Abstract
Phthalates are solvents and plasticizers found in consumer products including cosmetics, food/beverage containers, housing materials, etc. Phthalates are known endocrine-disrupting chemicals that can directly target the ovary, potentially causing defects in ovulation and fertility. Women are exposed to multiple different phthalates daily, therefore this study investigated the effects of an environmentally relevant phthalate mixture (PHTmix) on ovulation. Ovulation is initiated by the luteinizing hormone (LH) surge, which induces prostaglandin (PG) production, progesterone (P4)/progesterone receptor (PGR) signaling, and extracellular matrix (ECM) remodeling. We hypothesized that the PHTmix would directly inhibit ovulation by altering the levels of PGs, P4/PGR, and enzymes involved in ECM remodeling. Antral follicles from CD-1 mice were treated with vehicle control alone (dimethylsulfoxide, DMSO), hCG alone (LH analog), and hCG+PHTmix (1–500μg/ml), and samples were collected across the ovulatory period. The PHTmix decreased ovulation rates at all doses tested in a dose-dependent manner when compared to hCG. PG levels were decreased by the PHTmix when compared to hCG, which was potentially mediated by altered levels of PG synthesis (Ptgs2) and transport (Slco2a1) genes. The PHTmix altered P4 and Pgr levels when compared to hCG, leading to decreases in downstream PGR-mediated genes (Edn2, Il6, Adamts1). ECM remodeling was potentially dysregulated by altered levels of ovulatory mediators belonging to the matrix metalloproteases and plasminogen activator families. These data suggest that phthalate exposure inhibits ovulation by altering PG levels, P4/PGR action, and ECM remodeling.
Keywords: phthalates, ovulation, ovary, mixture, fertility, follicle
Phthalates are a class of chemicals used as solvents, additives, and plasticizers in many common consumer products including cosmetics, food and beverage containers, building materials, medical tubing, and more (Zhou and Flaws, 2017). Due to this widespread use, humans are ubiquitously exposed to a mixture of different phthalates via ingestion, inhalation, and dermal contact (Zhou and Flaws, 2017). Measurable levels of phthalate metabolites have been detected in follicular fluid from women, meaning that these chemicals can directly target the ovary (Du et al., 2016). This is concerning because studies show that phthalates act as endocrine-disrupting chemicals whereby exposure can alter the ovarian function and have negative effects on female reproductive health (Hannon and Flaws, 2015). Specifically, studies have found that exposure to phthalates reduces antral follicle growth, induces oocyte fragmentation, decreases steroid hormone production, and induces follicle death at different stages of folliculogenesis in the mouse ovary (Hannon et al., 2015b; Hannon and Flaws, 2015; Zhou and Flaws, 2017). Further, epidemiological studies suggest that increased phthalate exposure is associated with poor in vitro fertilization outcomes (decrease in the number of eggs retrieved and decrease in live births), lower levels of sex steroid hormones, and earlier onset of menopause (Du et al., 2019; Grindler et al., 2015; Hauser et al., 2016; Machtinger et al., 2018). Taken together, these findings underscore the importance of further investigating the effects of phthalates on ovarian function.
The vast majority of phthalate toxicology studies are focused on studying single phthalate exposures, which is not an environmentally relevant approach as humans are exposed to a mixture of phthalates daily. Thus, the effects of a physiological relevant mixture of phthalates on female reproduction are largely unknown (Zhou and Flaws, 2017). To best mimic human exposure, this study utilized an environmentally relevant phthalate mixture to examine the effects of phthalates on the ovulatory process. This mixture was derived from urinary phthalate levels in pregnant women enrolled in the Children's Environmental Health Research Center study (Zhou and Flaws, 2017). This mixture contained the most commonly used phthalates including diethyl phthalate (DEP), di(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), diisononyl phthalate (DiNP), diisobutyl phthalate (DiBP), and butyl benzyl phthalate (BBzP) (Johns et al., 2015).
Ovulation is a strictly coordinated process that is initiated by the luteinizing hormone (LH) surge or clinical/experimental treatment with human chorionic gonadotrophin (hCG; a potent LH analog), whereby LH and hCG cause oocyte release and luteinization (the transformation of the ruptured follicle into a corpus luteum [CL]) (Duffy et al., 2019). Ovulation and luteinization are regulated by a combination of various biological processes, signaling cascades, and hormones including prostaglandins and progesterone (Duffy et al., 2019).
Prostaglandins (PGs) are eicosanoids that are produced by the follicle following the LH surge and are vital mediators of ovulation. Ovulatory PG accumulation occurs due to LH/hCG-induced increases in PG synthases (PLA2G4A, PTGS2) and transporters (SLCO2A1) along with decreases in PG metabolic enzymes (HPGD) (Duffy et al., 2019). PGs are crucial for fertility as demonstrated by studies where inhibiting the synthesis of PGs, using PG synthase inhibitors and knockout approaches, results in anovulation (Duffy and Stouffer, 2002; Lim et al., 1997). PGs regulate ovulation by causing oocyte maturation, cumulus-oocyte complex expansion, follicle rupture, angiogenesis, and other inflammatory responses during the periovulatory period (Duffy et al., 2019; Liu et al., 2009; Takahashi et al., 2018). Therefore, the impact of phthalates on the ovulatory PG pathway were investigated in this study.
Progesterone (P4) is a sex steroid hormone that is produced in the ovary and is essential for ovulation. P4 production occurs via LH/hCG-induced increases in steroidogenic proteins (STAR, CYP11A1, HSD3B1, PARM1) (Andersen and Ezcurra, 2014). When bound to its nuclear receptor (progesterone receptor [PGR]), P4 action facilitates fertility by inducing oocyte release and the initiation/maintenance of pregnancy. As a transcription factor, PGR up-regulates several downstream ovulatory mediators in the ovary (EDN2, IL6, ADAMTS1, among several others) (Kim et al., 2009). Studies show that deficient P4 levels negatively impact women’s health by contributing to multiple reproductive disorders often resulting in infertility (Fauser et al., 2011). Further, studies show that inhibition of P4 synthesis, antagonists of PGR, and knocking out Pgr, Edn2, and Adamts1 results in anovulation (Brown et al., 2010; Kim et al., 2009; Lydon et al., 1995; Tanaka et al., 1991). Thus, the effect of phthalates on the ovulatory P4/PGR pathway were explored in this study.
Degradation of the follicle wall is accomplished by remodeling of the extracellular matrix (ECM) via LH/hCG-induced increases in matrix metalloproteases (MMPs), tissue inhibitors of MMPs (TIMPs), a disintegrin and metalloproteinase with thrombospondin-like motifs (ADAMTS), plasminogen activators (PLAT and PLAU), and inhibitors of the plasminogen activator (PA) system (Serpins). Increases in these ovulatory mediators involved in tissue remodeling are essential for successful ovulation because Adamts1 knockout mice expressed an infertile phenotype (Brown et al., 2010). Further, studies show that deficient expression of genes involved in the PA system resulted in reduced ovulation rates (Leonardsson et al., 1995). The effects of phthalates on the ovulatory mediators involved in ECM remodeling were explored in this study.
The present study was designed to investigate the direct effects of phthalate mixture exposure on the crucial ovulatory process by using an in vitro mouse antral follicle culture system (Hannon et al., 2015a,b; Skory et al., 2015). We hypothesized that the phthalate mixture would directly inhibit ovulation by altering the levels of PGs, P4/PGR, and enzymes involved in ECM remodeling. We further sought to elucidate the mechanism by which phthalate exposure alters the levels of these vital ovulatory mediators.
MATERIALS AND METHODS
Chemicals
The environmentally relevant phthalate mixture (PHTmix) used in this study was derived from urinary phthalate levels in pregnant women enrolled in a study by our colleagues at the University of Illinois at Urbana-Champaign (Zhou and Flaws, 2017). The PHTmix was comprised of 35% diethyl phthalate (DEP; Sigma-Aldrich), 21% di(2-ethylhexyl) phthalate (DEHP; Sigma-Aldrich), 15% dibutyl phthalate (DBP; Sigma-Aldrich), 15% diisononyl phthalate (DiNP; Sigma-Aldrich), 8% diisobutyl phthalate (DiBP; Sigma-Aldrich), and 5% butyl benzyl phthalate (BBzP; Sigma-Aldrich), each with greater than 98% purity. Stock solutions of the PHTmix were created with the vehicle control (dimethyl sulfoxide, DMSO; Sigma-Aldrich) in various concentrations (1.33, 13.3, 133, and 655 mg/ml) to ensure that the concentration of the vehicle control was 0.75 µg/ml. Final concentrations of the PHTmix that were used in culture were 1, 10, 100, and 500 µg/ml.
These concentrations were chosen based on previous research, which supports that such concentrations of this phthalate mixture can induce oocyte fragmentation, decrease sex steroid hormone production, and reduce antral follicle growth (Zhou and Flaws, 2017). Other studies show that such concentrations of individual phthalates in the PHTmix have negative effects on ovarian folliculogenesis and steroidogenesis in mouse ovaries (Craig et al., 2013; Hannon et al., 2015b). Studies also show that follicular fluid from in vitro fertilization patients has measurable phthalate metabolite levels that correspond with the parent phthalates incorporated in the PHTmix used in this study (Du et al., 2016, 2019; Krotz et al., 2012). When looking at the concentration of some individual phthalates (DBP and DEHP) relative to its percentage of the mixture, the lowest dose of the PHTmix (1 µg/ml) is still below the highest level of that same phthalate found in follicular fluid from women (Du et al., 2016). However, it cannot be discounted that medical products and procedures involved in in vitro fertilization contain phthalates, which potentially can lead to contamination in measurements and limits the ability to truly mimic human ovarian exposures.
Mouse antral follicle culture
Adult female CD-1 mice (postnatal day 34–39) were acquired from Charles River Laboratories (Wilmington, MA) and housed in the University of Kentucky’s Division of Laboratory Animal Resources (DLAR). All animal procedures involving animal care, euthanasia, and tissue collection were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky. All the animals in these experiments were housed in a controlled animal room environment (temperature at 22°C ± 1°C and 12L:12D cycles) and were provided food and water ad libitum. Each culture utilized 3 mice. Mice were humanely euthanized, both ovaries were aseptically removed, and early antral follicles (250–350μm) were isolated and cleaned of interstitial tissue.
Isolated follicles were then transferred into individual wells of a 96-well plate. Each treatment group contained 10–12 follicles for RNA experiments or 20–24 follicles for the ovulation assay. All follicles were first treated with supplemented α-MEM (Life Technologies) containing 10 ng/ml insulin, 5.5 ng/ml transferrin, 5.5 ng/ml selenium, 100 units/ml penicillin, 100 mg/ml streptomycin (Sigma-Aldrich), 5 units/ml human recombinant follicle-stimulating hormone (FSH; Dr. A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center), and 5% fetal bovine serum (Atlanta Biologicals) (Hannon et al., 2015a). Follicles were simultaneously treated with DMSO or PHTmix according to the assigned treatment group. The follicles were then cultured for 96 h to allow for pre-ovulatory development. The ovulatory cascade was then induced by treating the follicles with α-MEM supplemented with 1.5 IU/ml human chorionic gonadotrophin (hCG; Sigma-Aldrich), 5 ng/ml epidermal growth factor (EGF; BD Biosciences), 100 units/ml penicillin, 100 mg/ml streptomycin, 3 mg/ml BSA, 5 µg/ml insulin, 5 µg/ml transferrin, and 5 ng/ml selenium (Sigma-Aldrich), (Skory et al., 2015). Follicles were simultaneously treated with DMSO or PHTmix, with the final treatment groups being DMSO alone, hCG alone ovulatory control group, and hCG+PHTmix (1–500μg/ml).
Ovulation was visually assessed under a light microscope at 18 h post-hCG treatment (ovulation occurs at approximately 12 h post-hCG). Briefly, successful ovulation was noted when the oocyte was extruded from the follicle wall and when spindle-like granulosa cells surrounding the follicle were differentiated into cuboidal luteal cells (Skory et al., 2015). Failure of ovulation was noted when the oocyte remained intact in the follicle with no/minimal differentiation of granulosa cells to luteal cells. Media and follicles/CLs were collected across different time-points in the ovulatory period (4 h, 11 h, and 18 h post-hCG) and stored at −80°C for prostaglandin measurements, progesterone measurements, and gene expression analysis.
Prostaglandin measurements
Active prostaglandin levels in the conditioned culture media were measured via enzyme-linked immunosorbent assay (ELISA) kits that were purchased from Cayman Chemical. Both PGE2 and PGF2α were measured according to the manufacturer's protocol for each kit, respectively. The PGE2 kit had an assay range of 7.8–1000 pg/ml and sensitivity of 15 pg/ml. The PGF2α kit had an assay range of 3.9–500 pg/ml and sensitivity of 10 pg/ml. All undiluted samples were ran in duplicates.
Progesterone measurements
Progesterone (P4) levels in the conditioned culture media were measured via an Immulite1000 using an Immulite Progesterone Kit (Diagnostic Products Corp). The sensitivity of the assay was 0.02 ng/ml and the intraassay and interassay coefficients of variation were 7% and 12% respectively (Al-Alem et al., 2015). Samples were diluted 1:4 and were analyzed as singlets, as is routine in our clinically accurate, College of American Pathologists certified laboratory.
Gene expression analysis
After follicles/CLs were collected at each time-point and frozen at −80°C, RNA was extracted using the RNeasy Mini Kit (Qiagen, Inc.), according to the manufacturer's protocol. The maximum amount of cDNA (100 or 200 ng) was reverse transcribed according to the manufacturer’s protocol using the iScript RT kit from Bio-Rad Laboratories, Inc. All samples were diluted to the desired concentration needed for quantitative real-time polymerase chain reactions (qPCR) (1.67 ng/μl). The AriaMx Real-Time PCR system and the comprehensive data analysis software were used in this study. Data were generated by using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Inc.) or the TaqMan Gene Expression Master Mix (Invitrogen Life Technologies, Inc.) reagents, which quantify the amount of PCR product produced by measuring fluorescence. For SYBR reactions, primers were designed (Integrated DNA Technologies) and stocks were reconstituted with nuclease-free water to a concentration of 500 pmol/μl. Genes of interest and reference gene (Actb) primer sequences used can be found in Table 1. All reactions included a no template control and a no reverse transcriptase control. The SsoAdvanced program ran on the PCR system followed the manufacturer's protocol, which consisted of a single enzyme activation step (95°C for 30 s), 45 cycles of amplification and quantification (95°C for 10 s, 60°C for 10 s) with single fluorescence reading, and a melt curve (65°C–95°C heating 0.5°C per s) with continuous fluorescence readings. For TaqMan reactions, TaqMan primers were purchased from Invitrogen Life Technologies, Inc. and can be seen in Table 1. The TaqMan program ran on the PCR system followed the manufacturer's protocol, which consisted of 2 minutes at 50°C to permit AmpErase uracil-N-glycosylase optimal activity, denaturation step for 10 min at 95°C, 15 s at 95°C, and 1 min at 60°C for 50 cycles, followed by 1 min at 95°C, 30 s at 58°C, and 30 s at 95°C for ramp dissociation. The reference gene used in this study was Actb because each treatment group did not alter Actb gene expression.
Table 1.
Primer Information
| SYBR Primer Sequences | |||
|---|---|---|---|
| Gene Symbol | Gene Name | F/R | Primer Sequence |
| Abcc4 | ATP-binding cassette, sub-family C (CFTR/MRP), member 4 | F | 5′-TTCTGGTTATTCTTCTGCCTCTG-3′ |
| R | 5′-CCCACGCATACATCTTTATTATCC-3′ | ||
| Actb | Actin, beta | F | 5′-GGGCACAGTGTGGGTGAC-3′ |
| R | 5′-CTGGCACCACACCTTCTAC-3′ | ||
| Adamts1 | A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 1 | F | 5′-CAGTACCAGACCTTGTGCAGACCTT-3′ |
| R | 5′-CACACCTCACTGCTTACTGGTTTGA-3′ | ||
| Cdkn1a | Cyclin-dependent kinase inhibitor 1 A (P21) | F | 5′-TTAGGCAGGCTCCAGTGGCAACC-3′ |
| R | 5′-ACCCCCACCACCACACACCATA-3′ | ||
| Cyp11a1 | Cytochrome P450, family 11, subfamily a, polypeptide 1 | F | 5′-AGATCCCTTCCCCTGGTGACAATG-3′ |
| R | 5′-CGCATGAGAGTATCGACGCATC-3′ | ||
| Edn2 | Endothelin 2 | F | 5′-CTCCTGGCTTGACAAGGAATG-3′ |
| R | 5′-GCTGTCTGTCCCGCAGTGTT-3′ | ||
| Hpgd | Hydroxyprostaglandin dehydrogenase 15 (NAD) | F | 5′-CACCTCCGTTTTGCTTACTCA-3′ |
| R | 5′-GTTCGTCCAGTGTGATGTGG-3′ | ||
| Hsd3b1 | Hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 | F | 5′-CAGGAGAAAGAACTGCAGGAGGTC-3′ |
| R | 5′-GCACACTTGCTTGAACACAGGC-3′ | ||
| Il6 | interleukin 6 | F | 5′-GATGCTACCAAACTGGATATAATC-3′ |
| R | 5′-GGTCCTTAGCCACTCCTTCTGTG -3′ | ||
| Mmp9 | Matrix metallopeptidase 9 | F | 5′-GATCCCCAGAGCGTCATTC-3′ |
| R | 5′-CCACCTTGTTCACCTCATTTTG-3′ | ||
| Mmp14 | Matrix metallopeptidase 14 (membrane-inserted) | F | 5′-CAGTATGGCTACCTACCTCCAG-3′ |
| R | 5′-GCCTTGCCTGTCACTTGTAAA-3′ | ||
| Mmp16 | Matrix metallopeptidase 16 | F | 5′-TTACTCGCATTCAGCTCTGGA-3′ |
| R | 5′-CCGCAGACTGTAGCACATAAAA-3′ | ||
| Mmp19 | Matrix metallopeptidase 19 | F | 5′- GACAGCAAAGACCTGGAGGATTA -3′ |
| R | 5′- CTGACCGGAAATGGGCAGT -3′ | ||
| Parm1 | Prostate androgen-regulated mucin-like protein 1 | F | 5′-ACCTGAAGATCAGGCACTCC-3′ |
| R | 5′-CCTCAGCCACCTTTCTTCGT-3′ | ||
| Pla2g4a | Phospholipase A2, group IVA (cytosolic, calcium-dependent) | F | 5′-CAGCAGGAAGCGAACGAGAC-3′ |
| R | 5′-GACGTAGTTGGCATCCATCAGT-3′ | ||
| Plat | Plasminogen activator, tissue | F | 5‘- AGGAGGACTCTACACAGACATCACCTC -3′ |
| R | 5′-ATCGTCATCAAATTCCTCATGGACTATG-3′ | ||
| Plau | Plasminogen activator, urokinase | F | 5′-GTTCAGACTGTGAGATCACTGG -3′ |
| R | 5′-CAGAGAGGACGGTCAGCATGG-3′ | ||
| Ptges | Prostaglandin E synthase | F | 5′-ATCAAGATGTACGCGGTGGCT-3′ |
| R | 5′-GATTGTCTCCATGTCGTTGCG-3′ | ||
| Serpinb2 | Serine (or cysteine) peptidase inhibitor, clade B, member 2 | F | 5′-CCGCTCAGAAGATAACGAGATTG-3′ |
| R | 5′-TGGCCAATGTTGATGAGATGC-3′ | ||
| Serpine1 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 | F | 5′-CCCACACAGCCCATCAGG-3′ |
| R | 5′-CCGAGGACACGCCATAGG-3′ | ||
| Slco2a1 | Solute carrier organic anion transporter family, member 2a1 | F | 5′-TCGCCTCTGTATATCTCCATC-3′ |
| R | 5′-GTAGCCGTGTCCACTCTG-3′ | ||
| Star | Steroidogenic acute regulatory protein | F | 5′-CAGGGAGAGGTGGCTATGCA-3′ |
| R | 5′-CCGTGTCTTTTCCAATCCTCTG-3′ | ||
| Timp1 | Tissue inhibitor of metalloproteinase 1 | F | 5′-CGAGACCACCTTATACCAGCG-3′ |
| R | 5′-ATGACTGGGGTGTAGGCGTA-3′ | ||
| Wnt4 | Wingless-type MMTV integration site family, member 4 | F | 5′-CAGGAAGGCCATCTTGACACACA-3′ |
| R | 5′-TGGCACCGTCAAACTTCTCC-3′ | ||
| TaqMan Primer Information | |||
| Gene Symbol | Gene Name | Assay ID | |
| Pgr | Progesterone receptor | Mm00435628_m1 | |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 | Mm00478374_m1 | |
Genes of interest included genes involved in luteinization (Cdkn1a, Wnt4); PG production (Pla2g4a, Ptgs2, Ptges), metabolism (Hpgd), and transport (Slco2a1, Abcc4) genes; the progesterone receptor (Pgr), P4 steroidogenic genes (Star, Cyp11a1, Hsd3b1, Parm1), and PGR-regulated genes (Edn2, Il6); and genes involved in tissue remodeling (Mmp9, Mmp14, Mmp16, Mmp19, Timp1, Adamts1, Plat, Plau, Serpine1, Serpinb2). Gene expression data were generated by subtracting the reference gene Ct value from the gene of interest Ct value (ΔCt). The ΔΔCt was calculated subtracting the average DMSO ΔCt from the ΔCt. Fold change was then calculated by evaluating 2−ΔΔCT for each sample. Data are represented as percent fold change relative to the hCG alone treatment group. Genes involved in luteinization (Cdkn1a, Wnt4) were measured at 18 h due to their induction after ovulation. Progesterone steroidogenic genes (Star, Cyp11a1, Hsd3b1, Parm1) were measured at 4, 11, and 18 h due to their known increases throughout the entire ovulatory period. Genes involved in the PG pathway (Pla2g4a, Ptgs2, Hpgd, Slco2a1), PGR signaling pathway (Pgr, Il6, Edn2), and tissue remodeling (Mmp9, Mmp14, Mmp16, Mmp19, Timp1, Adamts1, Plat, Plau, Serpine1, Serpinb2) were measured at 4 and 11 h because of their known increases leading up to ovulation (ovulation occurs at approximately 12 h).
Statistical analysis
IBM SPSS 24 Statistical Software was used for data analysis. Data were analyzed via one-way ANOVA followed by a post hoc test (Tukey) with multiple comparisons and were deemed significant when p ≤ .05. Final data were represented as bar graphs with each treatment group mean, error bars (representing the SEM), and letters (denoting statistical difference).
RESULTS
Effect of PHTmix on Ovulation and Luteinization
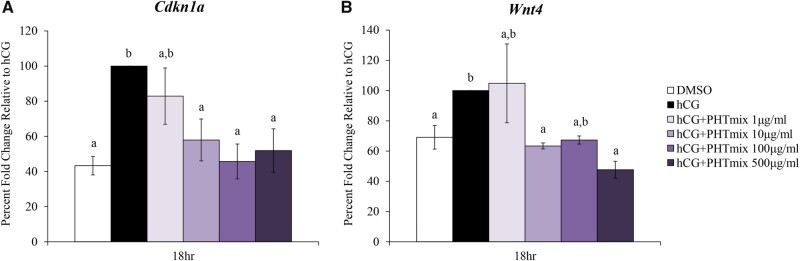
Ovulation was first measured after 18 h of treatment with maturation media containing hCG. When treated with hCG alone, the ovulation rate (percentage of successful ovulations per total follicles plated) was 78%. However, with increasing doses of PHTmix, a dose-dependent decrease in ovulation rates was observed, with the highest dose of PHTmix (500 µg/ml) being statistically equivalent to the DMSO group that did not receive hCG and did not ovulate (Figure 1). Further, the expression of genes involved in luteinization (Cdkn1a, Wnt4) were measured at 18 h post-hCG administration. Treatment with hCG+PHTmix decreased both Cdkn1a (10, 100, and 500 μg/ml doses) (Figure 2A) and Wnt4 (10 and 500 μg/ml doses) (Figure 2B) expression when compared to hCG alone.
Figure 1.
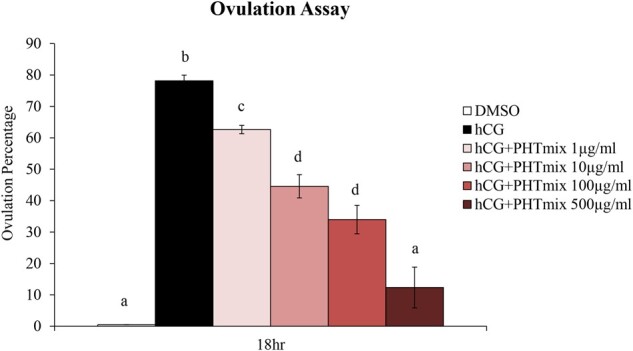
Effect of phthalate mixture exposure on ovulation. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Ovulation was visually assessed at 18 h post-hCG treatment. Data are presented as a percentage of successful ovulations per culture. Graph represents mean ± SEM from 3–9 experiments, with 20–24 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
Figure 2.
Effect of phthalate mixture exposure on luteinization. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Follicles were collected at 18 h post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Cdkn1a (A) and Wnt4 (B). Data are presented as a percent fold change relative to the hCG alone control group. Graphs represent mean ± SEM from 3–9 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
Effect of PHTmix on Ovulatory Prostaglandin Production
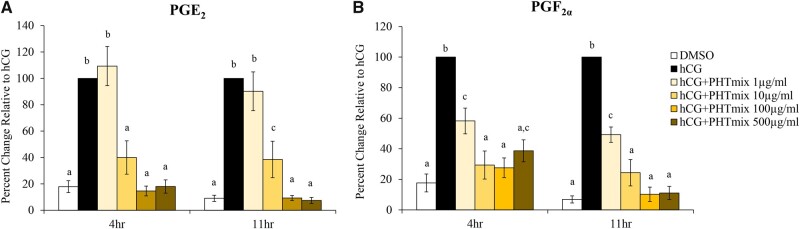
Prostaglandins, PGE2 and PGF2α, are essential mediators of ovulation (Duffy et al., 2019; Liu et al., 2009). The present study examined the enzymes known to be regulated by the LH surge/hCG treatment that are involved in prostaglandin production (Pla2g4a, Ptgs2, Ptges), transport (Slco2a1, Abcc4), and metabolism (Hpgd). Expected increases of PGE2 and PGF2α levels were observed at 4 and 11 h when treated with hCG alone compared to DMSO (Figs. 3A and 3B). However, when treated with hCG+PHTmix, PGE2 levels are decreased at the 10, 100, and 500 μg/ml doses at both time-points when compared to hCG alone (Figure 3A). PGF2α levels were decreased at all doses of the hCG+PHTmix tested at both time-points when compared to hCG alone (Figure 3B).
Figure 3.
Effect of phthalate mixture exposure on prostaglandin (PG) levels. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Media were collected at multiple time-points (4 and 11 h) post-hCG treatment, and PGE2 (A) and PGF2α (B) levels were measured via an ELISA. Data are presented as a percent change relative to the hCG alone control group. Graphs represent mean ± SEM from 3 to 10 experiments, with 10 to 12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
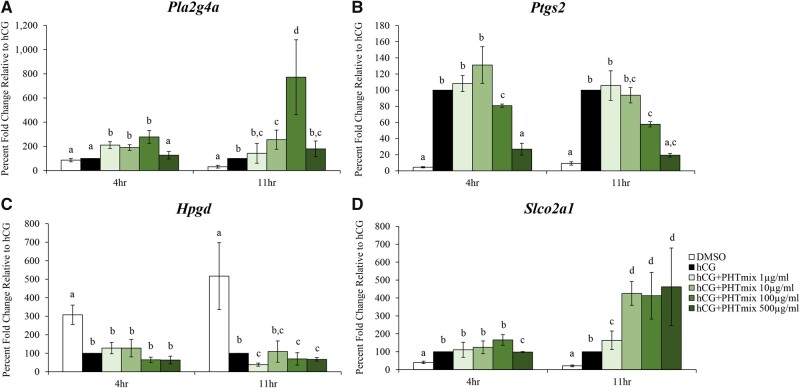
Due to these decreases in PGs, the mRNA levels of the enzymes that drive prostaglandin production, transport, and metabolism were also measured. Expression of Pla2g4a was increased by the PHTmix at 4 h (1, 10, 100 μg/ml doses) and 11 h (10 and 100 μg/ml doses) when compared to hCG alone (Figure 4A). Exposure to the PHTmix decreased expression of Ptgs2 at 4 hr (100 and 500 μg/ml doses) and 11 h (100 and 500 μg/ml doses) relative to hCG alone (Figure 4B). Expression of Hpgd was not changed at 4 h when exposed to the PHTmix, but was decreased at 11 h (1, 100, and 500 μg/ml doses) when compared to hCG alone (Figure 4C). Expression of Slco2a1 was decreased by the PHTmix at 4 h (500 μg/ml) but increased at 11 h (1–500μg/ml doses) when compared to the hCG alone group (Fig. 4D). The mRNA levels of other enzymes in the prostaglandin pathway were measured (Ptges and Abcc4), but exposure to the PHTmix did not alter their levels when compared to hCG alone (data not shown).
Figure 4.
Effect of phthalate mixture exposure on prostaglandin synthesis, metabolism, and transport. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 hr prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Follicles were collected at multiple time-points (4 and 11 h) post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Pla2g4a (A), Ptgs2 (B), Hpgd (C), and Slco2a1 (D). Data are presented as a percent fold change relative to the hCG alone control group. Graphs represent mean ± SEM from 3–10 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
Effect of PHTmix on the Ovulatory Progesterone/Progesterone Receptor Pathway
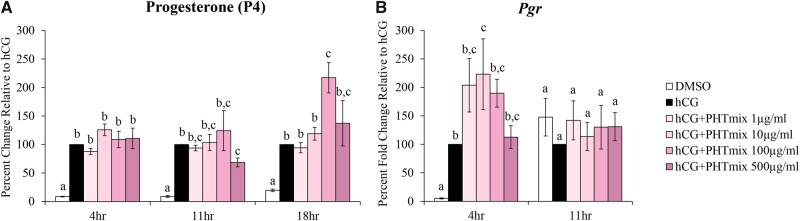
Increases in P4 levels as well as the LH/hCG-induced increase in progesterone receptor (Pgr) is required for successful oocyte release, the initiation/maintenance of pregnancy, and is therefore vital for fertility (Choi et al., 2017). At all time-points, we see an anticipated increase in progesterone production with hCG alone when compared to DMSO (Figure 5A). There was no change with hCG+PHTmix treatment at 4 h but decreases at 11 h (500 μg/ml), and further increases at 18 h (100 μg/ml) were observed when compared to hCG alone (Figure 5A). The mRNA levels of Pgr were increased by exposure to the PHTmix at 4 h (10 μg/ml) but were unchanged at 11 h when compared to hCG alone (Figure 5B).
Figure 5.
Effect of phthalate mixture exposure on progesterone (P4) and progesterone receptor (Pgr) levels. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 hr prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Media were collected at multiple time-points (4, 11, and 18 h) post-hCG treatment, and P4 levels were measured using an Immulite kit (A). Follicles were collected at multiple time-points (4 and 11 h) post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Pgr (B). Data are presented as a percent change (P4) or percent fold change (Pgr) relative to the hCG alone control group. Graphs represent mean ± SEM from 3–9 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
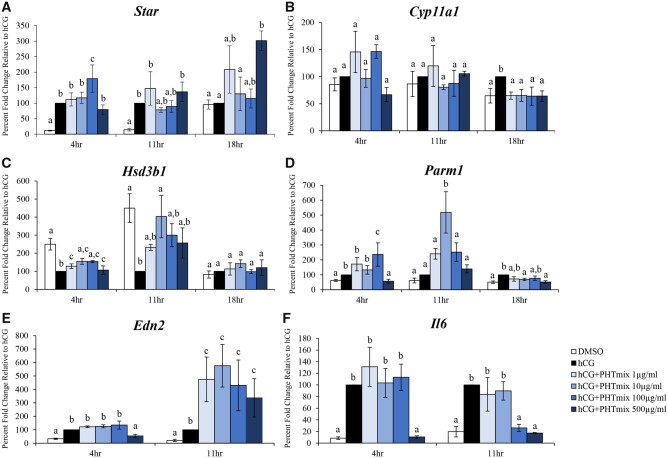
To elucidate the mechanism by which the PHTmix altered P4 levels, the mRNA levels of progesterone steroidogenic genes were also measured. The PHTmix increased expression of Star at 4 h (100 μg/ml), did not change expression at 11 h, but further increased expression at 18 h (500 μg/ml) when compared to hCG alone (Figure 6A). Expression of Cyp11a1 was unchanged by the PHTmix at 4 and 11 h but decreased expression at 18 h (1–500μg/ml doses) relative to hCG alone (Figure 6B). Exposure to the PHTmix increased expression of Hsd3b1 at 4 h (1–500μg/ml doses) and 11 h (10 μg/ml) but did not change expression at 18 h when compared to hCG alone (Figure 6C). Expression of Parm1 was increased (100 μg/ml) and decreased (500 μg/ml) at 4 h, increased at 11 h (10 μg/ml), and decreased at 18 h (10 and 500 μg/ml doses) by the PHTmix relative to hCG alone (Figure 6D).
Figure 6.
Effect of phthalate mixture exposure on the progesterone/progesterone receptor pathway. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Follicles were collected at multiple time-points (4, 11, and 18 h) post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Star (A), Cyp11a1 (B), Hsd3b1 (C), Parm1 (D), Edn2 (E), and Il6 (F). Data are presented as a percent fold change relative to the hCG alone control group. Graphs represent mean ± SEM from 3–9 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
Downstream P4/PGR signaling results in the upregulation of several genes, such as Edn2 and Il6 (Kim et al., 2009). Expression of Edn2 was decreased by the PHTmix at 4 h (500 μg/ml) but increased at 11 h (1–500μg/ml doses) when compared to hCG alone (Figure 6E). Exposure to the PHTmix decreased expression of Il6 at 4 h (500 μg/ml) and 11 h (100 and 500 μg/ml doses) when compared to hCG alone (Figure 6F).
Effect of PHTmix on Ovulatory Mediators Involved in Extracellular Matrix Remodeling
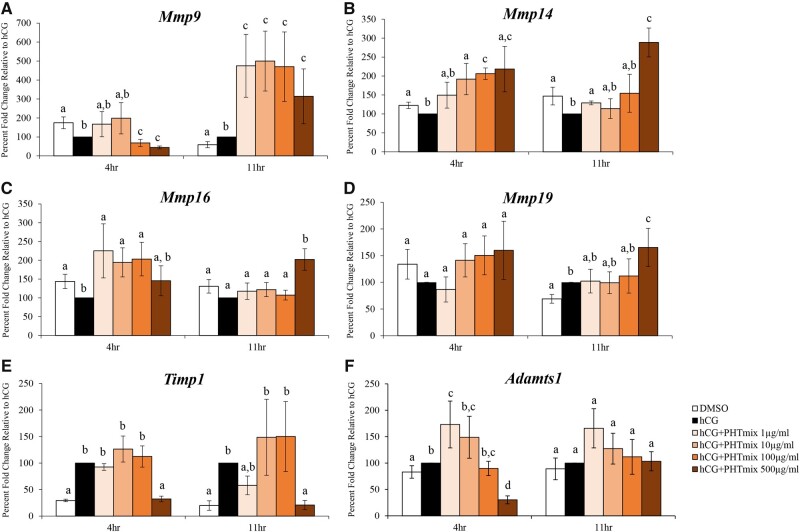
The success of ovulation requires remodeling of the ECM, which is conducted by the upregulation of several matrix metalloproteases (MMPs; Mmp9, Mmp14, Mmp16, Mmp19), ADAMTS (a disintegrin and metalloproteinase with thrombospondin-like motifs; Adamts1), and metalloproteinase inhibitors (TIMPs; Timp1) (Curry and Smith, 2006). Expression of Mmp9 was decreased at 4 h (100 and 500 μg/ml doses) and increased at 11 h (1–500μg/ml doses) by exposure to the PHTmix when compared to hCG alone (Figure 7A). The PHTmix increased expression of Mmp14 at 4 h (10, 100, and 500 μg/ml doses) and 11 h (1 and 500 μg/ml doses) when compared to hCG alone (Figure 7B). Expression of Mmp16 was increased by the PHTmix at 4 hr (1, 10, and 100 μg/ml doses) and 11 h (500 μg/ml) relative to hCG alone (Figure 7C). When treated with the PHTmix, expression of Mmp19 was unchanged at 4 h but increased at 11 h (500 μg/ml) when compared to hCG alone (Figure 7D). Expression of Timp1 was decreased by the PHTmix at 4 h (500 μg/ml) and 11 h (500 μg/ml) relative to hCG alone (Figure 7E). Exposure to the PHTmix both increased (1 μg/ml) and decreased (500 μg/ml) expression of Adamts1 at 4 h but did not change the expression of Adamts1 at 11 h when compared to hCG alone (Figure 7F).
Figure 7.
Effect of phthalate mixture exposure on ovulatory mediators involved in tissue remodeling. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Follicles were collected at multiple time-points (4 and 11 h) post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Mmp9 (A), Mmp14 (B), Mmp16 (C), Mmp19 (D), Timp1 (E), and Adamts1 (F). Data are presented as a percent fold change relative to the hCG alone control group. Graphs represent mean ± SEM from 4–10 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
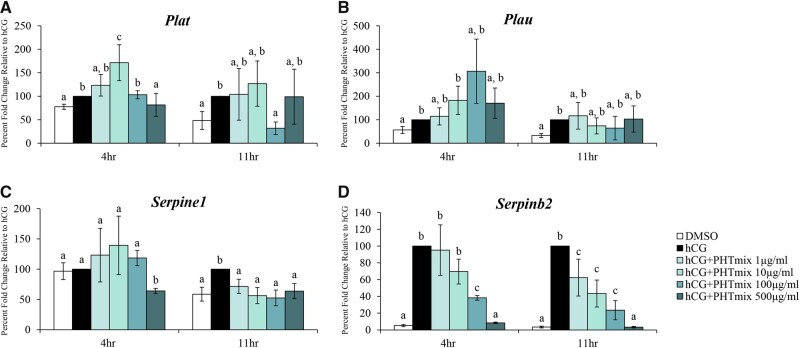
Similar to the genes above, the functioning PA system assists in ECM remodeling and follicle wall breakdown. This is carried out by the regulation of the enzyme plasmin via increases in tissue-type (Plat) and urokinase-type (Plau) plasminogen activators and their inhibitors (PA inhibitor type-1 [Serpine1] and PA inhibitor type-2 [Serpinb2]) (Liu, 2004). The PHTmix increased (10 μg/ml) and decreased (500 μg/ml) expression of Plat at 4 h, but decreased Plat at 11 h (100 μg/ml) relative to hCG alone (Figure 8A). Expression of Plau was unchanged by exposure to the PHTmix at 4 h and 11 h relative to hCG alone (Figure 8B). Expression of Serpine1 was decreased by the PHTmix at 4 h (500 μg/ml) and 11 h (1–500μg/ml doses) relative to hCG alone (Figure 8C). Exposure to the PHTmix decreased expression of Serpinb2 at 4 h (100 and 500 μg/ml doses) and 11 h (1–500μg/ml doses) relative to hCG alone (Figure 8D).
Figure 8.
Effect of phthalate mixture exposure on the PA system. Early antral follicles from adult CD-1 mice were cultured in FSH supplemented media and treated with DMSO (vehicle control) or phthalate mixture (PHTmix; 1–500µg/ml) for 96 h prior to hCG treatment. Follicles were then treated with or without hCG maturation media and with or without PHTmix. Follicles were collected at multiple time-points (4 and 11 h) post-hCG treatment and were subjected to qPCR to measure the mRNA levels of Plat (A), Plau (B), Serpine1 (C), and Serpinb2 (D). Data are presented as a percent fold change relative to the hCG alone control group. Graphs represent mean ± SEM from 3–10 experiments, with 10–12 follicles/treatment group in each experiment. Bars that do not share a letter designation are significantly different (p ≤ .05).
DISCUSSION
This study utilized an in vitro mouse antral follicle culture system to investigate the effects of an environmentally relevant phthalate mixture on ovulation and potential mechanisms by which ovulation is hindered. Our data demonstrated that this model is effective at mimicking ovulatory outcomes, as hCG treatment abundantly increased ovulation rates, P4 and PG levels, and several other ovulatory mediators when compared to DMSO. Our main findings suggest that ovulation rates are directly decreased by exposure to the PHTmix, which was potentially mediated by decreases in prostaglandin levels, alternations in progesterone/PGR signaling, and dysregulation of ECM remodeling.
The PHTmix directly decreased ovulation rates in a dose-dependent manner. We expect that these decreases were most likely due to the dramatic decreases observed in PGE2 and PGF2α levels when exposed to the PHTmix. Unlike other pathways explored in this study, each dose of the PHTmix decreased PG levels, which correlates with each dose causing decreased ovulation rates. These decreases in PG levels were most likely due to decreased PG synthesis (Ptgs2) and altered transport (Slco2a1), which mediates the influx and efflux of PGs. Further, the unanticipated decrease in the PG metabolic enzyme (Hpgd) is potentially compensatory due to the overall decrease in PG production. However, these compensatory decreases are not functional because we still observe decreased PG levels and inhibited ovulation. Our results are consistent with other studies, which found that exposure to different phthalates significantly reduced PGE2 (DEHP, DBP, BBP) and PGF2α (DEHP) release in human luteal cells (Romani et al., 2014).
In addition to decreased PG levels, we also observed decreases in other ovulatory mediators which include those in the P4/PGR signaling pathway. P4/PGR-induced increases in Edn2 and Il6 are needed for successful ovulation (Cacioppo et al., 2017; Kim et al., 2009); however, exposure to the PHTmix altered expression of these genes relative to hCG alone. Although Adamts1 aids in ECM remodeling, Adamts1 is also downstream of PGR. Further, disruptions in PGR signaling are also supported by the observed decrease in Adamts1 in this study. This dysregulation of PGR signaling is consistent with other studies where maternal exposure to DEHP down-regulated ovarian Pgr expression in mice (Pocar et al., 2012).
ECM remodeling is regulated by various proteinases including MMPs, TIMPS (MMP tissue inhibitors), ADAMTS, plasminogen activators (PAs), and PA inhibitors (PA-Is), all of which are required for oocyte release [28]. In the present study, ECM remodeling is potentially being dysregulated by the observed decreases in Adamts1, Timp1, Plat, Serpine1, and Serpinb2. Additionally, ECM remodeling is also potentially dysregulated by the increases in multiple MMPs at both 4 (Mmp14, Mmp16) and 11 h (Mmp9, Mmp14, Mmp16, Mmp19). It is possible that exposure to the PHTmix is causing compensatory increases in MMPs, or these MMP increases could be due to decreases in their inhibitors. Increases in Timp1 are needed to terminate MMP activity postovulation and to initiate follicle repair (Goldman and Shalev, 2004). Interestingly, we observed decreases in Timp1 (4 and 11 h) when exposed to the PHTmix compared to hCG. This could indicate that Timp1 is unable to terminate MMP activity because of PHTmix exposure.
Observed increases in the mRNA for MMPs measured in this study may coincide with previous research that suggests that frequent changes in MMP expression may contribute to increases in pro-apoptotic factors, resulting in atresia (Goldman and Shalev, 2004). This was supported by a study that observed increases of Mmp9 in normal sheep follicles undergoing atresia following hypophysectomy, as well as elevated MMP9 expression in PCOS patients (Goldman and Shalev, 2004). Thus, the increases we observed in MMP expression could also be inducing apoptosis in the follicles, resulting in atresia and decreased ovulation rates. Other studies investigating single phthalate exposure to mouse follicles have shown that both DEHP and DBP cause atresia via apoptosis (Craig et al., 2013; Hannon et al., 2015b). However, one study that investigated the effects of this PHTmix on mouse follicles suggests that the mix decreases apoptosis but causes oocyte fragmentation (Zhou and Flaws, 2017). It is currently unknown if this mix causes atresia during the periovulatory period, thus contributing to the ovulatory defects observed in this study. Future studies will investigate this possibility.
Because ovulation was inhibited, we observed decreases in classic luteal markers (Cdkn1a, Wnt4). Decreases in these markers suggest some impairment in luteal transformation and can possibly be explained by the dysregulation of ECM remodeling, which is supported by the observed decreases in Timp1, Serpine1, and Serpinb2 expression. Without increases in these MMP and PA inhibitors, the follicle cannot undergo proper CL formation. Based on our findings, however, we suspect that there is some level of luteal functionality because P4 is still produced at levels comparable to hCG, and even further increased at 18 h. Yet, ovulation still does not occur at hCG levels. In this sense, exposure to PHTmix may be causing luteinized unruptured follicular follicle syndrome (LUFS), which will be explored histologically in our future studies.
In addition to the potential compensatory increases in MMPs, we also observed further increases in P4 levels and P4 steroidogenic genes when follicles were treated with the PHTmix. These findings differ from other studies that have investigated the effects of a phthalate mixture on cultured mouse antral follicles, where the PHTmix did not alter P4 levels, but reduced expression of Star, Cyp11a1, and Hsd3b1 relative to controls (Zhou and Flaws, 2017). Whereas we observed increases in P4 levels, Star, and Hsd3b1 when treated with varying doses of hCG+PHTmix. These conflicting results could be due to the different exposure paradigms. Our study investigated ovulatory steroidogenesis following hCG treatment, while the previous study focused on follicular steroidogenesis following FSH treatment.
With the knowledge that several of these key ovulatory mediators are altered by PHTmix exposure, future studies will provide a more in-depth analysis of the impact of phthalates on each of these pathways. Specifically, these studies will also measure protein and enzyme activity levels in order to circumvent the limitations of measuring gene expression. PGs, P4/PGR, and factors involved in ECM remodeling all contribute to ovulatory success by inducing several different biological processes, including oocyte meiosis resumption and maturation, cumulus-oocyte complex expansion, angiogenesis, granulosa cell differentiation, and direct follicle wall breakdown (Duffy et al., 2019). These future studies will also further investigate how the PHTmix inhibits these biological processes required for ovulation.
In conclusion, our results indicated that exposure to an environmentally relevant phthalate mixture directly decreases ovulation rates in cultured mouse antral follicles. The mechanism by which the PHTmix potentially decreased ovulation rates is via decreases in PG levels, downstream PGR ovulatory mediators, and factors that drive ECM remodeling. Further, the unanticipated increases in other ovulatory mediators likely lead to dysregulation of the entire PGR signaling network and the ECM remodeling/repair system, which is possibly inhibiting oocyte release and impairing luteinization. Such effects from the PHTmix are of concern because our study suggests that phthalate exposure can possibly contribute to ovulatory defects (a leading cause of infertility in women) and overall negatively impact female reproductive health.
DECLARATION OF CONFLICTING INTERESTS
The author/authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This publication was made possible by grants R00 ES028748 and P30 ES026529 from the National Institute of Environmental Health Sciences (NIEHS).
ACKNOWLEDGMENTS
The authors thank all members in the Dr. Patrick Hannon, Dr. Thomas Curry, and Dr. Misung Jo laboratories for technical assistance, and Dr. Jodi Flaws for information about the PHTmix.
REFERENCES
- Al-Alem L., Puttabyatappa M., Rosewell K., Brännström M., Akin J., Boldt J., Muse K., Curry T. E. Jr. (2015). Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology 156, 3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen C. Y., Ezcurra D. (2014). Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod. Biol. Endocrinol. 12, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. M., Dunning K. R., Robker R. L., Boerboom D., Pritchard M., Lane M., Russell D. L. (2010). Adamts1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 83, 549–557. [DOI] [PubMed] [Google Scholar]
- Cacioppo J. A., Lin P. P., Hannon P. R., McDougle D. R., Gal A., Ko C. (2017). Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci. Rep. 7, 817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Wilson K., Hannon P. R., Rosewell K. L., Brännström M., Akin J. W., Curry T. E. Jr.,, Jo M. (2017). Coordinated regulation among progesterone, prostaglandins, and egf-like factors in human ovulatory follicles. J. Clin. Endocrinol. Metab. 102, 1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig Z. R., Hannon P. R., Wang W., Ziv-Gal A., Flaws J. A. (2013). Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol. Reprod. 88, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry T. E. Jr.,, Smith M. F. (2006). Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin. Reprod. Med. 24, 228–241. [DOI] [PubMed] [Google Scholar]
- Du Y., Guo N., Wang Y., Teng X., Hua X., Deng T., Yao Y., Yuan X., Li Y. (2019). Follicular fluid concentrations of phthalate metabolites are associated with altered intrafollicular reproductive hormones in women undergoing in vitro fertilization. Fertil. Steril. 111, 953–961. [DOI] [PubMed] [Google Scholar]
- Du Y. Y., Fang Y. L., Wang Y. X., Zeng Q., Guo N., Zhao H., Li Y. F. (2016). Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod. Toxicol. 61, 142–150. [DOI] [PubMed] [Google Scholar]
- Duffy D. M., Ko C., Jo M., Brannstrom M., Curry T. E. (2019). Ovulation: parallels with inflammatory processes. Endocr. Rev. 40, 369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy D. M., Stouffer R. L. (2002). Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum. Reprod. 17, 2825–2831. [DOI] [PubMed] [Google Scholar]
- Fauser B. C., Laven J. S., Tarlatzis B. C., Moley K. H., Critchley H. O., Taylor R. N., Berga S. L., Mermelstein P. G., Devroey P., Gianaroli L., et al. (2011). Sex steroid hormones and reproductive disorders: Impact on women's health. Reprod. Sci. 18, 702–712. [DOI] [PubMed] [Google Scholar]
- Goldman S., Shalev E. (2004). Mmps and timps in ovarian physiology and pathophysiology. Front. Biosci. 9, 2474–2483. [DOI] [PubMed] [Google Scholar]
- Grindler N. M., Allsworth J. E., Macones G. A., Kannan K., Roehl K. A., Cooper A. R. (2015). Persistent organic pollutants and early menopause in U. S. Women. PLoS One 10, e0116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Flaws J. A. (2015. a). Mono(2-ethylhexyl) phthalate accelerates early folliculogenesis and inhibits steroidogenesis in cultured mouse whole ovaries and antral follicles. Biol. Reprod. 92, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Brannick K. E., Wang W., Gupta R. K., Flaws J. A. (2015. b). Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 284, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon P. R., Flaws J. A. (2015). The effects of phthalates on the ovary. Front. Endocrinol. (Lausanne) 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R., Gaskins A. J., Souter I., Smith K. W., Dodge L. E., Ehrlich S., Meeker J. D., Calafat A. M., Williams P. L.; for the EARTH Study Team (2016). Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the earth study. Environ. Health Perspect. 124, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns L. E., Cooper G. S., Galizia A., Meeker J. D. (2015). Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 85, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Bagchi I. C., Bagchi M. K. (2009). Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol. Hum. Reprod. 15, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotz S. P., Carson S. A., Tomey C., Buster J. E. (2012). Phthalates and bisphenol do not accumulate in human follicular fluid. J. Assist. Reprod. Genet. 29, 773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardsson G., Peng X. R., Liu K., Nordström L., Carmeliet P., Mulligan R., Collen D., Ny T. (1995). Ovulation efficiency is reduced in mice that lack plasminogen activator gene function: Functional redundancy among physiological plasminogen activators. Proc. Natl. Acad. Sci. U.S.A. 92, 12446–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H., Paria B. C., Das S. K., Dinchuk J. E., Langenbach R., Trzaskos J. M., Dey S. K. (1997). Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208. [DOI] [PubMed] [Google Scholar]
- Liu Y. X. (2004). Plasminogen activator/plasminogen activator inhibitors in ovarian physiology. Front. Biosci. 9, 3356–3373. [DOI] [PubMed] [Google Scholar]
- Liu Z., de Matos D. G., Fan H. Y., Shimada M., Palmer S., Richards J. S. (2009). Interleukin-6: An autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology 150, 3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon J. P., DeMayo F. J., Funk C. R., Mani S. K., Hughes A. R., Montgomery C. A. Jr., Shyamala G., Conneely O. M., O'Malley B. W. (1995). Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9, 2266–2278. [DOI] [PubMed] [Google Scholar]
- Machtinger R., Gaskins A. J., Racowsky C., Mansur A., Adir M., Baccarelli A. A., Calafat A. M., Hauser R. (2018). Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 111, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocar P., Fiandanese N., Secchi C., Berrini A., Fischer B., Schmidt J. S., Schaedlich K., Borromeo V. (2012). Exposure to di(2-ethyl-hexyl) phthalate (dehp) in utero and during lactation causes long-term pituitary-gonadal axis disruption in male and female mouse offspring. Endocrinology 153, 937–948. [DOI] [PubMed] [Google Scholar]
- Romani F., Tropea A., Scarinci E., Federico A., Dello Russo C., Lisi L., Catino S., Lanzone A., Apa R. (2014). Endocrine disruptors and human reproductive failure: the in vitro effect of phthalates on human luteal cells. Fertil. Steril. 102, 831–837. [DOI] [PubMed] [Google Scholar]
- Skory R. M., Xu Y., Shea L. D., Woodruff T. K. (2015). Engineering the ovarian cycle using in vitro follicle culture. Hum. Reprod. 30, 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Hagiwara A., Ogiwara K. (2018). Prostaglandins in teleost ovulation: A review of the roles with a view to comparison with prostaglandins in mammalian ovulation. Mol. Cell Endocrinol. 461, 236–247. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Espey L. L., Kawano T., Okamura H. (1991). Comparison of inhibitory actions of indomethacin and epostane on ovulation in rats. Am. J. Physiol. 260, E170–174. [DOI] [PubMed] [Google Scholar]
- Zhou C., Flaws J. A. (2017). Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol. Sci. 156, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]