions to the first draft; all authors: reviewed draft versions of the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Bill & Melinda Gates Foundation, the US Centers for Disease Control and Prevention, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the French National Research Institute for Sustainable Development (IRD).
Author disclosures: The authors report no conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Supplemental Tables 1–6 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: aPR, adjusted prevalence ratio; AGP, α-1 acid glycoprotein; BIS, body iron stores; BRINDA, Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia; CRP, C-reactive protein; Hb, hemoglobin; PSC, preschool children; sTfR, soluble transferrin receptor; WRA, women of reproductive age.
Contributor Information
Valerie Greffeuille, Alimentation, Nutrition, Sante (E6), Qualisud, Université Montpellier, Université Avignon, CIRAD, Institut Agro, IRD, Université de la Reunion, Montpellier, France.
Sonia Fortin, Alimentation, Nutrition, Sante (E6), Qualisud, Université Montpellier, Université Avignon, CIRAD, Institut Agro, IRD, Université de la Reunion, Montpellier, France.
Rosalind Gibson, Department of Human Nutrition, University of Otago, Dunedin, New Zealand; South Australian Health and Medical Institute, Adelaide, Australia.
Fabian Rohner, GroundWork, Fläsch, Switzerland.
Anne Williams, Division of Nutrition, Physical Activity and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA; McKing Consulting Corporation, Atlanta, GA, USA.
Melissa F Young, Hubert Department of Global Health, Emory University, Atlanta, GA, USA.
Lisa Houghton, Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
Jiangda Ou, McKing Consulting Corporation, Atlanta, GA, USA.
Marjoleine A Dijkhuizen, Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark.
James P Wirth, GroundWork, Fläsch, Switzerland.
Rebecca L Lander, Department of Human Nutrition, University of Otago, Dunedin, New Zealand.
Christine M McDonald, UCSF Benioff Children's Hospital Oakland, Children's Hospital Oakland Research Institute, Oakland, CA, USA.
Parminder S Suchdev, Division of Nutrition, Physical Activity and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA; Hubert Department of Global Health, Emory University, Atlanta, GA, USA.
Jacques Berger, Alimentation, Nutrition, Sante (E6), Qualisud, Université Montpellier, Université Avignon, CIRAD, Institut Agro, IRD, Université de la Reunion, Montpellier, France.
Frank T Wieringa, Alimentation, Nutrition, Sante (E6), Qualisud, Université Montpellier, Université Avignon, CIRAD, Institut Agro, IRD, Université de la Reunion, Montpellier, France.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TPet al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, Collaborators GBDA . The global burden of anemia. Hematol Oncol Clin North Am. 2016;30:247–308. [DOI] [PubMed] [Google Scholar]
- 3.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman MM, Abe SK, Rahman MS, Kanda M, Narita S, Bilano V, Ota E, Gilmour S, Shibuya K. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495–504. [DOI] [PubMed] [Google Scholar]
- 5.Nutritional anaemias: tools for effective prevention and control. Geneva: World Health Organization; 2017. [Accessed 2021 Feb 02]. Available from: https://www.who.int/publications/i/item/9789241513067. [Google Scholar]
- 6.Petry N, Olofin I, Hurrell RF, Boy E, Wirth JP, Moursi M, Donahue Angel M, Rohner F. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients. 2016;8(11):693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engle-Stone R, Aaron GJ, Huang J, Wirth JP, Namaste SM, Williams AM, Peerson JM, Rohner F, Varadhan R, Addo OYet al. Predictors of anemia in preschool children: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:402S–15S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirth JP, Woodruff BA, Engle-Stone R, Namaste SM, Temple VJ, Petry N, Macdonald B, Suchdev PS, Rohner F, Aaron GJ. Predictors of anemia in women of reproductive age: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:416S–27S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wieringa FT, Dahl M, Chamnan C, Poirot E, Kuong K, Sophonneary P, Sinuon M, Greuffeille V, Hong R, Berger Jet al. The high prevalence of anemia in Cambodian children and women cannot be satisfactorily explained by nutritional deficiencies or hemoglobin disorders. Nutrients. 2016;8(6):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton LA, Parnell WR, Thomson CD, Green TJ, Gibson RS. Serum zinc is a major predictor of anemia and mediates the effect of selenium on hemoglobin in school-aged children in a nationally representative survey in New Zealand. J Nutr. 2016;146:1670–6. [DOI] [PubMed] [Google Scholar]
- 11.Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assessment of the risk of zinc deficiency in populations and options for its control . IZINCG technical document #1. Supplement 2 ed. International Nutrition Foundation for United Nations University Press; 2004. [PubMed] [Google Scholar]
- 13.Namaste SM, Aaron GJ, Varadhan R, Peerson JM, Suchdev PS, Group BW. Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:333S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald CM, Suchdev PS, Krebs NF, Hess SY, Wessells KR, Ismaily S, Rahman S, Wieringa FT, Williams AM, Brown KHet al. Adjusting plasma or serum zinc concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2020;111:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO . Haemoglobin Concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 16.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 17.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet North Am Ed. 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 18.Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJet al. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJet al. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106:372S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101:3359–64. [DOI] [PubMed] [Google Scholar]
- 21.Hess SY, Peerson JA, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007;28:S403–S29. [DOI] [PubMed] [Google Scholar]
- 22.Wessells KR, King JC, Brown KH. Development of a plasma zinc concentration cutoff to identify individuals with severe zinc deficiency based on results from adults undergoing experimental severe dietary zinc restriction and individuals with acrodermatitis enteropathica. J Nutr. 2014;144:1204–10. [DOI] [PubMed] [Google Scholar]
- 23.Beard JL, Murray-Kolb LE, Rosales FJ, Solomons NW, Angelilli ML. Interpretation of serum ferritin concentrations as indicators of total-body iron stores in survey populations: the role of biomarkers for the acute phase response. Am J Clin Nutr. 2006;84:1498–505. [DOI] [PubMed] [Google Scholar]
- 24.Gibson RS, Abebe Y, Stabler S, Allen RH, Westcott JE, Stoecker BJ, Krebs NF, Hambidge KM. Zinc, gravida, infection, and iron, but not vitamin B-12 or folate status, predict hemoglobin during pregnancy in Southern Ethiopia. J Nutr. 2008;138:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein BL. The anemia of inflammation. J Clin Rheumatol. 2012;18:437–42. [DOI] [PubMed] [Google Scholar]
- 26.Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr. 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 27.Fiorentino M, Perignon M, Kuong K, Chamnan C, Berger J, Wieringa FT. Subclinical inflammation affects iron and vitamin A but not zinc status assessment in Senegalese children and Cambodian children and women: ERRATUM. Public Health Nutr. 2018;21:2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen YH, Feng HL, Jeng SS. Zinc supplementation stimulates red blood cell formation in rats. Int J Mol Sci. 2018;19:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen YH, Shiu JR, Ho CL, Jeng SS. Zinc as a signal to stimulate red blood cell formation in fish. Int J Mol Sci. 2017;18:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konomi A, Yokoi K. Zinc deficiency decreases plasma erythropoietin concentration in rats. BTER. 2005;107:289–92. [DOI] [PubMed] [Google Scholar]
- 31.Osawa M, Yamaguchi T, Nakamura Y, Kaneko S, Onodera M, Sawada K, Jegalian A, Wu H, Nakauchi H, Iwama A. Erythroid expansion mediated by the Gfi-1B zinc finger protein: role in normal hematopoiesis. Blood. 2002;100:2769–77. [DOI] [PubMed] [Google Scholar]
- 32.Ohno H, Doi R, Yamamura K, Yamashita K, Iizuka S, Taniguchi N. A study of zinc distribution in erythrocytes of normal humans. Blut. 1985;50:113–6. [DOI] [PubMed] [Google Scholar]
- 33.O'Dell BL. Role of zinc in plasma membrane function. J Nutr. 2000;130:1432S–6S. [DOI] [PubMed] [Google Scholar]
- 34.O'Dell BL, Browning JD, Reeves PG. Zinc deficiency increases the osmotic fragility of rat erythrocytes. J Nutr. 1987;117:1883–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


